Abstract
Objective
The objectives of this study are to compare the relative use of different postacute care settings in different countries and to compare three important outcomes as follows: total expenditure, total days of care in different care settings, and overall longevity over a 1‐year period following a hip fracture.
Data Sources
We used administrative data from hospitals, institutional and home‐based long‐term care (LTC), physician visits, and medications compiled by the International Collaborative on Costs, Outcomes, and Needs in Care (ICCONIC) from five countries as follows: Canada, France, Germany, the Netherlands, and Sweden.
Data Extraction Methods
Data were extracted from existing administrative data systems in each participating country.
Study Design
This is a retrospective cohort study of all individuals admitted to acute care for hip fracture. Descriptive comparisons were used to examine aggregate institutional and home‐based postacute care. Care trajectories were created to track sequential care settings after acute‐care discharge through institutional and community‐based care in three countries where detailed information allowed. Comparisons in patient characteristics, utilization, and costs were made across these trajectories and countries.
Principal Findings
Across five countries with complete LTC data, we found notable variations with Germany having the highest days of home‐based services with relatively low costs, while Sweden incurred the highest overall expenditures. Comparisons of trajectories found that France had the highest use of inpatient rehabilitation. Germany was most likely to discharge hip fracture patients to home. Over 365 days, France averaged the highest number of days in institution with 104, Canada followed at 94, and Germany had just 87 days of institutional care on average.
Conclusion
In this comparison of LTC services following a hip fracture, we found international differences in total use of institutional and noninstitutional care, longevity, and total expenditures. There exist opportunities to organize postacute care differently to maximize independence and mitigate costs.
Keywords: care trajectories, hip fracture, international comparison, long‐term care
What is known about this topic
International comparisons of long‐term care mostly rely on global comparisons of national expenditure.
Little comparative work has examined variations in the care settings used for long‐term care or associated expenditures across countries.
Using a patient vignette and tracing the trajectory of care settings provide a useful way to compare variations in treatment for similar patients across countries.
What this study adds
This study presents a novel approach to examining trajectories of care across several postacute care settings including inpatient rehabilitation, institutional, and home‐based long‐term care.
The care trajectories for patients vary across countries and are associated with notable differences in the number of days spent in institutional compared to home‐based care settings.
There continues to exist substantial gaps in nationally representative data to enable rigorous comparisons of long‐term care utilization and expenditure patterns.
1. INTRODUCTION
A tremendously under‐studied area in international comparisons is that of long‐term care (LTC). LTC comprises a wide range of services that can include (but is not limited to) physical and cognitive rehabilitation services, nursing care, and personal support services for bathing, dressing, or other activities of self‐care. These medical and nonmedical services can be delivered by a range of professional and nonprofessional caregivers in institutional, community, and home‐based care settings. Informal or unpaid caregivers including family and friends often participate in LTC provision. The extent to which LTC services are provided for through health insurance systems varies widely with many countries considering these services to be part of social care systems related to poverty and infirmity. 1 , 2 , 3 , 4 As populations continue to age, health care systems across high‐income countries will continue to experience rapid growth in health expenditures, especially related to services in the LTC setting. Therefore, it is important for policy makers to evaluate the value of the services they provide relative to other countries. One approach is by assessing the intensity of LTC services among frail older adults with functional limitations.
International comparisons of care and costs offer an opportunity to explore how different health systems result in better or worse value for care. Determining whether some countries are able to achieve equivalent gains with lower expenditures prompts examination regarding the structures of health delivery systems and considers whether there are approaches that might improve the overall efficiency of health services. Most existing international comparisons are either entirely aggregated (comparing health expenditures as a proportion of gross domestic product [GDP]) or focus on sector‐specific costs such as acute care. 3 , 5 , 6 While these generally provide some insights into health system performance, they do not allow for the identification of actionable opportunities that can improve total and allocative efficiency.
The use of “personas” as promoted by the National Academy of Medicine to compare countries' use of LTC provides an opportunity for an identifiable and actionable focus. 7 , 8 For the purpose of LTC comparisons, a condition such as hip fracture offers a strong opportunity. Hip fractures for individuals older than age 65 years are a marker of frailty and a strong indicator to identify individuals who need LTC. Hip fractures are highly likely to be comparably presented to acute‐care hospitals with robust and comparable diagnostic measurement across all high‐income countries. 9 , 10 Hip fractures are also very costly and can be associated with high levels of subsequent mortality. Incremental health system costs for a full year of treatment following a hip fracture has been estimated in Canada at $36,929 for women and $39,479 (CAD) for men alongside associated mortality rates of 22% and 33%, respectively. 11 Direct medical cost for hip fractures in the United States have been estimated to range from $34,509 to $54,054 (USD). 12 Hip fractures most often require acute surgical treatment followed by postacute rehabilitation care with an extended duration of recovery such that use of institutional and home‐based LTC is a viable alternative and potential substitute.
The International Collaborative on Costs, Outcomes and Needs in Care (ICCONIC) is a group of institutions and researchers who have come together to examine health system costs and outcomes internationally. 7 ICCONIC selected a number of “personas” as a way to compare how different health systems manage patients with similar care needs. In this article, we focus on the hip fracture persona, which is an emblematic condition experienced by frail older adults. 7 Comparing differences in LTC utilization and costs of frail older people can help to understand the needs and resource use of patients in an array of care settings.
To contrast LTC utilization and potential substitution between care settings, it is most useful to examine where people are discharged to at key transition points. Trajectories of care that follow patient transitions between care settings enable a comparison of outcomes according to different care pathways. 13 We identified only one study that provided a 12‐month observation of subsequent care settings following hip fracture from a single‐center study of 254 patients. 14 In this study, we report on the 1‐year trajectory for hip fracture patients following acute‐care discharge through to postacute care settings including inpatient rehabilitation, institutional LTC, and home‐based LTC with comprehensive data from three countries. The objectives of this study are to compare the relative use of different care settings in different countries and to compare three important outcomes as follows: total cost, total days of care in different care settings, and overall longevity over a 1‐year period following a hip fracture. We examine these outcomes across temporal transitions from acute through postacute care settings until the patient returns to the community.
2. METHODS
The overall design was a retrospective cohort study of all individuals admitted to acute care for hip fracture in five countries (Canada, France, Germany, the Netherlands, and Sweden) that were able to collect patient‐level data on postacute care settings including inpatient rehabilitation as well as institutional and home‐based LTC. We were able to track transitions for the first three countries only, due to differences in the ways in which data are collected and reported across countries.
2.1. Sample population
Our sample population was determined following the ICCONIC methodology for the hip fracture persona. 10 Individuals in each country were identified in 2016–2017 based on an acute‐care admission with a diagnosis of hip fracture (ICD10 codes S72.0, S72.1, and S72.2) with total or partial hip replacement or pinning procedures. ICD10 codes were used in all countries except in the Netherlands where national experts verified comparable diagnostic codes. 10 For the trajectory analysis, we made two further restrictions. First, we excluded those who died during the initial acute hospitalization. Second, we excluded those who were living in LTC institutions at the time of admission to acute care because nearly all individuals who live in institutional LTC prior to an acute‐care admission will be returned to that care setting.
2.2. Data sources
We used routinely available linked data sources in each country. The data have been compiled by the ICCONIC collaborative, which has worked for 2 years to develop comparable health services utilization and cost measures that can be ascribed to specific patients with specific conditions. The sources of data for the five countries included in this study are summarized in Supplementary Table 1. Data include utilization and expenditure data in different settings: (1) acute hospital care; (2) postacute inpatient rehabilitative care; (3) institutional LTC; (4) home‐based rehabilitation, nursing, and LTC; (5) primary care; (6) outpatient‐ambulatory specialty care; and (7) outpatient pharmaceuticals. Much of our analysis in this article focused on the second, third, and fourth categories.
2.3. Measurement of health care use
Each country used linked individual encounter data to create and provide aggregate data tables according to the categories required for summary analyses. We measured health care use in each category of utilization and expenditure from the date of admission to an acute hospital for an incident hip fracture and up to 365 days following this admission date. Institutional care categories were created for each of acute inpatient care, inpatient rehabilitation care, and institutional LTC. We created three categories of home‐based care as follows: home‐ and community‐based rehabilitation services (inclusive of at‐home and in‐clinic services when available), home‐based nursing services, and all other home‐based LTC services (personal support with activities of daily living, dressing, bathing, feeding, etc). Utilization was measured in service‐days irrespective of the number of different services of each type provided on a given day. Notably in all three countries, noninstitutional rehabilitation services could be provided within a patient's home or in a rehabilitation clinic though we refer to these generally as home‐based services.
2.4. Measurement of expenditures
Expenditure was measured within each country using the most appropriate cost per unit measurement (generally based on case mix systems for acute care; either episode or per‐diem based for inpatient rehabilitation; per‐diem based for institutional LTC; per‐service for home‐based rehabilitation; nursing and other home‐based LTC services; and per‐unit costs for physician, pharmacy, and other ancillary payments). These correspond to insurance expenditures for these services and do not include out‐of‐pocket spending by patients. In Canada, expenditures for services were based on well‐described methodology for determining costs from administrative data sources. 15 In France, the national average inpatient rehabilitation and LTC expenditures were calculated from the estimations of average national expenditures. In Germany, utilization and expenditure measures were derived from insurance claims. All expenditures were converted to 2017 USD equivalents using the Organization for Economic Co‐operation and Development's (OECD) Actual Individual Consumption Purchasing Power Parities (AIC PPPs) to individual country‐reported expenditure data. AIC PPPs, rather than GDP PPPs, are currently used by the OECD as the most reliable economy‐wide conversion rates for health expenditure. This makes the expenditures reported in this article directly comparable to the ICCONIC overall hip fracture cohort study. 10
2.5. Other measures
We also captured patient characteristics including age and sex, date of death (days alive), and the number of chronic conditions captured in administrative data in the 1 year prior to hip fracture using Elixhauser definitions. 16
2.6. Analyses
First, we sought to compare overall institutional and home‐based LTC utilization and expenditure within 1 year after hip fracture across the five countries for which comparable aggregate data were available. We calculated the total number of institutional days other than for acute hospitalization as the sum of inpatient rehabilitation and institutional LTC days. We calculated total postacute home‐based care days as the sum of postacute rehabilitation, nursing, and LTC services provided at home and in community settings. We also calculated total expenditure for each of these two categories. Finally, we created a summary of the two sources of expenditure. For each country, we plotted the total annual average institutional expenditure against the total home‐based expenditure and reported the total annual average expenditures across both categories of care.
Second, we compared patient pathways by creating trajectories of care (Supplementary Figure 1). Because this required sequential alignment of individual encounter data on a daily basis, we were only able to undertake this analysis with the following three countries: Canada, France, and Germany. In each case, we sequentially aligned each postacute care service following discharge from the initial acute‐care hospitalization for hip fracture. We created a hierarchy to manage rapid transitions between care settings in the first 30 days after discharge (e.g., if someone was discharged to home for 3 days before admission to inpatient rehabilitation care, we allocated the patient to the latter care setting). We set the following prioritization rules: (1) inpatient rehabilitation care, (2) institutional LTC, (3) home‐based rehabilitation, (4) home‐based nursing, and (5) other home‐based LTC or no care within 30 days. We searched to find the first of these care settings that occurred within 30 days of postacute discharge. If there was no such utilization with 30 days of acute discharge, then we made the fifth category (other home‐based care) as the absorbing category to limit the number of possible destinations. This also acknowledges that most individuals will require some personal support after a hip fracture regardless of whether this is provided by formal paid services (as recorded here), by informal caregivers, or through other public programs or privately paid supportive services, all of which are common across all three countries.
For individuals admitted to inpatient rehabilitation or to institutional LTC as a first postacute destination, we followed them to their second postacute destination (using the same five categories) that occurred within 30 days of discharge from the initial postacute care setting. We based this prioritization and the 30‐day decision criteria on prior work to create episodes of care for high‐cost users. 17 We also identified whether individuals were never discharged from inpatient rehabilitation or died in that care setting, whether individuals were never discharged from institutional LTC or died in that care setting, and whether individuals discharged to each of the home‐based service trajectories died within 365 days of the initial hip fracture. In total, there were 18 possible trajectories as depicted in Supplementary Figure 1.
We report summary comparisons across the three countries with complete trajectory capabilities including the proportions of patients discharged to each of the trajectories, the total annual comprehensive expenditures according to each trajectory (inclusive of all expenditure categories). Finally, we examine the number of days that patients spent in each care setting.
3. RESULTS
Initially, we looked at total days of care over the 365‐day period after discharge from the index hip fracture hospitalization contrasting postacute institutional care (inpatient rehabilitation and institutional LTC) with home‐based care (including days with rehabilitation, nursing, or LTC services). In Figure 1, we plotted each country on a graph with the average total postacute institutional expenditure against the total noninstitutional (home‐based) expenditure for each country and report (by size of bubble) the total average annual postacute expenditure per person (not including outpatient physician and pharmaceutical) for each country. In this summary, we include nonphysician medical (e.g., nursing), rehabilitation, and personal support services as home‐based service days. (Exact utilization and expenditures are reported in Supplementary Table 2.)
FIGURE 1.
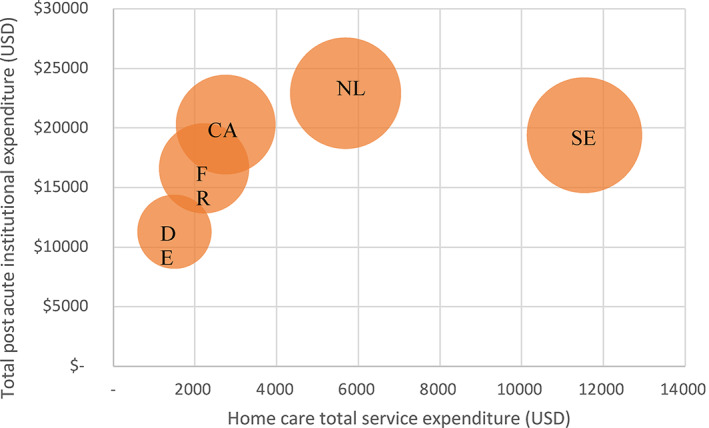
Average postacute home care, institutional, and total expenditure within 365 days for hip fracture patients aged 65 years and older in 2016/2017. CA, Canada; DE, Germany; FR, France; NL, the Netherlands; SE, Sweden. Size of bubbles reflects total average postacute annual expenditure [Color figure can be viewed at wileyonlinelibrary.com]
Germany reported the lowest average total expenditure for both home‐based and postacute institutional care. Sweden reported similar institutional postacute expenditures as Canada and France, but expenditures for services in home‐based settings were a little more than four times greater. Overall spending for both institutional and home‐based service expenditures combined was fairly similar for Sweden and the Netherlands at approximately $30,000 USD in the year following hip fracture. These were followed by Canada, then, the Netherlands, and finally, Germany that reported total expenditures of less than half that of the highest spending countries (the Netherlands and Sweden). Considering the underlying expenditure and utilization data (Supplementary Table 2), the Netherlands appears to have the highest home‐based unit costs, while Germany has the lowest. Canada and France have relatively similar unit costs for both institutional and home‐based services.
3.1. Analysis of trajectories
Table 1 provides detailed summary of the characteristics of populations in each of the three countries for which we have data, across the 18 trajectories. A few key observations can be drawn from this table. First is that in all countries, the top trajectories are to inpatient rehabilitation and then home with rehabilitation followed by direct discharge from acute to home with rehabilitation. In Canada, these trajectories capture 64% and 20% of the population, respectively; in France, it is 78% and 13%, while in Germany, these trajectories capture 47% and 25%, respectively, indicating a much higher likelihood of being discharge to community in Germany. It is rare, but only Canada has any individuals who were admitted to inpatient rehabilitation and stayed there for the entire year. The average age patterns are similar across all countries with a few notable patterns. The most striking is that the youngest individuals in all countries are discharged to home and survive the full year whilst the oldest individuals are discharged to institutional LTC and die in that setting. In Canada, it seems notable that fewer females are discharged to inpatient rehabilitation and more are discharged to institutional LTC, in spite of the fact that those discharged to LTC appear to be younger than in the other countries. The number of comorbidities is systematically higher in Germany. It is more likely that this is related to coding practices and a stronger relationship between diagnostic coding and remuneration than to true differences in the health status of older German adults. In general, the number of days alive has a nonsystematic variation across trajectories except that those who died obviously have fewer days alive than other categories. Because the total number of days alive is lowest and mortality within 365 days is highest in Canada in the most populous trajectory, this weighs on the overall longevity of Canadian hip fracture patients. Generally, total annual expenditure for all care (inclusive of all seven categories of utilization) is highest in Canada, followed by Germany and then France. The resultant expenditure per day alive is therefore much higher in Canada in all trajectories. France has generally higher expenditures than Germany for postacute trajectories beginning with inpatient rehabilitation. In other trajectories, Germany generally has higher expenditures compared with France.
TABLE 1.
Population descriptive statistics for Canada, France, and Germany by care trajectory
| Inpatient rehabilitation trajectory | LTC trajectory | Home rehab trajectory | Home nursing trajectory | Home trajectory | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathway | To LTC | To home rehab | To home nursing | To home | Died a | No discharge | To IR | To home rehab | To home nursing | To home | *Died | No dis‐charge | H‐Rehab + died | H‐Rehab | H‐Nursing + died | H‐Nursing | Home + died | Home | |
| Population: N (row %) | |||||||||||||||||||
| CA | N = 8162 | 5% | 40% | 1% | 14% | 2% | 0% | 0% | 1% | 0% | 3% | 3% | 2% | 3% | 17% | 0% | 1% | 2% | 5% |
| FR | N = 31,650 | 11% | 35% | 10% | 21% | 1% | 0% | 0% | 0% | 0% | 0% | 0% | 1% | 1% | 12% | 0% | 3% | 1% | 3% |
| DE | N = 10,368 | 3% | 26% | 4% | 14% | 0% | 0% | 0% | 2% | 0% | 3% | 2% | 0% | 4% | 21% | 2% | 4% | 4% | 11% |
| Age: mean | |||||||||||||||||||
| CA | 85.9 | 83.4 | 82.5 | 82.2 | 87.7 | 84.9 | 82.3 | 84.2 | 81.9 | 85.6 | 87.3 | 85.2 | 84.3 | 79.4 | 84.8 | 79.5 | 84.6 | 78.8 | |
| FR | 7.1 | 7.5 | 8.2 | 8.1 | 7.5 | 8.5 | 10.1 | 7.5 | 9.3 | 7.4 | 6.4 | 7.1 | 8.2 | 8.3 | 7.5 | 9.3 | 8.2 | 8.8 | |
| DE | 87.3 | 83.2 | 83.4 | 83.8 | 87.2 | 0.0 | 85.8 | 87.4 | 83.3 | 87.9 | 88.0 | 57.1 | 85.0 | 78.3 | 82.6 | 76.5 | 84.8 | 83.8 | |
| Sex (% female) | |||||||||||||||||||
| CA | 76% | 73% | 61% | 67% | 57% | 63% | 93% | 75% | n.r. | 75% | 74% | 74% | 70% | 73% | 56% | 79% | 51% | 65% | |
| FR | 80% | 79% | 78% | 73% | 61% | n.a. | 80% | 87% | 75% | 69% | 73% | 82% | 68% | 73% | 61% | 70% | 62% | 78% | |
| DE | 81% | 80% | 80% | 75% | 62% | n.a. | 78% | 81% | 85% | 82% | 68% | 88% | 66% | 76% | 67% | 79% | 69% | 73% | |
| # Comorbidities: mean | |||||||||||||||||||
| CA | 2.6 | 2.7 | 3.2 | 2.7 | 3.4 | 2.9 | 2.8 | 2.5 | 4.0 | 2.7 | 2.4 | 2.3 | 3.3 | 2.2 | 3.7 | 2.2 | 3.5 | 1.9 | |
| FR | 1.8 | 2.0 | 2.1 | 2.1 | 2.4 | 1.9 | 2.1 | 1.7 | 2.0 | 2.1 | 2.1 | 1.8 | 2.1 | 1.9 | 1.9 | 1.9 | 2.6 | 1.6 | |
| DE | 2.6 | 2.4 | 2.7 | 3.1 | 2.4 | 0.0 | 5.4 | 2.1 | 1.8 | 3.3 | 3.3 | 2.4 | 3.6 | 1.7 | 4.0 | 1.5 | 3.7 | 2.3 | |
| Total days in institutional care: mean | |||||||||||||||||||
| CA | 230 | 47 | 43 | 76 | 72 | 328 | 123 | 95 | 173 | 184 | 210 | 305 | 13 | 16 | 7 | 7 | 25 | 60 | |
| FR | 121 | 55 | 36 | 94 | 108 | n.a. | 87 | 67 | 146 | 132 | 141 | 84 | 40 | 59 | 30 | 25 | 73 | 117 | |
| DE | 280 | 60 | 70 | 88 | 31 | n.a. | 110 | 83 | 109 | 163 | 52 | 358 | 19 | 13 | 17 | 8 | 15 | 43 | |
| Total days alive: mean | |||||||||||||||||||
| CA | 338 | 348 | 331 | 325 | 90 | 365 | 347 | 354 | 282 | 304 | 240 | 365 | 162 | 365 | 112 | 365 | 100 | 365 | |
| FR | 69 | 59 | 85 | 96 | 106 | n.a. | 56 | 50 | 121 | 106 | 139 | 0 | 98 | 0 | 83 | 0 | 111 | 0 | |
| DE | 334 | 354 | 347 | 300 | 46 | n.a. | 303 | 352 | 365 | 278 | 156 | 365 | 192 | 365 | 154 | 365 | 102 | 365 | |
| 365‐day Mortality: column % | |||||||||||||||||||
| CA | 16% | 10% | 16% | 18% | 100% | 0% | n.r. | 7% | n.r. | 31% | 100% | 0% | 100% | 0% | 100% | 0% | 100% | 0% | |
| FR | 14% | 7% | 10% | 19% | 100% | n.a. | 40% | 9% | 0% | 34% | 100% | 0% | 100% | 0% | 100% | 0% | 100% | 0% | |
| DE | 26% | 6% | 16% | 14% | 100% | n.a. | 11% | 11% | 5% | 20% | 100% | 0% | 100% | 0% | 100% | 0% | 100% | 0% | |
| Total annual expenditure/day alive | |||||||||||||||||||
| CA | $236 | $154 | $191 | $208 | $654 | $457 | $181 | $135 | $225 | $207 | $229 | $227 | $295 | $86 | $404 | $101 | $489 | $136 | |
| FR | $152 | $105 | $116 | $145 | $436 | n.a. | $168 | $80 | $79 | $105 | $146 | $78 | $160 | $60 | $196 | $56 | $240 | $70 | |
| DE | $153 | $86 | $136 | $103 | $555 | n.a. | $160 | $147 | $141 | $140 | $301 | $137 | $245 | $106 | $298 | $134 | $294 | $92 | |
Abbreviations: CA, Canada; DE, Germany; FR, France; H, home with; IR, inpatient rehabilitation; LTC, long‐term care; n.a. not applicable; n.r. not reportable due to small cells.
Died within inpatient rehabilitation, and LTC trajectories indicates died before next transition.
Graphical summaries of the primary and secondary discharge trajectories are shown in Figure 2. We first examined the first postacute destination and subsequently examined the next destination for those that were admitted to inpatient rehabilitation or institutional LTC. From the top‐most section of Figure 2 summarizing postacute discharge destinations, in Canada, the dominant postacute discharge destination was to inpatient rehabilitation, while Germany had the largest proportion of hip fracture patients discharged to home including a total of 15% of all patients with no record of any postacute nursing or rehabilitation care within 30 days of discharge from acute care. Compared to Canada and Germany, fewer patients in France were discharged directly to institutional LTC (2% in France contrasted with 8% and 6% for Canada and Germany), but relatively more patients in France are discharged to LTC after treatment in an inpatient rehabilitation facility.
FIGURE 2.
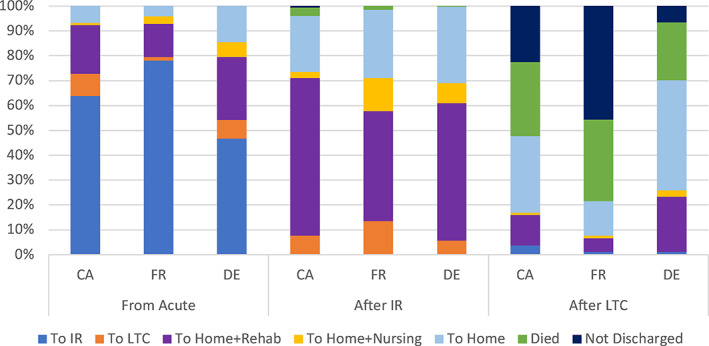
Discharge destinations by source institution. CA, Canada; DE, Germany; FR, France; IR, inpatient rehabilitation; LTC, long‐term care [Color figure can be viewed at wileyonlinelibrary.com]
For those admitted first to inpatient rehabilitation, there was greater similarity with continued rehabilitation in the community after discharge being the dominant subsequent care setting followed by home without rehabilitation or nursing services. France had slightly more individuals whose next care setting after inpatient rehabilitation was in an LTC institution but still only marginally above 10%. Finally, we examined the trajectory for individuals who were discharged from acute care to institutional LTC settings. In France, we observe that many of these individuals (46%) continued to live in these facilities throughout the year, while about 33% died within the year. In Canada, there was a relatively equal distribution among those discharged from institutional LTC to home without care, those who remained in institutional LTC throughout the remainder of the year, and slightly fewer that died in these institutions. In Germany, the largest proportion of this cohort were discharged from institutional LTC back to home where 22% received further rehabilitation supports and 44% received no further professional care services (within 30 days). Still 23% of the German population who were transferred to institutional LTC died in these institutions, a proportion similar to that of France.
We next examined the total annual expenditure (by public payers) inclusive of all care beginning from the index hip fracture acute‐care admission through all subsequent care settings according to the trajectories described above. Unlike in Figure 1 where we examined expenditures only for postacute supports for rehabilitation and LTC (across five countries with comparable data), here we include the entirety of all expenditures inclusive of all care settings including acute, physician, and other expenditure categories. This allows us to consider offsets in other care settings and provides a comprehensive expenditure view. Total expenditure by trajectory in Figure 3 correspond to the trajectories shown in Figure 2 and adds the results for those who were discharged directly home from the acute‐care setting. In Figure 3, we observe that in all countries, the highest expenditure was incurred for those who were admitted to inpatient acute care and subsequently discharged to institutional LTC. This could be expected given the number of individuals who resided in LTC institutions for the remainder of the year. There are other notable patterns. Those that were discharged from inpatient rehabilitation to home with further rehabilitation care had relatively lower total expenditure than other home‐based care trajectories. There were relatively few individuals who died before discharge from inpatient rehabilitation, and we do not place credible assertions on the expenditure results for these individuals. Overall, Canada had the highest annual expenditures, while France was marginally higher than Germany except for those discharged home with nursing care. Overall, there is some consistency in the relative expenditures according to trajectories across all countries.
FIGURE 3.
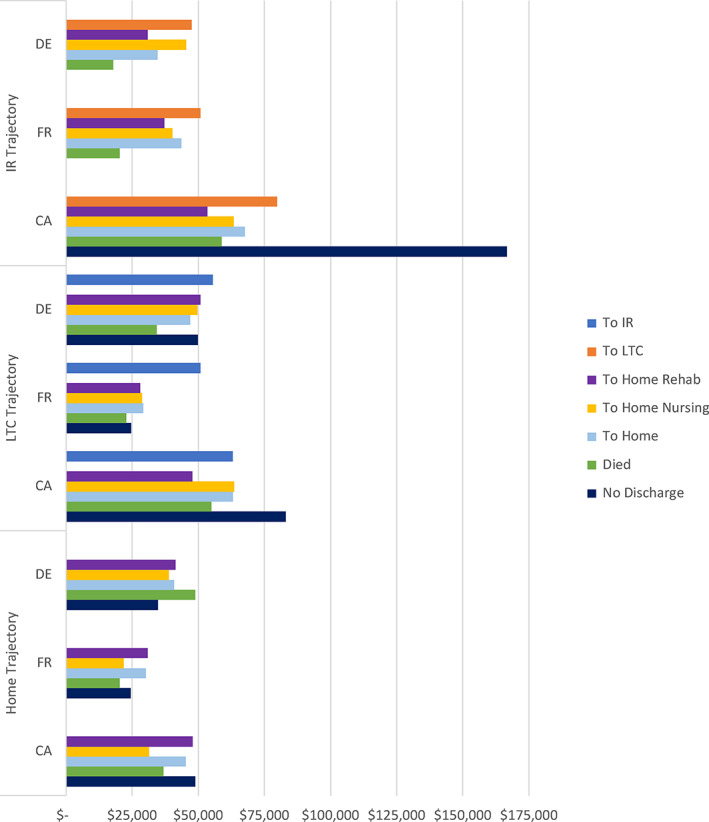
Average total expenditure within 365 days by trajectory. CA, Canada; DE, Germany; FR, France; IR, inpatient rehabilitation; LTC, long‐term care. Currency in 2017 USD [Color figure can be viewed at wileyonlinelibrary.com]
The total annual expenditures for those who were discharged from their initial hip fracture surgery to institutional LTC had relatively little variation across trajectories in France and Germany. Although the expenditure for those discharged from institutional LTC to inpatient rehabilitation in France was markedly higher, this represented very few individuals and is generally an unusual care trajectory. Overall, Canada generally had higher total expenditures with Germany second and France incurring the lowest average total expenditures per individual.
Our final analysis of expenditures compared those who were discharged directly from acute to home with either in‐home rehabilitation services, in‐home nursing services, or neither. We stratify these results by whether the individual died in the 365 days to disaggregate those who may have had incremental expenditures associated with the end of life. Here, we observe very similar patterns in Canada and France. For those discharged with rehabilitation or nursing care, total expenditures were higher for those who died compared with those who did not. Among those discharged home from acute care with no rehabilitation or nursing care (within 30 days) of acute discharge, average total annual expenditures were relatively similar. Germany generally had more uniform expenditures across trajectories, and, for those individuals who received rehabilitation or nursing services and survived the entire year, the expenditures were uniquely higher than those of Canada (the only observation where Canada's expenditures were not highest). Expenditures in Canada were still higher for those who died and for those who did not receive home‐based rehabilitation or nursing services.
We end our graphical summaries by examining which services individuals received in the full year following a hip fracture across all major trajectories and countries. We simplify this analysis by collapsing those discharged to any home‐based setting and by collapsing all home‐based services including rehabilitation, nursing, and LTC services. In Figure 4, we observe that the care types and locations are relatively similar for individuals who were discharged from acute care to inpatient rehabilitation facilities. After acute and inpatient rehabilitation hospital stays, combining to last approximately 75 days (slightly less in Germany), individuals spend another 120 days receiving rehabilitation and/or nursing care at home (closer to 90 days in France). Individuals then have about another 110–140 days (3–4 months) at home with only personal support (or no) services. On average, individuals have died after 11 months in Canada and France and after 11½ months in Germany. There is more variation for other trajectory patterns. In the institutional LTC trajectory, Canada and Germany have similar acute hospital days. But then, Germany has slightly more institutional LTC days and substantially more days with home‐based services, while more Canadians die in LTC. Germany has the lowest mortality rate in this trajectory. People in France spend less time in acute care, more time in institutional LTC, receive less home‐based services, and have slightly lower survival than in Canada. In the acute to home trajectory, Germany has more acute days and more days of care at home in comparison with Canada and France which are similar, notably in the lower levels of any form of home‐care service. Over the course of the full year and across all trajectories, France averaged the highest number of days in institution with 104, Canada followed at 94, and Germany had just 87 days of institutional care on average.
FIGURE 4.
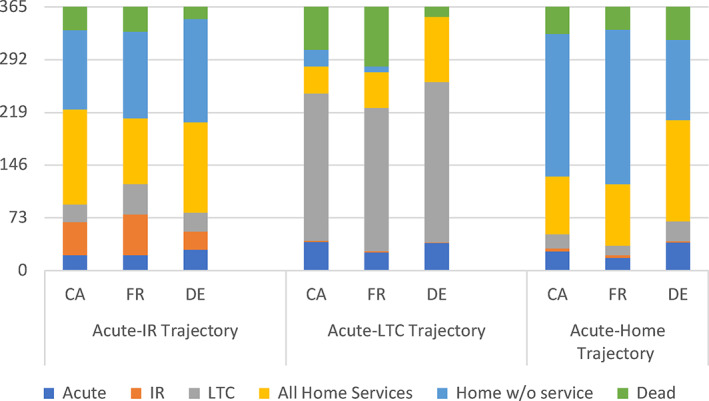
Total days in each care setting by care trajectory over 365 days. CA, Canada; DE, Germany; FR, France; IR, inpatient rehabilitation; LTC, long‐term care [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This study provides a unique perspective on the care management of older adults who experiences a hip fracture and how their care trajectories varied across different health care systems, all with universal health insurance. Among a group of five high‐income countries, we find that Germany had the lowest aggregate associated expenditures, which was achieved by relatively low levels of institutional postacute care and high levels of home‐based care. Sweden, the Netherlands, and Canada all have comparably high total postacute expenditures but with different allocations. Canada has relatively high institutional expenditure and low expenditures for home‐based services. France is closest to Germany in terms of total expenditures.
This study also provides unique information on the comparative trajectories of care following acute treatment for a hip fracture. There are both differences in the prevalence of different trajectories and in associated expenditures across countries. When individuals are admitted to inpatient rehabilitation care following a hip fracture, the overall tendency is to have relatively similar trajectories of care across all countries. Most patients are discharged to home with further rehabilitation or other nursing care and most survive for at least 365 days after their hip fracture. However, the preponderance of this trajectory is not uniform across countries.
While Canada and France made the highest use of inpatient rehabilitation, Germany favored home‐based rehabilitation care. In other analyses from the ICCONIC collaborative on the acute stage of care for hip fracture patients, Germany was shown to have the longest inpatient acute stays, which may also be associated with the increased likelihood to discharge home. 10 When patients in Germany were admitted to institutional LTC, they were most likely to be discharged to home. While in France, institutional LTC becomes the permanent residence for older patients who rarely return home after being admitted to institutional LTC. The number of days in institutional LTC and in death was fairly similar in France and Canada.
One other finding that was particularly troubling was the tendency shown in Table 1 that women (often younger) had a higher overall prevalence among those discharged to institutional LTC as compared to institutional rehabilitation. Given the higher mortality and lower services levels available in institutional LTC, it is worrisome that there may be a bias against rehabilitative care for women. This may relate to differential supports available to women in the community after inpatient rehabilitation as the vast majority of those treated in inpatient rehabilitation are then transferred to home in all countries. Women who generally survive longer than men may no longer have supports at home as compared to men who may still have spouses to support their return to home.
Generally, across all trajectories, total expenditures were highest in Canada and lowest in France. This was true both for the detailed hip fracture trajectory analysis and the aggregate LTC summary expenditures presented in Table 1 and Figure 1. The latter results indicate that these expenditures arise from differences in sector‐specific prices and overall utilization. Particularly among individuals who were discharged home, costs within Canada were markedly higher among individuals who died. Such differences were less notable in France and Germany. End‐of‐life expenditures among hip fracture patients are explored in more detail in an associated ICCONIC study. 18
This study has provided a novel extension to other international comparisons of hip fracture patients. International standards through OECD‐led harmonization efforts have provided substantial comparability in acute‐care settings. On the other hand, because the positionality and coverage of LTC (within or outside of health and insurance systems) vary across countries, similar standards have not been widely accepted. In the present study, this meant that only 5 of 11 countries involved in the ICCONIC study were able to provide any data on LTC expenditures and only three on specific utilization. Tracing trajectories of care requires accurate dates of admission and discharge, which may not be required for billing purposes. In the Netherlands for example, monthly claims are provided without reference to admission and discharge dates. In Sweden, such data should be available, but as these data were not originally requested for the initial analysis, the approval process for accessing the data is relatively long and data were not available in time for this study. Ontario has very accurate information because acute, LTC, and home care are funded from the same source, and Ontario requires daily service provision reporting for payment. The specifics of the payment systems (per diem for LTC and per visit for home care) are also contributing factors to accurate data. On the other hand, community‐based outpatient clinic visits for physiotherapy rehabilitation care are insured services and systematically tracked in Germany and France, while such visits are largely paid for privately in Canada and therefore not part of the data reported here. In France, care utilization (visits and days in facility) is well tracked in integrated health data system, but spending in rehabilitation facilities was difficult to construct as these are mostly paid by global budgets. It is hoped that the novel creation and reporting of trajectories of care as developed here is the starting point for greater standardization and analyses to address a myriad of questions relating to the overall quality and efficiency of continuing care services internationally.
The value of international comparisons such as those represented here highlight the value and also data requirements. First, we have learned that Germany is able to discharge a larger proportion of patients to home earlier and at lower cost and achieves equal or lesser mortality. Whether this is true of other health insurance systems in Germany would require having pooled data across insurers. Having a single payer as in Ontario (and Sweden) enables a population‐based approach, while acknowledging that Canada's data systems are provincial and the federalist system must overcome regional isolationist policies to enable national data representation. This has largely been overcome in France where data from the national health insurance fund, which acts as a single payer, represents 12/18 regions (capturing two thirds of the population). Overall, across many countries, data linkage between acute and LTC is challenged by having different payers. Appreciating the common good of pooled data for health system management and creating the capability through legislation is thought to be the most promising opportunity for all interested countries.
The OECD reports on total expenditure patterns in LTC and identified the rank order of LTC expenditures as a proportion of GDP in 2019 for these five countries as the Netherlands (2.9%), Sweden (2.9%), France (1.8%), Germany (2.2%), and Canada (2.0%). 19 The notable difference in this study is that Canada had higher expenditures than Germany or France in LTC for hip fracture patients. This relates to a primacy of prioritizing home‐care services for those with acute as compared to long‐term conditions in Canada. In detailed comparisons for the hip fracture persona, Canada's expenditures per case and per‐day alive is notably higher than that of Germany and France, reflecting somewhat intensity of services but also higher prices per unit service considering that Canada's days of care were commonly lower than that of Germany and France. Complete data were not available for the Netherlands and Sweden in this comparison. We have included additional contextual data on insurance and copayments from each of the countries in Supplementary Table 3.
This study cannot assert equivalency of service quality across LTC settings. There is in fact no directly comparable information about quality for hip fracture patients in postacute settings. The accompanying article on outcomes demonstrates some variability in inpatient and postacute mortality across countries, 10 but the variation cannot be clearly attributable to acute or postacute care. The OECD does report on the proportion of hip fracture patients who receive surgery within 48 h, which is documented as 91%, 96%, and 93% in Germany, the Netherlands, and Sweden, respectively. 20 While the OECD does not report data for Canada or France, studies within those countries indicate equivalent rates of 90% in Ontario 21 and single‐site variation in France ranging from 41% within 48 h 22 to 90% within 22 h. 23 Much more work should be done to be able to estimate measures such as functional status on admission and discharge from postacute care settings. International tools such as the Functional Independence Measure or interRAI‐based Activities of Daily Living indices would be suitable candidates.
5. LIMITATIONS
This study was developed as part of the ICCONIC collaboration. In this work, great efforts were made to align and systematically specify comparable data sources for utilization and expenditure and to create directly comparable data across all countries. In the area of long‐term institutional and home‐based care, this was especially problematic. Of the 11 countries in the collaborative, only five could provide comparable aggregate data and only three could provide comparable patient‐level day‐by‐day measurement of the care locations and utilization of institutional and home‐based LTC services. In all three countries, we have only included the expenditures of the national or regional health insurers (France and Germany) or regional government (Canada). While the sources of data represent approximately 67%, 40%, and 30%, respectively, the samples are considered to be reasonable representations of national care standards. 7 Many home‐based LTC services are provided outside of insurance systems through local municipalities in France (as well as Sweden and the Netherlands), meaning that we are still missing potentially substantive expenditure and utilization of nonprofessional care. This study also leaves out important patient co‐payments, which are ubiquitous in all countries for lodging costs and may vary considerably between and within countries. We also do not measure the considerable economic contribution of informal caregivers in the home in any of these countries. There may be vast differences and considerable variation in the cost of informal care. There are also other sources of social support that can be provided, particularly for home‐based LTC services. In France, there is considerable local social care support funded by local authorities. In Canada, municipalities provide additional funding for select LTC institutions. The exact magnitude of these differences is unclear in the case of the hip fracture patient. OECD estimates indicate that the value of social care amounts to nearly 0.6% of GDP overall in France and 0.02% in Germany. 19 , 24 In addition, we do not have accurate information about supply levels and restrictions, which also vary within and across regions and may impact on the availability of services according to setting (inpatient rehabilitation, LTC, or home care).
In spite of these limitations, we have demonstrated important similarities and differences across countries that were able to quantify at an individual level the amount of care provided through a range of postacute care setting with a particular emphasis on LTC services. These costs can accumulate and represent considerable investments on the part of health systems, particularly for individuals with complex health needs such as for hip fractures. Improving the data capture and comparability of LTC across countries would serve to enhance policy makers' ability to assess the opportunities for system improvements internationally. A key recommendation from this work is to build on the work of ICCONIC by developing a classification system to describe different intensities and locations of residential health care in different countries. This would be a really important step to make meaningful comparisons across a wider range of countries.
6. CONCLUSION
We provide a detailed comparison of postacute expenditures in inpatient rehabilitation, institutional LTC, home‐based rehabilitation, nursing care, and LTC for a population of hip fracture patients using representative data from Canada, France, and Germany. We find both similarities and differences across countries. When individuals are discharged from acute care to inpatient rehabilitation, the overall trajectories and outcomes are relatively similar across countries. However, there is considerable variation in the use of institutional LTC as well as home‐based rehabilitation, nursing, and other LTC services at home. The specific sequential care settings also vary across countries as well as overall mortality differences, total days alive, and the number of days in an institution. The moderate expenditure levels, fewer days in an institution, and apparently higher survival in Germany suggest that they may have a more efficient health system for patients who experience a hip fracture relative to the other countries.
Supporting information
Supplementary Table 1 Country dataset information
Supplementary Table 2. Comparison of international utilization and expenditure per person in postacute long‐term care settings (2017 USD)
Supplementary Table 3. System characteristics19,24
Supplementary Figure 1. Trajectories
ACKNOWLEDGMENTS
This work was partially funded by The Commonwealth Fund and the Health Foundation. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Canadian data management was supported in part by a grant from the Ontario Ministry of Health to the Health System Performance Network and by a grant to ICES. Walter P Wodchis is supported by a Research Chair funded by the Trillium Health Partners Foundation. We like to thank BARMER for providing access to the researcher data warehouse for the German data of this project, especially Joachim Saam and Martial Mboulla‐Nzomo who provided data analysis. This research in Sweden acknowledges cooperation on data collection by the Socialstyrelsen in Sweden and especially Lena Jörgensen for computations on the Swedish data. We would like to acknowledge all members of the ICCONIC collaborative and the international Advisory Board for their contributions to this project.
Wodchis WP, Or Z, Blankart CR, et al. An international comparison of long‐term care trajectories and spending following hip fracture. Health Serv Res. 2021;56(Suppl. 3):1383‐1393. doi: 10.1111/1475-6773.13864
Funding information Commonwealth Fund; Ontario Ministry of Health; The Health Foundation
REFERENCES
- 1. French E, McCauley J, Aragon M, et al. End‐of‐life medical spending in last twelve months of life is lower than previously reported. Health Aff. 2017;36(7):1211‐1217. [DOI] [PubMed] [Google Scholar]
- 2. Siegel E, Backman A, Cai Y, et al. Understanding contextual differences in residential LTC provision for cross‐national research: identifying internationally relevant CDEs. Gerontol Geriatr Med. 2019;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. OECD . Long‐term Care and Health Care Insurance in OECD and Other Countries; 2020. Accessed March 15, 2020. https://www.oecd.org/daf/fin/insurance/Long-Term-Care-Health-Care-Insurance-in-OECD-and-Other-Countries.pdf
- 4. OECD . Public and private sector relationships in long‐term care and healthcare insurance; 2021. www.oecd.org/finance/insurance/long-term-care-health-care-insurance-in-oecd-and-other-countries.htm
- 5. Busse R. Do diagnosis‐related groups explain variations in hospital costs and length of stay? Analyses from the EuroDRG project for 10 episodes of care across 10 European countries. Health Econ. 2012;21(Suppl 2):1–5. [DOI] [PubMed] [Google Scholar]
- 6. Bekelman J, Halpern S, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315(3):272‐283. [DOI] [PubMed] [Google Scholar]
- 7. Figueroa J, Horneffer K & Riley K et al. A methodology for identifying high‐need, high‐cost patient personas for international comparisons: The International Collaborative on Costs, Outcomes, and Needs in Care (ICCONIC) Project; 2021. [DOI] [PMC free article] [PubMed]
- 8. National Academy of Medicine . Effective Care for High‐Need Patients: Opportunities for Improving Outcomes, Value, and Health. Washington, DC: National Academy of Sciences; 2017. [PubMed] [Google Scholar]
- 9. Kassim Javaid M, Chana J, Cooper C. Hip fracture as the tracer condition. Best Pract Res Clin Rheumatol. 2013;27(6):711‐715. [DOI] [PubMed] [Google Scholar]
- 10. Papanicolas I, Figueroa J & Schoenfeld A et al. Differences in Health Care Spending & Utilization Among Frail Elders in High‐Income Countries: ICCONIC Hip Fracture Persona. Health Serv Res. 2021. 10.1111/1475-6773.13739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikitovic M, Wodchis WP, Krahn MD, Cadarette SM. Direct health‐care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int. 2013;24(2):659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu Q, Koenig L, Mather RC, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536‐3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall RE, Sondergaard D, Wodchis WP, Fang J, Mondal P, Bayley MT. Trajectories of stroke care in Ontario: which path to best care? Can J Neurol Sci. 2017;44(3):261‐266. [DOI] [PubMed] [Google Scholar]
- 14. Beauchamp‐Chalifour P, Belzile EL, Racine LC, et al. The long‐term postoperative trajectory of geriatric patients admitted for a hip fracture: a prospective observational cohort study. Orthop Traumatol Surg Res. 2020;106(4):621‐625. [DOI] [PubMed] [Google Scholar]
- 15. Wodchis W, Bushmeneva K, Nikitovic M, McKillop I. Guidelines on Person‐Level Costing Using Administrative Databases in Ontario. Toronto, Canada: Health System Performance Research Network; 2013. [Google Scholar]
- 16. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 17. Guilcher SJ, Bronskill SE, Guan J, Wodchis WP. Who are the high‐cost users? A method for person‐Centred attribution of health care spending. PLoS One. 2016;11(3):e0149179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blankart CR, van Gool K, Papanicolas I, et al. International Comparison of Spending and Utilization at the End of Life for Hip Fracture Patients. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. OECD . Health at a Glance 2019: OECD Indicators. Paris: OECD; 2019. 10.1787/4dd50c09-en. [DOI] [Google Scholar]
- 20. Union OE. Health at a glance: Europe 2020: state of health in the EU cycle. Waiting Times for Hip Fracture. Paris: OECD Publishing; 2020. 10.1787/82129230-en [DOI] [Google Scholar]
- 21. Sheehan KJ, Filliter C, Sobolev B, et al. Time to surgery after hip fracture across Canada by timing of admission. Osteoporos Int. 2018;29(3):653‐663. 10.1007/s00198-017-4333-4 [DOI] [PubMed] [Google Scholar]
- 22. Huette P, Abou‐Arab O, Djebara AE, et al. Risk factors and mortality of patients undergoing hip fracture surgery: a one‐year follow‐up study. Sci Rep. 2020;10(1):9607. 10.1038/s41598-020-66614-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delaveau A, Saint‐Genez F, Gayet LE, Paccalin M, Ounajim A. T. V. impact of time to surgery in upper femoral fracture in orthogeriatrics. Orthop Traumatol Surg Res. 2019;105(5):975‐978. 10.1016/j.otsr.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 24. OECD . Health expenditure and financing: Health expenditure indicators. OECD Health Statistics (database). Paris: OECD; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Country dataset information
Supplementary Table 2. Comparison of international utilization and expenditure per person in postacute long‐term care settings (2017 USD)
Supplementary Table 3. System characteristics19,24
Supplementary Figure 1. Trajectories


