Abstract
Objective
To identify and explore differences in spending and utilization of key health services at the end of life among hip fracture patients across seven developed countries.
Data Sources
Individual‐level claims data from the inpatient and outpatient health care sectors compiled by the International Collaborative on Costs, Outcomes, and Needs in Care (ICCONIC).
Study Design
We retrospectively analyzed utilization and spending from acute hospital care, emergency department, outpatient primary care and specialty physician visits, and outpatient drugs. Patterns of spending and utilization were compared in the last 30, 90, and 180 days across Australia, Canada, England, Germany, New Zealand, Spain, and the United States. We employed linear regression models to measure age‐ and sex‐specific effects within and across countries. In addition, we analyzed hospital‐centricity, that is, the days spent in hospital and site of death.
Data Collection/Extraction Methods
We identified patients who sustained a hip fracture in 2016 and died within 12 months from date of admission.
Principal Findings
Resource use, costs, and the proportion of deaths in hospital showed large variability being high in England and Spain, while low in New Zealand. Days in hospital significantly decreased with increasing age in Canada, Germany, Spain, and the United States. Hospital spending near date of death was significantly lower for women in Canada, Germany, and the United States. The age gradient and the sex effect were less pronounced in utilization and spending of emergency care, outpatient care, and drugs.
Conclusions
Across seven countries, we find important variations in end‐of‐life care for patients who sustained a hip fracture, with some differences explained by sex and age. Our work sheds important insights that may help ongoing health policy discussions on equity, efficiency, and reimbursement in health care systems.
Keywords: administrative data, age inequalities, end‐of‐life care, gender inequalities, health care spending, health care utilization, international comparison
What is known on this topic
Countries follow different approaches to providing end‐of‐life care.
Health care spending and utilization increases near death.
International studies of health systems mainly compare acute hospital care and therefore lack to account for different organization of end‐of‐life care.
What this study adds
End‐of‐life care in the United States occurs more with specialists than in primary care, which is in fundamental contrast to other countries.
In most countries, health care spending and utilization decreases with age and is lower for women.
1. INTRODUCTION
Health policy makers have a strong interest in developing a better understanding of how they can improve end‐of‐life care. 1 , 2 , 3 Prior studies have found that in the months leading to death, the need and the costs of care increase substantially, and the bulk of these expenditures come from high‐acuity, high‐cost individuals, such as those with persistent chronic conditions. 4 , 5 , 6 In addition, there are serious concerns that the quality of care at the end of life often falls short of expectations. 7 , 8 , 9 As more people reach old age with chronic and disabling conditions, improving the quality and efficiency of care at the end of life will continue to grow as a priority policy issue. 10 As such, end‐of‐life care has become an important dimension of how we measure health system performance. 11
International comparisons on end‐of‐life care may yield important insights into how policy makers could improve the efficiency and the quality of care. Such research is vital in setting performance benchmarks and establishing best practice models from a system‐wide and policy perspective. However, to date, comparisons at the end of life across countries are quite limited, especially when it comes to comparing robust data across more than two countries and across different health care sectors.
In recent years, there have been significant advances in data infrastructure across many countries that enable international comparative research. This development is particularly true for patient‐level data, which is necessary to examine potential differences in end‐of‐life care. Much of the relevant data are routinely collected through administrative datasets, which are increasingly accessible for research and quality improvement purposes. 12 For example, claims data from health care payers, such as health insurers or national health services, provide a solid starting point for international comparisons as shown in several international projects such as Health Basket 13 and EuroDRG. 14
In this study, as part of the International Collaborative on Costs, Outcomes, and Needs in Care (ICCONIC) project, which is a research collaborative across a set of high‐income countries, we sought to evaluate differences in treatment at the end of life among frail, older adults who sustained a hip fracture across seven countries as follows: Australia, Canada, England, Germany, New Zealand, Spain, and the United States. Specifically, we sought to examine differences in utilization and spending across key health care services, including hospital care, emergency care, primary care and outpatient specialty care, and pharmaceuticals. Using a framework analyzing hip fracture patients, our goal was to provide insight into how health systems can optimally address the needs of the frail decedents by effectively accounting for resource constraints.
2. SUMMARY OF PREVIOUS INTERNATIONAL STUDIES ON END‐OF‐LIFE CARE
There is an extensive literature on the health care costs associated with end‐of‐life care. Numerous studies have shown that health care costs increase manifold in the time leading up to death. 15 , 16 , 17 Riley and Lubitz, 18 for example, find that although decedents account for only 5% of the US Medicare population in any given year, expenditure on this group explains more than 25% of total Medicare expenditure. As has been shown, the bulk of these expenditures come from high‐need high‐cost individuals, such as those with persistent chronic conditions. 19
The high costs at the end of life have led the policy makers to question if health systems are obtaining value for money in end‐of‐life care—particularly when considering that quality of care remains far from optimal. 20 , 21 , 22 , 23 At the same time, there is growing evidence that not all patients at the end of life face the same cost trajectory. 24 , 25 , 26 These studies show that there is considerable heterogeneity in the pattern in health care use and associated costs at the of end of life among different patients. Some patients face very high and persistent costs over an extended period of time, and others face a sudden decline in health status and associated rise in health care costs over a very short period of time. 27 As a result, there are widening calls to develop a greater understanding the drivers of end‐of‐life costs.
Another area of importance to health policy makers is awareness of gender and age disparities, including in end‐of‐life care. While literature found gender disparities in end‐of‐life spending and utilization, the direction of the disparity is not always consistent. 28 , 29 , 30 Some studies show that women receive less aggressive treatment than men receive when it comes to cancer or care in intensive care units. One potential reason is that women are more likely to have a do‐not‐resuscitate order than men. 31 , 32 , 33 In addition, other work has found that end‐of‐life spending at least in the United States declines with age, indicating declining treatment intensity. 28 Thus, while there is some evidence in the area of cancer care, the evidence for frail elders with a hip fracture, while similarly important, is less established. Further, cultural factors can influence utilization of health services. In some countries, there is variation in the proportion of older adults who live alone versus live with other family members. In the United States, evidence suggests that older adults are more likely to live alone than other countries and, therefore, may have a limited support system to care for themselves safely at home (and thus end up in a skilled nursing facility). 34 We therefore aim to expand on this literature and, importantly, show some consistency in patterns across countries by these important demographics.
Health policy makers who shape the health system have a strong interest in understanding how different countries provide end‐of‐life care, including current work at the Organisation for Economic Co‐operation and Development (OECD). The international evidence on end‐of‐life care is limited—especially when it comes to comparing robust data across more than two countries. There are two notable exceptions. The first is French et al. 5 who estimated the cost of care in the last 30 and 365 days of life across nine countries. This study found that mean cost in the last 12 months of life varied from US$ 50,000 in Germany to over US$80,000. Noting that the study was unable to control for the cause of death nor report on utilization. The second exception is Bekelman et al. 7 who examined hospital and chemotherapy use and cost in seven countries in the last 180 days of life for patients who died with cancer. The study found that mean per capita hospital expenditures were highest in Canada (US$ 21,840) and lowest in England (US$ 9342). Whilst Bekelman et al. 7 focused on a relatively homogenous patient group, the study was restricted to estimating costs in a sub‐set of the health care sector—albeit very important sector.
This project builds on previous work and makes a considerable contribution to developing a better understanding of internationally comparable end‐of‐life costs and health care use. To the best of our knowledge, this is the first study that has examined the end‐of‐life costs for frail elderly patients who have experienced a hip fracture in the last year of their life. This focus will provide new insights into end‐of‐life costs for a relatively homogeneous group of frail elderly patients who—due to their clinical condition—may be less likely to be cared for in a palliative care setting.
Frail elderly hip fracture patients are a vulnerable patient group with a high mortality rate following on from their hip fracture 35 and likely to incur significant health care costs. 36 Using standardized codes to identify a relatively frail and homogeneous group of patients, this article reports on a broad range of health care costs and utilization measures in seven countries and develops country‐specific estimates of these costs at various time points in the months leading up to death.
3. METHODS
3.1. Data sources
We used patient‐level data of seven out of the eleven countries participating in the ICCONIC collaborative, that is, all countries that could determine the exact date of death, to examine end‐of‐life utilization, and spending as follows: Australia (AU), Canada (CA), England (EN), Germany (DE), New Zealand (NZ), Spain (ES), and the United States (US). Extracted utilization and spending data of Australia, Canada, Germany, Spain, and the United States are based on individual‐level claims data from 2015 to 2017, while England and New Zealand used a longer identification period. For specific details of each country's dataset, please see Appendix 1 and Figueroa et al. 37
3.2. Patient selection
We followed a two‐step approach to identify a comparable set of frail elders who received treatment for a hip fracture. Hip fracture has been commonly used as a reliable marker of frailty among older adults, 38 and it accounts for the majority of fractures related to fragility globally. 39 Hip fracture is also highly associated with physical and mental disability, high mortality, and increased costs, thus requiring considerable health care resources from different parts of the health system. 39 , 40 , 41 , 42 As hip fractures almost always require a hospital admission and usually require surgery, the majority will be recorded in hospital admissions data and can thus serve as a robust and reliable tracer condition to explore differences in resource use across health systems. 38 We first identified a sample of comparable patients by examining all patients who received a primary diagnosis of hip fracture (S72.0‐2 according to the International Classification of Diseases version 10) in 2016 and obtained a total hip replacement, a partial hip replacement, or were treated with an osteosynthesis method such as screw, plate, pin osteosynthesis, or internal fixation (see Appendix 2). From this sample, we identified those who died within 365 days from the index hospitalization associated with the hip fracture.
3.3. Spending and utilization measures
We followed a federated data extraction approach due to data protection reasons. Each country produced means of utilization and spending by sex and age (65–69, 70–74, 75–79, 80–84, 85–89, 90–94, and older than 95 years), from individual patient‐level data. These aggregated nonidentifiable data were then collected in a central database for the analysis. It is important to note that we used the perspective of the health care payer across all countries. In most countries, this is either directly by an insurance or sickness fund (Germany and the Netherlands) or directly from a national form of health insurance (United States with Medicare program, Canada, etc.). Therefore, our study does not capture full costs (as it does not account for the fixed costs of all structures within a health system). It only captures were actually paid for the services, which across all countries, already included the fixed costs of the system. In order to compare spending reliably, we first applied the OECD Actual Individual Consumption Purchasing Power Parities (AIC PPPs) to the expenditure data. AIC PPPs, rather than gross domestic product–based purchasing power parities, are currently used by the OECD as the most reliable economy‐wide conversion rates for health expenditure. 43 Across each country, we applied 2017 AIC PPPs to all expenditures using the following exchange rates as follows: 1 AU$ ≙ US$ 0.69, 1 CA$ ≙ US$ 0.83, 1 GBP ≙ US$ 1.45, 1 EUR (Germany) ≙ US$ 1.33, 1 NZ$ ≙ US$ 0.69, 1 EUR (Spain) ≙ US$ 1.56, and US$ 1 ≙ US$ 1. 37 Similar to Bekelman et al., 7 we calculated average utilization and average spending for acute hospital care, emergency department admissions, excluding observation stays, primary and specialist outpatient physician services, including the services of nurse practitioners in Australia, Canada, and the United States and outpatient drugs for the periods of 30, 90, and 180 days before death (see Figure 1A). Utilization and spending were allocated proportionally to the observation periods in case of accruals (see Figure 1B).
FIGURE 1.
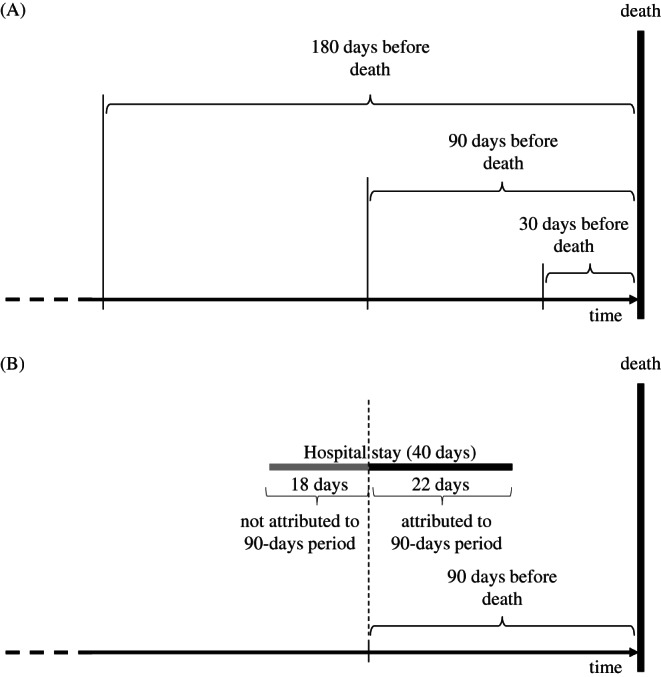
(A) Utilization and spending included in the analyses of 30, 90, and 180 days before death and (B) calculation of utilization and costs in case of accruals at the example of the 90‐day period
3.4. Data analysis
First, we described the number of decedents by country and sex. From each country's total sample of elderly patients who have experienced a hip fracture, we calculate the proportion of people who have died within 365 days of the fracture. 44 We also calculated the proportion of decedents in hospital relative to those who are discharged after their hip fracture admission.
To analyze within‐ and between‐country variation, we estimate utilization and spending as a function of country‐fixed effects to determine baseline utilization and baseline spending, respectively. In addition, we include the interaction of country and sex, the interaction of country and age group, which we coded ascendingly from 1 to 7 for the 65–70 years to the older than 95 group, and the interaction of country and days before death into the model to disentangle potential country‐specific effects of sex, age, and time. We fitted the model with no intercept term and, therefore, the age group and country estimates refer to zero, that is, we needed no reference group. We define the following six dependent variables for utilization as follows: acute hospital admissions, days in hospital, emergency department visits, medical doctor specialist visits, primary care visits, and outpatient prescription drugs. In addition, we measure the following five dependent variables spending as follows: acute hospital stays, emergency department, specialists, and primary care visits, as well as for outpatient prescription drugs. We estimate for each dependent variable the following linear regression:
where denotes a vector of country‐fixed effects and is a normally distributed error term. We obtaine indicating baseline utilization and spending, sex‐specific utilization and spending, an age gradient, and time‐dependent utilization and spending. We considered as statistically significant throughout the whole paper. All estimations were using the statistical program R 4.0.3 and the integrated lm function for linear regression. 45
4. RESULTS
4.1. Descriptive statistics
A total of 16,482 decedents were observed across the seven countries. In relation to the main cohort, 37 between 23.0% and 31.6% of all frail elders died within 365 days after a hip fracture across countries. Across all countries, more women (10,588) than men (5894) died after the event in absolute terms, but the relative mortality of women was lower than that of men. Mortality ranged for women between 20.3% and 28.2% and for men between 29.5% and 40%. Next, we observed distinct differences in hospital‐based mortality: while in Spain, 40% of patients died in hospital, in Australia, this occurred in only 6.7% of patients. Across all countries, men had a higher likelihood of dying in hospital (range 6.3%–49.7%) than women (range 7.1%–35.2%), while the variation across age groups was not pronounced (Table 1).
TABLE 1.
Number and percentage of decedents by country, age group, and site of death
| Australia | Canada | England | Germany | New Zealand | Spain | United States | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | F | M | All | F | M | All | F | M | All | F | M | All | F | M | All | F | M | All | F | M | |
| Baseline population (as reported in ICCONIC project) | |||||||||||||||||||||
| All age groups | 2511 | 1577 | 934 | 9872 | 6975 | 2897 | 2738 | 1943 | 795 | 13,998 | 10,628 | 3370 | 2940 | 2070 | 870 | 1849 | 1419 | 430 | 29,134 | 20,789 | 8345 |
| AG 65–69 | 141 | 93 | 48 | 695 | 425 | 270 | 166 | 102 | 64 | 759 | 516 | 243 | 168 | 105 | 63 | 62 | 45 | 17 | 2099 | 1314 | 785 |
| AG 70–74 | 197 | 127 | 70 | 931 | 603 | 328 | 239 | 158 | 81 | 1085 | 761 | 324 | 246 | 165 | 81 | 81 | 57 | 24 | 3024 | 2032 | 992 |
| AG 75–79 | 298 | 191 | 107 | 1341 | 898 | 443 | 387 | 285 | 102 | 2455 | 1810 | 645 | 369 | 255 | 114 | 164 | 123 | 41 | 4180 | 2877 | 1303 |
| AG 80–84 | 455 | 281 | 174 | 1946 | 1361 | 585 | 627 | 432 | 195 | 2902 | 2137 | 765 | 573 | 411 | 165 | 404 | 305 | 99 | 5711 | 4028 | 1683 |
| AG 85–89 | 751 | 460 | 291 | 2498 | 1815 | 683 | 666 | 480 | 186 | 3385 | 2567 | 818 | 831 | 579 | 249 | 602 | 467 | 135 | 6840 | 4960 | 1880 |
| AG 90–94 | 523 | 320 | 203 | 1832 | 1374 | 458 | 462 | 335 | 127 | 2578 | 2120 | 458 | 555 | 399 | 156 | 412 | 317 | 95 | 5221 | 3929 | 1292 |
| AG 95+ | 146 | 105 | 41 | 629 | 499 | 130 | 191 | 151 | 40 | 834 | 717 | 117 | 198 | 156 | 42 | 124 | 105 | 19 | 2059 | 1649 | 410 |
| Number of decedents | |||||||||||||||||||||
| All age groups | 640 | 324 | 316 | 2272 | 1416 | 856 | 865 | 547 | 318 | 3662 | 2505 | 1157 | 798 | 522 | 276 | 473 | 318 | 155 | 7772 | 4956 | 2816 |
| AG 65–69 | 10 | 4 | 6 | 72 | 34 | 38 | 22 | ‐ | ‐ | 76 | 40 | 36 | ‐ | ‐ | ‐ | 11 | 8 | 3 | 312 | 168 | 144 |
| AG 70–74 | 18 | 9 | 9 | 123 | 69 | 54 | 47 | ‐ | ‐ | 136 | 69 | 67 | ‐ | ‐ | ‐ | 9 | 7 | 2 | 454 | 264 | 190 |
| AG 75–79 | 53 | 29 | 24 | 220 | 120 | 100 | 75 | 50 | 25 | 434 | 271 | 163 | 66 | 36 | 30 | 28 | 16 | 12 | 789 | 447 | 342 |
| AG 80–84 | 97 | 48 | 49 | 408 | 236 | 172 | 171 | 99 | 72 | 647 | 411 | 236 | 135 | 84 | 51 | 71 | 43 | 28 | 1359 | 816 | 543 |
| AG 85–89 | 208 | 102 | 106 | 592 | 363 | 229 | 237 | 146 | 91 | 988 | 642 | 346 | 255 | 171 | 84 | 155 | 103 | 52 | 2103 | 1323 | 780 |
| AG 90–94 | 186 | 87 | 99 | 582 | 388 | 194 | 201 | 128 | 73 | 971 | 734 | 237 | 216 | 144 | 72 | 145 | 99 | 46 | 1847 | 1251 | 596 |
| AG 95+ | 68 | 45 | 23 | 275 | 206 | 69 | 112 | 84 | 28 | 410 | 338 | 72 | 84 | 63 | 21 | 54 | 42 | 12 | 908 | 687 | 221 |
| Percentage of decedents | |||||||||||||||||||||
| All age groups | 25.5% | 20.5% | 33.8% | 23.0% | 20.3% | 29.5% | 31.6% | 28.2% | 40.0% | 26.2% | 23.6% | 34.3% | 27.1% | 25.2% | 31.7% | 25.6% | 22.4% | 36.0% | 26.7% | 23.8% | 33.7% |
| AG 65–69 | 7.1% | 4.3% | 12.5% | 10.4% | 8.0% | 14.1% | 13.3% | ‐ | ‐ | 10.0% | 7.8% | 14.8% | ‐ | ‐ | ‐ | 17.7% | 17.8% | 17.6% | 14.9% | 12.8% | 18.3% |
| AG 70–74 | 9.1% | 7.1% | 12.9% | 13.2% | 11.4% | 16.5% | 19.7% | ‐ | ‐ | 12.5% | 9.1% | 20.7% | ‐ | ‐ | ‐ | 11.1% | 12.3% | 8.3% | 15.0% | 13.0% | 19.2% |
| AG 75–79 | 17.8% | 15.2% | 22.4% | 16.4% | 13.4% | 22.6% | 19.4% | 17.5% | 24.5% | 17.7% | 15.0% | 25.3% | 17.9% | ‐ | ‐ | 17.1% | 13.0% | 29.3% | 18.9% | 15.5% | 26.2% |
| AG 80–84 | 21.3% | 17.1% | 28.2% | 21.0% | 17.3% | 29.4% | 27.3% | 22.9% | 36.9% | 22.3% | 19.2% | 30.8% | 23.6% | 20.4% | 30.9% | 17.6% | 14.1% | 28.3% | 23.8% | 20.3% | 32.3% |
| AG 85–89 | 27.7% | 22.2% | 36.4% | 23.7% | 20.0% | 33.5% | 35.6% | 30.4% | 48.9% | 29.2% | 25.0% | 42.3% | 30.7% | 29.5% | 33.7% | 25.7% | 22.1% | 38.5% | 30.7% | 26.7% | 41.5% |
| AG 90–94 | 35.6% | 27.2% | 48.8% | 31.8% | 28.2% | 42.4% | 43.5% | 38.2% | 57.5% | 37.7% | 34.6% | 51.7% | 38.9% | 36.1% | 46.2% | 35.2% | 31.2% | 48.4% | 35.4% | 31.8% | 46.1% |
| AG 95+ | 46.6% | 42.9% | 56.1% | 43.7% | 41.3% | 53.1% | 58.6% | 55.6% | 70.0% | 49.2% | 47.1% | 61.5% | 42.4% | 40.4% | 50.0% | 43.5% | 40.0% | 63.2% | 44.1% | 41.7% | 53.9% |
| Percentage of decedents in hospital | |||||||||||||||||||||
| All age groups | 6.7% | 7.1% | 6.3% | 21.3% | 20.4% | 22.9% | 36.5% | 34.6% | 39.9% | 21.3% | 19.4% | 25.4% | 12.0% | 10.3% | 15.2% | 40.0% | 35.2% | 49.7% | 20.5% | 19.2% | 22.9% |
| AG 65–69 | 10.0% | 0.0% | 16.7% | 20.8% | 14.7% | 26.3% | ‐ | ‐ | ‐ | 25.0% | 15.0% | 36.1% | ‐ | ‐ | ‐ | 36.4% | 25.0% | 66.7% | 27.2% | 25.6% | 29.2% |
| AG 70–74 | 0.0% | 0.0% | 0.0% | 13.0% | 11.6% | 14.8% | ‐ | ‐ | ‐ | 22.1% | 21.7% | 22.4% | ‐ | ‐ | ‐ | 44.4% | 42.9% | 50.0% | 27.3% | 29.5% | 24.2% |
| AG 75–79 | 7.5% | 6.9% | 8.3% | 18.6% | 15.8% | 22.0% | ‐ | ‐ | ‐ | 20.7% | 20.3% | 21.5% | 9.1% | ‐ | ‐ | 42.9% | 25.0% | 66.7% | 24.2% | 22.6% | 26.3% |
| AG 80–84 | 6.2% | 6.3% | 6.1% | 21.3% | 18.6% | 25.0% | 33.9% | 32.3% | 36.1% | 18.7% | 18.5% | 19.1% | 17.8% | 17.9% | 17.6% | 39.4% | 44.2% | 32.1% | 21.2% | 20.1% | 22.8% |
| AG 85–89 | 5.8% | 6.9% | 4.7% | 20.9% | 21.2% | 20.5% | 38.4% | 36.3% | 41.8% | 19.8% | 16.2% | 26.6% | 8.2% | 5.3% | 14.3% | 43.9% | 35.9% | 59.6% | 20.7% | 19.7% | 22.4% |
| AG 90–94 | 6.5% | 6.9% | 6.1% | 23.0% | 22.4% | 24.2% | 42.3% | 39.8% | 46.6% | 22.6% | 20.6% | 28.7% | 10.6% | 10.4% | 12.5% | 37.2% | 31.3% | 50.0% | 18.1% | 16.3% | 22.0% |
| AG 95+ | 11.8% | 11.1% | 13.0% | 24.4% | 23.3% | 27.5% | 40.2% | 38.1% | 46.4% | 25.6% | 23.4% | 36.1% | 14.3% | 19.0% | ‐ | 35.2% | 38.1% | 25.0% | 15.2% | 14.6% | 17.2% |
Note: AG = age group; F = female; M = male; "‐" = not reported due to data protection reasons.
Abbreviation: ICCONIC, International Collaborative on Costs, Outcomes, and Needs in Care.
The frail older adult with hip fracture was characterized by high multimorbidity and high utilization of services across all countries. Within the last 180 days before death, they received on average across countries 11.4 different drug substances (range: 7.3 [AU] to 15.8 [NZ]). On average, they had 1.81 hospitalizations (range: 1.56 [AU] to 2.20 [EN]) and spent 21.5 days in hospital (range: 13.1 [NZ] to 32.5 [EN]). During the same period, the decedents visited specialists, on average, 3.5 times (range: 0.95 [NZ] to 6.4 [ES]), primary care doctors, 7.3 times (range: 2.2 [US] to 11.7 [ES]), and emergency departments, 0.75 times (range: 0.15 [NZ] to 2.24 [ES]; see Table 2; Appendices 3 and 4 show numbers by sex).
TABLE 2.
Utilization and spending by country during 30, 90, and 180 days before death
| Utilization | Spending [in US$] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute hospital admissions | Days in hospital | Emergency department visits | Specialist visits | Primary care visits | Drug prescriptions | Hospital spending | Emergency department spending | Specialist spending | Primary care spending | Drug spending | |
| 30 days before death | |||||||||||
| Australia | 0.45 | 4.2 | 0.07 | 0.78 | 2.1 | 2.0 | 6937 | 39 | 112 | 107 | 120 |
| Canada | 0.84 | 9.2 | 0.16 | 0.78 | 2.5 | 6.6 | 11,611 | 60 | 94 | 187 | 219 |
| England | 0.94 | 12.7 | 0.08 | 0.40 | 2.1 | 6.0 | 7982 | 48 | 83 | 87 | 212 |
| Germany | 0.93 | 9.6 | ‐ | 0.58 | 0.6 | 3.6 | 8094 | ‐ | 88 | 102 | 238 |
| New Zealand | 0.68 | 4.9 | 0.03 | 0.13 | ‐ | 8.7 | 4942 | 8 | 21 | ‐ | 143 |
| Spain | 1.03 | 14.8 | 1.05 | 2.10 | 2.7 | 5.2 | 9089 | 226 | 284 | 174 | 236 |
| United States | 0.77 | 5.4 | 0.24 | 0.71 | 0.3 | 3.6 | 11,109 | 276 | 241 | 87 | 267 |
| 90 days before death | |||||||||||
| Australia | 0.97 | 8.7 | 0.16 | 1.73 | 5.2 | 4.4 | 13,805 | 79 | 209 | 247 | 372 |
| Canada | 1.28 | 17.1 | 0.40 | 2.14 | 5.9 | 10.1 | 19,356 | 135 | 242 | 389 | 648 |
| England | 1.54 | 24.2 | 0.18 | 1.40 | 4.7 | 10.0 | 12,909 | 97 | 259 | 187 | 660 |
| Germany | 1.51 | 19.5 | ‐ | 1.61 | 1.5 | 7.9 | 14,092 | ‐ | 249 | 318 | 688 |
| New Zealand | 1.18 | 9.7 | 0.08 | 0.46 | ‐ | 13.1 | 9485 | 19 | 88 | ‐ | 323 |
| Spain | 1.38 | 21.7 | 1.68 | 4.22 | 6.8 | 9.1 | 13,802 | 363 | 467 | 423 | 609 |
| United States | 1.31 | 9.7 | 0.52 | 2.29 | 1.0 | 7.5 | 18,867 | 625 | 977 | 321 | 914 |
| 180 days before death | |||||||||||
| Australia | 1.56 | 13.3 | 0.28 | 2.85 | 9.4 | 7.3 | 20,200 | 129 | 329 | 422 | 722 |
| Canada | 1.72 | 24.0 | 0.70 | 4.15 | 9.5 | 12.7 | 25,839 | 229 | 456 | 626 | 1291 |
| England | 2.20 | 32.5 | 0.31 | 2.65 | 7.9 | 12.2 | 16,503 | 158 | 504 | 312 | 1325 |
| Germany | 2.07 | 27.5 | ‐ | 3.13 | 2.8 | 10.8 | 18,931 | ‐ | 492 | 594 | 1274 |
| New Zealand | 1.64 | 13.1 | 0.15 | 0.95 | ‐ | 15.8 | 12,827 | 33 | 190 | ‐ | 576 |
| Spain | 1.71 | 26.6 | 2.24 | 6.40 | 11.7 | 10.9 | 17,427 | 483 | 689 | 712 | 1193 |
| United States | 1.80 | 13.2 | 0.82 | 4.66 | 2.2 | 10.4 | 25,336 | 997 | 2156 | 696 | 1923 |
Across all countries, we observed relatively higher expenses in the time interval closer to the date of death. Canada had the highest hospital costs within 180 days (mean of US$ 25,839) with 44.9% of this amount incurred in the last 30 days before death. All other countries similarly incurred the highest costs related to acute hospital care within the last 30 days before death, ranging from 34.3% in Australia to 52.2% in Spain. For the other spending categories, concentration of costs in the last 30 days before death was much less pronounced.
4.2. Regression results
Regression results with country‐specific baseline utilization and spending, effects of sex, age gradients, and time effects are presented in Table 3. Root mean squared error ranges between 0.20 for emergency department visits and 33,120 for hospital spending. Adjusted R‐squared ranges between 0.66 for specialist visits and 0.98 for drug prescriptions. The estimates for baseline utilization and spending were mainly positive and, for most countries, across all estimations, significantly different from zero. This suggests that utilization and spending not only depend on the number of days, but that there is a nonlinear relationship between days before death and utilization and spending. In some cases, females seem to receive less care than men. Females had significantly less hospitalizations in New Zealand (−0.25) and spent less days in hospital in Canada (−3.11) and Spain (−8.87). The sex effect on hospital expenditures is statistically significantly negative in Canada (US$ −3618), Germany (US$ −2963), and the United States (US$ −4030), while the other countries also show a negative but not significant coefficient at the 5% level. This suggests that although women have an equally high utilization, they tend to receive less expensive treatments in those countries. In the outpatient sector, the situation is ambiguous: in the United States, specialist spending is significantly lower for women (US$ −393.4), while primary care spending is higher for women in Canada (US$ 96.2) and Spain (US$ 90.8). The age gradient is almost always significantly negative for both utilization and spending, suggesting significantly lower utilization before death among older than younger people. The time effect is consistently significantly positive as utilization and spending increase with a longer the time horizon (see Table 3).
TABLE 3.
Regression results by utilization and spending categories
| Utilization | Acute hospital admissions (SE) | Days in hospital (SE) | Emergency department visits (SE) | Specialist visits (SE) | Primary care visits (SE) | Drug prescriptions (SE) |
|---|---|---|---|---|---|---|
| Baseline utilization | ||||||
| Australia | 0.41 (0.24) . | 6.05 (3.73) | 0.04 (0.16) | 2.79 (1.7) | 0.02 (1.96) | 1.98 (1.07) . |
| Canada | 1.17 (0.15)*** | 16.3 (2.27)*** | 0.22 (0.09)* | 1.37 (1.03) | 1.65 (0.62)** | 5.6 (0.65)*** |
| England | 1.27 (0.15)*** | 8.99 (2.27)*** | 0.1 (0.09) | 1.79 (1.03) . | 1.05 (0.62) . | 6.53 (0.65)*** |
| Germany | 1.32 (0.15)*** | 16.43 (2.27)*** | ‐ | 0.29 (1.03) | 0.05 (0.62) | 2.8 (0.65)*** |
| New Zealand | 0.6 (0.15)*** | 5.45 (2.27)* | 0 (0.09) | 0.28 (1.03) | ‐ | 7.72 (0.65)*** |
| Spain | 0.89 (0.15)*** | 22.98 (2.27)*** | 1.14 (0.09)*** | 3.2 (1.03)** | 1.12 (0.62) . | 3.93 (0.65)*** |
| United States | 1.17 (0.24)*** | 11.27 (3.73)** | 0.4 (0.16)* | 2.1 (1.7) | 0.18 (1.01) | 4.47 (1.07)*** |
| Sex effect (female = 1) | ||||||
| Australia | −0.09 (0.17) | −1.45 (2.64) | −0.07 (0.111) | 2.18 (1.202) . | −0.77 (0.719) | −0.57 (0.756) |
| Canada | −0.06 (0.1) | −3.11 (1.55)* | 0.07 (0.065) | −0.23 (0.703) | 0.6 (0.42) | 0.82 (0.442) . |
| England | 0.12 (0.1) | −0.46 (1.55) | 0 (0.065) | −0.83 (0.703) | 0.66 (0.42) | 1.11 (0.442)* |
| Germany | −0.09 (0.1) | −2.52 (1.55) | ‐ | −0.36 (0.703) | −0.01 (0.42) | 0.35 (0.442) |
| New Zealand | −0.25 (0.1)* | −2.27 (1.55) | −0.03 (0.065) | −0.21 (0.703) | ‐ | −0.56 (0.442) |
| Spain | 0.01 (0.1) | −8.87 (1.55)*** | 0.07 (0.065) | 0.78 (0.703) | 1.28 (0.42)** | 1.17 (0.442)** |
| United States | −0.15 (0.17) | −2.03 (2.64) | −0.05 (0.111) | −0.41 (1.202) | −0.02 (0.719) | 0.07 (0.756) |
| Age effect | ||||||
| Australia | −0.04 (0.02) . | −0.38 (0.39) | 0.01 (0.02) | −1.122 (0.18)*** | 0.017 (0.02) | −0.11 (0.11) |
| Canada | −0.1 (0.02)*** | −1.71 (0.39)*** | −0.05 (0.02)** | −0.294 (0.18) . | −0.185 (0.11) . | −0.13 (0.11) |
| England | −0.16 (0.02)*** | 0.18 (0.39) | −0.015 (0.02) | −0.358 (0.18)* | −0.065 (0.11) | −0.45 (0.11)*** |
| Germany | −0.11 (0.02)*** | −1.76 (0.39)*** | ‐ | −0.019 (0.18) | 0.024 (0.11) | −0.1 (0.11) |
| New Zealand | 0.01 (0.02) | −0.1 (0.39) | 0 (0.02) | −0.034 (0.18) | ‐ | 0.07 (0.11) |
| Spain | 0.01 (0.02) | −1.46 (0.39)*** | −0.071 (0.02)*** | −0.534 (0.18)** | −0.145 (0.11) | 0.06 (0.11) |
| United States | −0.09 (0.02)*** | −1.03 (0.39)** | −0.048 (0.02)** | −0.439 (0.18)* | −0.059 (0.11) | −0.44 (0.11)*** |
| Time effect | ||||||
| Australia | 0.009 (0.001)*** | 0.06 (0.02)** | 0.001 (0.001) | 0.041 (0.01)*** | 0.044 (0.006)*** | 0.034 (0.006)*** |
| Canada | 0.006 (0.001)*** | 0.11 (0.01)*** | 0.004 (0.001)*** | 0.025 (0.006)*** | 0.05 (0.003)*** | 0.042 (0.004)*** |
| England | 0.011 (0.001)*** | 0.13 (0.01)*** | 0.002 (0.001)** | 0.02 (0.006)*** | 0.037 (0.003)*** | 0.043 (0.004)*** |
| Germany | 0.008 (0.001)*** | 0.13 (0.01)*** | ‐ | 0.018 (0.006)** | 0.015 (0.003)*** | 0.048 (0.004)*** |
| New Zealand | 0.007 (0.001)*** | 0.06 (0.01)*** | 0.001 (0.001) . | 0.006 (0.006) | ‐ | 0.047 (0.004)*** |
| Spain | 0.004 (0.001)*** | 0.09 (0.01)*** | 0.007 (0.001)*** | 0.027 (0.006)*** | 0.054 (0.003)*** | 0.03 (0.004)*** |
| United States | 0.006 (0.001)*** | 0.04 (0.02)* | 0.004 (0.001)*** | 0.029 (0.01)** | 0.013 (0.006)* | 0.047 (0.006)*** |
| Root mean squared error | 0.30 | 4.76 | 0.20 | 2.17 | 1.31 | 1.36 |
| Adjusted R‐squared | 0.96 | 0.93 | 0.92 | 0.67 | 0.93 | 0.98 |
| Spending in US$ PPP | Hospital spending (SE) | Emergency department spending (SE) | Specialist spending (SE) | Primary care spending (SE) | Drug spending (SE) |
|---|---|---|---|---|---|
| Baseline utilization | |||||
| Australia | 12,681 (2593.5)*** | 31.9 (46.4) | 198.5 (279.7) | 117.9 (76.4) | 496.9 (263.5) . |
| Canada | 21,305 (1578.3)*** | 73.6 (28.2)** | 152.8 (170.2) | 102.2 (46.5)* | 403.1 (160.4)* |
| England | 8062 (1578.3)*** | 56.7 (28.2)* | 288.6 (170.2) . | 99.4 (46.5)* | 93.2 (160.4) |
| Germany | 17,322 (1578.3)*** | ‐ | 265.3 (170.2) | −67.8 (46.5) | 619.1 (160.4)*** |
| New Zealand | 5350 (1578.3)*** | 16 (28.2) | 82 (170.2) | ‐ | 143.6 (160.4) |
| Spain | 3837 (1578.3)* | 244.7 (28.2)*** | 374.6 (170.2)* | 50.6 (46.5) | 215.3 (160.4) |
| United States | 22,508 (2593.5)*** | 519.9 (46.4)*** | 1977.4 (279.7)*** | 226.1 (76.4)** | 1130.6 (263.5)*** |
| Sex effect (female = 1) | |||||
| Australia | −2811 (1836.9) | −25.6 (32.8) | 213.2 (198.1) | −45.4 (54.1) | 2.1 (186.7) |
| Canada | −3618 (1074.4)*** | 16.9 (19.2) | −1.6 (115.9) | 96.2 (31.6)** | −26.5 (109.2) |
| England | −1223 (1074.4) | 0.6 (19.2) | −55.2 (115.9) | 25.4 (31.6) | 92.3 (109.2) |
| Germany | −2963 (1074.4)** | ‐ | −56.5 (115.9) | 24.2 (31.6) | 70.8 (109.2) |
| New Zealand | −2044 (1074.4) . | −10.2 (19.2) | −49.2 (115.9) | ‐ | −65.1 (109.2) |
| Spain | −771 (1074.4) | 15 (19.2) | 75.2 (115.9) | 90.8 (31.6)** | 131.6 (109.2) |
| United States | −4030 (1836.9)* | −60.8 (32.8) . | −393.4 (198.1)* | 20.1 (54.1) | −156.1 (186.7) |
| Age effect | |||||
| Australia | −1152.6 (268.6)*** | 1.8 (4.8) | −87.4 (29)** | −4.9 (7.9) | −137.2 (27.3)*** |
| Canada | −2214 (268.6)*** | −14.2 (4.8)** | −33.5 (29) | −11.3 (7.9) | −105.9 (27.3)*** |
| England | −215.6 (268.6) | −7.2 (4.8) | −66.2 (29)* | −14.8 (7.9) . | −35.3 (27.3) |
| Germany | −1981.8 (268.6)*** | ‐ | −58.9 (29)* | 13.7 (7.9) . | −160 (27.3)*** |
| New Zealand | −57.9 (268.6) | −0.8 (4.8) | −14 (29) | ‐ | −15.4 (27.3) |
| Spain | 1184 (268.6)*** | −15.2 (4.8)** | −49.5 (29) . | −2.9 (7.9) | −55.3 (27.3)* |
| United States | −1925.1 (268.6)*** | −68.2 (4.8)*** | −434.6 (29)*** | −77.9 (7.9)*** | −266.3 (27.3)*** |
| Time effect | |||||
| Australia | 80.3 (14.9)*** | 0.5 (0.3) . | 4.1 (1.6)* | 1.9 (0.4)*** | 6.2 (1.5)*** |
| Canada | 105.7 (8.7)*** | 1.3 (0.2)*** | 2.8 (0.9)** | 3.1 (0.3)*** | 8.4 (0.9)*** |
| England | 61.6 (8.7)*** | 0.8 (0.2)*** | 3.7 (0.9)*** | 1.5 (0.3)*** | 7.4 (0.9)*** |
| Germany | 81.3 (8.7)*** | ‐ | 3.3 (0.9)*** | 3.2 (0.3)*** | 8.9 (0.9)*** |
| New Zealand | 52.9 (8.7)*** | 0.1 (0.2) | 1 (0.9) | ‐ | 3.1 (0.9)*** |
| Spain | 48.7 (8.7)*** | 1.6 (0.2)*** | 2.6 (0.9)** | 3.2 (0.3)*** | 5.7 (0.9)*** |
| United States | 77.2 (14.9)*** | 4.8 (0.3)*** | 15.2 (1.6)*** | 5.1 (0.4)*** | 12.7 (1.5)*** |
| Root mean squared error | 3311.5 | 59.7 | 357.1 | 98.2 | 336.5 |
| Adjusted R‐squared | 0.96 | 0.97 | 0.84 | 0.93 | 0.88 |
Note: “‐” utilization/spending category not available in this country; significance codes: “***” = 0.001, “**” = 0.01, “*” = 0.05, “.” = 0.1.
Abbreviation: SE, standard errors.
5. DISCUSSION
International comparisons of end‐of‐life care for frail older adults provide important insights as to how health systems manage complex populations at the end of life. Using patient‐level data linked across inpatient and outpatient health care sectors, we found important differences in how older adults with hip fracture are managed across a group of seven high‐income countries. We also shed insights into how treatment intensity differences according to age, sex, and time may explain some of the variation across countries. By comparing expenditures relative to proximity of death, policy makers may be able to identify opportunities to better allocate scarce health care resources.
A key finding of this work is the large difference across countries in the site of death, specifically whether it occurred in a hospital setting or not. Site of death is increasingly becoming an important measure of health system performance, especially as prior studies have shown that patients prefer dying at home, 46 , 47 something that is not the reality in most countries. 48 In our study, England (36.5%) and Spain (40%) are among the countries with the most decedents dying in hospital. Australia and New Zealand, on the other hand, observed the lowest rate of in hospital‐based deaths at 6.7% and 12%. Similar patterns regarding site of care have also been found elsewhere. 7 , 49 The high rate in England seems to be related to unplanned hospital care at the end of life, 50 while the low rate in New Zealand may be attributed to a historically strong focus on hospice and end‐of‐life care, which limits the number of days a patient may spend in a hospital setting in their final days of life. 51 Indeed, end‐of‐life care in New Zealand tends to be provided in the home with the support of homecare, medical, and hospice services. In cases where a higher level of care may be needed, patients are cared for in a hospice facility or an elderly care facility designed to provide specialized end‐of‐life support services. 52 Furthermore, it is also important to not only consider the site of death but also where the majority of end‐of‐life care took place prior to the death. 53 According to our data, the number of days spent in hospital within the last month is strongly correlated with the rate of decedents dying in the hospital, that is, the more days spent in hospital care, the higher the in‐hospital mortality rate. Further support for specialized and community palliative care services may offer patients and their carers more choice in deciding the most appropriate site of death for them. 47
We also observed important differences in the treatment by health care sector at the end of life, which is a novel contribution to previous international comparisons on end‐of‐life care. It was interesting to observe that primary care visits play a much smaller role in Germany (2.8) and the United States (2.2) than in the other countries, where the average number of visits across Australia, Canada, England, and Spain was 9.6 visits (range 9.4–11.7). With regard to specialist visits, the United States is second with 4.7 visits, while in Germany, an average of 3.1 specialist visits take place within the last 180 days of life. The low number in Germany can be attributed to the reimbursement system, where many services in the outpatient sector are flat rate and bundled over a quarter. In the United States, however, it seems that care at the end of life occurs more with specialists than with primary care, which is a fundamental contrast to other countries (e.g., Australia, Canada, England, and Spain) where the primary care physician appears to assume a more prominent role. This is consistent with overall patterns of utilization. 54 Prescription drug use was high across all countries, ranging from 7.3 unique type of scripts in Australia to 15.8 in New Zealand. Spending on drugs, however, was relatively modest and ranged from US$ 576 in New Zealand to US$ 1923 in the United States in last 180 days of life.
Hospice use is organized very differently across countries and difficult to capture with our administrative data that are underlined by the divergent data that are provided by Germany and the United States (see Appendix 5 for utilization and spending). According to our data, end‐of‐life patients spend, on average, 0.16 days in hospice in Germany, while they spend 8.3 days in hospice in the United States. In terms of hospital days, the situation is the opposite: in Germany, decedents spend 9.6 days, and in the United States, only 5.4 days in hospital during their last phase of life. In Australia, England, Germany, and Spain, the decedents are very much supported by palliative care teams that are based in the reference hospitals or are part of the primary care organization and therefore included in the inpatient and specialist costs. 55 Similarly, in New Zealand, end‐of‐life care is provided in the home, with the support of homecare, medical, and hospice services, in a hospice if there are comorbidities that require active care, or in an aged care facility, which is designed to provide specialized end‐of‐life support services. However, the cost of aged care accommodation is often serviced by the patient, subject to an asset assessment, with only limited costs coming from the public sector. These different organizational and reimbursement arrangements make a direct comparison difficult, and our study based on administrative data should be complemented by qualitative research to explore the variation in the organization of hospice care further.
In most international comparisons, the United States typically has the highest health care costs. 56 , 57 Interestingly, this seems not to be the case for end‐of‐life care in the acute hospital setting, while the United States still has the highest health care costs across all other spending categories analyzed in this study. This may also be reflective of the fact that the use of advance care planning, and of end‐of‐life directives, may be more limited in the United States as compared to other countries. In previous work, Gupta et al. 58 reported that among US Medicare beneficiaries, the presence of an advanced care plan was associated with less intensive health care utilization around the end of life. Similar to our current findings reported, Bekelman et al. 7 found in their study of cancer patients that Canada had the highest end‐of‐life hospital costs, while Germany had slightly lower costs than Canada. This is not because the United States has fundamentally lower hospital costs but may reflect fewer inpatient hospital days in the United States prior to death and likely more time during end‐of‐life care spent in postacute rehabilitative facilities. 54
We also encountered important differences across some countries in the treatment of people by age. Canada, Germany, and the United States had the highest negative age gradients, that is, on average, these countries spend around US$ 2000 less per older age group. In contrast, the age effect on spending did not play a role in New Zealand and England across all health care services. This negative age gradient indicates that countries discriminate against age—whether this addresses the patient needs to or due to efficiency considerations. It is likely that spending is managed by differentiation of the place of service delivery during the trajectories of care. The study by Wodchis et al. 59 on trajectories of care suggests that patients are primarily transferred to home settings, that is, home, home rehab, home nursing, and so forth, prior to death. Inpatient rehab plays a subordinate role for decedents because if there is no chance of recovery, they are not moved to these facilities; in Australia, Canada, and Germany, for example, a decedent spends on average a very short time in an inpatient rehabilitation unit during the last 30 days before death (AU 0.86, CA 1.06, and DE 0.37 days). In the United States, however, a decedent spends on average 7.41 days in inpatient rehabilitation, especially in skilled nursing homes. This effect, however, converges when viewed over 90 and 180 days (see Appendix 5 for utilization and spending). There are several reasons why this might be the case. Patients have different accessibility to postacute rehabilitative care and/or long‐term care, which may influence the amount of time spent in the hospital setting by age. In England, the National Health Service does not cover these services, which limits the ability of hospitals to discharge patients needing this type of care. In the United States, patients are likely to be quickly discharged to postacute rehabilitative care, while in Canada, patients have access to long‐term care. New Zealand, on the other hand, had relatively consistent spending through the age groups, which as noted above, may be due to the country's concerted and coordinated application of elder care assessment services and support.
In part, the variation in use and costs across countries in each health care sector (e.g., hospitals) may be driven by differences in the roles and functions of that sector. For example, rehabilitation in some countries may be a part of a hospital's routine functions—whereas in other countries, much of this care may be provided in specialized clinics or in the community. This implies that rehabilitation costs will be incurred in the hospital sector for some countries, but in other countries, it may be registered as an outpatient care. Such differences in the roles and functions may be important drivers of the variation seen between countries. The results reported in this article provide an accurate description of costs and use within each sector, but further work is needed to examine the reasons for that variation—which includes further exploration of the roles and functions of each sector as well as full data capture.
Finally, we observed that some countries spend less on treating women before death. Specifically, while men and women generally receive similar care in the outpatient setting, there appears to be some differences in the type of care provided in the acute care setting, with males receiving more expensive acute hospital end‐of‐life care across countries. There are some potential factors that might explain this finding. First, prior work has suggested that women may have a lower biological age than men and may therefore be less frail. 60 There is also some evidence that suggests men enter the last phase of life sicker than women. For example, in Germany, male decedents scored a higher Elixhauser mortality score 61 than women. Second, it is possible that there may be differences in preference of high‐intensity treatments by gender or in the offering of such treatments by clinicians. This effect has been shown for other conditions, for example, Sharma et al. 29 find that men with advanced cancers are more likely than women to receive aggressive, nonbeneficial care in the intensive care unit near death. Due to the paucity of international studies on this topic, there is a limited capacity to compare our results to others. In the few studies available, there is some agreement in the results. In comparison to cancer patients, for example, there were similar costs associated with acute care costs but, perhaps not surprisingly, a much higher costs on outpatient care compared to the patients in our sample. 7 Further, in our study, we find that the United States spends substantially more for patients at the end of life after a hip fracture, which is consistent with the fact that they spend more overall for this population and other populations like people hospitalized with heart failure, even after adjusting for similar levels of utilization. Our findings confirm that the United States has high prices per unit across multiple care domains.
5.1. Limitations
The study has limitations. First, we had to rely on a federated analysis approach due to data protection regulations. Across countries, there are differences in how data are structured and collected, which may yield differences in the variables examined in this study. However, we used a patient vignette with specific diagnostic codes that are commonly used that should limit potential biases and deviations across countries. Second, the federated approach did not allow us to perform the estimations with individual‐level data, which lower the efficiency of our model; however, the coefficients should remain unbiased. The small number of data points may also lead to a technical overfitting of the regression. However, given that the data points are based on thousands of observations, it still allows for a generalizability of the conclusions. Third, the method applied ignores that spending may be frontloaded during hospital stays in case of accruals (as illustrated in Figure 1B). This leads to an overestimation of the actual hospital costs incurred, most notably in the last 30 days before death. This is a problem of almost all claims data analyses and fades the longer the observation period is. Fourth, we used only a limited number of utilization and spending categories, focusing on the most important categories available and comparable across countries. Further research should invest additional effort in detailing and identifying further categories and making them comparable to complement our picture of the provision of end‐of‐life care. Fifth, when comparing absolute spending across countries, we have to acknowledge that we compare prices but do not account for the different economics of care delivery. We explored the economic implications of different patterns of resource use in the study of Lorenzoni et al. 62 Lastly, we were unable to determine whether the differences in spending and utilization within and between countries are due to differences in responsiveness to the populations' demands, better allocative efficiency by countries, or whether the end‐of‐life spending was potentially wasteful. 25 Further, we must be very careful with any causal conclusions based on the data. Without taking the endogeneity issue and the health system structures into account, it is very difficult to interpret the effects. 37 Despite our significant progress in making the results as comparable as possible, great care still needs to be taken in drawing conclusions on the reasons for the cross‐country variations.
6. CONCLUSION
Across a group of seven high‐income countries, we found important variations in end‐of‐life care for patients who sustained a hip fracture, with some differences explained by sex and age. Our work sheds important insights that may help ongoing health policy discussions on equity, efficiency, and reimbursement in health care systems. However, while our analysis has limitations, improved data capture and availability might substantially increase the explanatory value of international comparisons in the future.
DISCLAIMER
The opinions, results, and conclusions reported in this paper are those of the authors and are independent from funding sources. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The 45 and Up survey data used to represent Australia oversample people older than 80 years and residents of rural and remote areas (45 and Up Study Collaborators; 2008). The 45 and Up Study had a response rate of 18%, so the cohort might not be representative of the NSW population. Also, the survey focuses on NSW and may not be representative of the national sample for the same age group.
The results from New Zealand are not official statistics. They have been created for research purposes from the Integrated Data Infrastructure (IDI), which is carefully managed by Stats NZ. For more information about the IDI, please visit www.stats.govt.nz/integrated-data.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
This research representing Australia was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council New South Wales (NSW); and partners as follows: the National Heart Foundation of Australia (NSW Division); NSW Ministry of Health; NSW Department of Communities and Justice; and the Australian Red Cross Lifeblood. We thank the many thousands of people participating in the 45 and Up Study. We also acknowledge Services Australia (formerly the Australian Government Department of Human Services) for the provision of Medicare Benefits Schedule (MBS) and the Pharmaceutical Benefits Schedule (PBS) datasets. The Admitted Patient Data Collection (APDC) and Emergency Department Data Collection (EDDC) data were provided by the NSW Ministry of Health and linked to the 45 and Up Study data by the Centre for Health Record Linkage (CHeReL). We capture the administrative health datasets of each survey participant by linkage through the CHeReL unique identifier using continuous health data records from MBS, PBS, APDC, and EDDC over time within the Secured Unified Research Environment (SURE) provided by the Sax Institute (see http://www.cherel.org.au/ for more details).
We like to thank BARMER for providing access to the researcher data warehouse for the German data of this project, especially Joachim Saam and Martial Mboulla‐Nzomo who were incredibly supportive in analyzing and extracting the data. The work representing England uses data provided by patients and collected by the National Health Service (NHS) as part of their care and support. We thank the Data Management Team at the Health Foundation and Dr. Will Parry for their work in preparing the data. The research representing Spain acknowledges the biocomputing unit at the IACS, in particular, Beatriz Gonzalez and Carlos Telleria who implemented the ETL processes to fit Aragonese data into the International Collaborative on Costs, Outcomes, and Needs in Care (ICCONIC) data schema. Further, we like to thank Vishahan Suntharam for his background search on end‐of‐life care and Katharina Blankart for discussing the methods part.
This work representing England is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare Products Regulatory Agency and in part from Hospital Episode Statistics Copyright © (2020), reused with the permission of the Health and Social Care Information Centre. All rights reserved. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. We thank the Data Management Team at the Health Foundation and Dr. Will Parry for their work in preparing the data.
Blankart CR, van Gool K, Papanicolas I, et al. International comparison of spending and utilization at the end of life for hip fracture patients. Health Serv Res. 2021;56(Suppl. 3):1370‐1382. 10.1111/1475-6773.13734
Funding information: The overall work was partially funded by The Commonwealth Fund and the Health Foundation. Canadian data management was supported in part by a grant from the Ontario Ministry of Health to the Health System Performance Network and by a grant to Institute for Clinical Evaluative Sciences (ICES). Walter P. Wodchis is supported by a Research Chair funded by the Trillium Health Partners Foundation. Research representing Spain has been partially financed by the European Regional Development Fund, ISCIII, REDISSEC, grant number RD16/0001/0007
Funding information Commonwealth Fund; European Regional Development Fund, Grant/Award Number: RD16/0001/0007; Health Foundation; Ontario Ministry of Health
REFERENCES
- 1. Higginson IJ. End‐of‐life care: lessons from other nations. J Palliat Med. 2005;8(suppl 1):S161‐S173. 10.1089/jpm.2005.8.s-161 [DOI] [PubMed] [Google Scholar]
- 2. van Beek K, Woitha K, Ahmed N, et al. Comparison of legislation, regulations and national health strategies for palliative care in seven European countries (Results from the Europall Research Group): a descriptive study. BMC Health Serv Res. 2013;13(1):1‐11. 10.1186/1472-6963-13-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson J, Gott M, Gardiner C, Ingleton C. The ‘problematisation’ of palliative care in hospital: an exploratory review of international palliative care policy in five countries. BMC Palliat Care. 2016;15(1):64. 10.1186/s12904-016-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wammes JJG, van der Wees PJ, Tanke MAC, Westert GP, PPT J. Systematic review of high‐cost patients' characteristics and healthcare utilisation. BMJ Open. 2018;8(9):e023113. 10.1136/bmjopen-2018-023113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. French EB, McCauley J, Aragon M, et al. End‐of‐life medical spending in last twelve months of life is lower than previously reported. Health Aff. 2017;36(7):1211‐1217. 10.1377/hlthaff.2017.0174 [DOI] [PubMed] [Google Scholar]
- 6. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high‐need, high‐cost patients – an urgent priority. N Engl J Med. 2016;375(10):909‐911. 10.1056/NEJMp1608511 [DOI] [PubMed] [Google Scholar]
- 7. Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315(3):272‐283. 10.1001/jama.2015.18603 [DOI] [PubMed] [Google Scholar]
- 8. Langton JM, Blanch B, Drew AK, Haas M, Ingham JM, Pearson S‐A. Retrospective studies of end‐of‐life resource utilization and costs in cancer care using health administrative data: a systematic review. Palliat Med. 2014;28(10):1167‐1196. 10.1177/0269216314533813 [DOI] [PubMed] [Google Scholar]
- 9. Faes K, de Frène V, Cohen J, Annemans L. Resource use and health care costs of COPD patients at the end of life: a systematic review. J Pain Symptom Manage. 2016;52(4):588‐599. 10.1016/j.jpainsymman.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 10. Virdun C, Luckett T, Lorenz KA, Phillips J. National quality indicators and policies from 15 countries leading in adult end‐of‐life care: a systematic environmental scan. BMJ Support Palliat Care. 2018;8(2):145. 10.1136/bmjspcare-2017-001432 [DOI] [PubMed] [Google Scholar]
- 11. Singer PA, Bowman KW. Quality care at the end of life. Should be recognised as a global problem for public health and health systems. BMJ. 2002;324(7349):1291‐1292. 10.1136/bmj.324.7349.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7):S51‐S55. http://www.jstor.org/stable/40221969 [DOI] [PubMed] [Google Scholar]
- 13. Busse R, Schreyögg J, Smith PC. Variability in healthcare treatment costs amongst nine EU countries ‐ results from the HealthBASKET project. Health Econ. 2008;17(1 suppl):S1–S8. 10.1002/hec.1330 [DOI] [PubMed] [Google Scholar]
- 14. Busse R. Do diagnosis‐related groups explain variations in hospital costs and length of stay? Analyses from the EuroDRG project for 10 episodes of care across 10 European countries. Health Econ. 2012;21(suppl 2):1–5. 10.1002/hec.2861 [DOI] [PubMed] [Google Scholar]
- 15. Zweifel P, Felder S, Meiers M. Ageing of population and health care expenditure: a red herring? Health Econ. 1999;8(6):485‐496. [DOI] [PubMed] [Google Scholar]
- 16. Seshamani M, Gray A. Time to death and health expenditure: an improved model for the impact of demographic change on health care costs. Age Ageing. 2004;33(6):556‐561. 10.1093/ageing/afh187 [DOI] [PubMed] [Google Scholar]
- 17. Stearns SC, Norton EC. Time to include time to death? The future of health care expenditure predictions. Health Econ. 2004;13(4):315‐327. 10.1002/hec.831 [DOI] [PubMed] [Google Scholar]
- 18. Riley GF, Lubitz JD. Long‐term trends in medicare payments in the last year of life. Health Serv Res. 2010;45(2):565‐576. 10.1111/j.1475-6773.2010.01082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fassbender K, Fainsinger RL, Carson M, Finegan BA. Cost trajectories at the end of life: the Canadian experience. J Pain Symptom Manage. 2009;38(1):75‐80. 10.1016/j.jpainsymman.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 20. Emanuel EJ. Where are the health care cost savings? JAMA. 2012;307(1):39‐40. 10.1001/jama.2011.1927 [DOI] [PubMed] [Google Scholar]
- 21. Pivodic L, Smets T, van den Noortgate N, et al. Quality of dying and quality of end‐of‐life care of nursing home residents in six countries: an epidemiological study. Palliat Med. 2018;32(10):1584‐1595. 10.1177/0269216318800610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pivodic L, Pardon K, Miccinesi G, et al. Hospitalisations at the end of life in four European countries: a population‐based study via epidemiological surveillance networks. J Epidemiol Community Health. 2016;70(5):430‐436. 10.1136/jech-2015-206073 [DOI] [PubMed] [Google Scholar]
- 23. Cohen J, Pivodic L, Miccinesi G, et al. International study of the place of death of people with cancer: a population‐level comparison of 14 countries across 4 continents using death certificate data. Br J Cancer. 2015;113(9):1397‐1404. 10.1038/bjc.2015.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis MA, Nallamothu BK, Banerjee M, Bynum JPW. Identification of four unique spending patterns among older adults in the last year of life challenges standard assumptions. Health Aff. 2016;35(7):1316‐1323. 10.1377/hlthaff.2015.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Einav L, Finkelstein A, Mullainathan S, Obermeyer Z. Predictive modeling of U.S. health care spending in late life. Science. 2018;360(6396):1462. 10.1126/science.aar5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aldridge MD, Kelley AS. The myth regarding the high cost of end‐of‐life care. Am J Public Health. 2015;105(12):2411‐2415. 10.2105/AJPH.2015.302889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rolden HJA, van Bodegom D, Westendorp RGJ. Variation in the costs of dying and the role of different health services, socio‐demographic characteristics, and preceding health care expenses. Soc Sci Med. 2014;120:110‐117. 10.1016/j.socscimed.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 28. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in medicare expenditures and service utilization at the end of life for lung cancer decedents. Womens Health Issues. 2008;18(3):199‐209. 10.1016/j.whi.2008.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma RK, Prigerson HG, Penedo FJ, Maciejewski PK. Male‐female patient differences in the association between end‐of‐life discussions and receipt of intensive care near death. Cancer. 2015;121(16):2814‐2820. 10.1002/cncr.29417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miesfeldt S, Murray K, Lucas L, Chang C‐H, Goodman D, Morden NE. Association of age, gender, and race with intensity of end‐of‐life care for medicare beneficiaries with cancer. J Palliat Med. 2012;15(5):548‐554. 10.1089/jpm.2011.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakagawa K, Vento MA, Seto TB, et al. Sex differences in the use of early do‐not‐resuscitate orders after intracerebral hemorrhage. Stroke. 2013;44(11):3229‐3231. 10.1161/STROKEAHA.113.002814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Temkin‐Greener H, Li Q, Li Y, Segelman M, Mukamel DB. End‐of‐life care in nursing homes: from care processes to quality. J Palliat Med. 2016;19(12):1304‐1311. 10.1089/jpm.2016.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crosby MA, Cheng L, DeJesus AY, Travis EL, Rodriguez MA. Provider and patient gender influence on timing of do‐not‐resuscitate orders in hospitalized patients with cancer. J Palliat Med. 2016;19(7):728‐733. 10.1089/jpm.2015.0388 [DOI] [PubMed] [Google Scholar]
- 34. Ausubel J. Older people are more likely to live alone in the U.S. than elsewhere in the world. https://www.pewresearch.org/fact-tank/2020/03/10/older-people-are-more-likely-to-live-alone-in-the-u-s-than-elsewhere-in-the-world/. Accessed June 11, 2021.
- 35. Huette P, Abou‐Arab O, Djebara A‐E, et al. Risk factors and mortality of patients undergoing hip fracture surgery: a one‐year follow‐up study. Sci Rep. 2020;10(1):9607. 10.1038/s41598-020-66614-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364‐370. 10.1046/j.1532-5415.2003.51110.x [DOI] [PubMed] [Google Scholar]
- 37. Figueroa JF, Horneffer KE, Riley K, Abiona O, Arvin M, Atsma F. A methodology for identifying high‐need, high‐cost patient personas for international comparisons: The International Collaborative on Costs, Outcomes, and Needs in Care (ICCONIC) Project; 2021. [DOI] [PMC free article] [PubMed]
- 38. Kassim Javaid M, Chana J, Cooper C. Hip fracture as the tracer condition. Best Pract Res Clin Rheumatol. 2013;27(6):711‐715. 10.1016/j.berh.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 39. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726‐1733. 10.1007/s00198-006-0172-4 [DOI] [PubMed] [Google Scholar]
- 40. Leal J, Gray AM, Prieto‐Alhambra D, Arden NK, Cooper C, Javaid MK. Impact of hip fracture on hospital care costs: a population‐based study. Osteoporos Int. 2016;27(2):549‐558. 10.1007/s00198-015-3277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williamson S, Landeiro F, McConnell T, et al. Costs of fragility hip fractures globally: a systematic review and meta‐regression analysis. Osteoporos Int. 2017;28(10):2791‐2800. 10.1007/s00198-017-4153-6 [DOI] [PubMed] [Google Scholar]
- 42. Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103(2 suppl 1):S12‐S19. 10.1016/S0002-9343(97)90022-X [DOI] [PubMed] [Google Scholar]
- 43. European Union, OECD . Eurostat‐OECD Methodological Manual on Purchasing Power Parities. Luxembourg: Publications Office of the European Union; 2012. https://www.oecd.org/sdd/prices-ppp/PPP%20manual%20revised%202012.pdf. [Google Scholar]
- 44. Papanicolas I, Riley K, Abiona O, Arvin M, Atsma F, Bernal‐Delgado E. Differences in health outcomes for high‐need, high‐cost patients across high‐income countries; 2021. https://onlinelibrary.wiley.com/doi/10.1111/1475-6773.13735 [DOI] [PMC free article] [PubMed]
- 45. R Core Team . R: A Language and Environment for Statistical Computing: Version 4.0.3 (2020‐10‐10) – “Bunny‐Wunnies Freak Out”. Vienna, Austria: R Foundation for Statistical Comput Secur; 2020. https://www.R-project.org. [Google Scholar]
- 46. Gomes B, Higginson IJ, Calanzani N, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol. 2012;23(8):2006‐2015. 10.1093/annonc/mdr602 [DOI] [PubMed] [Google Scholar]
- 47. Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12(1):7. 10.1186/1472-684X-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Broad JB, Gott M, Kim H, Boyd M, Chen H, Connolly MJ. Where do people die? An international comparison of the percentage of deaths occurring in hospital and residential aged care settings in 45 populations, using published and available statistics. Int J Public Health. 2013;58(2):257‐267. 10.1007/s00038-012-0394-5 [DOI] [PubMed] [Google Scholar]
- 49. Pivodic L, Pardon K, Morin L, et al. Place of death in the population dying from diseases indicative of palliative care need: a cross‐national population‐level study in 14 countries. J Epidemiol Community Health. 2016;70(1):17‐24. 10.1136/jech-2014-205365 [DOI] [PubMed] [Google Scholar]
- 50. Jayatunga W, Lewer D, Shand J, Sheringham J, Morris S, George J. Health and social care costs at the end of life: a matched analysis of linked patient records in East London. Age Ageing. 2020;49(1):82‐87. 10.1093/ageing/afz137 [DOI] [PubMed] [Google Scholar]
- 51. Clark D, Wright M. The international observatory on end of life care: a global view of palliative care development. J Pain Symptom Manage. 2007;33(5):542‐546. 10.1016/j.jpainsymman.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 52. Mann S, Galler D, Williams P, Frost P. Caring for patients and families at the end of life: withdrawal of intensive care in the patient's home. N Z Med J. 2004;117(1196):U935. [PubMed] [Google Scholar]
- 53. Pollock K. Is home always the best and preferred place of death? BMJ. 2015;351:h4855. 10.1136/bmj.h4855 [DOI] [PubMed] [Google Scholar]
- 54. Papanicolas I, Figueroa JF, Schoenfeld AJ, Riley K, Abiona O, Atsma F. Differences in health care spending & utilization among frail elders in high‐income countries: ICCONIC Hip Fracture Persona; 2021. [DOI] [PMC free article] [PubMed]
- 55. López‐Valcárcel BG, Pinilla J, Barber P. Dying at home for terminal cancer patients: differences by level of education and municipality of residence in Spain. Gac Sanit. 2019;33(6):568‐574. 10.1016/j.gaceta.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 56. Lorenzoni L, Belloni A, Sassi F. Health‐care expenditure and health policy in the USA versus other high‐spending OECD countries. Lancet. 2014;384(9937):83‐92. 10.1016/S0140-6736(14)60571-7 [DOI] [PubMed] [Google Scholar]
- 57. Papanicolas I, Woskie LR, Jha AK. Health care spending in the united states and other high‐income countries. JAMA. 2018;319(10):1024‐1039. 10.1001/jama.2018.1150 [DOI] [PubMed] [Google Scholar]
- 58. Gupta A, Jin G, Reich A, et al. Association of billed advance care planning with end‐of‐life care intensity for 2017 medicare decedents. J Am Geriatr Soc. 2020;68(9):1947‐1953. 10.1111/jgs.16683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wodchis W, Or Z, Blankart CR, Atsma F, Janlov N, Bai YQ. An International Comparison of Long‐Term Care Trajectories and Spending Following Hip Fracture; 2021. https://onlinelibrary.wiley.com/doi/10.1111/1475-6773.13864 [DOI] [PMC free article] [PubMed]
- 60. Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late‐life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2(1):1. 10.1186/1471-2318-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in‐hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698‐705. 10.1097/MLR.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 62. Lorenzoni L, Marino A, Or Z, Shatrov K, Blankart CR, Figueroa JF. International comparison of health care consumed by highneed, high‐cost individuals using pathway‐specific purchasing power parities; 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


