Key Points
Question
Is myocardial ischemia provoked by mental stress associated with adverse cardiovascular events in patients with coronary heart disease?
Findings
In this pooled analysis of 2 prospective cohort studies that included 918 participants, the presence of ischemia with mental stress, compared with no ischemia with mental stress, was significantly associated with an increased risk of cardiovascular death or nonfatal myocardial infarction (hazard ratio, 2.5).
Meaning
Mental stress–induced myocardial ischemia was significantly associated with an increased risk of cardiovascular events in patients with coronary heart disease, but further research is needed to assess whether testing for mental-stress ischemia has utility in clinical practice.
Abstract
Importance
Mental stress–induced myocardial ischemia is a recognized phenomenon in patients with coronary heart disease (CHD), but its clinical significance in the contemporary clinical era has not been investigated.
Objective
To compare the association of mental stress–induced or conventional stress–induced ischemia with adverse cardiovascular events in patients with CHD.
Design, Setting, and Participants
Pooled analysis of 2 prospective cohort studies of patients with stable CHD from a university-based hospital network in Atlanta, Georgia: the Mental Stress Ischemia Prognosis Study (MIPS) and the Myocardial Infarction and Mental Stress Study 2 (MIMS2). Participants were enrolled between June 2011 and March 2016 (last follow-up, February 2020).
Exposures
Provocation of myocardial ischemia with a standardized mental stress test (public speaking task) and with a conventional (exercise or pharmacological) stress test, using single-photon emission computed tomography.
Main Outcomes and Measures
The primary outcome was a composite of cardiovascular death or first or recurrent nonfatal myocardial infarction. The secondary end point additionally included hospitalizations for heart failure.
Results
Of the 918 patients in the total sample pool (mean age, 60 years; 34% women), 618 participated in MIPS and 300 in MIMS2. Of those, 147 patients (16%) had mental stress–induced ischemia, 281 (31%) conventional stress ischemia, and 96 (10%) had both. Over a 5-year median follow-up, the primary end point occurred in 156 participants. The pooled event rate was 6.9 per 100 patient-years among patients with and 2.6 per 100 patient-years among patients without mental stress–induced ischemia. The multivariable adjusted hazard ratio (HR) for patients with vs those without mental stress–induced ischemia was 2.5 (95% CI, 1.8-3.5). Compared with patients with no ischemia (event rate, 2.3 per 100 patient-years), patients with mental stress–induced ischemia alone had a significantly increased risk (event rate, 4.8 per 100 patient-years; HR, 2.0; 95% CI, 1.1-3.7) as did patients with both mental stress ischemia and conventional stress ischemia (event rate, 8.1 per 100 patient-years; HR, 3.8; 95% CI, 2.6-5.6). Patients with conventional stress ischemia alone did not have a significantly increased risk (event rate, 3.1 per 100 patient-years; HR, 1.4; 95% CI, 0.9-2.1). Patients with both mental stress ischemia and conventional stress ischemia had an elevated risk compared with patients with conventional stress ischemia alone (HR, 2.7; 95% CI, 1.7-4.3). The secondary end point occurred in 319 participants. The event rate was 12.6 per 100 patient-years for patients with and 5.6 per 100 patient-years for patients without mental stress–induced ischemia (adjusted HR, 2.0; 95% CI, 1.5-2.5).
Conclusions and Relevance
Among patients with stable coronary heart disease, the presence of mental stress–induced ischemia, compared with no mental stress–induced ischemia, was significantly associated with an increased risk of cardiovascular death or nonfatal myocardial infarction. Although these findings may provide insights into mechanisms of myocardial ischemia, further research is needed to assess whether testing for mental stress–induced ischemia has clinical value.
This study examines whether mental stress–induced ischemia is related to adverse cardiovascular events in 2 parallel modern cohorts of patients with stable coronary heart disease.
Introduction
There is growing evidence of a link between psychological stress and the risk of coronary heart disease (CHD).1 In approximately 17% patients with clinically stable CHD, acute mental stress in the laboratory can trigger myocardial ischemia detected with myocardial perfusion imaging.2,3,4,5 In contrast to myocardial ischemia provoked by a conventional test, such as exercise stress testing, mental stress–induced myocardial ischemia is usually asymptomatic, occurs at a lower hemodynamic workload, and can occur in patients who do not have a positive conventional stress test result.2,5 Factors that can occur in patients with mental stress–induced ischemia include abnormal vasomotion, psychological conditions, and platelet reactivity, although the exact mechanisms remain unclear.6,7
Although mental stress–induced ischemia has been recognized as a common phenomenon in stable patients with CHD, little information is available on its prognostic significance. In a limited number of studies, mental stress–induced ischemia was associated with increased risk of adverse cardiovascular events.8 These studies were small, most were conducted prior to 1995, and largely enrolled White men. Furthermore, none of these investigations used myocardial perfusion imaging, the current standard for ischemia detection.7 In addition, the majority of previous studies enrolled only patients with conventional stress ischemia; thus, they were unable to examine the role of mental stress–induced ischemia in combination or in contrast with conventional stress ischemia.
This study was conducted to examine whether mental stress–induced ischemia is related to adverse cardiovascular events in 2 parallel modern cohorts of patients with stable CHD. All patients underwent measurement of mental stress–induced ischemia and conventional stress ischemia using myocardial perfusion imaging with single-photon emission computed tomography (SPECT).
Methods
Study Participants and Protocol Overview
The research protocol for both studies was approved by the Institutional Review Board of Emory University and all participants provided written informed consent.
Between June 2011 and March 2016, we enrolled individuals with stable CHD in 2 parallel studies with similar protocols, the Mental Stress Ischemia Prognosis Study (MIPS)9 and the Myocardial Infarction and Mental Stress Study 2 (MIMS2).3 Both studies recruited patients with stable CHD from hospitals and clinics affiliated with Emory University and shared protocols, staff, facilities, and equipment. For MIPS, patients were eligible to participate if they were 30 to 79 years of age and had a documented history of CHD.9 For MIMS2, patients were included if they were hospitalized for a verified myocardial infarction (MI) within the past 8 months and were 18 to 60 years of age at the time of the MI.3 MIMS2 also included 50% women by design. Patients were excluded from both studies if they were pregnant or if they had medical comorbidities expected to shorten life expectancy; other exclusion criteria are listed in eFigure 1 in the Supplement. A total of 57 patients enrolled in MIMS2 also participated in MIPS. To avoid overlap, these patients were excluded from the analysis.
At the baseline visit, patients underwent clinical and psychosocial assessments, a standardized mental stress test, and SPECT imaging with mental stress and with exercise or pharmacological stress (Table 1).
Table 1. Characteristics of the Study Populations.
| Patients with mental stress–induced ischemia, No. (%) | ||||
|---|---|---|---|---|
| MIPS | MIMS2 | |||
| Yes (n = 96) | No (n = 522) | Yes (n = 51) | No (n = 249) | |
| Demographic factors | ||||
| Age, mean (SD), y | 63.9 (8.4) | 63.9 (8.7) | 49.6 (8.1) | 51.1 (6.3) |
| Sex | ||||
| Women | 19 (20) | 145 (28) | 31 (61) | 119 (48) |
| Men | 77 (80) | 377 (72) | 20 (39) | 130 (52) |
| Racea | ||||
| American Indian or Alaska Native | 3 (3) | 4 (1) | 0 | 2 (1) |
| Asian | 1 (1) | 19 (4) | 1 (2) | 6 (2) |
| Black or African American | 31 (32) | 141 (27) | 37 (72) | 161 (65) |
| ≥1 Race | 0 | 0 | 3 (6) | 5 (2) |
| Pacific Islander | 0 | 1 (<1) | 0 | 0 |
| White | 61 (64) | 357 (68) | 10 (20) | 75 (30) |
| Cardiovascular risk factors | ||||
| Hyperlipidemia | 80 (83) | 431 (83) | 41 (80) | 199 (80) |
| Hypertension | 75 (78) | 387 (74) | 44 (86) | 200 (80) |
| Diabetes | 38 (40) | 165 (32) | 18 (35) | 78 (31) |
| Smoking status | ||||
| Ever | 63 (66) | 308 (59) | 25 (49) | 142 (57) |
| Current | 10 (10) | 63 (12) | 11 (22) | 61 (25) |
| BMI, mean (SD) | 30.2 (5.2) | 29.5 (5.3) | 31.3 (9.4) | 31.2 (6.8) |
| Clinical characteristics | ||||
| ≥1 Coronary vessel with 70% stenosis, No./total. (%) | 68/80 (85) | 374/451 (83) | 42/47 (89) | 194/233 (83) |
| Coronary artery revascularization | 79 (82) | 389 (75) | 44 (86) | 198 (79) |
| LVEF, mean (SD), %b | 62.4 (14.5) | 67.5 (14.1) | 45.0 (14.8) | 52.0 (11.0) |
| MI | 34 (35) | 161 (31) | 51 (100) | 249 (100) |
| Heart failure | 33 (34) | 112 (22) | 8 (16) | 23 (9) |
| Selected medications | ||||
| Aspirin | 82 (85) | 451 (86) | 44 (86) | 199 (80) |
| Statins | 81 (84) | 447 (86) | 45 (88) | 210 (84) |
| β-Blockers | 72 (75) | 379 (73) | 47 (92) | 211 (85) |
| ACE inhibitors | 51 (53) | 228 (44) | 28 (55) | 111 (45) |
| Clopidogrel | 33 (34) | 163 (31) | 36 (71) | 175 (70) |
| Antidepressants | 21 (22) | 131 (25) | 8 (16) | 44 (18) |
| Conventional stress testing, No./total (%) | ||||
| Conventional stress–induced ischemiac | 68/94 (72) | 139/510 (27) | 28/51 (55) | 46/244 (19) |
| Underwent exercise stress testing | 54/94 (57) | 356/410 (70) | 36/51 (71) | 171/244 (70) |
| Time on the treadmill, mean (SD), min | 7.2 (2.3) | 7.7 (2.4) | 6.8 (2.5) | 7.7 (2.7) |
| No. | 54 | 356 | 36 | 170 |
| Underwent pharmacological stress testing | 40/94 (43) | 154/410 (30) | 15/51 (29) | 73/244 (30) |
| Mental stress testing, mean (SD) | ||||
| Blood pressure, change, mm Hgd | ||||
| Systolic | 29.3 (17.8) | 25.7 (15.3) | 40.3 (17.6) | 40.5 (16.4) |
| Diastolic | 14.2 (9.9) | 12.6 (8.2) | 30.9 (12.8) | 28.0 (11.3) |
| Heart rate, change, beats/mind | 13.6 (13.2) | 10.4 (7.9) | 24.6 (16.8) | 23.0 (14.2) |
| RPP, change, per 1000d | 4.2 (3.1) | 3.3 (2.1) | 6.4 (3.2) | 6.2 (3.0) |
| Subjective Units of Distress Scale, changee | 9.4 (14.7) | 10.5 (19.3) | 24.5 (32.9) | 26.2 (30.2) |
| Psychological factors, mean (SD) | ||||
| Beck Depression Inventory IIf | 7.8 (8.4) | 8.2 (8.4) | 13.7 (9.6) | 12.1 (10.7) |
| Cohen Perceived Stress Scale 10g | 11.7 (8.2) | 12.2 (7.3) | 18.8 (8.5) | 16.0 (8.5) |
| State anxietyh | 30.2 (11.7) | 31.0 (10.8) | 40.4 (13.7) | 35.2 (12.7) |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study; RPP, rate-pressure product.
Race was self-reported using predetermined categories.
In MIPS, LVEF was derived from myocardial perfusion imaging; in MIMS2, from echocardiography, coronary angiography, or myocardial perfusion imaging (whichever was available in this order) at the time of the hospitalization for MI.
Patients underwent a standard Bruce protocol. Time on treadmill included the time from the beginning to the end of the Bruce protocol or if the patients developed fatigue, or serious symptoms or complications, according to prespecified stopping rules.
Difference between maximum value during mental stress (the 3 minutes of speaking task) and minimum value during rest.
Subjective rating of distress on a linear scale of 0 to 100, with 100 being most distressed.
Measures depressive symptoms on a continuous scale, using 21 questions, each scored on a scale of 0 to 3 (total scale score range, 0-63); a higher score denotes more depressive symptoms; a score of 14, mild depression.
Measures general stress on a continuous scale using 10 questions, each scored on a scale of 0 to 4 (total score range, 0-40; a higher score denotes more distress).
Measures state anxiety on a continuous scale, using 20 questions, each scored on a scale of 1 to 4 (total score range, 1-80; a higher score denotes more anxiety).
Mental Stress Procedure
Patients were tested in the morning after a 12-hour fast. Mental stress was induced by a standardized public speaking task as previously described.9 Patients were given 2 minutes to prepare a speech and 3 minutes to deliver it in front of an evaluative audience of at least 4 people. Blood pressure and heart rate were recorded throughout the test, and the rate-pressure product was calculated. Subjective ratings of distress were obtained with the Subjective Units of Distress Scale.10
Myocardial Perfusion Imaging
We performed 3 technetium Tc 99m (99mTC) sestamibi SPECT scans at rest, during mental stress, and during conventional stress, following standard protocols.9 Testing was performed on 2 separate days up to 1 week apart on a dedicated SPECT camera (Philips Cardio MD). Antianginal medications were withheld for 24 hours prior to stress testing. On the mental stress day, 99mTC sestamibi was injected 1 minute into the speech task. On the conventional stress day, participants underwent a standard Bruce protocol, or, if unable to exercise, a pharmacological stress test with regadenoson (Astellas).
SPECT scans were interpreted by 2 experienced readers blinded to stress test type (mental or exercise or pharmacological stress) and other clinical data. Discrepancies were resolved by consensus or, if needed, a third reader. Rest and stress images were visually compared using a 17-segment model. Each segment was scored from 0 to 4, with 0 being normal uptake, and 4 no uptake. Current guidelines define ischemia as at least 1 reversible defect with 2 or more points of improvement at rest or improvement to a score of 1.11 We defined ischemia more conservatively by segment, as a defect with 2 or more points of improvement in a single segment or 1 or more in contiguous segments. This allowed the use of a computer algorithm applied to the visually interpreted images. To evaluate the robustness of the results, a summed difference score was calculated through the entire myocardium11 and ischemia was defined as a summed difference score of 4 or more.12 The interrater reliability and reproducibility for the interpretation of SPECT images was previously published.13 Bland-Altman plots for assessing reproducibility of mental stress–induced ischemia using 99mTc sestamibi SPECT demonstrated 90% agreement for repeated testing 2 weeks apart, and 94% agreement between readers.
Other Measures
Information on sociodemographic and clinical factors was obtained using standardized questionnaires and chart reviews. Depressive symptoms were assessed with the Beck Depression Inventory II,14 general stress with the 10-item Cohen Perceived Stress Scale,15 and state anxiety with the Spielberger State-Trait Anxiety Inventory.16 Height and weight were measured to calculate body mass index (BMI). Angiographic data were obtained from the most recent coronary angiogram in the patient’s chart. Race and ethnicity were reported by participants according to investigator-defined categories. Because few participants were of race other than non-Hispanic Black or non-Hispanic White, only 2 categories were used in the models, non-Hispanic Black vs all others. Race was included in the analysis to serve as a proxy for complex social exposures that may influence both stress responses and cardiovascular risk (Table 1).
Follow-up and Study Outcomes
Follow-up data were collected through patient contacts, medical record review, and the Social Security Death Index. If hospitalizations or procedures were reported, patients’ physicians were contacted and hospital records obtained. MIPS patients were contacted at 6-month intervals for the first 3 years and then at 5 years. MIMS2 patients were contacted at their approximate 3- and 5-year anniversary from the initial visit. First and recurrent events were adjudicated by study cardiologists blinded to other study data.
Because CHD is characterized by recurrent nonfatal cardiovascular events, considering all such events is a more accurate reflection of the burden of disease.17 Thus, the primary end point was a composite of cardiovascular death or nonfatal MI (first and recurring MI). The secondary end point was a composite of cardiovascular death, nonfatal MI, and hospitalizations for heart failure (first and recurring events).
Statistical Analysis
Patient characteristics, including demographic, clinical and psychosocial factors, and hemodynamic responses to mental stress, were described by mental stress–induced ischemia status for each cohort. We plotted mean cumulative function curves for the study end points in patients with and without mental stress–induced ischemia. For multivariable analysis, we used the Wei-Lin-Weissfeld model for recurrent events,18 which allows a separate underlying hazard for each event, and is more flexible than other methods. Model diagnostics were performed by inspecting Schoenfeld residuals and by testing interactions with follow-up time to examine the proportional hazards assumption, which was met. Martingale residuals were used to assess nonlinearity of continuous terms, which was also found to be acceptable. Sequential models were constructed to derive hazards ratios (HRs) and 95% CIs after adjusting for prespecified variables. The first model adjusted for demographic factors, including age, sex, and race. In model 2 we added cardiovascular risk factors and other relevant medical history, including ever smoking, BMI, history of hypertension, diabetes, heart failure (MIPS) or left ventricular ejection fraction (MIMS2), and previous MI (MIPS) or MI type, ie, ST-elevation MI (STEMI) or non-STEMI (MIMS2). In model 3, we added current medications (β-blockers, statins, angiotensin-converting enzyme inhibitors, and aspirin). In model 4, we added psychological factors, including depressive symptoms (Beck Depression Inventory II score) and perceived stress (Perceive Stress Scale score).
Given the similarity of results in MIPS and MIMS2, we pooled the 2 cohorts to provide an overall estimate of effect. We used an individual patient data meta-analysis approach with random effects to preserve the clustering within studies.19 In the pooled data, we also examined whether the association of mental stress–induced ischemia with outcome varied according to a priori selected strata, including age (≤60 vs >60 years), sex, race (Black vs non-Black), previous MI, history of diabetes, history of heart failure, and presence or absence of conventional stress ischemia, by adding the corresponding interactions in the models. Because of the potential for type I error due to multiple comparisons, findings for secondary analyses and end points should be interpreted as exploratory.
We conducted a number of sensitivity analyses. To address a possible concern of model overfitting, we ran a reduced model that adjusted for only 7 variables: sex, age, race, previous MI, and a history of diabetes, hypertension, or heart failure. We used the summed difference score as a continuous measure of ischemic burden, as well as a summed difference score of 4 or more as an alternative definition of ischemia. We also assessed whether the association for mental stress–induced ischemia persisted after adjusting for conventional stress ischemia, and among those who had undergone an exercise stress test, whether the association persisted after adjustment for duration of exercise. In an additional sensitivity analysis, we used Cox proportional hazards regression for first events to examine consistency of results.
We also examined the prognostic value of different ischemia phenotypes: only mental stress–induced ischemia, only conventional stress ischemia, or both compared with the group without ischemia. Missing data were rare (<3%); thus, no imputation was done. As a consequence, some models included fewer observations. We used SAS (version 9.4; SAS Institute Inc). Two-sided P values <.05 were considered statistically significant.
Results
Study Sample and Baseline Characteristics
Of the 638 patients in MIPS, 13 were excluded because of missing or inadequate quality of SPECT images, 5 were lost to follow-up, and 2 withdrew before completion of baseline assessments, leaving 618 patients for analysis (eFigure 1 in the Supplement). Of the 313 patients in MIMS2, 7 were excluded because of inadequate imaging data, and 6 were lost to follow-up, resulting in 300 patients for analysis. For analyses involving conventional stress testing, 14 patients in MIPS and 5 in MIMS2 were further excluded for lack of imaging data. Other missing data were rare; the most missing values were among psychosocial factors: 16 (2%) in MIPS and 5 (2%) in MIMS2.
By design, participants in MIMS2 were younger and more often women than those in MIPS. Of the MIPS patients, 96 (15%) demonstrated mental stress–induced ischemia, and in MIMS2, 51 (17%) demonstrated mental stress–induced ischemia. The corresponding frequency of conventional stress ischemia was 207 (34%) and 74 (25%). Patients with mental stress–induced ischemia had more frequent history of heart failure and a lower ejection fraction than those without, but other demographic and clinical data were similar. In the MIPS cohort those with mental stress–induced ischemia developed higher blood pressure and heart rate during mental stress than those without, but these differences were not observed in the MIMS2 sample (Table 1).
Association of Mental Stress–Induced Ischemia With Adverse Cardiovascular Events
The median follow-up time was 6.0 years (IQR, 5.6-6.0 years) in MIPS and 4.6 years (IQR, 3.8-5.3 years) in MIMS2. The follow-up was completed in February 2020.
For the primary end point, there were 90 total (first and repeated) events in MIPS, and 66 in MIMS2. Corresponding numbers for the secondary end point were 166 and 153. Both first and recurrent events occurred more often in patients with mental stress–induced ischemia than without it (Table 2; eFigure 2 in Supplement).
Table 2. Incidence of Cardiovascular Outcomes by Mental Stress–Induced Myocardial Ischemia.
| Patients with mental stress–induced ischemia, No. (%) | ||||
|---|---|---|---|---|
| MIPS | MIMS2 | |||
| Yes (n = 96) | No (n = 522) | Yes (n = 51) | No (n = 249) | |
| Eventsa | ||||
| Cardiovascular death or MI | ||||
| Patients | ||||
| With first events | 16 (16.7) | 54 (10.3) | 14 (27.5) | 33 (13.3) |
| With recurrent events | 6 (6.3) | 7 (1.3) | 7 (13.7) | 5 (2.0) |
| Total No. of events (first and recurrent) | 26 | 64 | 27 | 39 |
| Cardiovascular death, MI, or heart failure | ||||
| Patients | ||||
| With first events | 22 (22.9) | 76 (14.5) | 20 (39.2) | 52 (20.9) |
| With recurrent events | 12 (12.5) | 25 (4.8) | 11 (21.6) | 18 (7.2) |
| Total No. of events (first and recurrent) | 43 | 123 | 54 | 99 |
| Cardiovascular death | 6 (6.3) | 23 (4.4) | 6 (11.7) | 9 (3.6) |
| All-cause death | 10 (10.4) | 45 (8.6) | 7 (9.3) | 13 (2.5) |
| MI | ||||
| Patients | ||||
| With first events | 10 (10.4) | 31 (5.9) | 11 (21.6) | 26 (10.4) |
| With recurrent events | 4 (4.2) | 4 (0.8) | 3 (5.9) | 3 (1.2) |
| Total No. of events (first and recurrent) | 16 | 36 | 14 | 29 |
| Hospitalization for heart failure | ||||
| Patients | ||||
| With first events | 7 (7.9) | 33 (6.3) | 12 (23.5) | 25 (10.0) |
| With recurrent events | 5 (5.2) | 13 (2.5) | 6 (11.8) | 14 (5.6) |
| Total No. of events (first and recurrent) | 17 | 59 | 27 | 60 |
| Total observation period, mo (IQR)a | 72.3 (70.2-72.3) | 72.3 (67.8-72.3) | 56.7 (46.0-68.7) | 55.5 (46.2-63.4) |
Abbreviations: MI, myocardial infarction; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study.
The total observation period was the same for all end points. The median (IQR) observation was 6.0 years (IQR, 5.6-6.0 years) for MIMPS and 4.6 years (IQR, 3.8 to 5.3 years) for MIMS2.
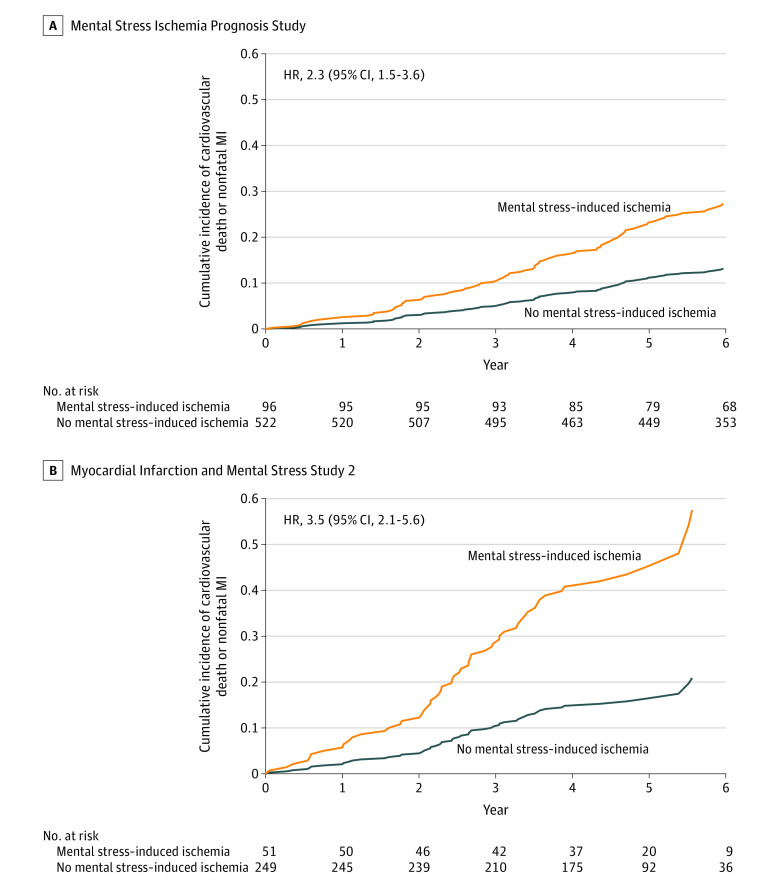
In both populations, individuals with mental stress–induced ischemia had a significantly elevated risk of both study end points compared with those without it (Table 3, Figure 1; eFigure 3 in Supplement). In MIPS, patients with mental stress–induced ischemia had an event rate for the primary end point of 4.9 per 100 patient-years vs 2.2 per 100 patient-years for those without. In MIMS2, the corresponding event rates were 11.5 and 3.7. The unadjusted HR for the primary end point was 2.3 (95% CI, 1.5-3.6) in MIPS and 3.5 (95% CI, 2.1-5.6) in MIMS2. Adjustment for demographic, clinical, and psychological factors did not eliminate the association (Table 3).
Table 3. Association Between Mental Stress–Induced Myocardial Ischemia and the Study End Points in Each of the 2 Study Populations.
| MIPS | MIMS2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mental stress–induced ischemia | Rate difference per 100 patient-years (95% CI) | HR (95% CI)a | Mental stress–induced ischemia | Rate difference per 100 patient-years (95% CI) | HR (95% CI)a | |||
| Yes (n = 96) | No (n = 522) | Yes (n = 51) | No (n = 249) | |||||
| Cardiovascular death or nonfatal MI b | ||||||||
| Total No. of events | 26 | 64 | 27 | 39 | ||||
| Rate per 100 patient-years | 4.9 | 2.2 | 2.7 (0.7-4.7) | 2.29 (1.45-3.62) | 11.5 | 3.7 | 7.8 (3.3-12.3) | 3.45 (2.11-5.64) |
| Adjusted for demographic factorsc | 2.30 (1.46-3.64) | 3.57 (2.17-5.89) | ||||||
| Above variables + clinical risk factorsd | 2.11 (1.33-3.36) | 2.88 (1.70-4.88) | ||||||
| Above variables + medicationse | 2.02 (1.26-3.23) | 2.80 (1.63-4.81) | ||||||
| Above variables + psychological factorsf | 2.04 (1.27-3.25) | 2.71 (1.56-4.71) | ||||||
| Cardiovascular death, nonfatal MI, or heart failure hospitalization b | ||||||||
| Total No. of events | 43 | 123 | 54 | 99 | ||||
| Rate per 100 patient-years | 8.1 | 4.3 | 3.8 (1.3-6.3) | 2.01 (1.42-2.85) | 22.9 | 9.3 | 13.6 (7.2-20.0) | 2.78 (1.99-3.87) |
| Adjusted for demographic factorsc | 1.96 (1.38-2.77) | 2.77 (1.98-3.88) | ||||||
| Above variables + clinical risk factorsd | 1.75 (1.23-2.50) | 2.04 (1.43-2.92) | ||||||
| Above variables + medicationse | 1.71 (1.19-2.44) | 2.02 (1.42-2.89) | ||||||
| Above variables + psychological factorsf | 1.62 (1.13-2.32) | 2.01 (1.39-2.91) | ||||||
Abbreviations: HR, hazard ratio; MI, myocardial infarction; MIMS2, Myocardial Infarction and Mental Stress Study 2; MIPS, Mental Stress Ischemia Prognosis Study.
The HRs compare the incidence of outcome events between patients who were mental stress–ischemia positive and those who were mental stress–ischemia negative (reference) at baseline.
The median observation period was 6.0 years (IQR, 5.6-6.0 years) for MIPS and 4.6 years (IQR, 3.8-5.3 years) for MIMS2.
Age, sex, and race (Black vs non-Black participants).
Ever smoking, body mass index, history of hypertension, history of diabetes, history of dyslipidemia, history of heart failure (MIPS) or left ventricular ejection fraction (MIMS2), and previous MI (MIPS) or MI type, ie, ST-segment elevation MI or non- ST-segment elevation MI (MIMS2).
β-Blockers, statins, angiotensin-converting enzyme inhibitors, and aspirin.
Depressive symptoms (Beck Depression Inventory II score) and perceived stress (Cohen Perceive Stress Scale 10 score). These scales were treated as continuous variables. (See footnotes to Table 1 for descriptions of these scales).
Figure 1. Cumulative Incidence of the Primary Study End Point in Each of the 2 Study Populations.
Because of the repeated events analysis, patients were removed from the risk set at each time point only if they died or were censored.
A, The median observation period was 72.3 months (IQR, 70.2-72.3 months) for patients with mental stress ischemia and 72.3 months (IQR, 67.8-72.3 months) for patients without mental stress ischemia.
B, The median observation period was 56.7 months (IQR, 46.0-68.7 months) for patients with mental stress ischemia and 55.5 months (IQR, 46.2-63.4 months) for patients without mental stress ischemia.
HR indicates hazard ratio; MI, myocardial infarction.
Pooling the 2 study populations with meta-analysis yielded a total sample of 918 participants with a mean age of 60 years (SD, 10 years), 40% Black, 55% White, and 34% women. Of the total sample, 147 (16%) had mental stress–induced ischemia, 281 (31%) had conventional stress ischemia, and 96 (10%) had both. The pooled event rate was 6.9 per 100 patient-years for patients with and 2.6 per 100 patient-years for those without mental stress–induced ischemia; the pooled adjusted HR comparing patients with vs without for the primary end point was 2.5 (95% CI, 1.8-3.5). For the secondary end point, the event rate was 12.6 per 100 patient-years for patients with mental stress–induced ischemia and 5.6 per 100 patient-years for those without mental stress–induced ischemia (adjusted HR, 2.0 (95% CI, 1.5-2.5). The corresponding absolute rate difference was 4.3 (95% CI, 2.4-6.2) per 100 patient-years for the primary end point and 7.0 (95% CI, 4.4-9.6) per 100 patient-years for the secondary end point. In the reduced model, the results remained similar: The HR was 2.4 (95% CI, 1.7-3.4) for the primary end point and was 2.0 (95% CI, 1.5-2.5) for the secondary end point.
In analyses stratified on baseline factors, most of the interactions were not significant (Figure 2). The association, however, was stronger among men than women (P = .01 for interaction) for the primary end point. A similar interaction with sex, however, was not present for the secondary end point (eFigure 4 in the Supplement).
Figure 2. Pooled Estimates and Plots for the Association of Mental Stress–Induced Myocardial Ischemia With the Primary End Point of Cardiovascular Death or Myocardial Infarction.
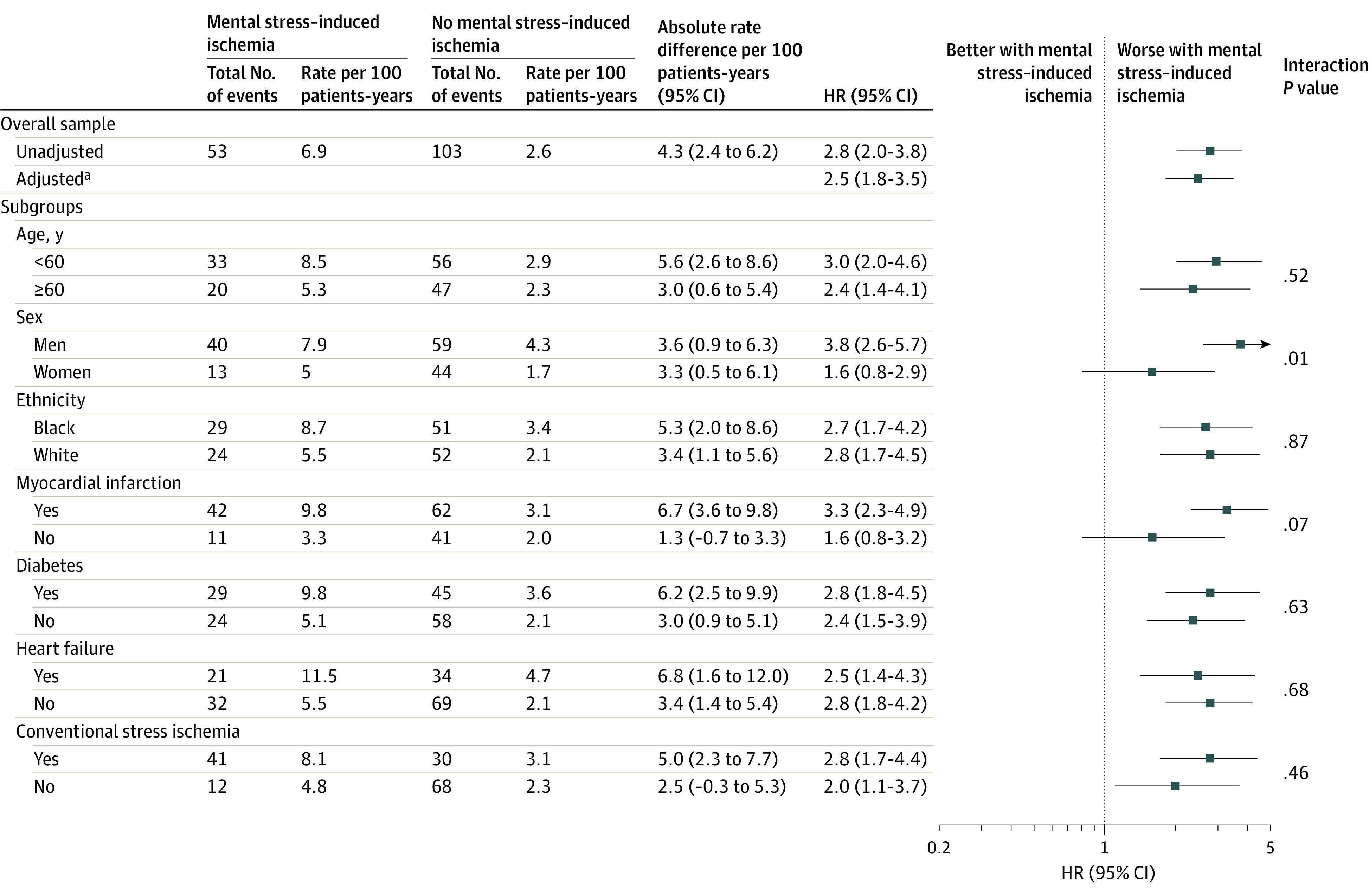
The 2 study populations were pooled using individual patient data meta-analysis. For the overall sample, both unadjusted and fully adjusted estimates are shown. For the subgroups, the estimates are shown as unadjusted.
aAdjusted for demographic factors, clinical factors, and medications
The distribution of the summed difference score of myocardial perfusion was similar for mental stress–induced ischemia and conventional stress ischemia (median, 2; IQR, 1-2). When using this score as a continuous measure and adopting the alternative definition of ischemia of a summed difference score of 4 or more, the results remained consistent (eTable 1 in the Supplement). The adjusted HR per point increase in the summed difference score with mental stress was 1.14 (95% CI, 1.08-1.21), and for a score of 4 or more compared with a score of less than 4, it was 2.3 (95% CI, 1.6-3.4). Furthermore, adjustment for duration of exercise among patients who had undergone an exercise stress test did not diminish the association between mental stress–induced ischemia and outcome (HR, 3.0; 95% CI, 1.8-5.1). The results of Cox proportional hazards regression for first events yielded consistent results, with an adjusted HR of 1.7 (95% CI, 1.1-2.6) for the primary end point and 1.5 (95% CI, 1.1-2.2) for the secondary end point.
Conventional stress ischemia was significantly associated with the study outcomes when considered irrespective of presence of mental stress–induced ischemia. For the primary end point, the event rate for patients with conventional stress ischemia was 4.8 per 100 patient-years; for those without conventional stress ischemia, it was 2.5 per 100 patient-years, with an adjusted HR of 2.0 (95% CI, 1.5-2.8). For the secondary end point, respective event rates were 8.4 and 5.6, with an adjusted HR of 1.6 (95% CI, 1.3-2.0). However, mental stress–induced ischemia remained significant after adding conventional stress ischemia to the model (adjusted HR, 2.1; 95% CI, 1.5-3.1).
When examining separate ischemia phenotypes, mental stress–induced ischemia alone (event rate, 4.8 per 100 patient-years) vs no ischemia (event rate, 2.3 per 100 patient-years) was significantly associated with the primary end point, with an HR of 2.0 (95% CI, 1.1-3.7), whereas conventional stress ischemia alone (event rate, 3.1 per 100 patient-years) vs no ischemia was not significant (HR, 1.4; 95% CI, 0.9-2.1; Figure 3; eTable 2 in the Supplement). Patients with both mental stress–induced ischemia and conventional stress ischemia (event rate, 8.1 per 100 patient-years) had an HR of 3.8 (95% CI, 2.6-5.6), compared with patients with no ischemia (Figure 3; eTable 2 in the Supplement). The risk associated with having both mental stress–induced and conventional stress ischemia was also significantly higher than having conventional stress ischemia alone (HR, 2.7; 95% CI, 1.7-4.3), although it was not significantly higher than having mental stress–induced ischemia alone (HR, 1.9; 95% CI, 0.98-3.6). Results for the secondary end point were consistent (eFigure 5 and eTable 2 in the Supplement). Model diagnostics demonstrated that model assumptions were met.
Figure 3. Cumulative Incidence of the Primary Study End Point for Separate Ischemia Phenotypes in the Pooled Sample.
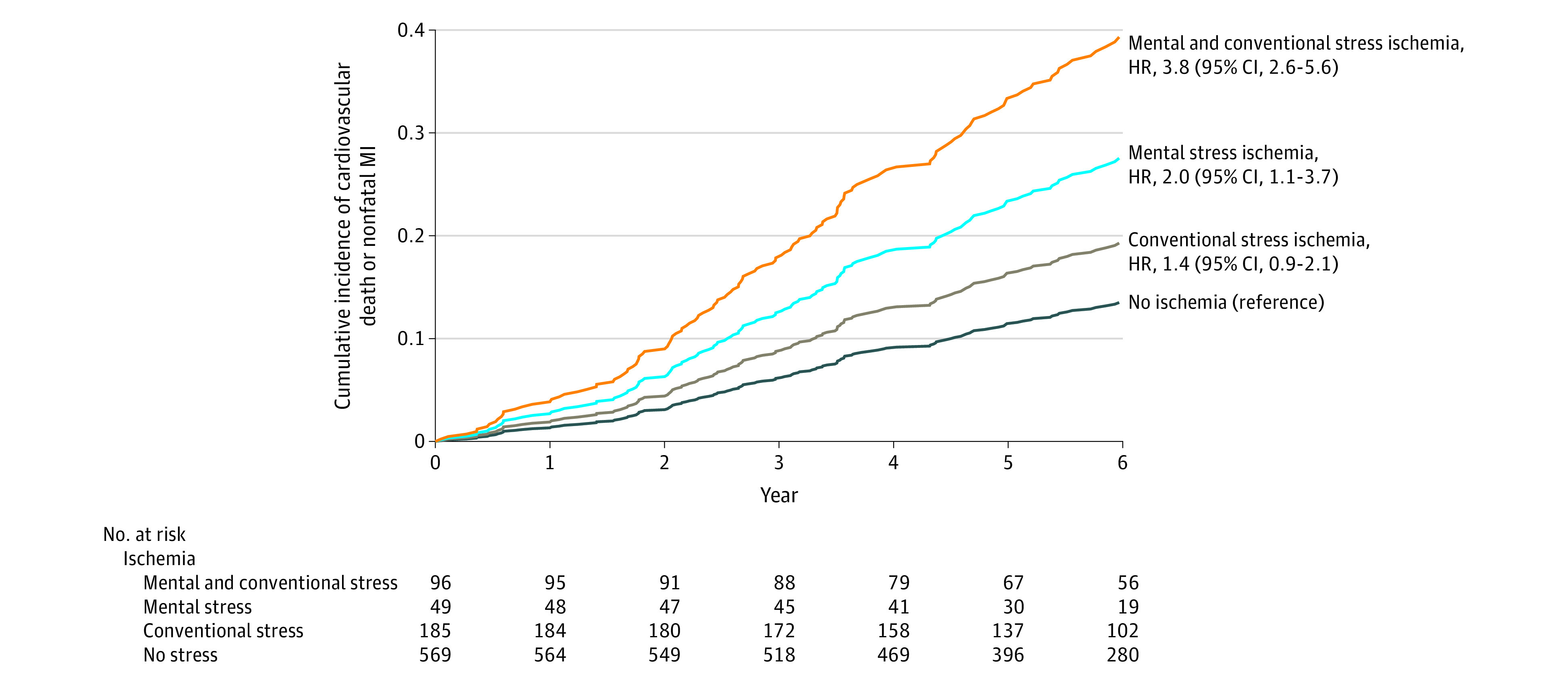
Because of the repeated events analysis, patients were removed from the risk set at each time point only if they died or were censored. The median observation period was 72.3 months (IQR, 56.5-72.3 months) for patients with both mental stress and conventional stress ischemia; 68.6 months (IQR, 53.9-72.3 months) for patients with mental stress ischemia only; 72.3 months (IQR, 58.8-72.3 months) for patients with conventional stress ischemia only; and 70.7 months (IQR, 55.8-72.3 months) for patients with no ischemia.
MI indicates myocardial infarction.
Discussion
In this pooled analysis of 2 prospective studies of patients with stable CHD, mental stress–induced ischemia measured with myocardial perfusion imaging was significantly associated with an elevated risk of adverse cardiovascular events. The findings were consistently observed in each of the 2 cohorts and applied to first and subsequent events. The presence of both ischemia phenotypes together (mental stress–induced ischemia and conventional stress ischemia) was associated with a further increased risk. When examined separately, mental stress–induced ischemia alone but not conventional stress ischemia alone was significantly associated with an increased risk. The known association of conventional stress ischemia with outcomes in patients with CHD may be in part explained by its co-occurrence with mental stress–induced ischemia.
The association of mental stress–induced ischemia with adverse cardiovascular events has been examined in 6 small prospective studies.20,21,22,23,24,25 Five of the 6 studies exclusively enrolled patients with exercise–induced myocardial ischemia20,21,22,23,24; thus, their results may not be applicable to most patients with CHD. Four of the 6 studies used radionuclide ventriculography to assess ischemia,20,21,23,24 a technique no longer used in clinical practice. The remaining 2 used either echocardiography25 or a mix of radionuclide ventriculography and echocardiography.22 All of these investigations assessed changes in left ventricular function during mental stress (wall motion abnormalities or a decrease in left ventricular ejection fraction). The study populations were predominantly White and men and were largely recruited at a time when the clinical management of CHD was different from current standards.
Although both mental stress and conventional stress can provoke ischemia by increasing myocardial oxygen demand, nonsystemic hemodynamic factors may contribute to mental stress–induced ischemia. This is highlighted by the MIMS2 cohort because no significant difference existed in hemodynamic parameters with stress by mental stress–induced ischemia status, possibly related to greater use of β-blockers. Mental stress–induced ischemia develops at a lower myocardial oxygen demand than exercise-stress ischemia, and although it is less strongly related to severity of coronary atherosclerosis than conventional stress ischemia,4,7 presence of endothelial dysfunction and plaques can disturb the vasomotor response to mental stress causing paradoxical vasoconstriction.26 While systemic vascular resistance falls in response to exercise, it rises with mental stress due to peripheral vasoconstriction,6 which contributes to mental stress–induced ischemia.2 Inflammation also increases acutely with mental stress in patients with CHD,27 although its relationship with mental stress–induced ischemia has not been demonstrated.27 Ultimately, the reasons some individuals and not others develop mental stress–induced ischemia under stress are likely related to the neurobiology of stress. Brain imaging studies have linked mental stress–induced ischemia to alterations in brain reactivity to stress in areas involved in the regulation of emotions and autonomic function.28 Brain responses in this circuit are also associated with cardiovascular events in patients with CHD.29
Although the association of mental stress–induced ischemia with adverse outcomes was similar in most a priori selected strata, it was stronger in men than in women. It is possible that the assessment of mental stress–induced ischemia was less precise in women than in men due to possible breast artifacts, given that no attenuation correction was performed. Thus, measurement error may have driven the estimate toward the null among women. However, given the large number of secondary and subgroup analyses, along with an absence of difference in the association by sex for the secondary end point, this could be a chance finding.
These findings are consistent with a role of psychological stress in the outcome of patients with CHD. Additional studies have linked stress-related changes in microvascular tone,30 brachial endothelial function,31 and brain activity29 to outcomes in patients with CHD. As a whole, this evidence suggests that the value of stress and mental health factors for CHD risk stratification should be investigated, given that they are amenable to medical and lifestyle intervention,32,33,34 including aerobic exercise and stress management training,35 antidepressants,36 and β-blockers and antianginal drugs.37,38
This study is strengthened by the use of myocardial perfusion imaging and by the consideration of first and repeated events for an evaluation of the burden of morbidity in patients with CHD. Two independent populations produced similar results. Pooling these 2 samples provided what is, to our knowledge, the largest prospective study of mental stress–induced ischemia to date.
Limitations
This study has several limitations. First, mental stress was assessed in the laboratory, so its relationship with stress in everyday life needs further evaluation. However, in previous studies, there was a reasonable correlation between mental stress–induced ischemia and ischemia in the ambulatory setting.39 Second, a single mental stressor was used; yet, multiple stressors in sequence may cause undesirable cumulative effects.5 A single stressor is necessary when using myocardial perfusion imaging with 99mTc; this provides a measure of perfusion at the time of mental stress, with the advantage that the patient does not need to lie in a scanner during the public speaking task. Third, this study was conducted at a single institution and generalizability to other populations or settings needs further study. Fourth, this study does not establish the clinical value or feasibility of mental stress–induced ischemia testing in patient care.
Conclusions
Among patients with stable coronary heart disease, the presence of mental stress–induced ischemia, compared with no mental stress–induced ischemia, was significantly associated with an increased risk of cardiovascular death or nonfatal myocardial infarction. Although these findings may provide insights into mechanisms of myocardial ischemia, further research is needed to assess whether testing for mental stress–induced ischemia has clinical value.
eFigure 1. Flow chart for cohort construction
eFigure 2. Distribution of first and subsequent events for each of the study endpoints
eFigure 3. Cumulative incidence of the secondary study endpoint (cardiovascular death, MI, or hospitalizations for heart failure)
eFigure 4. Pooled estimates and plots for the association of mental stress-induced myocardial ischemia with the secondary endpoint of cardiovascular death, MI, or hospitalizations for heart failure
eFigure 5. Cumulative incidence of secondary study endpoint (cardiovascular death, MI, or hospitalizations for) for separate ischemia phenotypes in the pooled sample
eTable 1. Association of ischemia provoked by mental stress and by conventional (pharmacological or exercise) stress with the primary study endpoint in the pooled sample
eTable 2. Association of ischemia phenotypes with the primary and the secondary endpoints in the pooled sample
References
- 1.Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15(4):215-229. doi: 10.1038/nrcardio.2017.189 [DOI] [PubMed] [Google Scholar]
- 2.Hammadah M, Alkhoder A, Al Mheid I, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47-53. doi: 10.1016/j.ijcard.2017.05.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarino V, Sullivan S, Hammadah M, et al. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137(8):794-805. doi: 10.1161/CIRCULATIONAHA.117.030849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheps DS, Soufer R, Freedland KE. Psychological stress and myocardial ischemia: understanding the link and the implications. Psychosom Med. 2007;69(6):491-492. doi: 10.1097/PSY.0b013e31814527c2 [DOI] [PubMed] [Google Scholar]
- 5.Burg MM, Soufer R. Psychological stress and induced ischemic syndromes. Curr Cardiovasc Risk Rep. 2014;8(4):377. doi: 10.1007/s12170-014-0377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102(6):472-480. doi: 10.1136/heartjnl-2014-307306 [DOI] [PubMed] [Google Scholar]
- 7.Krantz DS, Burg MM. Current perspective on mental stress-induced myocardial ischemia. Psychosom Med. 2014;76(3):168-170. doi: 10.1097/PSY.0000000000000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J, Rooks C, Ramadan R, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114(2):187-192. doi: 10.1016/j.amjcard.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammadah M, Al Mheid I, Wilmot K, et al. The Mental Stress Ischemia Prognosis Study: objectives, study design, and prevalence of inducible ischemia. Psychosom Med. 2017;79(3):311-317. doi: 10.1097/PSY.0000000000000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolpe J. The Practice of Behavior Therapy . Pergamon Press; 1969. [Google Scholar]
- 11.Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846. doi: 10.1007/s12350-018-1283-y [DOI] [PubMed] [Google Scholar]
- 12.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535-543. doi: 10.1161/01.CIR.97.6.535 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan S, Hammadah M, Al Mheid I, et al. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol. 2018;38(2):473-480. doi: 10.1161/ATVBAHA.117.309535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA, Brown GK. Beck Depression Inventory: BDI-II: Manual. Harcourt Brace & Co; 1996. [Google Scholar]
- 15.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, eds. The Social Psychology of Health. Sage; 1988:31-67. [Google Scholar]
- 16.Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety (STAI) Manual. Consulting Psychologists Press; 1970. [Google Scholar]
- 17.Solomon SD, Pfeffer MA. The future of clinical trials in cardiovascular medicine. Circulation. 2016;133(25):2662-2670. doi: 10.1161/CIRCULATIONAHA.115.020723 [DOI] [PubMed] [Google Scholar]
- 18.Therneau TM, Grambsch PM. The Cox Model. In: Modeling Survival Data: Extending the Cox Model. Statistics for Biology and Health. Springer; 2000. doi: 10.1007/978-1-4757-3294-8_3 [DOI] [Google Scholar]
- 19.de Jong VMT, Moons KGM, Riley RD, et al. Individual participant data meta-analysis of intervention studies with time-to-event outcomes: a review of the methodology and an applied example. Res Synth Methods. 2020;11(2):148-168. doi: 10.1002/jrsm.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain D, Burg M, Soufer R, Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76(1):31-35. doi: 10.1016/S0002-9149(99)80796-6 [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA. 1996;275(21):1651-1656. doi: 10.1001/jama.1996.03530450041030 [DOI] [PubMed] [Google Scholar]
- 22.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84(11):1292-1297. doi: 10.1016/S0002-9149(99)00560-3 [DOI] [PubMed] [Google Scholar]
- 23.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105(15):1780-1784. doi: 10.1161/01.CIR.0000014491.90666.06 [DOI] [PubMed] [Google Scholar]
- 24.Babyak MA, Blumenthal JA, Hinderliter A, et al. Prognosis after change in left ventricular ejection fraction during mental stress testing in patients with stable coronary artery disease. Am J Cardiol. 2010;105(1):25-28. doi: 10.1016/j.amjcard.2009.08.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JL, Boyle SH, Samad Z, et al. Mental stress-induced left ventricular dysfunction and adverse outcome in ischemic heart disease patients. Eur J Prev Cardiol. 2017;24(6):591-599. doi: 10.1177/2047487316686435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325(22):1551-1556. doi: 10.1056/NEJM199111283252205 [DOI] [PubMed] [Google Scholar]
- 27.Hammadah M, Sullivan S, Pearce B, et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun. 2018;68:90-97. doi: 10.1016/j.bbi.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremner JD, Campanella C, Khan Z, et al. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med. 2018;80(6):515-525. doi: 10.1097/PSY.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moazzami K, Wittbrodt MT, Lima BB, et al. Higher activation of the rostromedial prefrontal cortex during mental stress predicts major cardiovascular disease events in individuals with coronary artery disease. Circulation. 2020;142(5):455-465. doi: 10.1161/CIRCULATIONAHA.119.044442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Almuwaqqat Z, Hammadah M, et al. Peripheral vasoconstriction during mental stress and adverse cardiovascular outcomes in patients with coronary artery disease. Circ Res. 2019;125(10):874-883. doi: 10.1161/CIRCRESAHA.119.315005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima BB, Hammadah M, Kim JH, et al. Association of transient endothelial dysfunction induced by mental stress with major adverse cardiovascular events in men and women with coronary artery disease. JAMA Cardiol. 2019;4(10):988-996. doi: 10.1001/jamacardio.2019.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott-Sheldon LAJ, Gathright EC, Donahue ML, et al. Mindfulness-based interventions for adults with cardiovascular disease: a systematic review and meta-analysis. Ann Behav Med. 2020;54(1):67-73. doi: 10.1093/abm/kaz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JM, Stewart R, Lee YS, et al. Effect of escitalopram vs placebo treatment for depression on long-term cardiac outcomes in patients with acute coronary syndrome: a randomized clinical trial. JAMA. 2018;320(4):350-358. doi: 10.1001/jama.2018.9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells A, Reeves D, Capobianco L, et al. Improving the effectiveness of psychological interventions for depression and anxiety in cardiac rehabilitation: PATHWAY-a single-blind, parallel, randomized, controlled trial of group metacognitive therapy. Circulation. 2021;144(1):23-33. doi: 10.1161/CIRCULATIONAHA.120.052428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. 2005;293(13):1626-1634. doi: 10.1001/jama.293.13.1626 [DOI] [PubMed] [Google Scholar]
- 36.Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2149. doi: 10.1001/jama.2013.5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bairey CN, Krantz DS, DeQuattro V, Berman DS, Rozanski A. Effect of beta-blockade on low heart rate-related ischemia during mental stress. J Am Coll Cardiol. 1991;17(6):1388-1395. doi: 10.1016/S0735-1097(10)80152-4 [DOI] [PubMed] [Google Scholar]
- 38.Andrews TC, Parker JD, Jacobs S, et al. Effects of therapy with nifedipine GITS or atenolol on mental stress-induced ischemic left ventricular dysfunction. J Am Coll Cardiol. 1998;32(6):1680-1686. doi: 10.1016/S0735-1097(98)00445-8 [DOI] [PubMed] [Google Scholar]
- 39.Blumenthal JA, Jiang W, Waugh RA, et al. ; Association and Hemodynamic Features . Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life: association and hemodynamic features. Circulation. 1995;92(8):2102-2108. doi: 10.1161/01.CIR.92.8.2102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow chart for cohort construction
eFigure 2. Distribution of first and subsequent events for each of the study endpoints
eFigure 3. Cumulative incidence of the secondary study endpoint (cardiovascular death, MI, or hospitalizations for heart failure)
eFigure 4. Pooled estimates and plots for the association of mental stress-induced myocardial ischemia with the secondary endpoint of cardiovascular death, MI, or hospitalizations for heart failure
eFigure 5. Cumulative incidence of secondary study endpoint (cardiovascular death, MI, or hospitalizations for) for separate ischemia phenotypes in the pooled sample
eTable 1. Association of ischemia provoked by mental stress and by conventional (pharmacological or exercise) stress with the primary study endpoint in the pooled sample
eTable 2. Association of ischemia phenotypes with the primary and the secondary endpoints in the pooled sample