Abstract
To assess airway and lung parenchymal damage noninvasively in cystic fibrosis (CF), chest MRI has been historically out of the scope of routine clinical imaging because of technical difficulties such as low proton density and respiratory and cardiac motion. However, technological breakthroughs have emerged that dramatically improve lung MRI quality (including signal-to-noise ratio, resolution, speed, and contrast). At the same time, novel treatments have changed the landscape of CF clinical care. In this contemporary context, there is now consensus that lung MRI can be used clinically to assess CF in a radiation-free manner and to enable quantification of lung disease severity. MRI can now achieve three-dimensional, high-resolution morphologic imaging, and beyond this morphologic information, MRI may offer the ability to sensitively differentiate active inflammation vs scarring tissue. MRI could also characterize various forms of inflammation for early guidance of treatment. Moreover, functional information from MRI can be used to assess regional, small-airway disease with sensitivity to detect small changes even in patients with mild CF. Finally, automated quantification methods have emerged to support conventional visual analyses for more objective and reproducible assessment of disease severity. This article aims to review the most recent developments of lung MRI, with a focus on practical application and clinical value in CF, and the perspectives on how these modern techniques may converge and impact patient care soon.
Key Words: cystic fibrosis, imaging, lung, magnetic resonance
Abbreviations: ABPA, allergic bronchopulmonary aspergillosis; CF, cystic fibrosis; HAM, high-attenuation mucus; IMIS, inverted mucus impaction signal; PFT, pulmonary function test; T1w, T1 weighted; T2w, T2 weighted; TE, echo time; TR, repetition time; UTE, ultrashort echo time; ZTE, zero echo time
Cystic fibrosis (CF) is one of the most frequent genetic conditions in Caucasians, and lung involvement is responsible for the vast majority of morbidity and death.1 Although MRI is a preferred modality over CT scans for imaging most organs, lung MRI has not been widely adopted for routine use.2 For airway and lung parenchyma damage assessment, MRI has been historically out of the scope of lung imaging because of technical difficulties such as low proton density and artifacts due to respiratory and cardiac motion.3 MRI also has longer acquisition time and is less accessible and more expensive than either chest radiograph or CT imaging. However, technical breakthroughs have recently emerged to dramatically improve lung MRI quality,4 including better signal-to-noise ratio, resolution, speed, and contrast. At the same time, clinical care has demonstrated significant advances, mainly due to novel treatments.5 Therefore, nowadays, the majority of patients are not children but adults.6 It is expected that the improvement in patient care will further increase the ratio of patients with mild disease. Thus, there is a growing need for tools to sensitively detect, precisely characterize, and follow up the disease process longitudinally.7 MRI, as a modality free from ionizing radiation,8 is particularly appropriate for repeated imaging over time in a patient population with increased life expectancy.9,10 Over the last decade, significant technical developments have been reported, facilitating its clinical use.11 Owing to these developments, position papers have been published to recognize that lung MRI can be part of the routine follow-up of patients with CF.4,12, 13, 14 Lung MRI has proven more sensitive to assess the variation in the disease process than pulmonary function tests and allows for both visual and quantitative assessments. Nevertheless, the advent of MRI as a tool to assess CF lung disease is recent, and treating physicians may not be aware of the new tools that are now accessible to manage their patients. To help the physician navigate this maze of modern lung MRI technical developments, the most recent advancements are reviewed here. An emphasis is made on practical clinical application to CF and on perspectives concerning how these techniques may converge and ultimately impact care for patients living with CF.
Morphologic MRI of the Lung
High-Resolution Morphologic Imaging of the Lung: What For?
For clinical management of CF-related lung disease to be effective, the onset and progression of the lung disease should be closely monitored. This can be done either indirectly by monitoring lung function or more directly by monitoring lung structure. Pulmonary function tests (PFTs) and the lung clearance index are considered essential tools for measuring lung function. However, there is no doubt that the treatment of patients with CF cannot rely on a single parameter but is always based on a combination of information, including imaging. For instance, imaging is part of the diagnostic criteria to define an exacerbation15 and allergic bronchopulmonary aspergillosis (ABPA).16 The German CF community has also considered imaging to manage chronic Pseudomonas aeruginosa infection.17 These examples of disease-modifying conditions imply that, without an accurate imaging modality, one piece of the puzzle may be missing for decision-making. Moreover, imaging is an objective criterion. A summary of the CF structural alterations that can be identified by lung imaging is given in e-Appendix 1. The main hallmarks in CF are, however, bronchiectasis (ie, irreversible dilatation of the airway lumen), wall thickening (ie, enlargement of the bronchial wall thickness), mucus plugs (ie, secretion of viscosity), and consolidations (ie, exudate that replaces alveolar air).18
Nevertheless, there used to be a debate on whether high-resolution morphologic imaging should be used as a standard tool in CF.19,20 Opponents stated that chest radiography is widely available, inexpensive, and easy to do, and that reproducible scoring methods are available. In addition, there was a lack of evidence that imaging might affect standard management. There was also a need to balance the benefits and risks of radiation exposure.19
Supporters advocated that lung structure and function represent two different types of information. The sensitivity of chest radiography is low. In the study by Bhalla et al,21 chest radiography missed 91% of mucus plugs and 44% of bronchiectasis compared with CT imaging. Tiddens20 reported that, despite some correlations, a discrepancy between PFT and CT imaging may be found in up to 50% of patients with CF. Recently, Bortoluzzi et al22 showed that CT imaging but not chest radiography induced a CF management change, including the need for antifungal treatment, bronchoscopy, and hospitalization. Most importantly, with the advent of novel therapies, there are new possibilities to change the course of the disease outcome.5 Using repeat imaging to assess the response to treatment objectively, the reversibility of structural abnormalities has been proven.10 Thus, contemporary data suggest that radiation-free imaging at high resolution is desirable.
High-Resolution Morphologic Imaging, Using MRI: Is It Possible?
Broadly, the basic principle of conventional MRI is to use radiofrequency pulses to manipulate the nuclear spins of protons in the body’s tissues and acquire the resulting resonance signal. Protons are plentiful in the human body, except the lung, which is primarily filled with air. For years, low proton density, rapid relaxation time (T2∗, ∼0.8 ms at 1.5 T), and motion artifacts all limited the quality of morphologic lung MRI.23 Thus, CF-specific, MRI scoring systems were developed at a lobar level of precision, where wall thickening, bronchiectasis, and mucus plugs were considered a mixed item, as a concession to the lack of spatial resolution.24,25 Also, many systems assume injected gadolinium contrast agents to enhance the low lung MRI signal.
Novel MR sequences have been demonstrated to address the issue of a rapidly decaying MR signal from the lung parenchyma, using so-called ultrashort (UTE) or zero (ZTE) echo-time techniques.26, 27, 28, 29 In CF, the image quality of UTE has been consistently reported to be similar to that of low-dose CT imaging, at a millimeter to submillimeter spatial resolution. It can depict all CF structural features necessary to assess disease severity, such as bronchial wall thickening, bronchial dilation, central and peripheral mucus plugs, consolidation, atelectasis, emphysema, cysts, abscesses, or a mosaic pattern.27,30 Conversely, all of these features were underestimated by conventional MR sequences, especially mucus-free bronchiectasis.25,30 Still, the detection of normal airways in the lung periphery, using UTE, is inferior to that of CT imaging. However, when the airways are diseased, the visibility of airway wall thickening up to the peripheral lung has been shown.29
Therefore, three-dimensional (3D)-UTE MRI is now generally accepted for in vivo assessment of the severity of structural alterations, with the possibility to apply CT imaging scoring systems27,30,31 at a segmental level of precision. Technical solutions have reduced the acquisition time down to 4 to 6 min26,31 in free-breathing. Novel respiratory synchronization systems allow removing motion artifacts, standardizing the lung volume of acquisition without needing an external device, or repeat breath-hold maneuvers. Furthermore, no gadolinium contrast injection is required. Images in all planes can be reconstructed from the same single 3D isotropic MR acquisition (Fig 1). Also, variant sequence schemes have been published, but these technical differences were shown not to modify the assessment of a visual CT imaging score.31
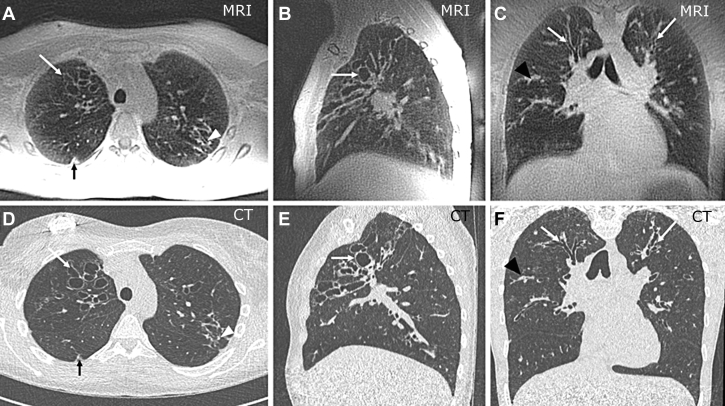
Figure 1.
A-F, 3D-UTE MRI (A-C) and CT imaging (D-F) in a 16-year-old girl with CF. Sagittal (B, E) and coronal (C, F) reconstructions can be generated from the axial (A, C) isotropic acquisitions. Open arrows indicate mucus-free bronchiectasis; open arrowheads (A, C) show peripheral mucus plugs; solid arrows (A, C) show a small nodular consolidation; solid arrowheads (C, F) indicate central mucus plugs. 3D = three-dimensional; CF = cystic fibrosis; UTE = ultrashort echo time.
In the past, a consensus surrounding morphologic imaging in CF could not be reached regarding a high-resolution or a radiation-free modality. 3D-UTE MRI might solve this problem because CT imaging scoring systems can be applied to MRI.4 Because the nonionizing nature may promote more frequent use, one could discuss that imaging is recommended at least once per year in CF,19 whereas specific conditions are prone to increase the need for imaging, such as exacerbation or ABPA.
Automated Quantification of Structural Abnormalities
Despite its clinical usefulness, any visual reader score in CF is inherently subjective, that is, dependent on the training of the reader, and only moderately reproducible.32 Therefore, complementary software-based quantitative tools have been emphasized to enable objective and reproducible assessment for more standardized evaluation. Three-dimensional measurement of the central bronchial tree, at functional residual capacity,33 or the amount of emphysema34 (Fig 2) has been proven using 3D-UTE. A quantitative volumetric measurement of structural abnormalities by UTE also demonstrated clinical relevance to discriminate CF with exacerbation and to detect their longitudinal improvement after a 1-year follow-up, although PFT results were found to be stable.35
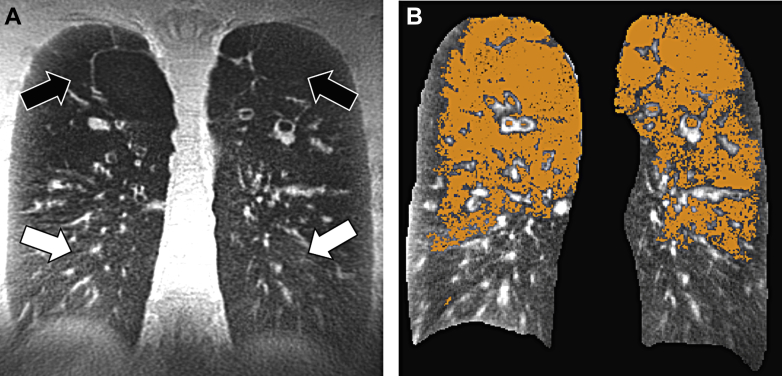
Figure 2.
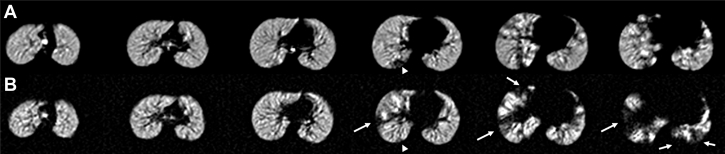
3D-UTE MRI using a coronal reformation in a 32-year-old man with CF. A, Solid arrows show areas of advanced emphysema, and open arrows show normal lung parenchyma areas. B, The volume of hyposignal intensities can be automatically quantified by a curve-fitting method, as described in Benlala et al,34 and shown as the orange mask. 3D = three-dimensional; CF = cystic fibrosis; UTE = ultrashort echo time.
Perspectives
First, MRI acquisition time could be reduced in the near future. Using clinical 1.5-T MRI scanners and newly developed metamaterials, Duan et al36 have reported a dramatic decrease in acquisition time of up to 13.9-fold. The promising application of this potentially game-changing technology to lung MRI has not been reported yet. Moreover, reports have shown that, at a field strength of 0.55 T, there are fewer susceptibility artifacts than at standard field strengths.37
MRI of Airway Inflammation
Beyond Morphologic Imaging
Studies using BAL showed that considerable inflammation exists in patients with CF early in life compared with control subjects.38 However, lung structural abnormalities are shared commonly across the various forms of inflammation requiring different treatments.39 Besides, PFT results correlate poorly with the inflammatory status.40 BAL is the standard of reference to assess airway inflammation, but it is invasive.40 Sputum biomarkers have been proposed, but their variability is high.41 Moreover, PET-CT imaging has been described, but its use may be limited to research investigations.42
An interesting feature of MRI could be its ability to characterize inflammatory processes to help guide treatment. Tissue contrast in MRI is obtained by varying two main parameters, the repetition time (TR) and the echo time (TE).43 Typically, MR images with short TR and TE are considered T1 weighted (T1w), whereas images with a long TR and TE are T2 weighted (T2w). Combining these two allows more sensitive and/or specific characterization of the tissue components by considering their endogenous contrast information. Another technical possibility is diffusion imaging.44 Using this technique, an acute consolidation is prone to have a high diffusion coefficient,45 whereas tissues within a highly cellularized environment, such as pus within an abscess, may display lower coefficients of diffusion.46
MRI to Track Active Inflammation
The more recent literature indicates that MRI could target, and be used to follow up, areas of active inflammation4,13,14,47, 48, 49 (Fig 3). This paradigm has become relevant in the context of novel CF treatments to target potentially reversible lesions. For instance, using visual scores, it has been shown that abnormalities with high T2w signal intensity are increased in patients with CF experiencing a pulmonary exacerbation47,49 and, conversely, are decreased after antibiotic treatment.47,49 A correlation with biological markers of inflammation has been reported.49 High T2w signal intensity is mediated by an increase in either water and/or cellular content. Although this cannot discriminate between mucus plugs, vasogenic edema, cytotoxic edema, cellular infiltration, or pneumonia, all of these features are likely related to CF inflammation49 (Fig 4). It was also shown that an inflammatory hyper-T2 signal intensity can be seen without any associated visible structural abnormality48 (Fig 3).
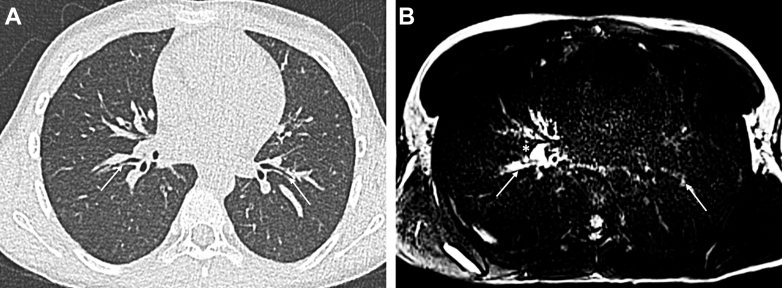
Figure 3.
A and B, Axial CT imaging (A) and T2-weighted MRI (B) in a 9-year-old girl with CF and a period of exacerbation. A, Using CT imaging, no structural difference can be seen in the right and left segmental bronchi (arrows). B, Using MRI, there is a higher right-sided T2 signal intensity of the bronchial walls (arrows) and a hilar lymph node (asterisk), showing the localization of inflammation. CF = cystic fibrosis.
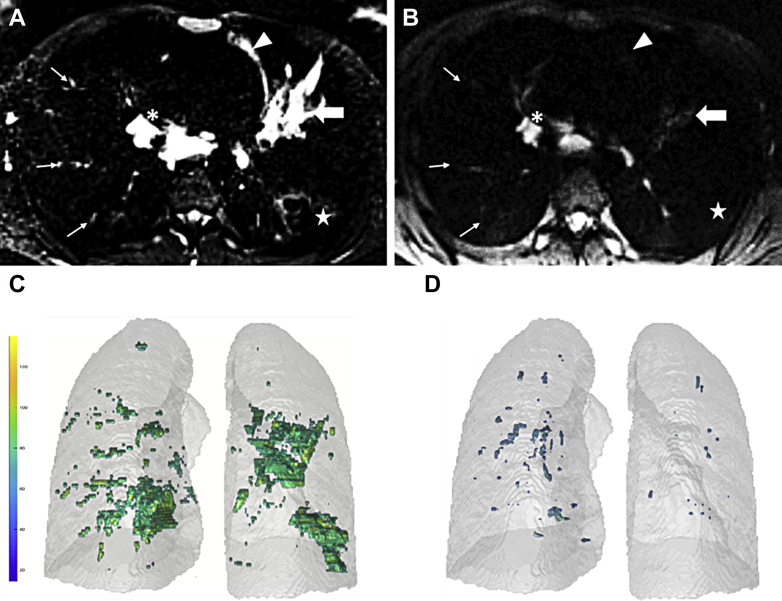
Figure 4.
A-D, T2-weighted MRI in a 28-year-old woman during pulmonary exacerbation (A, C) and then 4 weeks after antibiotic treatment (B, D). A and B, Samples of 2D axial slices; C and D, volume of lesions. The vertical bar in (C) represents the signal intensity range, in milliseconds. In (A), the large arrow indicates bronchoceles, the thin arrows show peripheral mucus plugs, the arrowhead indicates atelectasis, and the star indicates bronchial wall thickening, and asterisk indicates a hilar lymph node. After treatment, there is a marked reduction of the volume and signal intensity of the reversible inflammatory lesions (B-D). 2D = two-dimensional.
Although bronchiectasis has been emphasized as a pivotal feature to assess the severity of CF and its worsening,2 studies have demonstrated that bronchiectasis is mainly a nonreversible alteration, highlighted in studies of ivacaftor.10 Therefore, from a therapeutic point of view, bronchiectasis may not be the most relevant feature to follow up as compared with the reversible alterations, which are accurately detected by T2w MRI. Nevertheless, the signal intensity of a lesion may be subjective in evaluation.49 A recent report has shown that fully automated quantification can be achieved (Fig 4).48
MRI to Characterize Bronchial Inflammation
MRI seems able to differentiate various forms of inflammation, such as infectious inflammation4,13,44,47,48 and allergic inflammation,2,4,11,50, 51, 52, 53 and this discrimination may have direct clinical implications for treatment (ie, antibiotics vs corticosteroids16,54). This has been demonstrated in the context of allergic bronchopulmonary aspergillosis (ABPA), which affects up to 15% of patients with CF. Assessment of ABPA status is internationally recommended during the routine annual follow-up of CF.16 Nevertheless, the diagnosis of ABPA is often difficult and delayed because clinical, immunologic, and radiologic data may overlap with other infectious processes. Therefore, the lack of improvement after a therapeutic antibiotic test is considered part of the diagnostic criteria of ABPA in CF.16 However, guidelines indicate that by using CT imaging, it is possible to detect an alteration in mucus attenuation, the so-called high-attenuation mucus (HAM), a 100% specific criterion for ABPA.55 Using a combination of T1w and T2w MRI sequences, the so-called inverted mucoid impaction signal (IMIS) has been described50,51 and was consistently recognized as a surrogate for HAM in both CF2,11,50,52,56 and non-CF conditions.51,53,57 Indeed, infectious inflammation displays water-like signal intensity (low T1w and high T2w signal intensity43). Conversely, IMIS refers to mucus impactions with a complete reversal of MR appearance, with high T1w and low T2w signal intensity50,51 (Fig 5). In a study performed in 18 CF patients with ABPA and 90 CF patients without ABPA, the sensitivity of IMIS to diagnose CF with ABPA was shown to be 94% (95% CI, 73%-99%) and the specificity was shown to be 100% (95% CI, 96%-100%).50 Interestingly, IMIS was absent in CF patients without ABPA but in whom colonization or sensitization to Aspergillus fumigatus was present. Importantly, IMIS was cleared after oral corticosteroid treatment. The high prevalence of mucus abnormalities in ABPA was also confirmed in another CT imaging study,39 further indicating that the prevalence is higher in CF than in non-CF asthma. To date, Agarwal et al53 have recently introduced IMIS as the MR counterpart of HAM to diagnose ABPA. However, further refinement of the diagnostic accuracy, and notably the sensitivity, should be allowed by an ongoing multicenter trial.58
Figure 5.
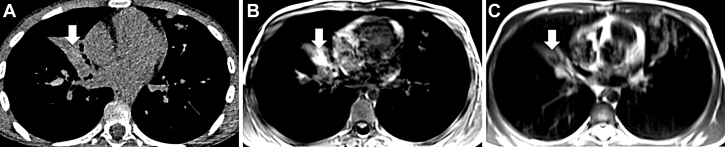
A-C, Axial CT imaging (A), axial T1-weighted MRI (B), and T2-weighted MRI (C) in a 10-year-old boy with CF. A, Using CT imaging, there is an infiltrate in the middle lobe (arrow). B and C, Using MRI, a “V”-shaped bronchocele with high T1-weighted signal intensity (B) and low T2-weighted signal intensity (C) can be seen. This IMIS sign is specific to ABPA. ABPA = allergic bronchopulmonary aspergillosis; CF = cystic fibrosis; IMIS = inverted mucus impaction signal.
Diffusion MRI has also been reported to detect inflammatory modifications of the lung.44 However, whether a reduction in the diffusion coefficient may be specific to active inflammation remains unproven.45 In some instances, diffusion images can help characterize lung abscesses44,45 requiring intensive antibiotic therapy (Fig 6).
Figure 6.
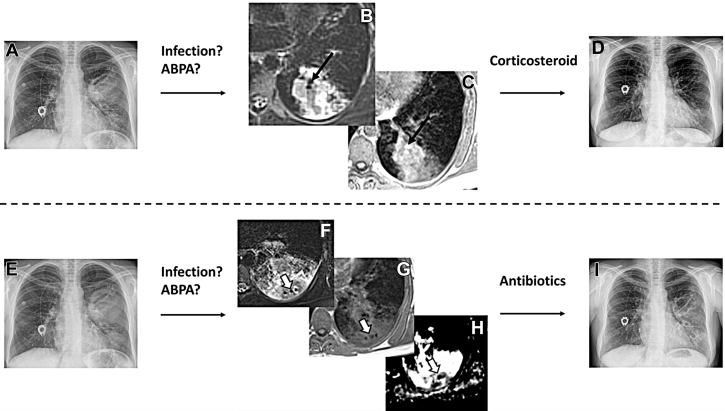
A-I, Coronal chest radiographs (A, D, E, I) and axial MRI using T2 weighting (B, F), T1 weighting (C, G), and mapping of the coefficient of diffusion (H) in a 30-year-old woman with CF. Panels from the top and bottom rows were acquired during two different exacerbations. In the top row, there is a lung infiltrate in the left lung (A) with an IMIS sign, which was cured with oral corticosteroids (D). In the bottom row, there is another infiltrate in the left lower lobe (E). This time, MRI shows typical features of an abscess with hypointense air surrounded by a restriction of diffusion (open arrows in F, G, H). The abscess was cured by antibiotic therapy (I). ABPA = allergic bronchopulmonary aspergillosis; CF = cystic fibrosis; IMIS = inverted mucus impaction signal.
Functional MRI of the Lung
Imaging of Lung Function
Structural imaging and its characterization using endogenous contrast relate mainly to central airways. To obtain a full picture of CF lung disease, additional techniques are required to assess the small airways, an important site of involvement in CF, which is beyond the spatial resolution of any imaging modality for a direct assessment. These alterations were proven to occur early in the onset and progression of CF.47 However, many young children with CF have only small, early structural changes, with normal spirometry, and will have spirometric declines only after puberty.59
Contrast-Enhanced MRI of Lung Ventilation
One of the early strategies in pulmonary MRI to sidestep the low proton density of the lung parenchyma was the use of hyperpolarized noble gases (eg, 3He and 129Xe) as inhaled contrast agents.60,61 In the absence of airway obstruction, the inhaled gas distributes equally throughout both lungs; however, with airway obstruction, the gas flow is lessened or completely blocked during a near-tidal inspiration (Fig 7). These ventilation deficits can be quantified, most often as a ventilation defect percentage, that is, the percentage of lung volume below a determined signal intensity threshold. The field has pivoted to the more readily available 129Xe gas.62 Most importantly, numerous groups have shown that hyperpolarized gas MRI is exquisitely sensitive to early airway obstruction in patients with CF and normal spirometry, and has higher sensitivity than PFT or the lung clearance index.59,63
Figure 7.
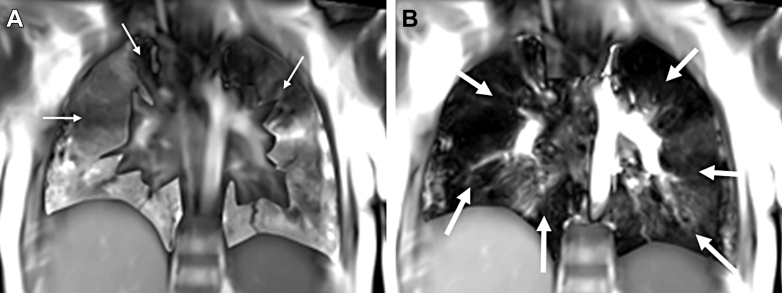
A and B, Axial hyperpolarized 129Xe ventilation MR images in a pediatric patient with CF at baseline (A) and 224 days later (B). Ventilation in the superior lungs is uniform and remains unchanged; however, ventilation deficits in the inferior lungs worsen at follow-up (arrows). A small defect in the posterior right lung at baseline (arrowhead) appears to improve at follow-up. CF = cystic fibrosis.
Nevertheless, the process of hyperpolarization requires costly hardware and skilled personnel. Therefore, other inhalational techniques have been investigated, although at an early stage of development, such as oxygen-enhanced64 proton MRI or perfluorinated compounds.65 However, as for hyperpolarized gases, the latter requires additional dedicated coils and multinuclear acquisition, which is not supported on all clinical MR scanners.
Contrast-Enhanced MRI of Perfusion
One of the most documented applications of functional MRI has been MRI of perfusion in chronic airway disease, using an injection of gadolinium contrast agent. It has been proposed that such evaluation may be part of assessing CF disease severity. Variations under treatment and applicability to children down to 2 to 3 years of age have been consistently demonstrated.47 Nevertheless, developing a non-contrast-enhanced MR technique would increase clinical applicability.66
Non-Contrast-Enhanced Functional MRI
A promising development of functional proton MRI is via Fourier decomposition and related methods.67 This technique is done with a free-breathing patient in normal room air, and allows the generation of ventilation- and perfusion-weighted maps without any contrast agent (Fig 8). The technique has been validated vs hyperpolarized 3He and dynamic contrast-enhanced MRI.68
Figure 8.
A and B, Ventilation (A) and perfusion (B) maps, using a 2D nonuniform Fourier decomposition MR technique, in a 12-year-old boy with CF. The 2D coronal slice was acquired in 2 min. Entire lung coverage would have required 12 slices. No contrast product injection or inhalation was done. Note the possibility of mismatches between the maps, indicating two different kinds of information. To date, the implications of this observation have not been fully elucidated. In panel A, white arrows indicate ventilation defects. In panel B, white arrows indicate perfusion defects. (Courtesy of David Bondesson and Julien Dinkel, University Hospital, Munich, Germany.)
Elastic registration of proton density-weighted sequences between inspiration and expiration has also shown a promising application to quantify parenchymal involvement.69 However, the sensitivity to detect early forms of the disease and variations over time may require further evaluation.
Functional MRI of the Lung: Which One?
It is noticeable that all of the functional techniques eventually aim at assessing the small airways. Although perfusion and ventilation maps have a significant but imperfect correlation between each other,70 there is no evidence that using perfusion, ventilation, or both may be superior for management. Thus, a single modality is generally used.
The use of a contrast agent is also a matter of specific regulations or varies among countries (Table 1, footnote). To date, gadolinium chelate injection and 129Xe MRI are the most well-studied functional imaging modalities, with the most well-documented evidence of their clinical meaningfulness.
Table 1.
Proposal for Flexible Lung Magnetic Resonance Protocol in Cystic Fibrosis
| Core Protocol | Sequence | Contrast Agent Enhancement | Acquisition Time | Expected Information | Typical Use |
|---|---|---|---|---|---|
| Morphology | 3D-UTE or 3D-ZTE | None | 4-6 min | High-resolution morphology with 3D reformations | Routine |
| Endogenous contrast | T1w | None | 15 s | T1 contrast Characterization of inflammation |
Routine |
| T2w | None | 2-4 min for 2D sequence | T2 contrast | Routine | |
| 8-10 min for 3D sequence | Detection, characterization, quantification of active inflammation | ||||
| Additional sequences | |||||
| Perfusion | Contrast- or non-contrast-enhanced sequence | If yes, gadolinium chelate injection | 5-30 min | Quantification of perfusion defects | Research,a IRB approval needed for contrast product injection |
| Ventilation | Contrast- or non-contrast-enhanced sequence | If yes, 129Xe (or oxygen of 19F) | 5-30 min | Quantification of ventilation defects | Research,a IRB approval needed for contrast product inhalation |
| Diffusion | DWI | None | 5-20 min | Characterization of abscess | Routine, according to context |
| Quantification of inflammation | Researcha |
2D = two-dimensional; 3D = three-dimensional; DWI = diffusion-weighted imaging; IRB = institutional review board; T1w = T1 weighted; T2w = T2 weighted; UTE = ultrashort echo time; ZTE = zero echo time.
Lung MRI in cystic fibrosis is a recent, trained, and expert activity. Therefore, the classification as “routine” or “research” may vary according to country regulations, institutional review board policy, and local technical availability. Gadolinium chelate injection is used routinely in Germany. Xenon-129 inhalation is considered for clinical use in the United Kingdom, and Food and Drug Administration approval is pending in the United States. Conversely, oxygen and 19F are used for research purposes only. In this table, the core protocol does not require a hardware change, can be performed in < 15 min, and is likely to be applicable across institutions without any concern about radiation and contrast product safety.
Apart from ventilation and/or perfusion defects, 129Xe has demonstrated potential in the assessment of new MR-specific biomarkers such as gas diffusion71 and gas exchange parameters.72 However, no study related to these MR features has been reported in CF yet.
Using Lung MRI in CF
Proposal for a Framework for Lung MR Protocols
A proposal for a flexible MR protocol is summarized in Table 1. The rationale is to take advantage of all the most recent developments and to enhance MRI as a multiparameter tool of investigation, in reasonable acquisition time.
First, the core MR protocol relies on a 3D-UTE or 3D-ZTE sequence, which has been recognized as the modality of choice to obtain 3D high-resolution morphologic imaging using proton MRI.13
Second, additional information on airway inflammation can be obtained using T2w (4 min) and T1w (15 s) sequences.4 Of note, the use of a single weighting may miss important information, because an intrabronchial hypo-T2 structure may correspond to either air or IMIS. Conversely, a hyper-T1 structure may sometimes be challenging to detect when the same contrast is homogeneously distributed to all mucus plugs. Thus, a combination of these two features is mandatory.
Third, insight into small-airway involvement should be attained, using a functional imaging technique. This latter feature provides an opportunity to quantify alterations beyond the spatial resolution of any imaging modality.
Additional information about the lung MRI procedure, notably to avoid issues related to respiratory motions, is provided in e-Appendix 2.
Implementing MRI in CF: When, Pros and Cons?
The suggestion of a rationale for using lung MRI, notably the routine annual follow-up, is provided in Table 2. The aim of this review is not to build any recommendation or a guideline but to provide a state-of-the-art review of MRI. However, it may be worth discussing how a practical implementation of lung MRI in CF could be envisioned, considering both the advantages and drawbacks of the technique. Three main fields of clinical application can be distinguished: the yearly follow-up, emergency situations, and clinical research. To assess disease severity, several visual scoring methods have been proposed in the literature.2,21,24,27,32 However, none of them has been recognized worldwide. Thus, we would strongly encourage the development and adoption of fully automated quantitative measurements, for standardization of results and practices. Therefore, reporting both a visual score and quantitative automated measurements may be the best complementary approach.
Table 2.
Suggestions for Clinical Use of Lung MRI in Cystic Fibrosis
| When? | Pros and Cons | Rationale for Clinical Use |
|---|---|---|
| Yearly follow-up | Radiation exposure | |
| + | Radiation-free lifelong management | |
| Availability | ||
| +/– | Anticipation of a yearly occurrence needed | |
| Cost | ||
| – | More expensive than chest radiography or CT imaging | |
| Clinical relevance | ||
| + | High-resolution scoring of structural abnormalities | |
| + | Characterization of airway inflammation | |
| + | Annual evaluation of ABPA status | |
|
||
|
||
| + | Functional evaluation of small airways | |
| Emergency situation | Radiation exposure | |
| + | Radiation-free short-term follow-up | |
| Availability | ||
| – | Lack of availability of MRI in ICU | |
| Cost | ||
| – | More expensive than chest radiography or CT imaging | |
| Clinical relevance | ||
| +/– | Chest radiography may be sufficient for large, acute abnormalities | |
| + | Secondary characterization, according to context | |
| Clinical research | Radiation exposure | |
| + | Longitudinal study design | |
| + | Imaging of healthy control subjects | |
| Availability | ||
| +/– | Anticipation of the research protocol timing needed | |
| Cost | ||
| – | More expensive than chest radiography or CT imaging | |
| Clinical relevance | ||
| + | MRI-specific, noninvasive multiparameter tools | |
| + | Sensitivity to detect longitudinal variation | |
| + | Automated reproducible quantifications |
+, pro; –, con; +/–, neutral consideration; ABPA = allergic bronchopulmonary aspergillosis; HAM = high-attenuation mucus; IMIS = inverted mucus impaction signal.
In addition, other imaging modalities, such as CT imaging, are experiencing significant breakthroughs, including novel sensitive, quantitative scorings, which were proven better than the conventional visual scores73,74 and standardization of evaluations.75 Also, the spatial resolution of MRI remains much less than that of CT imaging. For instance, CT imaging can quantify airway wall thickening up the tenth bronchial generation.74 Moreover, CT imaging is less expensive, more widely available than MRI, and faster in acquisition. A description of the extent to which various imaging modalities may prove complementary in the years to come is beyond the scope of this MRI review.
Conclusion/Perspective: Toward Five-Dimensional Lung MRI
In the early 1900s, cubism was a revolutionary approach to represent reality in painting. A single vanishing point to create an illusion of three dimensions was rejected, and different views were brought together to develop novel representations of reality. An analogy to cubism can be made with MRI of CF, where the assessment can be performed by 3D imaging, but adding functional temporal information (4D) and regional signal intensity contrasts (5D) for more comprehensive clinical evaluation.
Expected advances include larger MR scanners, 0.55-T MR scans, noiseless sequences, improved resolution, lower acquisition times, and automated quantification. Although multicenter standardization trials are ongoing,76 lung MRI is still at the fringe of routine applications, and its recommendation in the clinical setting of CF is new.12, 13, 14 Thus, a clinical application may require specific knowledge, training, and expertise. Soon, 5D-MRI of the lung may benefit from the advent of artificial intelligence, where combinations of complex multiparameter information may be possible, beyond standard human capacity, to help better predict outcomes.
In summary, lung MRI is clinically applicable and yet still rapidly developing. The results of ongoing trials and continued improvements in pulmonary MRI methods will likely prompt updated modifications of current position statements.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Solenn Toupin, PhD, for technical support from Siemens Healthineers.
Additional information: The e-Appendixes can be found in the Supplemental Materials section of the online article.
Footnotes
This study was performed within the context of the Laboratory of Excellence TRAIL, ANR-10-LABX-57.
FUNDING/SUPPORT: G. D. received academic funding from the French Society of Radiology [research grant Alain Rahmouni 2019-2020] and IdeX Bordeaux [Grant ANR-10-IDEX-03-02]. J. C. W. received funding from the National Institutes of Health [Grant HL131012 and HL123299].
Supplementary Data
References
- 1.Jain M., Goss C.H. Update in cystic fibrosis 2013. Am J Respir Crit Care Med. 2014;189(10):1181–1186. doi: 10.1164/rccm.201402-0203UP. [DOI] [PubMed] [Google Scholar]
- 2.Tiddens H.A.W.M., Kuo W., van Straten M., Ciet P. Paediatric lung imaging: the times they are a-changin’. Eur Respir Rev. 2018;27(147):170097. doi: 10.1183/16000617.0097-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauczor H.-U., Ley-Zaporozhan J., Ley S. Imaging of pulmonary pathologies: focus on magnetic resonance imaging. Proc Am Thorac Soc. 2009;6(5):458–463. doi: 10.1513/pats.200901-002AW. [DOI] [PubMed] [Google Scholar]
- 4.Woods J.C., Wild J.M., Wielpütz M.O., et al. Current state of the art MRI for the longitudinal assessment of cystic fibrosis. J Magn Reson Imaging. 2020;52(5):1306–1320. doi: 10.1002/jmri.27030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton P.G., Mall M.A., Dřevínek P., et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgel P.-R., Bellis G., Olesen H.V., et al. Future trends in cystic fibrosis demography in 34 European countries. Eur Respir J. 2015;46(1):133–141. doi: 10.1183/09031936.00196314. [DOI] [PubMed] [Google Scholar]
- 7.Joshi D., Ehrhardt A., Hong J.S., Sorscher E.J. Cystic fibrosis precision therapeutics: emerging considerations. Pediatr Pulmonol. 2019;54(suppl 3):S13–S17. doi: 10.1002/ppul.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dournes G., Marthan R., Berger P., Laurent F. Excess risk of cancer from computed tomography scan is small but not so low as to be incalculable. Am J Respir Crit Care Med. 2015;192(11):1396–1397. doi: 10.1164/rccm.201507-1467LE. [DOI] [PubMed] [Google Scholar]
- 9.Leuraud K., Richardson D.B., Cardis E., et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2(7):e276–e281. doi: 10.1016/S2352-3026(15)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronan N.J., Einarsson G.G., Twomey M., et al. CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with ivacaftor. Chest. 2018;153(2):395–403. doi: 10.1016/j.chest.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Liszewski M.C., Ciet P., Lee E.Y. MR imaging of lungs and airways in children: past and present. Magn Reson Imaging Clin N Am. 2019;27(2):201–225. doi: 10.1016/j.mric.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Centre de Référence Mucoviscidose de Lyon Protocole National de Diagnostic et de Soin (PNDS) Mucoviscidose. Haute Autorité de Santé, France. 2017. https://www.has-sante.fr/portail/upload/docs/application/pdf/2017-09/pnds_2017_vf1.pdf
- 13.Hatabu H., Ohno Y., Gefter W.B., et al. Fleischner Society Expanding applications of pulmonary MRI in the clinical evaluation of lung disorders: Fleischner Society position paper. Radiology. 2020;297(2):286–301. doi: 10.1148/radiol.2020201138. [DOI] [PubMed] [Google Scholar]
- 14.Schiebler M.L., Parraga G., Gefter W.B., et al. Synopsis from expanding applications of pulmonary MRI in the clinical evaluation of lung disorders: Fleischner Society position paper. Chest. 2021;159(2):492–495. doi: 10.1016/j.chest.2020.09.075. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs H.J., Borowitz D.S., Christiansen D.H., et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis: the Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 16.Stevens D.A., Moss R.B., Kurup V.P., et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(suppl 3):S225–S264. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz C., Schulte-Hubbert B., Bend J., et al. [CF lung disease—a German S3 guideline: Module 2: Diagnostics and treatment in chronic infection with Pseudomonas aeruginosa] [article in German] Pneumologie. 2018;72(5):347–392. doi: 10.1055/s-0044-100191. [DOI] [PubMed] [Google Scholar]
- 18.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 19.Cooper P., MacLean J. High-resolution computed tomography (HRCT) should not be considered as a routine assessment method in cystic fibrosis lung disease. Paediatr Respir Rev. 2006;7(3):197–201. doi: 10.1016/j.prrv.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Tiddens H.A.W.M. Chest computed tomography scans should be considered as a routine investigation in cystic fibrosis. Paediatr Respir Rev. 2006;7(3):202–208. doi: 10.1016/j.prrv.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Bhalla M., Turcios N., Aponte V., et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179(3):783–788. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 22.Bortoluzzi C.F., Pontello E., Pintani E., et al. The impact of chest computed tomography and chest radiography on clinical management of cystic fibrosis lung disease. J Cyst Fibros. 2019;19(4):641–646. doi: 10.1016/j.jcf.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Altes T.A., Eichinger M., Puderbach M. Magnetic resonance imaging of the lung in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):321–327. doi: 10.1513/pats.200611-181HT. [DOI] [PubMed] [Google Scholar]
- 24.Eichinger M., Optazaite D.-E., Kopp-Schneider A., et al. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol. 2012;81(6):1321–1329. doi: 10.1016/j.ejrad.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 25.Ciet P., Serra G., Bertolo S., et al. Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol. 2016;26(3):780–787. doi: 10.1007/s00330-015-3850-9. [DOI] [PubMed] [Google Scholar]
- 26.Willmering M.M., Robison R.K., Wang H., Pipe J.G., Woods J.C. Implementation of the FLORET UTE sequence for lung imaging. Magn Reson Med. 2019;82(3):1091–1100. doi: 10.1002/mrm.27800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roach D.J., Crémillieux Y., Fleck R.J., et al. Ultrashort echo-time magnetic resonance imaging is a sensitive method for the evaluation of early cystic fibrosis lung disease. Ann Am Thorac Soc. 2016;13(11):1923–1931. doi: 10.1513/AnnalsATS.201603-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson K.M., Fain S.B., Schiebler M.L., Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70(5):1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dournes G., Grodzki D., Macey J., et al. Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology. 2015;276(1):258–265. doi: 10.1148/radiol.15141655. [DOI] [PubMed] [Google Scholar]
- 30.Dournes G., Menut F., Macey J., et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol. 2016;26(11):3811–3820. doi: 10.1007/s00330-016-4218-5. [DOI] [PubMed] [Google Scholar]
- 31.Dournes G., Yazbek J., Benhassen W., et al. 3D ultrashort echo time MRI of the lung using stack-of-spirals and spherical k-space coverages: evaluation in healthy volunteers and parenchymal diseases. J Magn Reson Imaging. 2018;48(6):1489–1497. doi: 10.1002/jmri.26212. [DOI] [PubMed] [Google Scholar]
- 32.Calder A.D., Bush A., Brody A.S., Owens C.M. Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr Radiol. 2014;44(12):1496–1506. doi: 10.1007/s00247-013-2867-y. [DOI] [PubMed] [Google Scholar]
- 33.Baldacci F, Laurent F, Berger P, Dournes G. 3D human airway segmentation from high resolution MR imaging. Proc SPIE. 11041, Eleventh International Conference on Machine Vision (ICMV 2018), 110410Y (March 15, 2019). 10.1117/12.2522948. Accessed January 2, 2021. [DOI]
- 34.Benlala I., Berger P., Girodet P.-O., et al. Automated volumetric quantification of emphysema severity by using ultrashort echo time MRI: validation in participants with chronic obstructive pulmonary disease. Radiology. 2019;292(1):216–225. doi: 10.1148/radiol.2019190052. [DOI] [PubMed] [Google Scholar]
- 35.Benlala I., Point S., Leung C., et al. Volumetric quantification of lung MR signal intensities using ultrashort TE as an automated score in cystic fibrosis. Eur Radiol. 2020;30(10):5479–5488. doi: 10.1007/s00330-020-06910-w. [DOI] [PubMed] [Google Scholar]
- 36.Duan G., Zhao X., Anderson S.W., Zhang X. Boosting magnetic resonance imaging signal-to-noise ratio using magnetic metamaterials. Commun Phys. 2019;2(1):35. doi: 10.1038/s42005-019-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell-Washburn A.E., Ramasawmy R., Restivo M.C., et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology. 2019;293(2):384–393. doi: 10.1148/radiol.2019190452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong D.S., Grimwood K., Carlin J.B., et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156(4):1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 39.Refait J., Macey J., Bui S., et al. CT evaluation of hyperattenuating mucus to diagnose allergic bronchopulmonary aspergillosis in the special condition of cystic fibrosis. J Cyst Fibros. 2019;18(4):e31–e36. doi: 10.1016/j.jcf.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Wainwright C.E., Grimwood K., Carlin J.B., et al. Safety of bronchoalveolar lavage in young children with cystic fibrosis. Pediatr Pulmonol. 2008;43(10):965–972. doi: 10.1002/ppul.20885. [DOI] [PubMed] [Google Scholar]
- 41.Ordoñez C.L., Henig N.R., Mayer-Hamblett N., et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(12):1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 42.Chen D.L., Atkinson J.J., Ferkol T.W. FDG PET imaging in cystic fibrosis. Semin Nucl Med. 2013;43(6):412–419. doi: 10.1053/j.semnuclmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Biederer J., Beer M., Hirsch W., et al. MRI of the lung (2/3). Why … when … how? Insights Imaging. 2012;3(4):355–371. doi: 10.1007/s13244-011-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciet P., Bertolo S., Ros M., et al. Detection and monitoring of lung inflammation in cystic fibrosis during respiratory tract exacerbation using diffusion-weighted magnetic resonance imaging. Eur Respir J. 2017;50(1):1601437. doi: 10.1183/13993003.01437-2016. [DOI] [PubMed] [Google Scholar]
- 45.Dournes G., Laurent F. Restricted magnetic resonance diffusion of lung consolidation is not specific for respiratory exacerbation. Eur Respir J. 2017;50(5):1701621. doi: 10.1183/13993003.01621-2017. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch F.W., Sorge I., Vogel-Claussen J., et al. The current status and further prospects for lung magnetic resonance imaging in pediatric radiology. Pediatr Radiol. 2020;50(5):734–749. doi: 10.1007/s00247-019-04594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wielpütz M.O., Puderbach M., Kopp-Schneider A., et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189(8):956–965. doi: 10.1164/rccm.201309-1659OC. [DOI] [PubMed] [Google Scholar]
- 48.Benlala I., Hocke F., Macey J., et al. Quantification of T2 high signal volume in cystic fibrosis: a pilot study. Radiology. 2020;294(1):186–196. doi: 10.1148/radiol.2019190797. [DOI] [PubMed] [Google Scholar]
- 49.Renz D.M., Scholz O., Böttcher J., et al. Comparison between magnetic resonance imaging and computed tomography of the lung in patients with cystic fibrosis with regard to clinical, laboratory, and pulmonary functional parameters. Invest Radiol. 2015;50(10):733–742. doi: 10.1097/RLI.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 50.Dournes G., Berger P., Refait J., et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology. 2017;285(1):261–269. doi: 10.1148/radiol.2017162350. [DOI] [PubMed] [Google Scholar]
- 51.Garg M.K., Gupta P., Agarwal R., Sodhi K.S., Khandelwal N. MRI: a new paradigm in imaging evaluation of allergic bronchopulmonary aspergillosis? Chest. 2015;147(2):e58–e59. doi: 10.1378/chest.14-2347. [DOI] [PubMed] [Google Scholar]
- 52.Kapur S., Bhalla A.S., Jana M. Pediatric chest MRI: a review. Indian J Pediatr. 2019;86(9):842–853. doi: 10.1007/s12098-018-02852-w. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal R., Sehgal I.S., Dhooria S., et al. Allergic bronchopulmonary aspergillosis. Indian J Med Res. 2020;151(6):529–549. doi: 10.4103/ijmr.IJMR_1187_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellani C., Duff A.J.A., Bell S.C., et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17(2):153–178. doi: 10.1016/j.jcf.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R., Chakrabarti A., Shah A., et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43(8):850–873. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 56.Savant A.P., McColley S.A. Cystic fibrosis year in review 2017. Pediatr Pulmonol. 2018;53(9):1307–1317. doi: 10.1002/ppul.24081. [DOI] [PubMed] [Google Scholar]
- 57.Sodhi K.S., Gupta P., Shrivastav A., et al. Evaluation of 3 T lung magnetic resonance imaging in children with allergic bronchopulmonary aspergillosis: pilot study. Eur J Radiol. 2019;111:88–92. doi: 10.1016/j.ejrad.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health Clinical Center MR imaging of lung in the follow-up assessment of cystic fibrosis (CFMR-lung). NCT03357562. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health. 2017. https://clinicaltrials.gov/ct2/show/NCT03357562 Updated 2020.
- 59.Smith L.J., Horsley A., Bray J., et al. The assessment of short and long term changes in lung function in CF using 129Xe MRI. Eur Respir J. 2020:2000441. doi: 10.1183/13993003.00441-2020. [DOI] [PubMed] [Google Scholar]
- 60.Woods J.C., Choong C.K., Yablonskiy D.A., et al. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med. 2006;56(6):1293–1300. doi: 10.1002/mrm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walkup L.L., Thomen R.P., Akinyi T.G., et al. Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol. 2016;46(12):1651–1662. doi: 10.1007/s00247-016-3672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomen R.P., Walkup L.L., Roach D.J., Cleveland Z.I., Clancy J.P., Woods J.C. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros. 2017;16(2):275–282. doi: 10.1016/j.jcf.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bannier E., Cieslar K., Mosbah K., et al. Hyperpolarized 3He MR for sensitive imaging of ventilation function and treatment efficiency in young cystic fibrosis patients with normal lung function. Radiology. 2010;255(1):225–232. doi: 10.1148/radiol.09090039. [DOI] [PubMed] [Google Scholar]
- 64.Zha W., Nagle S.K., Cadman R.V., Schiebler M.L., Fain S.B. Three-dimensional isotropic functional imaging of cystic fibrosis using oxygen-enhanced MRI: comparison with hyperpolarized 3He MRI. Radiology. 2019;290(1):229–237. doi: 10.1148/radiol.2018181148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Couch M.J., Ball I.K., Li T., Fox M.S., Biman B., Albert M.S. 19 F MRI of the lungs using inert fluorinated gases: challenges and new developments. J Magn Reson Imaging. 2019;49(2):343–354. doi: 10.1002/jmri.26292. [DOI] [PubMed] [Google Scholar]
- 66.Mall M.A., Stahl M., Graeber S.Y., Sommerburg O., Kauczor H.-U., Wielpütz M.O. Early detection and sensitive monitoring of CF lung disease: prospects of improved and safer imaging. Pediatr Pulmonol. 2016;51(S44):S49–S60. doi: 10.1002/ppul.23537. [DOI] [PubMed] [Google Scholar]
- 67.Veldhoen S., Weng A.M., Knapp J., et al. Self-gated non-contrast-enhanced functional lung MR imaging for quantitative ventilation assessment in patients with cystic fibrosis. Radiology. 2017;283(1):242–251. doi: 10.1148/radiol.2016160355. [DOI] [PubMed] [Google Scholar]
- 68.Bauman G., Scholz A., Rivoire J., et al. Lung ventilation- and perfusion-weighted Fourier decomposition magnetic resonance imaging: in vivo validation with hyperpolarized 3He and dynamic contrast-enhanced MRI. Magn Reson Med. 2013;69(1):229–237. doi: 10.1002/mrm.24236. [DOI] [PubMed] [Google Scholar]
- 69.Pennati F., Salito C., Borzani I., et al. Quantitative multivolume proton-magnetic resonance imaging in patients with cystic fibrosis lung disease: comparison with clinical indicators. Eur Respir J. 2019;53(5):1702020. doi: 10.1183/13993003.02020-2017. [DOI] [PubMed] [Google Scholar]
- 70.Kaireit T.F., Sorrentino S.A., Renne J., et al. Functional lung MRI for regional monitoring of patients with cystic fibrosis. PLOS ONE. 2017;12(12) doi: 10.1371/journal.pone.0187483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan H.-F., Stewart N.J., Norquay G., Collier G.J., Wild J.M. 3D diffusion-weighted 129Xe MRI for whole lung morphometry. Magn Reson Med. 2018;79(6):2986–2995. doi: 10.1002/mrm.26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z., Bier E.A., Swaminathan A., et al. Diverse cardiopulmonary diseases are associated with distinct xenon MRI signatures. Eur Respir J. 2019;54(6):1900831. doi: 10.1183/13993003.00831-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenow T., Oudraad M.C.J., Murray C.P., et al. PRAGMA-CF: a quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med. 2015;191(10):1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 74.Kuo W., Soffers T., Andrinopoulou E.-R., et al. Quantitative assessment of airway dimensions in young children with cystic fibrosis lung disease using chest computed tomography. Pediatr Pulmonol. 2017;52(11):1414–1423. doi: 10.1002/ppul.23787. [DOI] [PubMed] [Google Scholar]
- 75.Kuo W., Kemner-van de Corput M.P.C., Perez-Rovira A., et al. Multicentre chest computed tomography standardisation in children and adolescents with cystic fibrosis: the way forward. Eur Respir J. 2016;47(6):1706–1717. doi: 10.1183/13993003.01601-2015. [DOI] [PubMed] [Google Scholar]
- 76.Wielpütz M.O., Stackelberg O von, Stahl M., et al. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J Cyst Fibros. 2018;17(4):518–527. doi: 10.1016/j.jcf.2018.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.