Abstract
Background
Physiologic and symptom responses at the ventilatory threshold (Tvent) during incremental cardiopulmonary exercise testing (CPET) can provide important prognostic information.
Research Question
This study aimed to develop an updated normative reference set for physiologic and symptom responses at Tvent during cycle CPET (primary aim) and to evaluate previously recommended reference equations from a 1985 study for predicting Tvent responses (secondary aim).
Study Design and Methods
Participants were adults 40 to 80 years of age who were free of clinically relevant disease from the Canadian Cohort Obstructive Lung Disease. Rate of oxygen consumption (O2) at Tvent was identified by two independent raters; physiologic and symptom responses corresponding to O2 at Tvent were identified by linear interpolation. Reference ranges (5th-95th percentiles) for responses at Tvent were calculated according to participant sex and age for 29 and eight variables, respectively. Prediction models were developed for nine variables (oxygen pulse, O2, rate of CO2 production, minute ventilation, tidal volume, inspiratory capacity, end-inspiratory lung volume [in liters and as percentage of total lung capacity], and end-expiratory lung volume) using quantile regression, estimating the 5th (lower limit of normal), 50th (normal), and 95th (upper limit of normal) percentiles based on readily available participant characteristics. The two one-sided test of equivalence for paired samples evaluated the measured and 1985-predicted O2 at Tvent for equivalence.
Results
Reference ranges and equations were developed based on 96 participants (49% men) with a mean ± SD age of 63 ± 9 years. Mean O2 at Tvent was 50% of measured O2 peak; the normal range was 33% to 66%. The 1985 reference equations overpredicted O2 at Tvent: mean difference in men, −0.17 L/min (95% CI, −0.25 to −0.09 L/min); mean difference in women, −0.19 L/min (95% CI, −0.27 to −0.12 L/min).
Interpretation
A contemporary reference set of CPET responses at Tvent from Canadian adults 40 to 80 years of age is presented that differs from the previously recommended and often used reference set from 1985.
Trial Registry
ClinicalTrials.gov; No.: NCT00920348; URL: www.clinicaltrials.gov;
Key Words: anaerobic threshold, cardiopulmonary exercise testing, CPET, exercise responses, normal values, reference set, ventilatory threshold
Abbreviations: CanCOLD, Canadian Cohort Obstructive Lung Disease; CPET, cardiopulmonary exercise testing; FRIEND, Fitness Registry and the Importance of Exercise; IC, inspiratory capacity; LLN, lower limit of normal; SHIP, Study of Health in Pomerania; Tvent, ventilatory threshold; ULN, upper limit of normal; CO2, rate of carbon dioxide production; O2, rate of oxygen consumption; VT, tidal volume
Cardiopulmonary exercise testing (CPET) is unique in its ability to identify pathophysiologic mechanisms underlying exercise intolerance and exertional symptoms noninvasively.1 Importantly, CPET permits evaluation of both submaximal and maximal physiologic and symptom responses to exercise, both relevant in diagnostic and prognostic evaluation.1,2 Compared with peak CPET responses (eg, peak rate of oxygen consumption [O2]), submaximal physiologic and symptom responses are not dependent on participant effort or motivation, nor are they susceptible to underestimation because of premature termination of exercise by the supervisor (eg, because of cardiac-related events). Therefore, evaluation of submaximal physiologic and symptom responses to CPET become particularly important in the individual who is unable or unwilling to provide maximal effort and to achieve a peak CPET response.
The ventilatory threshold (Tvent) is a submaximal metabolic inflection point during exercise that has shown particular importance for clinical decision-making and health-related outcomes.1,2 For example, a low O2 at Tvent is a strong independent predictor of mortality in people with chronic heart failure,3,4 after surgery,5,6 and after organ transplantation.7,8 A low Tvent has been defined as a O2 of less than a critical threshold (eg, < 11 mL/kg/min),3 less than a certain percentage of the predicted normal O2 peak (eg, < 40%), or both.1,9 Although absolute thresholds are important in specific contexts, such as surgery recovery, clinical CPET guidelines define a normal Tvent to be a O2 that is 50% to 60% of the predicted normal O2 peak, with a value of < 40% indicating potential underlying pathophysiologic features.1 However, these normative reference ranges were based on studies from more than three decades ago.9,10 We recently demonstrated that these historical reference sets by Hansen et al9 in 1984 and Jones et al10 in 1985 overpredicted peak cardiac CPET responses (oxygen pulse, heart rate) in a contemporary population-based sample of Canadian adults11 and likely are no longer applicable to today’s population, who generally are less fit and active than adults from the 1980s.12,13
The primary aim of this study was to develop a contemporary normative reference set (including normative reference ranges [5th-95th percentiles] and prediction models based on readily available participant characteristics) for physiologic and symptom responses at Tvent during incremental cycle CPET in Canadian adults 40 to 80 years of age. In addition to providing a reference set for O2 at Tvent, we aimed to provide a comprehensive reference set for cardiac, ventilatory, operating lung volume, gas exchange, and symptom responses at Tvent, where several of these parameters also have demonstrated importance for predicting health outcomes.6 Our secondary aim was to compare the O2 at Tvent measured in contemporary Canadian adults with the O2 at Tvent predicted using reference equations published by Jones et al10 more than 30 years ago.
Methods
This study was a secondary analysis of data from the Canadian Cohort Obstructive Lung Disease (CanCOLD) study, a prospective, random-sampled, population-based study conducted across nine sites in Canada (ClinicalTrials.gov Identifier: NCT00920348).14 Participants were noninstitutionalized male and female adults 40 years of age or older identified with random telephone digit dialing. All participants provided written informed consent before completing study assessments. The research ethics board for each participating institution approved the study protocol.
Participants
For the current study, 1,367 participants who completed a CPET with a Vmax (SensorMedics) CPET system at the initial CanCOLD cross-sectional assessment phase (visit 1) were considered for inclusion. Breath-by-breath (raw) CPET data collected at visit 1 using other CPET systems in Montréal (Ergocard, Medisoft [n = 26]), Toronto (Ergocard, Medisoft [n = 5]), and Calgary (TrueOne, Parvo Medics [n = 15]) were inaccessible and unavailable for analyses; therefore, these participants (n = 46) were excluded from the current study (e-Table 1 presents participant characteristics and peak O2 responses compared with the final sample). Specific details on further participant eligibility criteria are outlined in e-Appendix 1. Briefly, participants were excluded if they: (1) had a cigarette smoking history of > 5 pack-years; (2) self-reported cardiovascular, pulmonary, or metabolic disease; (3) showed abnormal pulmonary function at rest15, 16, 17; (4) did not achieve a symptom or physiologic limitation to CPET; or (5) showed an abnormal CPET response determined by the supervising physician. Participants older than 80 years were also excluded because few (n = 4) participants older than 80 years met the abovementioned eligibility criteria.
Measures
Body mass and height were measured and postbronchodilator spirometry, diffusing capacity of the lungs for carbon monoxide, and body plethysmography were performed according to current standards.18 Sociodemographic and health characteristic information (eg, smoking history, self-reported health conditions) were obtained during a structured interview with a trained researcher. Self-reported physical activity levels were obtained via questionnaire.19
CPET
CPET was performed in accordance with recommended guidelines1,20 on an electronically braked cycle ergometer using a computerized CPET system (Vmax, SensorMedics). The CPET protocol was standardized across sites, consisting of a steady-state rest period of 3 to 10 min, 1 min of unloaded pedaling, and then stepwise increases in power output of 10 W/min, starting at 10 W, until symptom limitation.
Gas exchange and breathing pattern responses were collected breath-by-breath while participants breathed through a mouthpiece with a low-resistance flow transducer wearing a nose clip. Heart rate was assessed with a 12-lead ECG and peripheral oxygen saturation via a pulse oximeter. During the steady-state rest period, every 2 min throughout exercise, and at end exercise, maximal inspiratory capacity (IC) maneuvers were performed, BP was assessed, and participants rated the intensity of their breathing discomfort (breathlessness) and leg discomfort using the 0-10 modified Borg scale.21 Physiologic variables were averaged in 30-s epochs and linked with contemporaneous symptom intensity ratings and IC-derived variables (e-Appendix 1).
Tvent Identification and Analysis of Exercise Responses
The O2 at Tvent was identified by two independent raters (O. E., F. N.) using the V-Slope22, 23, 24, 25, 26 and Dual Criterion27 methods, as recommended by CPET guidelines1 and described in detail in e-Appendix 1. Interrater acceptability criteria were defined a priori as ≤ 10% or a ≤ 0.15-L/min difference between the two O2 values at Tvent. If interrater criteria were met, the average of the two O2 values was taken as the final O2 at Tvent. If interrater criteria were not met, a third blinded rater (D. J.) evaluated Tvent, and the two closest O2 values meeting interrater criteria were averaged and taken as the final O2 at Tvent. In the case where the interrater criteria were still not met after evaluation by the third reviewer, the Tvent was considered indeterminant. When the final O2 at Tvent was identified, other physiologic and symptom responses corresponding to this level of O2 were estimated via linear interpolation using the closest available data points (ie, the 30-s averaged O2 values recorded immediately before and after the newly identified Tvent and the physiologic and symptom variables recorded at these same time points).
Statistical Analysis
Participant baseline demographic and health characteristics were summarized. The absolute (L/min) and relative (percentage) difference between O2 at Tvent identified by the two raters was evaluated for each participant, with mean ± SD and range of differences reported. To assess the level of agreement between the two raters for O2 (L/min) at Tvent, the intraclass correlation coefficient and 95% CI were calculated based on a mean-rating (raters, k = 2), absolute-agreement, 2-way mixed-effects model.28 The two-way mixed-effects model was used because each participant’s Tvent was assessed by the same set of raters (two-way), with these raters being the only raters of interest (mixed-effects). To define normative reference ranges for physiologic and symptom responses at Tvent, the mean ± SD and 5th (lower limit of normal [LLN]) and 95th percentiles (upper limit of normal [ULN]) were estimated overall and by participant sex. For CPET variables demonstrating significant (P < .05) bivariate associations with age, the 5th to 95th percentiles also were estimated in age groups of 40 to 55 years, 56 to 69 years, and 70 to 80 years, providing additional aged-based normative reference ranges. To develop prediction models, correlation matrices and scatterplots were generated to assess strength and shape of crude associations between readily available participant characteristics of sex, age, height, and body mass and CPET responses at Tvent (e-Fig 1); generalized linear models estimated crude bivariate relationships. Explanatory variables were included in the prediction model if (1) crude bivariate associations were significant (P < .05) or, (2) if not significant, inclusion in the model improved model fit indicated by an increase in the adjusted R2 value. Interactions between explanatory variables also were explored using product terms and were included in the final models when significant (P < .05). Prediction models were presented for a given CPET variable based on the overall adjusted R2 value (≥ 0.30), the authors’ consideration of the residual SE clinical significance, or both. The final multivariate prediction models were developed using quantile regression. Quantile regression enables prediction of the median response (50th percentile) in addition to other specific quantiles (percentiles), of which we predicted the 5th percentile (LLN) and the 95th percentile (ULN).
The two one-sided test of equivalence for paired-samples evaluated statistical equivalence between the O2 measured at Tvent in the CanCOLD study participants and the O2 predicted at Tvent using reference equations from Jones et al.10 Upper and lower equivalence bounds were set as raw scores for O2, predefined as ± 0.674 SD. A Z score of ± 0.674 represents a change in O2 from the 50th to the 25th (−0.674 SD) or 75th (+0.674 SD) percentile in a normal distribution. Data are reported as mean ± SD unless otherwise indicated. Significance was considered at α < 0.05. Analyses were performed in R version 3.6.0 software (R Foundation for Statistical Computing).
Results
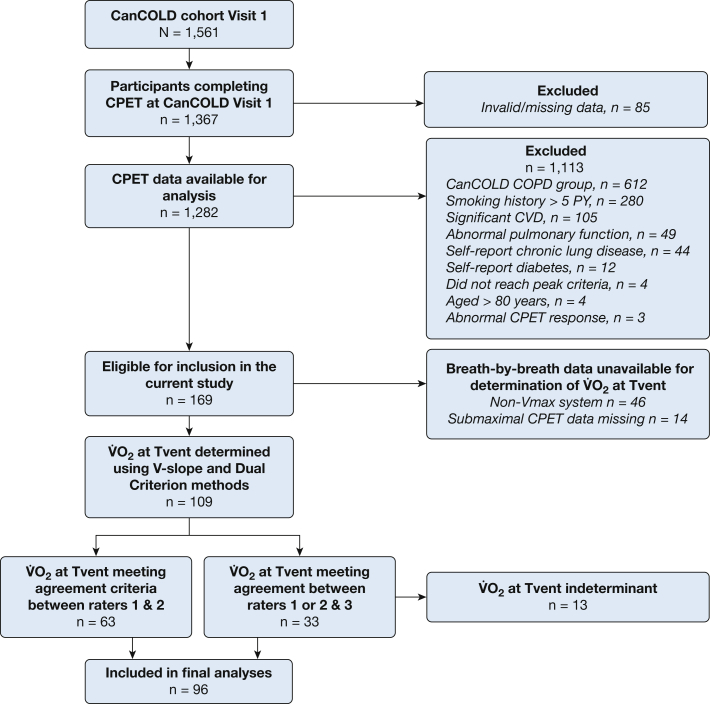
Of the 1,367 participants who completed CPET as part of the initial cross-sectional assessment phase of CanCOLD study, 109 were eligible for inclusion in the current study. Thirteen participants were excluded because the O2 at Tvent was indeterminate (Fig 1). Therefore, the final sample included 96 participants, 47 (49%) men and 49 (51%) women, with a mean age of 63 ± 9 years (Table 1). Most participants were between 60 and 70 years of age (men, n = 19 [40%]; women, n = 22 [45%]) (e-Fig 2).
Figure 1.
Participant flow diagram. CanCOLD = Canadian Cohort Obstructive Lung Disease study; CPET = cardiopulmonary exercise test; CVD = cardiovascular disease; PY = pack-years; Tvent = ventilatory threshold; O2 = rate of oxygen consumption.
Table 1.
Participant Sociodemographic and Health Characteristics
| Participant Characteristic | Total Sample (N = 96) | Men (n = 47) | Women (n = 49) |
|---|---|---|---|
| Age, y | 63 ± 9 | 63 ± 9 | 63 ± 9 |
| Range | 42-79 | 47-79 | 42-78 |
| Height, cm | 167.9 ± 9.0 | 174.2 ± 7.5 | 161.8 ± 5.4 |
| Body mass, kg | 76.3 ± 14.1 | 83.5 ± 12.5 | 69.3 ± 11.9 |
| BMI, kg/m2 | 27.0 ± 4.2 | 27.5 ± 3.8 | 26.5 ± 4.5 |
| FEV1, L | 2.95 ± 0.71 | 3.39 ± 0.64 | 2.54 ± 0.44 |
| FEV1, % predicted | 104 ± 14 | 102 ± 14 | 106 ± 14 |
| FVC, L | 3.80 ± 0.91 | 4.37 ± 0.84 | 3.26 ± 0.59 |
| FVC, % predicted | 104 ± 14 | 101 ± 13 | 107 ± 15 |
| TLC, % predicted | 102 ± 12 | 101 ± 12 | 102 ± 13 |
| Dlco, % predicted | 98 ± 18 | 99 ± 24 | 97 ± 12 |
| Cigarette smoking, pack-y | 0.6 ± 1.4 | 0.8 ± 1.7 | 0.5 ± 1.0 |
| Cigarette smoking status | |||
| Never | 71 (74) | 35 (74) | 36 (73) |
| Former | 25 (26) | 12 (26) | 13 (27) |
| MVPA, h/wk | 2.7 ± 2.5 | 3.1 ± 2.8 | 2.3 ± 2.1 |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. Dlco = diffusing capacity of the lungs for carbon monoxide; MVPA = moderate to vigorous intensity physical activity assessed by self-report with the Community Healthy Activities Models Program for Seniors questionnaire20; TLC = total lung capacity.
Participants included in the current study were comparable with Canadian adults 40 years of age or older (2017 and 2018 Statistics Canada data) for standing height (mean CanCOLD: 174.2 cm [men], 161.8 cm [women]; mean Canadian population: 174.4 cm [men], 161.2 cm [women]29) and physical activity levels (mean CanCOLD: 3.1 h/wk [men], 2.3 h/wk [women]; mean Canadian population: 2.7 h/wk [men], 2.5 h/wk [women]30), but had a slightly lower body mass (mean CanCOLD: 83.5 kg [men], 69.3 kg [women]; mean Canadian population: 86.5 kg [men], 73.7 kg [women]29).
The mean ± SD and range of O2 values at Tvent identified by the two raters, as well as the mean absolute and relative differences between rater 1 and rater 2, is presented in e-Table 2. High agreement was found between the two raters (intraclass correlation coefficient, 0.99; 95% CI, 0.98-0.99; P < .001).
The O2 at Tvent was, on average, 48 ± 11% (LLN, 33%; ULN, 66%) of the measured O2 peak and 51 ± 14% (LLN, 33%; ULN, 75%) of the predicted11 normal O2 peak. The complete set of normative reference ranges (mean ± SD and 5th and 95th percentiles) for cardiac, ventilatory, operating lung volume, gas exchange, and symptom variables at Tvent are presented in Table 2 by participant sex. For eight CPET variables demonstrating significant associations with age, normative reference ranges also are presented in the age groups of 40 to 55 years (men, n = 10; women, n = 9), 56 to 69 years (men, n = 25; women, n = 30), and 70 to 80 years (men, n = 12; women, n = 10) (Table 3). Prediction models to estimate the average (50th percentile), lower (5th percentile), and upper (95th percentile) limits of normal for nine CPET variables at Tvent : rate of carbon dioxide production (CO2), oxygen pulse, minute ventilation (e), tidal volume (VT), IC, end-expiratory lung volume (in liters and as percentage of total lung capacity), and end-inspiratory lung volume (in liters)—based on participant sex, age, height, and body mass are presented in Table 4.
Table 2.
Normative Reference Values and Ranges for Physiologic and Symptom Responses at the Tvent During Symptom-Limited Incremental CPET According to Participant Sex
| CPET Parameter | Total Sample (N = 96) |
Men (n = 47) |
Women (n = 49) |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | LLN-ULN | Mean ± SD | LLN-ULN | Mean ± SD | LLN-ULN | |
| O2 at Tvent, L/min | 0.95 ± 0.31 | 0.51-1.56 | 1.11 ± 0.28 | 0.75-1.60 | 0.80 ± 0.25 | 0.49-1.23 |
| O2 at Tvent, mL/kg/min | 12.6 ± 3.9 | 7.7-18.3 | 13.7 ± 3.9 | 8.7-21.2 | 11.6 ± 3.6 | 6.6-16.3 |
| O2 at peak, L/min | 2.01 ± 0.58 | 1.20-2.96 | 2.38 ± 0.51 | 1.63-3.40 | 1.66 ± 0.40 | 1.05-2.23 |
| O2 at peak, mL/kg/min | 26.7 ± 7.2 | 17.0-42.7 | 28.8 ± 6.7 | 20.5-43.0 | 24.5 ± 7.2 | 16.0-37.5 |
| O2 at Tvent, %O2 peak | 48.3 ± 10.6 | 32.6-65.6 | 47.6 ± 9.5 | 31.6-63.0 | 49.0 ± 11.6 | 32.8-65.7 |
| O2 at Tvent, % predictedaO2 peak | 51.2 ± 13.5 | 32.9-75.4 | 48.5 ± 11.2 | 33.5-67.2 | 53.8 ± 15.1 | 33.1-82.0 |
| CO2, L/min | 0.83 ± 0.31 | 0.41-1.39 | 0.98 ± 0.28 | 0.64-1.45 | 0.69 ± 0.27 | 0.35-1.14 |
| RER | 0.85 ± 0.10 | 0.69-1.02 | 0.86 ± 0.09 | 0.72-0.99 | 0.84 ± 0.11 | 0.68-1.03 |
| Power output, W | 51 ± 24 | 16-94 | 60 ± 23 | 29-97 | 42 ± 20 | 15-71 |
| HR, beats/min | 101 ± 16 | 77-123 | 100 ± 19 | 75-121 | 102 ± 13 | 84-123 |
| Oxygen pulse, mL O2/beat | 9.5 ± 3.0 | 5.5-14.6 | 11.2 ± 2.9 | 7.5-14.8 | 7.8 ± 2.0 | 5.3-11.1 |
| BP, mm Hg | ||||||
| Systolic | 147 ± 32 | 112-188 | 153 ± 32 | 117-188 | 142 ± 31 | 105-187 |
| Diastolic | 77 ± 14 | 62-95 | 77 ± 14 | 61-95 | 76 ± 14 | 65-93 |
| e/CO2 | 30.2 ± 3.8 | 25.1-37.9 | 29.2 ± 2.7 | 25.4-33.9 | 31.2 ± 4.5 | 25.2-39.5 |
| e/O2 | 25.8 ± 3.3 | 20.8-32.3 | 25.5 ± 3.0 | 21.4-31.6 | 26.2 ± 3.6 | 20.7-32.3 |
| PETCO2, mm Hg | 40.6 ± 4.2 | 34.5-47.1 | 41.0 ± 4.4 | 35.1-47.5 | 40.2 ± 4.1 | 34.5-46.8 |
| PETO2, mm Hg | 101.6 ± 7.7 | 90.6-111.4 | 100.8 ± 9.1 | 89.3-112.4 | 102.2 ± 6.0 | 93.4-111.0 |
| SpO2, % | 97 ± 2 | 95-99 | 97 ± 3 | 94-99 | 98 ± 1 | 96-99 |
| e, L/min | 24.4 ± 8.0 | 13.7-38.7 | 28.2 ± 7.3 | 18.7-40.7 | 20.8 ± 6.8 | 12.1-34.3 |
| e, % MVV | 24 ± 8 | 14-36 | 25 ± 8 | 14-36 | 24 ± 8 | 14-37 |
| VT, L | 1.22 ± 0.36 | 0.68-1.88 | 1.41 ± 0.31 | 0.98-2.02 | 1.04 ± 0.31 | 0.62-1.50 |
| VT, % IC | 41.5 ± 9.7 | 25.8-61.0 | 43.0 ± 8.3 | 29.0-60.7 | 40.1 ± 11.2 | 25.0-60.6 |
| Bf, breaths/min | 20 ± 4 | 16-94 | 20 ± 4 | 15-27 | 21 ± 5 | 13-28 |
| IC, L | 2.98 ± 0.67 | 2.01-4.01 | 3.34 ± 0.66 | 2.10-4.17 | 2.63 ± 0.46 | 2.01-3.52 |
| IRV, L | 1.76 ± 0.56 | 0.90-2.66 | 1.93 ± 0.61 | 0.94-2.84 | 1.59 ± 0.45 | 0.89-2.36 |
| EILV, L | 4.20 ± 0.97 | 2.82-5.78 | 4.77 ± 0.93 | 3.21-6.53 | 3.69 ± 0.68 | 2.68-4.74 |
| EELV, L | 2.98 ± 0.80 | 1.86-4.42 | 3.35 ± 0.84 | 2.06-4.57 | 2.66 ± 0.61 | 1.78-3.73 |
| EILV, % TLC | 70 ± 7 | 58-82 | 70 ± 8 | 58-83 | 69 ± 6 | 61-81 |
| EELV, % TLC | 49 ± 7 | 38-62 | 49 ± 8 | 36-60 | 50 ± 6 | 39-62 |
| Breathlessness, Borg 0-10 | 1 ± 1 | 0-3 | 1 ± 1 | 0-3 | 1 ± 1 | 0-3 |
| Leg discomfort, Borg 0-10 | 1 ± 1 | 0-3 | 2 ± 1 | 0-4 | 1 ± 1 | 0-3 |
Bf = breathing frequency; CPET = cardiopulmonary exercise testing; EELV = end-expiratory lung volume; EILV = end-inspiratory lung volume; HR = heart rate; IC = inspiratory capacity; IRV = inspiratory reserve volume; LLN = lower limit of normal; MVV = maximum voluntary ventilation; PETCO2 = end-tidal Pco2; PETO2 = end-tidal Po2; RER = respiratory exchange ratio; SpO2 = peripheral oxygen saturation; TLC = total lung capacity; Tvent = ventilatory threshold; ULN = upper limit of normal; CO2 = rate of CO2 production; e = minute ventilation; e/CO2 = ventilatory equivalent for CO2; e/O2 = ventilatory equivalent for oxygen; O2 = rate of oxygen consumption; VT = tidal volume.
Predicted using reference equations by Lewthwaite et al.11
Table 3.
Age Based Normative Reference Ranges for Physiologic Responses at the Tvent During Symptom-Limited Incremental CPET in Men and Women
| Variable | LLN (5th Percentile) | Average (50th Percentile) | ULN (95th Percentile) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age group, y | 40-55 | 56-69 | 70-80 | 40-55 | 56-69 | 70-80 | 40-55 | 56-69 | 70-80 |
| Men (n = 47) | … | … | … | n = 10 | n = 25 | n = 12 | … | … | … |
| Power output, W | 22 | 31 | 37 | 58 | 54 | 57 | 111 | 96 | 85 |
| e/O2 | 20.6 | 21.9 | 22.6 | 24.3 | 25.1 | 27.6 | 25.9 | 29.5 | 32.8 |
| e/CO2 | 25.0 | 25.4 | 27.3 | 28.6 | 28.9 | 30.0 | 32.0 | 33.2 | 34.5 |
| PETO2, mm Hg | 88.9 | 94.0 | 97.1 | 99.6 | 99.0 | 106.0 | 104.8 | 109.2 | 119.4 |
| PETCO2, mm Hg | 37.0 | 34.9 | 35.5 | 41.8 | 40.8 | 40.2 | 46.7 | 46.9 | 46.4 |
| Bf, breaths/min | 13 | 15 | 18 | 21 | 19 | 22 | 25 | 26 | 27 |
| IC, L | 2.84 | 2.50 | 2.01 | 3.80 | 3.56 | 2.92 | 4.15 | 4.18 | 3.90 |
| IRV, L | 1.35 | 1.05 | 0.75 | 2.48 | 2.14 | 1.60 | 2.86 | 2.80 | 2.39 |
| Women (n = 49) | … | … | … | n = 9 | n = 30 | n = 10 | … | … | … |
| Power output, W | 11 | 16 | 15 | 50 | 42 | 31 | 65 | 75 | 62 |
| e/O2 | 19.9 | 21.4 | 23.5 | 26.5 | 25.1 | 28.6 | 30.1 | 31.8 | 33.4 |
| e/CO2 | 25.6 | 25.0 | 28.6 | 28.7 | 30.6 | 32.7 | 34.9 | 38.4 | 41.5 |
| PETO2, mm Hg | 92.6 | 93.4 | 94.3 | 101.4 | 102.1 | 104.2 | 109.5 | 111.1 | 109.8 |
| PETCO2, mm Hg | 37.4 | 34.9 | 31.8 | 41.5 | 39.8 | 37.7 | 47.9 | 46.6 | 41.5 |
| Bf, breaths/min | 13 | 12 | 18 | 19 | 20 | 23 | 24 | 29 | 26 |
| IC, L | 2.23 | 2.14 | 1.92 | 2.52 | 2.59 | 2.39 | 3.20 | 3.59 | 3.11 |
| IRV, L | 0.96 | 0.93 | 0.95 | 1.49 | 1.55 | 1.40 | 2.13 | 2.33 | 2.28 |
Bf = breathing frequency; CPET = cardiopulmonary exercise testing; IC = inspiratory capacity; IRV = inspiratory reserve volume; LLN = lower limit of normal; PETCO2 = end-tidal Pco2; PETO2 = end-tidal Po2; Tvent = ventilatory threshold; ULN = upper limit of normal; e/CO2 = ventilatory equivalent for CO2; e/O2 = ventilatory equivalent for oxygen.
Table 4.
Reference Equations for Estimating the 5th, 50th, and 95th Percentiles for CPET Responses at the Tvent During Symptom-Limited Peak Incremental CPET in Canadian Adults 40 to 80 y of Age
| Variable | Percentile | Prediction Model | RSE | Adjusted R2 |
|---|---|---|---|---|
| Oxygen pulse, mL/beat | 5th | (−0.03533121 × A) + (0.09011971 × H) + (0.05225060 × BM) + (0.55640862 × S) − 10.98937749 | 2.29 | |
| 50th | (0.005140994 × A) + (0.127872626 × H) + (0.067945521 × BM) + (0.971916363 × S) − 18.014483023 | 2.23 | 0.44 | |
| 95th | (−0.100909091 × A) + (0.072918660 × H) + (0.003899522 × BM) + (4.019066986 × S) + 5.697559809 | 2.55 | ||
| O2, L/min | 5th | (0.003400268 × A) + (0.001307452 × H) + (0.002167187 × BM) + (0.217925033 × S) − 0.108384650 | 0.26 | |
| 50th | (−0.004884696 × A) + (0.005723270 × H) + (0.007316562 × BM) + (0.125324948 × S) − 0.330859539 | 0.24 | 0.36 | |
| 95th | (−0.016777814 × A) + (0.013341037 × H) + (0.005153516 × BM) + (0.094767250 × S) − 0.211875206 | 0.27 | ||
| CO2, L/min | 5th | (0.007850010 × A)—(0.000689250 × H) + (0.004826708 × BM) + (0.188562757 × S) − 0.356657529 | 0.28 | |
| 50th | (−0.004115224 × A) + (0.004663891 × H) + (0.006672310 × BM) + (0.181162769 × S) − 0.302360774 | 0.26 | 0.27 | |
| 95th | (−0.014787252 × A) + (0.017253462 × H) + (0.008488236 × BM) + (0.014583681 × S) − 1.294810612 | 0.30 | ||
| e, L/min | 5th | (0.2623758 × A) + (0.2588337 × H) + (0.1332613 × BM) + (3.1646220 × S) − 55.8834557 | 6.7 | |
| 50th | (−0.006474397 × A) + (0.152884049 × H) + (0.090700412 × BM) + (4.063095939 × S) − 10.601589170 | 6.6 | 0.30 | |
| 95th | (−0.03091813 × A) + (0.59708755 × H) + (0.32857397 × BM) − (1.09069437 × S) − 86.33411472 | 7.8 | ||
| VT, L | 5th | (0.005076481 × A) − (0.001306902 × H) + (0.002103524 × BM) + (0.349656143 × S) + 0.369177068 | 0.32 | |
| 50th | (−0.006037296 × A) + (0.003076923 × H) + (0.002703963 × BM) + (0.285454545 × S) + 0.725920746 | 0.30 | 0.29 | |
| 95th | (−0.008875830 × A) + (0.003832397 × H) + (0.003362289 × BM) + (0.421486970 × S) + 1.244828820 | 0.31 | ||
| IC, L | 5th | (−0.0228894428 × A) − (0.0006870968 × H) + (0.0022351906 × BM) − (2.0071149560 × S) + (0.0278563050 × BM × S) + 3.4489973607 | 0.52 | |
| 50th | (−0.01409342 × A) + (0.01630130 × H) + (0.00536711 × BM) − (0.96017019 × S) + (0.01855641 × BM × S) + 0.39616067 | 0.47 | 0.49 | |
| 95th | (−0.018708633 × A) + (0.021608276 × H) + (0.000502157 × BM) − (0.130127032 × S) + (0.006159484 × BM × S) + 1.037483841 | 0.48 | ||
| EILV, L | 5th | (0.006772217 × A) + (0.067003745 × H) − (0.020371127 × BM) + (0.084525026 × S) − 6.942032686 | 0.67 | |
| 50th | (0.01523658 × A) + (0.09257164 × H) − (0.03361260 × BM) + (0.31317782 × S) − 9.84548408 | 0.62 | 0.59 | |
| 95th | (−0.001598174 × A) + (0.067899543 × H) − (0.037031963 × BM) + (0.520593607 × S) − 3.462739726 | 0.64 | ||
| EELV, L | 5th | (0.005558408 × A) + (0.071604621 × H) − (0.018048780 × BM)—(0.210436457 × S) − 8.836469833 | 0.57 | |
| 50th | (0.01302074 × A) + (0.07131571 × H) − (0.03290423 × BM) + (0.30861324 × S) − 7.40879795 | 0.53 | 0.55 | |
| 95th | (0.03236735 × A) + (0.06967347 × H) − (0.03081633 × BM) + (0.45330612 × S) − 7.61955102 | 0.56 | ||
| EELV, % TLC | 5th | (0.10161365 × A) + (0.25263672 × H) + (0.03069661 × BM) + (47.47714565 × S) − (0.60105097 × BM × S) − 9.69830357 | 6.0 | |
| 50th | (0.1196350 × A) + (0.3994345 × H) − (0.1885312 × BM) + (26.7201143 × S) − (0.3433643 × BM × S) − 9.8796655 | 5.7 | 0.39 | |
| 95th | (0.3716889 × A) + (0.7825651 × H) − (0.2659657 × BM) + (29.4583111 × S) − (0.4273232 × BM × S) − 72.8113778 | 6.1 |
A = age (y); BM = body mass (kg); CPET = cardiopulmonary exercise testing; EELV = end-expiratory lung volume; EILV = end-inspiratory lung volume; H = height (cm); IC = inspiratory capacity; RSE = residual SE in the units of the given variable, where residual SE is the SD of the residuals; S = sex, where male = 1 and female = 0; TLC = total lung capacity; Tvent = ventilatory threshold; CO2 = rate of CO2 production; e = minute ventilation; O2 = rate of oxygen consumption; VT = tidal volume.
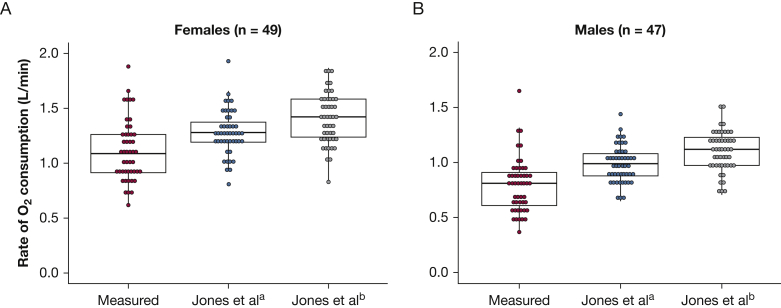
In the CanCOLD study participants, the Jones et al10 reference equations overpredicted O2 at Tvent, particularly for women (Fig 2, Table 5). Differences between the Jones et al10 predicted O2 at Tvent and the measured O2 at Tvent both were statistically significant and outside the predefined acceptable equivalence bounds (ie, not statistically equivalent) (Fig 2, Table 5).
Figure 2.
Graphs showing the rate of oxygen consumption during symptom-limited incremental cardiopulmonary cycle exercise testing at the ventilatory threshold measured in the Canadian Cohort Obstructive Lung Disease study participants and predicted using reference equations published by Jones et al10 in 1985. Jones et ala reference equations are based on participant height and age, whereas Jones et alb reference equations additionally include participant body mass.
Table 5.
O2 at the Tvent and at the Symptom-Limited Peak of Incremental CPET Measured in the Canadian Cohort Obstructive Lung Disease Study Participants and Predicted Using the Equations of Jones et al10 (1985)
| Variable | Measured | Jones et al10 (1985) Mean ± SD | Measured–Jones Mean (95% CI) | Equivalence Margin | TOST-P 90% CI |
|---|---|---|---|---|---|
| Men | |||||
| O2 at Tvent, L/min | 1.11 ± 0.28 | 1.28 ± 0.20 | −0.17 (−0.25 to −0.09) | ± 0.19 | −0.24 to −0.10 |
| O2 at peak, L/min | 2.38 ± 0.51 | 2.37 ± 0.42 | a | a | a |
| O2 at Tvent, % peak | 48 ± 10 | 54 ± 2 | a | a | a |
| Women | |||||
| O2 at Tvent, L/min | 0.80 ± 0.25 | 0.99 ± 0.16 | −0.19 (−0.27 to −0.12) | ± 0.17 | −0.25 to −0.13 |
| O2 at peak, L/min | 1.66 ± 0.40 | 1.20 ± 0.36 | a | a | a |
| O2 at Tvent, % peak | 49 ± 12 | 87 ± 15 | a | a | a |
CPET = cardiopulmonary exercise testing; TOPT-P = two one-sided test of equivalence for paired samples; Tvent = ventilatory threshold; O2 = rate of oxygen consumption.
No comparisons were made with peak data.
Discussion
This study developed a contemporary normative reference set for cardiac, ventilatory, operating lung volume, gas exchange, and symptom responses at Tvent during incremental cycle CPET in Canadian adults 40 to 80 years of age. Although normative reference sets for peak cycle CPET responses are more widely available,31,32 including a recently published reference set for Canadian adults 40 years of age and older,11 few normative reference sets have been published for physiologic and symptom responses at Tvent.31,32
It could be argued that the normal reference ranges for symptom (breathing and leg discomfort) responses at Tvent seem low (Table 2). However, previous studies in healthy volunteers 40 to 80 years of age have shown that, on average, intensity ratings of breathing discomfort do not increase beyond 1 to 2 on the 0-to-10 Borg scale until a O2 corresponding to > 50% of the achieved O2 peak, or just more than Tvent.33 Breathing discomfort during exercise has been shown to correlate with inspiratory reserve volume and VT (%IC), with intensity ratings increasing at and beyond previously defined critical thresholds (ie, inspiratory reserve volume ≤ 0.7 L; VT [%IC] ≥ 73%).34,35 In the CanCOLD participants, the inspiratory reserve volume and VT (%IC) had not yet reached these critical thresholds at Tvent (Table 2).
Jones et al10 and Hansen et al9 were among the first to provide normative values for O2 at Tvent during cycle CPET, which form the basis of current recommendations by clinical CPET guidelines.1 In each of these studies, Tvent was identified using just the dual criterion method presumably by a single rater.9,10 Normative reference values developed by Jones et al10 were based on 50 men and 50 women 15 to 71 years of age sampled conveniently from the university and general population in Hamilton, Ontario, a younger age group than in this multicenter CanCOLD study. The average O2 at Tvent for participants in the Jones et al10 study was 1.64 ± 0.42 L/min (56 ± 12% measured peak O2) in men and 1.23 ± 0.30 L/min (74 ± 14% measured peak O2) in women. Hansen et al9 included only men (n = 77) who were former shipyard workers 34 to 74 years of age. The O2 at Tvent for participants in the Hansen et al9 study was similar to male participants in the Jones et al10 study: 56 ± 8% (range, 40%-78%) measured O2 peak. These values for O2 at Tvent are considerably higher than in the current study (men: 1.11 ± 0.28 L/min, 48 ± 10% measured O2 peak; women: 0.80 ± 0.25 L/min, 49 ± 12% measured O2 peak).
Since these early studies by Jones et al10 and Hansen et al,9 a number of publications have provided updated reference sets for normative responses at Tvent during cycle CPET. Perhaps the most comprehensive reference set, not including the current study, is that published in 1994 by Meyer et al.36 This study provided normative reference ranges by participant age decade as the mean ± SD and 95% CI for power output, O2, CO2, respiratory exchange ratio, HR, oxygen pulse, e, ventilatory equivalent for O2, ventilatory equivalent for CO2, end-tidal Po2, end-tidal Pco2, and dead space to VT ratio.36 However, these normative reference ranges were based on only male participants (n = 69) who were volunteer clinical staff at the testing site in Germany. No prediction models were provided for estimating CPET responses at Tvent based on readily available participant characteristics. Physiologic responses at Tvent reported by Meyer et al36 were higher than male participants in the current study of comparable age (eg, average O2 of Meyer et al36 participants 40 to 49 and 50 to 59 years of age: 18.4 and 16.4 mL/kg/min, respectively vs 13.7 mL/kg/min, of male participants in the current study).
More recently, normative responses at Tvent from large cycle CPET databases have been published, including the Fitness Registry and the Importance of Exercise (FRIEND) in the United States37 and the Study of Health in Pomerania (SHIP) in Germany.38,39 The FRIEND study included 8,155 participants (81% men) 46 ± 12 years of age (range, 20-79 years),37 whereas the SHIP study included 534 participants (47% men) 25 to 80 years of age, most of whom (88%) were younger than 65 years.38 In both of these studies, as with the current study, normative reference ranges for O2 at Tvent were presented as 5th to 95th percentiles by participant sex; however, only the SHIP study provided reference equations for estimating O2 at Tvent based on readily available participant characteristics. We evaluated the SHIP reference equations38,39 for predicting O2 at Tvent in the CanCOLD study cohort (e-Fig 3). The SHIP equations38,39 slightly overpredicted O2 at Tvent in the CanCOLD study participants (measured − SHIP predicted mean difference, −0.10 L/min [95% CI, −0.65 to 0.44 L/min]). The SHIP study38 also presented reference equations for estimating end-tidal Pco2 and ventilatory equivalent for CO2 at Tvent; however, no reference equations were provided for estimating other CPET variables.
Absolute values for O2 at Tvent reported by FRIEND were comparable with those of the current study (mean range for participants 40 to 80 years of age: men, 15.3-11.8 mL/kg/min; women, 13.2-10.3 mL/kg/min).37 However, the O2 at Tvent expressed as a percentage of the measured O2 peak was higher in FRIEND compared with the current study (FRIEND participants 40-80 years of age mean range: men, 57%-61%; women, 61%-67% vs current study mean: men, 48%; women, 49%). This also was true for the SHIP study (SHIP range of LLN [5th percentile] 52%-55% measured O2 peak, with no participant achieving < 41%).38 It is possible that these higher relative values are the result of FRIEND and SHIP participants achieving a lower measured O2 peak, particularly for FRIEND participants who achieved a similar absolute O2 at Tvent as the current study participants; however, no measured values for O2 peak were reported in either the FRIEND or SHIP studies.37,38
Differences between studies for the expected normal physiologic responses at Tvent during incremental cycle CPET may be the result of differences in sampling methods (convenience based10,36 vs population based in the current study); participants in previous studies being (1) younger; (2) taller with lower body mass, ie, leaner; (3) more physically active than the contemporary CanCOLD sample, or a combination thereof; For example, participants in the Meyer et al36 study were 20 to 59 years of age (vs 42-79 years of age in the current study), with an average (range in mean across age groups) standing height of 179 to 181 cm (vs mean ± SD in current study of 167.9 ± 9.0 cm) and a BMI of 23 to 26 kg/m2 (vs mean ± SD in current study of 27.0 ± 4.2 mg/m2).
Strengths and Limitations
An important strength of this study is development of prediction models that enable estimation of normative CPET responses at Tvent (beyond just O2) for an individual patient, including individualized estimation of the LLN and ULN. Further, to the best of our knowledge, we provide, for the first time, a comprehensive set of normative reference ranges for multiple cardiac, ventilatory, operating lung volume, gas exchange, and symptom responses at Tvent. The O2 at Tvent was identified by two independent raters using established and recommended methods, with a third independent rater to resolve discrepancies. Strict a priori criteria for interrater reliability also were defined. The CPET protocol was standardized across testing sites and aligned with clinical CPET guidelines.1,2
This study is not without its limitations. Although this study was limited by a relatively small sample size (n = 96) of Canadian adults 40 to 80 years of age, this group represents the most commonly tested population within clinical CPET laboratories for identification of exercise intolerance and problematic symptoms. The reference set may not be applicable to adults younger than 40 years or older than 80 years of age. All participant testing for this study was performed using a Vmax SensorMedics CPET system. Because of known differences among metabolic analysis systems, particularly regarding measurement of O2,40 it is not known whether the current reference set is applicable for use with other CPET systems; previous estimates of differences in O2 at the peak of exercise between CPET systems have ranged from 10% to 17%41 and at different submaximal time points during exercise from 6% to 22%.42 The prediction models presented in this study need to be validated externally in a separate population-based random sample of participants with comparable sociodemographic characteristics and using similar CPET methodology.
Conclusions
This study presents an updated comprehensive normative reference set for physiologic and symptom responses at Tvent during incremental cycle CPET in Canadian adults 40 to 80 years of age. This updated reference set facilitates, in the clinical and research settings, comprehensive interpretation of integrative CPET responses at Tvent, particularly useful in people who are unable or unwilling to provide maximal effort and exercise until their symptom-limited peak.
Acknowledgments
Author contributions: All of the authors reviewed the manuscript, contributed to the concept and design and to data interpretation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: H. L. reports personal fees for consulting from Boehringer Ingelheim during the conduct of the study. J. A. G. reports personal fees from Agartee Technology, Inc., during the conduct of the study. D. D. M. reports consulting fees from AstraZeneca, Boehringer-Ingelheim, GSK, and Novartis; consulting fees from the Canadian Foundation for Healthcare Improvement, Health Canada, Mylan, and Yukon Health and Social Services; and personal fees from Lung Association of Saskatchewan; and other from the Canadian Thoracic Society. J. B. reports other from GlaxoSmithKline during the conduct of the study; personal fees from the Canadian Thoracic Society and CHEST; and personal fees from AstraZeneca, Boehringer Ingelheim, Grifols, GlaxoSmithKline, Novartis, and Trudell outside the submitted work. W. C. T. reports industry partners Astra Zeneca Canada, Ltd., Boehringer-Ingelheim Canada, Ltd., GlaxoSmithKline Canada, Ltd., Merck, Novartis Pharma Canada, Inc., Nycomed Canada, Inc., and Pfizer Canada, Ltd., during the conduct of the study. F. M. reports personal fees for serving on speaker bureaus and consultation panels from Boehringer Ingelheim, Grifols, and Novartis outside the submitted work, and is financially involved with Oxynov, a company that is developing an oxygen delivery system. D. J. reports personal fees for consulting from Boehringer Ingelheim during the conduct of the study; personal fees from AstraZeneca; None declared (O. E., F. N., M. K. S., D. E. O., B. M. S.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
∗CanCOLD Collaborative Research Group Collaborators: Shawn E. Aaron (The Ottawa Hospital Research Institute, Ottawa, ON, Canada); Kenneth R. Chapman (Asthma & Airway Centre, University Health Network and University of Toronto, Toronto, ON, Canada); Mark J. FitzGerald (University of British Columbia, Vancouver, BC, Canada); Paul Hernandez (Faculty of Medicine, Division of Respirology, Dalhousie University, Halifax, NS, Canada); Donald D. Sin (University of British Columbia Centre for Heart Lung Innovation, St. Paul’s Hospital, Vancouver, BC, Canada); and Brandie Walker (University of Calgary, Calgary, AB, Canada).
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The Canadian Cohort Obstructive Lung Disease (ClinicalTrials.gov Identifier: NCT00920348) study currently is funded by the Canadian Respiratory Research Network, the Canadian Institutes of Health Research [CIHR/Rx&D Collaborative Research Program Operating Grant 93326], and the industry partners AstraZeneca Canada Ltd., Boehringer Ingelheim Canada Ltd., GlaxoSmithKline (GSK) Canada Ltd., and Novartis. Previous funding partners were the Respiratory Health Network of the Fonds de la Recherche en Santé du Québec, the Foundation of the McGill University Health Centre, and the following industry partners: Almirall, Merck, Nycomed, Pfizer Canada Ltd., and Theratechnologies. The funders had no role in any aspect of the manuscript. H. L. was supported by a Fonds de Recherche du Québec Santé Postdoctoral Training Fellowship. J. B. holds a GlaxoSmithKline (GSK)/Canadian Institutes of Health Research (CIHR) Research Chair on Better Understanding of COPD and Intervention Guides at McGill University. J. A. G. was supported by a Scholar Award from the Michael Smith Foundation for Health Research and a Clinical Rehabilitation New Investigator Award from the CIHR. F. M. holds a GSK Research Chair on COPD at Université Laval. B. M. S. is supported by Québec Health Research Fund, the Canadian Institutes of Health Research, the National Institutes of Health (USA), the Respiratory Health Network of Québec, and Québec Lung Association. D. J. holds a Canada Research Chair, Tier II, in Clinical Exercise & Respiratory Physiology from the CIHR.
Contributor Information
Hayley Lewthwaite, Email: Hayley.Lewthwaite@mcgill.ca.
Canadian Respiratory Research Network:
S.D. Aaron, K.R. Chapman, P. Hernandez, D.D. Sin, and B. Walker
Supplementary Data
References
- 1.ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Radtke T., Crook S., Kaltsakas G., et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev. 2019;28(154):180101. doi: 10.1183/16000617.0101-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gitt A.K., Wasserman K., Kilkowski C., et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106(24):3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 4.Carriere C., Corra U., Piepoli M., et al. Anaerobic threshold and respiratory compensation point identification during cardiopulmonary exercise tests in chronic heart failure. Chest. 2019;156(2):338–347. doi: 10.1016/j.chest.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Moran J., Wilson F., Guinan E., McCormick P., Hussey J., Moriarty J. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth. 2016;116(2):177–191. doi: 10.1093/bja/aev454. [DOI] [PubMed] [Google Scholar]
- 6.Colson M., Baglin J., Bolsin S., Grocott M.P. Cardiopulmonary exercise testing predicts 5 yr survival after major surgery. Br J Anaesth. 2012;109(5):735–741. doi: 10.1093/bja/aes263. [DOI] [PubMed] [Google Scholar]
- 7.Ney M., Haykowsky M.J., Vandermeer B., Shah A., Ow M., Tandon P. Systematic review: pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther. 2016;44(8):796–806. doi: 10.1111/apt.13771. [DOI] [PubMed] [Google Scholar]
- 8.Epstein S.K., Freeman R.B., Khayat A., Unterborn J.N., Pratt D.S., Kaplan M.M. Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transpl. 2004;10(3):418–424. doi: 10.1002/lt.20088. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J.E., Sue D.Y., Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 10.Jones N.L., Makrides L., Hitchcock C., Chypchar T., McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131(5):700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 11.Lewthwaite H., Benedetti A., Stickland M.K., et al. Normative peak cardiopulmonary exercise test responses in Canadian adults aged ≥40 years. Chest. 2020;158(6):2532–2545. doi: 10.1016/j.chest.2020.06.074. [DOI] [PubMed] [Google Scholar]
- 12.Colley R.C., Garriguet D., Janssen I., Craig C.L., Clarke J., Tremblay M.S. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22(1):7–14. [PubMed] [Google Scholar]
- 13.Hallal P.C., Andersen L.B., Bull F.C., et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 14.Bourbeau J., Benedetti A., Tan W.C., et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014;11(2):125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer P.H., Stanojevic S., Cole T.J., et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanojevic S., Graham B.L., Cooper B.G., et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50(3):1700010. doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 17.Crapo R.O., Morris A.H., Clayton P.D., Nixon C.R. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18(3):419–425. [PubMed] [Google Scholar]
- 18.Miller M.R., Crapo R., Hankinson J., et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 19.Stewart A.L., Mills K.M., King A.C., Haskell W.L., Gillis D., Ritter P.L. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Radtke T., Crook S., Puhan M.A., et al. ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev. 2019;28(154):180101. doi: 10.1183/16000617.0101-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borg G.A. Psychophysical bases of perceived exertion. J Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 22.Beaver W.L., Wasserman K., Whipp B.J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 23.Balady G.J., Arena R., Sietsema K., et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 24.Beaver W.L., Wasserman K., Whipp B.J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 2016;121(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 25.Sue D.Y., Wasserman K., Moricca R.B., Casaburi R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. Chest. 1988;94(5):931–938. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- 26.Schneider D.A., Phillips S.E., Stoffolano S. The simplified V-slope method of detecting the gas exchange threshold. Med Sci Sports Exerc. 1993;25(10):1180–1184. [PubMed] [Google Scholar]
- 27.Caiozzo V.J., Davis J.A., Ellis J.F., et al. A comparison of gas exchange indices used to detect the anaerobic threshold. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(5):1184–1189. doi: 10.1152/jappl.1982.53.5.1184. [DOI] [PubMed] [Google Scholar]
- 28.Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Statistics Canada. Anthropometry measures of the household population. Table 13-10-0319-01. Statistics Canada website. 10.25318/1310031901-eng. Accessed September 24, 2020. [DOI]
- 30.Statistics Canada Physical activity, self reported, adult, by age group. Table 13-10-485 0096-13. Statistics Canada website. https://www150.statcan.gc.ca/t1/tb11/en/tv.action?pid=1310009613 Accessed September 24, 2020.
- 31.Paap D., Takken T. Reference values for cardiopulmonary exercise testing in healthy adults: a systematic review. Expert Rev Cardiovasc Ther. 2014;12(12):1439–1453. doi: 10.1586/14779072.2014.985657. [DOI] [PubMed] [Google Scholar]
- 32.Takken T., Mylius C.F., Paap D., et al. Reference values for cardiopulmonary exercise testing in healthy subjects—an updated systematic review. Expert Rev Cardiovasc Ther. 2019;17(6):413–426. doi: 10.1080/14779072.2019.1627874. [DOI] [PubMed] [Google Scholar]
- 33.Ofir D., Laveneziana P., Webb K.A., Lam Y.M., O’Donnell D.E. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol. 2008;104(6):1583–1593. doi: 10.1152/japplphysiol.00079.2008. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell D.E., Hamilton A.L., Webb K.A. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol. 2006;101(4):1025–1035. doi: 10.1152/japplphysiol.01470.2005. [DOI] [PubMed] [Google Scholar]
- 35.Chin R.C., Guenette J.A., Cheng S., et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;187(12):1315–1323. doi: 10.1164/rccm.201211-1970OC. [DOI] [PubMed] [Google Scholar]
- 36.Meyer K., Hajric R., Samek L., et al. Cardiopulmonary exercise capacity in healthy normals of different age. Cardiology. 1994;85(5):341–351. doi: 10.1159/000176733. [DOI] [PubMed] [Google Scholar]
- 37.Vainshelboim B., Arena R., Kaminsky L.A., Myers J. Reference standards for ventilatory threshold measured with cardiopulmonary exercise testing: the Fitness Registry and the importance of exercise: a national database. Chest. 2020;157(6):1531–1537. doi: 10.1016/j.chest.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Koch B., Schaper C., Ittermann T., et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J. 2009;33(2):389–397. doi: 10.1183/09031936.00074208. [DOI] [PubMed] [Google Scholar]
- 39.Glaser S., Ittermann T., Schaper C., et al. [The Study of Health in Pomerania (SHIP) reference values for cardiopulmonary exercise testing] Pneumologie. 2013;67(1):58–63. doi: 10.1055/s-0032-1325951. [DOI] [PubMed] [Google Scholar]
- 40.Hodges L.D., Brodie D.A., Bromley P.D. Validity and reliability of selected commercially available metabolic analyzer systems. Scand J Med Sci Sports. 2005;15(5):271–279. doi: 10.1111/j.1600-0838.2005.00477.x. [DOI] [PubMed] [Google Scholar]
- 41.Babineau C., Léger L., Long A., Bosquet L. Variability of maximum oxygen consumption measurement in various metabolic systems. J Strength Cond Res. 1999;13(4):318–324. [Google Scholar]
- 42.Hiilloskorpi H., Manttari A., Pasanen M. The comparison between three different respiratory gas analysers. Med Sci Sports Exerc. 2000;31(suppl 5):S354. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.