Abstract
Total pancreatectomy with islet autotransplantation (TPIAT) is used to treat debilitating chronic pancreatitis (CP) and acute recurrent pancreatitis (ARP) that has failed medical and endoscopic therapy. We performed a retrospective review of TPIAT patients at a free-standing children’s hospital to evaluate perioperative outcomes. Twenty patients (median age 13, 65% female) underwent TPIAT (2015 through 2017). Of the 20 patients, 95% had CP and 1 patient (5%) had ARP alone. Seventy-five percent of the patients had a pancreatitis-associated genetic mutation; 40% had pancreas divisum. The median surgical time was 757 (IQR 657 to 835) minutes. Median islet equivalents per kg of body weight (IEQ/kg) were 6404 (IQR 5018 to 7554). At 90 days postoperatively vs preoperatively, significantly fewer patients were receiving parenteral nutrition (0% vs 25%, P = .006) and opioids (45% vs 75%, P = .01). Short Form 36-Item Health Survey (SF-36) physical health module scores and total scores improved (34.0 preoperatively vs 54.6 at 90 days, P = .008, and 47.1 vs 65.3, P = .007, respectively); SF-10 physical health scores also improved (13.4 vs 33.1, P = .02). Insulin requirement decreased from 0.5 unit/kg/day to 0.4 unit/kg/day between discharge and 90 days (P = .02). TPIAT is an effective option when debilitating disease persists despite maximal medical and endoscopic therapy. Opioid, parenteral nutrition, and exogenous insulin use can successfully be weaned within 90 days after TPIAT, with gains in health-related quality of life.
Keywords: autotransplantation, clinical research/practice, health services and outcomes research, insulin/C-peptide, islet transplantation, nutrition, pediatrics, quality of life (QOL)
1 |. INTRODUCTION
Chronic pancreatitis (CP) and acute recurrent pancreatitis (ARP) cause significant morbidity for both adult and pediatric patients, with recurrent inflammation resulting in irreversible parenchymal damage (eg, fibrosis, atrophy) and significant symptomatology. While the overall incidence of CP is approximately 0.5% in the United States,1,2 the annual health care expenditure of $2.6 billion is a significant financial burden.3 The incidence of acute pancreatitis (AP) in children has been estimated between 3.6 and 13.2 cases per 100 000 children, with a notable increase during the past 2 decades.4–7 This observed rise in pediatric AP has mirrored the increase in CP admissions during the past decade.8 Similar to adult disease patterns, pediatric CP may follow a course of progressive pain severity and opioid dependence, with the progression of disease to exocrine and endocrine insufficiency.9 Children with CP have health-related quality of life (HRQOL) scores that are 1 to 2 SDs below the average, consistent with HRQOL scores for cancer, end‐stage renal disease, and rheumatologic diseases in children,10,11 indicating the profound effect of the disease on their daily functioning, with recurrent hospitalizations, school absences, and impaired interactions with family and peers.12 CP results in malnutrition and feeding challenges, further highlighting the need to recognize it and intervene promptly.13
Total pancreatectomy with islet autotransplantation (TPIAT) has been performed in adult populations for >4 decades, having first been described in 1977.14 In adult populations, post-TPIAT patients have demonstrated significant decreases in opioid requirements and improved quality of life.15–18 The first pediatric TPIAT was performed in 1989,19 yet data on TPIAT in pediatric populations remain limited.20 In this study, we report post-TPIAT outcomes from a single free-standing pediatric academic medical center, focusing on immediate preoperative, in-hospital, and 90-day postoperative outcomes, including opioid, parenteral nutrition (PN), and exogenous insulin use; C-peptide levels as a marker of β-cell function; and HRQOL. Additionally, we are the first to report on the early benefits of TPIAT on supplemental nutritional needs in children.
2 |. MATERIALS AND METHODS
A retrospective review was performed of pediatric patients who underwent TPIAT at Cincinnati Children’s Hospital Medical Center (CCHMC) between April 2015 and August 2017. Twenty consecutive patients were included in the review. Demographic information, preoperative characteristics (including genetic risk factors, nutritional supplementation, and opioid requirements), in-hospital course (including operative duration, length of stay, and islet yields), and 90-day postoperative outcomes (including complications, changes in opioid and nutritional supplementation, and HRQOL) were abstracted from the clinical record. The study was approved by the Institutional Review Board at CCHMC.
2.1 |. Patient selection
Potential patients with CP and ARP were evaluated by a multidisciplinary team that included pediatric subspecialists in the following areas: transplant surgery, gastroenterology, pain management, endocrinology, and psychology. The appropriateness of recommending a TPIAT to a patient was based on a comprehensive review and assessment of the child’s prior clinical course, response to treatments and interventions, and their current level of impairment of quality of life and daily functioning. CP and ARP in the pediatric population were defined as published by the International Study Group of Pediatric Pancreatitis: In Search for a Cure (INSPPIRE) consortium.21 Exocrine pancreatic insufficiency was defined as fecal elastase level <200 μg/g stool or an endoscopic pancreatic function test consistent with exocrine insufficiency. Patients and their families underwent thorough preoperative counseling regarding the nature of the operation, as well as the potential risks and the benefits. In addition, they participated in extensive education and preparation with regard to pain management (including opioid use) before, during, and after TPIAT. Given the inclusion of splenectomy as part of the surgical intervention, all patients underwent a preoperative assessment of their vaccination status for encapsulated organisms; if determined to be deficient, the appropriate vaccinations were administered before surgery.
2.2 |. Operative procedure
TPIAT included resection of the pancreas, duodenum (pylorus pre-serving), gallbladder, spleen, and appendix. Pancreatic blood supply was dissected and preserved until shortly before removal of the pancreas to minimize ischemia time for the islets. Islet isolation was performed via mechanical and enzymatic degradation as previously described.22 The processing of juvenile pancreata for autologous islet transplant was performed through developmental therapeutics in cooperation with University of Cincinnati, Cellular Therapies. Immediately after total pancreatectomy, the pancreas was taken to a sterile field in the operating room, where it was flushed with cold University of Wisconsin solution, trimmed, and packaged in fresh cold preservation solution for immediate transport to the islet processing facility. The pancreas was perfused with Liberase MTF (Roche, Indianapolis, IN) and digested by using the Ricordi Isolator III (Biorep Technologies, Miami, FL). Processed cells were not purified. The cell volume was suspended in serum albumin with 70 units/kg heparin, packaged, and immediately transported to the operating room for fresh infusion. Isolated islets were infused into the portal circulation via cannulation of the splenic vein stump. During infusion, mean arterial pressure, portal venous pressure, central venous pressure, and blood glucose were monitored. Islet infusion was paused if there was a significant rise in portal venous pressure from preinfusion measurements (>20 mm Hg change from starting pressure or absolute value >25 mm Hg). Dextran infusion was initiated before islet transplant to reduce the instant blood-mediated inflammatory response,23 and patients were maintained on an insulin infusion for glycemic control (goal blood glucose level of 80 to 120 mg/dL) throughout the operation to maximize survival and engraftment of the islets. Biliary reconstruction was performed via Roux-en-Y choledochojejunostomy or hepaticojejunostomy, and alimentary tract reconstruction was performed via Roux-en-Y duodenojejunostomy. All patients had a gastrojejunostomy tube placed at the time of surgery to allow for early initiation of distal enteral feeds postoperatively.
2.3 |. Postoperative care
All patients were admitted to the intensive care unit (ICU) postoperatively for a standard 7-day ICU stay for administration and monitoring of insulin and heparin infusions. Abdominal ultrasound was performed immediately postoperatively to evaluate for portal vein thrombosis. Blood glucose levels were closely monitored with hourly blood glucose checks and a subcutaneous continuous glucose monitoring system. Insulin infusion was titrated to keep blood glucose levels in the range of 80 to 120 mg/dL by using a standardized guideline. Continuous glucose monitoring via the use of a Dexcom G4 Platinum Professional monitor (Dexcom, San Diego, CA) was used throughout the hospital stay to allow for early identification of deviation in blood glucose. Patients were maintained on a low-dose heparin infusion (10 units/kg/hr) for 7 days, before transition to low-molecular-weight heparin subcutaneous prophylaxis. Patients were subsequently transitioned from insulin infusion to insulin pumps and underwent extensive teaching for the maintenance of glycemic control postoperatively. Patients were managed by the acute pain service intraoperatively and postoperatively. Perioperative pain management included extensive use of nonopioid analgesics beginning in the operating room and continuing into the ICU stay. All patients were seen in close follow-up with surgery, gastroenterology, pain management, and endocrine providers after hospital discharge. All patients underwent standard mixed meal testing at 90 days postoperatively to evaluate islet function.24
2.4 |. Health-related quality of life
All patients 12 years and older were asked to complete the Short Form 36-Item Health Survey (SF-36), and parents of all patients <12 years of age were asked to complete the Short Form 10-Item Health Survey (SF-10) Health Survey for Children. The SF-36 is a 36-item, patient-reported survey of patient health that includes 8 modules composing a physical health score and a mental health score.25 The SF-10 is a parent-completed survey of patient health and includes physical and psychosocial component scores.26
2.5 |. Statistical analysis
Data were analyzed by using SAS, version 9.4 (SAS Institute, Cary, NC). Due to sample sizes, continuous data were summarized as median values (IQR: 25th-75th percentiles), while categorical data were summarized as frequency counts and percentages. Exact binomial tests were used to compare proportions, and Wilcoxon signed rank tests were used to compare continuous variables preoperatively and 90 days postoperatively. A value of P < .05 was considered statistically significant.
3 |. RESULTS
Twenty patients underwent TPIAT between April 2015 and August 2017. Clinical and demographic characteristics of the cohort are described in Table 1. Indication for TPIAT was CP in 95% of patients, whereas 1 patient had debilitating ARP alone without meeting diagnostic criteria for CP. Within this cohort, 75% had an identified genetic risk factor for their pancreatitis, and 45% of patients had an anatomic abnormality, either pancreas divisum or annular pancreas. Seventy-five percent of patients had used opioid medications for management of chronic pain in the year before surgery, and 25% were receiving partial or full parenteral nutritional support, with an additional three patients requiring enteral feeding supplementation due to failure to thrive and/or intolerance of oral feeds. Sixty percent of patients had preoperative exocrine insufficiency, and 15% had preoperative endocrine insufficiency with diabetes. Patients undergoing TPIAT had severe debilitating disease with a median of 4.5 hospitalizations in the preceding year.
TABLE 1.
Preoperative demographic and patient characteristics of children undergoing TPIAT
| Characteristic | No. of children (N = 20) |
|---|---|
| Age at TPIAT, y | 13.5 (10.4 to 16.2) |
| Sex | |
| Male | 7 (35) |
| Female | 13 (65) |
| BMI percentile | 84.8 (63.8 to 93.6) |
| Weight percentile | 81.5 (49.5 to 93.9) |
| Classification of pancreatitis | |
| Chronic | 19 (95) |
| Acute recurrent alone | 1 (5) |
| Etiology of chronic pancreatitis | |
| Genetic risk factor | 16 (80) |
| PRSS1 | 4 (20) |
| SPINK1 | 5 (25) |
| CFTR | 7 (35) |
| CTRC | 1 (5) |
| CPA1 | 1 (5) |
| Anatomic association | 9 (45) |
| Pancreas divisum | 8 (40) |
| Annular pancreas | 1 (5) |
| No. of hospitalizations in preceding year | 4.5 (3.5 to 7.5) |
| No. of ERCPs in preceding year | 1.0 (0.0 to 2.0) |
| Preoperative opioid use in preceding year | |
| Yes | 15 (75) |
| No | 5 (25) |
| Exocrine pancreatic insufficiency | |
| Yes | 12 (60) |
| No | 6 (30) |
| Unknown | 2 (10) |
| Endocrine pancreatic dysfunction: diabetes | |
| Yes | 3 (15) |
| No | 17 (85) |
| Preoperative nutrition | |
| Total parenteral nutrition only | 4 (20) |
| Parenteral and oral feeds | 1 (5) |
| Enteral and oral feeds | 3 (15) |
| Oral diet only | 12 (60) |
Data presented as median (25th–75th percentiles) or n (%).
BMI, body mass index; TPIAT, total pancreatectomy with islet autotransplantation; ERCP, endoscopic retrograde cholangiopancreatography.
3.1 |. Operative details
Median operative duration was 757.5 (IQR: 657.5 to 835.5) minutes. Eighty-five percent of patients had all isolated islets infused into the liver via portal vein via cannulation of the splenic vein stump. Three patients had to have portal vein infusion stopped for persistently elevated portal pressures, with the remainder of those islets infused into the peritoneal cavity. The median islet equivalents transplanted per kg body weight (IEQ/kg) was 6404 (IQR: 5018 to 7554; Table 2).
TABLE 2.
Operative details and hospital stay
| Operative details | |
|---|---|
| Operative duration, min | 757.5 (657.5 to 835.5) |
| Islets | |
| Total IEQ | 323 100 (226 350 to 449 400) |
| Total lEQ/kg | 6404 (5018 to 7554) |
| Site of islet infusion | |
| Liver/intraportal only | 17 (85) |
| Liver/intraportal plus peritoneal cavity | 3 (15) |
| ICU length of stay, days | 10.0 (8.5 to 11.0) |
| Overall length of stay, d | 26.5 (22.5 to 31.0) |
Data presented as median (25th–75th percentiles) or n (%).
IEQ, islet equivalents, ICU, intensive care unit.
3.2 |. In-hospital and 90-day outcomes
Median length of stay was 26 (IQR: 23 to 31) days, with a median ICU length of stay of 10 (IQR: 9 to 11) days. Six patients experienced postoperative complications during their initial hospitalization, 5 of which were fluid collections requiring percutaneous drainage. One patient required immediate reexploration for bleeding due to omental venous bleeding related to suction trauma from an internal drain. One additional patient developed an adhesive bowel obstruction necessitating exploration after initial hospital discharge but within the 90 days after surgery (Table 3). At 90 days, all patients were off PN, and 70% were tolerating full nutrition via mouth with 30% still requiring enteral supplementation via jejunal tube feeds. The difference in PN use between the preoperative and postoperative periods was statistically significant (P = .006, Figure 1). Patients were significantly less likely to be using opioids at 90 days postoperatively (45%), compared with 75% preoperatively (P = .01, Figure 2). As a measure of opioid use, among patients who were taking opioids preoperatively, the median use was 20 morphine equivalents/day preoperatively, which decreased at 90 days to a median of 7.5 morphine equivalents/day. This difference was not statistically significant (P = .16). Median stimulated C-peptide, a reflection of endogenous insulin secretion, was 1.9 (IQR: 1.1–2.5) ng/mL at 90 days. The median exogenous insulin requirement was 0.4 (IQR: 0.3–0.6) unit/kg/day at 90 days, down from 0.5 (IQR: 0.4–0.8) unit/kg/day at the time of discharge. This decrease in exogenous insulin requirements was statistically significant (P = .02). One patient was no longer requiring exogenous insulin at 90 days.
TABLE 3.
Ninety-day outcomes
| Outcome | No. of children (N = 20) |
|---|---|
| Nutrition at 90 days | |
| Any parenteral nutrition | 0 |
| Enteral and oral feeds | 6 (30) |
| Oral diet only | 14 (70) |
| Opioid use at 90 days | |
| Yes | 9 (45) |
| No | 11 (55) |
| Postoperative complications | |
| Percutaneous drainage of fluid collection | 5 (25) |
| Reexploration for bleeding | 1 (5) |
| Bowel obstruction | 1 (5) |
| Time to cessation of opioid use, wka | 12.0 (6.0 to 20.0), n = 11 |
| Stimulated C-peptide, ng/mL | 1.9 (1.1 to 2.5), n = 18 |
| Insulin requirement, units/kg/d | 0.4 (0.3 to 0.6) |
Data presented as median (25th–75th percentiles) or n (%).
Calculated among patients off opioids at the end of the study period (90 days postoperatively).
FIGURE 1.
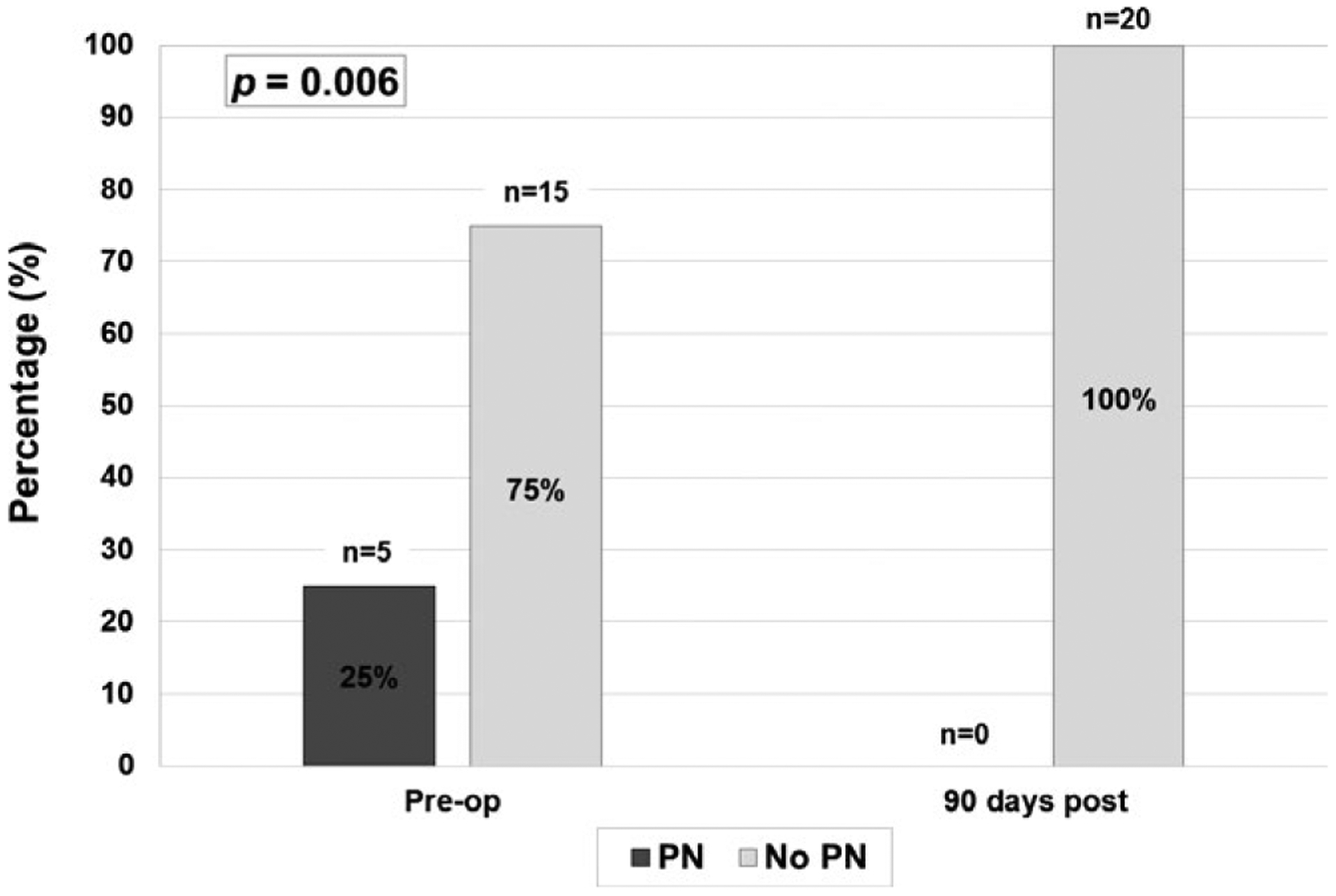
Parenteral nutrition (PN) use preoperatively and postoperatively. This figure compares the percentage of patients requiring PN preoperatively with the percentage requiring PN postoperatively. By 90 days postoperatively, no patient required PN support (P = .006)
FIGURE 2.
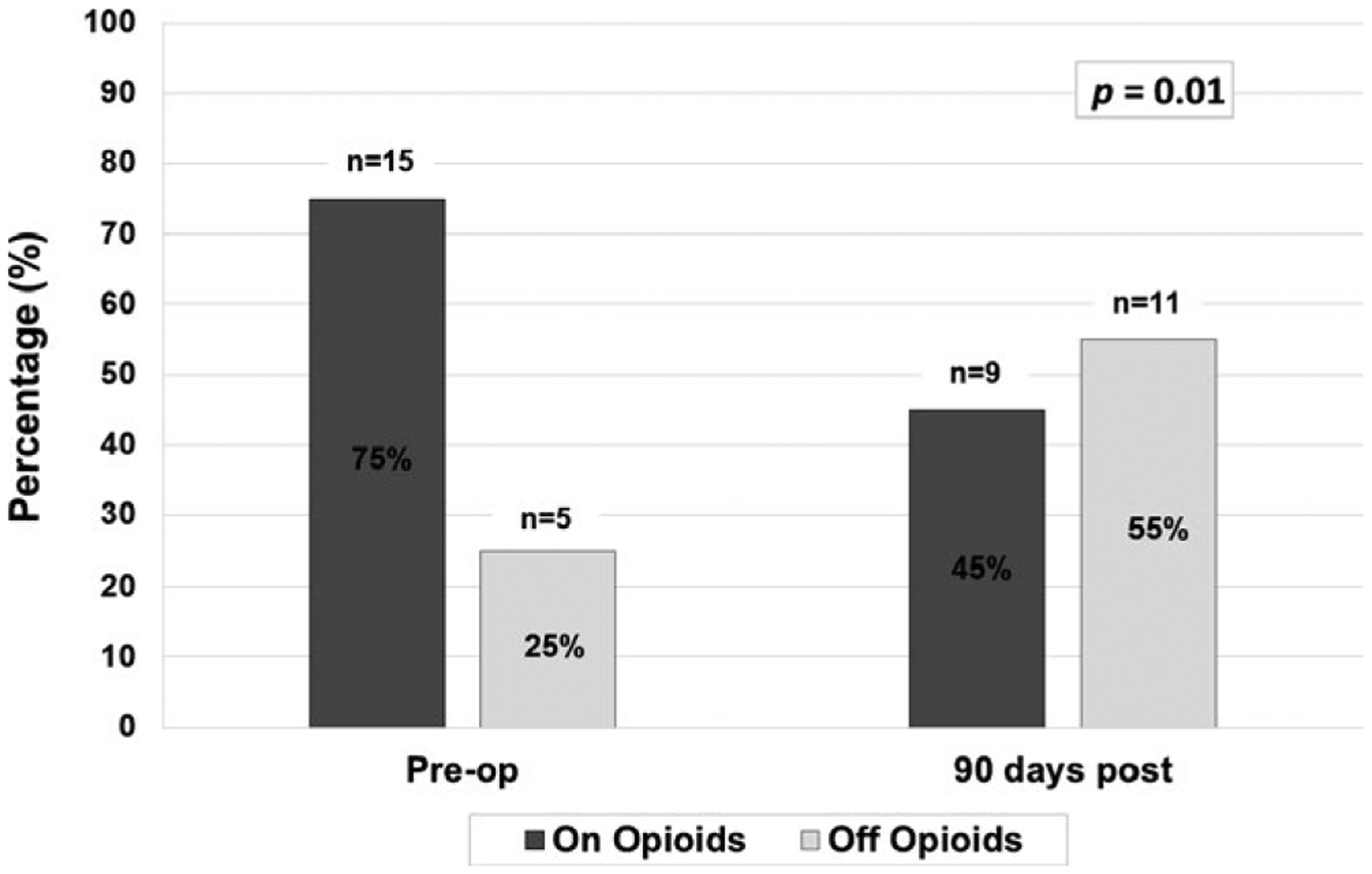
Opioid use preoperatively and postoperatively. This figure compares the percentage of patients on and off opioids preoperatively and at 90 days postoperatively, with a significant decrease in the proportion of patients requiring opioids at 90 days
3.3 |. Health-related quality of life
The SF-36 was completed by 11 patients preoperatively and at 90 days postoperatively. Physical health and total SF-36 scores were significantly improved at 90 days (Figure 3). Preoperatively, the median SF-36 physical health score was 34.0, and it improved to 54.6 at 90 days (P = .008). Total SF-36 scores improved from a median of 47.1 to 65.3 (P = .007). The median mental health score increased at 90 days but was not statistically significant (P = .12). The SF-10 was completed by parents of 13 patients. The physical health score was significantly improved at 90 days postoperatively (13.4 vs 33.1, P = .01). The median psychosocial component also increased, but the increase was not statistically significant (43.6 vs 51.6, P = .38).
FIGURE 3.
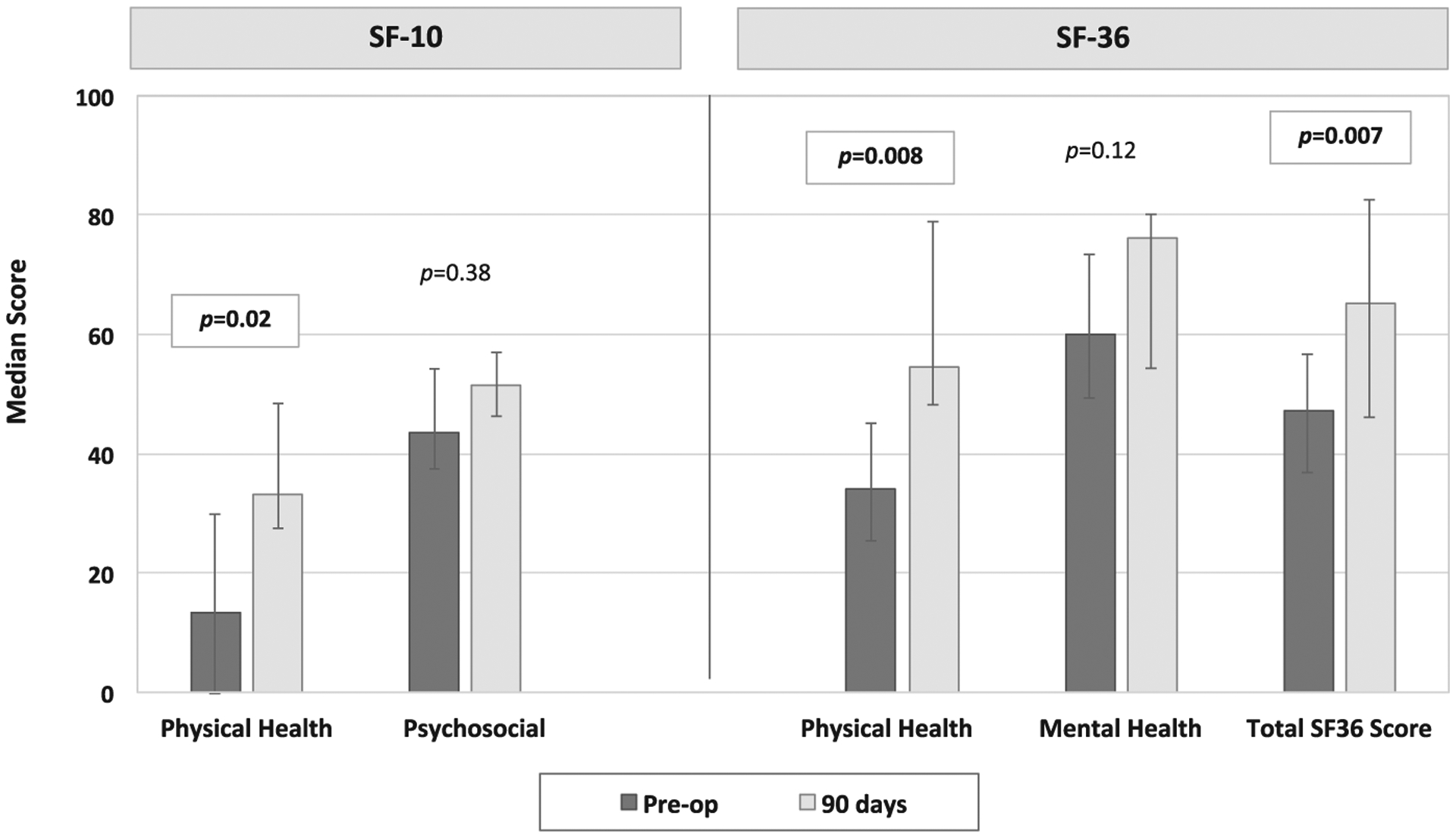
Health-related quality of life preoperatively and postoperatively. This figure compares health-related quality of life scores in patients preoperatively and at 90 days postoperatively. The SF-10 was completed for patients <12 years of age. The SF-36 was completed for patients 12 years and older. The bars in the figure represent median scores, and the error bars demonstrate the IQR. SF-10 physical health scores and SF-36 physical health module scores and total scores significantly improved at 90 days postoperatively
4 |. DISCUSSION
Pediatric CP and ARP are debilitating diseases that can effectively be treated with TPIAT when significant pain and impaired quality of life persist despite maximal medical and endoscopic therapy. Patients are carefully selected, recognizing that the operative intervention itself has significant risk and short-term morbidity, but overall outcomes, both in-hospital and at 90 days postoperatively, remain excellent. This study is the first to demonstrate that pediatric patients with ARP/CP who undergo TPIAT have significant improvements in opioid and PN dependence at 90 days postoperatively. Stimulated C-peptide levels are measurable at 90 days, demonstrating active β-cell function, and exogenous insulin requirements are significantly decreased at 90 days compared with the time of discharge. Importantly, HRQOL scores significantly improved in this population, as early as 3 months postoperatively.
While TPIAT has been performed in adults for 40 years, its introduction and broad implementation in children are significantly more recent. The pediatric population presents additional technical challenges with smaller bile ducts for reconstruction, as well as a theoretical increased risk of portal vein thrombus given smaller-caliber vasculature. However, surgical intervention in pediatric patients with CP who have had a shorter duration of disease relative to adult patients with CP may allow for isolation and autotransplant of islets before progressive destruction of the gland with advanced fibrosis, islet cell loss, and consequent endocrine insufficiency. Genetic risk factors are known to result in the development of CP, including cationic trypsinogen gene (PRSS1), serine protease inhibitor Kazal type 1 (SPINK1), cystic fibrosis transmembrane conductance regulator (CFTR), and chymotrypsin C (CTRC) mutations, with 73% of children possessing at least 1 mutation in a pancreatitis-related gene in the INSPPIRE cohort.21,27,28 In these patients specifically, with the high likelihood of progressive CP, the absence of anatomic characteristics that would allow surgical drainage procedures or local resections, and the life-time risk of pancreatic adenocarcinoma,29 early intervention in pediatric patients may have a significant long-term impact.
The benefits of TPIAT in pediatric patients include the ability to wean off of opioid medications as well as PN.30,31 PN has significant risk and cost associated with it, including the risk of central line–associated bloodstream infections.32 However, at present, the overarching indication for TPIAT is for the treatment of chronic debilitating symptoms. Chronic pain has been associated with depression, anxiety, and decreased quality of life in pediatric populations.33,34 Our study confirms previous reports of significant improvement in quality of life, with our outcomes demonstrating significant improvement as early as 90 days postoperatively. Bellin et al, in their 2011 series of 19 consecutive pediatric patients undergoing TPIAT, found that patients had below average HRQOL before surgery based on SF-36, with significant improvement in the mean physical health score and a strong trend toward improvement in the mean mental health score at 1 year postoperatively.12 Wilson et al reported their experience with 14 adolescent patients who underwent TPIAT and found that SF-36 scores at 9 months were significantly increased compared with preoperative baseline scores for this population.30 Our study is the first to demonstrate that HRQOL scores, as measured with both SF-36 and SF-10, improved in as short a period as 90 days after surgery, indicating the profound social and quality of life benefit pediatric patients experience from the elimination of chronic pain.
In addition, we found that although the operation itself is a major undertaking, requiring reconstruction of the alimentary and biliary systems, in addition to splenectomy, cholecystectomy, and appendectomy, the postoperative complications have been minimal. One patient required reexploration for a bowel obstruction after the initial hospital course, and an additional patient required immediate reexploration for bleeding. Otherwise, 5 patients underwent percutaneous drain placement into a fluid collection identified on a computed tomography scan obtained for postoperative fevers but required no further intervention and did not develop clinically significant infection. These complication rates are consistent with previously reported pediatric TPIAT complication rates.30,31 Optimal outcomes of TPIAT require careful patient selection based on rigorous multidisciplinary evaluation with surgery, gastroenterology, endocrinology, pain management physicians, and psychologists.
Our study does have limitations. It is a retrospective review of a small cohort of pediatric patients undergoing TPIAT with short-term follow-up. However, even with this small sample size and 90-day follow-up, we have been able to demonstrate significant improvements in opioid and PN use as well as quality of life. The quality of life data were collected prospectively, both preoperatively and at 90 days, reducing recall bias inherent in asking patients about changes in their quality of life retrospectively. The SF-36, used in this study, has not been validated in pediatric populations. However, it has previously been used in these adolescent CP populations to evaluate for improvements in quality of life.12,30 Last, our sample size for this study was small, which may have affected our ability to detect statistical significance. For example, we did not find the amount of morphine equivalents used by patients taking opioids to be statistically different at 90 days compared with preoperatively, although the median decreased from 20 to 7.5 morphine equivalents per day, which may be a reflection of the small sample size. As the number of pediatric patients undergoing TPIAT continues to grow and the current post-TPIAT patients extend further out from their surgical intervention, we hope to be able to assess these outcomes with larger sample size, as well assessing their long-term postoperative outcomes.
ARP and CP are debilitating diseases that are increasing in frequency in the pediatric population and can be effectively treated with TPIAT, with significant improvements in opioid and PN use as well as quality of life. Recovery of β-cell function has a longer expected postoperative course, but even at 90 days, patients show evidence of islet function and a decrease in exogenous insulin needs. Improvement in all of these parameters—opioid, parenteral nutrition, and exogenous insulin use, as well as HRQOL—can be demonstrated over a short period of time postoperatively.
ACKNOWLEDGMENTS
This work was supported by funding from the Academic Research Committee at Cincinnati Children’s Hospital Medical Center.
Abbreviations:
- ARP
acute recurrent pancreatitis
- BMI
body mass index
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CFTR
cystic fibrosis transmembrane conductance regulator
- CPA1
carboxypeptidase A1
- CP
chronic pancreatitis
- CTRC
chymotrypsin C
- ERCP
endoscopic retrograde cholangiopancreatography
- HRQOL
health-related quality of life
- ICU
intensive care unit
- IEQ
islet equivalents
- INSPPIRE
International Study Group of Pediatric Pancreatitis: In Search for a Cure
- PN
parenteral nutrition
- PRSS1
cationic trypsinogen gene
- SF-10
Short Form 10-Item Health Survey
- SF-36
Short Form 36-Item Health Survey
- SPINK1
serine protease inhibitor Kazal type 1
- TPIAT
total pancreatectomy with islet autotransplantation
Footnotes
Meeting Presentations: This work was presented as an abstract at the American Pediatric Surgical Association (APSA) Meeting, Palm Springs, CA, May 2018.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Tom K. Lin, MD: Medtronic, payment for lecture on capsule endoscopy. John K. Rose, MD: Data Safety Monitoring Board for Grunethal, pediatric Tapentadol trials. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence and survival of chronic pancreatitis: a population‐based study. Am J Gastroenterol. 2011;106:2192–2199. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keim V, Witt H, Bauer N, et al. The course of genetically determined chronic pancreatitis. JOP. 2003;4:146–154. [PubMed] [Google Scholar]
- 5.Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut. 2009;58:97–103. [DOI] [PubMed] [Google Scholar]
- 6.Morinville VD, Barmada MM, Lowe ME. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas. 2010;39:5–8. [DOI] [PubMed] [Google Scholar]
- 7.Srinath AI, Lowe ME. Pediatric pancreatitis. Pediatr Rev. 2013;34:79–89. [DOI] [PubMed] [Google Scholar]
- 8.Abu-el-Haija M, El-Dika S, Hinton A, et al. Acute pancreatitis admission trends: a national estimate through the Kids’ Inpatient Database. J Pediatr. 2018;194:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252–261. [DOI] [PubMed] [Google Scholar]
- 10.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing PedsQL 4.0 Generic Core Scales. Health Qual Outcomes. 2007;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingerski LM, Modi AC, Hood KK, et al. Health-related quality of life across pediatric chronic conditions. J Pediatr. 2010;156:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez-Munoz JE, Phillips M. Nutritional therapy in chronic pancreatitis. Gasteroenterol Clin North Am. 2018;47:95–106. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 2007;87:1477–1501. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad SA, Lowy AM, Wary CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. [DOI] [PubMed] [Google Scholar]
- 16.Wahoff DC, Paplois BE, Najarian JS, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg. 1995;222:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrman SW, Mulloy M. Total pancreatectomy for the treatment of chronic pancreatitis: indications, outcomes and recommendations. Am Surg. 2006;72:297–302. [PubMed] [Google Scholar]
- 18.Chinnakotla S, Radosevich DM, Dunn TB, et al. Long term outcomes of total pancreatectomy and islet autotransplantation for hereditary/genetic pancreatitis. J Am Coll Surg. 2014;218:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahoff DC, Paplois BE, Najarian JS, et al. Islet autotransplantation after total pancreatectomy in a child. J Pediatr Surg. 1996;31:132–135. [DOI] [PubMed] [Google Scholar]
- 20.Chinnakotla S, Bellin MD, Schwarzenberg SJ. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis. Ann Surg. 2014;260:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morinville VD, Hussain SZ, Bai H, et al. Definitions of pediatric pancreatitis and survey of current clinical practices: report from INSPPIRE (International Study Group of Pediatric Pancreatitis: in Search for a Cure). Pediatr Gastroenterol Nutr. 2012;55:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. [DOI] [PubMed] [Google Scholar]
- 23.Goto M, Johansson H, Maeda A, et al. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77:741–747. [DOI] [PubMed] [Google Scholar]
- 24.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF‐36). I. Conceptual Framework And Item Selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 26.Saris-Baglama RN, DeRosa MA, Raczel AE, et al. The SF-10 Health Survey for Children: A User’s Guide. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 27.Whitcomb DC. Hereditary pancreatitis: new insights into acute and chronic pancreatitis. Gut. 1999;45:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S, Ooi CY, Werlin S, et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr. 2016;170:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebours V, Boutron-Ruault M, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111–119. [DOI] [PubMed] [Google Scholar]
- 30.Wilson GC, Sutton JM, Salehi M, et al. Surgical outcomes after total pancreatectomy and islet cell autotransplantation in pediatric patients. Surgery. 2013;154:777–784. [DOI] [PubMed] [Google Scholar]
- 31.Bellin MD, Forlenza GP, Majumder K, et al. Total pancreatectomy with islet autotransplantation resolves pain in young children with severe chronic pancreatitis. JPGN. 2017;64:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: a cohort study. Infect Control Hosp Epidemiol. 2016;37:939–945. [DOI] [PubMed] [Google Scholar]
- 33.Howard RF. Current status of pain management in children. JAMA. 2003;290:2464–2469. [DOI] [PubMed] [Google Scholar]
- 34.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. [DOI] [PubMed] [Google Scholar]


