Abstract
Background:
Hemophagocytic lymphohistiocytosis (HLH) is a severe or even fatal inflammatory status. Lymphoma associated hemophagocytic lymphohistiocytosis (LAHS) is a kind of secondary HLH (sHLH). It suffers the worst outcome among sHLH. Allo-HSCT is often considered necessary. Autologous stem cell transplantation (auto-SCT) is widely used in the treatment of lymphoma, especially for high-risk NHL. There have been no clinical reports on the use of auto-SCT in LAHS in the past 20 years.
Methods:
We retrospectively evaluated 12 LAHS patients who received auto-SCT at our center from January 2013 to January 2020. Follow-up started at the date of LAHS diagnosis and ended at the date of death or last examination. Overall survival (OS) was calculated from the diagnosis of HLH to death of any cause.
Results:
The median period between diagnosis and auto-SCT is 6.7 months. All 12 patients achieved remission after transplantation. Follow-up to 1 January 2021, 8 patients remained disease-free, 4 patients relapsed and 2 of them died eventually. The median follow-up time is 20.9 months, and the median overall survival time has not been reached yet. The 3-year OS rates was 71%. Compared with LAHS patients who did not undergo transplantation during the same period (median OS time is 3.4 months), patients who underwent auto-SCT had a significantly better prognosis (P=0.001). Even if the lymphoma reaches CR after treatment, auto-SCT still provides a better prognosis compared to CR patients without transplantation (P=0.037). Compared with lymphoma patients without HLH who underwent auto-SCT during the same period, they had a similar prognosis (P=0.350).
Conclusion:
LAHS, as a common type in secondary HLH, may have a better prognosis after removing the trigger of HLH. In this study, the autologous transplantation in LAHS can significantly improve the prognosis, and provide LAHS a similar prognosis as high-risk lymphoma without HLH.
Keywords: hematopoietic stem cells, hemophagocytic lymphohistiocytosis, autologous transplantation, lymphoma, prognosis
Background
Hemophagocytic lymphohistiocytosis (HLH) is a severe or even fatal inflammatory state caused by hereditary or acquired immunoregulatory abnormalities. It is characterized by non-malignant proliferation of lymphocytes and histiocytes and secretion of a large number of inflammatory factors. HLH can be divided into primary HLH due to gene mutation and secondary HLH, which is secondary to infection, autoimmune disease, malignancy and others. Malignancy HLH (M-HLH) is a kind of sHLH, and lymphoma associated (LAHS) is the most common kind of M-HLH. The prognosis of LAHS is very poor. The median survival time of LAHS is considered to be less than 2 months 1 . There are often problems in treatment such as difficulty in controlling HLH, difficulty in relieving lymphomas, and high recurrence rate. The reasons may be associated with the pathogenesis of LAHS.
Autologous stem cell transplantation (auto-SCT) is widely used in the treatment of lymphoma, especially for high-risk NHL. Salvage chemotherapy followed by auto-SCT stands the standard of care for relapsed/refractory lymphoma 2 , and auto-SCT is also recommended as a first-line treatment in PTCL 3 . In the recommendations of histiocyte society for adult HLH in 2019, LAHS patients in remission who are eligible for treatment intensification may be candidates for autologous PBSCT 4 . This suggestion is basing on the assumption demonstrated in a study of peripheral T-cell lymphoma, that the poor prognosis of lymphoma complicated with HLH can be overcome by high-dose etoposide-containing chemotherapy followed by autologous-PBSCT 3 . But this is only an assumption, and there is not enough clinical evidence to support it. So far, reports of the use of auto-SCT in LAHS have been largely case reports. The one with the largest number of cases was reported in Japan in 2001 with auto-SCT in 5 cases of B-LAHS 5 . There have been no clinical reports on the use of auto-SCT in LAHS in the past 20 years. This study presented the first organized study to investigate the role of auto-SCT in the treatment of LAHS, and whether the use of auto-SCT can improve the short-term efficacy and long-term prognosis of LAHS.
Patients and Methods
Patients
We retrospectively evaluated 12 LAHS patients who received auto-SCT at our center from January 2013 to January 2020.
Eligibility criteria for this study were as follows: (1) patients met HLH-04 diagnostic criteria [4]; (2) pathology confirmed as lymphoma; (3) a diagnosis of primary HLH was excluded; (4) LAHS caused by chemotherapy during lymphoma treatment were excluded.
The pathological criteria of the diagnosis of the lymphoma were according to the World Health Organization classification of lymphoid neoplasms. All pathological biopsies were reviewed double blinded by two pathologists.
All patients were treated with the HLH-94/04 regimen 6 . Patients who showed no response to HLH-94 treatment accepted DEP regimen (liposomal doxorubicin with etoposide and high-dose methylprednisolone) as salvage therapy 7 . Response of HLH to treatment was assessed based on criteria proposed by Marsh et al and revised by Yini Wang et al 7,8 . Response of lymphoma to treatment was assessed base on the “Revised response criteria for malignant lymphoma” presented by The International Working Group in 2007 9 .
Conditioning Regimen and Assessment of Hematopoietic Reconstitution
All patients were conditioned with CBV regimen: cyclophosphamide 1.5 g/m2/ d -5, -4, -3 and -2, BCNU 150 mg/m2/ d -8, -7 and etoposide 300 mg/m2/ d -8, -7 and -6. PBSCs were thawed rapidly and reinfused on day 0. The mean number of infused CD34+ cell was 5.025*106/kg (3.344–13.8*106/kg). Neutrophil engraftment was defined as an absolute neutrophil count of >0.5*109/L for three consecutive measurements on different days. Platelet engraftment was defined as a platelet count of >20*109/L for consecutive measurements over 7 days, without requiring platelet transfusions. The median numbers of days to reach neutrophil engraftment and platelet engraftment were 11 (range 9–13) and 12 (range 10–18), respectively.
Survival Time and Statistical Analysis
Follow-up started at the date of LAHS diagnosis and ended at the date of death or last examination. Overall survival (OS) was calculated from the diagnosis of HLH to death of any cause. When the latter date was not reached, the date was censored at the time of the last follow-up evaluation or end of follow-up (1 January 2021).
SPSS 22.0 (IBM, New York/USA) statistical software was adopted, and data that did not fit a normal distribution are presented as median and range. T-test was used for data that fit a normal distribution and homogeneity of variance, and Wilcoxon rank sum test was used for others. Kaplan–Meier survival curves were used to analyze the patients’ survival. Survival duration differences between different groups were compared with log-rank tests. P<0.05 was considered to denote a significant difference.
Results
Patients Characteristics
In the 12 patients, 6 were male, and 6 were female. The median age of the patients was 37.5 years (18–57 years). As for the type of lymphoma, 6 patients were with non-Hodgkin B cell lymphomas (5 Diffuse large B cell lymphoma and 1 follicular lymphoma), 5 patients were with T/NK cell lymphomas (2 NK/T cell lymphoma, 2 ALK+ anaplastic large cell lymphoma, 1 subcutaneous panniculitis like T cell lymphoma), and 1 patient were with Hodgkin’s Lymphoma. Three patients were combined with EBV infection.
All patients (100%) presented with fever and elevated ferritin (>500 ng/mL). 11 patients (92%) presented with elevated soluble CD25, ten patients (83%) presented hepato/splenomegaly, and nine patients (75%) presented cytopenia in two or more lineages. Nine patients (75%) presented with abnormal liver function. 8 (67%) were with low NK-cell activity and 11 (92%) were with hemophagocytosis in bone marrow. (Table 1)
Table 1.
Patient’s Characteristics before Transplantation.
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 43 | 27 | 57 | 18 | 39 | 57 | 36 | 40 | 27 | 46 | 34 | 36 |
| Gender | F | F | F | M | M | M | F | M | M | F | F | M |
| Lmmunophenotype of lymphoma | B | NK/T | B | T | T | B | B | NK/T | B | B | B | T |
| International Prognostic Index | 4 | 1 | 4 | 2 | 2 | 3 | 3 | 4 | 3 | 3 | 2 | 2 |
| Previous therapy | RE-CHOP | E-CHOP | RE-CHOP | LE-CHOP | E-CHOP | RE-CHOP | RE-CHOP | L-CHOP | E-CHOP | R-CHOP | RE-CHOP | E-CHOP |
| HLH’s presentation | ||||||||||||
| Fever (T>38.5°C) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Hepato/splenomegaly (n) | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Hemophagocytosis | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y |
| WBC (*109/L) | 2.5 | 2.4 | 1 | 0.5 | 2.96 | 5.7 | 1.65 | 5.32 | 2.8 | 7.31 | 1.71 | 6.03 |
| HGB (g/L) | 128 | 85 | 59 | 102 | 116 | 132 | 79 | 97 | 121 | 76 | 63 | 127 |
| PLT (*109/L) | 27 | 171 | 61 | 61 | 10 | 55 | 71 | 25 | 171 | 239 | 4 | 328 |
| Ferritin (ng/ml) | 1218 | 601 | 4097.8 | 1500 | 7816 | 1046 | 26169 | 1500 | 5726 | 2685 | 2694 | 2000 |
| sCD25 (pg/mL) | 44000 | 7202.2 | 29678 | 44000 | 44000 | 41419 | 39008 | 44000 | 6983 | 31820 | 44000 | 2317 |
| NK-cell activity (%) | 9.05 | 14.06 | 3.68 | 14.28 | 25.87 | 14.17 | 13.94 | 16.53 | 15.33 | 14.91 | 17.91 | 14.71 |
| ALT (U/L) | 207 | 111 | 15 | 95 | 124 | 146 | 35 | 48 | 94 | 10 | 20 | 59 |
| AST (U/L) | 249 | 99.2 | 12.5 | 204 | 117 | 265 | 30 | 61 | 100 | 18 | 47 | 26 |
| Fbg (g/L) | 0.89 | 0.84 | 3.88 | 2.12 | 2.31 | 4.13 | 5.53 | 1.7 | 2.6 | 4.29 | 4.81 | 4.97 |
| TG (mmol/L) | 5.68 | 3 | 1.07 | 2.03 | 5.93 | 5.2 | 6.48 | 2.71 | 2 | 2.7 | 4.92 | 2.56 |
| EBV-DNA (PBMC) copies/ml | 0 | <500 | <500 | 0 | <500 | <500 | 0 | - | <500 | 5.7*105 | 0 | 0 |
| EBV-DNA (plasma) copies/ml | 0 | <500 | 0 | 0 | 0 | <500 | 0 | 4.60*104 | 3.30*104 | 4.50*104 | 0 | 0 |
All patients received HLH-directed therapy, including HLH-94/04 regimen and DEP regimen. After all patients achieved remission of HLH, they began to go through lymphoma-directed chemotherapy, including R-CHOP, E-CHOP, RE-CHOP, L-CHOP, and LE-CHOP. All patients achieved at least partial remission of lymphoma before transplantation (8 CR and 4 PR). The median period between diagnosis and auto-SCT is 6.7 months.
Outcome
All 12 patients achieved remission after transplantation. Follow-up to 1 January 2021, 8 patients remained disease-free, 4 patients relapsed and 2 of them died eventually 14.7 and 3.4 months after auto-SCT. 1 patient were lost to follow-up after 7.1 months. The median follow-up time is 20.9 months [95% CI 7.1, 34.7], and the median overall survival time has not been reached yet. The 3-year OS rates was 71%. The overall survival curve is shown in Fig. 1. The disease-free survival (DFS) of these 12 patients is 33.5 [95% CI 0.6, 66.4]. The DFS survival curve is shown in Fig. 2. The characteristics of transplantation were presented in Table 2.
Figure 1.
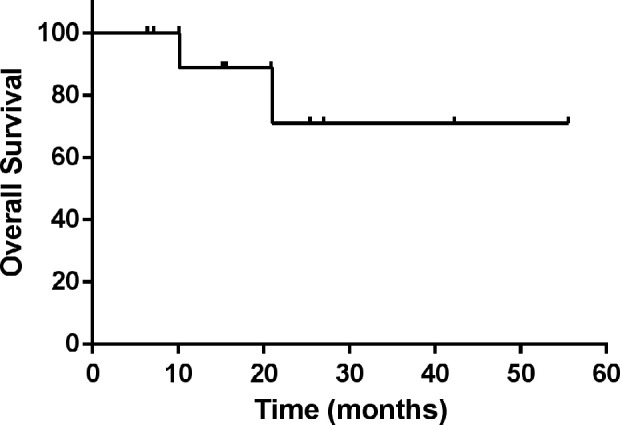
The overall survival curve of 12 LAHS patients.
Figure 2.
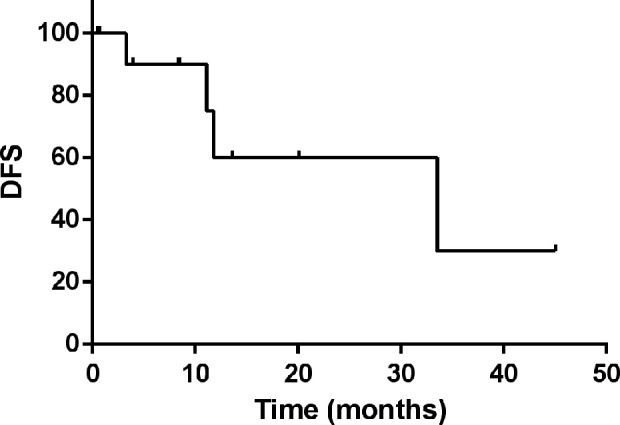
The DFS survival curve of 12 LAHS patients.
Table 2.
Characteristics of Transplantation.
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease status at transplantation | CR | CR | CR | CR | PR | CR | CR | PR | PR | CR | CR | PR |
| Conditioning regimen | CVB | CVB | CVB | CVB | CVB | CVB | CVB | CVB | CVB | CVB | CVB | CVB |
| Infused CD34+ cells (´106/kg) | 3.52 | 3.344 | 8.098 | 3.928 | 5.44 | 11.31 | 3.5 | 3.1 | 7.1 | 4.84 | 5.21 | 13.8 |
| Date of engraftment of neutrophils (days) | 10 | 11 | 9 | 9 | 12 | 11 | 11 | 12 | 13 | 13 | 11 | 11 |
| Date of engraftment of platelets (days) | 11 | 11 | 10 | 9 | 12 | 11 | 12 | 18 | 16 | 17 | 11 | 16 |
| Relapse (months from HSCT) | - | 33.5 | - | - | - | - | 11.1 | 11.8 | - | 3.3 | - | - |
| Final outcome | Survival | Survival | Lost | Survival | Survival | Survival | Survival | Death | Survival | Death | Survival | Survival |
There were multi reasons why some of LAHS patients didn’t undergo auto-HSCT, such as financial considerations, patient and/or families’ refusal, age limitation and poor performance status. Compared with LAHS patients who did not undergo transplantation during the same period (median OS time is 3.4 months [95% CI 1.5, 5.4]), LAHS patients who underwent auto-SCT had a significantly better prognosis (P=0.001; Fig. 3). There was no significant difference in the age comparison of LAHS patients who received and did not receive HSCT (38.3 vs 45.5, P=0.134). The performance status was evaluated by ECOG score standard, and there was no difference between them (P=0.660).
Figure 3.
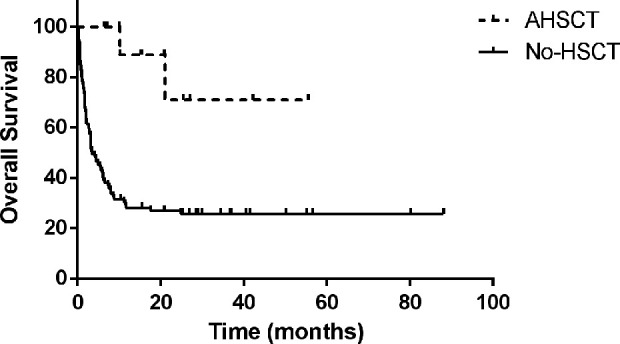
Compared with LAHS patients who did not undergo transplantation during the same period (median OS time is 3.4 months [95% CI 1.5, 5.4]), LAHS patients who underwent auto-SCT had a significantly better prognosis (P=0.001).
Considering the influence of lymphoma remission status on the prognosis, the survival analysis of LAHS patients who achieved lymphoma-CR after chemotherapy and LAHS patients who underwent AHSCT were also compared. The results showed that the OS of LAHS patients who achieved lymphoma-CR (LAHS-CR) after chemotherapy (median OS time is 11.7 months [95% CI 0, 27.5]) was significantly better than those who didn’t (11.7 months [95% CI 0, 27.5] vs 2.1 months [95% CI 1.4, 2.9], P<0.001), but LAHS patients who underwent AHSCT still have a better prognosis than LAHS-CR patients (P=0.037; Fig. 4).
Figure 4.
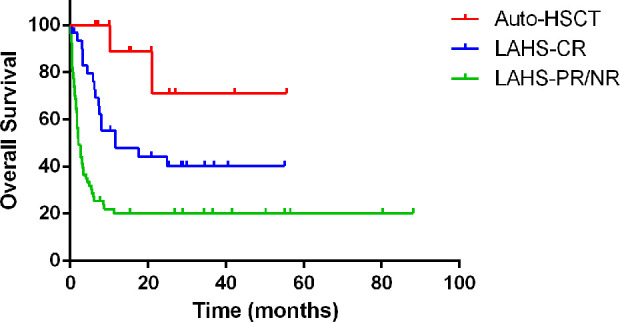
The OS of LAHS patients who achieved lymphoma-CR (LAHS-CR) after chemotherapy (median OS time is 11.7 months [95% CI 0, 27.5]) was significantly better than those who didn’t (11.7 months [95% CI 0, 27.5] vs 2.1 months [95% CI 1.4, 2.9], P<0.001), but LAHS patients who underwent AHSCT still have a better prognosis than LAHS-CR patients (P=0.037).
Compared with lymphoma patients without HLH who underwent auto-SCT during the same period, they had a similar prognosis (no statistically significant difference in overall survival, P=0.350; Fig. 5).
Figure 5.
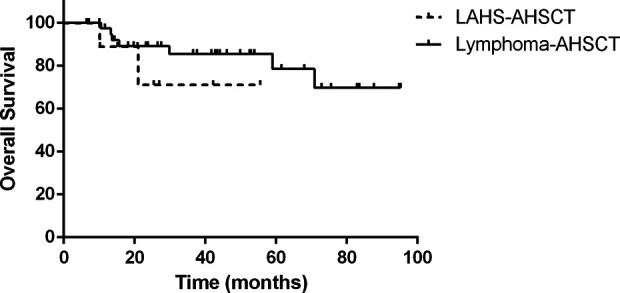
Compared with lymphoma patients without HLH who underwent auto-SCT during the same period, they had a similar prognosis (no statistically significant difference in overall survival, P=0.350).
Discussion
Malignancy associated HLH (M-HLH) may occur in up to 1% of patients with hematologic malignancies 10 . HLH is a severe or even fatal inflammatory status caused by a hereditary or acquired immunoregulatory abnormality, non-malignant proliferation of lymphocytes and histiocytic cells, and secretion of a large number of inflammatory cytokines. M-HLH is the most common type of secondary HLH, and the most common type of M-HLH is lymphoma associated HLH (LAHS). LAHS secondary to NK / T-cell lymphoma is more common, and the incidence of LAHS in NK / T-cell lymphoma can be as high as 20% 11 . The prognosis of M-HLH is extremely poor, which is the worst type of prognosis among HLH. The mortality of HLH secondary to s-JIA is only 8%, while the mortality of M-HLH is even> 80%, and the median survival time is only 2 months 12 . Obviously, many previous clinical observations have found out that only using chemotherapy to treat LAHS has a higher recurrence rate and a worse prognosis 1 . At present, the general opinion is that allo-SCT after remission of regular chemotherapy is the only way to achieve long-term survival for patients with LAHS 13 . However, after all, allo-SCT has difficulties such as high risks, high costs, and donor requirements. The role of auto-PBSCT in relapsed and refractory lymphoma has been confirmed by many studies, and it is also widely used in clinical practice. Especially in some certain high-risk lymphomas, it is even considered as a first-line solution 3 . There is a lack of study on the role of auto-SCT in patients with LAHS. Auto-SCT in the treatment of LAHS has only been reported as single case report in previous literature. In a report of B-LAHS in 1999, auto-SCT was used in LAHS for the first time. All 5 cases had a long-term disease-free survival of more than 2 years 14 . In the later period, there were also reports of complete remission and long-term survival using auto-SCT in T, NK / T-cell lymphoma HLH 15 –17 .
The reason for auto-SCT’s effectiveness in LAHS is closely related to the possible pathogenesis of LAHS. In the 2017 M-HLH consensus, the pathogenesis of HLH was considered to be based on the relationship between the occurrence of HLH and the primary malignancy. When HLH was triggered by malignancy, the hyperinflammation is triggered by the neoplasm due to an excessive secretion of pro-inflammatory cytokines and persistent antigen stimulation by the tumor cells. When HLH occur during chemotherapy, the immunodeficiency generated by the loss of immune homeostasis due to chemotherapy or infection, but not the malignancy itself 11 . All the 12 patients in this study, HLH appeared at the same time with lymphoma, indicating that the hyperinflammation of HLH in these patients was induced by tumor cells but not chemotherapy related immune homeostasis. Under this condition, avoiding tumor recurrence by reducing the residual disease of lymphoma, and thereby reducing the trigger of HLH recurrence, is obviously the key to the long-term disease-free survival of LAHS patients. Autologous HSCT has become a standard treatment for patients with relapsed and refractory Hodgkin lymphoma. Also, for relapsed or refractory (R/R) NHL patients, use of high dose chemotherapy (HDT) consolidation with autologous stem cell transplant (ASCT) can be curative 18 –20 . The main mechanism is to eradicate disease activity by high-dose chemotherapy prior to PBSCT, and then supporting by stem cells infusion 21 . The usage of high-dose etoposide is important 14 . The reason why AHSCT is effective for LAHS is that by reducing the residual tumor cells of lymphoma, avoiding the recurrence of lymphoma, and achieving the long-term disease-free remission of LAHS.
In this study, all 12 LAHS patients achieved remission after auto-SCT, and 10 patients survived until the follow-up. Once lymphoma complicated with HLH (LAHS), its prognosis is significantly worse than those without HLH, which has been widely recognized. The overall survival rate of patients undergoing auto-SCT was significantly better than that of LAHS patients who did not undergo auto-SCT. This suggests that undergoing auto-SCT can indeed improve the prognosis of LAHS in a certain extent. But to what extent? Can auto-SCT provide LAHS a similar prognosis as high-risk lymphoma without HLH? The results support this opinion by showing that patients with LAHS receiving auto-SCT can achieve the same survival as patients with simple high-risk lymphoma undergoing auto-SCT. After auto-SCT, the cause of HLH is removed, reducing the possibilities of HLH recurrence, and HLH as the high-risk factor for lymphoma is also removed. Of course, the small number of cases may limit the reliability of this conclusion. Considering the high transplantation-related mortality rate of allo-SCT (48% vs 4%) compared with auto-HSCT 22 , and the difficulties with lack of donors, financial issues and et al, LAHS patients in remission who are eligible for treatment intensification may be candidates for autologous PBSCT. Also, for LAHS patients whose lymphoma can achieve complete remission through chemotherapy, they will indeed have a significantly better prognosis than patients who cannot achieve CR. However, for these LAHS patients who have already achieved CR, auto-SCT can further improve survival.
One of the main concerns of auto-SCT is relapse. Compared with allo-SCT, its higher recurrence rate cannot be avoided. In this study, there was four cases of lymphoma recurrence. It has been reported that the use of high-dose VP-16 for myeloablative chemotherapy as a pretreatment before auto-SCT may reduce the recurrence rate by eliminating potential small residual lesions 14 . Cyclosporine and prednisolone used after auto-SCT as maintenance therapy may also reduce the relapse rate 21 . Even though this study was not involved, the remission status of lymphoma before transplantation is closely related to the probability of recurrence. Considering the important role of lymphoma recurrence for the long-term survival of LAHS patients, we recommend that it is best for LAHS patients with lymphoma to achieve complete remission to undergo auto-HSCT, while patients who do not achieve CR need to consider allo-HSCT.
Conclusion
LAHS, as a common type in secondary HLH, may have a better prognosis after removing the trigger of HLH. Unlike EBV-HLH or primary HLH, which must rely on allogeneic transplantation to remove incentives, autologous transplantation itself is an effective means in the treatment of lymphoma and can significantly prolong the disease-free survival of refractory relapsed lymphoma. In this study, the use of autologous transplantation in LAHS can significantly improve the prognosis, and provide LAHS a similar prognosis as high-risk lymphoma without HLH. Further research is still needed on recurrence and other issues, which has certain implications for the future direction of LAHS treatment.
Acknowledgments
We thank the patients and their families for participating in our study.
Footnotes
Authors’ Contributions: ZW contributed to the design of the study. JSW and QXY helped with the study design and data analyses. YS conducted the data analysis and wrote the manuscript. All authors approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: All Procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee at Beijing Friendship Hospital.
Data Availability Statements: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No.81871633); Beijing Natural Science Foundation (No.7181003). The funding body did not contribute to the design of the study and collection, analysis, and interpretation of data, or in writing the manuscript.
ORCID iD: Yue Song  https://orcid.org/0000-0003-0898-5281
https://orcid.org/0000-0003-0898-5281
References
- 1. Tamamyan GN, Kantarjian HM, Ning J, Jain P, Sasaki K, McClain KL, Allen CE, Pierce SA, Cortes JE, Ravandi F, Konopleva MY, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes. Cancer. 2016;122(18):2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zahid U, Akbar F, Amaraneni A, Husnain M, Chan O, Riaz IB, McBride A, Iftikhar A, Anwer F. A review of autologous stem cell transplantation in lymphoma. Curr Hematol Malig Rep. 2017;12(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilhelm M, Smetak M, Reimer P, Geissinger E, Ruediger T, Metzner B, Schmitz N, Engert A, Schaefer-Eckart K, Birkmann J. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016;6(7):e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. La Rosee P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. [DOI] [PubMed] [Google Scholar]
- 5. Hirai H, Shimazaki C, Hatsuse M, Okano A, Ashihara E, Inaba T, Murakami S, Saigo K, Nakagawa M. Autologous peripheral blood stem cell transplantation for adult patients with B-cell lymphoma-associated hemophagocytic syndrome. Leukemia. 2001;15(2):311–312. [DOI] [PubMed] [Google Scholar]
- 6. Henter JI, Samuelsson-Horne A, Arico M, Egeler RM, Elinder G, Filipovich AH, Gadner H, Imashuku S, Komp D, Ladisch S, Webb D, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367–2373. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Huang W, Hu L, Cen X, Li L, Wang J, Shen J, Wei N, Wang Z. Multicenter study of combination DEP regimen as a salvage therapy for adult refractory hemophagocytic lymphohistiocytosis. Blood. 2015;126(19):2186–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, Lee ND, Khan SP, Lawrence J, Mo JQ, Bleesing JJ, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60(1):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, et al. International Harmonization Project on L. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 10. Machaczka M, Vaktnas J, Klimkowska M, Hagglund H. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: a retrospective population-based analysis from a single center. Leuk Lymphoma. 2011;52(4):613–619. [DOI] [PubMed] [Google Scholar]
- 11. Daver N, McClain K, Allen CE, Parikh SA, Otrock Z, Rojas-Hernandez C, Blechacz B, Wang S, Minkov M, Jordan MB, La Rosee P, et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer. 2017;123(17):3229–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220–224. [DOI] [PubMed] [Google Scholar]
- 13. Bigenwald C, Fardet L, Coppo P, Meignin V, Lazure T, Fabiani B, Kohn M, Oksenhendler E, Boutboul D, Uzzan M, Lambotte O, et al. A comprehensive analysis of Lymphoma-associated haemophagocytic syndrome in a large French multicentre cohort detects some clues to improve prognosis. Br J Haematol. 2018;183(1):68–75. [DOI] [PubMed] [Google Scholar]
- 14. Shimazaki C, Inaba T, Nakagawa M. B-cell lymphoma-associated hemophagocytic syndrome. Leuk Lymphoma. 2000;38(1–2):121–130. [DOI] [PubMed] [Google Scholar]
- 15. Inoue D, Nagai Y, Takiuchi Y, Nagano S, Arima H, Kimura T, Shimoji S, Mori M, Togami K, Tabata S, Yanagita S, et al. Successful treatment of extranodal natural killer/T-cell lymphoma, nasal type, complicated by severe hemophagocytic syndrome, with dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide chemotherapy followed by autologous stem cell transplant. Leuk Lymphoma. 2010;51(4):720–723. [DOI] [PubMed] [Google Scholar]
- 16. Han AR, Lee HR, Park BB, Hwang IG, Park S, Lee SC, Kim K, Lim HY, Ko YH, Kim SH, Kim WS. Lymphoma-associated hemophagocytic syndrome: clinical features and treatment outcome. Ann Hematol. 2007;86(7):493–498. [DOI] [PubMed] [Google Scholar]
- 17. Uehara T, Yokota A, Onoda M, Yamamoto K, Terano T. Successful autologous peripheral blood stem cell transplantation for a patient with primary adrenal lymphoma with hemophagocytic syndrome. Clin Lymphoma Myeloma. 2008;8(3):184–187. [DOI] [PubMed] [Google Scholar]
- 18. David KA, Mauro L, Evens AM. Relapsed and refractory Hodgkin lymphoma: transplantation strategies and novel therapeutic options. Curr Treat Options Oncol. 2007;8(5):352–374. [DOI] [PubMed] [Google Scholar]
- 19. Chin CK, Lim KJ, Lewis K, Jain P, Qing Y, Feng L, Cheah CY, Seymour JF, Ritchie D, Burbury K, Tam CS, et al. Autologous stem cell transplantation for untreated transformed indolent B-cell lymphoma in first remission: an international, multi-centre propensity-score-matched study. Br J Haematol. 2020;191(5):806–815. [DOI] [PubMed] [Google Scholar]
- 20. Laport GG. Peripheral T-cell lymphoma: autologous hematopoietic cell transplantation as first-line therapy. Curr Opin Oncol. 2010;22(5):409–413. [DOI] [PubMed] [Google Scholar]
- 21. Ohga S, Nomura A, Kai T, Matsuzaki A, Inaba S, Suda M, Ueda K. Prolonged resolution of hemophagocytic lymphohistiocytosis following myeloablative chemotherapy and subsequent autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997;19(6):633–635. [DOI] [PubMed] [Google Scholar]
- 22. Suzuki R, Suzumiya J, Nakamura S, Kagami Y, Kameoka JI, Sakai C, Mukai H, Takenaka K, Yoshino T, Tsuzuki T, Sugimori H, et al. Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant. 2006;37(4):425–431. [DOI] [PubMed] [Google Scholar]


