Abstract
Objective
Studies focusing on Kawasaki disease (KD) in adolescents are lacking in Southwest China. We systematically summarized the clinical characteristics of KD in adolescents to improve pediatricians’ recognition of this condition.
Methods
The clinical data of patients with adolescent-onset KD in our center were retrospectively analyzed. The patients were divided into Group A (n = 7), whose first hospitalization was at our hospital, and Group B (n = 10), who were transferred from their local hospital or community health center.
Results
Seventeen patients with adolescent-onset KD were identified (constituent ratio of 0.8%). Seven patients had an intermittent fever for >10 days. The incidence of incomplete KD was 52.9%. These patients had a high incidence of other atypical clinical manifestations. Fifteen patients were initially misdiagnosed with other infectious diseases. Although the incidence of typical KD was higher in Group B, the overall misdiagnosis rate at the initial stages was higher and the average fever duration on arrival and before IVIG administration were much longer in Group B than A.
Conclusions
KD in adolescents was frequently misdiagnosed, which might be associated with its atypical, diverse clinical features and pediatricians’ poor recognition. Pediatricians must be aware of the possibility of KD in adolescents.
Keywords: Kawasaki disease, adolescent, misdiagnosis, infectious disease, fever, China
Introduction
Kawasaki disease (KD) is an acute, self-limiting systemic vasculitis that predominately affects children aged 6 months to 5 years. It is characterized by fever, bilateral non-exudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities such as erythema or desquamation, rash, and cervical lymphadenopathy. 1 Coronary artery lesions (CALs), severe sequelae of KD that develop in 20% to 25% of children without treatment, have surpassed acute rheumatic fever as the leading cause of acquired heart disease in children. 2 However, it has been proven that timely therapy with intravenous immunoglobulin (IVIG) can significantly reduce the incidence of CALs to about 4.5%. 3 Currently, the identification of KD mainly relies on the principle clinical manifestations after excluding other clinically similar entities with known etiologies. However, incomplete KD is difficult for pediatricians to recognize and is often misdiagnosed as other similar entities, particularly infectious diseases such as cervical lymphangitis, respiratory tract infection, and infectious diarrhea. This results in a delayed diagnosis of KD, impeding prompt IVIG treatment and thereby increasing the risk of CALs.
Prior studies have shown that incomplete KD is more frequently found in the extreme age spectrum, particularly in children aged <6 months and 5 to 10 years. 4 One study showed that more than 50% of general pediatricians and about 25% of infectious disease specialists did not consider the diagnosis of KD in patients younger than 6 months and older than 8 years. 5 However, pediatricians’ recognition and awareness of KD in patients younger than 6 months and 5 to 10 years of age improved after a series of studies summarized the clinical manifestations of KD among patients in those age spectrums.6–8 Nonetheless, adolescent-onset KD (defined as onset from 10 to 18 years of age), which has a much lower estimated incidence of 0.10 to 1.45 per 100,000 population, 9 has gained much less attention from clinicians. Only limited studies are available in the literature. Most pediatricians, particularly from undeveloped areas, do not consider the diagnosis of KD in adolescents, and they lack recognition and awareness of KD in this age group. The present study was therefore performed to retrospectively summarize the clinical characteristics of adolescent-onset KD in our center and review all available literature reporting KD in adolescents with an aim to improve the recognition and outcomes of KD in this extreme age spectrum.
Methods and materials
We retrospectively reviewed the data of all patients (n = 2118) who were diagnosed with KD from January 2007 to July 2017 at the West China Second University Hospital of Sichuan University (WCSUH-SCU), the largest pediatric medical center in Southwest China. Among these 2118 patients, 17 adolescent patients aged 10 to 18 years were included in the present study. To explore the effect of pediatricians’ awareness on the early diagnosis and prognosis of KD in this age group, these patients were divided into two groups based on their first hospitalization: Group A (n = 7), whose first hospitalization was at our hospital, and Group B (n=10), who were transferred from their local hospital or community health center to our hospital. The patients’ clinical details, laboratory examination parameters, echocardiography data, treatment effectiveness, and follow-up results were systematically collected and analyzed. Informed written consent was obtained from the patients’ parents after the nature of this study had been fully explained to them. The University Ethics Committee on Human Subjects at Sichuan University approved the study. Our study followed the relevant EQUATOR Network guidelines. 10
Classic KD is diagnosed in patients who have had a fever for at least 5 days and exhibit at least four of the five principal clinical features. 11 Incomplete KD is suspected in patients with a prolonged unexplained fever, cervical lymphadenitis, or retropharyngeal/parapharyngeal phlegmon that is nonresponsive to antibiotics but whose condition does not fulfill the classic criteria. Laboratory testing and/or echocardiography are further performed in such cases. If the patients’ laboratory results show an increased C-reactive protein concentration of ≥30 mg/L and/or an erythrocyte sedimentation rate of ≥40 mm/hour along with more than three additional laboratory criteria including an albumin concentration of ≤30 g/L, elevated alanine aminotransferase concentration, ≥10 white blood cells per high-power field in the urine, white blood cell count of ≥15 × 109/L, platelet count of ≥450 × 109/L after 7 days, anemia for age, or fewer than three additional laboratory criteria but with positive echocardiography, the diagnosis of incomplete KD is established with reference to the incomplete KD diagnostic algorithm proposed in the 2017 American Heart Association scientific statement for the diagnosis, treatment, and long-term management of KD. 1 The diagnoses of the patients in the present study were confirmed by two pediatricians (including a KD specialist).
All patients underwent the same treatment program after the diagnosis of KD was established. IVIG (2 g/kg) and aspirin (30–50 mg/kg/day) were administered during the acute phase of KD. After the patient defervesced, the aspirin dosage was decreased to 3 to 5 mg/kg/day and continued for 6 to 8 weeks. If the patient had CALs, the aspirin administration was continued until the patient showed no evidence of coronary changes. If the patient had a recurrent or persistent fever for ≥36 hours after the first IVIG administration, IVIG (1 g/kg) was administered a second time. Furthermore, methylprednisolone and/or prednisone were administered if the patient had a recurrent or persistent fever even after the second IVIG administration. IVIG resistance was defined as a persistent or recurrent fever (oral temperature of ≥38.0°C) or other clinical signs of KD for ≥36 hours but ≤7 days after the first IVIG infusion. 12
CALs are defined based on a coronary artery branch internal lumen diameter that meets the following Japanese Ministry of Health criteria: any coronary artery branch internal lumen diameter of ≥3 mm for children <5 years of age or ≥4 mm for children >5 years of age, or the internal diameter of any branch being 1.5 times greater than that of any adjacent segment. 13 According to our institutional protocol, patients with CALs underwent standardized echocardiography by two pediatric ultrasonologists during the acute phase and 6 to 8 weeks later during their cardiology clinic follow-up evaluations until resolution of the CALs.
Statistical analysis
All data analyses were conducted with IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). Quantitative data are presented as mean and range or mean ± standard deviation, and qualitative data are expressed as n (%). The Shapiro–Wilk test and homogeneity test of variance were applied to confirm that the quantitative data from different groups came from a normal distribution and met the homogeneity of variance. Differences in quantitative data between Group A and Group B were determined by the independent-samples t-test or Mann–Whitney U test. Fisher’s exact test was applied to compare proportions because the sample size was <40 (n = 17 patients). Statistical significance was defined as a two-tailed P value of <0.05.
Results
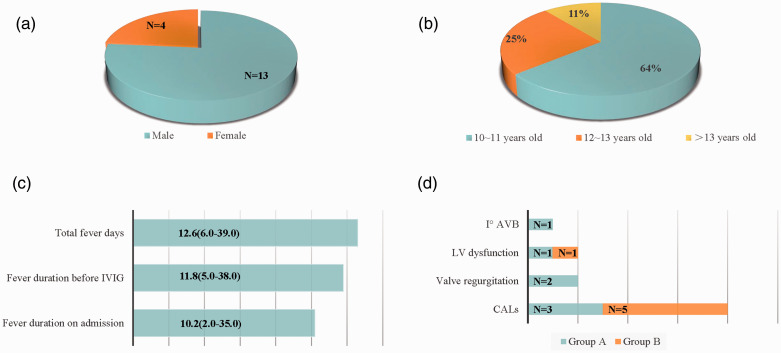
Seventeen adolescents were diagnosed with KD, including 13 male and 4 female patients (male:female ratio of 3.25:1.00). Their ages ranged from 10.1 to 13.5 years (median age, 11.8 years); 10 (58%) were 10 to 11 years of age, 3 (18%) were 12 to 13 years of age, and 4 (24%) were >13 years of age (Figure 1(a), (b)). On admission, the mean fever duration was 10.2 days (range, 2.0–35.0) (Figure 1(c)). Notably, seven patients had experienced an intermittent fever for >10 days before being transferred to our hospital. The longest fever duration of one child (Patient 10) was 35 days (Table 1).
Figure 1.
Sex, age distribution, fever duration, and cardiac complications of adolescents with KD. (a) Sex distribution of adolescents with KD. (b) Age distribution of adolescents with KD. (c) Fever duration in adolescents with KD. (d) Cardiac complications in adolescents with KD. KD, Kawasaki disease; IVIG, intravenous immunoglobulin; Iº AVB, primary atrioventricular block; LV, left ventricular; CALs, coronary artery lesions.
Table 1.
Clinical features, treatment, cardiac complications, and follow-up of adolescents with KD.
| Patient No. | Age (years)/sex | Fever duration, days |
Primary misdiagnosis | Principle clinical featuresc | Other clinical findings | IVIG resistance | Aspirin/sec-IVIG/steroid | Cardiac complications | Follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| On admission | Before IVIG | After IVIG | |||||||||
| Group Aa | |||||||||||
| 1 | 11.0/M | 5 | 6 | 1 | Cervical lymphadenitis | 1,2,3,6 | Cough, dizziness, left knee arthritis, SOM | − | +/−/− | CAD (LCA 3.8 mm, RCA 3.5 mm) | Normal 1 week later |
| 2 | 10.1/M | 5 | 6 | 3 | Amygdalitis | 1,2,3,5,6 | Diarrhea, vomiting | + | +/+/+ | TR, MR | Normal 3 weeks later |
| 3 | 11.8/M | 3 | 5 | 1 | Cervical lymphadenitis | 1,2,5,6 | Cough, diarrhea, dizziness | − | +/−/− | CAA (LCA 5.0 mm), LVEF↓ | Normal 6 weeks later |
| 4 | 13.1/M | 7 | 10 | 3 | Bronchitis, infectious diarrhea, pyohemia | 1,2,3,5 | Cough, diarrhea, vomiting, fatigue, dizziness, tinnitus, proteinuria, sterile pyuria, pleural effusion | + | +/+/+ | CAD (RCA 3.3 mm), TR, I°AVB | Normal 2 months later |
| 5 | 10.8/M | 2 | 7 | 0 | Cervical lymphadenitis | 1,2,3,4,6 | − | − | +/−/− | NA | N |
| 6 | 12.1/M | 7 | 9 | 0 | Suspected KD | 1,2,3,5 | Vomiting, headache, proteinuria, sterile pyuria | − | +/−/− | NA | N |
| 7 | 11.3/M | 6 | 7 | 0 | Suspected KD | 1,2,3,5 | Cough, arthritis, arthralgia of limbs | − | +/−/− | NA | N |
| Group Bb | |||||||||||
| 8 | 11.1/M | 15 | 15 | 4 | RTI | 1,3,4,5 | Cough, pharyngalgia, diarrhea, fatigue, drowsiness, proteinuria | + | +/+/− | CAD (LCA 3.6 mm) | Normal 4 weeks later |
| 9 | 11.8/M | 6 | IVIG allergy | / | RTI, infectious diarrhea | 1,2,3,5 | Diarrhea, vomiting, abdominal pain | IVIG allergy | +/−/− | CAD (LAD 3.4 mm) | Normal 1 week later |
| 10 | 12.8/M | 35 | 38 | 1 | Mesenteric lymphadenitis, infective skin rashes | 1,2,3,5,6 | Cough, vomiting, abdominal pain, ankle joint edema, swelling of right epididymis | − | +/−/− | CAA (LCA 8.2 mm) | CAA still present 11 months later |
| 11 | 10.9/F | 14 | 15 | 0 | Pneumonia | 1,2,3,4,5 | Cough, vomiting, arthritis, arthralgia | − | +/−/− | CAA (RCA 5.7 mm) | CAA still present at 1 year later |
| 12 | 11.2/F | 10 | 11 | 0 | Suppurative tonsillitis, scarlatina | 1,2,4,5 | − | − | +/−/− | CAD (LCA 3.1 mm) | Normal 5 days later |
| 13 | 13.0/M | 17 | 17 | 7 | Tonsillitis | 1,2,3,4,5 | − | + | +/+/− | NA | N |
| 14 | 13.5/M | 10 | 10 | 0 | Cervical lymphadenitis | 1,2,3 | Fatigue, sterile pyuria | − | +/−/− | NA | N |
| 15 | 11.8/F | 10 | 10 | 0 | Bronchitis | 1,2,3,4,5 | Cough | − | +/−/− | NA | N |
| 16 | 13.3/F | 11 | 11 | 0 | Intracranial infection | 1,2,3,4,5 | Cough, diarrhea, vomiting, sterile pyuria | − | +/−/− | NA | N |
| 17 | 13.3/M | 10 | 11 | 0 | Pyohemia | 1,2,3,5,6 | Cough, abdominal pain | − | +/−/− | LVEF↓ | Normal 2 weeks later |
aGroup A: first hospitalization was at our hospital.
bGroup B: transferred from their local hospital or community health center.
cPrincipal clinical features of KD: 1. Fever for at least 5 days, 2. Bilateral non-exudative conjunctival injection, 3. Erythema of lips/oral mucosa, 4. Extremity changes, 5. Polymorphous skin rash, 6. Cervical lymphadenopathy.
CAA, coronary artery aneurysm; CAD, coronary artery dilation; IVIG, intravenous immunoglobulin; I°AVB, first-degree atrioventricular block; F, female; KD, Kawasaki disease; LCA, left coronary artery; LVEF, left ventricular ejection fraction; M, male; MR, mitral regurgitation; NA, not available; RCA, right coronary artery; RTI, respiratory tract infection; TR, tricuspid regurgitation; Sec-IVIG, second intravenous immunoglobulin; SOM, secretory otitis media.
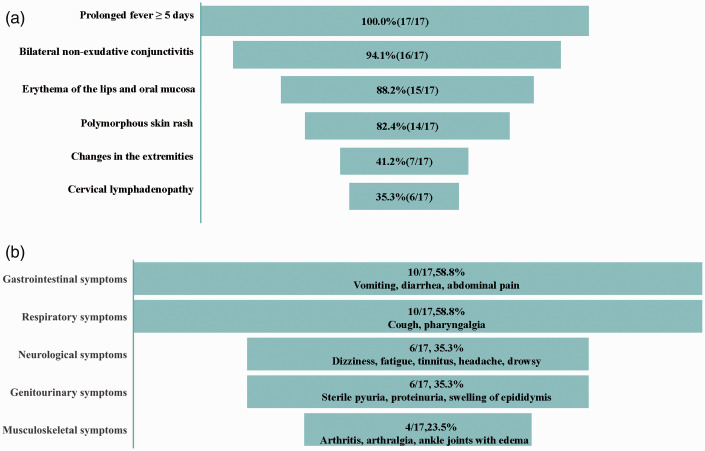
Of the 17 patients, 9 (52.9%) were diagnosed with incomplete KD. All patients had a prolonged fever for >5 days. Sixteen (94.1%) patients had bilateral non-exudative conjunctivitis, 15 (88.2%) had erythema of the lips and oral mucosa, 14 (82.4%) had a polymorphous skin rash, 7 (41.2%) had changes in the extremities, and 6 (35.3%) had cervical lymphadenopathy (Figure 2(a)). Remarkably, other atypical clinical findings were observed. The gastrointestinal system was the most commonly affected; gastrointestinal symptoms were found in 10 (58.8%) patients, presenting as vomiting (n = 7), diarrhea (n = 6), and abdominal pain (n = 3). Ten (58.8%) patients had respiratory symptoms, namely cough (n = 10) and pharyngalgia (n = 1). Neurological symptoms were also observed in six (35.3%) patients and included dizziness (n = 3), fatigue (n = 3), tinnitus (n = 1), headache (n = 1), and drowsiness (n = 1). The genitourinary system was affected in six (35.3%) patients, manifesting as sterile pyuria (n = 4), proteinuria (n = 3), and swelling of the right epididymis (n = 1). Additionally, four (23.5%) patients had musculoskeletal system involvement, including arthritis (n = 3), arthralgia (n = 2), and ankle joint edema (n = 1) (Figure 2(b)). The diagnosis of KD was only proposed in two patients at the initial stage of illness. The remaining patients were misdiagnosed as having other similar entities, including cervical lymphangitis (n = 4), tonsillitis (n = 2), respiratory tract infection (n = 2), infectious diarrhea (n = 2), bronchitis (n = 2), pyohemia (n = 1), pneumonia (n = 1), and intracranial infection (n = 1) (Table 1). Generally, the time interval from fever onset to the diagnosis of KD was 11.8 days.
Figure 2.
Typical and atypical clinical manifestations of KD in adolescents. (a) Typical clinical manifestations of KD in adolescents. (b) Atypical clinical findings of KD in adolescents. KD, Kawasaki disease.
One patient developed anaphylaxis after IVIG administration; the remaining 16 (94.1%) patients received IVIG (2 g/kg) and aspirin (30–50 mg/kg) after the diagnosis of KD. However, seven of these patients received the IVIG therapy at >10 days from fever onset. Four children presented with initial IVIG resistance, and two of them defervesced after additional IVIG infusion (1 g/kg). The other two children had a normal body temperature until receiving methylprednisolone pulse infusion (10 mg/kg/day for 3 days).
Ten patients had cardiac complications, including CALs (n = 8), valve regurgitation (n = 2), left ventricular dysfunction (n = 2), and first-degree atrioventricular block (n = 1) (Figure 1(d)). No cardiac enlargement or pericardial effusion was found. All cases of valve regurgitation, left ventricular dysfunction, and first-degree atrioventricular block resolved 2 months later. After a mean follow-up of 2 years, six of the eight children with CALs completely recovered to normal. One patient (Patient 11) developed right coronary artery dilation (5.7 mm) at the initial stage of illness, and the dilation further progressed to 7.9 mm and segmental stenosis at the latest follow-up. The left coronary artery of another patient (Patient 10) was found to be dilated (8.2 mm) on admission but gradually decreased to 6.7 mm 11 months later.
There was no significant difference in the baseline clinical and laboratory data between Group A and Group B. More patients in Group B came from rural areas (90.0% vs. 14.3%, P = 0.004), where the population’s medical condition was relatively poor and pediatricians were more unlikely to be aware of the possibility of KD in adolescents. Indeed, although the incidence of typical KD was higher in Group B than Group A (60.0% vs. 29.6%), Group B had a higher overall misdiagnosis rate at the initial stage (100.0% vs. 71.4%) as well as a longer average fever duration on arrival (13.8 days; range, 6.0–35.0 days vs. 5.0 days; range, 2.0–7.0 days; P = 0.013) and before IVIG administration (15.3 days; range, 10.0–38.0 days vs. 7.1 days; range, 5.0–10.0 days; P = 0.032). All patients in Group A were treated with IVIG within 10 days from fever onset, whereas only 3 of 10 patients in Group B received IVIG therapy in a timely manner. The rate of IVIG resistance and steroid use did not differ between the two groups. Cardiac complications including CALs, valve regurgitation, left ventricular dysfunction, and arrhythmia were more common in Group B (60.0% vs. 57.1%) (Table 2). Only the CALs of two children in Group B (Patients 10 and 11) did not resolve and even became more severe during follow-up.
Table 2.
Demographic data and clinical characteristics of adolescents with Kawasaki disease.
| Group Aa | Group Bb | P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Patients, n | 7 | 10 | – |
| Sex, male:female | 7:0 | 6:4 (1.5:1.0) | 0.103 |
| Age, years | 11.5 (10.1–13.1) | 12.3 (10.9–13.5) | 0.120 |
| From urban areas/rural areas | 6/1 | 1/9 | 0.004* |
| Total fever duration, days | 9.0 (6.0–13.0) | 15.6 (6.0–39.0) | 0.044* |
| Fever duration on admission, days | 5.0 (2.0–7.0) | 13.8 (6.0–35.0) | 0.013* |
| Fever duration before IVIG, days | 7.1 (5.0–10.0) | 15.3 (10.0–38.0) | 0.032* |
| ≥10 days from fever onset to IVIG therapy | 1 (14.3) | 7 (70.0) | 0.060 |
| IVIG therapy | 7 (100.0) | 9 (90.0) | <0.001* |
| Failure to respond to initial IVIG therapy | 2 (28.6) | 2 (22.2) | 1.000 |
| Intravenous and/or oral steroid treatment | 2 (28.6) | 1 (11.1) | 0.550 |
| Incomplete Kawasaki disease | 5 (71.4) | 4 (40.0) | 0.335 |
| Cardiac complication | 4 (57.1) | 6 (60.0) | 1.000 |
| Coronary artery lesions | 3 (42.9) | 5 (50.0) | 1.000 |
| Valve regurgitation | 2 (28.6) | 0 (0.0) | 0.154 |
| Left ventricular dysfunction | 1 (14.3) | 1 (10.0) | 1.000 |
| Laboratory features | |||
| C-reactive protein, mg/L | 136.6 (18.0–298.0) | 89.2 (5.0–179.0) | 0.204 |
| White blood cell count, ×109/L | 15.8 (10.7–25.4) | 13.2 (4.6–29.7) | 0.485 |
| Erythrocyte sediment rate, mm/hour | 39.1 (22.0–100.0) | 60.4 (21.0–111.0) | 0.207 |
| Platelet, ×109/L | 216.7 (112.0–272.0) | 282.0 (152.0–495.0) | 0.157 |
| Hemoglobin, g/L | 124.4 (113.0–136.0) | 113.1 (96.0–134.0) | 0.077 |
| Alanine transaminase, U/L | 58.4 (20.0–100.0) | 52.0 (17.0–96.0) | 0.655 |
| Aspartate transaminase, U/L | 35.6 (14.0–50.0) | 41.6 (15.0–78.0) | 0.526 |
| Albumin, g/L | 30.0 (22.9–34.0) | 33.6 (23.6–39.7) | 0.956 |
| Total bilirubin, µmol/L | 9.6 (6.6–13.1) | 7.5 (3.1–18.1) | 0.308 |
| LDH, U/L | 351.4 (152.0–562.0) | 359.2 (129.0–728.0) | 0.933 |
| Na+, mmol/L | 137.9 (130.0–151.0) | 136.4 (128.0–143.7) | 0.286 |
Data are presented as n, n (%), or mean (range).
Differences in quantitative data between Group A and Group B were determined by an independent-samples t-test. Fisher’s exact test was applied for comparison. *P < 0.05.
aGroup A: first hospitalization was at our hospital.
bGroup B: transferred from their local hospital or community health center.
IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; Na+, serum sodium.
Discussion
The estimated incidence of KD in adolescents is reportedly significantly lower (0.10–1.45 per 100,000 population) than that of KD in patients of other ages, 9 which is consistent with the findings in our study (with a low constituent ratio of 0.8%). Therefore, most pediatricians, particularly from undeveloped areas, lack recognition and awareness of KD in this age group and may not even consider the diagnosis of KD in adolescents. A study of physicians’ practices in diagnosing KD in the United States showed that ≥50% of general pediatricians and 25% of infectious disease specialists always ignore the possibility of KD in children >8 years of age. 5 The present study is the largest series of adolescent-onset KD in China to date and has summarized all available literature reporting KD in adolescents. As shown in Table 3, the four largest series of adolescent-onset KD merely reported 46, 28, 17, and 10 cases from India, 14 the United States, 15 Indonesia, 16 and Canada, 17 respectively. Another 18 cases were documented in other case reports.18–33 Among the 136 reported cases, including ours, the oldest patient was 17 years old,27,32 suggesting that KD can affect patients of any age because adult-onset KD in patients from 18 to 68 years of age has also been previously identified. KD in adolescents is more likely to affect male patients. The incidence of incomplete KD was 52.9% in our study cohort and 46% to 59% in previous studies,15,16 which is much higher than that in the general KD population. 34 Most patients were misdiagnosed as having other conditions, such as cervical lymphangitis, and the mean duration from disease onset to diagnosis of KD was 10.8 days (range, 4–38 days). Accordingly, IVIG infusion was delayed (>10 days from fever onset) in most patients (30%–61%).15,16 Notably, 47.1% to 77.8% of patients had coronary artery abnormalities, 17 which most likely resulted from the delayed diagnosis and treatment. These findings strongly suggest that the recognition and awareness of KD in adolescents are extremely limited worldwide, and further studies are needed to clarify the clinical profiles of these patients.
Table 3.
Summary of patients with adolescent-onset KD reported in the literature.
| First author, year | Age (years)/ Sex | Fever duration, daysa | Primary diagnosis | iKD | Treatment | IVIG resistance | Coronary artery lesions | Prognosis |
|---|---|---|---|---|---|---|---|---|
| Bello, 24 1995 | 15/M | 6/8 | Pneumonia | No | IVIG, aspirin, dipyridamole | No | Diffuse CAD 2 weeks later | – |
| Davies, 28 1996 | 13/M | 10/14 | TSS | No | IVIG, aspirin | No | CAD (RCA 4 mm, LCA 5 mm, LAD 3 mm) | – |
| Von Xylander, 22 1997 | 14/M | 18/21 | Streptococcus infection | Yes | Prednisone, salicylate | – | CAA (LAD 8 mm, LCA 8 mm, RCA 7 mm) at 3 weeks | Persistent aneurysm of LCA 3 years later |
| Callaway, 18 2002 | 15/M | 2/12 | Anterior uveitis | No | IVIG, aspirin | No | Left ventricular systolic dysfunction | Normal |
| Marchetto, 26 2004 | 15/M | 5/9 | –c | Yes | IVIG, aspirin | No | Normal | Recurrent KD 9 days later |
| Yang, 20 2006 | 11.75/M | 7/7 | – | Yes | IVIG, aspirin, intravenous methylprednisolone | No | CAD (LCA 4.5 mm, RCA 2.8 mm) | CAA 2 weeks later |
| Guven, 23 2010 | 12/F | 4/4 | – | No | IVIG, aspirin, dexamethasone | CAD (LCA 5.6–5.8 mm) | Normal 4 weeks later | |
| Hadid, 29 2011 | 13/M | 9/− | Cytomegalovirus hepatitis | Yes | No treatment | – | CAA 1 year later | CABG performed and ICD inserted |
| Hyams, 30 2012 | 15/M | 9/21 | Vasculitis, infection | Yes | IVIG, aspirin, prednisolone | Yes | CAD (RCA 4.6 mm, LCA 4.7 mm, LAD 4.2 mm, LCX 4.2 mm) | – |
| Yamauchi, 21 2012 | 16/F | 4/12 | Acute myocarditis | Yes | IVIG, aspirin | No | CAA (LAD 10 mm) | Aneurysm and occlusion of CAA 1 year later |
| Fradin, 19 2013 | 16/M | 6/9 | – | Yes | IVIG, aspirin | No | CAD (Z-score 2.9) at 9 days after fever onset | CAD (Z-score 3.0) |
| Sileikiene, 27 2013 | 17/M | 12/13 | Pneumonia, pleurisy | No | IVIG, aspirin, steroid, infliximab, warfarin | Yes | CAA (LCA 8 mm, RCA 7 mm) | LCA 7.8 mm, LAD 14.4 mm, RCA 8.6 mm 6 months later |
| Sinhabahu, 25 2016 | 12/F | 3/8 | Septic shock | Yes | IVIG, aspirin | No | CAD (LAD 3.5 mm) | Dilated LAD 2 months later |
| Momenah, 17 1998 | ≥9/(M:F, 5:5) | – | – | 0 | IVIG, aspirin | 2/10 | CAD (6/10), CAA (2/10) | – |
| Stockheim, 15 2000 | ≥8/(M:F, 20:8)b | 9/10.5 | – | 46% | IVIG, aspirin | 1/28 | CAA (6/28, all were male) | Remaining CAA in two patients |
| Advani, 16 2019 | ≥10/(M:F, 14:3) | – | – | 59% | IVIG, aspirin | – | CAD (8/17) | – |
| Jindal, 14 2020 | ≥10/(M:F, 26:20) | 10/− | – | IVIG, aspirin | 5/46 | CAA (6/46) | – | |
| Silveira, 31 2021 | 16/F | 7/14 | Influenza B infection | No | IVIG, aspirin | Yes | Normal | Normal |
| El Haddar 33 , 2021 | 11/M | 10/17 | Typhoid fever | Yes | IVIG, aspirin | No | CAD | Normal 3 months later |
| Sakai 32 , 2021 | 17/F | 7/− | Acute tonsillitis | No | Infliximab | – | CAA | Normal |
| 17/F | 2/6 | Viral infection | Yes | IVIG | No | Normal | Normal | |
| 16/M | 2/− | – | No | IVIG | No | Normal | Normal |
aFever durations on admission and before IVIG.
bThere were 28 patients with KD aged ≥8 years in that study, including 13 patients aged >10 years (range, 10–15 years).
cNot mentioned in the study.
KD, Kawasaki disease; iKD, incomplete Kawasaki disease; IVIG, intravenous immunoglobulin; ICD, implantable cardioverter defibrillator; M, male; F, female; CAD, coronary artery dilation; CAA, coronary artery aneurysm; LCA, left coronary artery; LCX, left circumflex artery; LAD, left anterior descending artery; RCA, right coronary artery; CABG, coronary artery bypass grafting; TSS, toxic shock syndrome.
There are two possible reasons for KD in adolescents being usually diagnosed and treated later than in patients of other ages. First, some reports have indicated that older children, who are more likely to present with atypical clinical manifestations, have a higher incidence of incomplete KD. In older patients, the typical clinical presentations are often dispersed throughout the course of KD, in contrast to the close clustering of presentations seen in younger patients. In our cohort, nine (52.9%) patients did not fulfill the diagnostic criteria of classic KD. Their clinical presentations were dispersed throughout the course of KD; the criteria may have been met only fleetingly and therefore missed. In contrast, the primary clinical manifestations were variable and nonspecific in our adolescent patients with KD (i.e., cough, vomiting, abdominal pain, diarrhea, fatigue, and drowsiness), similar to previous studies.4,17,19,24,35–37 This has commonly led to misdiagnosis of KD as other infectious diseases such as tonsillitis, respiratory tract infection, lymphadenitis, pneumonia, infectious diarrhea, and intracranial infection.
The second possible reason why KD in adolescents is usually diagnosed and treated later than in patients of other ages is that many pediatricians are subjectively unfamiliar with the clinical features of KD in adolescents and are reluctant to consider KD, particularly those from underdeveloped areas. General pediatricians, especially primary care physicians, mainly focus on only one or two signs of illness, even in patients with typical clinical features of KD; namely, a diagnosis of cervical lymphadenopathy causing fever. The present study suggests that even with the similar baseline information and laboratory data between patients in Groups A and B, patients from Group B (who were transferred from a local hospital or community health center) had a longer fever duration on admission and before IVIG treatment despite them being more likely to present with typical KD than patients in Group A. Seven (41.2%) patients had received IVIG therapy at >10 days from fever onset, and all seven of these patients were transferred from their local hospital or community health center, where pediatricians were more likely to be familiar with common diseases and rarely deal with KD, especially in older children. Notably, one patient from Group B was considered to have an infectious disease and was finally diagnosed with KD only after a fever duration of 38 days, even with classic clinical manifestations of KD at the initial stage of illness.
A new syndrome known as multisystem inflammatory syndrome in children (MIS-C), characterized by a systemic inflammatory status that seems to be related to SARS-CoV-2 infection, was recently identified.33,38,39 It is essential to distinguish adolescent-onset KD from MIS-C during the COVID-19 pandemic because several common symptoms are common to both KD and MIS-C, and MIS-C predominantly affects older children. 40 Moreover, the prognosis of MIS-C is worse than that of KD. The mortality rate of MIS-C is 1.7% in the United States and 1.4% in Europe, 41 which is much higher than the mortality rate of 0.01% reported in children with KD. 40 Although MIS-C seems to significantly overlap with KD, these two syndromes appear to have some distinct differences, including racial and ethnic disparities, clinical manifestations, cardiac involvement, and inflammatory markers. First, the incidence of KD is significantly higher in East Asia34,42 but lower in the United States and Europe. 1 In contrast to KD, MIS-C has mostly been reported in Europe and the United States. 40 Intriguingly, many fewer cases have been reported in Asian countries.34,42 To date, no cases of MIS-C have been reported in China or Japan. 40 Underlying genetic discrepancies are considered to be a factor resulting in these epidemiologic differences. Second, the underlying mechanisms of hyperinflammation differ between KD and MIS-C. In KD, interleukin-1 (IL-1) has direct inflammatory effects on coronary endothelial cells, 1 whereas in MIS-C, the myocardial dysfunction and higher severity of SAR-CoV-2 infection are predominantly driven by IL-6 and IL-10. 43 Third, compared with KD, patients with MIS-C likely develop a higher incidence of gastrointestinal symptoms, multisystem organ involvement, lower levels of platelet and lymphocytes, and higher ferritin and procalcitonin levels. 43
The symptoms of MIS-C have greater resemblance to those of macrophage activation syndrome. Finally, unlike in classic KD, myocardial dysfunction is common in MIS-C. A recent study showed that 80% of patients with MIS-C who developed acute left ventricular failure required inotropic support, and 28% received extracorporeal membrane oxygenation support. 39 In contrast, coronary artery involvement, which is the cardiac hallmark of KD, is less common in MIS-C. In a case series of 503 patients with MIS-C, the incidence of coronary artery aneurysms was only 13%, and 93% of these aneurysms were mild; no large or giant aneurysm were observed. 44 Overall, altough it is challenging to distinguish these two syndromes because of their overlapping clinical presentations and the lack of a specific diagnostic test for either MIS-C or KD, 40 the aforementioned points provide some references for clinical management. Pediatricians should bear in mind that MIS-C must be regarded as a differential diagnosis for adolescent-onset KD, particularly for children with an epidemiological history of COVID-19.
This study must be viewed in light of some potential limitations inherent in its retrospective design. Additionally, the prevalence of adolescent-onset KD in our study compared with other series may naturally reflect some selection bias. Because our hospital is the largest children’s medical center in Southwest China, we encounter a greater proportion of complicated cases of KD, and the nature of KD in adolescents suggests that these patients would be transferred to our hospital.
Conclusions
The constituent ratio of KD in adolescents was relatively low. Their clinical features were atypical, diverse, and frequently misdiagnosed. The high incidence of misdiagnosis might be associated with atypical clinical manifestations and pediatricians’ poor recognition, leading to a subsequent delay of therapy or administration of non-standardized IVIG treatment and a higher occurrence of CALs in this age spectrum. For adolescents with a prolonged fever of >5 days, the diagnosis of KD should still be highly suspected. Primary care physicians must be aware of the occurrence of KD in adolescents.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Kaiyu Zhou https://orcid.org/0000-0002-4783-4243
Chuan Wang https://orcid.org/0000-0001-5165-8273
References
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135: e927–e999. [DOI] [PubMed] [Google Scholar]
- 2.Taubert KA Rowley AH andShulman ST.. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr 1991; 119: 279–282. [DOI] [PubMed] [Google Scholar]
- 3.Durongpisitkul K, Gururaj VJ, Park JM, et al. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics 1995; 96: 1057–1061. [PubMed] [Google Scholar]
- 4.Manlhiot C, Yeung RSM, Clarizia NA, et al. Kawasaki Disease at the extremes of the age spectrum. Pediatrics 2009; 124: e410. [DOI] [PubMed] [Google Scholar]
- 5.Pannaraj PS, Turner CL, Bastian JF, et al. Failure to diagnose Kawasaki disease at the extremes of the pediatric age range. Pediatr Infect Dis J 2004; 23: 789–791. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Agarwal S, Bhattad S, et al. Kawasaki disease in infants below 6 months: a clinical conundrum? Int J Rheum Dis 2016; 19: 924–928. [DOI] [PubMed] [Google Scholar]
- 7.Chang FY, Hwang B, Chen SJ, et al. Characteristics of Kawasaki disease in infants younger than six months of age. Pediatr Infect Dis J 2006; 25: 241–244. [DOI] [PubMed] [Google Scholar]
- 8.Lee KY, Hong JH, Han JW, et al. Features of Kawasaki disease at the extremes of age. J Paediatr Child Health 2006; 42: 423–427. [DOI] [PubMed] [Google Scholar]
- 9.Kim GB, Park S, Eun LY, et al. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012-2014. Pediatr Infect Dis J 2017; 36: 482–485. [DOI] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 11.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004; 114: 1708–1733. [DOI] [PubMed] [Google Scholar]
- 12.Bayers S Shulman ST andPaller AS.. Kawasaki disease: Part II. Complications and treatment. J Am Acad Dermatol 2013; 69: 513.e511-513.e518. [DOI] [PubMed] [Google Scholar]
- 13.Group JJW. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Circ J 2014; 78: 2521–2562. [DOI] [PubMed] [Google Scholar]
- 14.Jindal AK, Pilania RK, Guleria S, et al. Kawasaki disease in children older than 10 years: a clinical experience from Northwest India. Front Pediatr 2020; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockheim JA Innocentini N andShulman ST.. Kawasaki disease in older children and adolescents. J Pediatr 2000; 137: 250–252. [DOI] [PubMed] [Google Scholar]
- 16.Advani N Santoso LA andSastroasmoro S.. Profile of Kawasaki disease in adolescents: is it different? Acta Med Indones 2019; 51: 42–46. [PubMed] [Google Scholar]
- 17.Momenah T, Sanatani S, Potts J, et al. Kawasaki disease in the older child. Pediatrics 1998; 102: e7. [DOI] [PubMed] [Google Scholar]
- 18.Callaway LK andBennett CJ.. Adolescent Kawasaki disease with uveitis. Intern Med J 2002; 32: 421–422. [DOI] [PubMed] [Google Scholar]
- 19.Fradin KN andRhim HJ.. An adolescent with fever, jaundice, and abdominal pain: an unusual presentation of Kawasaki disease. J Adolesc Health 2013; 52: 131–133. [DOI] [PubMed] [Google Scholar]
- 20.Yang SW Qin YM andCao LM.. [A case of adolescent Kawasaki disease with coronary artery aneurysm, shock and multi-organ dysfunction]. Zhonghua Er Ke Za Zhi 2006; 44: 917. [PubMed] [Google Scholar]
- 21.Yamauchi A, Nakagawa N, Kawamura Y, et al. Incomplete Kawasaki disease manifesting as transient heart failure in a previously healthy adolescent. Intern Med 2012; 51: 2169–2173. [DOI] [PubMed] [Google Scholar]
- 22.Von Xylander S, Mudra H, Rieber J, et al. Intravascular ultrasonography of an adolescent boy with a coronary artery aneurysm due to Kawasaki disease. Pediatr Cardiol 1997; 18: 437–439. [DOI] [PubMed] [Google Scholar]
- 23.Guven B, Tavli V, Mese T, et al. Isolated abducens palsy in adolescent girl with Kawasaki disease. Pediatr Int 2010; 52: 334. [DOI] [PubMed] [Google Scholar]
- 24.Bello MB. Kawasaki disease in a 15-year-old adolescent. J Am Board Fam Pract 1995; 8: 465–468. [PubMed] [Google Scholar]
- 25.Sinhabahu VP Suntharesan J andWijesekara DS.. Kawasaki shock syndrome in a 12-year-old girl mimicking septic shock. Case Rep Infect Dis 2016; 2016: 4949036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchetto S, Chiappini E, Simonini G, et al. Lupus-like onset of recurrent Kawasaki disease in an adolescent boy. Clin Exp Rheumatol 2004; 22: 377. [PubMed] [Google Scholar]
- 27.Sileikiene R, Kudzyte J, Jankauskas A, et al. Rare refractory Kawasaki disease in an adolescent boy with cardiac and diffuse coronary artery involvement. Medicina (Kaunas) 2013; 49: 341–345. [PubMed] [Google Scholar]
- 28.Davies HD, Kirk V, Jadavji T, et al. Simultaneous presentation of Kawasaki disease and toxic shock syndrome in an adolescent male. Pediatr Infect Dis J 1996; 15: 1136–1138. [DOI] [PubMed] [Google Scholar]
- 29.Hadid D, Plambeck CJ, Nicolosi AC, et al. Sudden cardiac death resulting from acute coronary artery aneurysm occlusion: successful resuscitation and treatment of an adolescent boy with previously unrecognized Kawasaki disease. Int Anesthesiol Clin 2011; 49: 42–51. [DOI] [PubMed] [Google Scholar]
- 30.Hyams C, Day TG, Ramroop S, et al. An unusual case of incomplete Kawasaki disease in an adolescent returning from holiday in Montana. Pediatr Cardiol 2012; 33: 1196–1199. [DOI] [PubMed] [Google Scholar]
- 31.Silveira JO, Pegoraro MG, Ferranti JF, et al. Influenza B infection and Kawasaki disease in an adolescent during the COVID-19 pandemic: a case report. Rev Bras Ter Intensiva 2021; 33: 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai H, Minami-Hori M, Shimamura T, et al. Kawasaki disease in adolescence and adulthood: Report of four cases. J Dermatol 2021. [DOI] [PubMed] [Google Scholar]
- 33.El Haddar Z, El Ouali A, Ghanam A, et al. Atypical Kawasaki disease in an adolescent with multivisceral involvement. Case Rep Pediatr 2021; 2021: 8941847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukazawa R, Kobayashi J, Ayusawa M, et al. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J 2020; 84: 1348–1407. [DOI] [PubMed] [Google Scholar]
- 35.Binder E, Griesmaier E, Giner T, et al. Kawasaki disease in children and adolescents: clinical data of Kawasaki patients in a western region (Tyrol) of Austria from 2003–2012. Pediatr Rheumatol Online J 2014; 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Z Zuo R andLiu Y.. Characteristics of Kawasaki disease in older children. Clin Pediatr (Phila) 2011; 50: 952–956. [DOI] [PubMed] [Google Scholar]
- 37.Granel B, Serratrice J, Ene N, et al. Painful jaundice revealing Kawasaki disease in a young man. J Gastroenterol Hepatol 2004; 19: 713–715. [DOI] [PubMed] [Google Scholar]
- 38.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395: 1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142: 429–436. [DOI] [PubMed] [Google Scholar]
- 40.Lee MS, Liu YC, Tsai CC, et al. Similarities and differences between COVID-19-related multisystem inflammatory syndrome in children and Kawasaki disease. Front Pediatr 2021; 9: 640118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaushik A, Gupta S, Sood M, et al. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J 2020; 39: e340–e346. [DOI] [PubMed] [Google Scholar]
- 42.Chen JJ, Ma XJ, Liu F, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J 2016; 35: 7–12. [DOI] [PubMed] [Google Scholar]
- 43.Shulman ST. Pediatric Coronavirus Disease-2019-associated multisystem inflammatory syndrome. J Pediatric Infect Dis Soc 2020; 9: 285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325: 1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]