Abstract
Objective
Inflammation is an important factor in the pathological process of cerebral ischemia. Artesunate exhibits a broad range of anti-inflammatory properties in many diseases. We investigated the potential protective effect of artesunate against cerebral ischemia and the related mechanisms.
Methods
Mice were divided into distal middle cerebral artery occlusion (dMCAO), sham, low dose, and high dose groups and subjected to dMCAO, except for the sham group. The low and high dose groups were administered artesunate (15 and 30 mg/kg), and the neuroprotective effects were analyzed by evaluating infarct volumes and neurological deficits. Microglial activation and neutrophil infiltration were evaluated by immunofluorescence, immunohistochemical staining, and western blotting. Inflammatory mediators were measured by enzyme-linked immunosorbent assays. Nuclear factor (NF)-κB nuclear translocation was detected by immunofluorescence and western blotting.
Results
Compared with the dMCAO group, artesunate significantly improved neurological deficit scores and infarct volumes and ameliorated inflammation by reducing neutrophil infiltration, suppressing microglial activation, and downregulating tumor necrosis factor-α and interleukin-1β expression. Furthermore, artesunate inhibited nuclear translocation of NF-κB and inhibitor protein α proteolysis.
Conclusions
Artesunate protected against inflammatory injury by reducing neutrophil infiltration and microglial activation, suppressing inflammatory cytokines, and inhibiting the NF-κB pathway. Therefore, artesunate is a potential ischemic stroke treatment.
Keywords: Artesunate, ischemic stroke, inflammation, NF-κB, microglia, neuroprotective effect
Introduction
Ischemic cerebrovascular disease is one of the most common causes of disability and death. Both secondary damage caused by neuroinflammation and primary damage following arterial occlusion can cause brain damage. Abundant evidence has shown that neuroinflammation has a long therapeutic time window. Therefore, control of inflammation might be a promising therapeutic target. Cerebral ischemia activates endogenous immune cells (such as microglia) and induces the adhesion and infiltration of leukocytes.1,2 These activated inflammatory cells generate various pro-inflammatory cytokines including interleukin (IL)-1 and tumor necrosis factor (TNF)-α, which can lead to cellular damage and death. Nuclear factor (NF)-κB is an important transcription factor in inflammatory processes. NF-κB can be activated by IL-1, TNF-α, oxidative stress, and hypoxia. During cerebral ischemia, NF-κB is activated in neurons, endothelial cells, astrocytes, neutrophils, and microglia.3–5 Activated NF-κB regulates numerous genes, including TNF-α, IL-6, IL-1β, intercellular adhesion molecule 1, matrix metallopeptidase 9, and inducible nitric oxide synthase,3,4 which can increase inflammatory damage.
Artesunate is recommended for treatment of cerebral and other types of severe malaria by the World Health Organization Guidelines. 6 It is a derivative of artemisinin and has high water solubility and thus can pass through the blood-brain barrier. Artesunate can also be maintained at a high concentration in the nervous system and exhibits high efficacy and low toxicity.7–10 Increasing evidence has shown that artesunate may exert multiple functions including anti-inflammatory, immune regulatory, blood-brain barrier protective, antimicrobial, and antitumor effects.9,10 Some anti-inflammatory effects of artesunate were reported to be mediated by NF-κB and inflammatory cytokine suppression. A study by Lai et al. reported that artesunate could alleviate hepatic fibrosis and inflammation by inhibiting the lipopolysaccharide/toll-like receptor 4/NF-κB pathway. 11 In another study by Okorji and Olajide, artesunate was shown to decrease pro-inflammatory cytokine production by inhibiting the p38 MAPK-NF-κB signaling pathway in activated BV2 microglia. 12 Artesunate also exerted protective effects In cerebral ischemia/reperfusion injury and suppressed TNF-α and IL-1β expression. 13
Artesunate may act as a potential anti-neuroinflammatory agent by inhibiting the NF-κB pathway in diseases involving cerebral ischemia. We aimed to investigate whether artesunate had neuroprotective and anti-inflammatory effects in a mouse model of distal middle cerebral artery occlusion (dMCAO) and explore the potential mechanisms of these effects.
Methods
Animals and the dMCAO model
Vital River (Beijing Vital River Laboratory Animal Technology, China) provided specific pathogen-free grade adult male C57BL/6 mice. The experimental animals were housed in controlled conditions with 12-hour light/dark cycles, a temperature of 22 ± 3°C, and 60 ± 5% humidity. The mice were housed for at least 1 week before the experiment. All mice had ad libitum access to water and a standard rodent diet. The experimental procedures were approved by the Experimental Animal Ethics Committee of Hebei Medical University (Shijiazhuang, China, Permit No. HMUSHC-130318). All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edition) and the ARRIVE guidelines. 14
Focal cerebral cortical ischemia was induced in mice, with the body temperature maintained at 37 ± 0.5°C. After anesthesia was induced with isoflurane (induction dose 3%; maintenance dose 1.5%), the right common carotid artery was ligated, and the right middle cerebral artery distal to the striatal branch was coagulated with a cauterizer. 15 The exclusion criteria were as follows: cerebral hemorrhage or subarachnoid hemorrhage during the operation, death before the experimental observation time point, or recanalization after two electrocoagulation attempts. None of the mice exhibited these symptoms in our study. Sham-operated mice were operated in the same manner, except occlusion of the distal middle cerebral artery and common carotid artery was not performed.
Groups and drug administration
A total of 168 mice were divided into four groups with a random number table that was generated with IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA), including the dMCAO group (42 mice), sham group (42 mice), low dose group (42 mice), and high dose group (42 mice). Seven groups of mice were used for different studies, including 2,3,5-triphenyltetrazolium chloride (TTC) staining (n = 6), the rotarod test (n = 6), immunofluorescence staining (n = 6), a myeloperoxidase (MPO) activity assay (n = 6), western blotting (n = 6), an enzyme-linked immunosorbent assay (ELISA) (n = 6), and immunohistochemistry (n = 6). All efforts were made to minimize animal suffering and the number of animals used.
Artesunate (Meilun Biotechnology Co. Ltd, Dalian, China) was dissolved in 5% sodium bicarbonate solution at a concentration of 100 mg/mL. After the drug was completely dissolved, 0.9% sodium was added to dilute the concentration to 2 mg/mL. Artesunate (15 and 30 mg/kg, with saline added to produce a total volume of 0.5 mL) was administered once daily to the low dose and high dose groups, respectively, by intraperitoneal injection immediately after cerebral ischemia. The same volume of saline was injected in the dMCAO and sham groups. At 72 hours after cerebral ischemia, mice were sacrificed by rapid decapitation under deep anesthesia. Samples were collected for further studies. Investigators who were blinded to the experimental group assignment evaluated the results.
Infarct volume measurement
Mice were euthanized at 72 hours after cerebral ischemia was induced. The brains were then removed and dissected into four 2-mm coronal sections, which were stained with 2% TTC. Normal tissues were stained red, and the infarcted areas exhibited a pale color. The infarct volume was calculated using the following formula: % hemisphere lesion volume = (total infarct volume − (ipsilateral hemisphere volume − contralateral hemisphere volume))/contralateral hemisphere volume. The volume was calculated with ImageJ software (National Institutes of Health, Bethesda, MD, USA, RRID: SCR_003070).
Rotarod test
Sensorimotor deficits of the mice were evaluated using the rotarod test. An examiner who was blinded to the experimental groups administered the test. Before the test, mice were placed on the rotating rod and practiced the procedure three times a day. All mice were trained 5 days before the operation. After cerebral ischemia was induced, mice were tested on rotating rods that accelerated from 4 rpm to 40 rpm in 4 minutes. The results were recorded as the latency until falling from the rod.
Immunofluorescence
After perfusion with 0.01 M phosphate-buffered saline, mice were transcardially perfused with 4% paraformaldehyde. First, mouse brains were placed in 30% sucrose for approximately 24 hours at 4°C. Then, the brains were cut into 30-μm coronal sections. Coronal slices were obtained from the center of the ischemic tissue (approximately −1.5 mm to +0.5 mm relative to bregma). Three slices were randomly selected from one mouse corresponding to every 10th coronal slice. 16 The brain slices were exposed to 0.3% Triton X-100 and incubated with blocking solution (10% donkey serum, Solarbio, Beijing, China) for 1 hour at room temperature. Then, the slices were incubated with primary antibodies, including rabbit anti-NF-κB (1:100, Bioworld Biotechnology, Nanjing, China) and goat anti-Iba1 (1:100, Abcam, Cambridge, MA, USA), overnight at 4°C. The following morning, the final incubation was conducted using secondary antibodies (1:500, Alexa Fluor 488 or 594, Jackson Immuno Research, West Grove, PA, USA). After counterstaining the nuclei with Hoechst stain, a laser scanning confocal microscope (LSM880, Zeiss, Gottingen, Germany) was used to capture immunofluorescence images. A researcher who was blinded to the experimental groups counted the cells in three peri-infarct regions. A peri-infarct region consisted of the cortical region (1 mm wide) around the core of the infarct region. 17 The cells that exhibited NF-κB in the nuclei were counted as NF-κB-positive cells.
Western blotting
The nuclear and cytosolic extracts used for western blotting were prepared from the peri-infarct region using a kit according to the manufacturer’s instructions (Applygen, Beijing, China). Cytosolic protein was prepared to detect inhibitor protein α (Iκ-Bα), MPO, and Iba-1. Nuclear protein was prepared to detect NF-κB. Bicinchoninic acid and protein assay reagent kits (Thermo Scientific, Rockford, IL, USA) were used to identify the protein contents of the samples. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed to separate the protein samples. Then, they were transferred to polyvinylidene difluoride membranes. Nonfat dried milk (5%) was used to block the membranes. The membranes were then incubated with primary antibodies, including rabbit anti-NF-κB (1:1000; Abcam), rabbit anti-Iκ-B (1:1000; Abcam), rabbit anti-MPO (1:1000; Abcam), rabbit anti-Iba1 (1:1000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000; Bioworld Technology), and rabbit anti-H3 (1:1000; Santa Cruz, CA, USA) overnight at 4°C. GAPDH and H3 were used as internal controls. On the following day, the membranes were incubated with fluorescently-labeled secondary antibodies (1:10,000, rabbit anti-goat, Rockland, Gilbertsville, PA, USA) for 1 hour. A researcher who was blinded to the experimental groups analyzed the relative density of each band using an imaging densitometer (LI-COR Biosciences UK Ltd., Cambridge, UK).
Immunohistochemical staining
Paraffin-embedded sections were used to histologically evaluate MPO expression at 72 hours after cerebral ischemia. The sections were incubated with anti-MPO antibodies (1:100 rabbit, Abcam). MPO-positive cells indicated infiltrating neutrophils. The secondary biotinylated conjugates, diaminobenzidine, and the secondary antibodies were supplied in a Vect ABC kit (Zhong Shan Biology Technology Company, Beijing, China). Cells exhibiting immunofluorescence were counted in three separate areas of the peri-infarct region from three slides per mouse by a researcher who was blinded to the experimental groups.
MPO activity assay
Seventy-two hours after cerebral ischemia was induced, brain tissues were collected from the peri-infarct region for an MPO activity assay. The brains were rinsed, weighed, and homogenized. The MPO activity in the homogenate was measured by spectrophotometry using a commercial diagnostic kit (A003, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
ELISA
The levels of the inflammatory cytokines TNF-α and IL-1β in brain homogenates from the peri-infarct region were measured using ELISAs, following the manufacturer’s instructions (Joyee Biotechnics, China), by a researcher who was blinded to the experimental groups.
Data analysis
IBM SPSS Statistics for Windows, version 21, was used for the statistical analysis. The data are presented as the mean ± SEM. One-way analysis of variance was used for data analysis. The Student–Newman–Keuls test was used for intergroup comparisons. A value of P < 0.05 was considered to be statistically significant.
Results
Artesunate reduced neurological deficits and the infarct volume
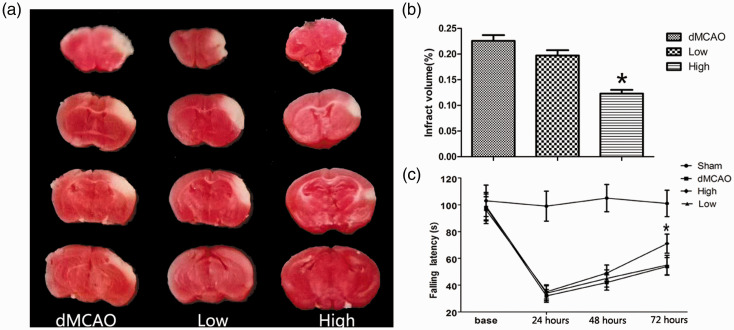
On the first day after cerebral ischemia, no difference was observed between the dMCAO and artesunate groups regarding the functional outcomes. At 72 hours after cerebral ischemia, the latency to fall times in the sham, dMCAO, low dose, and high dose groups were 99.83 ± 3.71, 50.5 ± 4.68, 57.33 ± 7.52, and 63.17 ± 6.65 s, respectively. The brain infarct sizes in the sham, dMCAO, low dose, and high dose groups were 0%, 23 ± 3%, 20 ± 3%, and 12 ± 2%, respectively. A significant decrease in the infarct volume was observed in the high dose group (P < 0.05, Figure 1a, b). The low dose group tended to have a lower volume, but the value did not reach statistical significance. Furthermore, mice treated with 30 mg/kg artesunate remained on the rotarod for a significantly longer time than those in the dMCAO group (P < 0.05, Figure 1c). These results indicate that 30 mg/kg artesunate improved the neurological recovery and decreased the infarct volume after cerebral ischemia.
Figure 1.
Artesunate ameliorated neurological deficits and the infarct volume. (a) 2,3,5-Triphenyltetrazolium chloride staining. (b) Infarct volumes of mice at 72 hours after distal middle cerebral artery occlusion (dMCAO) (*P < 0.05 vs the dMCAO group). (c) Rotarod test on the first, second, and third days after dMCAO (*P < 0.05 vs the dMCAO group).
Artesunate reduced post-ischemic neutrophil infiltration
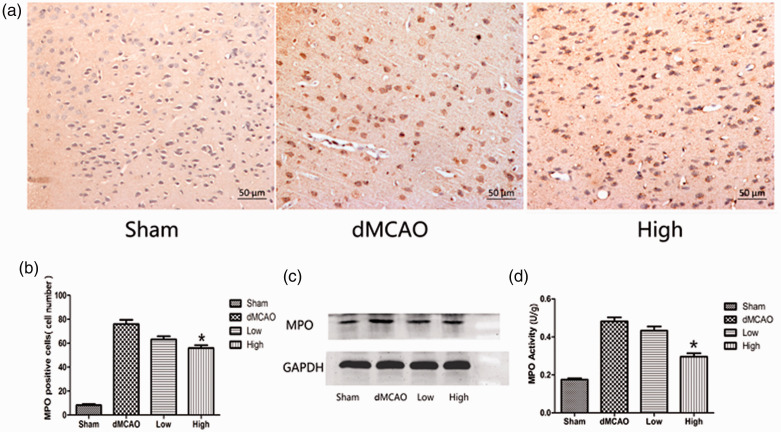
MPO is an important indicator of neutrophil accumulation and inflammation. The MPO activity, number of MPO-positive cells, and MPO expression were quantified in the ischemic cortex. The numbers of MPO-positive cells in the sham, dMCAO, low dose, and high dose groups were 8.33 ± 2.16, 76 ± 8.76, 63.17 ± 6.43, and 56 ± 5.87, respectively. The brains in the sham group exhibited little neutrophil infiltration. After cerebral ischemia, the number of MPO-positive cells was obviously increased. The number of MPO-positive cells was significantly lower in the group administered 30 mg/kg artesunate than that in the dMCAO group (P < 0.05, Figure 2a, b). This result was consistent with the western blotting analysis. Ischemia led to an increase in the MPO level in the right cortex, whereas this increase was attenuated by 30 mg/kg artesunate, (MPO/GAPDH density ratio: 0.46 ± 0.05 vs 0.7 ± 0.12, P < 0.05, Figure 2c). Meanwhile, administration of 30 mg/kg artesunate reduced MPO activity in the ischemic cortex compared with that in the dMCAO group (0.30 ± 0.04 vs 0.48 ± 0.05 U/g, P < 0.05, Figure 2d). In the group administered 15 mg/kg artesunate, MPO activity tended to be lower but did not reach statistical significance (0.43 ± 0.05 vs 0.48 ± 0.05 U/g).
Figure 2.
Effect of artesunate on myeloperoxidase (MPO) expression and activity. (a) MPO staining in the cerebral cortex in different groups 72 hours after cerebral ischemia (200× magnification). (b) Measurement of MPO-positive cells in the cerebral cortex in different groups after cerebral ischemia. (c) Western blotting analysis of MPO in the cerebral cortex in different groups. (d) Artesunate reduced MPO activity after cerebral ischemia. The bar graph shows MPO activity in the cerebral cortex in different groups after cerebral ischemia (*P<0.05 vs the distal middle cerebral artery occlusion (dMCAO) group).
Artesunate suppressed microglial activation
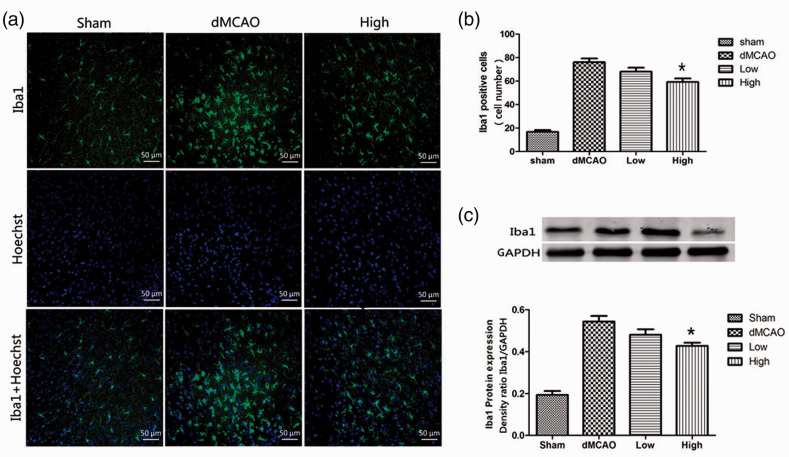
After ischemia, activated microglia transform into round amoeboid-like cells. In normal brains, microglia have a small soma and exhibit a ramified morphology. Immunofluorescence staining was performed to evaluate the morphological changes in microglia. Iba1-positive cells were counted to quantify microglia. Few microglia with a ramified morphology were detected in the sham group. At 72 hours after ischemia, the number of Iba1 positive cells in the sham, dMCAO, low dose, and high dose groups were 16.83 ± 3.49, 76.17 ± 7.7, 68.17 ± 8.01, and 59.33 ± 7.23, respectively, indicating that the number of microglia was significantly increased in the peri-infarct region after cerebral ischemia (P < 0.05). Furthermore, the majority of Iba1-positive cells had transformed into an amoeboid-like morphology. Compared with the dMCAO group, the number of Iba1-positive cells was decreased by administration of 30 mg/kg artesunate. Meanwhile, most of the Iba1-positive cells exhibited a ramified morphology (Figure 3a, b). The western blot results were consistent with those of immunofluorescence staining. After ischemia, Iba1 expression was obviously increased in the dMCAO group compared with that in the sham group (Iba1/GAPDH density ratio: 0.54 ± 0.03 vs 0.19 ± 0.02, P < 0.05). Artesunate (30 mg/kg) markedly reduced Iba1 expression (Iba1/GAPDH density ratio: 0.43 ± 0.01 vs 0.54 ± 0.03, P < 0.05, Figure 3c) compared with that in the dMCAO group, indicating that artesunate decreased microglial activation in the ischemic brain.
Figure 3.
Artesunate suppressed microglial activation induced by cerebral ischemia. (a) Brain sections were double‐stained with anti-Iba-1 (green) and Hoechst (blue) to label microglia in the cerebral cortex in different groups at 72 hours after distal middle cerebral artery occlusion (dMCAO). (b) Quantification of Iba1-immunopositive cells. *P < 0.05 vs the dMCAO group. (c) Western blotting analysis and quantification of the Iba1 protein level in the cerebral cortex in different groups (*P < 0.05 vs the dMCAO group).
Artesunate deregulated inflammatory cytokines
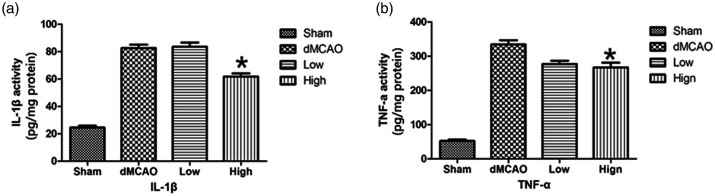
TNF-α and IL-1β are the most important inflammatory mediators at the onset of ischemia. The levels of both cytokines were measured via ELISAs. Compared with the sham group, the TNF-α (334.02 ± 30.96 vs 52.15 ± 10.59 pg/mg protein, P < 0.05) and IL-1β (82.64 ± 5.84 vs 24.57 ± 3.16 pg/mg protein, P < 0.05) levels in the dMCAO group were elevated. However, treatment with 30 mg/kg artesunate decreased TNF-α (267.24 ± 33.33 vs 334.02 ± 30.96 pg/mg protein, P < 0.05) and IL-1β (61.78 ± 5.55 vs 82.64 ± 5.84 pg/mg protein, P < 0.05) expression compared with that in the dMCAO group (Figure 4a, b). These results indicated that administration of 30 mg/kg artesunate ameliorated the inflammatory response after cerebral ischemia.
Figure 4.
Artesunate downregulated pro-inflammatory cytokines in the ischemic brain. The expression of interleukin (IL)-1β (a) and tumor necrosis factor (TNF)-α (b) in the cerebral cortex in different groups at 72 hours after distal middle cerebral artery occlusion (dMCAO) was detected by enzyme-linked immunosorbent assays (*P<0.05 vs the dMCAO group).
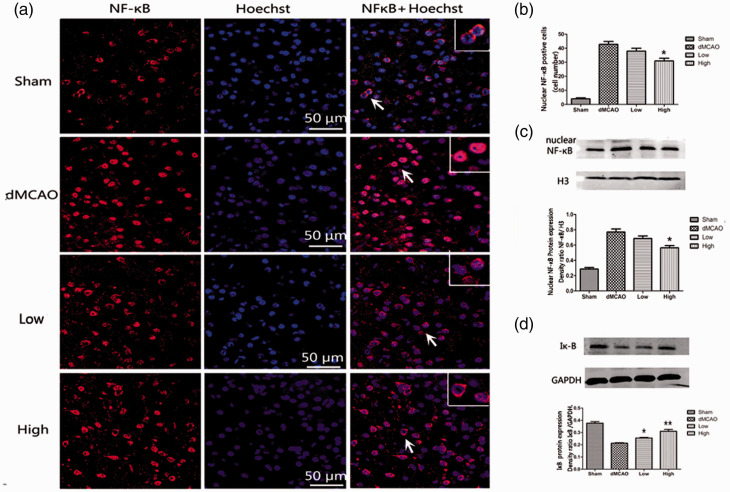
Artesunate inhibited the nuclear translocation of NF-κB
To observe the NF-κB p65 distribution, brain sections were double-stained with anti-NF-κB (red) antibodies and Hoechst (blue). The merged area indicated nuclear translocation. According to Figure 5a, NF-κB p65 was mostly present in the cytoplasm in the sham group. At 72 hours after ischemia, NF-κB p65 translocated into the nucleus. The distribution of NF-κB p65 in the nucleus was partly reversed by administration of 30 mg/kg artesunate according to a comparison with the dMCAO group (number of cells with NF-κB in the nucleus 31 ± 4.7 vs 42.83 ± 5, P < 0.05; Figure 5a, b). These results showed that artesunate inhibited translocation of NF-κB from the cytosol to the nucleus in the ischemic cortex.
Figure 5.
Artesunate inhibited the translocation of nuclear factor (NF)-κB. (a) Brain sections were stained with anti‐NF-κB (red) and Hoechst (blue) to label NF-κB and the nucleus, respectively. The merged area indicates active transcription. (b) Quantification of cells labeled with anti-NF-κB in the nucleus. (c) Western blotting analysis and quantification of the nuclear NF-κB protein level in the cerebral cortex in different groups at 72 hours after distal middle cerebral artery occlusion (dMCAO). (d) Western blotting analysis and quantification of the protein levels of cytosolic inhibitor protein alpha (Iκ-B) in different groups at 72 hours after dMCAO (*P < 0.05 vs the dMCAO group, **P < 0.001 vs the dMCAO group).
When NF-κB p65 is activated, Iκ-Bα, which binds to NF-κB and prevents its nuclear translocation, is degraded. In our study, we evaluated NF-κB protein in the nucleus and Iκ-B protein in the cytoplasm. Artesunate (30 mg/kg) produced an obvious decrease in the nuclear protein level of NF-κB compared with that in the dMCAO group (NF-κB/H3 density ratio: 0.56 ± 0.07 vs 0.77 ± 0.1, P < 0.05; Figure 5c). Meanwhile, we evaluated the impact of artesunate on Iκ-B degradation by western blotting analysis. As shown in Figure 5d, the Iκ-B level was markedly decreased in the dMCAO group compared with that in the sham group. Artesunate at doses of 15 mg/kg (Iκ-B/GAPDH density ratio: 0.26 ± 0.01 vs 0.21 ± 0.01, P < 0.05) and 30 mg/kg (Iκ-B/GAPDH density ratio: 0.31 ± 0.04 vs 0.21 ± 0.01, P < 0.001) markedly increased the Iκ-B level compared with that in the dMCAO group. In brief, this result showed that artesunate decreased the degradation of Iκ-B in the cytoplasm and suppressed the increase in NF-κB in the nucleus.
Discussion
Numerous studies have shown that artesunate has substantial protective effects against neurological disorders, including anti-neuroinflammatory, anti-parasite, anti-tumor, and anti-microbial effects. Artesunate also prevented cerebral ischemia-reperfusion injury-induced neurological impairments in mice and protected MCAO rats from imminent death.13,18 Our results were consistent with these studies. Our study demonstrated that artesunate ameliorated ischemic injury in mice after dMCAO by reducing the infarct volume and ameliorating neurological deficits. However, the possible mechanism is unknown. A study by Lu et al. found that artesunate exerted antioxidant effects by activating the Nrf2 pathway and suppressing reactive oxygen species. 13 In a study by Shao et al., the protective effect of artesunate was speculated to be associated with increased autophagy via regulation of mTOR activity. 18 However, regarding the anti-inflammatory effect, the above studies only evaluated proinflammatory cytokine expression. Inflammatory cells and cytokines both contribute to injury. Post-ischemic inflammation is characterized by infiltration of immune cells (leukocytes), activation of endogenous immune cells such as microglia, cytokine release, and transcription factor activation. 1 Therefore, our study evaluated the expression and activation of leukocytes, microglia, inflammatory cytokines, and the inflammatory transcription factor NF-κB.
Recruitment of leukocytes is an important feature of cerebral ischemia. Leukocytes block the microvasculature and produce inflammatory mediators. Neutrophils are the earliest leukocytes that invade the ischemic brain. They induce injury through production of inflammatory mediators, which are often harmful to the brain.1,19,20 The expression of MPO, an important inflammatory enzyme, is elevated in neutrophils after ischemia. 21 MPO can produce oxygen species, which cause additional damage after cerebral ischemia. Numerous studies have shown that inhibition of MPO activity and expression is beneficial for cerebral ischemic injury.5,21–23 In our study, MPO activation and expression were observed by immunohistochemical, biochemical, and western blotting assays. The results demonstrated that artesunate significantly inhibited the increase in MPO at 72 hours after cerebral ischemia. Therefore, the neuroprotective effect of artesunate against cerebral ischemic injury might be associated with suppression of neutrophil infiltration.
Microglia are the resident immune cells of the central nervous system. In cerebral ischemia, activated microglia transform from a “ramified” state to an “amoeboid” state.2,24 The activated microglia release various toxic chemicals, leading to cell injury and death.25,26 Accumulating evidence has demonstrated that inhibition of microglial activation has a beneficial effect on ischemic injury.22,27–29 In our study, artesunate suppressed microglial growth and reversed the morphological change in microglia from a normal state to an activated state. Thus, the results showed that artesunate might be a potential therapy for cerebral ischemia via inhibition of microglial activation.
After cerebral ischemia, the levels of many inflammatory cytokines, including IL-1β and TNF-α, are increased. The neurotoxicity of IL-1β has been verified in the ischemic brain.30,31 Treatment with recombinant TNF-α protein exacerbated brain damage after cerebral ischemia. 32 According to many studies, decreasing TNF-α and IL-1β expression has a beneficial effect on ischemic injury.27,33,34 Our study showed that artesunate could be an effective treatment to alleviate inflammatory damage by limiting the increase in TNF-α and IL-1β in the ischemic brain. The results are consistent with those of Lu et al. 13 To further explore the mechanism, we studied the upstream transcription factors of TNF-α and IL-1β.
When NF-κB is inactive, the p65/p50 heterodimer remains in the cytoplasm and binds Iκ-Bα. After cerebral ischemia, Iκ-Bα is phosphorylated and undergoes proteolysis.27,34–36 The p65/p50 dimer translocates into the nucleus and enhances the expression of inflammatory cytokines, such as TNF-α and IL-1β. Many neuroprotective agents inhibit NF-κB signaling by preventing its nuclear translocation and reducing Iκ-Bα degradation. NF-κB plays a central role in promoting the inflammatory cascade. The upregulation of inflammatory cytokines causes further neuronal injury. Studies have shown that NF-κB activation has detrimental effects in cerebral ischemia.3,4 In previous studies, artesunate had a strong effect on alleviating activation of the NF-κB signaling pathway in hepatic fibrosis and activated BV2 microglia.11,12 Our western blot results showed that artesunate reduced the ischemia-induced increase in NF-κB in the nucleus and inhibited Iκ-Bα proteolysis in the cytoplasm. Our immunofluorescence results also showed that artesunate reversed the ischemia-induced nuclear translocation of NF-κB. These results were consistent with the changes in the inflammatory cytokines TNF-α and IL-1β, suggesting that artesunate might ameliorate the enhanced inflammatory response by inhibiting NF-κB activation.
Limitations
There are several limitations of our study. The dMCAO model only simulates ischemia but not hypertension and diabetes, which are common in humans with stroke. In our study, we found that artesunate ameliorated ischemic injury and the inflammatory response and inhibited the NF-κB pathway. However, further investigation is needed to determine whether the NF-κB pathway is the only pathway involved in this process.
Conclusions
Artesunate ameliorated ischemic injury and the inflammatory response after cerebral ischemia. It also decreased the inflammatory response by suppressing the infiltration and activation of inflammatory cells, inhibiting NF-κB activation, and suppressing inflammatory cytokine expression. Artesunate has some side effects when it is used clinically. It occasionally causes dizziness, nausea, vomiting, and mild liver damage. In the acute phase of cerebral infarction, many patients have dysphagia, and some severe patients are comatose; therefore, the use of oral medication is limited. Artesunate injection has been widely used in the treatment of malaria; therefore, artesunate injection may be feasible for clinical application in patients with cerebral infarction.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was generously supported by grants from the National Natural Science Foundation of China (No. 81974184) and the Scientific Research Project of Hebei Health Commission (20170098).
ORCID iD: Ying Liu https://orcid.org/0000-0002-5691-1538
References
- 1.Kawabori M andYenari M.. Inflammatory responses in brain ischemia. Curr Med Chem 2015; 22: 1258–1277. 10.2174/0929867322666150209154036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J Kawabori M andYenari M.. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem 2014; 21: 2076–2097. 10.2174/0929867321666131228205146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurmi A, Lindsberg P, Koistinaho M, et al. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke 2004; 35: 987–991. 10.1161/01.str.0000120732.45951.26 [DOI] [PubMed] [Google Scholar]
- 4.Ridder D andSchwaninger M.. NF-kappaB signaling in cerebral ischemia. Neuroscience 2009; 158: 995–1006. 10.1016/j.neuroscience.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Zhang X, Dong L, et al. Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischaemia. Br J Pharmacol 2015; 172: 5009–5023. 10.1111/bph.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the Treatment of Malaria. Geneva: World Health Organization Copyright© World Health Organization 2015: 2015. [Google Scholar]
- 7.Zuo S, Ge H, Li Q, et al. Artesunate protected blood-brain barrier via sphingosine 1 phosphate receptor 1/phosphatidylinositol 3 kinase pathway after subarachnoid hemorrhage in rats. Mol Neurobiol 2017; 54: 1213–1228. 10.1007/s12035-016-9732-6 [DOI] [PubMed] [Google Scholar]
- 8.Clemmer L, Martins YC, Zanini GM, et al. Artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria and rapidly decrease leukocyte accumulation in the brain. Antimicrob Agents Chemother 2011; 55: 1383–1390. 10.1128/aac.01277-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho WE, Peh HY, Chan TK, et al. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 2014; 142: 126–139. 10.1016/j.pharmthera.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Zuo S, Li Q, Liu X, et al. The potential therapeutic effects of artesunate on stroke and other central nervous system diseases. Biomed Res Int 2016; 2016: 1489050. 10.1155/2016/1489050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai L, Chen Y, Tian X, et al. Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-κB signaling pathway in rats. Eur J Pharmacol 2015; 765: 234–241. 10.1016/j.ejphar.2015.08.040 [DOI] [PubMed] [Google Scholar]
- 12.Okorji UP andOlajide OA.. A semi-synthetic derivative of artemisinin, artesunate inhibits prostaglandin E2 production in LPS/IFNγ-activated BV2 microglia. Bioorg Med Chem 2014; 22: 4726–4734. 10.1016/j.bmc.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Wang B, Cui N, et al. Artesunate suppresses oxidative and inflammatory processes by activating Nrf2 and ROS‑dependent p38 MAPK and protects against cerebral ischemia‑reperfusion injury. Mol Med Rep 2018; 17: 6639–6646. 10.3892/mmr.2018.8666 [DOI] [PubMed] [Google Scholar]
- 14.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ open science 2020; 4: e100115. 10.1136/bmjos-2020-100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, et al. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci 2015; 35: 12446–12464. 10.1523/jneurosci.1641-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song D, Zhang X, Chen J, et al. Wnt canonical pathway activator TWS119 drives microglial anti-inflammatory activation and facilitates neurological recovery following experimental stroke. J Neuroinflammation 2019; 16: 256. 10.1186/s12974-019-1660-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J Alkayed N andHurn P.. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab 2007; 27: 1553–1562. 10.1038/sj.jcbfm.9600457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao M, Shen Y, Sun H, et al. Protectiveness of artesunate given prior ischemic cerebral infarction is mediated by increased autophagy. Front Neurol 2018; 9: 634. 10.3389/fneur.2018.00634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz G andGranger DN.. Cell adhesion molecules and ischemic stroke. Neurol Res 2008; 30: 783–793. 10.1179/174313208x341085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies CA, Loddick SA, Stroemer RP, et al. An integrated analysis of the progression of cell responses induced by permanent focal middle cerebral artery occlusion in the rat. Exp Neurol 1998; 154: 199–212. 10.1006/exnr.1998.6891 [DOI] [PubMed] [Google Scholar]
- 21.Barone FC, Hillegass LM, Price WJ, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res 1991; 29: 336–345. 10.1002/jnr.490290309 [DOI] [PubMed] [Google Scholar]
- 22.McCarter KD, Li C, Jiang Z, et al . Effect of low-dose alcohol consumption on inflammation following transient focal cerebral ischemia in rats. Sci Rep 2017; 7: 12547. 10.1038/s41598-017-12720-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gad S, Nofal S, Raafat E, et al. Lixisenatide reduced damage in hippocampus CA1 neurons in a rat model of cerebral ischemia-reperfusion possibly via the ERK/P38 signaling pathway. J Mol Neurosci 2020; 70: 1026–1037. 10.1007/s12031-020-01497-9 [DOI] [PubMed] [Google Scholar]
- 24.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312–318. 10.1016/0166-2236(96)10049-7 [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Dai Q, Han K, et al. JNK-IN-8, a c-Jun N-terminal kinase inhibitor, improves functional recovery through suppressing neuroinflammation in ischemic stroke. J Cell Physiol 2020; 235: 2792–2799. 10.1002/jcp.29183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai AY andTodd KG.. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol 2006; 84: 49–59. 10.1139/y05-143 [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Huang Y, Lin Y, et al. Anti-inflammatory effect of cholera toxin B subunit in experimental stroke. J Neuroinflammation 2016; 13: 147. 10.1186/s12974-016-0610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Xiang B, Shen T, et al. Anti-neuroinflammatory effect of 3,4-dihydroxybenzaldehyde in ischemic stroke. Int Immunopharmacol 2020; 82: 106353. 10.1016/j.intimp.2020.106353 [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Xu N, Yi W, et al. Electroacupuncture attenuates inflammation after ischemic stroke by inhibiting NF-κB-mediated activation of microglia. Evid Based Complement Alternat Med 2020; 2020: 8163052. 10.1155/2020/8163052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamasaki Y, Matsuura N, Shozuhara H, et al. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 1995; 26: 676–680; discussion 681. 10.1161/01.str.26.4.676 [DOI] [PubMed] [Google Scholar]
- 31.Boutin H, LeFeuvre RA, Horai R, et al. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci 2001; 21: 5528–5534. 10.1523/jneurosci.21-15-05528.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barone FC, Arvin B, White RF, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke 1997; 28: 1233–1244. 10.1161/01.str.28.6.1233 [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Tang L, Li Y, et al. Biochanin a protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J Neurol Sci 2015; 348: 121–125. 10.1016/j.jns.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 34.Zhan J, Qin W, Zhang Y, et al. Upregulation of neuronal zinc finger protein A20 expression is required for electroacupuncture to attenuate the cerebral inflammatory injury mediated by the nuclear factor-kB signaling pathway in cerebral ischemia/reperfusion rats. J Neuroinflammation 2016; 13: 258. 10.1186/s12974-016-0731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sapkota A, Gaire BP, Cho KS, et al. Eupatilin exerts neuroprotective effects in mice with transient focal cerebral ischemia by reducing microglial activation. PLoS One 2017; 12: e0171479. 10.1371/journal.pone.0171479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Yao N, Zhang T, et al. Ability of post-treatment glycyrrhizic acid to mitigate cerebral ischemia/reperfusion injury in diabetic mice. Med Sci Monit 2020; 26: e926551. 10.12659/msm.926551 [DOI] [PMC free article] [PubMed] [Google Scholar]