Abstract
Introduction
Intraventricular pressure gradient is regarded as a non-invasive indicator of diastolic function. Salvianolic acid B (Sal-B), a traditional Asian medicine, revealed its usefulness in myocardial infarction models; however, the hemodynamic effect of salvianolic acid B is still unknown. The present study aimed to investigate the intraventricular pressure gradient changes during the development of left ventricular hypertrophy with or without salvianolic acid B and a beta-blocker.
Methods
In total, 48 rats were divided into four groups; Sham, Non-treatment, salvianolic acid B, and Carvedilol. Aortic coarctation-induced left ventricular hypertrophy was done in three groups and the treatment was started from the third to the sixth week. Blood pressure, conventional echocardiography, and color M-mode echocardiography for measurement of intraventricular pressure gradient were carried out for six consecutive weeks.
Results
At 4.5 weeks, the LV mass was elevated in the coarctation groups but the blood pressure was significantly lower in salvianolic acid B and Carvedilol groups (P < 0.05). In the Non-treatment group, the total intraventricular pressure gradient was increased at 4.5 and 6 weeks (2.60 and 2.65, respectively). Meanwhile, the basal intraventricular pressure gradient was elevated at 3 and 6 weeks (1.67 and 1.75) compared with the Sham group. Salvianolic acid B and Carvedilol significantly reduced the basal intraventricular pressure gradient at six weeks compared with the Non-treatment group (1.52 and 1.51 vs 1.75, respectively).
Conclusions
Salvianolic acid B and Carvedilol promote cardiac function by decreasing the elevated basal intraventricular pressure gradient. The current preclinical results revealed the efficacy of salvianolic acid B as a potential therapy for left ventricular hypertrophy because of the non-blood pressure lowering effect.
Keywords: Doppler echocardiography, diastolic function, intraventricular pressure gradients, left ventricular hypertrophy, salvianolic acid B
Introduction
Left ventricle hypertrophy (LVH) is a pathological consequence of chronic pressure overload, including systemic hypertension and valve diseases, which results in increased mortality in humans and animals. 1 Chronic pressure overload causes progressive cardiac remodeling, resulting in LVH, and subsequently leads to diastolic dysfunction and congestive heart failure. The LVH is thought to play a compensatory role in enhancing cardiac performance to compensate for pressure overload, but this maladaptive response is ultimately detrimental. 2 Beta-adrenoceptor is activated during this process, elevating the levels of cAMP, PKA, and Ca2+, leading to an increase in the heart rate and myocardial contractility. Thus, beta-blockers like Carvedilol and sotalol have been used in the treatment of LVH. Beta-blockers are widely prescribed for heart failure patients despite a lack of compelling indication for use. In addition, the association of beta-blocker use with heart failure and hospitalization increases the need for another medication to be identified, which may act as a replacement and overcome some of these disadvantages.3,4
Salvianolic acid B (Sal-B), a water-soluble extract from the traditional Chinese medicine Radix Salviae Miltiorrhizae, is a potent antioxidant and anti-inflammatory drug with cardioprotective properties in vitro. 5 Preclinical studies revealed that Sal-B could alleviate ischemia-induced myocardial injury and modulate angiogenesis in vivo. 6 Therefore, Sal-B may be a useful therapy to treat LVH. However, the hemodynamic effects of Sal-B are unclear. To evaluate Sal-B as a putative medicine for the treatment of LVH, its cardiovascular effects in this context will be monitored.
Traditional echocardiography allows in vivo cardiac functional evaluation for longitudinal studies but a more accurate measurement of chamber size and functional assessment of the left atrium (LA) and LV remains challenging.7,8
In diastole, the force sucking blood from the LA to the LV is correlated with Tau, the time constant of LV pressure decay. Tau is known as the gold standard for the measurement of diastolic function and it is a source of the intraventricular pressure difference (IVPD). IVPD has thus been considered as a potential indicator of diastolic function. In clinical settings, non-invasive measurement of IVPD using analysis of color M-mode images provides a practical means to evaluate diastolic function.9–11 Non-invasive IVPD (mid-to-apical IVPD) is strongly negatively correlated with Tau and is a reliable preload-independent index of ventricle relaxation, while basal IVPD has interfered with preload change.12,13 The intraventricular pressure gradient (IVPG) is defined as a parameter in which IVPD is divided by ventricular length and is a universal parameter beyond the influence of heart size. 14
The cardiac diastolic function relies on active myocardial relaxation and the passive property of the ventricle wall. Since IVPG reveals active relaxation status and LA pressure,15,16 investigating the IVPG status during the pathogenesis of LVH could yield new insights into the mechanisms of diastolic dysfunction. Even though the difference between heart failure patients and healthy humans are well described, the chronological order during the development of heart failure is still unknown.17,18 Identifying the chronological order during the development of diastolic dysfunction would contribute to a more accurate diagnosis, and may elucidate the relationship between hemodynamic status and morphological changes in cardiac remodeling.
Although therapeutic modalities for LVH have been thoroughly investigated, the therapeutic threshold of pharmacological interventions may vary between patients. Precise diastolic monitoring after therapeutic interventions using technologies such as IVPG could allow evaluation of treatment efficacy in clinical settings, 19 thereby allowing physicians to tailor treatments to each patient.
We hypothesized that the IVPG would change during the development of LVH in rats and that IVPG could potentially reflect the cardioprotective effects of Carvedilol and Sal-B in this context. We assume that Sal-B has a cardiac protective effect similar to a beta-blocker by preventing the elevation of LA pressure and maintaining active relaxation, which means decreasing the elevated basal IVPG and maintaining the mid-to-apical IVPG.
Materials and methods
Ethics approval
The study was approved by the Animal Care and Use Committee of the Tokyo University of Agriculture and Technology (30–56). All experiments were conducted under the National Guide for the Care and Use of Laboratory Animals, published in 1994.
Experimental animals
A total of 68 female Sprague Dawley rats 2 months old and weighing 230–260 g were included in this study. Rats were supplied with water and food ad libitum and housed at 22°C on a 12-hour light/dark cycle. Abdominal aorta coarctation surgery was successfully performed in 36 rats as described previously. 20 More details regarding study design are provided as Supplementary data (S1) but briefly the rats were anesthetized with 50 mg/kg intraperitoneal pentobarbital sodium and placed supine under an operating microscope (Leica M60, Wetzlar, Germany). The abdominal aorta was exposed using a cotton swab and sutured together with a blunt 22-gauge needle with 3-0 silk. The needle was then withdrawn to create appropriate stenosis in the abdominal aorta.
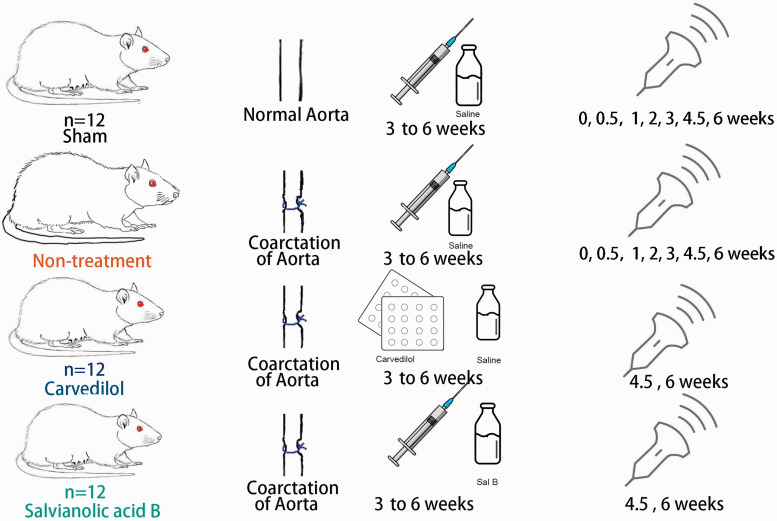
Three weeks postoperatively, the 36 successfully operated rats were randomly divided into three equal subgroups: Non-treatment group, Sal-B group, and Carvedilol group (n = 12). Another 12 Sham-operated rats were used as a Sham group. The treatment protocol includes administration of Sal-B (5 mg/kg/day, I.P, Danshen DuofensuanYan 100 mg, GreenValley Inc, Shanghai, China), Carvedilol (2 mg/kg/day, P.O, Artist Tablets 2.5 mg, Daiichi-Sankyo, Tokyo, Japan), and normal saline to the Non-treatment and Sham groups (Figure 1). After confirming the existence of LVH by elevated relative wall thickness (RWT), blood pressure, and left ventricular mass (LVM) three weeks postoperative, the treatments were administered once daily till the end of the six weeks to allow longitudinal evaluation.
Figure 1.
Schematic representation of experimental procedures and time frame of investigated groups.
Blood pressure
Blood pressure was monitored in the caged rats by the oscillometric method from the tail (BP monitor for rats, Muromachi, Japan). At least five consecutive measurements at each time interval were taken and the average of the systolic, diastolic and mean arterial blood pressure was reported.
Echocardiography
Sham and Non-treatment groups were sampled at 0, 0.5, 1, 2, 3, 4.5, and 6 weeks after the operation, while the two treatment groups Sal-B and Carvedilol were examined at 4.5 and 6 weeks after operation, as shown in Figure 1. The time frame of measurement was decided by the development of LVH and the effect of treatment as modified from previous reports. 21
Rats were positioned under an inspiratory anesthesia mask administering 2.5% isoflurane at 1 L/min oxygen. Noninvasive systemic vascular resistance measurements were obtained by abdominal aortic ultrasound in the prone decubitus position under anesthesia (ProSound F75 Premier CV, Hitachi Healthcare System Inc, Tokyo, Japan). A 1 mm sample volume was placed at the abdominal aortic stenosis to measure the degree of stenosis using a 5–15 MHz continuous-wave transducer as shown in Supplementary figure (S1).
Conventional echocardiography was also performed. Morphology data were sampled by M-mode tracing on the right parasternal short-axis image at the papillary muscle level of the LV. The following parameters were obtained: LV diastolic (d) and systolic (s) diameters (LVIDd and LVIDs, respectively), LV diastolic and systolic posterior wall thickness (LVPWd and LVPWs, respectively), and septal diastolic and systolic wall thickness (IVSd and IVSs). LVM and RWT were calculated with the formulae:
| (1) |
| (2) |
An apical four-chamber view was obtained for mitral valve (MV) inflow assessment and tissue Doppler imaging (TDI). The early diastolic (E) and atrial systolic (A) wave peak velocities of the mitral inflow were measured using pulsed-wave Doppler echocardiography. The early (E′) and late (A′) diastolic velocities of the posterior and anterior myocardial wall at the point of attachment to the MV were measured by pulsed-TDI. The TDI was calculated using the formula:
| (3) |
The LV function was evaluated by fractional shortening (FS) and the E/A velocity ratio of the mitral inflow. Every measurement was repeated five times at each experimental time interval, and the average value was used for data analysis.
IVPG measurement
Color M-mode echocardiography with simultaneous electrocardiography (ECG) was recorded with the cursor parallel to the mitral inflow in the apical four-chamber view, and the rat’s limbs were tightened with micropore paper tape. The sampling was only processed when the respiratory rate of rats was stable (25–35 cycles per minute). The heart rate was not controlled during the sampling to avoid the interference between anesthesia and circulation.
Color M-mode images were analyzed with the Euler equation by Matlab (The MathWorks, Natick, MA) to obtain the IVPD. IVPG was calculated using the formula:
| (4) |
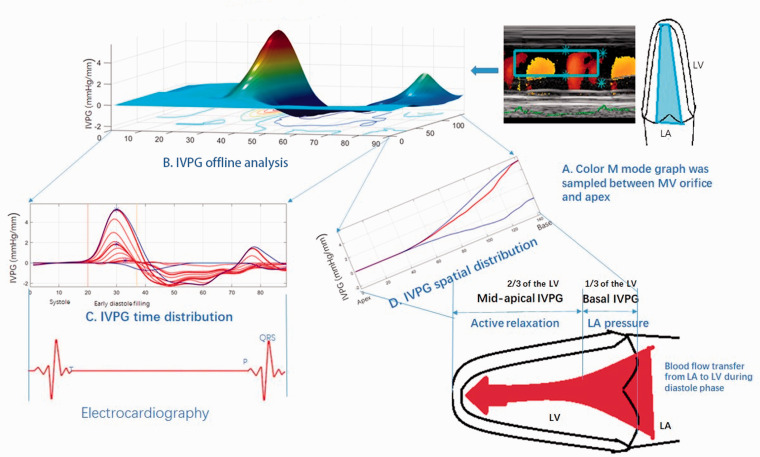
The total IVPG was divided into two segments based on one-third segments of LV length, where the smaller segment near the mitral valve was basal IVPG, and the mid-to-apical IVPG segment was the other two-thirds near the apex as shown in Figure 2. Two independent observers from Juntendo University analyzed the same images, and one blinded observer repeated the analysis on a different day. All data were measured at least five times at each interval time and the average data were reported.
Figure 2.
Procedures of IVPG analysis using the Euler equation by Matlab. Color M-mode echocardiography was sampled (a) and then analyzed using Matlab (b), the time distribution (c), and spatial distribution of intraventricular pressure gradients were gained from the Matlab. The spatial distribution could be calculated into basal IVPG and mid-to-apical IVPG (d).
Statistical analysis
All data measurements were tested by the Sidak test in the two-way factorial ANOVA while the two variables (group and time) were considered. Tukey’s test was used in the treatment reviewing two-way ANOVA multiple comparison test. Results are expressed as mean±SD and P-value < 0.05 was considered statistically significant. The data were analyzed and graphed using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA).
Results
Conventional echocardiography measurements and blood pressure
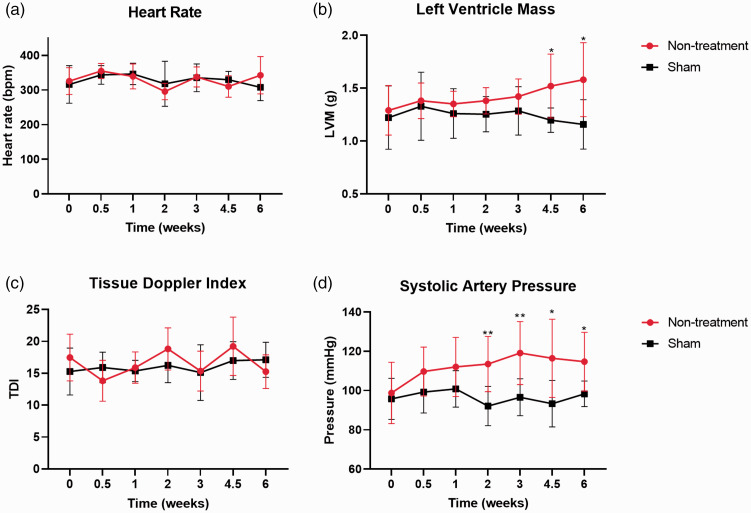
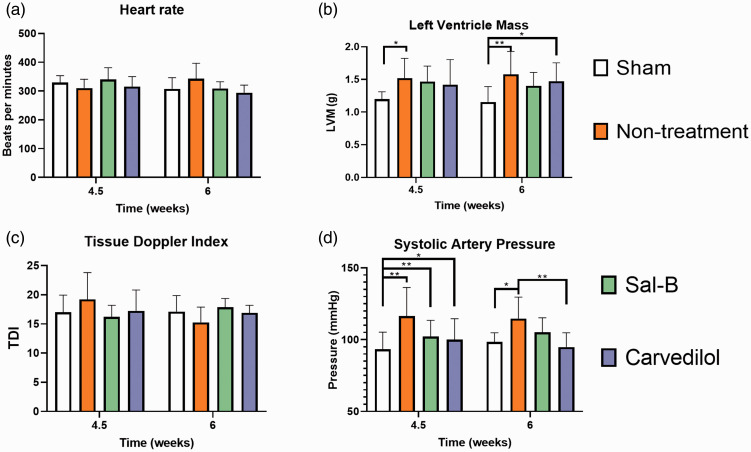
The conventional echocardiographic data and blood pressure throughout the experimental intervals are summarized (Tables 1 and 2, Figures 3 and 4). There was no significant difference in the HR and TDI among all four groups at 4.5 and 6 weeks (Figure 4(a) and (c)). LVH was confirmed by the elevated LVM after 4.5 weeks (Figure 4(b) and Table 1), and LVH was concentric because the RWT is higher than 0.42. The LVM of the Non-treatment group was higher than the Sham group at 4.5 weeks (1.52 vs. 1.20, P = 0.026) and 6 weeks (1.58 vs. 1.16, P = 0.002), respectively. Also, the LVM in the Carvedilol group was higher than Sham groups at 6 weeks (1.47 vs. 1.16, P = 0.030). Meanwhile, the LVM in the Sal-B group did not show a significant difference with the Sham group (Figure 4(b)).
Table 1.
Longitudinal echocardiographic examination of the sham and non-treatment groups throughout the entire experimental time-intervals.
| Sham |
Non-treatment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time/week | 0 | 0.5 | 1 | 2 | 3 | 4.5 | 6 | 0 | 0.5 | 1 | 2 | 3 | 4.5 | 6 |
| IVSd (mm) | 1.1 ± 0.13 | 1.12 ± 0.1 | 1.22 ± 0.13 | 1.19 ± 0.07 | 1.33 ± 0.2 | 1.19 ± 0.16 | 1.14 ± 0.23 | 1.15 ± 0.14 | 1.22 ± 0.12* | 1.20 ± 0.08 | 1.19 ± 0.11** | 1.54 ± 0.15** | 1.68 ± 0.28 | 1.73 ± 0.34** |
| LVIDd (mm) | 6.91 ± 0.47 | 6.86 ± 0.72 | 6.85 ± 0.41 | 6.86 ± 0.55 | 6.95 ± 0.51 | 6.91 ± 0.68 | 6.92 ± 0.61 | 6.92 ± 0.43 | 7.01 ± 0.38 | 7.03 ± 0.34 | 6.99 ± 0.63 | 7.08 ± 0.53 | 7.12 ± 0.53 | 7.12 ± 0.56 |
| LVPWd (mm) | 1.5 ± 0.25 | 1.43 ± 0.25 | 1.47 ± 0.25 | 1.47 ± 0.26 | 1.61 ± 0.36 | 1.54 ± 0.26 | 1.4 ± 0.33 | 1.55 ± 0.29 | 1.82 ± 0.28 | 1.67 ± 0.17 | 1.81 ± 0.43 | 1.53 ± 0.21 | 1.73 ± 0.31 | 1.89 ± 0.31* |
| IVSs (mm) | 2 ± 0.34 | 1.88 ± 0.28 | 2.03 ± 0.28 | 1.99 ± 0.44 | 2.01 ± 0.37 | 2.06 ± 0.23 | 2.21 ± 0.43 | 1.97 ± 0.4 | 2.18 ± 0.35 | 2.45 ± 0.16* | 2.56 ± 0.4* | 2.49 ± 0.23* | 2.51 ± 0.38 | 2.62 ± 0.43* |
| LVIDs (mm) | 4 ± 0.53 | 4.19 ± 0.42 | 4.22 ± 0.41 | 4.13 ± 0.41 | 4.42 ± 0.48 | 4.54 ± 0.72 | 4.23 ± 0.43 | 3.88 ± 0.4 | 4.22 ± 0.4 | 4.14 ± 0.34 | 4.01 ± 0.55 | 3.94 ± 0.44 | 4.01 ± 0.39 | 4.03 ± 0.53 |
| LVPWs (mm) | 2.3 ± 0.24 | 2.27 ± 0.27 | 2.34 ± 0.31 | 2.42 ± 0.37 | 2.33 ± 0.45 | 2.23 ± 0.29 | 2.47 ± 0.29 | 2.22 ± 0.17 | 2.5 ± 0.38 | 2.58 ± 0.23 | 2.67 ± 0.59** | 2.41 ± 0.16 | 2.63 ± 0.36 | 2.67 ± 0.46* |
| FS(%) | 42.11 ± 5.1 | 38.92 ± 4.46 | 38.42 ± 5.21 | 39.79 ± 6.25 | 36.40 ± 7.94 | 34.3 ± 5.85 | 38.87 ± 3.82 | 43.93 ± 4.11 | 39.80 ± 5.11 | 41.11 ± 4.35 | 42.63 ± 5.23 | 44.35 ± 3.74 | 43.68 ± 7.29 | 43.40 ± 5.43 |
| E wave velocity | 92.9 ± 11.31 | 99.67 ± 5.31 | 90.42 ± 11.88 | 91.68 ± 9.34 | 95.03 ± 9.55 | 96.38 ± 14.58 | 97.72 ± 16.27 | 96.88 ± 11.01 | 95.33 ± 16.99 | 102.67 ± 7.23 | 94.67 ± 8.12 | 101.53 ± 11.2 | 101.76 ± 19.3 | 110.67 ± 23.6 |
| E′ IVS | 6.1 ± 1.71 | 6.13 ± 1.21 | 5.68 ± 1.05 | 5.45 ± 1.09 | 6.43 ± 1.88 | 5.34 ± 0.77 | 5.4 ± 1.1 | 5.23 ± 0.65 | 6.4 ± 1.83 | 6.92 ± 1.2 | 4.78 ± 1.26 | 6.01 ± 1.09 | 5.15 ± 1.16 | 6.23 ± 1.52 |
| E′ FW | 6.7 ± 1.98 | 6.73 ± 1.32 | 6.28 ± 1.41 | 6.07 ± 0.9 | 6.98 ± 2.07 | 6.31 ± 1.2 | 6.23 ± 1.53 | 6.15 ± 1.04 | 8.2 ± 1.26** | 7.17 ± 1.66 | 5.98 ± 1.28 | 7.19 ± 2.03 | 5.95 ± 1.85 | 7.72 ± 1.93 |
| DAP (mmHg) | 51.42 ± 5.57 | 53.5 ± 6.26 | 58 ± 13.72 | 54.67 ± 6.02 | 53.58 ± 9.35 | 57.3 ± 14.16 | 56.17 ± 7.91 | 54.25 ± 19.99 | 56.5 ± 12.8 | 63 ± 8.54 | 70 ± 13.51 | 67.67 ± 10.14 | 70.3 ± 10.61 | 59.33 ± 12.46 |
| MAP (mmHg) | 66.19 ± 4.9 | 68.75 ± 3.54 | 72.28 ± 9.62 | 67.14 ± 6.12 | 67.92 ± 6.52 | 69.3 ± 10.5 | 72.11 ± 5.15 | 69.08 ± 17.6 | 74.25 ± 8.65 | 77.57 ± 6.71 | 84.47 ± 10.41 | 80.25 ± 14.7 | 85.6 ± 11.9** | 77.75 ± 11.46 |
| RWT | 0.38 ± 0.05 | 0.37 ± 0.08 | 0.39 ± 0.06 | 0.39 ± 0.05 | 0.42 ± 0.04 | 0.4 ± 0.04 | 0.4 ± 0.03 | 0.39 ± 0.04 | 0.43 ± 0.03 | 0.41 ± 0.05 | 0.43 ± 0.06 | 0.43 ± 0.08 | 0.48 ± 0.05 | 0.51 ± 0.09* |
Note: Values are expressed as mean ± SD (n = 12). IVSd: interventricular septum diastolic diameter. LVIDd: left ventricular internal diastolic diameter; LVPWd: left ventricular posterior wall diastolic diameter; IVSs: interventricular septum systolic diameter; LVIDs: left ventricular internal systolic diameter; LVPWs: left ventricular posterior wall systolic diameter; FS: fraction shortening; E wave velocity: velocity of early mitral inflow ; E′ IVS: early diastolic velocity of the septum; E′ FW: early diastolic velocity of the free wall; DAP: diastolic artery pressure; MAP: mean artery pressure; RWT: relative wall thickness.
*p < 0.05 and **p < 0.01 refer to the significance of the comparison along the study period (in weeks) in the non-treatment and sham groups using two-way ANOVA, n = 12.
Table 2.
Evaluation of Salvianolic acid B and Carvedilol on conventional echocardiographic data in the investigated groups at 4.5 and 6 weeks.
| Time | 4.5 weeks | 6 weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Sham | Non-treatment | Sal B | Carvedilol | Sham | Non-treatment | Sal B | Carvedilol |
| IVSd (mm) | 1.19 ± 0.16 | 1.68 ± 0.28 | 1.63 ± 0.16 | 1.41 ± 0.21 | 1.14 ± 0.23 | 1.73 ± 0.33** | 1.57 ± 0.19* | 1.55 ± 0.15 |
| LVIDd (mm) | 6.91 ± 0.68 | 7.12 ± 0.53 | 7.08 ± 0.56 | 7.09 ± 0.51 | 6.92 ± 0.61 | 7.12 ± 0.45 | 7.03 ± 0.58 | 7.09 ± 0.55 |
| LVPWd (mm) | 1.54 ± 0.26 | 1.73 ± 0.31 | 1.63 ± 0.23 | 1.69 ± 0.23 | 1.4 ± 0.33 | 1.89 ± 0.37 | 1.78 ± 0.34 | 1.77 ± 0.18 |
| IVSs (mm) | 2.06 ± 0.23 | 2.51 ± 0.38** | 2.55 ± 0.47** | 2.26 ± 0.20 | 2.21 ± 0.43 | 2.62 ± 0.48** | 2.58 ± 0.23** | 2.36 ± 0.21 |
| LVIDs (mm) | 4.54 ± 0.72 | 4.01 ± 0.39 | 4.06 ± 0.47 | 4.14 ± 0.51 | 4.23 ± 0.43 | 4.03 ± 0.33 | 3.8 ± 0.52 | 3.93 ± 0.6 |
| LVPWs (mm) | 2.23 ± 0.29 | 2.63 ± 0.36 | 2.52 ± 0.44 | 2.64 ± 0.41 | 2.47 ± 0.29 | 2.67 ± 0.23 | 2.77 ± 0.54 | 2.75 ± 0.34 |
| FS (%) | 34.3 ± 5.85 | 43.68 ± 7.29* | 42.66 ± 5.49 | 41.61 ± 6.76 | 38.87 ± 3.82 | 43.4 ± 3.71 | 45.95 ± 4.02 | 44.57 ± 5.24 |
| RWT | 0.40 ± 0.04 | 0.48 ± 0.05 | 0.46 ± 0.07 | 0.44 ± 0.07 | 0.40 ± 0.04 | 0.51 ± 0.08* | 0.48 ± 0.06 | 0.47 ± 0.05 |
| E flow velocity | 96.38 ± 14.58 | 101.76 ± 19.35 | 99.42 ± 14.41 | 95.02 ± 17.64 | 97.7 ± 15.58 | 100.3 ± 18.7 | 105.4 ± 11.77 | 97.5 ± 11.41 |
| E′ IVS | 5.34 ± 0.77 | 5.15 ± 1.16 | 5.78 ± 1.04 | 5.42 ± 1.14 | 5.4 ± 1.05 | 6.6 ± 1.75* | 5.4 ± 0.87 | 5.5 ± 0.49 |
| E′ FW | 6.31 ± 1.2 | 5.95 ± 1.85 | 6.78 ± 1.25 | 5.9 ± 1.24 | 6.2 ± 1.46 | 7 ± 1.73 | 6.6 ± 0.87 | 6.1 ± 0.76 |
| DAP (mmHg) | 57.3 ± 14.16 | 70.3 ± 10.61 | 59.8 ± 15.83 | 59 ± 15.72 | 56.2 ± 7.57 | 59.3 ± 11.93 | 66.2 ± 13.33 | 53.2 ± 12.27 |
| MAP (mmHg) | 69.3 ± 10.5 | 85.6 ± 11.9** | 73.9 ± 13.08 | 71.4 ± 12.56 | 72.1 ± 4.93 | 77.8 ± 10.97 | 79.5 ± 11.84 | 70 ± 11.57 |
Note: Values are expressed as mean ± SD (n=12). IVSd: interventricular septum diastolic diameter. LVIDd: left ventricular internal diastolic diameter; LVPWd: left ventricular posterior wall diastolic diameter; IVSs: interventricular septum systolic diameter; LVIDs: left ventricular internal systolic diameter; LVPWs: left ventricular posterior wall systolic diameter; FS: fraction shortening; E wave velocity: velocity of early mitral inflow; E′ IVS: early diastolic velocity of the septum; E′ FW: early diastolic velocity of the free wall; DAP: diastolic artery pressure; MAP: mean artery pressure; RWT: relative wall thickness.
*p<0.05 and **p<0.01 refer to the significance of the comparison between all groups using two-way ANOVA, n=12.
Figure 3.
Basic measurements of the Sham group and Non-treatment group throughout the experimental intervals. *p < 0.05 and **p < 0.01 refer to comparisons between Sham and Non-treatment groups.
Figure 4.
Basic measurements in all groups at 4.5 and 6 weeks.
The systolic artery pressure in the Non-treatment group from two to six weeks was significantly higher than that of the Sham group (Figure 4(d)). Besides, the systolic artery pressure in the Sham group was significantly lower than other groups at 4.5 weeks and did not show a significant difference with the Sal-B group at six weeks (Figure 4(d)). This indicates that the blood pressure-lowering effect of Sal-B is less than Carvedilol. Other conventional echocardiographic parameters are listed in Tables 1 and 2.
IVPG measurements
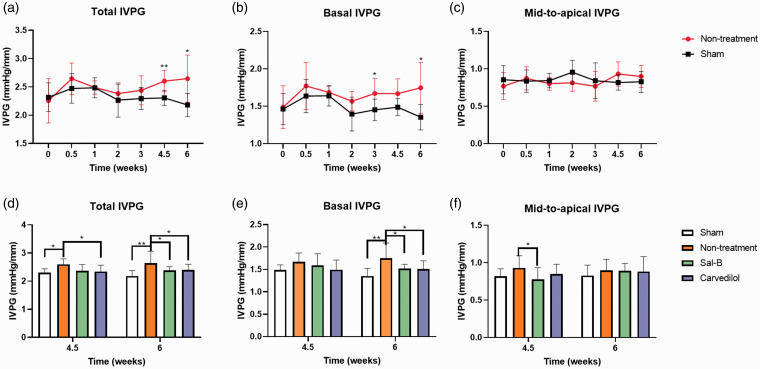
IVPG characteristics in the Sham group and Non-treatment group at different time points are shown in Figure 5(a) to (c). The total IVPG was significantly different among different time points and groups (P < 0.001). In the Non-treatment group, the total IVPG was increased at approximately 0.5 weeks and then decreased until two postoperative weeks in the aortic coarctation groups, followed by a significant increase at 4.5 and 6 weeks compared with the Sham group.
Figure 5.
Comparison of IVPG results in different groups at different time intervals. A, B, & C graphs, respectively, represent the Total, Basal, and Mid-to-apical IVPG parts in Sham and Non-treatment groups throughout the entire experiment. D, E & F graphs, respectively, summarized the Total, Basal, and Mid-to-apical IVPG parts at 4.5 and 6 weeks in all groups. *p < 0.05 and **p < 0.01 used to compare the significance between groups.
The basal IVPG of the Non-treatment group was significantly higher than the Sham group at three and six weeks. No significant difference was detected in mid-to-apical IVPG between Sham and Non-treatment groups.
The total IVPG was significantly different among the four groups at 4.5 weeks (P < 0.0001, Figure 5(d)) but mid-to-apical IVPG of the non-treatment group was higher than the Sal-B group at 4.5 weeks, indicating that the active relaxation of the Non-treatment group was higher than the Sal-B group, which may be connected with the lower LVM in the Sal-B group.
The total IVPG (2.646) and basal IVPG (1.747) in the Non-treatment group were higher than that of the Sham group (2.182 and 1.354) at six weeks (P < 0.001, P < 0.001, respectively). Carvedilol showed a therapeutic effect by a significantly lower total IVPG (2.395) and basal IVPG (1.512) value at six weeks compared with the Non-treatment group (P = 0.001 and 0.026, respectively).
Similar to Carvedilol, the Sal-B also exhibited a treatment effect by decreasing the total IVPG (2.395) and basal IVPG (1.521) value at six weeks compared with the Non-treatment group (P = 0.0296 and 0.0469, respectively). As expected, no difference was detected in the mid-to-apical IVPG among groups at six weeks (P = 0.1034) as shown in Figure 5(d) to (f).
Discussion
The clinical application of IVPG requires an explicit understanding of IVPG spatial distribution. Iwano et al. demonstrated that basal IVPG correlated with LA pressure,15 and mid-to-apical IVPG has been demonstrated to represent active relaxation during diastole.16 Thus, IVPG provides a tool for evaluating cardiac function, which can be used clinically to distinguish between different pathological features of diastolic dysfunction.
Abdominal aorta coarctation surgery dramatically increases the afterload and leads to LVH,22 which was consistent with the results in the present study. RWT higher than 0.42 and elevated LVM in the Non-treatment group at 4.5 and 6 weeks indicates that the heart eventually developed concentric LVH.23 Besides, hypertrophy in the two treatment groups was not as severe as the Non-treatment group as shown in Table 2.
The increased total IVPG at 0.5 weeks postoperative in the Non-treatment group was caused by the surgery, as the Sham group had a similar fluctuation. The increased total IVPG was primarily contributed by elevated basal IVPG, although no difference in the basal IVPG was observed. Total IVPG was significantly higher in the Non-treatment group than in the Sham group from 4.5 to 6 weeks. This has been attributed to higher basal IVPG, which reflects the increased LA pressure at this time. Also, the elevated LA pressure and left ventricle end-diastolic pressure (LVEDP) was reported in this model.24
The mid-to-apical IVPG in the Non-treatment group stayed at the same level as observed in the Sham group over the whole study period. This finding indicates that the active myocardial relaxation and the passive property of the ventricular wall are not dramatically changed at the early stage of compensated LVH.20
Differing from our primary hypothesis, severe diastolic dysfunction, decreased myocardium movement, and reduced mid-to-apical IVPG was not evidenced in the present study. Mild Diastolic dysfunction was observed at two weeks in elevated basal IVPG, but was not significant. Besides, the systolic artery pressure in the Non-treatment group was significantly higher than in Sham groups at two weeks. In contrast, the myocardial hypertrophy did not appear until three weeks as indicated by increased RWT. These results revealed that the hemodynamic changes precede the LVH-related morphological changes in the current study.25
Generally, the chronological order of the IVPG is explained as elevated basal IVPG with maintained mid-to-apical IVPG in the early stages of LVH, which implies elevated LA pressure and maintained active relaxation. We speculate that the basal and mid-to-apical IVPG may decrease in the late stage of LVH, since the heart may lose its ability to maintain the high LA pressure and active relaxation and compensated by the fibrosis and scarred myocardium in the late stage of LVH.26 This requires further research of IVPG changes in the late-stage of LVH.
LA pressure reflects LA function but it was difficult to measure in rats directly by catheterization. This makes the indirect measurement of LA pressure by echocardiography the only practical method to estimate LA function. In this regard, TDI, a cardiac performance assessment combined with both hemodynamic and myocardium movement, was considered a non-invasive method for evaluating LA function. However, the sensitivity of TDI remains questionable because the longitudinal regional strain is heterogeneous between humans and rats.27 Moreover, myocardial movement in rats is different from humans due to their higher heart rate and smaller heart size. This explains why the TDI shows no difference between the four groups at 4.5 and 6 weeks, while IVPG indicates elevated LA pressure.
Although LA pressure was elevated during the development of LVH, the elevation was not dramatic enough to be recognized by TDI. A significant increase in basal IVPG was observed that confirmed elevated LA pressure, while the TDI fluctuated and did not change along with the experiment. Therefore, IVPG may be more promising in cardiac function evaluation than TDI as it avoids the species difference in myocardial movement.14
Beta-blockers are the first-line medicine in treating LVH by relieving the stress of the myocardium, which is achieved by slowing the heart rate and blood pressure as shown in Figure 4. The elevated blood pressure in the Sal-B group at six weeks indicates that the treatment mechanism was different from Carvedilol. Using beta-blockers has been associated with higher mortality and elevated brain natriuretic peptide (BNP) levels in heart failure patients.4,28 In addition, use of beta-blockers may be poorly tolerated and require physician follow-up.29
The therapeutic effects of Carvedilol and Sal-B seem difficult to evaluate from conventional parameters. In our model, many echocardiographic parameters did not change between groups. Nevertheless, the new parameter, IVPG, seems useful to evaluate the effect of the used drugs. Total IVPG was increased by basal IVPG elevation, which indicated that the treatments effectively reduce the LA pressure in compensatory hypertrophy. In the present study, Sal-B and Carvedilol did not reverse the cardiac function and LVH to a normal level, but further studies are necessary to evaluate the response of IVPG and LVH to different dosages of Sal-B.
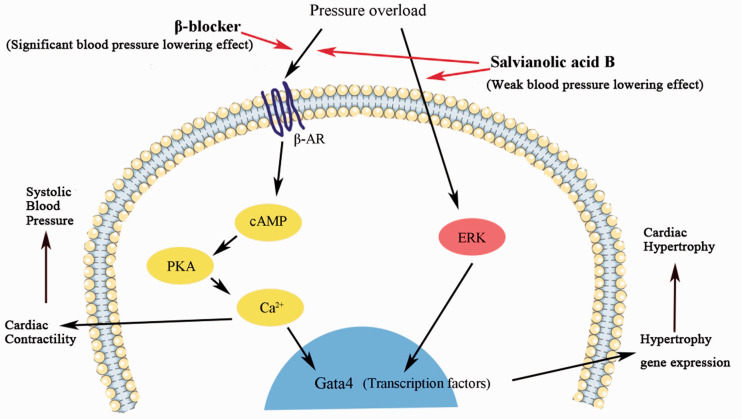
Carvedilol offers cardiac protection by blocking beta-1, 2, and alpha-1 adrenergic receptors, which reduce the IVPG in physiological heart remodeling.30 A similar effect was observed in the present study in the Sal-B group (Figure 5). This could be explained by improving cardiac contractibility, attenuating hyper contraction, and reducing LVEDP after Sal-B administration.31 Pressure overload activates the metabolism of ERK (extracellular signal-regulated kinase), which promotes the expression of Gata4 and results in heart failure. Besides, Sal-B has been proven to decrease myocardial fibrosis and hypertrophy in vivo and in vitro by reducing levels of ERK and Gata4,24,32 which might also be the therapeutic mechanism in Sal-B together with the beta-blocker effect.5,33,34 Although no evidence has suggested that Sal-B blocks beta-adrenoceptors directly, Lu et al. demonstrated that Sal-B had similar effects to a beta-blocker in decreasing Ca2+ and cAMP and inhibiting PKA activation.35 Scientists also proved that Sal-B owns the cardioprotective effect by inhibiting Gata4 gene expression, which controls the expression of atrial natriuretic peptide and BNP.26,36
Therefore, although the cardioprotective effects of Sal-B and Carvedilol did not differ significantly in our model, Sal-B may have therapeutic advantages over Carvedilol, as part of its effect is similar to beta-blockers and the other part is related to ERK/Gata4 to alleviate the LVH as shown in Figure 6.26,37 Salvia Miltiorrhiza Depside Salt for infusion, which contains 60% Sal-B is well tolerated and with fewer side effects than beta-blockers.38,39 Furthermore, beta-blockers are not recommended in hypertrophic obstructive cardiomyopathy (HOCM) by guidelines, and the refractory was reported in beta-blocker treatment.38,40 Consequently, Sal-B might have potential therapeutic effects to treat HOCM because of low side effects and non-blood pressure lowering effect.39 However, further study in this regard is needed.
Figure 6.
Sal-B and Carvedilol therapeutic pathway. Pressure overload activates the beta-adrenergic receptors and ERK, promotes the expression of Gata4 and results in hypertrophy. Sal B could inhibit the ERK and beta-adrenergic receptors at the same time while Carvedilol only works as a beta-blocker.
As observed in the present study, the IVPG status has been evaluated in aortic coarctation-induced LVH in the rat model with and without therapeutic interventions. First, we discussed the clinical meaning of IVPG spatial distribution. Second, we evaluated the chronological order of IVPG during the development of LVH. Third, we described the hemodynamic effects of Sal-B and Carvedilol in vivo.
Limitations and strengths
There are potential limitations. First, we could not measure the LA pressure invasively because of the unrepeatability in rats. Second, the heart rate was not controlled during anesthesia since different isoflurane doses lead to different sympathetic suppression.
Conclusion
IVPG changes precede morphological disruption during the development of LVH. Sal-B and Carvedilol promote cardiac function by preventing the progressive elevation of basal IVPG in the LVH model. Sal-B could be a potential therapy for LVH, as its efficacy for treatment promotes cardiac function without lowering the blood pressure, which is different from Carvedilol.
Supplemental Material
Supplemental material, sj-pdf-1-ult-10.1177_1742271X20987584 for Intraventricular pressure gradients change during the development of left ventricular hypertrophy: Effect of salvianolic acid B and beta-blocker by Danfu Ma, Ahmed S Mandour, Tomohiko Yoshida, Katsuhiro Matsuura, Kazumi Shimada, Pitipat Kitpipatkun, Akiko Uemura, Mayumi Ifuku, Ken Takahashi and Ryou Tanaka in Ultrasound
Supplemental material, sj-pdf-2-ult-10.1177_1742271X20987584 for Intraventricular pressure gradients change during the development of left ventricular hypertrophy: Effect of salvianolic acid B and beta-blocker by Danfu Ma, Ahmed S Mandour, Tomohiko Yoshida, Katsuhiro Matsuura, Kazumi Shimada, Pitipat Kitpipatkun, Akiko Uemura, Mayumi Ifuku, Ken Takahashi and Ryou Tanaka in Ultrasound
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: Animal Care and Use Committee of the Tokyo University of Agriculture and Technology approved this study (ID: 30-56).
Guarantor: Tanaka Ryou.
Contributorship: DM, ASM, KM and TY researched literature. DM, KS, PK, ASM, AU, MI and KT conceived the study. DM designed the audit. DM, ASM and KM did the data analysis. DM wrote the first draft of the manuscript. ASM, RT wrote the final version of the manuscript. RT was the project supervisor. All authors reviewed and approved the final version of the manuscript.
ORCID iDs: Danfu Ma https://orcid.org/0000-0002-6557-7703
Ahmed S Mandour https://orcid.org/0000-0001-6256-4539
Tomohiko Yoshida https://orcid.org/0000-0003-4311-9311
Kazumi Shimada https://orcid.org/0000-0002-2514-695X
Akiko Uemura https://orcid.org/0000-0003-2671-5074
Ryou Tanaka https://orcid.org/0000-0001-9948-6490
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Lazzeroni D, Rimoldi O, Camici PG. From left ventricular hypertrophy to dysfunction and failure. Circ J 2016; 80: 555–564. [DOI] [PubMed] [Google Scholar]
- 2.Crozatier B, Ventura-Clapier RJC. Is inhibition of hypertrophy a good therapeutic strategy in ventricular pressure overload?: Inhibition of hypertrophy, per se, may not be a good therapeutic strategy in ventricular pressure overload. Circulation 2015; 131: 1448–1457. [DOI] [PubMed] [Google Scholar]
- 3.Yum B, Archambault A, Levitan EB, et al. Indications for β‐blocker prescriptions in heart failure with preserved ejection fraction. J Am Geriatrics Soc 2019; 67: 1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman DN, Plante TB, Infeld M, et al. Association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Network Open 2019; 2: e1916598-e1916598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Luo H, Xu Y, et al. Salvianolic acid B-alleviated angiotensin II induces cardiac fibrosis by suppressing NF-κB pathway in vitro. Med Sci Monitor 2018; 24: 7654–7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Zheng Y, Liu X, et al. Metabolomic profiles of myocardial ischemia under treatment with salvianolic acid B. Chinese Med 2012; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edvardsen T, Smiseth OA. Evaluation of diastolic function by echocardiography: important progression, but issues to be resolved. Eur Heart J-Card Imag 2018; 19: 387–388. [DOI] [PubMed] [Google Scholar]
- 8.Orvalho JS. Real-time three-dimensional echocardiography: from diagnosis to intervention. Vet Clin N Am-Small Anim Pract 2017; 47: 1005–1019. [DOI] [PubMed] [Google Scholar]
- 9.Bermejo J, Antoranz JC, Yotti R, et al. Spatio-temporal mapping of intracardiac pressure gradients. A solution to Euler’s equation from digital postprocessing of color Doppler M-mode echocardiograms. Ultrasound Med Biol 2001; 27: 621–630. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg NL, Vandervoort PM, Firstenberg MS, et al. Estimation of diastolic intraventricular pressure gradients by Doppler M-mode echocardiography. Am J Physiol-Heart C 2001; 280: H2507–H2515. [DOI] [PubMed] [Google Scholar]
- 11.Stewart KC, Kumar R, Charonko JJ, et al. Evaluation of LV diastolic function from color M-mode echocardiography. JACC Cardiovasc Imag 2011; 4: 37–46. [DOI] [PubMed] [Google Scholar]
- 12.Popovic ZB, Prasad A, Garcia MJ, et al. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. 2006; 290: H1454–H1459. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura K, Shiraishi K, Sato K, et al. Left ventricular vortex and intraventricular pressure difference in dogs under various loading conditions. Am J Physiol Heart Circ Physiol 2019; 316: H882–H888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popovic ZB, Richards KE, Greenberg NL, et al. Scaling of diastolic intraventricular pressure gradients is related to filling time duration. Am J Physiol-Heart C 2006; 291: H762–H769. [DOI] [PubMed] [Google Scholar]
- 15.Iwano H, Kamimura D, Fox E, et al. Altered spatial distribution of the diastolic left ventricular pressure difference in heart failure. J Am Soc Echocardiogr 2015; 28: 597–605, e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notomi Y, Popovic ZB, Yamada H, et al. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol-Heart C 2008; 294: H505–H513. [DOI] [PubMed] [Google Scholar]
- 17.Ohara T, Niebel CL, Stewart KC, et al. Loss of adrenergic augmentation of diastolic intra-LV pressure difference in patients with diastolic dysfunction: evaluation by color M-mode echocardiography. JACC Cardiovasc Imag 2012; 5: 861–870. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Takahashi K, Yamada M, et al. Assessment of early diastolic intraventricular pressure gradient in the left ventricle among patients with repaired tetralogy of Fallot. Heart Vessels 2017; 32: 1364–1374. [DOI] [PubMed] [Google Scholar]
- 19.Rovner A, Smith R, Greenberg NL, et al. Improvement in diastolic intraventricular pressure gradients in patients with HOCM after ethanol septal reduction. Am J Physiol-Heart C 2003; 285: H2492–H2499. [DOI] [PubMed] [Google Scholar]
- 20.Ni M, Yang Z-W, Li D-J, et al. A potential role of alpha-7 nicotinic acetylcholine receptor in cardiac angiogenesis in a pressure-overload rat model. J Pharmacol Sci 2010; 114: 311–319. [DOI] [PubMed] [Google Scholar]
- 21.Fan Z, Gao Y, Huang Z, et al. Protective effect of hydrogen-rich saline on pressure overload-induced cardiac hypertrophyin rats: possible role of JAK-STAT signaling. BMC Cardiovasc Disorders 2018; 18: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kai H, Kuwahara F, Tokuda K, et al. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 2005; 28: 483--490. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Xue H, Zou Y, et al. Left ventricular hypertrophy, abnormal ventricular geometry and relative wall thickness are associated with increased risk of stroke in hypertensive patients among the Han Chinese. Hypertension Res 2014; 37: 870–874. [DOI] [PubMed] [Google Scholar]
- 24.Kuwahara F, Kai H, Tokuda K, et al. Transforming growth factor-β function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002; 106: 130–135. [DOI] [PubMed] [Google Scholar]
- 25.Dai C, Li Q, May HI, et al. Lactate dehydrogenase A governs cardiac hypertrophic growth in response to hemodynamic stress. Cell Reports 2020; 32: 108087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Chen R, Tan Y, et al. Salvianolic acid B alleviates heart failure by inactivating ERK1/2/GATA4 signaling pathway after pressure overload in mice. PLoS One 2016; 11: e0166560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachner-Hinenzon N, Ertracht O, Leitman M, et al. Layer-specific strain analysis by speckle tracking echocardiography reveals differences in left ventricular function between rats and humans. Am J Physiol-Heart C 2010; 299: H664–H672. [DOI] [PubMed] [Google Scholar]
- 28.Bergström A, Andersson B, Edner M, et al. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler‐echocardiographic study (SWEDIC). Eur J Heart Failure 2004; 6: 453–461. [DOI] [PubMed] [Google Scholar]
- 29.Edelmann F, Musial-Bright L, Gelbrich G, et al . Tolerability and feasibility of beta-blocker titration in HFpEF versus HFrEF: insights from the CIBIS-ELD trial. JACC: Heart Failure 2016; 4: 140–149. [DOI] [PubMed] [Google Scholar]
- 30.Cotrim C, Lopes LR, Almeida AR, et al. Efficacy of beta-blocker therapy in symptomatic athletes with exercise-induced intra-ventricular gradients. Cardiovasc Ultrasound 2010; 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao Z, Xu Y. Salvianolic acid B alleviating myocardium injury in ischemia reperfusion rats. Afr J Tradit Complement Altern Med 2016; 13: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto K, Masuyama T, Sakata Y, et al. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res 2002; 55: 76–82. [DOI] [PubMed] [Google Scholar]
- 33.Gao H, Bo Z, Wang Q, et al. Salvanic acid B inhibits myocardial fibrosis through regulating TGF-β1/Smad signaling pathway. Biomed Pharmacother 2019; 110: 685–691. [DOI] [PubMed] [Google Scholar]
- 34.Qiu H, Liu W, Lan T, et al. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine 2018; 51: 255–265. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Zheng Y, Liu X, et al. Metabolomic profiles of myocardial ischemia under treatment with salvianolic acid B. Chin Med 2012; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molkentin JDJJoBC. The zinc finger-containing transcription factors GATA-4,-5, and-6 ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem 2000; 275: 38949–38952. [DOI] [PubMed] [Google Scholar]
- 37.Bernardo BC, Weeks KL, Pretorius L, et al. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 2010; 128: 191–227. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki R, Mochizuki Y, Yuchi Y, et al. Assessment of myocardial function in obstructive hypertrophic cardiomyopathy cats with and without response to medical treatment by carvedilol. BMC Vet Res 2019; 15: 376–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Y-Y, Yang Y-H, Wang W-W, et al. Post-marketing safety surveillance of the Salvia miltiorrhiza Depside Salt for infusion: a real world study. PloS One 2017; 12: e0170182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ammirati E, Contri R, Coppini R, et al. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Failure 2016; 18: 1106–1118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ult-10.1177_1742271X20987584 for Intraventricular pressure gradients change during the development of left ventricular hypertrophy: Effect of salvianolic acid B and beta-blocker by Danfu Ma, Ahmed S Mandour, Tomohiko Yoshida, Katsuhiro Matsuura, Kazumi Shimada, Pitipat Kitpipatkun, Akiko Uemura, Mayumi Ifuku, Ken Takahashi and Ryou Tanaka in Ultrasound
Supplemental material, sj-pdf-2-ult-10.1177_1742271X20987584 for Intraventricular pressure gradients change during the development of left ventricular hypertrophy: Effect of salvianolic acid B and beta-blocker by Danfu Ma, Ahmed S Mandour, Tomohiko Yoshida, Katsuhiro Matsuura, Kazumi Shimada, Pitipat Kitpipatkun, Akiko Uemura, Mayumi Ifuku, Ken Takahashi and Ryou Tanaka in Ultrasound