Abstract
Epidemiological surveys suggest that excessive drinking is associated with higher risk of Alzheimer’s disease (AD). The present study utilized data from the National Alzheimer’s Coordinating Center data set to examine cognition as well as gray/white matter and ventricular volumes among participants with AD and alcohol use disorder (AD/AUD, n=52), AD only (n=701), AUD only (n=67), and controls (n=1283). AUD diagnosis was associated with higher Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) in AD than in non-AD. AD performed worse on semantic fluency and Trail Making Test A + B (TMT A + B) and smaller total GMV, WMV, and larger ventricular volume than non-AD. AD had smaller regional GMV in the inferior/superior parietal cortex, hippocampal formation, occipital cortex, inferior frontal gyrus, posterior cingulate cortex, and isthmus cingulate cortex than non-AD. AUD participants had significantly smaller somatomotor cortical GMV and showed a trend towards smaller volume in the hippocampal formation, relative to non-AUD participants. Misuse of alcohol has an additive effect on dementia severity among AD participants. Smaller hippocampal volume is a common feature of both AD and AUD. Although AD is associated with more volumetric deficits overall, AD and AUD are associated with atrophy in largely distinct brain regions.
1. Introduction
1.1. Alcohol use, AD risk and cognitive function
Epidemiological studies suggest that excessive drinking and life-time alcohol use are associated with the risk of Alzheimer’s disease (AD) (Rehm et al., 2019; Xu et al., 2017). Among patients with possible or probable AD, a history of heavy drinking was associated with an earlier onset of AD (Harwood et al., 2010). A prospective 7-year study of over 3,000 men showed that daily drinking was associated with a hazard ratio of 2.14 of AD (Zhou et al., 2014). A study evaluating 1394 participants with mild cognitive impairment (MCI) showed that, among the AD risk factors, including depression, obesity, and hypercholesterolemia, alcohol use most significantly elevated the risk of cognitive decline, regardless of AD pathology (Bos et al., 2017). Heavy drinkers dually diagnosed with AD demonstrated faster cognitive decline on the Mini-Mental State Examination, relative to mild-moderate drinkers or abstainers during 19-year follow-up (Heymann et al., 2016). On the other hand, some studies suggest that lower-level drinking can be protective against AD. For example, individuals who consumed less than one drink per week showed lower Modified Mini-Mental State Examination scores at follow-ups, compared to abstainers (Koch et al., 2019). The adjusted odds for dementia were lower in individuals consuming less than one (0.65), one to six (0.46), seven to 13 (0.69) drinks but higher for those with 14 or more drinks (1.22) per week, relative to non-drinkers (Mukamal et al., 2003). Thus, how alcohol use impacts the brain and influences the onset of AD likely depends on the severity of alcohol consumption (Rehm et al., 2019). A meta-analysis of eleven studies with 73,330 participants and 4,586 cases of all-cause dementia (AD and vascular) reported that modest (≤12.5 g/day) and excessive (≥38 g/day) alcohol consumption are associated with a reduced and elevated risk of dementia respectively (Xu et al., 2017).
Despite this literature suggesting potential effects of heavy alcohol use on the development of AD, few studies have systematically investigated cognition in AD, relative to AUD. An earlier work reported that individuals with AD relative to those with AUD exhibited worse performance on all cognitive measures, including attention, naming, immediate and delayed recall, visuo-constructive ability, semantic fluency, and executive function (Liappas et al., 2007). Further, it remains unclear whether AD and AUD share or show distinct neural pathology at the systems level.
1.2. Alcohol use and AD pathology
Experimental research has aimed to elucidate the mechanisms underlying alcohol use as a risk factor for AD. AD pathogenesis is driven by abnormal extracellular β-amyloid plaques and intracellular neurofibrillary tangles of tau proteins, leading to neurodegeneration and progressive cognitive impairment. Post-mortem studies have revealed early neurodegenerative changes in the entorhinal cortex, followed by the hippocampal formation and isocortex (Braak et al., 2006; Braak et al., 1993). β-amyloid is produced by β-secretase (BACE1) and γ-secretase via proteolytic cleavage of amyloid precursor protein (APP) (Heneka et al., 2015; Hooper, 2005; Tiraboschi et al., 2004). Animal research suggests that chronic alcohol administration accentuates AD pathogenesis by increasing the expression of APP, BACE1, and γ-secretase subunits in the hippocampus, cerebellum, and striatum (Kim et al., 2011). Chronic alcohol administration increases levels of APP and BACE1 and promotes β-amyloid production both in vitro and in vivo in transgenic AD model mice (Huang et al., 2018). Furthermore, chronic alcohol administration increases β-amyloid deposition and neuritic plaque formation in the brain and worsens learning and memory impairments (Huang et al., 2018). In addition to these effects on the primary disease process of AD, alcohol alone exerts neurotoxic effects and reduces neuroreceptor densities in the hippocampal formation and other brain regions (Freund and Ballinger, 1988, 1989a, b, 1992; Laukkanen et al., 2013; Nordberg et al., 1983), which can produce AD-like cognitive deficits, though less severe and reversible (Sullivan and Pfefferbaum, 2005). Thus, AD and AUD may demonstrate shared and distinct neuropathologies.
1.3. Brain imaging studies of AD and AUD
Structural brain imaging provides a venue to investigate the neuropathology shared by and potentially distinct to AD and AUD. A recent study found that frequent alcohol use was independently associated with diminished gray matter volumes (GMV) in the posterior cingulate cortex (PCC), thalamus, hippocampus, and orbitofrontal cortex, brain regions widely implicated in the progression of AD (Suzuki et al., 2019). Alcohol-dependent patients showed smaller GMV in the medial frontal and lateral prefrontal cortex as well as posterior cortical regions, and the extent of GMV reduction predicted relapse to heavy drinking (Rando et al., 2011). Recent mega-analyses/meta-analyses identified smaller and/or thinner anterior/posterior cingulate, superior frontal, lateral orbitofrontal, and temporal cortex as well as the hippocampus, insula, thalamus, and striatum in alcohol-dependent individuals (Hahn et al., 2020; Mackey et al., 2019; Yang et al., 2016). A recent meta-analysis revealed deficits in the frontal white matter and corpus callosum in AUD relative to healthy participants (Nowaczyk, 2019). These volumetric deficits have also been reported in AD. A meta-analysis showed significantly smaller GMV in the parahippocampal gyrus, PCC, fusiform and superior frontal gyri in AD versus controls (Wang et al., 2015). Another meta-analysis reported smaller white matter volumes in the inferior temporal gyrus, splenium of the corpus callosum, parahippocampal gyrus, and hippocampus in AD relative to controls (Wang et al., 2015). These data suggest both shared and distinct gray and white matter volumetric deficits in AD and AUD.
However, very few imaging studies have directly compared individuals with AD and AUD, and none have compared AD-alone to participants dually diagnosed with AD and AUD. An earlier work reported higher spin lattice relaxation times (T1) – indicative of higher water content and atrophy – in the frontal and temporal gray and white matter as well as the parietal and occipital white matter in AD, and higher T1 only in the frontal white matter in alcohol-related dementia, as compared to healthy individuals (Besson et al., 1989). Further, AD participants had higher T1 in the parietal and temporal white matter, relative to those with alcohol-related dementia. Another study reported larger ventricles and disrupted integrity of the corpus callosum in both AD and AUD vs. controls, and in AD vs. AUD (Pitel et al., 2010). More studies are warranted to investigate whether AD and AUD may demonstrate different cerebral volumetric deficits as well as the volumetric bases of cognitive dysfunction.
1.4. The present study
The present study examined cognition and brain volumes in participants with AD and AUD (AD+AUD+), AD only (AD+AUD−), AUD only (AD−AUD+), and controls (AD−AUD−). Our goal was to explore both shared and distinct volumetric changes and how these structural brain deficits may relate to cognitive dysfunction in AD and AUD. In particular, we examined whether AUD would add significantly to cognitive dysfunction and volumetric deficits observed in AD.
2. Methods
2.1. Dataset: participants and clinical assessments
We included participants from the National Alzheimer’s Coordinating Center (NACC) data set (https://naccdata.org/; September 2020 data freeze). The NACC data are contributed by approximately 39 past and present Alzheimer’s Disease Research Centers (ADRCs) supported by the U.S. National Institute on Aging. Since 2005, ADRCs have contributed standardized cognitive, behavioral, and functional data from approximately annual study visits to a common database, known as the NACC-Uniform Data Set or UDS (Beekly et al., 2004; Besser et al., 2018; Morris et al., 2006; Weintraub et al., 2009). A subset of ADRCs have also submitted structural MRI data (Alosco et al., 2018) to NACC to include with the UDS. The clinic-based population includes participants with AD and related disorders and MCI as well as cognitively normal participants. The recruitment and data collection procedures have been described previously (Beekly et al., 2007; Morris et al., 2006). All ADRCs that contribute data to NACC are approved by their local Institutional Review Boards and participants provided informed consent at the ADRC where they were enrolled.
Figure 1 shows the flow chart of participant inclusion and exclusion. We included participants with AD and a history of alcohol abuse (AD+AUD+ group; n=52), AD without a history of alcohol abuse (AD+AUD−; n=701), alcohol abuse without AD (AD−AUD+; n=67), and non-demented non-AUD controls (AD−AUD−; n=1283). MRI scans were not always performed at the time of UDS visits, when neuropsychological, neurological, and neuropsychiatric data were collected. Thus, MRI visits were matched ± 6 months within a UDS visit. When multiple MRI visits were available, the latest visit was chosen. The diagnosis of alcohol abuse was based on DSM-IV criteria. The diagnoses of AD were based on available UDS data, including neuropsychological, neurological, and neuropsychiatric, and imaging findings. Clinical research diagnoses of cognitive status and disease etiology were made at each UDS visit using established criteria for MCI and AD dementia. The NACC variable ALCOHOL was used to identify participants with (1=recent/active; 2=remote/inactive) and without a history of alcohol abuse (0=absent). It is defined as clinically significant impairment occurring over a 12-month period and manifested in one of the following areas: work, driving, legal, or social. The UDS protocol does not exclude participants with alcohol dependence. Thus, participants with [ALCOHOL] may be presumed to have a history of AUDs (i.e., alcohol abuse or dependence). Among those with an AUD, the ratio of current to past to unknown alcohol abuse history was 13:37:2 in AD+AUD+ group and 10:57:0 in AD-AUD+ group (χ2=4.833; p=0.09). Due to missing data on the ALCOHOL variable (n=584), additional participants without alcohol abuse were identified using the coding ALCABUSE = 8 (participants with normal cognition or who are cognitively impaired without an etiologic diagnosis of alcohol abuse).
Figure 1:
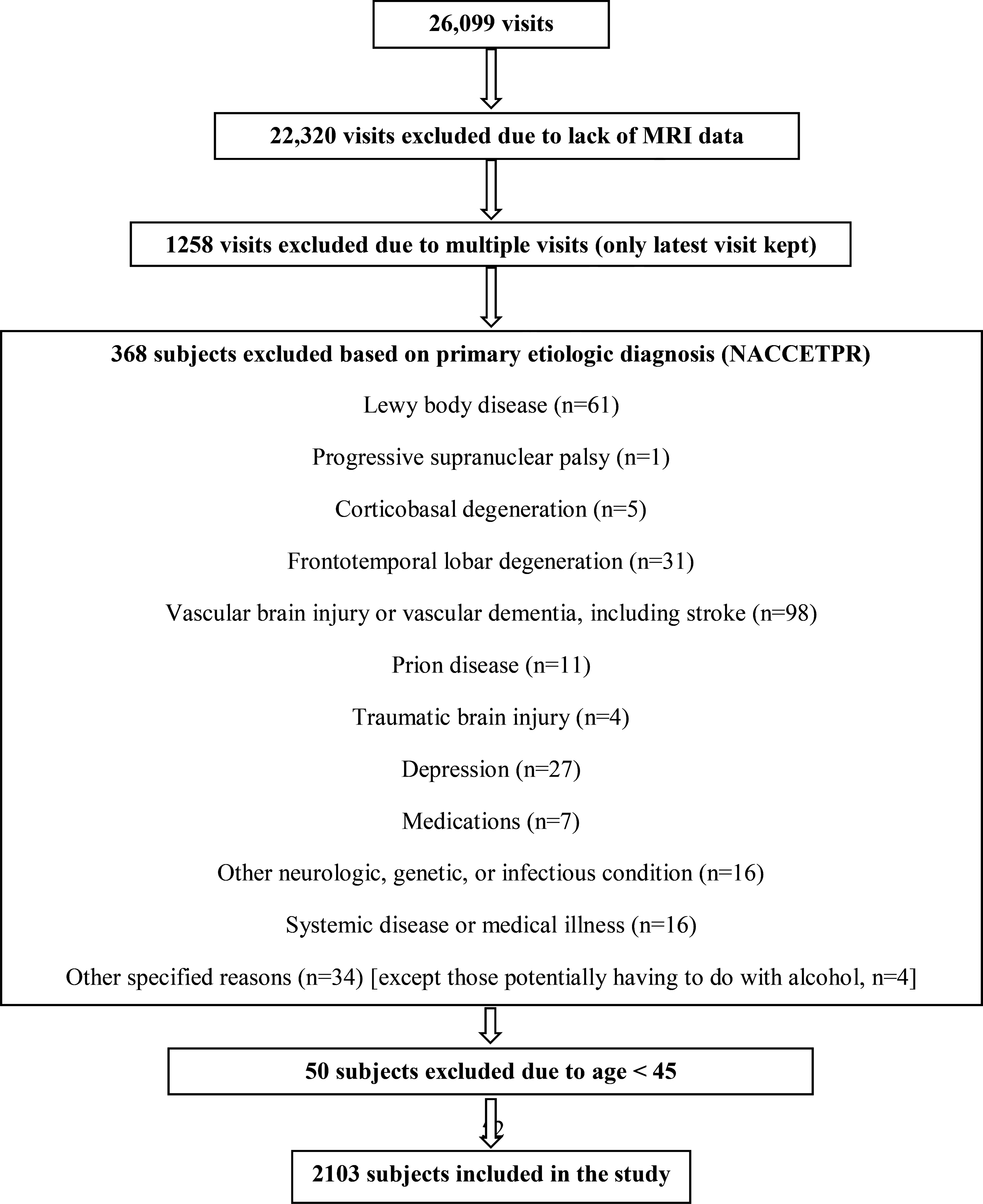
A flow chart of inclusion/exclusion of the data set. NACCETPR = primary etiologic diagnosis variable.
As shown in Table 1, AD participants were older than non-AD (F=40.7; p<0.001) participants, and AUD participants were older than non-AUD participants (F=7.0; p=0.008). The ratio of men vs. women among AD and AUD participants was higher than among non-AD (χ2=47.8; p<0.001) and non-AUD (χ2=27.8; p<0.001) participants, respectively. The level of education among AD and AUD participants was lower than among non-AD (F=6.9; p=0.009) and non-AUD (F=20.7; p<0.001) participants, respectively. Thus, we included age, sex, and years of education in all data analyses, including the analysis of variance and stepwise linear regression (See Results).
Table 1:
Socio-demographic, cognitive, and clinical characteristics of the subjects
| AD+ AUD+ (N=52) | AD+ AUD− (N=701) | AD− AUD+ (N=67) | AD− AUD− (N=1283) | ANCOVA |
|||
|---|---|---|---|---|---|---|---|
| AD group main effect | AUD group main effect | AD × AUD interaction | |||||
|
| |||||||
| Age (yr) | 74.7 (9.6) | 77.0 (8.6) | 68.5 (10.0) | 71.2 (10.5) | F=40.7; p<0.0001 | F=7.0; p=0.008 | F=0.0; p=0.8 |
| Male % | 75% | 48% | 54% | 35% | χ2=47.8; p<0.0001 | χ2=27.8; p<0.0001 | --- |
| Education (yr) | 13.4 (4.2) | 15.0 (3.5) | 14.4 (4.3) | 15.7 (3.2) | F=6.9; p=0.009 | F=20.7; p<0.0001 | F=0.4; p=0.5 |
| CDR-SB | 6.1 (4.8) | 4.6 (3.8) | 0.4 (0.7) | 0.2 (0.5) | F=473.4; p<0.0001 | F=10.2; p=0.001 | F=7.6; p=0.006 |
| Semantic fluency | 18.4 (9.0) | 19.8 (8.6) | 33.9 (9.7) | 36.2 (9.0) | F=295.6; p<0.0001 | F=1.0; p=0.3 | F=0.9; p=0.3 |
| Trails A (s) | 62.5 (33.2) | 60.5 (36.3) | 36.8 (23.7) | 31.6 (14.9) | F=100.4; p<0.0001 | F=0.6; p=0.4 | F=0.9; p=0.4 |
| Trails B (s) | 193.7 (79.2) | 183.8 (79.8) | 104.7 (77.9) | 84.7 (48.3) | F=212.7; p<0.0001 | F=3.7; p=0.06 | F=1.4; p=0.2 |
| Cognitive PC1 | 1.2 (0.9) | 1.0 (0.9) | −0.3 (0.7) | −0.6 (0.5) | F=490.4; p<0.0001 | F=5.7; p=0.02 | F=0.0 p=0.9 |
Data presented as mean +/− SD. Age, sex, and education were used as covariates in the ANCOVA for CDR-SB, semantic fluency, and TMT A and B. R = right; L= left; AD+AUD+ = AD with history of alcohol use disorder; AD+AUD− = AD without a history of alcohol use disorder; AD−AUD+ = history of alcohol use disorder without AD; AD−AUD− = no history of AD or AUD. PC1: first principal component of the PCA of CDR-SB, semantic fluency, and TMTs. P values that met p<0.05, FDR-corrected are highlighted in bold.
All participants were administered a standardized battery of neuropsychological tests at each study visit. These NACC-UDS tests are described in detail elsewhere (Beekly et al., 2004; Besser et al., 2018; Monsell et al., 2016; Weintraub et al., 2009). Dementia severity was examined using the CDR® Dementia Staging Instrument Sum of Boxes (CDR-SB) (Morris, 1993). Participants were asked to name as many 1) animals and 2) vegetables as they could in 60 seconds and scores were combined into a composite measure of semantic fluency. Speed of information processing and executive function were examined using Trail-making tests (TMT) A and B, respectively. Higher scores reflect worse performance on the CDR-SB and TMT A and B but better performance on semantic fluency.
2.2. MRI procedures and data processing
NACC MRI data used in the current study were acquired at fifteen different ADRCs using fifteen different scanner models of three different manufactures at 1.5 or 3 Tesla. The distribution of participants scanned on various models among the groups was skewed: AD+AUD+ were scanned on 8 of the 15 models; AD+AUD− on all of the 15 models; AD−AUD+ on 9 of the 15 models; and AD−AUD− on 14 of the 15 models. MRI data at NACC are best characterized as a convenience sample of images. Imaging data collection and acquisition protocols varied by ADRC. Each individual was scanned with a number of sequences but for this study we only used the baseline T1-weighted volumetric scans. GMV, WMV, and ventricular volume were computed by the IDeA lab at UC Davis following Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocols (Supplementary Methods).
2.3. Data analyses
Data were analyzed using IBM SPSS Statistics 26.0. Chi-square was used to analyze categorical data. A two-way analysis of covariance (ANCOVA) was used to analyze continuous data between the groups with sociodemographic, cognitive and MRI data as dependent variables and groups AD and AUD as fixed factors. Age and sex were entered as covariates in the analyses of clinical and cognitive test data.
The data of total intracranial volume (TIV), total GMV, WMV, lateral and third ventricular volume as well as the GMVs of 62 distinct brain regions were derived from data processed by IDeA lab (Aljabar et al., 2009; Fletcher et al., 2012a; Leung et al., 2011). The hippocampal volume was noted to comprise both gray and white matters. In the analyses of the 64 regional volumes, we performed principal component analysis (PCA) with promax rotation (K=4) and factor scores saved using a regression method. Component extraction was based on the latent root criterion where all factors with eigenvalues < 1 were discarded as insignificant. We set a minimum loading value at 0.4 for inclusion in a component for interpretative purposes (Stevens, 1992). Age, sex, and TIV were entered as covariates in the AD (+ vs. −) × AUD (+ vs. −) ANCOVA, and false discovery rate (FDR, p<0.05) was employed to correct for multiple comparisons.
To identify the “predictors” of cognitive performance, we first performed PCA on CDR-SB, TMT-A, TMT-B, and semantic fluency score to identify potentially distinct cognitive metrics. The PCA identified only one component with an eigenvalue > 1 and the weight of this PC served as the dependent variable of cognition. In stepwise linear regression with GMV components that differed significantly between the groups (see Results) and covariates (age, sex, years of education, and TIV) as the regressors, we identified the variables that best predicted this PC. Stepwise regression generates consecutive models in which significant predictors are sorted according to the amount of variance they account for in explaining a given dependent variable. An independent variable is added if the F test yields a p<0.05 and is removed if p>0.10. This is done until the model contains only the significant variables.
3. Results
3.1. Clinical characteristics
AD and AUD participants had higher CDR-SB than non-AD (F=473.4; p<0.0001) and non-AUD (F=10.2; p=0.001) participants, respectively. Further, there was a greater effect of AUD in AD than in non-AD participants (F=7.6; p=0.006). In the semantic fluency test, AD participants were able to name fewer animals and vegetables in 60 seconds than non-AD participants (F=295.6; p<0.0001). Finally, AD participants took more time to complete TMT A (F=100.4; p<0.0001) + B (F=212.7; p<0.0001) than non-AD participants. There were no significant interaction effects in semantic fluency or TMTs.
PCA of CDR-SB, semantic fluency and TMTs identified a single component with an eigenvalue > 1, which accounted for 70.1% of the variance. ANCOVA on this PC showed that AD and AUD participants had poorer cognitive performance than non-AD (F=490.4; p<0.0001) and non-AUD (F=5.7; p=0.02) participants, respectively, without a significant interaction effect.
3.2. Brain volumes
In ANCOVA with age, sex, years of education, and TIV as covariates, AD participants had smaller total GMV (F=70.5; p<0.0001) and WMV (F=5.2; p=0.02) than non-AD participants. AD participants also had larger lateral (F=83.2; p<0.0001) and third ventricular volumes (F=25.9; p<0.0001) than non-AD participants (Table 2). There were no significant AUD group main or AD × AUD interaction effect in any of these volumetric measures.
Table 2:
Total gray matter, white matter, and ventricular volume
| AD+ AUD+ (N=52) | AD+ AUD− (N=701) | AD− AUD+ (N=67) | AD− AUD− (N=1283) | ANCOVA |
|||
|---|---|---|---|---|---|---|---|
| AD group main effect | AUD group main effect | AD × AUD interaction | |||||
|
| |||||||
| Total GMV | 567.6 (61.9) | 564.1 (61.8) | 597.8 (63.6) | 595.9 (62.6) | F=70.5; p<0.0001 | F=2.7; p=0.1 | F=0.0; p=0.9 |
| Total WMV | 441.6 (61.0) | 426.1 (56.0) | 460.3 (65.3) | 445.1 (64.4) | F=5.2; p=0.02 | F=0.1; p=0.8 | F=0.0; p=0.9 |
| Total LVV | 52.0 (29.7) | 49.3 (24.8) | 29.2 (15.4) | 29.8 (17.7) | F=83.2; p<0.0001 | F=0.7; p=0.4 | F=0.5; p=0.5 |
| Total TVV | 1.7 (0.6) | 1.7 (0.6) | 1.3 (0.6) | 1.2 (0.5) | F=25.9; p<0.0001 | F=0.1; p=0.7 | F=1.1; p=0.3 |
Data presented as mean +/− SD. Age, sex, education, and total intracranial volume were used as covariates in the ANCOVA. AD+AUD+ = AD with history of alcohol use disorder; AD+AUD− = AD without a history of alcohol use disorder; AD−AUD+ = history of alcohol use disorder without AD; AD−AUD− = no history of AD or AUD. GMV = gray matter volume; WMV = white matter volume; LVV = lateral ventricle volume; TVV = third ventricle volume. P values that met p<0.05, FDR-corrected are highlighted in bold.
PCA of the 64 regional brain volumes revealed 8 components with an eigenvalue > 1 (Supplementary Table S1), with heaviest loadings on regions of the somatomotor cortex for component 1, inferior/superior parietal cortex (IPC/SPC) for component 2, the hippocampal formation for component 3, the occipital cortex for component 4, the inferior frontal gyrus (IFG) for component 5, the anterior cingulate cortex for component 6, the PCC for component 7, and the isthmus cingulate cortex (ICC) for component 8.
For each of the volumetric PCs, we performed an ANCOVA with age, sex, years of education and TIV as covariates. AUD vs. non-AUD showed smaller GMV for the somatomotor cortex (F=5.8; p=0.02) and a trend towards smaller hippocampal formation (F=4.7; p=0.03). AD vs. non-AD showed smaller GMV for the IPC/SPC (F=83.1; p<0.0001), hippocampal formation (F=235.6; p<0.0001), occipital cortex (F=9.6; p=0.002), IFG (F=23.4; p<0.0001), PCC (F=62.4; p<0.0001), and ICC (F=6.8; p=0.009) (Table 3). None of the interaction effects were significant.
Table 3:
ANCOVA of the eight principal components identified of regional brain volumes
| AD+ AUD+ (N=52) | AD+ AUD− (N=701) | AD− AUD+ (N=67) | AD− AUD− (N=1283) | ANCOVA |
|||
|---|---|---|---|---|---|---|---|
| AD group main effect | AUD group main effect | AD × AUD interaction | |||||
|
| |||||||
| Component 1 | |||||||
| Somatomotor c. | −0.3 (1.0) | −0.2 (1.0) | −0.1 (1.0) | 0.1 (1.0) | F=0.0; p=0.9 | F=5.8; p=0.02 | F=2.0; p=0.2 |
| Component 2 | |||||||
| Inferior / superior parietal c. | −0.3 (1.0) | −0.4 (1.0) | 0.3 (0.9) | 0.2 (0.9) | F=83.1; p<0.0001 | F=0.6; p=0.4 | F=0.0; p=0.9 |
| Component 3 | |||||||
| Hippocampal c. | −0.7 (1.1) | −0.6 (1.0) | 0.4 (0.9) | 0.4 (0.8) | F=235.6; p<0.0001 | F=4.7; p=0.03 | F=1.6; p=0.2 |
| Component 4 | |||||||
| Occipital c. | −0.2 (1.0) | 0.2 (1.0) | 0.1 (0.9) | 0.1 (1.0) | F=9.6; p=0.002 | F=2.0; p=0.2 | F=0.6; p=0.4 |
| Component 5 | |||||||
| Inferior frontal g. | −0.2 (0.9) | −0.3 (1.0) | 0.3 (0.9) | 0.2 (1.0) | F=23.4; p<0.0001 | F=0.0; p=0.9 | F=0.1; p=0.7 |
| Component 6 | |||||||
| Anterior cingulate c. | 0.2 (1.1) | 0.1 (1.0) | −0.0 (0.9) | −0.1 (1.0) | F=2.1; p=0.1 | F=0.1; p=0.7 | F=0.2; p=0.7 |
| Component 7 | |||||||
| Posterior cingulate c. | −0.2 (0.9) | −0.4 (1.0) | 0.3 (1.0) | 0.2 (0.9) | F=62.4; p<0.0001 | F=0.1 p=0.8 | F=0.0; p=0.9 |
| Component 8 | |||||||
| Isthmus cingulate c. | −0.2 (1.0) | −0.2 (1.0) | 0.1 (1.0) | 0.1 (1.0) | F=6.8; p=0.009 | F=1.8; p=0.2 | F=1.0; p=0.3 |
Data presented as mean +/− SD. Age, sex, education, and total intracranial volume were used as covariates in the ANCOVA. AD+AUD+ = AD with history of alcohol use disorder; AD+AUD− = AD without a history of alcohol use disorder; AD−AUD+ = history of alcohol use disorder without AD; AD−AUD− = no history of AD or AUD. P values that met p<0.05, FDR-corrected are highlighted in bold.
3.3. Relationship between GMVs and cognitive performance
As described earlier, PCA on CDR-SB, semantic fluency and TMTs revealed one component with an eigenvalue > 1 and explaining 70.1% of the variance. The stepwise regression produced the best model with five volumetric predictors—hippocampal formation, IPC/SPC, PCC, somatomotor cortex, and ICC, in that order — which along with age, sex, education and TIV accounted for 50% of the variance (F=232.8; p<0.0001) (Table 4). Smaller volumes in the hippocampal formation (t= −6.1; p<0.0001), IPC/SPC (t= −14.9; p<0.0001), PCC (t= −3.9; p<0.0001), and somatomotor cortex (t= −3.8; p=0.0001) were associated with worse cognitive performance. Conversely, larger ICC volumes were associated with worse performance, though only at marginal statistical significance (t=2.2; p=0.02).
Table 4:
Predictors of cognitive performance in stepwise regression
| r2 | β | Statistics | |
|---|---|---|---|
|
| |||
| Model | 0.50 | F=232.8; p<0.0001 | |
| Hippocampal complex | −0.39 | t=−16.1; p<0.0001 | |
| Inferior / superior parietal cortex | −0.38 | t=−14.9; p<0.0001 | |
| Posterior cingulate cortex | −0.09 | t=−3.9; p<0.0001 | |
| Somatomotor complex | −0.08 | t=−3.8; p=0.0001 | |
| Isthmus cingulate | 0.05 | t=2.2; p=0.02 | |
Components 1–5, 7, 8, sex, age, education and total intracranial volume were entered into the model.
4. Discussion
Both AD and AUD are associated with cognitive deficits, with an interaction effect showing a significantly higher CDR-SB in AD+AUD+ than in AD+AUD−. Whereas AD is associated with widespread reduction in GMVs and white matter volumes (WMV), AUD is associated more specifically with GMV deficits in the somatomotor cortex. After accounting for age, sex, education and TIV, volume of the hippocampal formation represents the most significant predictor of cognitive function for the entire cohort. We highlighted the main findings in discussion.
4.1. Cognitive dysfunction
AD and AUD showed higher CDR-SB, relative to non-AD and non-AUD participants, respectively, and the effects of AUD diagnosis showed significant influences on CDR-SB in AD participants. This finding is consistent with epidemiological studies showing that heavy drinking represents a major risk factor of AD (Bos et al., 2017; Harwood et al., 2010; Xu et al., 2017; Zhou et al., 2014) and an earlier report that patients with AD and AUD dual diagnosis demonstrated faster cognitive decline, compared to those with AD who were mild-moderate drinkers or abstainers (Heymann et al., 2016). In contrast, patients with AD+AUD+ who were alcohol abstainers did not appear to demonstrate disproportionate cognitive deficits compared with those with AD alone (Rosen et al., 1993; Toda et al., 2013). These findings together suggest that the effects of AUD on cognitive functioning of AD may not be permanent (Sullivan and Pfefferbaum, 2005). In the present study, we included both past and current AUD participants, which may explain the lack of an interaction effect for semantic fluency and TMT performance.
AD had poorer semantic fluency, performance on TMT A + B, and cognitive PC1 than non-AD participants. Semantic processes are distinctively disrupted early in the course of AD, likely due to parietal/temporal cortical pathology (Baldo et al., 2006; Clark et al., 2009; Eastman et al., 2013; Jutten et al., 2020). Semantic fluency declined the fastest in individuals at high risk for AD, including apolipoprotein E e4 carriers and those with amnesic MCI (Vonk et al., 2020). Semantic fluency captured significant one-year decline in AD as early as Stage 1 (no evidence of clinical impact) in the National Institute of Aging – Alzheimer’s Association clinical staging scheme, suggesting that it is disrupted early in the course of the disease (Jutten et al., 2020). A meta-analysis of 15,990 AD participants found that semantic but not phonemic fluency was significantly more impaired than measures of verbal intelligence and psychomotor speed (Henry et al., 2004). Structural MRI revealed that poorer semantic fluency was associated with bilateral atrophy of the IPC, frontal lobe, and temporal lobe in AD (Baldo et al., 2006). Lower baseline semantic fluency in AD was associated with less hippocampal volume as well as more cortical thinning and reduced glucose metabolism in the IPC, entorhinal cortex, ICC, and precuneus/PCC (Vonk et al., 2020). Further, some studies reported lower semantic fluency in alcohol misusers (Dao-Castellana et al., 1998; Heffernan et al., 2019; Villa et al., 2019), but others did not (Green et al., 2010; Nowakowska-Domagała et al., 2017; Topiwala et al., 2017). We also observed that AD took more time to complete TMT A + B than non-AD, consistent with previous studies (Jutten et al., 2020; Shindo et al., 2013; Terada et al., 2013). The TMT A and B each evaluates visuo-perceptual abilities and graphomotor speed, and task switching, respectively (Misdraji and Gass, 2010; Sánchez-Cubillo et al., 2009). AD who scored poorly vs. those who did well on the TMT-A showed hypoperfusion in the SPC (Shindo et al., 2013). AD patients with poor vs. those with good TMT-B scores exhibited hypoperfusion in the anterior cingulate, caudate, putamen, and thalamus (Terada et al., 2013). In contrast, the findings were mixed for AUD, with some (Cordovil De Sousa Uva et al., 2010; Moggi et al., 2020; Scholey et al., 2019) but not other (Choi et al., 2014; Konrad et al., 2012) studies showing worse performance on TMTs, a discrepancy likely related to alcohol use severity and abstinence time, as reported in a meta-analysis of cognitive function in dependent drinkers (Stavro et al., 2013). Taken together, these data suggest that semantic fluency, visuo-perceptual abilities/graphomotor speed, and task switching are disrupted in AD. We did not find any AUD effect on these individual cognitive tests, but AUD participants scored worse than non-AUD participants in PC1, which is likely to be driven by the significant differences in CDR-SB between AUD and non-AUD.
4.2. Brain volumes
AD but not AUD showed significantly smaller total GMV, WMV, and larger lateral and third ventricular volumes, indicating greater brain atrophy, than non-AD. Meta-analyses have reported GMV and WMV reductions in AD and AUD (Nowaczyk, 2019; Wang et al., 2015; Yang et al., 2016; Yin et al., 2015). A previous study observed higher T1 – a measure of brain water content and tissue atrophy – among AD participants than controls in frontal and temporal gray and white matter, whereas individuals with alcohol-related dementia showed higher T1 only in the frontal white matter (Besson et al., 1989). Another imaging study found that AD and AUD participants had larger lateral ventricles than controls, and AD had larger ventricles than AUD participants, which indicates more significant brain atrophy in AD relative to AUD (Pitel et al., 2010). Moreover, both AD and AUD groups exhibited disruptions in the white matter integrity of the corpus callosum. It is possible that we did not observe smaller brain volume in AUD in the present study because our AUD group comprised a large number of individuals who were in abstinence. Indeed, a previous longitudinal MRI study of AUD participants showed that one-month abstinence and relapse is each associated with recovery of cortical GMV and further shrinkage of the third ventricle, respectively (Pfefferbaum et al., 1995). In addition, the literature has highlighted changes in white matter microstructure rather than volumetric changes in relation to the effect of aging on white matter (Giorgio et al., 2010; Shokri-Kojori et al., 2021), which may be why we did not find any differences in WMV between AUD participants and controls. Overall, our data suggest that AD has a more deleterious effect on the brain than normal aging, as evidenced by lower gray and white matter volume as well as larger ventricular volume.
Volumetric deficits may also be limited to specific brain regions. Indeed, we observed smaller somatomotor cortical GMV in AUD, but not AD. The pre, para- and post-central gyri are part of the primary motor and somatosensory cortices, which are responsible for movement control and somatic sensation. Alcohol is well-known for its deleterious effects on motor and somatosensory function (Bogart et al., 1992; Chu and Yang, 1987; Neiman et al., 1990; Zhornitsky et al., 2010). Reduced cortical thickness was previously found among abstinent alcohol dependent individuals with frontal and temporal regions, including the pre- and post-central gyri, relative to controls (Fortier et al., 2011). A meta-analysis revealed gray matter reductions in the precentral gyrus in people with AUD, relative to controls (Yang et al., 2016). In contrast, AD pathology appeared to affect the primary sensory and motor cortices only during the advanced stages of the illness (Braak et al., 2006; Braak et al., 1993). These findings together suggest that GMV reductions in the somatomotor cortex may represent a specific marker of AUD, even in individuals who abstain from alcohol use. On the other hand, previous studies have reported volumetric and other morphometric markers in younger adults with AUD (Cao et al., 2021; Chye et al., 2020; Grace et al., 2021; Hahn et al., 2020; Ide et al., 2017; Mackey et al., 2019; Yang et al., 2020). It is likely that, in addition to potential recovery during abstinence, alcohol use-related structural brain changes may become less evident in older individuals, who have already manifested structural brain changes due to aging.
AD had smaller volume in the hippocampal formation than non-AD, and AUD trended towards smaller volume in the hippocampal formation, relative to non-AUD participants. Moreover, after accounting for age, sex, education and TIV, volume of the hippocampal formation represented the most significant predictor of cognitive function for the entire cohort. Meta-analyses have consistently reported hippocampal and parahippocampal GMV reductions in MCI and AD (Chen et al., 2020; Wang et al., 2015). The hippocampus, entorhinal, and parahippocampal gyri are part of a network of brain regions that support memory encoding and retrieval, and the pathology of this hippocampal formation is evident in early stages of AD (Braak et al., 2006; Braak et al., 1993). The hippocampal formation demonstrates functional changes in healthy aging, MCI and AD (Hu and Li, 2020; Li et al., 2018). Post-mortem data showed that sector CA1 of the hippocampus, subiculum, entorhinal cortex, and parahippocampal isocortex were among the brain regions with most significant atrophy, whereas the dentate gyrus was much less affected, in AD vs. controls (Narkiewicz et al., 1993). In AUD, post-mortem and imaging studies have shown neuronal loss (Bengochea and Gonzalo, 1990) as well as smaller volume (Fein and Fein, 2013; Kurth et al., 2004; Lee et al., 2016; Oscar-Berman and Song, 2011) and blood flow in the hippocampus (Suzuki et al., 2010). Further, a meta-analysis found that problem alcohol use was associated with significantly smaller hippocampal volume (Wilson et al., 2017). In our sample, the hippocampal volume was smaller in AUD vs. non-AUD, but the difference failed to reach significance after correction for multiple comparisons, likely because of recovery in abstinent drinkers (Agartz et al., 1999; Crews and Nixon, 2009; Harding et al., 1997; Korbo, 1999; Laakso et al., 2000). Further, we did not find an interaction effect, despite the fact that hippocampal function was disrupted in both AD and AUD. Nonetheless, low hippocampal formation volume was the most significant predictor of poor cognitive performance across all participants. This suggests a central role of hippocampal dysfunction in cognitive deficits in AD and AUD.
AD showed smaller regional GMV in the IPC/SPC vs. non-AD. Central to language functions, the left IPC has been implicated in deficits of semantic fluency in AD (Vonk et al., 2020). A previous study reported greater left and right IPC atrophy in participants who developed AD within 6 years of a baseline MRI scan, relative to those who were cognitively stable (Jacobs et al., 2011). Another longitudinal study showed faster atrophy of the IPC along with the hippocampus, entorhinal cortex, temporal pole, fusiform gyrus, and inferior and middle temporal gyri within 4–5 years in AD converters vs. non-converters (Desikan et al., 2006). The SPC is involved in spatial orientation, memory, and attention (Bagattini et al., 2019; Neufang et al., 2011; Wager and Smith, 2003; Wagner et al., 2005). Several studies have reported SPC atrophy in AD (Bakkour et al., 2013; Prvulovic et al., 2002; Teipel et al., 2007). Disconnection of the SPC from the precuneus was associated with worse memory capability in older relative to younger AD patients (Prawiroharjo et al., 2020). AD patients exhibited disconnection of the SPC from the middle frontal gyrus, in association with impairment in top-down attentional control (Neufang et al., 2011). Together, these earlier findings are consistent with IPC and SPC GMV loss and cognitive deficits in AD.
AD showed smaller regional GMV in the pericalcarine, cuneus, and lingual gyrus of the occipital cortex than non-AD participants. The occipital cortex processes visual information in the brain. Post-mortem data showed that the occipital cortex only begins to be affected in the last two stages of AD (Braak et al., 2006). AD patients with visual hallucinations have been shown to exhibit a smaller occipital/whole brain ratio on MRI than those without (Holroyd et al., 2000). Furthermore, constructional apraxia in AD was strongly related to early-stage tau hyperphosphorylation in occipital cortical post-mortem samples (Nielson et al., 1996; Smith et al., 2001). As a whole, these data suggest that occipital cortical dysfunction is related to poor visuospatial and visuomotor functions in AD.
AD showed smaller GMV than non-AD patients in the pars triangularis and pars orbitalis of the IFG, consistent with deficits in attention, memory, and executive function in AD (Cai et al., 2017; Hu et al., 2015). Repetitive transcranial magnetic stimulation of the IFG improved performance on TMT A + B in early AD (Eliasova et al., 2014). In a meta-analysis of 28 fMRI studies, MCI patients exhibited attenuated activation in the IFG during verbal retrieval, relative to controls (Nellessen et al., 2015). Another consequence of damage to frontal regions is a lack of insight, which is a common symptom of AD (Wilson et al., 2016). Further, in a review of 32 structural and functional imaging studies, IFG dysfunction was most consistently associated with anosognosia or a lack of awareness of one’s own illness in AD (Hallam et al., 2020).
AD patients showed smaller GMV in the PCC than non-AD patients, in accord with an earlier meta-analysis (Wang et al., 2015). The PCC is a key hub of the default mode network (DMN), a network of interacting brain regions that is active when a person is inwardly focused, as during self-reflection or recollection of autobiographical memory (Leech and Sharp, 2014). Reduced metabolism, β amyloid deposition, and atrophy in the DMN, including the PCC, have been identified as early signs of AD (Buckner et al., 2009; Buckner et al., 2005; Johnson et al., 1998; Minoshima et al., 1997). Additionally, functional connectivity is disrupted within the DMN, especially between the PCC and hippocampus, in AD (Greicius et al., 2004). The PCC has also been associated with anosognosia and deficits in meta-cognition in AD (Hallam et al., 2020).
4.3. Limitations of the study and conclusions
The present study has limitations. Firstly, we used cross-sectional data, so the current findings fall short in addressing causal relationship between brain volumes and cognition. Secondly, the sample comprised very different number of participants and showed significant differences in age, sex, and education between the groups. Although we considered these variables in data analyses, it remains a possibility that the differences may have introduced biases in the findings; thus, whether the findings generalize to groups with more balanced age and gender composition remains unclear. Thirdly, UDS does not include questions assessing severity or duration of alcohol misuse, and thus the cumulative alcohol effects could not be assessed for individuals. Further, the low sample size of AUD participants did not allow us to perform separate analyses on current versus past alcohol misuse. Finally, the participants were scanned on 15 different scanner models, which were not evenly distributed among the groups, and it remains unclear how scanning sites and models may have influenced the findings.
In conclusion, this is the first study of its kind to examine brain volumes among dual diagnosis AD and AUD patients, relative to individuals with AD or AUD alone and controls. We found that AUD contributed to cognitive deficits in AD patients. AD patients exhibited smaller total GMV, WMV, and larger ventricular volume, and all regional except somatomotor cortical GMVs, relative to non-AD patients. AUD demonstrated smaller GMV of the somatomotor cortex and a trend towards smaller volume in the hippocampal formation, compared to non-AUD participants. Further, hippocampal GMV represented the best predictor of cognitive dysfunction across all participants. Altogether, the findings suggest that chronic alcohol consumption may have an additive effect, leading to worse cognitive functioning, among AD participants. However, the magnitude of this effect is relatively small likely because the majority of our AUD participants were past users.
Supplementary Material
Highlights.
A dual diagnosis of Alzheimer’s disease and alcohol use disorder is associated with higher dementia severity than subjects with either disorder alone.
Subjects with Alzheimer’s disease had lower regional GMV in the inferior / superior parietal cortex, hippocampal complex, occipital cortex, inferior frontal gyrus, posterior cingulate cortex, and isthmus cingulate cortex than subjects without Alzheimer’s disease.
Subjects with alcohol use disorders had lower regional GMV in the somatomotor cortex, and trended towards lower volume in the hippocampal complex than subjects without alcohol use disorders.
Support and Disclosures
The current study is supported by NIH grants R21 AG067024, R01 AG072893, R01 CA218502, and P30 AG066508 as well as a VA Merit Award CX 001301. The NIH is otherwise not responsible for the design of the study or data analyses and interpretation or in the decision to publish the current results. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADRCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). We have no conflicts of interest in the current study.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW, 1999. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 56, 356–363. [DOI] [PubMed] [Google Scholar]
- Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D, 2009. Multi-atlas based segmentation of brain images: atlas selection and its effect on accuracy. Neuroimage 46, 726–738. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Sugarman MA, Besser LM, Tripodis Y, Martin B, Palmisano JN, Kowall NW, Au R, Mez J, DeCarli C, Stein TD, McKee AC, Killiany RJ, Stern RA, 2018. A Clinicopathological Investigation of White Matter Hyperintensities and Alzheimer’s Disease Neuropathology. J Alzheimers Dis 63, 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagattini C, Mutanen TP, Fracassi C, Manenti R, Cotelli M, Ilmoniemi RJ, Miniussi C, Bortoletto M, 2019. Predicting Alzheimer’s disease severity by means of TMS-EEG coregistration. Neurobiol Aging 80, 38–45. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC, 2013. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage 76, 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF, 2006. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 12, 896–900. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA, 2007. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 21, 249–258. [DOI] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA, 2004. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord 18, 270–277. [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM, 1990. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol 5, 349–357. [PubMed] [Google Scholar]
- Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, Jicha G, Carlsson C, Burns J, Quinn J, Sweet RA, Rascovsky K, Teylan M, Beekly D, Thomas G, Bollenbeck M, Monsell S, Mock C, Zhou XH, Thomas N, Robichaud E, Dean M, Hubbard J, Jacka M, Schwabe-Fry K, Wu J, Phelps C, Morris JC, 2018. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 32, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson JA, Crawford JR, Parker DM, Smith FW, 1989. Magnetic resonance imaging in Alzheimer’s disease, multi-infarct dementia, alcoholic dementia and Korsakoff’s psychosis. Acta Psychiatr Scand 80, 451–458. [DOI] [PubMed] [Google Scholar]
- Bogart CJ, Gonzalez LP, McNeill DL, 1992. Acute and chronic ethanol alter somatosensory-evoked potentials in conscious rats. Alcohol 9, 43–48. [DOI] [PubMed] [Google Scholar]
- Bos I, Vos SJ, Frölich L, Kornhuber J, Wiltfang J, Maier W, Peters O, Rüther E, Engelborghs S, Niemantsverdriet E, De Roeck EE, Tsolaki M, Freund-Levi Y, Johannsen P, Vandenberghe R, Lleó A, Alcolea D, Frisoni GB, Galluzzi S, Nobili F, Morbelli S, Drzezga A, Didic M, van Berckel BN, Salmon E, Bastin C, Dauby S, Santana I, Baldeiras I, de Mendonça A, Silva D, Wallin A, Nordlund A, Coloma PM, Wientzek A, Alexander M, Novak GP, Gordon MF, Wallin Å K, Hampel H, Soininen H, Herukka SK, Scheltens P, Verhey FR, Visser PJ, 2017. The frequency and influence of dementia risk factors in prodromal Alzheimer’s disease. Neurobiol Aging 56, 33–40. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K, 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, 1993. Staging of Alzheimer-related cortical destruction. Eur Neurol 33, 403–408. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA, 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29, 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA, 2005. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Ottino-Gonzalez J, Cupertino RB, Schwab N, Hoke C, Catherine O, Cousijn J, Dagher A, Foxe JJ, Goudriaan AE, Hester R, Hutchison K, Li CR, London ED, Lorenzetti V, Luijten M, Martin-Santos R, Momenan R, Paulus MP, Schmaal L, Sinha R, Sjoerds Z, Solowij N, Stein DJ, Stein EA, Uhlmann A, van Holst RJ, Veltman DJ, Wiers RW, Yücel M, Zhang S, Jahanshad N, Thompson PM, Conrod P, Mackey S, Garavan H, 2021. Mapping cortical and subcortical asymmetries in substance dependence: Findings from the ENIGMA Addiction Working Group. Addict Biol, e13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xu W, Xue C, Hu G, Ma W, Qi W, Dong L, Lin X, Chen J, 2020. Voxelwise Meta-Analysis of Gray Matter Abnormalities in Mild Cognitive Impairment and Subjective Cognitive Decline Using Activation Likelihood Estimation. J Alzheimers Dis 77, 1495–1512. [DOI] [PubMed] [Google Scholar]
- Choi SW, Kim HS, Kim GY, Jeon Y, Park SM, Lee JY, Jung HY, Sohn BK, Choi JS, Kim DJ, 2014. Similarities and differences among Internet gaming disorder, gambling disorder and alcohol use disorder: a focus on impulsivity and compulsivity. J Behav Addict 3, 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu NS, Yang SS, 1987. Somatosensory and brainstem auditory evoked potentials in alcoholic liver disease with and without encephalopathy. Alcohol 4, 225–230. [DOI] [PubMed] [Google Scholar]
- Chye Y, Mackey S, Gutman BA, Ching CRK, Batalla A, Blaine S, Brooks S, Caparelli EC, Cousijn J, Dagher A, Foxe JJ, Goudriaan AE, Hester R, Hutchison K, Jahanshad N, Kaag AM, Korucuoglu O, Li CR, London ED, Lorenzetti V, Luijten M, Martin-Santos R, Meda SA, Momenan R, Morales A, Orr C, Paulus MP, Pearlson G, Reneman L, Schmaal L, Sinha R, Solowij N, Stein DJ, Stein EA, Tang D, Uhlmann A, van Holst R, Veltman DJ, Verdejo-Garcia A, Wiers RW, Yücel M, Thompson PM, Conrod P, Garavan H, 2020. Subcortical surface morphometry in substance dependence: An ENIGMA addiction working group study. Addict Biol 25, e12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LJ, Gatz M, Zheng L, Chen YL, McCleary C, Mack WJ, 2009. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. Am J Alzheimers Dis Other Demen 24, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordovil De Sousa Uva M, Luminet O, Cortesi M, Constant E, Derely M, De Timary P, 2010. Distinct effects of protracted withdrawal on affect, craving, selective attention and executive functions among alcohol-dependent patients. Alcohol Alcohol 45, 241–246. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, 2009. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A, 1998. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med 28, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Eastman JA, Hwang KS, Lazaris A, Chow N, Ramirez L, Babakchanian S, Woo E, Thompson PM, Apostolova LG, 2013. Cortical thickness and semantic fluency in Alzheimer’s disease and mild cognitive impairment. Am J Alzheimers Dis (Columbia) 1, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasova I, Anderkova L, Marecek R, Rektorova I, 2014. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. J Neurol Sci 346, 318–322. [DOI] [PubMed] [Google Scholar]
- Fein G, Fein D, 2013. Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. Neuroimage Clin 3, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Carmichael O, Decarli C, 2012a. MRI non-uniformity correction through interleaved bias estimation and B-spline deformation with a template. Annu Int Conf IEEE Eng Med Biol Soc 2012, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, Williams V, Milberg WP, McGlinchey RE, 2011. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol Clin Exp Res 35, 2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund G, Ballinger WE Jr., 1988. Loss of cholinergic muscarinic receptors in the frontal cortex of alcohol abusers. Alcohol Clin Exp Res 12, 630–638. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WE Jr., 1989a. Loss of muscarinic and benzodiazepine neuroreceptors from hippocampus of alcohol abusers. Alcohol 6, 23–31. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WE Jr., 1989b. Neuroreceptor changes in the putamen of alcohol abusers. Alcohol Clin Exp Res 13, 213–218. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WE Jr., 1992. Alzheimer’s disease and alcoholism: possible interactions. Alcohol 9, 233–240. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H, 2010. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 51, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace S, Rossetti MG, Allen N, Batalla A, Bellani M, Brambilla P, Chye Y, Cousijn J, Goudriaan AE, Hester R, Hutchison K, Labuschagne I, Momenan R, Martin-Santos R, Rendell P, Solowij N, Sinha R, Li CR, Schmaal L, Sjoerds Z, Suo C, Terrett G, van Holst RJ, Veltman DJ, Yücel M, Thompson P, Conrod P, Mackey S, Garavan H, Lorenzetti V, 2021. Sex differences in the neuroanatomy of alcohol dependence: hippocampus and amygdala subregions in a sample of 966 people from the ENIGMA Addiction Working Group. Transl Psychiatry 11, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C, 2010. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol Clin Exp Res 34, 443–450. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V, 2004. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Mackey S, Cousijn J, Foxe JJ, Heinz A, Hester R, Hutchinson K, Kiefer F, Korucuoglu O, Lett T, Li CR, London E, Lorenzetti V, Maartje L, Momenan R, Orr C, Paulus M, Schmaal L, Sinha R, Sjoerds Z, Stein DJ, Stein E, van Holst RJ, Veltman D, Walter H, Wiers RW, Yucel M, Thompson PM, Conrod P, Allgaier N, Garavan H, 2020. Predicting alcohol dependence from multi-site brain structural measures. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam B, Chan J, Gonzalez Costafreda S, Bhome R, Huntley J, 2020. What are the neural correlates of meta-cognition and anosognosia in Alzheimer’s disease? A systematic review. Neurobiol Aging 94, 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM, 1997. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 7, 78–87. [DOI] [PubMed] [Google Scholar]
- Harwood DG, Kalechstein A, Barker WW, Strauman S, St George-Hyslop P, Iglesias C, Loewenstein D, Duara R, 2010. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry 25, 511–518. [DOI] [PubMed] [Google Scholar]
- Heffernan T, Samuels A, Hamilton C, McGrath-Brookes M, 2019. Alcohol Hangover Has Detrimental Impact Upon Both Executive Function and Prospective Memory. Front Psychiatry 10, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP, 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, Phillips LH, 2004. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia 42, 1212–1222. [DOI] [PubMed] [Google Scholar]
- Heymann D, Stern Y, Cosentino S, Tatarina-Nulman O, Dorrejo JN, Gu Y, 2016. The Association Between Alcohol Use and the Progression of Alzheimer’s Disease. Curr Alzheimer Res 13, 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd S, Shepherd ML, Downs JH 3rd, 2000. Occipital atrophy is associated with visual hallucinations in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 12, 25–28. [DOI] [PubMed] [Google Scholar]
- Hooper NM, 2005. Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans 33, 335–338. [DOI] [PubMed] [Google Scholar]
- Hu S, Li CR, 2020. Age-Related Structural and Functional Changes of the Hippocampus and the Relationship with Inhibitory Control. Brain Sci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Yu M, Yang S, Lou D, Zhou W, Zheng L, Wang Z, Cai F, Zhou W, Li T, Song W, 2018. Ethanol Alters APP Processing and Aggravates Alzheimer-Associated Phenotypes. Mol Neurobiol 55, 5006–5018. [DOI] [PubMed] [Google Scholar]
- Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Li CR, 2017. Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. Neuroimage Clin 14, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Van Boxtel MP, Uylings HB, Gronenschild EH, Verhey FR, Jolles J, 2011. Atrophy of the parietal lobe in preclinical dementia. Brain Cogn 75, 154–163. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS, 1998. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology 50, 1563–1571. [DOI] [PubMed] [Google Scholar]
- Jutten RJ, Sikkes SAM, Amariglio RE, Buckley RF, Properzi MJ, Marshall GA, Rentz DM, Johnson KA, Teunissen CE, Van Berckel BNM, Van der Flier WM, Scheltens P, Sperling RA, Papp KV, 2020. Identifying Sensitive Measures of Cognitive Decline at Different Clinical Stages of Alzheimer’s Disease. J Int Neuropsychol Soc, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Jeong HY, Yang S, Choi SP, Seo MY, Yun YK, Choi Y, Baik SH, Park JS, Gwon AR, Yang DK, Lee CH, Lee SM, Park KW, Jo DG, 2011. Effects of chronic alcohol consumption on expression levels of APP and Aβ-producing enzymes. BMB Rep 44, 135–139. [DOI] [PubMed] [Google Scholar]
- Koch M, Fitzpatrick AL, Rapp SR, Nahin RL, Williamson JD, Lopez OL, DeKosky ST, Kuller LH, Mackey RH, Mukamal KJ, Jensen MK, Sink KM, 2019. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults With or Without Mild Cognitive Impairment. JAMA Netw Open 2, e1910319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Lorscheider M, Bernow N, Thümmel M, Chai C, Pfeifer P, Stoeter P, Scheurich A, Fehr C, 2012. Broad disruption of brain white matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol Alcohol 47, 118–126. [DOI] [PubMed] [Google Scholar]
- Korbo L, 1999. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res 23, 164–168. [PubMed] [Google Scholar]
- Kurth C, Wegerer V, Reulbach U, Lewczuk P, Kornhuber J, Steinhoff BJ, Bleich S, 2004. Analysis of hippocampal atrophy in alcoholic patients by a Kohonen feature map. Neuroreport 15, 367–371. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J, 2000. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res 109, 177–186. [DOI] [PubMed] [Google Scholar]
- Laukkanen V, Storvik M, Häkkinen M, Akamine Y, Tupala E, Virkkunen M, Tiihonen J, 2013. Decreased GABA(A) benzodiazepine binding site densities in postmortem brains of Cloninger type 1 and 2 alcoholics. Alcohol 47, 103–108. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C, 2010. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and Alzheimer disease. Stroke 41, 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KK, Barnes J, Modat M, Ridgway GR, Bartlett JW, Fox NC, Ourselin S, 2011. Brain MAPS: an automated, accurate and robust brain extraction technique using a template library. Neuroimage 55, 1091–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jia X, Qi Z, Fan X, Ma T, Pang R, Ni H, Li CR, Lu J, Li K, 2018. Disrupted Functional Connectivity of Cornu Ammonis Subregions in Amnestic Mild Cognitive Impairment: A Longitudinal Resting-State fMRI Study. Front Hum Neurosci 12, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liappas I, Theotoka I, Kapaki E, Ilias I, Paraskevas GP, Soldatos CR, 2007. Neuropsychological assessment of cognitive function in chronic alcohol-dependent patients and patients with Alzheimer’s disease. In Vivo 21, 1115–1118. [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, Brooks S, Caparelli E, Chye YY, Cousijn J, Dagher A, Desrivieres S, Feldstein-Ewing S, Foxe JJ, Goldstein RZ, Goudriaan AE, Heitzeg MM, Hester R, Hutchison K, Korucuoglu O, Li CR, London E, Lorenzetti V, Luijten M, Martin-Santos R, May A, Momenan R, Morales A, Paulus MP, Pearlson G, Rousseau ME, Salmeron BJ, Schluter R, Schmaal L, Schumann G, Sjoerds Z, Stein DJ, Stein EA, Sinha R, Solowij N, Tapert S, Uhlmann A, Veltman D, van Holst R, Whittle S, Wright MJ, Yücel M, Zhang S, Yurgelun-Todd D, Hibar DP, Jahanshad N, Evans A, Thompson PM, Glahn DC, Conrod P, Garavan H, 2019. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry 176, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE, 1997. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42, 85–94. [DOI] [PubMed] [Google Scholar]
- Misdraji EL, Gass CS, 2010. The Trail Making Test and its neurobehavioral components. J Clin Exp Neuropsychol 32, 159–163. [DOI] [PubMed] [Google Scholar]
- Moggi F, Ossola N, Graser Y, Soravia LM, 2020. Trail Making Test: Normative Data for Patients with Severe Alcohol Use Disorder. Subst Use Misuse 55, 1790–1799. [DOI] [PubMed] [Google Scholar]
- Monsell SE, Dodge HH, Zhou XH, Bu Y, Besser LM, Mock C, Hawes SE, Kukull WA, Weintraub S, 2016. Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord 30, 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA, 2006. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20, 210–216. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT Jr., Mittleman MA, Siscovick DS, 2003. Prospective study of alcohol consumption and risk of dementia in older adults. Jama 289, 1405–1413. [DOI] [PubMed] [Google Scholar]
- Narkiewicz O, de Leon MJ, Convit A, George AE, Wegiel J, Morys J, Bobinski M, Golomb J, Miller DC, Wisniewski HM, 1993. Dilatation of the lateral part of the transverse fissure of the brain in Alzheimer’s disease. Acta Neurobiol Exp (Wars) 53, 457–465. [PubMed] [Google Scholar]
- Neiman J, Lang AE, Fornazzari L, Carlen PL, 1990. Movement disorders in alcoholism: a review. Neurology 40, 741–746. [DOI] [PubMed] [Google Scholar]
- Nellessen N, Rottschy C, Eickhoff SB, Ketteler ST, Kuhn H, Shah NJ, Schulz JB, Reske M, Reetz K, 2015. Specific and disease stage-dependent episodic memory-related brain activation patterns in Alzheimer’s disease: a coordinate-based meta-analysis. Brain Struct Funct 220, 1555–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Akhrif A, Riedl V, Förstl H, Kurz A, Zimmer C, Sorg C, Wohlschläger AM, 2011. Disconnection of frontal and parietal areas contributes to impaired attention in very early Alzheimer’s disease. J Alzheimers Dis 25, 309–321. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Cummings BJ, Cotman CW, 1996. Constructional apraxia in Alzheimer’s disease correlates with neuritic neuropathology in occipital cortex. Brain Res 741, 284–293. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Larsson C, Perdahl E, Winblad B, 1983. Changes in cholinergic activity in human hippocampus following chronic alcohol abuse. Pharmacol Biochem Behav 18 Suppl 1, 397–400. [DOI] [PubMed] [Google Scholar]
- Nowaczyk N, 2019. Frontal and callosal white matter changes and their potential effects on neurocognitive functioning in alcohol addicts: A meta-analysis. Acta Neuropsychologica 17, 189–205. [Google Scholar]
- Nowakowska-Domagała K, Jabłkowska-Górecka K, Mokros Ł, Koprowicz J, Pietras T, 2017. Differences in the verbal fluency, working memory and executive functions in alcoholics: Short-term vs. long-term abstainers. Psychiatry Res 249, 1–8. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Song J, 2011. Brain volumetric measures in alcoholics: a comparison of two segmentation methods. Neuropsychiatr Dis Treat 7, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO, 1995. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 19, 1177–1191. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Chanraud S, Sullivan EV, Pfefferbaum A, 2010. Callosal microstructural abnormalities in Alzheimer’s disease and alcoholism: same phenotype, different mechanisms. Psychiatry Res 184, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawiroharjo P, Yamashita KI, Yamashita K, Togao O, Hiwatashi A, Yamasaki R, Kira JI, 2020. Disconnection of the right superior parietal lobule from the precuneus is associated with memory impairment in oldest-old Alzheimer’s disease patients. Heliyon 6, e04516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prvulovic D, Hubl D, Sack AT, Melillo L, Maurer K, Frölich L, Lanfermann H, Zanella FE, Goebel R, Linden DE, Dierks T, 2002. Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage 17, 1403–1414. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R, 2011. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry 168, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M, 2019. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J, Colantonio A, Becker JT, Lopez OL, DeKosky ST, Moss HB, 1993. Effects of a history of heavy alcohol consumption on Alzheimer’s disease. Br J Psychiatry 163, 358–363. [DOI] [PubMed] [Google Scholar]
- Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, Rodríguez-Sánchez JM, Ríos-Lago M, Tirapu J, Barceló F, 2009. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc 15, 438–450. [DOI] [PubMed] [Google Scholar]
- Scholey A, Benson S, Kaufman J, Terpstra C, Ayre E, Verster JC, Allen C, Devilly GJ, 2019. Effects of Alcohol Hangover on Cognitive Performance: Findings from a Field/Internet Mixed Methodology Study. J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo A, Terada S, Sato S, Ikeda C, Nagao S, Oshima E, Yokota O, Uchitomi Y, 2013. Trail making test part a and brain perfusion imaging in mild Alzheimer’s disease. Dement Geriatr Cogn Dis Extra 3, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Bennett IJ, Tomeldan ZA, Krawczyk DC, Rypma B, 2021. Estimates of brain age for gray matter and white matter in younger and older adults: Insights into human intelligence. Brain Res 1763, 147431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MZ, Esiri MM, Barnetson L, King E, Nagy Z, 2001. Constructional apraxia in Alzheimer’s disease: association with occipital lobe pathology and accelerated cognitive decline. Dement Geriatr Cogn Disord 12, 281–288. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S, 2013. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol 18, 203–213. [DOI] [PubMed] [Google Scholar]
- Stevens JP, 1992. Applied multivariate statistics for the social sciences, 2nd ed. Erlbaum, Hillsdale, NJ. [Google Scholar]
- Sullivan EV, Pfefferbaum A, 2005. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 180, 583–594. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Venkataraman AV, Bai W, Guitton F, Guo Y, Dehghan A, Matthews PM, 2019. Associations of Regional Brain Structural Differences With Aging, Modifiable Risk Factors for Dementia, and Cognitive Performance. JAMA Netw Open 2, e1917257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Oishi M, Ogawa K, Mizutani T, 2010. Atrophy of the parahippocampal gyrus and regional cerebral blood flow in the limbic system in chronic alcoholic patients. Alcohol 44, 439–445. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Born C, Ewers M, Bokde AL, Reiser MF, Möller HJ, Hampel H, 2007. Multivariate deformation-based analysis of brain atrophy to predict Alzheimer’s disease in mild cognitive impairment. Neuroimage 38, 13–24. [DOI] [PubMed] [Google Scholar]
- Terada S, Sato S, Nagao S, Ikeda C, Shindo A, Hayashi S, Oshima E, Yokota O, Uchitomi Y, 2013. Trail making test B and brain perfusion imaging in mild cognitive impairment and mild Alzheimer’s disease. Psychiatry Res 213, 249–255. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J, 2004. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62, 1984–1989. [DOI] [PubMed] [Google Scholar]
- Toda A, Tagata Y, Nakada T, Komatsu M, Shibata N, Arai H, 2013. Changes in Mini-Mental State Examination score in Alzheimer’s disease patients after stopping habitual drinking. Psychogeriatrics 13, 94–98. [DOI] [PubMed] [Google Scholar]
- Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, Mahmood A, Fooks P, Singh-Manoux A, Mackay CE, Kivimäki M, Ebmeier KP, 2017. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. Bmj 357, j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa R, Espandian A, Sáiz PA, Astals M, Valencia JK, Martínez-Santamaría E, Álvarez S, García-Portilla MP, Bobes J, Flórez G, 2019. Cognitive functioning in patients with alcohol use disorder who start outpatient treatment. Adicciones 0, 1326. [DOI] [PubMed] [Google Scholar]
- Vonk JMJ, Bouteloup V, Mangin JF, Dubois B, Blanc F, Gabelle A, Ceccaldi M, Annweiler C, Krolak-Salmon P, Belin C, Rivasseau-Jonveaux T, Julian A, Sellal F, Magnin E, Chupin M, Habert MO, Chêne G, Dufouil C, 2020. Semantic loss marks early Alzheimer’s disease-related neurodegeneration in older adults without dementia. Alzheimers Dement (Amst) 12, e12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE, 2003. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 3, 255–274. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL, 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9, 445–453. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC, 2009. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 23, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Sytsma J, Barnes LL, Boyle PA, 2016. Anosognosia in Dementia. Curr Neurol Neurosci Rep 16, 77. [DOI] [PubMed] [Google Scholar]
- Wilson S, Bair JL, Thomas KM, Iacono WG, 2017. Problematic alcohol use and reduced hippocampal volume: a meta-analytic review. Psychol Med 47, 2288–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, Yu JT, 2017. Alcohol consumption and dementia risk: a dose-response meta-analysis of prospective studies. Eur J Epidemiol 32, 31–42. [DOI] [PubMed] [Google Scholar]
- Yang K, Yang Q, Niu Y, Fan F, Chen S, Luo X, Tan S, Wang Z, Tong J, Yang F, Le TM, Li CR, Tan Y, 2020. Cortical Thickness in Alcohol Dependent Patients With Apathy. Front Psychiatry 11, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Tian F, Zhang H, Zeng J, Chen T, Wang S, Jia Z, Gong Q, 2016. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: a voxel-based meta-analysis. Neurosci Biobehav Rev 66, 92–103. [DOI] [PubMed] [Google Scholar]
- Yin RH, Tan L, Liu Y, Wang WY, Wang HF, Jiang T, Radua J, Zhang Y, Gao J, Canu E, Migliaccio R, Filippi M, Gorno-Tempini ML, Yu JT, 2015. Multimodal Voxel-Based Meta-Analysis of White Matter Abnormalities in Alzheimer’s Disease. J Alzheimers Dis 47, 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Stip E, Pampoulova T, Rizkallah E, Lipp O, Bentaleb LA, Chiasson JP, Potvin S, 2010. Extrapyramidal symptoms in substance abusers with and without schizophrenia and in nonabusing patients with schizophrenia. Mov Disord 25, 2188–2194. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zhou R, Zhong T, Li R, Tan J, Zhou H, 2014. Association of smoking and alcohol drinking with dementia risk among elderly men in China. Curr Alzheimer Res 11, 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


