Abstract
Objectives
Although disparities in maternal vaccine acceptance among racial/ethnic groups are well documented, the reasons for these disparities are unclear. The objective of this study was to describe differences in pregnant women’s knowledge, attitudes, beliefs, intentions, and trust regarding maternal and infant vaccines by race/ethnicity.
Methods
We collected survey data from 1862 pregnant women from diverse prenatal care practices in Georgia and Colorado from June 2017 through July 2018. We performed multiple logistic regressions to determine differences in intentions, knowledge, attitudes, beliefs, and trust by race/ethnicity and calculated odds ratios (ORs) and 95% CIs.
Results
Compared with White women, Black and Hispanic women were less confident in vaccine safety and efficacy and less likely to perceive risk of acquiring vaccine-preventable diseases, report provaccine social norms, indicate having enough vaccine knowledge, and trust vaccine information from health care providers and public health authorities. Black women were the least confident in the safety of the maternal influenza vaccine (OR = 0.37; 95% CI, 0.27-0.49); maternal tetanus, diphtheria, and acellular pertussis vaccine (OR = 0.37; 95% CI, 0.27-0.52); and infant vaccines overall (OR = 0.40; 95% CI, 0.28-0.58), and were least likely to intend to receive both maternal vaccines (OR = 0.35; 95% CI, 0.27-0.47) or all infant vaccines on time (OR = 0.45; 95% CI, 0.34-0.61) as compared with White women.
Conclusions
Understanding differences in behavioral constructs integral to vaccine decision making among women of different races/ethnicities can lead to tailored interventions to improve vaccine acceptance.
Keywords: race, ethnicity, vaccine(s), pregnancy, attitudes
Two vaccines are routinely recommended during pregnancy in the United States: the influenza vaccine and the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. Although the influenza vaccine has been recommended during pregnancy since the 1960s, 1 interest in bolstering maternal influenza vaccine acceptance became a renewed public health priority during the 2009-2010 H1N1 influenza pandemic, when pregnant women were found to be at high risk for complications from infection with the pandemic strain. 2 The antenatal Tdap recommendation in the United States was expanded in 2012 to include administration at every pregnancy in an effort to curtail pertussis (whooping cough) mortality among infants; the first dose of infant diphtheria, tetanus, and acellular pertussis (DTaP) vaccine is not routinely administered until the infant is aged 2 months, leaving young infants vulnerable. 3 Since then, concerted efforts to promote maternal vaccination in the clinical obstetric setting have come to the fore.
The focus on promoting vaccines during pregnancy has not solely concentrated on maternal vaccine acceptance: interest is increasing in the promotion of childhood vaccines during pregnancy. 4 Studies exploring the origins of vaccine refusal show that many parents, especially those with negative attitudes toward childhood vaccines, begin to make decisions about vaccines before their children are born and soon after delivery. 5 Consequently, the prenatal period may be an optimal time for vaccine education, important as much for promoting maternal vaccine acceptance as for cultivating positive attitudes toward childhood vaccines, before misperceptions arise. 6
Despite efforts by public health officials and health care providers to promote vaccines during pregnancy, only about half of pregnant women in the United States receive influenza and Tdap vaccines. 7 Furthermore, disparities have emerged in coverage, the most notable of which is by race/ethnicity. In 2018, non-Hispanic Black women were the least likely racial/ethnic group to receive either influenza or Tdap vaccine during pregnancy. Although 53% and 59% of non-Hispanic White women received influenza and Tdap vaccines, respectively, 51% and 49% of Hispanic women and only 36% and 43% of non-Hispanic Black women, respectively, received them in 2018. 7 Despite these disparities, little attention has been given to understanding the reasons for their emergence, especially reasons related to behavior change.
One plausible explanation for these disparities is corresponding differences in trust (eg, of vaccine providers, manufacturers, researchers, and legislators), which likely plays a key role in vaccine confidence. Patients with considerable trust in their health care providers have improved outcomes across various health topics. 8 People who express vaccine hesitancy have lower levels of trust in government agencies and the pharmaceutical industry than people who do not express hesitancy. 9 Distrust in vaccine safety among Black people in the United States is stronger than it is among White people and is coupled with concerns about the government’s motives relating to racial/ethnic minority populations. 10,11 This distrust is understandable considering the history of experimentation on and abuse of Black people in the United States in medical research as well as the ongoing effects of structural racism and discrimination in health care. 12,13 Some researchers have posited that the mistrust of the medical community is central in explaining concerns about vaccines among Black women, 14 while others suggest negative attitudes toward their child’s health care provider as a cause. 15 Increasing trust in those who produce, require, recommend, and administer vaccines would likely also increase confidence in vaccines.
Beyond trust, confidence in vaccine effectiveness and knowledge of vaccines and vaccine-preventable diseases are strongly correlated with vaccine acceptance. 16 Risk perception is strongly associated with positive vaccination behaviors. 17 Confidence in vaccine safety also influences the self-reported vaccination behaviors of parents, 18 and social norms are linked to receipt of maternal influenza vaccine. 19 -21
Surveys assessing the attitudes and beliefs of pregnant women in the United States 22 -31 highlight their suboptimal vaccine knowledge, attitudes, and beliefs. Many surveys have demonstrated substantial differences in vaccine knowledge, attitudes, and beliefs by race/ethnicity. 32 -42 For example, Black people are less likely than White people to vaccinate against human papillomavirus 34,37 or to report trust in the influenza vaccine. 38,39 In addition, although Hispanic people are more likely than non-Hispanic White people to be concerned about serious adverse effects of human papillomavirus vaccines, they are also more likely to follow their physician’s recommendation to vaccinate. 40,42 However, these surveys were given to parents and focused on infant and adolescent vaccines, as opposed to pregnant women with a focus on maternal vaccines.
More must be learned about the racial/ethnic differences in the upstream factors that influence vaccine perceptions and acceptance among pregnant women. Understanding whether and how these constructs differ by race/ethnicity will inform the growing body of knowledge that supports the use of tailored and culturally sensitive vaccine promotion interventions among pregnant women. 43 Properly tailored vaccine messaging is a critical tool in addressing vaccine hesitancy and increasing vaccine acceptance. 44 This study explored whether and how vaccine knowledge, intentions, and perceptions differ by race/ethnicity and which factors may be most influential in vaccine decision making among pregnant women in the United States.
Methods
From June 2017 through July 2018, we administered a baseline survey to 2196 pregnant women as part of a larger study aimed at assessing the effectiveness of a multilevel intervention seeking to increase rates of maternal and childhood immunization. 45 Study staff members recruited pregnant women from waiting rooms of a geographically and sociodemographically diverse set of 23 prenatal care practices in Georgia and Colorado. We selected practices on the basis of a goal to capture diversity in patient demographic characteristics, urbanicity, practice size, and provider types. Eligibility criteria included gestational age of 8-26 weeks (because administration of Tdap vaccine is recommended to occur between 27 and 36 weeks of gestation), 3 aged 18-50, English speaking, and not having received a Tdap vaccine during their current pregnancy. After determining eligibility, recruiters obtained informed consent. Upon enrollment, we administered a baseline survey via electronic tablet in the office. We provided a $20 incentive for survey completion. This study was approved by the Emory University Institutional Review Board.
Survey Development
Survey development was informed by a review of relevant behavioral models, theories, scales, and previous survey items developed by our team and others. We selected several constructs based on the Parent Attitudes about Childhood Vaccines scale, developed and validated by Opel et al. 46,47 We also included other behavioral constructs related to vaccine behavior (eg, knowledge, beliefs, norms). 16 -21
The baseline survey included multiple-choice questions assessing the intention to receive recommended maternal and infant vaccines; it also asked about the number of previous pregnancies and included 58 Likert-type scale statements assessing various latent constructs related to maternal and infant vaccination. The statements measured the following constructs: confidence in vaccine safety and efficacy, perceived susceptibility to and severity of vaccine-preventable diseases, self-efficacy (an individual’s belief in the capacity to execute behaviors necessary to produce specific performance attainments), 48 descriptive norms (what people typically do) and injunctive norms (what people typically approve or disapprove), 49 perception of knowledge, and trust in information sources. Response options included strongly agree, agree, disagree, and strongly disagree; knowledge and trust statements included a “don’t know” option; and trust statements about pediatricians and naturopathic/chiropractic doctors included options for “I don’t have a pediatrician yet” and “I don’t see this type of doctor,” respectively. The survey also assessed information on race/ethnicity and educational attainment, although participants had the option of not providing this information. Mutually exclusive response options for race/ethnicity were Black, White, Hispanic, American Indian/Alaska Native, Asian, Native Hawaiian/other Pacific Islander, ≥2 races, and “other.” Mutually exclusive response options for highest education obtained were doctoral or professional degree, master’s degree, bachelor’s degree, associate’s degree, postsecondary nondegree award, some college but no degree, high school diploma or equivalent, and no formal educational credentials.
Data Analysis
As part of our initial analysis and to look for potential confounders, we used the Pearson χ2 test for independence to assess differences in race/ethnicity (reduced to Black, White, Hispanic, other) by other sociodemographic characteristics such as education (dichotomized as “high” for doctoral or professional degree, master’s degree, bachelor’s degree, or associate’s degree; otherwise “low,” unless missing), first pregnancy (yes or no), and state (Colorado or Georgia). All P values were 2-sided, and we considered P < .05 to be significant.
We dichotomized Likert scale responses as respondents who agreed or strongly agreed and respondents who did not. We performed multiple logistic regressions with dichotomous indicators for vaccine intentions or agreement with survey items as dependent variables and a dummy variable comparing each of Black and Hispanic with White race/ethnicity as the independent variable. Each of these regressions controlled for all of the sociodemographic characteristics described previously by including them as dependent variables in the analysis regardless of their significance. We calculated odds ratios (ORs) and 95% CIs for all logistic regressions. We considered CIs that did not overlap 1 to be significant.
We also created continuous construct summary scores to account for multiple survey items assessing each aforementioned latent construct. We encoded Likert scale responses as follows: 1, strongly disagree; 2, disagree; 3, don’t know (included only for items assessing knowledge or trust); 4, agree; and 5, strongly agree. We combined scores for items assessing each of the following constructs: confidence in vaccine safety (for the mother), confidence in vaccine safety (for the infant), confidence in vaccine efficacy (influenza), confidence in vaccine efficacy (whooping cough), self-efficacy, risk perception (maternal influenza), risk perception (maternal whooping cough), risk perception (infant whooping cough), perception of vaccine knowledge, social norms, trust in vaccine information (from obstetricians and pediatricians), trust in vaccine information (from naturopathic and/or chiropractic doctors), and trust in vaccine information (from federal agencies and academic institutions). We performed multiple linear regression models with construct summary scores as the independent variables and a dummy variable comparing each of Black and Hispanic with White race/ethnicity as the independent variable of interest, controlled for other sociodemographic characteristics as described previously. We performed all analyses using Stata/IC version 12.1 (StataCorp LLC).
Results
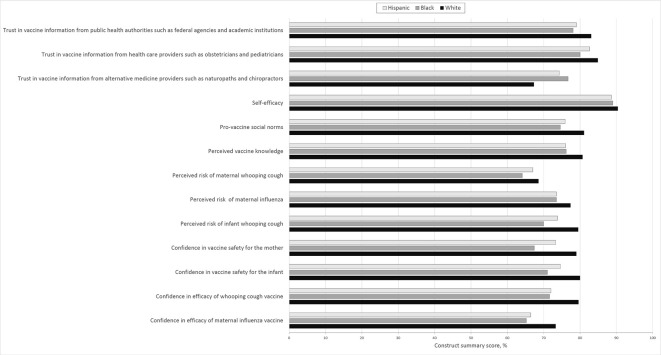
Of the 2196 pregnant women surveyed, 1862 (85%) provided information on race/ethnicity. Of these women, 63% were White, 17% were Black, and 11% were Hispanic (Table 1). Roughly half were from each state, and 45% were pregnant for the first time. Of the 1770 women who provided educational information, 71% had a high level of education. We found significant differences in race/ethnicity between Colorado participants and Georgia participants (P < .001): the percentage of White women was higher in Colorado (69%) than in Georgia (57%), the percentage of Hispanic women was higher in Colorado (16%) than in Georgia (6%), and the percentage of Black women was higher in Georgia (28%) than in Colorado (6%). We also found significant differences in educational attainment by race/ethnicity (P < .001): the percentage of women with a high level of education was lower among Hispanic women (45%) than among Black (63%) or White (77%) women. We found no significant differences in percentage of first-time mothers by race/ethnicity (P = .26). Black and Hispanic women had consistently lower construct summary scores than White women for almost every construct measured (Figure).
Table 1.
Race/ethnicity of participants (N = 1862) in a study on maternal and childhood immunization, stratified by state, whether first pregnancy, and educational attainment, June 2017–July 2018a
| Characteristic | Black | White | Hispanic | Other b | Total | P value c |
|---|---|---|---|---|---|---|
| All | 312 (17) | 1175 (63) | 209 (11) | 166 (9) | 1862 (100) | — |
| State of residence | <.001 | |||||
| Colorado | 56 (6) | 645 (69) | 150 (16) | 81 (9) | 932 (50) | |
| Georgia | 256 (28) | 530 (57) | 59 (6) | 85 (9) | 930 (50) | |
| First pregnancy | .26 | |||||
| Yes | 136 (44) | 525 (45) | 86 (41) | 85 (51) | 832 (45) | |
| No | 176 (56) | 650 (55) | 123 (59) | 81 (49) | 1030 (55) | |
| Educational attainment d | <.001 | |||||
| High | 183 (63) | 888 (77) | 82 (45) | 106 (69) | 1259 (71) | |
| Low | 106 (37) | 258 (23) | 100 (55) | 47 (31) | 511 (29) | |
aThe study team administered a survey to 2196 pregnant women in Colorado and Georgia as part of a larger study aimed at assessing the effectiveness of a multilevel intervention seeking to increase rates of maternal and childhood immunization 45 ; 1862 (85%) women provided information on race/ethnicity and completed the survey in its entirety. All values are number (percentage) unless otherwise indicated.
bIncludes American Indian/Alaska Native, Asian, Native Hawaiian/other Pacific Islander, ≥2 races, and “other.”
cPearson χ2 test for independence; significant at P < .05.
dHigh defined as having a doctoral or professional degree, master’s degree, bachelor’s degree, or associate’s degree; low defined as having a postsecondary nondegree award, some college but no degree, high school diploma or equivalent, or no formal educational credentials.
Figure.
Construct summary scores of pregnant White, Black, and Hispanic women participating in a survey among 2196 pregnant women as part of a larger study aimed at assessing the effectiveness of a multilevel intervention seeking to increase rates of maternal and childhood immunization 45 ; 1862 (85%) women provided information on race/ethnicity and completed the survey. Mean summary scores were standardized as the percentage of maximum possible value. Participants who stated they had not seen complementary or alternative medicine providers such as naturopathic and/or chiropractic doctors or pediatricians were removed from relevant analyses.
Intentions to Vaccinate
Black women (OR = 0.35; 95% CI, 0.27-0.47) and Hispanic women (OR = 0.52; 95% CI, 0.37-0.72) were less likely than White women to intend to receive both maternal vaccines (influenza and Tdap; Table 2). Black women (OR = 2.34; 95% CI, 1.60-3.41) and Hispanic women (OR = 1.67; 95% CI, 1.07-2.61) were more likely than White women to be unsure of their maternal vaccine intentions. Black women were less likely (OR = 0.45; 95% CI, 0.34-0.61) than White women to intend to receive all infant vaccines on time and more likely (OR = 3.99; 95% CI, 2.55-6.25) than White women to be unsure of their infant vaccine intentions (Table 3). Hispanic women were not significantly different from White women in their infant vaccine intentions.
Table 2.
Odds ratio (95% CI) for Black and Hispanic pregnant women intending to receive maternal vaccines and agreeing with maternal vaccine–related statements, compared with White pregnant women, controlled for state of residence, whether first pregnancy, and educational attainment (N = 1862), June 2017–July 2018a
| Maternal vaccine statements | Black b | Hispanic b |
|---|---|---|
| Maternal vaccine intentions | ||
| Influenza | 0.45 (0.34-0.60) c | 0.61 (0.44-0.85) c |
| Tdap | 0.33 (0.25-0.44) c | 0.41 (0.30-0.58) c |
| Influenza and Tdap | 0.35 (0.27-0.47) c | 0.52 (0.37-0.72) c |
| Influenza not Tdap | 2.57 (1.57-4.23) c | 2.11 (1.18-3.79) c |
| Tdap not influenza | 0.99 (0.65-1.50) | 0.63 (0.34-1.17) |
| Neither | 1.97 (1.37-2.84) c | 2.09 (1.37-3.19) c |
| Unsure | 2.34 (1.60-3.41) c | 1.67 (1.07-2.61) c |
| Confidence in maternal vaccine safety statements | ||
| I am confident that getting the flu vaccine during my pregnancy is safe for me. | 0.37 (0.27-0.49) c | 0.70 (0.48-1.02) |
| I am confident that getting the flu vaccine during my pregnancy is safe for my unborn baby. | 0.37 (0.27-0.49) c | 0.71 (0.49-1.03) |
| I am confident that getting the whooping cough vaccine during my pregnancy is safe for me. | 0.32 (0.24-0.44) c | 0.59 (0.40-0.86) c |
| I am confident that getting the whooping cough vaccine during my pregnancy is safe for my unborn baby. | 0.37 (0.27-0.52) c | 0.48 (0.33-0.70) c |
| Maternal vaccine knowledge, attitude, and belief statements | ||
| I worry that I could get the flu while I am pregnant. | 0.40 (0.29-0.55) c | 0.81 (0.55-1.19) |
| The flu is dangerous for pregnant women. | 1.25 (0.77-2.01) | 0.73 (0.45-1.18) |
| The flu is more dangerous for pregnant women than for women who are not pregnant. | 0.87 (0.60-1.27) | 0.62 (0.40-0.95) c |
| Getting the flu vaccine will reduce my risk of getting the flu during my pregnancy. | 0.38 (0.27-0.53) c | 0.54 (0.37-0.81) c |
| Getting the flu vaccine while I am pregnant will reduce my unborn baby’s risk of getting the flu. | 0.64 (0.47-0.88) c | 0.58 (0.40-0.86) c |
| I worry that I could get whooping cough while I am pregnant. | 0.47 (0.33-0.67) c | 1.06 (0.72-1.57) |
| I worry that I could give whooping cough to my baby after birth. | 0.42 (0.31-0.57) | 0.76 (0.52-1.12) |
| Whooping cough is dangerous for pregnant women. | 1.22 (0.83-1.79) | 0.67 (0.44-1.02) |
| Whooping cough vaccine will reduce my chances of getting whooping cough. | 0.35 (0.25-0.49) c | 0.48 (0.31-0.73) c |
| Whooping cough vaccine will reduce the chance of me giving whooping cough to my unborn baby. | 0.41 (0.30-0.57) c | 0.48 (0.32-0.71) c |
| Getting the whooping cough vaccine while I am pregnant will reduce my unborn baby’s risk of getting whooping cough. | 0.41 (0.30-0.56) c | 0.48 (0.33-0.71) c |
| It is in my control whether or not I get vaccines during my pregnancy. | 1.09 (0.35-3.39) | 0.59 (0.21-1.66) |
| The majority of my friends and family would get the vaccines that are recommended during pregnancy. | 0.45 (0.33-0.60) c | 0.59 (0.41-0.84) c |
| The majority of my friends and family would encourage me to get the vaccines that are recommended during pregnancy. | 0.47 (0.35-0.62) c | 0.56 (0.40-0.80) c |
| I have most of the important information I need to make a decision about vaccines given during pregnancy. | 0.45 (0.32-0.64) c | 0.64 (0.42-0.97) c |
| I know enough about the safety of the flu vaccine to make a decision about getting the vaccine for myself while pregnant. | 0.58 (0.39-0.86) c | 0.62 (0.39-0.98) |
| I know enough about the safety of the whooping cough vaccine to make a decision about getting the vaccine for myself while pregnant. | 0.60 (0.43-0.85) c | 0.69 (0.45-1.05) |
| Trust in maternal vaccine information source statements | ||
| I trust the information provided by my obstetrician or midwife about vaccines during pregnancy. | 0.59 (0.36-0.99) c | 0.68 (0.39-1.21) |
| I trust the information provided by my baby’s doctor about vaccines during pregnancy. d | 0.69 (0.42-1.16) | 0.65 (0.36-1.16) |
| I trust the information provided by naturopathic and/or chiropractic doctors about vaccines during pregnancy. d | 1.64 (1.12-2.39) c | 1.66 (1.06-2.60) c |
| I trust the information provided by federal agencies such as the Centers for Disease Control and Prevention (CDC) about vaccines during pregnancy. | 0.54 (0.39-0.75) c | 0.75 (0.50-1.11) |
| I trust the information provided by scientists and doctors at universities and academic institutions about vaccines during pregnancy. | 0.56 (0.40-0.78) c | 0.65 (0.43-0.99) c |
Abbreviation: Tdap, tetanus, diphtheria, and acellular pertussis.
aThe study team administered a survey to 2196 pregnant women in Colorado and Georgia as part of a larger study aimed at assessing the effectiveness of a multilevel intervention seeking to increase rates of maternal and childhood immunization. 45 Logistic regressions controlled for educational attainment (dichotomized as high for having a doctoral or professional degree, master’s degree, bachelor’s degree, or associate’s degree; low as having a postsecondary nondegree award, some college but no degree, high school diploma or equivalent, or no formal educational credentials), whether first pregnancy (yes/no), and state of residence (Colorado or Georgia).
bFor intention to receive maternal and infant vaccines or for agreeing with vaccine-related statements by race/ethnicity (a dummy variable comparing Black and Hispanic with White).
c95% CIs that did not overlap 1 were considered to be significantly different from White at P < .05.
dRespondents who stated they had not yet seen this type of provider were removed from analysis.
Table 3.
Odds ratio (95% CI) for Black and Hispanic pregnant women intending to receive infant vaccines and agreeing with infant vaccine–related statements, compared with White pregnant women, controlled for educational attainment, whether first child, and state of residence (N = 1862), June 2017–July 2018a
| Infant vaccine statements | Black b | Hispanic b |
|---|---|---|
| Infant vaccine intentions | ||
| All on time | 0.45 (0.34-0.61)c | 0.90 (0.63-1.28) |
| All but delayed | 0.79 (0.52-1.22) | 0.66 (0.39-1.11) |
| Some but on time | 3.11 (1.82-5.34)c | 1.93 (1.00-3.71) |
| Some but delayed | 1.52 (0.68-3.43) | 1.11 (0.44-2.80) |
| None | 0.92 (0.33-2.56) | 1.57 (0.62-3.95) |
| Unsure | 3.99 (2.55-6.25)c | 1.44 (0.78-2.66) |
| Confidence in infant vaccine safety statement | ||
| I am confident that vaccines are safe for my baby after birth. | 0.40 (0.28-0.58)c | 0.69 (0.43-1.09) |
| Infant vaccine knowledge, attitude, and belief statements | ||
| I worry that my baby could get whooping cough after birth. | 0.26 (0.19-0.36)c | 0.71 (0.48-1.05) |
| Whooping cough is dangerous for babies. | 0.49 (0.28-0.85)c | 0.40 (0.21-0.76)c |
| Whooping cough is more dangerous for babies than older children or adults. | 0.55 (0.36-0.85)c | 0.57 (0.34-0.98)c |
| Getting the whooping cough vaccine for my baby after birth will reduce my baby’s chances of getting whooping cough. | 0.55 (0.39-0.77)c | 0.50 (0.33-0.76)c |
| It is in my control whether or not my baby gets his/her vaccines. | 0.73 (0.36-1.47) | 1.02 (0.35-3.00) |
| I believe it is better for my baby to develop their own immunity by getting sick rather than by getting a vaccine. | 2.34 (1.67-3.27)c | 1.52 (1.01-2.29)c |
| The majority of my friends and family would get all of the vaccines recommended for their babies after birth. | 0.55 (0.39-0.77)c | 0.63 (0.43-0.93)c |
| The majority of my friends and family would encourage me to get all of the vaccines recommended for my baby after birth. | 0.56 (0.40-0.78)c | 0.60 (0.41-0.88)c |
| I have most of the important information I need to make a decision about vaccines for my baby after birth. | 0.54 (0.37-0.77)c | 0.68 (0.44-1.06) |
| I know enough about the safety of the whooping cough vaccine to make a decision about getting the vaccine for my baby after birth. | 0.53 (0.39-0.74)c | 0.60 (0.41-0.87)c |
| Trust in infant vaccine information source statements | ||
| I trust the information provided by my obstetrician or midwife about vaccines for babies after birth. | 0.67 (0.40-1.11) | 0.96 (0.51-1.82) |
| I trust the information provided by my baby’s doctor about vaccines for babies after birth. d | 0.64 (0.37-1.12) | 1.15 (0.54-2.43) |
| I trust the information provided by naturopathic and/or chiropractic doctors about vaccines for babies after birth. d | 1.82 (1.24-2.66)c | 1.66 (1.06-2.61)c |
| I trust the information provided by federal agencies such as the Centers for Disease Control and Prevention (CDC) about vaccines for babies after birth. | 0.56 (0.40-0.78)c | 0.68 (0.46-1.01) |
| I trust the information provided by scientists and doctors at universities and academic institutions about vaccines for babies after birth. | 0.56 (0.40-0.78)c | 0.73 (0.47-1.11) |
aThe study team administered a survey to 2196 pregnant women in Colorado and Georgia as part of a larger study aimed at assessing the effectiveness of a multilevel intervention seeking to increase rates of maternal and childhood immunization. 45 Logistic regressions controlled for educational attainment (dichotomized as high for having a doctoral or professional degree, master’s degree, bachelor’s degree, or associate’s degree; low as having a postsecondary nondegree award, some college but no degree, high school diploma or equivalent, or no formal educational credentials), whether first pregnancy (yes/no), and state of residence (Colorado or Georgia).
bFor intention to receive maternal and infant vaccines or for agreeing with vaccine-related statements by race/ethnicity (a dummy variable comparing Black and Hispanic with White).
c95% CIs that did not overlap 1 were considered to be significantly different from White at P < .05.
dRespondents who stated they had not yet seen this type of provider were removed from analysis.
Confidence in Vaccine Safety
Confidence in the safety of influenza and Tdap vaccines was lower among Black and Hispanic women than among White women (Tables 2 and 3). Among the 3 racial/ethnic groups, Black women had the least confidence in the safety of maternal vaccines (Table 2). Compared with White women, Black women had less confidence in the maternal influenza vaccine (OR = 0.37; 95% CI, 0.27-0.49), maternal Tdap (OR = 0.37; 95% CI, 0.27-0.52), and infant vaccines overall (OR = 0.40; 95% CI, 0.28-0.58). Both Hispanic women and Black women had lower levels of confidence than White women in the safety of infant vaccines overall, with Black women having the least confidence (OR = 0.40; 95% CI, 0.28-0.58; Table 3).
Vaccine Knowledge, Attitudes, and Beliefs
Compared with White women, Black and Hispanic women tended to be less confident in vaccine efficacy and less likely to perceive a risk of vaccine-preventable diseases, report provaccine social norms, perceive having enough vaccine knowledge to make an informed decision about infant vaccines, and trust vaccine information from health care providers and public health authorities (Tables 2 and 3). Among the 3 racial/ethnic groups, Black women were the least worried about their infant’s susceptibility to whooping cough (OR = 0.26; 95% CI, 0.19-0.36) and were most likely to report that they did not have all the information they needed to make an informed decision about infant vaccines (OR = 0.54; 95% CI, 0.37-0.77).
Trust in Sources of Vaccine Information
Thirty-eight percent of White women, 32% of Black women, and 26% of Hispanic women reported not seeing naturopathic and/or chiropractic doctors. Among those who did see naturopathic and/or chiropractic doctors, Black women and Hispanic women had 1.64-1.82 higher odds than White women of trusting the vaccine information provided by these health care providers.
Discussion
With the largest and most diverse sample of pregnant women recruited to date for a US-based study on maternal and infant vaccine acceptance, our study identified significant racial/ethnic disparities in the behavioral constructs that women use to make vaccine decisions. We found disparities in nearly all constructs measured, ranging from vaccine knowledge to vaccine attitudes to trust in information sources. The largest disparities appeared between Black women and White women, the most striking of which were within the constructs of vaccine confidence and perception of disease risk.
Our results mostly aligned with the results of previous surveys, 34,37 -40,42 although not completely, particularly among Hispanic women, whom we found to be more reticent than White women about vaccination. Compared with White women, Black women in our sample were less likely to intend to receive maternal and infant vaccines. Hispanic women were less likely than White women to intend to receive maternal vaccines; however, we found no difference for infant vaccines.
Black women in our sample perceived lower risk than White women of contracting vaccine-preventable diseases, but we observed no difference for Hispanic women. Both Black and Hispanic women perceived a lower level of danger than White women of whooping cough in infants. This disparity suggests that opportunities exist to bolster information and education about infectious diseases and vaccines among these groups. Others have suggested that immunization risks should be discussed in the context of the risks of infection of vaccine-preventable diseases. 50
Compared with White women, Black women in our sample were substantially less confident in the safety of both maternal and infant vaccines. Hispanic women also appeared less confident than White women, especially in the safety of maternal Tdap vaccine, although not to the extent of Black women. Both Black and Hispanic women were also much less confident than White women in the efficacy of both maternal and infant vaccines.
Both Black and Hispanic women in our sample were more likely than White women to trust information from alternative health care providers, whereas White women were more likely than Black women to trust information from obstetricians, federal agencies, and academic institutions. These findings align with research showing that people who seek care from complementary or alternative medicine (CAM) providers are less likely than people who do not seek such care to adhere to the recommended pediatric vaccination schedule 51 and that parents viewed CAM as largely free from the influence of the pharmaceutical industry. 52 However, these findings do not align with other data suggesting that White people use more CAM than Black people do. 53 Because the use of CAM has been increasing in the United States, it is important to continue monitoring the use of CAM and its possible influence on receipt of immunizations and to work hand-in-hand with provaccine CAM providers.
Limitations
Our analysis had several limitations. First, these data are not nationally generalizable. Although we selected the study sites to capture a wide range of demographic characteristics and vaccine perspectives, the sample consists solely of pregnant women willing to participate in a randomized controlled trial and recruited from 2 states. The women who chose to participate may differ from those who chose not to participate and the larger population of pregnant women generally. Second, our data are cross-sectional and, thus, are useful only for description and not for assessing causality or changes across time. Third, our sample size did not allow for meaningful analyses of races and ethnicities beyond the 3 largest groups. More surveys of vaccine intentions, attitudes, and beliefs among all age groups and demographic characteristics are needed, especially nationally representative, standardized surveys administered regularly over time. We would also suggest examining racial/ethnic disparities with larger and more robust sample sizes for other races and ethnicities not adequately captured in many studies, and we suggest mixed-methods research to understand more deeply the nuanced reasons for lack of confidence that may not be elucidated through survey data collection.
Conclusions
Addressing racial/ethnic disparities in vaccine uptake is crucial. Moving forward, we suggest the use of a framework that defines and diagnoses behaviorally related hesitancy determinants and proposes appropriate, tailored interventions, such as the Strategic Advisory Group of Experts on Immunization’s Guide to Tailoring Immunization Programs (TIP). 54 TIP is an evidence- and theory-based framework used to (1) identify vaccine-hesitant subgroups, (2) identify demand and supply-side immunization barriers and facilitators, and (3) design evidence-informed responses to hesitancy appropriate to the subgroup setting, context, and vaccine.
Other studies that have recruited pregnant women to prospectively assess the effect of vaccine promotion interventions have been too small 55 or homogeneous 56 to rigorously examine racial/ethnic differences in the vaccine constructs that may influence vaccine acceptance. With demographic data on more than 1800 women, our study was the first opportunity to explore these differences. Further research on such differences in vaccine attitudes among pregnant women is warranted, especially as it pertains to addressing disparities in vaccine uptake.
Although disparities in maternal vaccine acceptance among racial/ethnic groups are well documented, why these disparities exist is unclear. Our study shows that women of different racial/ethnic backgrounds differ markedly on important behavioral constructs integral to vaccine decision making. Understanding these differences can lead to tailored interventions that serve to appropriately address and overcome reticence and improve vaccine acceptance across all women.
Acknowledgment
The authors thank everyone who contributed to the survey design and/or participant recruitment in this study.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.Z.D. received some support from Walgreens. A.T.C. received a paid consultancy with the American College of Obstetricians and Gynecologists for provider-to-patient communications. D.A.S. received consulting and/or research support from Merck, Walgreens, and Pfizer.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grant number R01AI110482. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the article.
ORCID iD
Matthew Z. Dudley, PhD https://orcid.org/0000-0003-1201-4066
References
- 1. Burney LE. Influenza immunization: statement. Public Health Rep. 1960;75(10):944. 10.2307/4590965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jamieson DJ., Honein MA., Rasmussen SA. et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451-458. 10.1016/S0140-6736(09)61304-0 [DOI] [PubMed] [Google Scholar]
- 3. Sawyer M., Liang JL., Messonier N., Clark TA. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131-135. [PMC free article] [PubMed] [Google Scholar]
- 4. Salmon DA., Dudley MZ., Glanz JM., Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Am J Prev Med. 2015;49(6 suppl 4):391-398. 10.1016/j.amepre.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 5. O’Leary ST., Brewer SE., Pyrzanowski J. et al. Timing of information-seeking about infant vaccines. J Pediatr. 2018;203:125-130. 10.1016/j.jpeds.2018.07.046 [DOI] [PubMed] [Google Scholar]
- 6. Ellingson MK., Dudley MZ., Limaye RJ., Salmon DA., O’Leary ST., Omer SB. Enhancing uptake of influenza maternal vaccine. Expert Rev Vaccines. 2019;18(2):191-204. 10.1080/14760584.2019.1562907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn KE., Black CL., Ding H. et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2018. MMWR Morb Mortal Wkly Rep. 2018;67(38):1055-1059. 10.15585/mmwr.mm6738a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray B., McCrone S. An integrative review of promoting trust in the patient–primary care provider relationship. J Adv Nurs. 2015;71(1):3-23. 10.1111/jan.12502 [DOI] [PubMed] [Google Scholar]
- 9. Lee C., Whetten K., Omer S., Pan W., Salmon D. Hurdles to herd immunity: distrust of government and vaccine refusal in the US, 2002-2003. Vaccine. 2016;34(34):3972-3978. 10.1016/j.vaccine.2016.06.048 [DOI] [PubMed] [Google Scholar]
- 10. Quinn S., Jamison A., Musa D., Hilyard K., Freimuth V. Exploring the continuum of vaccine hesitancy between African American and White adults: results of a qualitative study. PLoS Curr. 2016;8 10.1371/currents.outbreaks.3e4a5ea39d8620494e2a2c874a3c4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamison AM., Quinn SC., Freimuth VS. “You don’t trust a government vaccine”: narratives of institutional trust and influenza vaccination among African American and White adults. Soc Sci Med. 2019;221:87-94. 10.1016/j.socscimed.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs EA., Rolle I., Ferrans CE., Whitaker EE., Warnecke RB. Understanding African Americans’ views of the trustworthiness of physicians. J Gen Intern Med. 2006;21(6):642-647. 10.1111/j.1525-1497.2006.00485.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scharff DP., Mathews KJ., Jackson P., Hoffsuemmer J., Martin E., Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21(3):879-897. 10.1353/hpu.0.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shui I., Kennedy A., Wooten K., Schwartz B., Gust D. Factors influencing African-American mothers’ concerns about immunization safety: a summary of focus group findings. J Natl Med Assoc. 2005;97(5):657-666. [PMC free article] [PubMed] [Google Scholar]
- 15. Shui IM., Weintraub ES., Gust DA. Parents concerned about vaccine safety: differences in race/ethnicity and attitudes. Am J Prev Med. 2006;31(3):244-251. 10.1016/j.amepre.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 16. Frew PM., Murden R., Mehta CC. et al. Development of a US trust measure to assess and monitor parental confidence in the vaccine system. Vaccine. 2019;37(2):325-332. 10.1016/j.vaccine.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brewer NT., Chapman GB., Gibbons FX., Gerrard M., McCaul KD., Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136-145. 10.1037/0278-6133.26.2.136 [DOI] [PubMed] [Google Scholar]
- 18. Lavail KH., Kennedy AM. The role of attitudes about vaccine safety, efficacy, and value in explaining parents’ reported vaccination behavior. Health Educ Behav. 2013;40(5):544-551. 10.1177/1090198112463022 [DOI] [PubMed] [Google Scholar]
- 19. Frew PM., Owens LE., Saint-Victor DS., Benedict S., Zhang S., Omer SB. Factors associated with maternal influenza immunization decision-making: evidence of immunization history and message framing effects. Hum Vaccin Immunother. 2014;10(9):2576-2583. 10.4161/hv.32248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frew PM., Saint-Victor DS., Isaacs MB. et al. Recruitment and retention of pregnant women into clinical research trials: an overview of challenges, facilitators, and best practices. Clin Infect Dis. 2014;59(suppl 7):S400-S407. 10.1093/cid/ciu726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frew PM., Saint-Victor DS., Owens LE., Omer SB. Socioecological and message framing factors influencing maternal influenza immunization among minority women. Vaccine. 2014;32(15):1736-1744. 10.1016/j.vaccine.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 22. Ahluwalia IB., Ding H., D’Angelo D. et al. Tetanus, diphtheria, pertussis vaccination coverage before, during, and after pregnancy—16 states and New York City, 2011. MMWR Morb Mortal Wkly Rep. 2015;64(19):522-526. [PMC free article] [PubMed] [Google Scholar]
- 23. Chamberlain AT., Seib K., Ault KA. et al. Factors associated with intention to receive influenza and tetanus, diphtheria, and acellular pertussis (Tdap) vaccines during pregnancy: a focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLoS Curr. 2015;7. 10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cunningham RM., Minard CG., Guffey D., Swaim LS., Opel DJ., Boom JA. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad Pediatr. 2018;18(2):154-160. 10.1016/j.acap.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 25. Gorman JR., Chambers CD. Pregnant women’s attitudes toward influenza vaccination while breastfeeding. Prev Med Rep. 2015;2:333-336. 10.1016/j.pmedr.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Healy CM., Rench MA., Montesinos DP., Ng N., Swaim LS. Knowledge and attitudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine. 2015;33(41):5445-5451. 10.1016/j.vaccine.2015.08.028 [DOI] [PubMed] [Google Scholar]
- 27. Henninger ML., Irving SA., Thompson M. et al. Factors associated with seasonal influenza vaccination in pregnant women. J Womens Health. 2015;24(5):394-402. 10.1089/jwh.2014.5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Halloran AC., Lu P-J., Williams WW., Ding H., Meyer SA. Tetanus, diphtheria, and acellular pertussis vaccination among women of childbearing age—United States, 2013. Am J Infect Control. 2016;44(7):786-793. 10.1016/j.ajic.2016.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Leary ST., Pyrzanowski J., Brewer SE. et al. Influenza and pertussis vaccination among pregnant women and their infants’ close contacts: reported practices and attitudes. Pediatr Infect Dis J. 2015;34(11):1244-1249. 10.1097/INF.0000000000000873 [DOI] [PubMed] [Google Scholar]
- 30. Payakachat N., Hadden KB., Ragland D. Promoting Tdap immunization in pregnancy: associations between maternal perceptions and vaccination rates. Vaccine. 2016;34(1):179-186. 10.1016/j.vaccine.2015.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiner JL., Fisher AM., Nowak GJ., Basket MM., Gellin BG. Childhood immunizations: first-time expectant mothers’ knowledge, beliefs, intentions, and behaviors. Am J Prev Med. 2015;33(6 Suppl 4):S426-S434. 10.1016/j.amepre.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adjei Boakye E., Tobo BB., Rojek RP., Mohammed KA., Geneus CJ., Osazuwa-Peters N. Approaching a decade since HPV vaccine licensure: racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Hum Vaccin Immunother. 2017;13(11):2713-2722. 10.1080/21645515.2017.1363133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooper DL., Hernandez ND., Rollins L., Akintobi TH., McAllister C. HPV vaccine awareness and the association of trust in cancer information from physicians among males. Vaccine. 2017;35(20):2661-2667. 10.1016/j.vaccine.2017.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De P., Budhwani H. Human papillomavirus (HPV) vaccine initiation in minority Americans. Public Health. 2017;144:86-91. 10.1016/j.puhe.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 35. Nonzee NJ., Baldwin SB., Cui Y., Singhal R. Disparities in parental human papillomavirus (HPV) vaccine awareness and uptake among adolescents. Vaccine. 2018;36(10):1243-1247. 10.1016/j.vaccine.2017.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okafor C., Hu X., Cook RL. Racial/ethnic disparities in HPV vaccine uptake among a sample of college women. J Racial Ethn Health Disparities. 2015;2(3):311-316. 10.1007/s40615-014-0074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Otanez S., Torr BM. Ethnic and racial disparities in HPV vaccination attitudes. J Immigr Minor Health. 2018;20(6):1476-1482. 10.1007/s10903-017-0685-2 [DOI] [PubMed] [Google Scholar]
- 38. Freimuth VS., Jamison AM., An J., Hancock GR., Quinn SC. Determinants of trust in the flu vaccine for African Americans and Whites. Soc Sci Med. 2017;193:70-79. 10.1016/j.socscimed.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santibanez TA., Kennedy ED. Reasons given for not receiving an influenza vaccination, 2011-12 influenza season, United States. Vaccine. 2016;34(24):2671-2678. 10.1016/j.vaccine.2016.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohammed KA., Vivian E., Loux TM., Arnold LD. Factors associated with parents’ intent to vaccinate adolescents for human papillomavirus: findings from the 2014 National Immunization Survey–Teen. Prev Chronic Dis. 2017;14:E45. 10.5888/pcd14.160314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freed GL., Clark SJ., Butchart AT., Singer DC., Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125(4):654-659. 10.1542/peds.2009-1962 [DOI] [PubMed] [Google Scholar]
- 42. Nuño VL., Gonzalez M., Loredo SM., Nigon BM., Garcia F. A cross-sectional study of human papillomavirus vaccine utilization among university women: the role of ethnicity, race, and risk factors. J Low Genit Tract Dis. 2016;20(2):131-134. 10.1097/LGT.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 43. DiClemente RJ., Murray CC., Graham T., Still J. Overcoming barriers to HPV vaccination: a randomized clinical trial of a culturally-tailored, media intervention among African American girls. Hum Vaccin Immunother. 2015;11(12):2883-2894. 10.1080/21645515.2015.1070996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dubé E, Gagnon D, MacDonald NE, SAGE Working Group on Vaccine Hesitancy . Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33(34):4191-4203. 10.1016/j.vaccine.2015.04.041 [DOI] [PubMed] [Google Scholar]
- 45. Salmon DA., Limaye RJ., Dudley MZ. et al. MomsTalkShots: an individually tailored educational application for maternal and infant vaccines. Vaccine. 2019;37(43):6478-6485. 10.1016/j.vaccine.2019.08.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Opel DJ., Mangione-Smith R., Taylor JA. et al. Development of a survey to identify vaccine-hesitant parents: the Parent Attitudes about Childhood Vaccines survey. Hum Vaccin. 2011;7(4):419-425. 10.4161/hv.7.4.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Opel DJ., Taylor JA., Mangione-Smith R. et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine. 2011;29(38):6598-6605. 10.1016/j.vaccine.2011.06.115 [DOI] [PubMed] [Google Scholar]
- 48. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191-215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 49. Cialdini RB. Crafting normative messages to protect the environment. Curr Dir Psychol Sci. 2003;12(4):105-109. 10.1111/1467-8721.01242 [DOI] [Google Scholar]
- 50. Betsch C., Sachse K. Debunking vaccination myths: strong risk negations can increase perceived vaccination risks. Health Psychol. 2013;32(2):146-155. 10.1037/a0027387 [DOI] [PubMed] [Google Scholar]
- 51. Downey L., Tyree PT., Huebner CE., Lafferty WE. Pediatric vaccination and vaccine-preventable disease acquisition: associations with care by complementary and alternative medicine providers. Matern Child Health J. 2010;14(6):922-930. 10.1007/s10995-009-0519-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Attwell K., Ward PR., Meyer SB., Rokkas PJ., Leask J. “Do-it-yourself”: vaccine rejection and complementary and alternative medicine (CAM). Soc Sci Med. 2018;196:106-114. 10.1016/j.socscimed.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 53. Upchurch DM., Wexler Rainisch BK. Racial and ethnic profiles of complementary and alternative medicine use among young adults in the United States: findings from the National Longitudinal Study of Adolescent Health. J Evid Based Complementary Altern Med. 2012;17(3):172-179. 10.1177/2156587212450713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Butler R, MacDonald NE, SAGE Working Group on Vaccine Hesitancy . Diagnosing the determinants of vaccine hesitancy in specific subgroups: the Guide to Tailoring Immunization Programmes (TIP). Vaccine. 2015;33(34):4176-4179. 10.1016/j.vaccine.2015.04.038 [DOI] [PubMed] [Google Scholar]
- 55. Chamberlain AT., Seib K., Ault KA. et al. Improving influenza and Tdap vaccination during pregnancy: a cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine. 2015;33(30):3571-3579. 10.1016/j.vaccine.2015.05.048 [DOI] [PubMed] [Google Scholar]
- 56. Kriss JL., Frew PM., Cortes M. et al. Evaluation of two vaccine education interventions to improve pertussis vaccination among pregnant African American women: a randomized controlled trial. Vaccine. 2017;35(11):1551-1558. 10.1016/j.vaccine.2017.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]