Abstract
A tetracycline-regulated reporter system was used to investigate the regulation of cyclooxygenase 2 (Cox-2) mRNA stability by the mitogen-activated protein kinase (MAPK) p38 signaling cascade. The stable β-globin mRNA was rendered unstable by insertion of the 2,500-nucleotide Cox-2 3′ untranslated region (3′ UTR). The chimeric transcript was stabilized by a constitutively active form of MAPK kinase 6, an activator of p38. This stabilization was blocked by SB203580, an inhibitor of p38, and by two different dominant negative forms of MAPK-activated protein kinase 2 (MAPKAPK-2), a kinase lying downstream of p38. Constitutively active MAPKAPK-2 was also able to stabilize chimeric β-globin–Cox-2 transcripts. The MAPKAPK-2 substrate hsp27 may be involved in stabilization, as β-globin–Cox-2 transcripts were partially stabilized by phosphomimetic mutant forms of hsp27. A short (123-nucleotide) fragment of the Cox-2 3′ UTR was necessary and sufficient for the regulation of mRNA stability by the p38 cascade and interacted with a HeLa protein immunologically related to AU-rich element/poly(U) binding factor 1.
Eicosanoids play a critical role in several physiological and pathophysiological processes, including blood clotting, wound healing, kidney function, acute inflammation, and cardiovascular disease (13, 19). The rate-limiting step in eicosanoid synthesis is catalyzed by cyclooxygenase (Cox) enzymes, which are encoded by two distinct genes. The Cox-1 gene is principally homeostatic in function and possesses a typical, GC-rich housekeeping promoter (55). In contrast the Cox-2 gene resembles an early response gene. It is strongly induced by mitogenic and proinflammatory stimuli, superinduced by inhibitors of protein synthesis, and acutely regulated at both transcriptional and posttranscriptional levels (17, 37–39, 46, 47). Posttranscriptional regulation is critical in determining the strength and duration of Cox-2 gene induction by external stimuli yet is poorly understood. Neither the sequences which regulate Cox-2 mRNA metabolism nor the trans-acting factors mediating this regulation have been identified.
Several proinflammatory treatments which induce Cox-2 gene expression also stimulate the mitogen-activated protein kinase (MAPK) p38. This kinase is activated by phosphorylation at threonine and tyrosine residues, catalyzed by the dual-specificity kinase MAPK kinase 6 (MKK6) (11, 23, 42). In turn, activated p38 phosphorylates numerous substrates which include kinases such as MAPK-activated protein kinase 2 (MAPKAPK-2) and -3 (18, 34, 49). Substrates of MAPKAPK-2 and -3 include the small heat shock protein hsp27, an abundant cytoplasmic protein of uncertain function (15, 18, 53). In a variety of cells treated with different inducing agents, specific inhibitors of p38 block the accumulation of Cox-2 mRNA (21, 29, 33, 41, 43, 45). In HeLa cells stimulated with interleukin 1 (IL-1) and in primary human monocytes stimulated with bacterial lipopolysaccharide inhibition of p38 results in a rapid and specific destabilization of Cox-2 mRNA but has little effect upon Cox-2 transcription (12, 44).
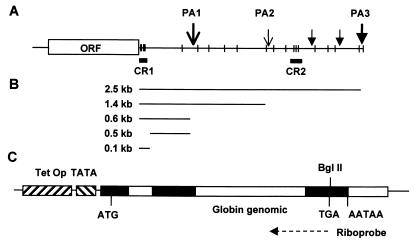
Regulation of mRNA stability is often mediated by sequences within the 3′ untranslated region (3′ UTR). Several Cox-2 transcripts differing in 3′ UTR length due to alternative polyadenylation site usage have been described, the two major transcripts being 4.6 and 2.8 kb long (38, 47). The most abundant (4.6-kb) transcript has a 3′ UTR of 2,515 nucleotides (nt), containing 22 copies of the pentamer sequence AUUUA (see Fig. 1). This sequence is found in the 3′ UTRs of numerous unstable cytokine- and protooncogene-encoding mRNAs and is a prominent feature of AU-rich elements (AREs) which regulate mRNA stability (6–8, 50). Two conserved regions (CR) have been noted within the Cox-2 3′ UTR (38). CR1 lies immediately 3′ to the translation termination codon and contains six AUUUA motifs, of which three are overlapping. CR2 lies 1,700 nt 3′ to the translation termination codon and contains three dispersed AUUUA motifs (Fig. 1).
FIG. 1.
Structure of Cox-2 mRNAs and construction of posttranscriptional reporters. (A) Structure of the major (4.6-kb) Cox-2 transcript. AUUUA motifs are shown as vertical bars. CR1 contains six of these motifs, and CR2 contains three. Polyadenylation sites (PA1 to PA3) are represented as vertical arrows, with the two major sites indicated by larger arrows. Canonical (AATAAA) polyadenylation sites are indicated by arrows with closed triangles heads; noncanonical (ATTAAA) sites are indicated by arrows with Vs for heads. The polyadenylation sites are discussed in the text. ORF, open reading frame. (B) Schematic of 3′ UTR-encoding fragments generated by PCR and cloned into the BglII site of pTetBBB. (C) Structure of the reporter construct pTetBBB. Rabbit β-globin exons and introns are shown as closed and open bars. Translation initiation (ATG), translation termination (TGA), and polyadenylation (AATAAA) signals are indicated. The antisense β-globin riboprobe is represented as a dashed arrow. TetOp, tetracycline operator sequences, TATA, minimal cytomegalovirus promoter.
Several proteins have been shown to interact specifically with AU-rich RNA stability determinants, and have been implicated in positive or negative regulation of mRNA stability. For example ARE/poly(U) binding factor 1 (AUF1), a member of the hnRNP D family of RNA binding proteins, is present in a cytosolic fraction which accelerates c-myc mRNA decay in vitro (3, 60). Increased AUF1 expression is associated with decreased mRNA stability in vivo (5, 40, 53), and AUF1 overexpression can antagonize mRNA stabilization in vivo (31). Furthermore the immunodepletion of AUF1 from cytoplasmic extracts increases the stability of an ARE-containing transcript in an in vitro degradation assay (4).
The tetracycline-responsive reporter system (20, 59) permits regulatory sequences and pathways to be mapped by performing RNA stability experiments in the absence of toxic transcriptional inhibitors. Reporter mRNAs are transcribed from a tetracycline-responsive promoter in a cell line expressing a chimeric, tetracycline-responsive transcription factor, tTA (20). In the presence of tetracycline, binding of tTA to the promoter is blocked and transcription is inhibited. Using this system, referred to herein as the TET-off system, we demonstrate that the effects of p38 are mediated by its downstream kinase MAPKAPK-2 and may involve the phosphorylation of the MAPKAPK-2 substrate hsp27. A conserved, 123-nucleotide ARE lying immediately 3′ to the translation termination codon (CR1) is necessary and sufficient for stabilization by the p38 pathway and interacts with a HeLa cell protein which is immunologically related to AUF1.
MATERIALS AND METHODS
Plasmids.
The MKK6E expression vector was a gift of J. Han. MAPKAPK-2 expression vectors were donated by C. Marshall. Human Cox-2 3′ UTR fragments were amplified by reverse transcription-PCR from IL-1-stimulated human gingival fibroblast RNA, using Moloney murine leukemia virus reverse transcriptase and Vent polymerase (NEB). Human tumor necrosis factor alpha (TNF-α) and c-myc 3′ UTR fragments were amplified from genomic DNA using TaqPlus polymerase (Stratagene). A mouse TNF-α 3′ UTR fragment was amplified from a mouse TNF-α genomic clone (gift of A. Shakhov), using Vent polymerase. PCR products were cloned into pCR-Blunt (Invitrogen) and then excised with BglII or BamHI and cloned into the BglII site of pTetBBB (gift of Ann-Bin Shyu).
Riboprobe vectors were constructed as follows. A 352-bp HincII-XbaI luciferase fragment was cloned from pGL3c (Promega) into pBluescript KS that had been digested with EcoRV and XbaI. A 256-bp BstYI-ClaI fragment was cloned from a human Cox-2 cDNA construct (gift of D. Fitzgerald) into pBluescript KS that had been digested with BamHI and ClaI. A 269-bp SspI-BglII fragment was cloned from pTetBBB into pBluescript KS that had been digested with BamHI and EcoRV. To construct hsp27 expression vectors, the hsp27 open reading frame was excised from pGEX2T-hsp27 (gift of S. Lumb), using BamHI, and cloned in frame at the BamHI site of pFlagCMV2 (Sigma-Aldrich). Mutagenesis was performed with the QuikChange kit (Stratagene). Sequences of oligonucleotides are available on request from the corresponding author. Novel DNA constructs were commercially sequenced by ABC (London, United Kingdom).
Cell culture and transfection.
HeLa-TO cells (Clontech) were maintained in Dulbecco's modified Eagle medium–10% fetal calf serum supplemented with G-418 (100 ng/ml; Life Technologies). Cells were seeded in six-well plates at a density of 1.5 × 105 cells/well. The following day cells were transfected using Superfect (Qiagen). The amount of total transfected DNA was kept constant within all experiments by addition of appropriate empty expression vectors and/or Bluescript plasmid (Stratagene). After 24 h, tetracycline (Sigma) was added at a final concentration of 100 ng/ml, and cells were harvested in guanidine thiocyanate lysis buffer (Ambion) at different intervals, as indicated in each figure. Lysates were passed through shredder columns (Qiagen) and stored frozen at −20°C. In some experiments 1 μM SB203580 (Calbiochem) or vehicle control (dimethyl sulfoxide [0.03%]) was added to cells 30 min prior to the addition of tetracycline.
Ribonuclease protection assay.
Riboprobe template constructs were linearized by appropriate restriction digestion and purified by phenol-chloroform extraction and ethanol precipitation. Riboprobes were synthesized using T7 polymerase (Boehringer Mannheim) in the presence of 50 μCi of [α-32P]UTP (800 Ci/mmol; Amersham). The final concentration of unlabeled UTP in in vitro transcription reactions was 12 μM, except in the case of GAPDH (glyceraldehyde-3-phosphate dehydrogenase), where it was 36 μM. Ribonuclease protection assays were carried out using the Direct Protect kit (Ambion). Under the conditions of hybridization DNA-RNA heteroduplexes are not detected. Protected RNA fragments were resolved by electrophoresis on denaturing 6% polyacrylamide gels and were visualized and quantified by phosphorimaging (Fuji FLA 2000) and autoradiography. Autoradiographs are shown in all figures. Each experiment was performed at least twice, and serial dilutions of lysates were used to check that quantitations were within the linear range of the assay.
Western blotting.
HeLa-TO cells in six-well dishes were transfected with 780 ng of wild-type or mutant hsp27 expression vector and 220 ng of pBluescript as described above. After 24 h proteins were harvested in radioimmunoprecipitation assay buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose (Sartorius). The membrane was probed with a mouse monoclonal antibody to the flag epitope (anti-Flag M2 [Sigma-Aldrich]) and then with a peroxidase-coupled second antibody (Dako). Proteins were detected using the enhanced chemiluminescence system (Amersham).
Preparation of HeLa extracts.
HeLa-TO cells were grown to confluence in 175-cm2 flasks. The cells were cooled on ice for 5 min, rinsed once with ice-cold phosphate-buffered saline, and lysed by addition of a buffer containing 10 mM HEPES (pH 7.6), 3 mM MgCl2, 40 mM KCl, 2 mM dithiothreitol, 5% glycerol, 0.5% NP-40, and protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 10 μM E64, and 4 μg of aprotinin and 4 μg of pepstatin per ml). After 15 min on ice, nuclei were removed by centrifugation at 600 × g for 10 min. The supernatant was aliquoted and snap frozen at −70°C.
RNA band shift assay.
RNA probes were synthesized essentially as described for the ribonuclease protection assay. RNA band shift assays were performed according to the method of Hel et al. (25). The protein extracts were incubated with the indicated RNA probes in a buffer containing 20 mM HEPES (pH 7.6), 3 mM MgCl2, 40 mM KCl, 2 mM dithiothreitol, and 5% glycerol. Typically, 10 μg of protein was incubated for 20 min with 400,000 to 500,000 cpm of α-32P-labeled RNA probe, corresponding to approximately 10 fmol of RNA. RNase T1 and heparin sulphate were added to final concentrations of 50 U/ml and 5 mg/ml, respectively, and the reaction was allowed to continue for a further 20 min on ice. Three microliters of loading buffer (90% glycerol, 0.025% bromophenol blue) was added to the samples which were then resolved by electrophoresis at 4°C on a 0.5× Tris-borate-EDTA nondenaturing 4% polyacrylamide gel. Gels were subjected to autoradiography and phosphorimaging. In some experiments binding reactions also contained homopolyribonucleotides (Pharmacia) as indicated, or 1 μl of polyclonal antiserum. HuR (gift of J. Steitz), Jun N-terminal protein kinase (JNK3), and AUF1 antisera were all raised in rabbits.
RESULTS
The Cox-2 3′ UTR mediates regulation of mRNA stability by the p38 pathway.
The tetracycline-responsive reporter construct pTetBBB (59), contains a rabbit β-globin genomic fragment downstream of tetracycline operator sequences and a minimal promoter (Fig. 1). Transcriptional activity of this construct was rapidly switched off by addition of 100 ng/ml tetracycline to the culture medium (unpublished data).
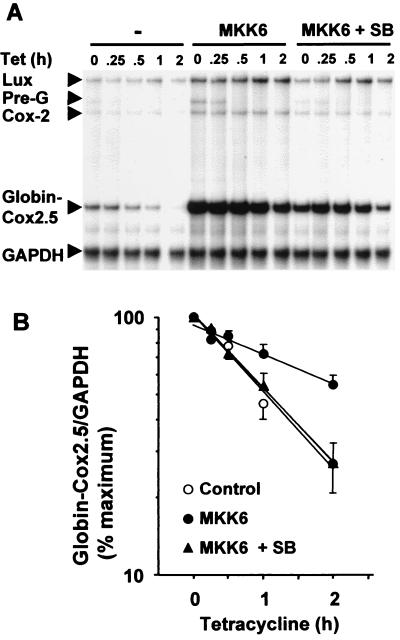
A cDNA fragment encoding the Cox-2 3′ UTR was inserted at the BglII site of pTetBBB (Fig. 1). The resulting construct (pTetBBB-Cox2.5) was transiently transfected into HeLa-TO cells with a luciferase expression vector and with or without a vector expressing constitutively active MKK6. After 24 h cells were treated with SB203580 or vehicle, and then tetracycline was added and cells were harvested at intervals as indicated in Fig. 2. A ribonuclease protection assay was used to quantify the β-globin–Cox2.5 reporter transcript, Cox-2, luciferase, and GAPDH mRNAs (the last two as controls for transfection efficiency and gel loading). In all such experiments endogenous Cox-2 expression was up-regulated by MKK6, and this was reversed by SB203580, confirming that the Cox-2 gene is a target for the p38 pathway in this particular HeLa cell line. Up-regulation of luciferase expression by MKK6 was not reproducible. The antisense β-globin probe spans an intron-exon boundary (Fig. 1), and detects an unspliced pre-mRNA species, which is indicated in Fig. 2A. Following the addition of tetracycline, this pre-mRNA rapidly disappeared, as transcription was inhibited and the pre-mRNA was processed.
FIG. 2.
The Cox-2 3′ UTR mediates regulation of mRNA stability by the p38 pathway. HeLa-TO cells were transfected with 200 ng of pGL3c, 20 ng of pTetBBB-Cox2.5, and 100 ng of MKK6 expression vector or empty vector control (pcDNA3). After 24 h vehicle control (dimethyl sulfoxide) or SB203580 (1 μM) was added. After a further 30 min, tetracycline was added to a final concentration of 100 ng/ml. Cells were harvested at the time intervals shown, and ribonuclease protection assays were performed to quantify luciferase, Cox-2, β-globin–Cox2.5, and GAPDH mRNAs. (A) A representative experiment. Ribonuclease protected luciferase (Lux), Cox-2, GAPDH, β-globin–Cox2.5 and pre-β-globin (Pre-G) fragments are indicated. (B) Graphical representation of means ± standard deviations (error bars) of seven independent experiments. β-globin/GAPDH ratios were plotted as percentage of the maximum value at the time of tetracycline addition.
Little or no decay of the β-globin transcript was detected over a 6-h tetracycline chase (see Fig. 4 for this and additional controls). In contrast the chimeric β-globin–Cox2.5 transcript decayed with a half-life of approximately 1 h. In the presence of constitutively active MKK6, the half-life of this transcript increased more than twofold. MKK6 coexpression increased the quantity of transcript detected at the start of the tetracycline chase, presumably because the increased transcript stability resulted in a greater mRNA accumulation during the 24 h prior to the addition of tetracycline. The addition of 1 μM SB203580 shortly before the addition of tetracycline reversed the MKK6-dependent stabilization of the chimeric transcript. In spite of very different starting levels, the transcripts decayed with identical half-lives in the absence of MKK6 or in the presence of both MKK6 and SB203580. Therefore, differences in rates of decay do not simply reflect limited capacity within the degradative machinery but are related to the activity of the p38 pathway. In HeLa cells phorbol myristate acetate is a potent activator of MAPKs p42 and p44 but not p38 and strongly induces expression of the Cox-2 gene. Treatment of HeLa-TO cells with phorbol myristate acetate had no impact upon the stability of the β-globin–Cox2.5 chimeric mRNA (unpublished data). The Cox-2 3′ UTR therefore confers instability and p38-dependent stabilization upon a heterologous mRNA.
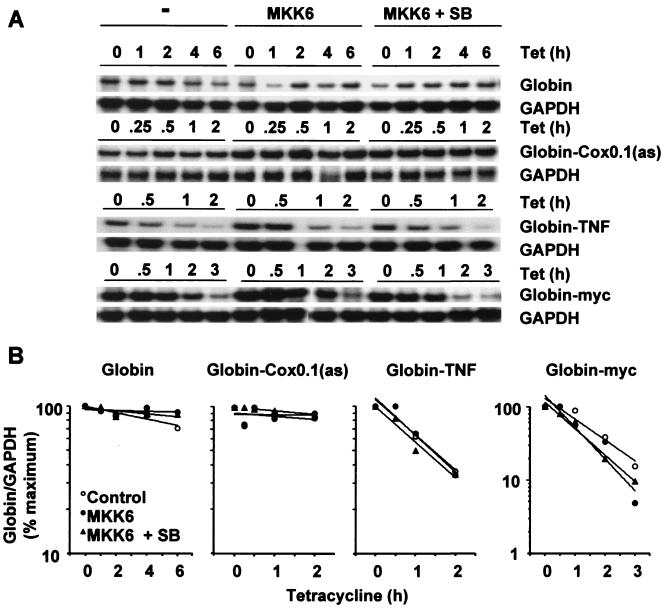
FIG. 4.
Sequence specificity of p38-regulated mRNA stability. Transfections and ribonuclease protection assays were performed as described in the legend to Fig. 2, using the reporter constructs pTetBBB, pTetBBB-Cox0.1(as), pTetBBB-TNF, and pTetBBB-myc. SB, 1 μM SB203580. (A) Representative experiments. Only the β-globin reporter and GAPDH loading control bands are shown. (B) Graphical representation of the experiments shown in panel A. Each transfection was performed at least twice, with qualitatively identical results.
CR1 of the Cox-2 3′ UTR is necessary and sufficient for the regulation of mRNA stability by the p38 pathway.
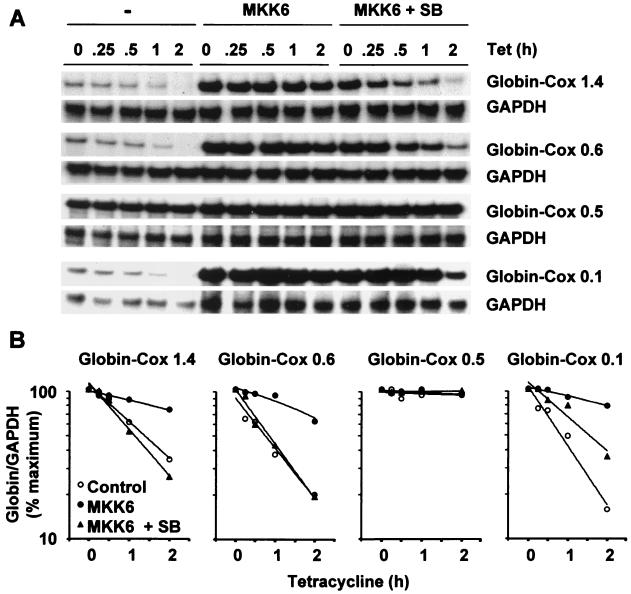
To determine what proportion of the 2,515-nt Cox-2 3′ UTR is required for p38-mediated regulation of stability, we initially cloned 1.4- and 0.6-kb 3′ UTR fragments into pTetBBB (Fig. 1). These fragments correspond to the 3′ UTRs present within two minor Cox-2 transcripts, which terminate at noncanonical ATTAAA polyadenylation signals (PA1 and PA2 in Fig. 1) (38, 47). The β-globin–Cox1.4 and β-globin–Cox0.6 transcripts behaved similarly to β-globin–Cox2.5 (Fig. 3). Therefore, only the first 600 nt of the Cox-2 3′ UTR is required for the regulation of mRNA stability by the p38 pathway and CR2 is dispensible. This is in agreement with our previous observation that endogenous 4.6- and 2.8-kb Cox-2 transcripts are identically destabilized by SB203580 in actinomycin D chase experiments (12, 44).
FIG. 3.
CR1 is necessary and sufficient for the regulation of mRNA stability by the p38 pathway. Transfections and ribonuclease protection assays were performed as described in the legend to Fig. 2, using the reporter constructs pTetBBB-Cox1.4, -Cox0.6, -Cox0.5, and -Cox0.1. SB, 1 μM SB203580. (A) Representative experiments. Only the β-globin reporter and GAPDH loading control bands are shown. (B) Graphical representation of the experiments shown in panel A. Each transfection was performed at least twice, with qualitatively identical results.
The 0.6-kb 3′ UTR fragment was further subdivided into a 0.1-kb fragment which contained six AUUUA repeats (equivalent to CR1) and a 0.5 kb fragment which contained only one AUUUA sequence (Fig. 1). The β-globin–Cox0.5 transcript was stable under all conditions (Fig. 3). In contrast the β-globin–Cox0.1 transcript was unstable and was very strongly stabilized by MKK6. This effect was significantly reversed in the presence of 1 μM SB203580 (Fig. 3). We conclude that CR1 contains all of the sequence elements required to mediate the regulation of mRNA stability by the p38 pathway. Under all p38-activating conditions tested, the β-globin–Cox0.1 transcript was stabilized more strongly than β-globin–Cox0.6 or any of the other chimeric mRNAs. In addition to CR1, the Cox-2 3′ UTR may therefore contain additional determinants of mRNA instability which do not respond to activation of the p38 pathway. The β-globin–Cox0.5 transcript is highly stable, suggesting that additional instability determinants function only in the presence of CR1.
Sequence specificity of p38-regulated mRNA stability.
CR1 contains overlapping AUUUA motifs and thus resembles a class II ARE (7). In contrast, class I AREs are characterized by dispersed AUUUA motifs in association with U-rich stretches. To investigate the sequence specificity of p38-mediated regulation of mRNA stability, the MKK6 responsiveness of several control transcripts was tested. These transcripts contained no insert, a class II ARE derived from the TNF-α 3′ UTR, a class I ARE derived from the c-myc 3′ UTR, or CR1 in reverse orientation [β-globin and β-globin–TNF, –myc and –Cox0.1 [as], respectively]. The β-globin–TNF and β-globin–myc transcripts were unstable but unresponsive to MKK6 or SB203580 (Fig. 4); therefore, the regulation of mRNA stability by p38 is not a general property of AREs. Neither synthesis nor degradation of the highly stable β-globin transcript from the tetracycline-regulated promoter was influenced by MKK6. The CR1 fragment did not confer instability or MKK6 responsiveness when placed in reverse orientation; therefore, it does not possess enhancer-like properties, and transcriptional effects of MKK6 coexpression can be discounted. Further, the regulation of mRNA stability by p38 is not simply a consequence of the high (78%) AU content of CR1, as this AU richness is preserved in β-globin–Cox0.1(as). Finally, all chimeric β-globin transcripts were assessed by Northern blotting and shown to be of the anticipated sizes (data not shown).
Regulation of mRNA stability is mediated by MAPKAPK-2.
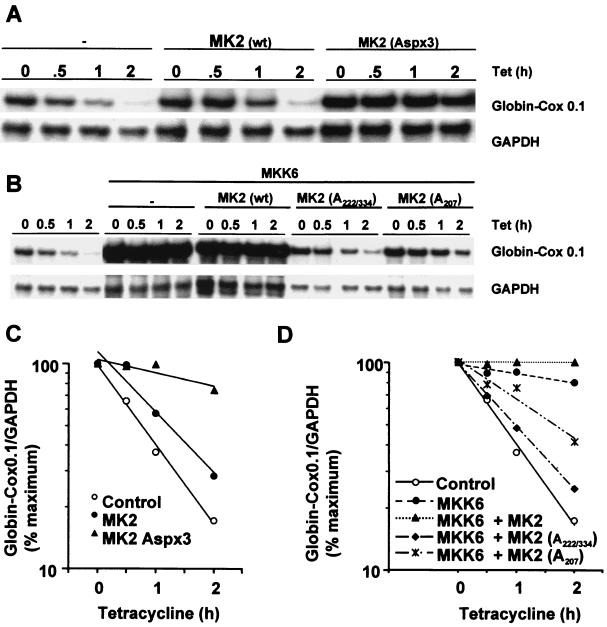
To further dissect the role of the p38 pathway in the regulation of Cox-2 mRNA stability, we used a series of mutants of MAPKAPK-2, a kinase which is phosphorylated and activated by p38 (18, 49). Wild-type MAPKAPK-2 had little or no impact upon the stability of β-globin–Cox0.1 transcripts; however, a constitutively active form (AspX3) (2) strongly stabilized the reporter transcript (Fig. 5A and 5C). Stabilization was also observed with β-globin–Cox2.5 and was not affected by 1 μM SB203580, consistent with the site of action of the drug in the p38 signal transduction cascade (unpublished data).
FIG. 5.
Regulation of stability of chimeric β-globin–Cox-2 transcripts is mediated by MAPKAPK-2. (A) HeLa-TO cells were transfected with 200 ng of pGL3c, 20 ng of pTetBBB-Cox0.1, and 780 ng of empty vector or MAPKAPK-2 expression vector as indicated. Ribonuclease protection assays were performed as described in the legend to Fig. 2. wt, wild type; Aspx3, constitutively active mutant. (B) HeLa-TO cells were transfected with 200 ng of pGL3c, 20 ng of pTetBBB-Cox0.1, 100 ng of MKK6 expression vector or the corresponding empty vector, and 500 ng of MAPKAPK-2 expression vector as indicated, or the corresponding empty vector. Ribonuclease protection assays were performed as described in the legend to Fig. 2. (C) Graphical representation of the experiment shown in panel A. (D) Graphical representation of the experiment shown in panel B. Each experiment was performed at least twice, with qualitatively identical results.
The stabilization of β-globin–Cox0.1 by MKK6 was blocked by two distinct dominant negative forms of MAPKAPK-2 (1), a nonphosphorylatable mutant (A222/334) and a kinase dead mutant (A207) (Fig. 5B and 5D). We observed a slight but reproducible additive stabilization of the reporter transcript by coexpression of MKK6 and wild-type MAPKAPK-2. Virtually identical results were obtained with β-globin–Cox2.5 (unpublished data). The results described here suggest that the regulation of mRNA stability by the p38 pathway is mediated largely or entirely by the p38 substrate MAPKAPK-2.
Chimeric β-globin–Cox transcripts are partially stabilized by hsp27 mutants.
The only known substrates of the kinase MAPKAPK-2 are the transcription factors ATF1, CREB, and SRF (24, 26, 54) and the small heat shock protein hsp27 (15, 18, 53). hsp27 is an abundant cytoplasmic protein thought to play a role in cell survival following stress (14, 28, 35). MAPKAPK-2 phosphorylates three serine residues (15, 78, and 82) in human hsp27 and two serine residues (15 and 90) in the rodent homologue hsp25. Phosphorylation is thought to regulate the functional properties of the small heat shock proteins in part by controlling their association into dimers or homopolymers (27, 48). Self-association is primarily regulated by S90 phosphorylation in hamster hsp25. Phosphorylation of S15 has little or no effect upon self-association but may regulate the interaction of hsp25 and/or hsp27 with other cellular proteins such as actin (27).
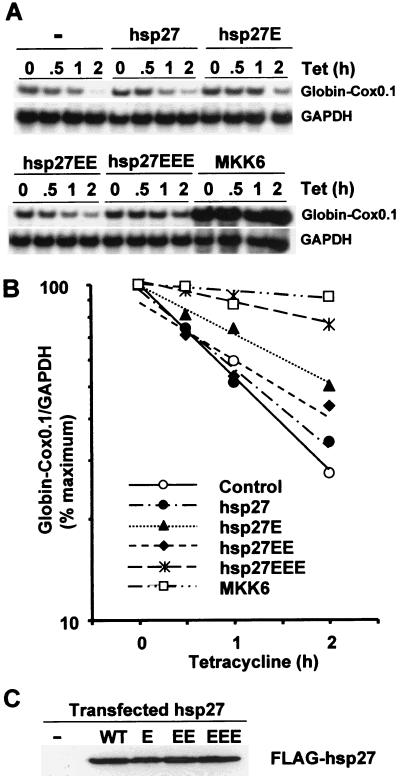
To investigate the potential role of hsp27 in the regulation of Cox-2 mRNA stability, we generated mutants in which serine 15, serines 78 and 82, or all three phospho-acceptor serines were mutated to alanine or glutamic acid. The triple glutamate substitution mutant hsp27EEE stabilized β-globin-Cox0.1 mRNA, while the single and double glutamate substitution mutants had less effect (Fig. 6A). Virtually identical results were obtained with β-globin–Cox2.5 (unpublished data). Equal expression of all mutants was confirmed by Western blotting (Fig. 6C).
FIG. 6.
Stabilization of a β-globin–Cox chimeric transcript by a mutant of hsp27. (A) HeLa-TO cells were transfected with 200 ng of pGL3c and 20 ng of pTetBBB-Cox0.1, plus 100 ng of MKK6 expression vector or 780 ng of Flag-hsp27 expression vector as indicated. Ribonuclease protection assays were performed as described in the legend to Fig. 2. (B) Graphical representation of the experiment shown in panel A. This experiment was performed three times, with qualitatively identical results. (C) HeLa-TO cells were transfected with 780 ng of pFlagCMV2 (first lane) or Flag-hsp27 expression vector as indicated. After 24 h cells were harvested and Western blotting was performed using an antibody against the Flag epitope.
The phosphorylation of hsp27 may contribute to the stabilization of mRNA by the MAPK p38 pathway, with a potential role for each of the sites in hsp27 phosphorylated by MAPKAPK-2. In this assay system the stabilizing effect of hsp27EEE was weak compared to that of MKK6 (Fig. 6) or MAPKAPK-2 itself (Fig. 5), and the alanine substitution mutants had no dominant negative effect on stabilization by MKK6 (unpublished data). However, the effects of phosphorylation site substitutions in a noncatalytic protein such as hsp27 are difficult to predict, especially within a cell which abundantly expresses the wild-type protein. In order to rule out or to prove more conclusively that hsp27 is involved in the regulation of mRNA stability, it may be necessary to use hsp27 knockout cells. It is possible that an unidentified substrate of MAPKAPK-2 plays a more significant role in this process.
CR1 interacts with AUF1 or an immunologically related protein.
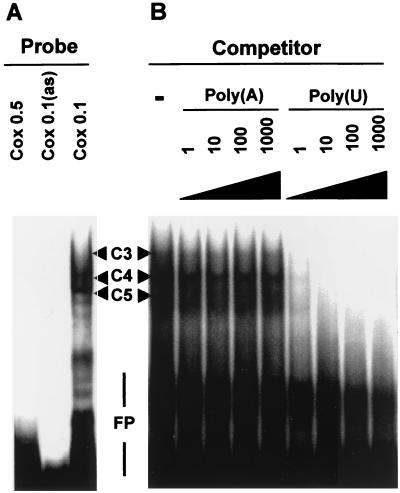
In electrophoretic mobility shift assays using a Cox0.1 probe and a HeLa-TO cytoplasmic extract three protein-RNA complexes were observed, with the lowest mobility complex (C3 in Fig. 7) being rather diffuse. No complexes were detected with the Cox0.5 or Cox0.1 antisense probes. All protein interactions with the Cox0.1 probe could be blocked by competition with an excess of unlabeled poly(U) but not with poly(A) (Fig. 7B), poly(C), or poly(G) (unpublished data). Complexes C3 and C4 were common to Cox0.1 and c-myc probes, while complex C5 was detected only with the Cox0.1 probe and complexes C1 and C2 were common to c-myc and TNF-α probes (Fig. 8B).
FIG. 7.
The p38-responsive region of the Cox-2 3′ UTR forms several complexes with HeLa-TO cytoplasmic proteins. Complexes C3 to C5 are discussed in the text. FP, free probe. (A) Electrophoretic mobility shift assays were performed using Cox0.1, Cox0.1 antisense, and Cox0.5 RNA probes and 10 μg of HeLa-TO cytoplasmic extract. (B) Electrophoretic mobility shift assays were performed using a Cox0.1 RNA probe and 10 μg of HeLa-TO cytoplasmic extract in the presence of a 0- to 1,000-fold excess (by mass) of homopolyribonucleotides poly(A) or poly(U), as indicated.
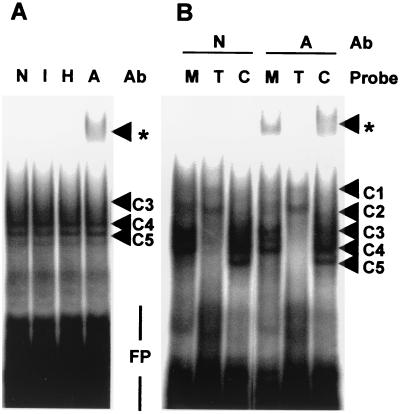
FIG. 8.
The p38-responsive region of the Cox-2 3′ UTR is able to bind AUF1. Complexes C1 to C5 are discussed in the text. Supershifted complexes are indicated by asterisks. FP, free probe; Ab, antibody. (A) Electrophoretic mobility shift assays were performed using a Cox0.1 RNA probe and 10 μg of HeLa-TO cytoplasmic extract in the presence of a nonimmune serum (N), an irrelevant immune serum raised against human JNK3 (I), HuR antiserum (H), or AUF1 antiserum (A). (B) Electrophoretic mobility shift assays were performed using c-myc (M), TNF-α (T) or Cox0.1 (C) RNA probes and 10 μg of HeLa-TO cytoplasmic extract, in the presence of nonimmune serum (N) or AUF1 antiserum (A).
The ARE-binding proteins AUF1 and HuR are both present in HeLa cells and bind to the c-myc 3′ UTR and to poly(U) RNA (3, 32, 36, 60). Because of the similarities between Cox0.1 and c-myc RNA-protein complexes, we hypothesized that AUF1, HuR, or both proteins might interact with the CR1 sequence. Therefore, electrophoretic mobility shift assays were carried out using HeLa-TO cytoplasmic extract and Cox0.1 probe in the presence of antibodies raised against HuR, AUF1, or an irrelevant protein (JNK3) (Fig. 8A). A supershifted complex was detected in the presence of the AUF1 antibody, suggesting that a protein which interacts with the CR1 sequence is identical (or closely related) to AUF1. No supershifted complexes were detected with a nonimmune serum or with HuR or JNK3 antiserum. An anti-AUF1 supershifted complex was also detected using the c-myc but not the TNF-α probe (Fig. 8B). The formation of supershifted bands was not accompanied by substantial reduction of RNA-protein complexes C3 to C5. Immunodepletion of AUF1 from the HeLa-TO cytoplasmic extract did not significantly inhibit the formation of complexes C3 to C5 (unpublished data). Therefore, an AUF1-related protein is present in HeLa-TO cells and is able to bind to the CR1 probe but represents only a small proportion of the CR1-binding activity detected in electrophoretic mobility shift assays.
DISCUSSION
We previously reported that Cox-2 mRNA stability is regulated by p38 in human monocytes and in HeLa cells (12, 44). Those studies employed pharmacological inhibitors of transcription and of p38, each of which is a potential source of artifacts. The transcriptional inhibitor actinomycin D is cytotoxic, induces nucleocytoplasmic shuttling of several RNA binding proteins (16, 22), and may artificially stabilize some mRNAs (9, 51, 58). At high concentrations SB203580 is able to inhibit some JNK isoforms (10, 56), although at the 1 μM concentration we have used there is little or no effect upon HeLa cell JNK activity (44). At the concentration used, tetracycline has no discernible effects upon HeLa-TO cells other than the regulation of the tetracycline-responsive promoter. The present study, using a tetracycline chase procedure, provides proof of p38-dependent Cox-2 mRNA stabilization, operating through the p38 substrate MAPKAPK-2, and possibly mediated in part by the phosphorylation of the small heat shock protein hsp27.
The p38-dependent stabilization of mRNA is sequence-specific, since 3′ UTR sequences derived from c-myc or TNFα destabilise the β-globin reporter transcript, but do not confer responsiveness to the p38 pathway. Using a similar system, it has recently been demonstrated that the p38 pathway regulates the stability of reporter transcripts containing IL-6, IL-8, c-fos, and granulocyte-macrophage colony-stimulating factor (GM-CSF) AREs (57). Thus, p38 is able to regulate the stability of a subset of mRNAs containing class I AREs and a subset of mRNAs containing class II AREs. For comparison, several of the relevant ARE sequences are illustrated in Fig. 9. The TNF-α ARE is strikingly similar to CR1, and we find endogenous TNF-α mRNA stability to be regulated by p38 in RAW264.7 mouse macrophage cells (unpublished data).
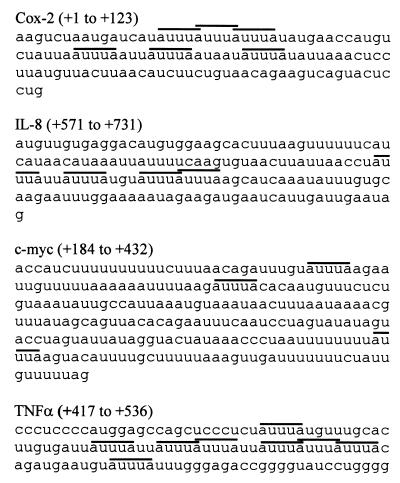
FIG. 9.
Comparison of 3′ UTR sequences. Partial sequences of Cox-2, IL-8, c-myc, and TNF-α 3′ UTRs are shown, with AUUUA motifs overlined. Coordinates are with respect to the translation termination codon in each case. The Cox-2 and IL-8 sequences are the smallest fragments shown to mediate regulation of stability by the p38 pathway (this work and reference 57).
The basis of selectivity of p38 action is not clear, but such selectivity most likely arises from specific protein-RNA interactions. We have shown that CR1 interacts with AUF1, a member of the hnRNP D family which may be involved in the degradation of labile mRNAs (4, 5, 30, 40, 52, 60). AUF1 is a relatively small proportion of the CR1-binding activity detected in electrophoretic mobility shift assays. However, the cell contains multiple ARE-binding factors which may not function in the control of degradation; therefore, this finding does not rule out an involvement of AUF1 in the regulation of Cox-2 mRNA stability by p38 (4, 5, 52). AUF1 is a relatively minor component of the binding activity detected using a GM-CSF 3′ UTR probe. However, the depletion of AUF1 from cytoplasmic extracts greatly increases the stability of GM-CSF RNA in an in vitro degradation system (4, 5). As AUF1 interacts with the c-myc 3′ UTR, which is not p38 responsive, the binding of AUF1 alone cannot account for p38 sensitivity. One possibility is that signal-responsive stabilization involves a p38-dependent displacement of AUF1 from its binding site. Further experiments are required to determine the involvement (or noninvolvement) of both AUF1 and HuR. We also hope to define determinants of p38 responsiveness by extending the comparison of p38-sensitive and -insensitive ARE-containing transcripts using the system described here. It will also be interesting to extend the comparison of RNA-protein complexes involving p38-sensitive and p38-insensitive AREs and to determine whether the formation of complex C5 is specific to p38-sensitive AREs.
The most-abundant (4.6-kb) Cox-2 transcript contains a 2,515-nt 3′ UTR, while the second-most-abundant (2.8-kb) transcript contains a 603-nt 3′ UTR (38, 47). Only 123 nt, immediately 3′ to the translation termination codon, are required for the regulation of mRNA stability by p38. Evolutionary conservation of p38-mediated stability regulation is suggested by the high degree of conservation of this region (between mouse and human transcripts: 77% for CR1, 100% for the AUUUA motifs within CR1, and 64% for the entire 3′ UTR). The 4.6- and 2.8-kb transcripts are similarly destabilized by inhibition of p38 (12, 44) but are differentially destabilized by the anti-inflammatory glucocorticoid dexamethasone (47), suggesting an involvement of distal sequences (possibly CR2). Regulation of stability by dexamethasone requires glucocorticoid receptor-mediated gene expression and could not be reconstituted using posttranscriptional reporter constructs (37, 46, 47). The p38 and glucocorticoid pathways therefore seem to employ distinct mechanisms and distinct cis-acting sequences to regulate Cox-2 mRNA stability. Thus, both transcriptional and posttranscriptional regulatory elements of the Cox-2 gene have evolved a capacity to respond to diverse extracellular signals.
ACKNOWLEDGMENTS
We are grateful to C. J. Marshall, A.-B. Shyu, J. Han, S. Lumb, J. A. Steitz, and D. Fitzgerald for provision of reagents and to J. L. Dean for helpful discussions.
M. Lasa is supported by a grant from the Nuffield Foundation Oliver Bird Fund. G. Brewer is supported by a grant from the Arthritis Foundation.
REFERENCES
- 1.Ben-Levy R, Hooper S, Wilson R, Paterson H F, Marshall C J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Levy R, Leighton I A, Doza Y N, Attwood P, Morrice N, Marshall C J, Cohen P. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. EMBO J. 1995;14:5920–5930. doi: 10.1002/j.1460-2075.1995.tb00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer G. An A+U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991;11:2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzby J S, Brewer G, Nugent D J, Buzby J S, Lee S M, Van Winkle P, DeMaria C T, Brewer G, Cairo M S. Developmental regulation of RNA transcript destabilization by A + U-rich elements is AUF1-dependent. J Biol Chem. 1999;274:33973–33978. doi: 10.1074/jbc.274.48.33973. [DOI] [PubMed] [Google Scholar]
- 5.Buzby J S, Lee S M, Van Winkle P, DeMaria C T, Brewer G, Cairo M S. Increased granulocyte-macrophage colony-stimulating factor mRNA instability in cord versus adult mononuclear cells is translation-dependent and associated with increased levels of A + U-rich element binding factor. Blood. 1996;88:2889–2897. [PubMed] [Google Scholar]
- 6.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C Y, Shyu A B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen C Y, Shyu A B. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol Cell Biol. 1994;14:8471–8482. doi: 10.1128/mcb.14.12.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C Y, Xu N, Shyu A B. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol Cell Biol. 1995;15:5777–5788. doi: 10.1128/mcb.15.10.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerk A, Sugden P H. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs) FEBS Lett. 1998;426:93–96. doi: 10.1016/s0014-5793(98)00324-x. [DOI] [PubMed] [Google Scholar]
- 11.Cuenda A, Alonso G, Morrice N, Jones M, Meier R, Cohen P, Nebreda A R. Purification and cDNA cloning of SAPKK3, the major activator of RK/p38 in stress- and cytokine-stimulated monocytes and epithelial cells. EMBO J. 1996;15:4156–4164. [PMC free article] [PubMed] [Google Scholar]
- 12.Dean J L, Brook M, Clark A R, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- 13.Dubois R N, Abramson S B, Crofford L, Gupta R A, Simon L S, Van De Putte L B, Lipsky P E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 14.Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel K, Ahlers A, Brach M A, Herrmann F, Gaestel M. MAPKAP kinase 2 is activated by heat shock and TNF-alpha: in vivo phosphorylation of small heat shock protein results from stimulation of the MAP kinase cascade. J Cell Biochem. 1995;57:321–330. doi: 10.1002/jcb.240570216. [DOI] [PubMed] [Google Scholar]
- 16.Fan X C, Steitz J A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher B S, Kujubu D A, Perrin D M, Herschman H R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992;267:4338–4344. [PubMed] [Google Scholar]
- 18.Freshney N W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 19.Goetzl E J, An S, Smith W L. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995;9:1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- 20.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan Z, Buckman S Y, Pentland A P, Templeton D J, Morrison A R. Induction of cyclooxygenase-2 by the activated MEKK1 → SEK1/MKK4 → p38 mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:12901–12908. doi: 10.1074/jbc.273.21.12901. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton B J, Burns C M, Nichols R C, Rigby W F C. Modulation of AUUUA response element binding by heterogeneous nuclear ribonucleoprotein A1 in human T lymphocytes. The roles of cytoplasmic location, transcription, and phosphorylation. J Biol Chem. 1997;272:28732–28741. doi: 10.1074/jbc.272.45.28732. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill M A, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- 25.Hel Z, Skamene E, Radzioch D. Two distinct regions in the 3′ untranslated region of tumor necrosis factor alpha mRNA form complexes with macrophage proteins. Mol Cell Biol. 1996;16:5579–5590. doi: 10.1128/mcb.16.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf H J, Herrlich P. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 1997;16:1009–1022. doi: 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert H, Charette S J, Bernier A F, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 28.Landry J, Chretien P, Lambert H, Hickey E, Weber L A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaPointe M C, Isenovic E. Interleukin-1β regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 30.Laroia G, Cuesta R, Brewer G, Schneider R J. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science. 1999;284:499–502. doi: 10.1126/science.284.5413.499. [DOI] [PubMed] [Google Scholar]
- 31.Loflin P, Chen C Y, Shyu A B. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13:1884–1897. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma W J, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 33.Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling T E, Dannenberg A J, Tanabe T, Inoue H, Arata J, Jetten A M. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role Of mitogen-activated protein kinases. J Biol Chem. 1999;274:29138–29148. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin M M, Kumar S, McDonnell P C, Van Horn S, Lee J C, Livi G P, Young P R. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 35.Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo A P. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- 36.Myer V E, Fan X C, Steitz J A. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997;16:2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton R, Seybold J, Kuitert L M, Bergmann M, Barnes P J. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998;273:32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- 38.Newton R, Seybold J, Liu S F, Barnes P J. Alternate COX-2 transcripts are differentially regulated: implications for post-transcriptional control. Biochem Biophys Res Commun. 1997;234:85–89. doi: 10.1006/bbrc.1997.6586. [DOI] [PubMed] [Google Scholar]
- 39.O'Banion M K, Sadowski H B, Winn V, Young D A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991;266:23261–23267. [PubMed] [Google Scholar]
- 40.Pende A, Tremmel K D, DeMaria C T, Blaxall B C, Minobe W A, Sherman J A, Bisognano J D, Bristow M R, Brewer G, Port J. Regulation of the mRNA-binding protein AUF1 by activation of the beta-adrenergic receptor signal transduction pathway. J Biol Chem. 1996;271:8493–8501. doi: 10.1074/jbc.271.14.8493. [DOI] [PubMed] [Google Scholar]
- 41.Pouliot M, Baillargeon J, Lee J C, Cleland L G, James M J. Inhibition of prostaglandin endoperoxide synthase-2 expression in stimulated human monocytes by inhibitors of p38 mitogen-activated protein kinase. J Immunol. 1997;158:4930–4937. [PubMed] [Google Scholar]
- 42.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiser C O, Lanz T, Hofmann F, Hofer G, Rupprecht H D, Goppelt-Struebe M. Lysophosphatidic acid-mediated signal-transduction pathways involved in the induction of the early-response genes prostaglandin G/H synthase-2 and Egr-1: a critical role for the mitogen-activated protein kinase p38 and for Rho proteins. Biochem J. 1998;330:1107–1114. doi: 10.1042/bj3301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridley S H, Dean J L, Sarsfield S J, Brook M, Clark A R, Saklatvala J. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 1998;439:75–80. doi: 10.1016/s0014-5793(98)01342-8. [DOI] [PubMed] [Google Scholar]
- 45.Ridley S H, Sarsfield S J, Lee J C, Bigg H F, Cawston T E, Taylor D J, DeWitt D L, Saklatvala J. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- 46.Ristimaki A, Garfinkel S, Wessendorf J, Maciag T, Hla T. Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation. J Biol Chem. 1994;269:11769–11775. [PubMed] [Google Scholar]
- 47.Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem J. 1996;318:325–331. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo A P, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 49.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 50.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 51.Shyu A B, Greenberg M E, Belasco J G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989;3:60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- 52.Sirenko O I, Lofquist A K, DeMaria C T, Morris J S, Brewer G, Haskill J S. Adhesion-dependent regulation of an A+U-rich element-binding activity associated with AUF1. Mol Cell Biol. 1997;17:3898–3906. doi: 10.1128/mcb.17.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stokoe D, Engel K, Campbell D G, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992;313:307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- 54.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb M J. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L H, Hajibeigi A, Xu X M, Loose-Mitchell D, Wu K K. Characterization of the promoter of human prostaglandin H synthase-1 gene. Biochem Biophys Res Commun. 1993;190:406–411. doi: 10.1006/bbrc.1993.1062. [DOI] [PubMed] [Google Scholar]
- 56.Whitmarsh A J, Yang S H, Su M S, Sharrocks A D, Davis R J. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen C Y, Shyu A B, Gaestel M I M M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisdom R, Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991;5:232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]
- 59.Xu N, Loflin P, Chen C Y, Shyu A B. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy. Nucleic Acids Res. 1998;26:558–565. doi: 10.1093/nar/26.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Wagner B J, Ehrenman K, Schaefer A W, DeMaria C T, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]