Abstract
OBJECTIVES
To examine the effects of the First 1000 Days intervention on the prevalence of infant overweight and maternal postpartum weight retention and care.
METHODS
Using a quasi-experimental design, we evaluated the effects of the First 1000 Days program among 995 term, low-income infants and their mothers receiving care in 2 intervention community health centers and 650 dyads in 2 comparison health centers. The program includes staff training, growth tracking, health and behavioral screening, patient navigation, text messaging, educational materials, and health coaching. Comparison centers implemented usual care. Infant outcomes were assessed at 6 and 12 months, including weight-for-length z score and overweight (weight for length ≥97.7th percentile). We also examined maternal weight retention and receipt of care 6 weeks’ post partum.
RESULTS
The mean birth weight was 3.34 kg (SD 0.45); 57% of infants were Hispanic; 66% were publicly insured. At 6 months, infants had lower weight-for-length z scores (β: −.27; 95% confidence interval [CI]: −.39 to −.15) and lower odds of overweight (adjusted odds ratio [OR]: 0.46; 95% CI: 0.28 to 0.76) than infants in comparison sites; differences persisted at 12 months (z score β: −.18; 95% CI: −.30 to −.07; adjusted OR for overweight: 0.60; 95% CI: 0.39 to 0.92). Mothers in the intervention sites had modestly lower, but nonsignificant, weight retention at 6 weeks’ post partum (β: −.51 kg; 95% CI: −1.15 to .13) and had higher odds (adjusted OR: 1.50; 95% CI: 1.16 to 1.94) of completing their postpartum visit compared with mothers in the comparison sites.
CONCLUSIONS
An early-life systems-change intervention combined with coaching was associated with improved infant weight status and maternal postpartum care.
What’s Known on This Subject:
Obesity disparities emerge in early life and are partially determined by risk factors in pregnancy, infancy, and early childhood. Despite the potential for obesity prevention, there are relatively fewer interventions focused on early life.
What This Study Adds:
A comprehensive program implementing changes across early-life systems was associated with reduced infant overweight and increased receipt of maternal postpartum care among low-income dyads. Such interventions hold promise to improve the population health of women and children.
Childhood obesity prevalence remains at historically high levels, and primary prevention strategies could have significant public health impacts. Substantial evidence suggests that the first 1000 days, the period from early pregnancy to age 2, is critical for both the development and prevention of childhood obesity and related health outcomes.1 This period is marked by developmental plasticity, during which environmental and nutritional cues can metabolically program body composition, increasing risk for obesity, adiposity, and associated diseases later in life.2 In particular, children’s early weight gain trajectories have been shown to modulate their long-term obesity risk,3 underscoring the importance of developing early interventions that promote healthy growth during this critical period.
Despite the potential for obesity prevention, fewer interventions are focused on early life than on other periods. Existing interventions reveal promising effects on improving pediatric weight outcomes4,5 and are generally focused on individual- or family-level behavior change through health professional–led clinical or in-home visits.6–16 Few interventions begin in pregnancy or are focused on low-income families, who have the highest risk for childhood obesity. Moreover, systems-level and community-based interventions are lacking, as are those that target social-contextual or upstream influences on obesity.
To address these gaps, we developed the First 1000 Days program, a comprehensive program implementing changes across early-life clinical and public health systems to reduce obesity risk factors among low-income mother-infant pairs. Mothers are enrolled during their first trimester and are supported throughout pregnancy and their infants’ first 2 years of life. Previously, we found that women enrolled in the First 1000 Days program with overweight at the start of their pregnancies experienced a modest reduction in excess gestational weight gain.17 In this study, we examine the effectiveness of the program on infant weight status in the first year of life and continued effects of the program on maternal postpartum outcomes. We hypothesized that infants in the program would have lower weight-for-length z scores, reduced prevalence of overweight, and reduced rates of weight gain at 6 and 12 months compared with infants who received care in comparison community health centers. As secondary outcomes, we examined program effects on maternal weight retention and receipt of care at 6 weeks’ post partum.
Methods
Study Overview
Pregnant women were recruited from 2 community health centers in eastern Massachusetts. The conceptual framework, intervention design, and maternal outcomes are described elsewhere.17,18 Briefly, the program is a systems-level initiative with the aim to reduce the prevalence of overweight by addressing individual, family, and socio-contextual factors across early-life clinical and public health systems. In contrast to individual-level interventions that target participants directly and individually, systems-level interventions are intended to change organizational practices and policies to ensure that intervention components are systematically implemented at every clinical and public health system encounter and delivered to all patients and clients of those organizations. Thus, systems-level interventions in the First 1000 Days program implemented organizational changes across early-life clinical and public health systems, such as Obstetrics, Pediatrics, and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
The primary outcome of the current analysis was weight status at 6 and 12 months among infants who received care in the 2 intervention community health centers versus infants in 2 comparison health centers. We also evaluated the program’s effects on maternal weight retention and receipt of care at 6 weeks’ post partum. Outcomes at 24 months of age are forthcoming. The study protocol was approved by the Institutional Review Board of Mass General Brigham. A waiver of informed consent was granted to collect longitudinal electronic health record (EHR) data for evaluation.
Setting
The intervention and comparison community health centers are affiliated with Mass General Brigham, an integrated health system in Massachusetts. All 4 health centers offer primary and specialty care for children, adults, and pregnant women in urban locations (Chelsea, MA; Revere, MA; and Jamaica Plain, Boston, MA) and are located in communities that primarily serve low-income and racial and ethnic minority patients.19–22
Program Components
The primary components of the program were evidence informed and focused on primary and secondary prevention of maternal-infant overweight, obesity, and related risk factors. Components of the pregnancy program have been previously described and are similar to the infancy and postpartum components described.17,18 Systems-level components included the following: (1) standardized early-childhood obesity prevention training for pediatric clinicians and staff, including nurses, medical assistants, and WIC and home visiting staff23,24; (2) enhanced tracking of infant weight gain, screening through clinical decision-support tools in the EHR, and surveillance by First 1000 Days health coaches; (3) universal screening for adverse health behaviors and socio-contextual factors at ages 1, 6, and 12 months25; and (4) educational materials and twice weekly text messaging to educate families on early-life health behaviors and to encourage behavior change (through 24 months of age). Individual-level supports included the following: (1) patient navigation to support parents’ adoption of infant health behaviors, assess social needs, and strengthen integration of clinical and public health services (up to 3 phone calls from birth to 24 months) and (2) health coaching and care coordination (up to 3 phone calls from birth to 24 months) for women and infants considered to be high-risk for obesity based on prepregnancy BMI, accelerated pregnancy weight gain, and/or rapid infant weight gain. Coaching was delivered virtually or by an embedded health coach at well-child care visits. At a minimum, coaches had academic degrees related to maternal-child health, community health, or nutrition. All coaches received training on program components and obesity prevention recommendations and participated in weekly supervisory conferences with a senior health educator.
Behavioral Targets
The program was focused on several behavioral targets for mothers both during pregnancy17,18 and in the postpartum period, including improving diet and beverage quality, guideline-adherent gestational weight gain, reducing postpartum weight retention, physical activity, sleep, and stress reduction. We also encouraged women to schedule appointments for their postpartum visits and enroll in WIC, if eligible. In infancy, the primary behavioral targets included infant feeding mode and behavior, responsive feeding, complementary food and beverage quality, sleep quality and quantity, avoidance of screen time, and developmentally appropriate play.
Educational materials encouraged consistent behavioral messaging across clinicians and public health services. Materials included posters in the health centers and infant age-specific booklets and a postpartum care booklet provided to families at well-child visits or mailed to their homes. Booklets were available in English, Spanish, Vietnamese, and Arabic. We sent 2 to 3 automated text messages per week throughout the child’s first 2 years of life.18 Messages reinforced the behavioral recommendations. Parents also had access to >50 short informational videos to reinforce the program’s goals. Web links to the videos were included in the booklets and text messages.
Analytic Sample
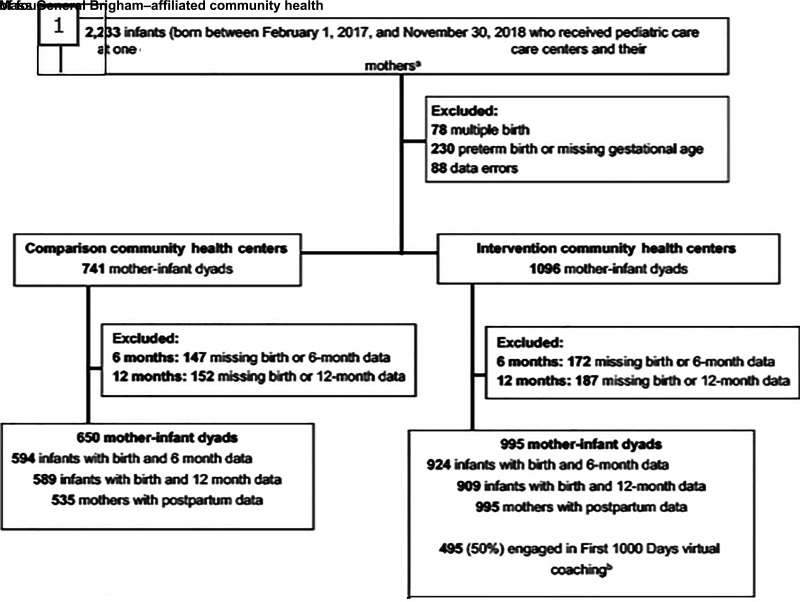
We extracted longitudinal weight, length, and vital demographic information from the EHR of the 2 intervention and 2 comparison sites for 2233 infants born February 1, 2017, to November 30, 2018. Data were collected from birth to age 15 months (Fig 1). We excluded infants who were multiples, those born preterm (<37 weeks), and those missing gestational age data. Infants were eligible for the longitudinal analyses if they had valid birth data within the first 15 days of life and 6-month (5.0–7.5 months) or 12-month (10.5–15 month) length and weight measurements. We excluded measurement and recording errors, such as implausible values and repeated measurements, and carried forward growth data using a validated algorithm.26 This yielded sample sizes of 995 infants in the intervention sites and 650 infants in the comparison sites. We also extracted height, weight, and vital demographic data on 1530 mothers of these infants (995 mothers in the intervention sites and 535 mothers in the comparison sites). Infants excluded from analyses did not differ from the analytic sample on race and/or ethnicity, gestational age, or birth weight. Infants excluded from the intervention sites because of missing 6- and 12-month outcomes were more likely to be publicly insured compared with the analytic sample; multivariate models were adjusted for public insurance.
FIGURE 1.
Intervention and comparison sample size flow for First 1000 Days pediatric program. a The sample size for intervention sites reflects infants who had mothers also receiving prenatal care within the Mass General Brigham health system. b One of the intervention sites embedded a health coach in person at well-child care visits. In-person coaching is not included.
Outcome Measures
The primary infant outcomes were (1) mean weight-for-length z score at 6 and 12 months and (2) prevalence of infant overweight at 6 and 12 months. We also examined conditional weight gain from birth to 6 and 12 months. Medical assistants measured infant weight and length at birth and at outpatient visits using written standardized protocols and entered the data into the EHR. Age- and sex-specific weight-for-length z scores were calculated by using World Health Organization growth charts.27 Infant overweight was defined as a weight for length ≥97.7th percentile. Conditional weight gain was calculated as the standardized residuals from the linear regression of weight for age at 6 or 12 months on weight for age and length for age at birth and length for age and infant age at 6 or 12 months.13 Conditional weight gain reveals the variation in child weight gain, which is not explained by child age, birth length, or birth weight. With a population mean score of 0, scores >0 reflect faster than average weight gain compared to the population average.
As secondary outcomes, we also examined postpartum weight retention at 6 weeks, defined as prepregnancy weight subtracted from weight at the postpartum visit, and the proportion of women who attended a postpartum follow-up visit (eg, 4–10 weeks after delivery).
Other Measures
We extracted race and/or ethnicity, public insurance, and maternal prepregnancy BMI from the EHR. Race and/or ethnicity missingness in the EHR ranged from 24% to 67% for infants and was 1% for mothers. We assumed these values were missing at random and performed multiple imputation in SAS version 9.4 (SAS Institute, Inc, Cary, NC). We created and analyzed 10 multiply imputed data sets in SAS using MI and MIANALYZE procedures.
Statistical Analysis
Descriptive statistics are summarized by health center as mean (SD) or n (%). To evaluate the associations of the First 1000 Days program with our main outcomes, we examined differences in mean weight-for-length z scores and prevalence of infant overweight at 6 and 12 months in the intervention sites compared with the comparison health centers. We examined outcomes for the combined sample and separately for each intervention site. We used PROC GLM to analyze unadjusted mean differences in infant weight-for-length z scores and postpartum weight retention and PROC LOGSITIC to calculate odds ratios (ORs) for infant overweight and postpartum visit completion. In adjusted models, we used PROC GENMOD and PROC LOGISTIC to assess outcomes, which were fitted with PROC MIANALYZE to generate results from race and ethnicity–imputed data sets. For infant outcomes, we ran models unadjusted and adjusted for infant race and/or ethnicity, public insurance, and birth weight. For postpartum outcomes, we ran models unadjusted and adjusted for maternal race and/or ethnicity and prepregnancy BMI. For maternal postpartum weight retention, we additionally adjusted for time post partum when the weight measurement was taken. The enrolled sample of 1645 infants was sufficient to detect a weight-for-length z score difference of 0.15 between intervention and nonintervention groups at a precision of 0.10 and power of 90%.
Results
Table 1 reveals demographic and clinical characteristics of mother-infant dyads in the intervention and comparison sites. Across sites, infant mean (SD) birth weight was 3.34 kg (0.45); 57% of infants were Hispanic, and 66% were publicly insured. Analyses were adjusted for infant race and/or ethnicity, public insurance, and birth weight given differences across sites (Table 1). Mean (SD) maternal age at delivery was 29.9 years (5.8), and mean (SD) prepregnancy BMI was 28.2 (7.3).
TABLE 1.
Baseline Characteristics of Mother-Infant Pairs in the First 1000 Days Community Health Centers and Comparison Health Centers, by Intervention Assignment
| Infant and Maternal Characteristics | Comparison Sites | Intervention Sites | P |
|---|---|---|---|
| Infant characteristics | n = 650 | n = 995 | — |
| Sex, n (%) | |||
| Male | 334 (51.4) | 469 (47.1) | .09 |
| Female | 316 (48.6) | 526 (52.9) | — |
| Race and/or ethnicity, n (%) | |||
| White, non-Hispanic | 222 (34.2) | 196 (19.7) | <.001 |
| Hispanic/Latino | 340 (52.3) | 597 (60.0) | — |
| Black, non-Hispanic | 33 (5.1) | 60 (6.0) | — |
| Asian or other | 55 (8.5) | 142 (14.3) | — |
| Public insurance, n (%) | 372 (57.2) | 718 (72.2) | <.001 |
| Infant birth wt, kg, mean (SD) | 3.2 (0.4) | 3.4 (0.4) | <.001 |
| Gestational age, wk, mean (SD) | 39.1 (1.2) | 39.6 (1.1) | <.001 |
| Maternal characteristics | n = 535 | n = 995 | — |
| Age at delivery,a y, mean (SD) | 30.3 (5.8) | 29.7 (5.8) | .04 |
| Parity,a mean (SD) | 1.9 (1.0) | 2.1 (1.2) | .001 |
| Race and/or ethnicity, n (%) | |||
| White, non-Hispanic | 103 (19.2) | 241 (24.2) | <.001 |
| Hispanic/Latino | 362 (67.7) | 569 (57.2) | — |
| Black, non-Hispanic | 42 (7.9) | 68 (6.9) | — |
| Asian or other | 28 (5.2) | 117 (11.8) | — |
| Public insurance,b n (%) | 261 (49.3) | 738 (74.2) | <.001 |
| Prepregnancy BMI,c mean (SD) | 28.1 (9.1) | 28.2 (6.1) | .78 |
—, not applicable.
N = 1528
N = 1525
N = 1504
Table 2 presents unadjusted and adjusted infant weight status outcomes at 6 and 12 months. In adjusted models, infants in the intervention sites had a −0.27 lower weight-for-length z score at 6 months (95% confidence interval [CI]: −0.39 to −0.15), and a −0.18 lower z score at 12 months (95% CI: −0.30 to −0.07) compared with children in the comparison sites. At 6 and 12 months, the prevalence of overweight was lower in the intervention sites (3.6% at 6 months and 6.2% at 12 months) than in the comparison sites (6.2% at 6 months and 7.8% at 12 months). In adjusted models, children had 0.46 (95%CI: 0.28 to 0.76) the odds of being overweight at 6 months and 0.60 (95% CI: 0.39 to 0.92) the odds of being overweight at 12 months compared with children in the comparison sites. Examining these outcomes for each of the intervention sites separately (Supplemental Table 4) revealed a stronger association among infants at intervention site 2.
TABLE 2.
Infant Anthropometric Outcomes in the First 1000 Days, by Intervention Assignment
| Main Outcome | Unadjusted n (%) or Mean (SD) | Unadjusted Mean Difference or OR (95% CI) | Adjusteda Mean Difference or OR (95% CI) |
|---|---|---|---|
| 6-mo outcomes (N = 1518) | |||
| Wt-for-length z score | |||
| Intervention sites | 0.16 (1.05) | −0.15 (−0.27 to −0.04)* | −0.27 (−0.39 to −0.15)* |
| Comparison sites | 0.31 (1.14) | 0.00 (reference) | 0.00 (reference) |
| Wt for length ≥97.7th percentile | |||
| Intervention sites | 33 (3.6%) | 0.56 (0.34 to 0.90)* | 0.46 (0.28 to 0.76)* |
| Comparison sites | 37 (6.2%) | 1.00 (reference) | 1.00 (reference) |
| 12-mo outcomes (N = 1498) | |||
| Wt-for-length z score | |||
| Intervention sites | 0.41 (1.03) | −0.06 (−0.17 to 0.05) | −0.18 (−0.30 to −0.07)* |
| Comparison sites | 0.47 (1.10) | 0.00 (reference) | 0.00 (reference) |
| Wt for length ≥97.7th percentile | |||
| Intervention sites | 56 (6.2%) | 0.77 (0.52 to 1.16) | 0.60 (0.39 to 0.92)* |
| Comparison sites | 46 (7.8%) | 1.00 (reference) | 1.00 (reference) |
Data are presented as mean (SD) with mean difference (95% CI) or n (%) with OR (95% CI).
All models were adjusted for birth weight, race and/or ethnicity (imputed), and public insurance.
Statistical significance at the P = .05 level.
The adjusted mean conditional weight gain score from birth to 6 months was lower among infants in the intervention sites compared with those in the comparison sites (Fig 2), suggesting that children in the intervention sites gained weight more slowly than those in the comparison sites. This effect was sustained through 12 months of age, with an adjusted mean conditional weight gain score from birth to 12 months that was 0.22 U (95% CI: −0.33 to −0.11) less compared with the comparison sites.
FIGURE 2.
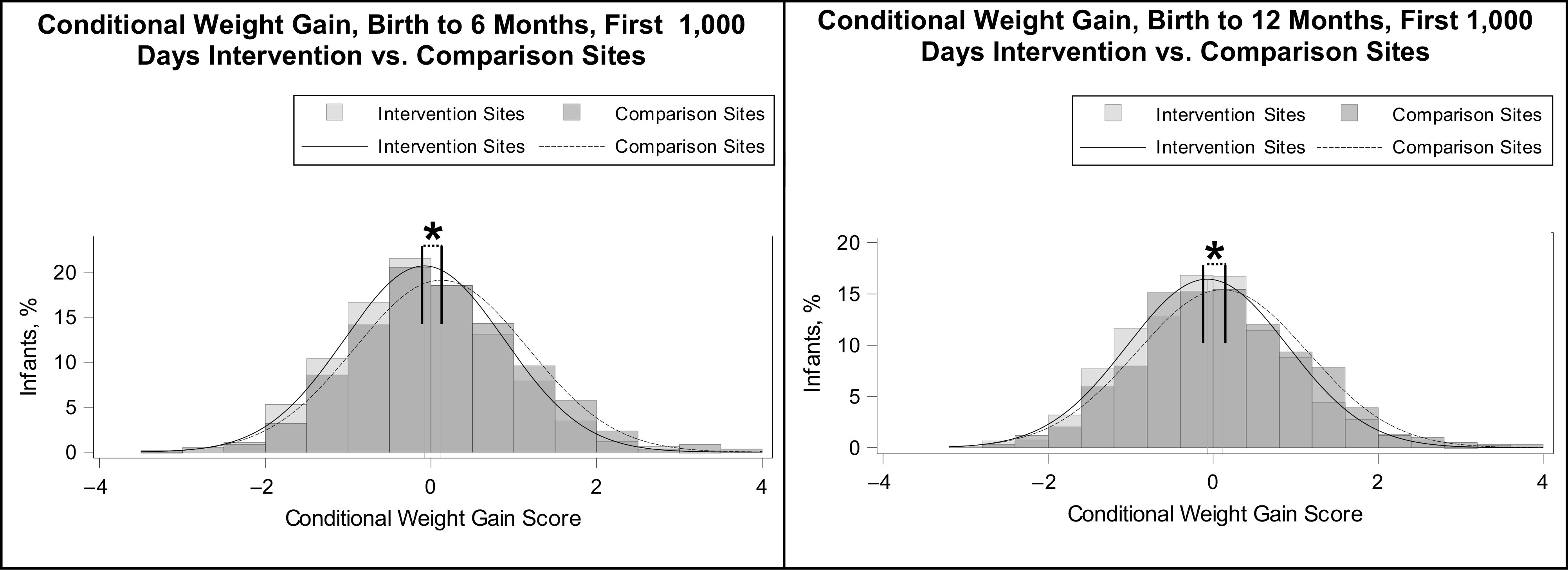
Conditional weight gain from birth to 6 and 12 months, by First 1000 Days program intervention assignment. a The unadjusted mean (SD) conditional weight gain scores were −0.08 (0.96) for the intervention sites and 0.12 (1.04) for the comparison sites, with an unadjusted difference of −0.20 (95% CI: −0.31 to −0.10) and an adjusted difference of −0.25 (95% CI: −0.36 to −0.15), indicating that children in the intervention sites gained weight more slowly than those in the comparison sites. b The unadjusted mean (SD) conditional weight gain scores were −0.07 (0.97) for the intervention sites and 0.11 (1.04) for the comparison sites, with an unadjusted difference of −0.18 (95% CI: −0.28 to −0.08) and an adjusted difference of −0.22 (95% CI: −0.33 to −0.11), indicating that children in the intervention sites gained weight more slowly than those in the comparison sites.
In secondary analyses (Table 3), we found that women from the intervention sites had higher adjusted odds (adjusted OR: 1.50; 95% CI: 1.16 to 1.94) of completing a postpartum visit than women from the comparison sites (adjusted for prepregnancy BMI and race and/or ethnicity). We observed a modestly lower, but nonsignificant, effect on maternal postpartum weight retention (β: −.51; 95% CI: −1.15 to .13) between the intervention and comparison sites.
TABLE 3.
Maternal Weight Retention and Health Care Visit Completion at 6 Weeks’ Post Partum in the First 1000 Days Program, by Intervention Assignment (N = 1530)
| Main Outcome | Unadjusted n (%) or Mean (SD) | Unadjusted Mean Difference or OR (95% CI) | Adjusteda Mean Difference or OR (95% CI) |
|---|---|---|---|
| Postpartum weight retention, kgb | |||
| Intervention sites | 3.10 (5.55) | −0.32 (−0.97 to 0.33) | −0.51 (−1.15 to 0.13) |
| Comparison sites | 3.43 (5.65) | 0.00 (reference) | 0.00 (reference) |
| Postpartum visit completion | |||
| Intervention sites | 818 (82.2%) | 1.56 (1.21 to 2.01)* | 1.50 (1.16 to 1.94)* |
| Comparison sites | 400 (74.8%) | 1.00 (reference) | 1.00 (reference) |
Data are presented as mean (SD) with mean difference (95% CI) or n (%) with OR (95% CI).
All models were adjusted for prepregnancy BMI and race and/or ethnicity. Postpartum weight retention models were additionally adjusted for time in weeks at postpartum weight measurement.
N = 1226
Statistical significance at the P = .05 level.
Discussion
In this quasi-experimental trial, we found that a systems-level intervention combined with individualized health coaching in the first year of life was associated with lower weight-for-length z scores, lower odds of overweight, and reduced rapid weight gain among infants from low-income communities. Infants who received care from a community health center that implemented systems-level changes, including clinician and staff training, growth tracking, parent education, social support, and individual behavioral counseling by a health coach, had 54% lower odds of overweight at 6 months and 40% lower odds of overweight at 12 months compared with infants who received usual infant care in comparison community health centers. The magnitude of improvement in infant weight status was robust to adjustment for birth weight, race and/or ethnicity, and public insurance. Overall, our findings suggest that systematic changes combined with individual-level coaching may help sustain behavior change over the first year of life.
Mothers in the intervention sites who were enrolled during pregnancy and provided with continued support in the postpartum period had modestly lower, but clinically nonsignificant, weight retention at 6 weeks’ post partum compared with mothers receiving usual care in the comparison sites. We also observed higher odds of a completed 6-week postpartum visit, suggesting that mothers also benefited from the program components in the postpartum period.
The First 1000 Days study had several features in common with previous interventions to prevent obesity in early life. Similar to the Healthy Beginnings Trial and the NOURISH study,6–10 both of which decreased mean BMI before 24 months, our study was focused on multiple diet and activity behaviors, particularly parenting practices focused on children’s early feeding experiences. We also targeted infant sleep, complementing findings from the SLIMTIME (Sleeping and Intake Methods Taught to Infants and Mothers Early in Life) study, a nurse-led randomized trial in which lower weights for length were reported among 12-month old infants who received an intervention focused on prolonging sleep duration in addition to dietary guidance.11 A follow-up randomized controlled trial in a similar population confirmed the effectiveness of targeting infant feeding and sleep for healthy weight gain in infancy.12,13 Unlike other obesity prevention interventions in which null findings were reported,28–40 First 1000 Days involved multiple intervention targets and was implemented during pregnancy and early infancy, suggesting that early, multicomponent interventions hold the most promise for obesity prevention.
Our study had several distinguishing features. First, unlike most studies that begin after birth, we enrolled women during their first trimester of pregnancy and provided support throughout their pregnancy and during the first 2 years of their infants’ lives. Second, we targeted both individual- and systems-level changes, whereas many interventions have been focused on individual behavior change. Third, whereas most interventions are clinic-based and nurse-led, the First 1000 Days program built collaborations for obesity prevention across early-life clinical and public health services. Finally, our program leveraged existing clinical health information technology to link families across these clinical disciplines and public health programs, resulting in more effective, efficient, and coordinated care for families. Our findings can inform the development of future interventions, including by implementing both systems- and individual-level interventions that are focused on the mother-child dyad to improve both maternal and child health outcomes.
Although our study had several strengths, including the systematic approach across pregnancy and infancy, the study also had limitations. First, given the nature of the systems-level intervention, we were not able to use a randomized or blinded design, which could lead to selection bias. Although a randomized controlled trial provides the highest level of internal validity, in many settings (particularly low-resource settings serving vulnerable populations) random assignment to a control group is not acceptable because it conflicts with the organization’s objective to serve vulnerable families.41 As a viable alternative, we used a longitudinal, quasi-experimental design with a comparison group, which allowed for more flexibility and adaptation in our community setting. The First 1000 Days program was implemented in 2 urban hospital-affiliated community health centers that predominantly serve low-income and racial and ethnic minority patients; generalizability of our findings to rural or other populations or settings may not be applicable. Additionally, given the systems- and individual-level components of our intervention, it is not possible to attribute the observed effects to a specific component of the intervention. For example, we were unable to isolate the impact of health coaching, although the stronger associations we observed for site 2 suggest that in-person health coaching may have been more impactful. Lastly, although Mass General Brigham has a comprehensive EHR capable of being queried at the individual level, there are limitations to the data quality and differences in documentation across sites, which we addressed through multiple imputation (eg, race and ethnicity) and chart reviews. The use of EHR data also means misclassification of outcomes is possible. Despite these limitations, we believe our study reflects best practice in the design and evaluation of a multilevel intervention, and our results add to the knowledge base in addressing childhood obesity and improving maternal postpartum outcomes in low-income communities.
Conclusions
The First 1000 Days program reveals that a systems-level intervention combined with individualized health coaching in pregnancy and infancy was associated with improved infant weight status and maternal postpartum health care. Such interventions hold promise to improve the population health of women and children through existing community health centers and public health programs that serve low-income and racially and ethnically diverse populations, the segments of the population who need it most.
Glossary
- CI
confidence interval
- EHR
electronic health record
- OR
odds ratio
- WIC
Special Supplemental Nutrition Program for Women, Infants, and Children
Footnotes
Dr Taveras conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Boudreau, Blake-Lamb, Matathia, and Kotelchuck designed the study and reviewed and revised the manuscript; Dr Cheng, Ms Perkins, Ms Price, and Ms Roche drafted the initial manuscript and reviewed and revised the manuscript; Ms Luo conducted the analyses and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Individual patient data will not be made available to others. Investigators may contact Senior Program Manager, Ms Perkins (meperkins@mgh.harvard.edu), to request access to deidentified data after approval from the principal investigator, and any required institutional review board approvals and data use agreements are executed.
This trial has been registered at www.clinicaltrials.gov (identifier NCT03191591).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by The Boston Foundation (G2015-0007), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K24DK105989 and K01DK114383), and Massachusetts General Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The sponsors had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit for publication. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Gillman MW. Early infancy as a critical period for development of obesity and related conditions. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koletzko B, Fishbein M, Lee WS, et al. Prevention of childhood obesity: a position paper of the global Federation of International Societies of Paediatric Gastroenterology, Hepatology and Nutrition (FISPGHAN). J Pediatr Gastroenterol Nutr. 2020;70(5):702–710 [DOI] [PubMed] [Google Scholar]
- 3. Zheng M, Lamb KE, Grimes C, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19(3):321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med. 2016;50(6):780–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Messito MJ, Mendelsohn AL, Katzow MW, Scott MA, Vandyousefi S, Gross RS. Prenatal and pediatric primary care-based child obesity prevention program: a randomized trial. Pediatrics. 2020;146(4):e20200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children’s BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen LM, Baur LA, Simpson JM, et al. Sustainability of effects of an early childhood obesity prevention trial over time: a further 3-year follow-up of the Healthy Beginnings Trial. JAMA Pediatr. 2015;169(6):543–551 [DOI] [PubMed] [Google Scholar]
- 8. Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int J Obes (Lond). 2012;36(10):1292–1298 [DOI] [PubMed] [Google Scholar]
- 9. Daniels LA, Mallan KM, Nicholson JM, Battistutta D, Magarey A. Outcomes of an early feeding practices intervention to prevent childhood obesity. Pediatrics. 2013;132(1). Available at: https://pediatrics.aappublications.org/content/132/1/e109 [DOI] [PubMed] [Google Scholar]
- 10. Daniels LA, Mallan KM, Nicholson JM, et al. An early feeding practices intervention for obesity prevention. Pediatrics. 2015;136(1). Available at: https://pediatrics.aappublications.org/content/136/1/e40 [DOI] [PubMed] [Google Scholar]
- 11. Paul IM, Savage JS, Anzman SL, et al. Preventing obesity during infancy: a pilot study. Obesity (Silver Spring). 2011;19(2):353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paul IM, Savage JS, Anzman-Frasca S, et al. Effect of a responsive parenting educational intervention on childhood weight outcomes at 3 years of age: the INSIGHT randomized clinical trial. JAMA. 2018;320(5):461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatr. 2016;170(8):742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor BJ, Gray AR, Galland BC, et al. Targeting sleep, food, and activity in infants for obesity prevention: an RCT. Pediatrics. 2017;139(3):e20162037. [DOI] [PubMed] [Google Scholar]
- 15. Taylor BJ, Heath ALM, Galland BC, et al. Prevention of Overweight in Infancy (POI.nz) study: a randomised controlled trial of sleep, food and activity interventions for preventing overweight from birth. BMC Public Health. 2011;11:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor RW, Gray AR, Heath ALM, et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the Prevention of Overweight in Infancy (POI) randomized controlled trial at ages 3.5 and 5 y. Am J Clin Nutr. 2018;108(2):228–236 [DOI] [PubMed] [Google Scholar]
- 17. Blake-Lamb T, Boudreau AA, Matathia S, et al. Association of the First 1,000 Days systems-change intervention on maternal gestational weight gain. Obstet Gynecol. 2020;135(5):1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blake-Lamb T, Boudreau AA, Matathia S, et al. Strengthening integration of clinical and public health systems to prevent maternal-child obesity in the first 1,000 days: a collective impact approach. Contemp Clin Trials. 2018;65:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Census Bureau . QuickFacts: Chelsea city, Massachusetts. 2018. Available at: https://www.census.gov/quickfacts/chelseacitymassachusetts. Accessed August 6, 2020
- 20. US Census Bureau . QuickFacts: Revere city, Massachusetts. 2018. Available at: https://www.census.gov/quickfacts/fact/table/reverecitymassachusetts/PST045219. Accessed August 6, 2020
- 21. City of Boston Department of Neighborhood Development . Jamaica Plain data profile: population demographics. 2010. Available at: https://www.cityofboston.gov/images_documents/Jamaica_Plain_Planning_District_Profile_tcm3-12993.pdf. Accessed August 6, 2020
- 22. Boston Redevelopment Authority Research Division . Poverty in Boston. 2014. Available at: www.bostonplans.org/getattachment/f1ecaf8a-d529-40b6-a9bc-8b4419587b86. Accessed August 6, 2020
- 23. Hagan JF Jr, Shaw JS, Duncan PM, eds.. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017 [Google Scholar]
- 24. Institute of Medicine . Early Childhood Obesity Prevention Policies. Washington, DC: The National Academies Press; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Medicare and Medicaid Services . The accountable health communities health-related social needs screening tool. Available at: https://innovation.cms.gov/files/worksheets/ahcm-screeningtool.pdf. Accessed October 29, 2020
- 26. Daymont C, Ross ME, Russell Localio A, Fiks AG, Wasserman RC, Grundmeier RW. Automated identification of implausible values in growth data from pediatric electronic health records. J Am Med Inform Assoc. 2017;24(6):1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization . WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization; 2006 [Google Scholar]
- 28. Bonuck K, Avraham SB, Lo Y, Kahn R, Hyden C. Bottle-weaning intervention and toddler overweight. J Pediatr. 2014;164(2):306–312.e2 [DOI] [PubMed] [Google Scholar]
- 29. Cameron AJ, Ball K, Hesketh KD, et al. Variation in outcomes of the Melbourne Infant, Feeding, Activity and Nutrition Trial (InFANT) Program according to maternal education and age. Prev Med. 2014;58:58–63 [DOI] [PubMed] [Google Scholar]
- 30. Campbell KJ, Lioret S, McNaughton SA, et al. A parent-focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics. 2013;131(4):652–660 [DOI] [PubMed] [Google Scholar]
- 31. French GM, Nicholson L, Skybo T, et al. An evaluation of mother-centered anticipatory guidance to reduce obesogenic infant feeding behaviors. Pediatrics. 2012;130(3). Available at: https://pediatrics.aappublications.org/content/130/3/e507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33(5):964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harvey-Berino J, Rourke J. Obesity prevention in preschool Native-American children: a pilot study using home visiting. Obes Res. 2003;11(5):606–611 [DOI] [PubMed] [Google Scholar]
- 34. Kramer MS, Matush L, Vanilovich I, et al. ; PROBIT Study Group . Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–1721 [DOI] [PubMed] [Google Scholar]
- 35. Louzada ML, Campagnolo PD, Rauber F, Vitolo MR. Long-term effectiveness of maternal dietary counseling in a low-income population: a randomized field trial. Pediatrics. 2012;129(6). Available at: https://pediatrics.aappublications.org/content/129/6/e1477.full [DOI] [PubMed] [Google Scholar]
- 36. Martin RM, Kramer MS, Patel R, et al. Effects of promoting long-term, exclusive breastfeeding on adolescent adiposity, blood pressure, and growth trajectories: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2017;171(7):e170698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mustila T, Raitanen J, Keskinen P, Luoto R. A pragmatic controlled trial to prevent childhood obesity within a risk group at maternity and child health-care clinics: results up to six years of age (the VACOPP study). BMC Pediatr. 2018;18(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheiwe A, Hardy R, Watt RG. Four-year follow-up of a randomized controlled trial of a social support intervention on infant feeding practices. Matern Child Nutr. 2010;6(4):328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanvig M, Vinter CA, Jørgensen JS, et al. Anthropometrics and body composition by dual energy X-ray in children of obese women: a follow-up of a randomized controlled trial (the Lifestyle in Pregnancy and Offspring [LiPO] study). PLoS One. 2014;9(2):e89590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wake M, Price A, Clifford S, Ukoumunne OC, Hiscock H. Does an intervention that improves infant sleep also improve overweight at age 6? Follow-up of a randomised trial. Arch Dis Child. 2011;96(6):526–532 [DOI] [PubMed] [Google Scholar]
- 41. Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA. Practical clinical trials for translating research to practice: design and measurement recommendations. Med Care. 2005;43(6):551–557 [DOI] [PubMed] [Google Scholar]