Abstract
Background
Fatigue is a main or secondary reason for 10–20% of all consultations with a primary care physician.
Methods
This review is based on pertinent publications retrieved by a comprehensive, selective literature search on the epidemiology, etiology, and diagnostic evaluation of fatigue as a leading symptom of disease, as well as on the treatment of its common causes. Information was also included from the literature search we conducted for the German clinical practice guideline on fatigue that was issued by the German College of General Practitioners and Family Physicians (Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin, DEGAM).
Results
Fatigue can be due to any of a broad spectrum of diseases, including decompensation of already known conditions. Sleep disorders and sleep-related disorders of breathing, depression (18.5%), and excessive psychosocial stress are the most common causes of persistent fatigue. Previously undiagnosed cancer is a rare cause, accounting for only 0.6% of cases (95% confidence interval [0.3; 1.3]). Anemia and other organic causes are rare as well (4.3% [2.7; 6.7]). Investigations beyond the history, physical examination, and simple laboratory tests are needed only in the presence of additional symptoms or findings. If the diagnosis remains unclear, watchful waiting and regularly scheduled follow-up help prevent an excessive focus on somatic causes, leading to overdiagnosis. Irrespective of specific causes, psychoeducative and psychotherapeutic approaches should be discussed with the patient, as well as an individually adapted exercise program.
Conclusion
The work-up of fatigue as a chief complaint should be guided by investigating common and/or potentially dangerous disorders. Since the latter are rare, an exclusively somatic focus should be avoided in order to prevent overdiagnosis.
In 2018, a 27-year-old woman was employed by the Hamburg Consumer Advice Center to be a simulated patient with persistent fatigue, presenting herself to the primary care practices of 28 randomly chosen general practitioners and internists. The quality of history-taking, physical examination, diagnosis, counseling, and the physician-patient relationship in the initial patient interview was assessed on the basis of the S3 guideline for fatigue issued by the German College of General Practitioners and Family Physicians (Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin, DEGAM).
Frequency.
Fatigue is the main reason or a secondary reason for 10–20% of patient consultations in primary care.
In the overall assessment, 14.3% of the physicians were judged as “very good,” but another 14.3% were “deficient.” 18%, 32%, and 21.4% were “good,” “satisfactory,” and “adequate,” respectively (1). Other studies, too, have shown that fatigue, a symptom that patients commonly report, is among those that often cause diagnostic difficulties in routine clinical practice, and that physicians perceive as difficult (2– 4).
Learning objectives
In view of the commonness of fatigue as a chief complaint, and the difficulties physicians face in its evaluation, this article is intended to help the reader do the following:
Causes.
A wide range of medical problems and decompensation processes of underlying diseases can cause fatigue. Sleep disorders, sleep-related respiratory disorders, depression, and psychosocial stress are the main causes of persistent fatigue.
learn a rational, evidence-based, somatic and psychosocial diagnostic and therapeutic approach to patients complaining of fatigue in primary care;
known the common causes of fatigue as a chief complaint, its dangerous but treatable causes, and its rarer causes
be able to apply the proper strategy of care for patients whose initial evaluation has not yielded any explanation for fatigue.
Methods
This CME article is based on a comprehensive selective literature search on the epidemiology, etiology, and diagnostic evaluation of fatigue as a chief complaint, as well as the treatment of its more common causes. The search was for publications from the years 2015 to 2019. In the Medline and Scopus databases, the searching terms “fatigue,” “tiredness,” “primary care,” “general practice,” “family medicine,” “Allgemeinmedizin,” and/or “Primaerarzt” were used; in the Cochrane Database of Systematic Reviews and the Cochrane Database of Clinical Trials, the searching terms were “fatigue” and “tiredness,” without any restriction to primary care. In the Current Content database, the search additionally included the tables of contents of German-language medical publications from the years 2015 to 2019. The comprehensive literature searches performed by the authors for the current edition of the German S3 guideline on fatigue (5) (searching period, up to July 2015) were also incorporated in this review, as were links to further publications.
Definition and types of fatigue
There is no uniform, generally accepted definition of fatigue. In one proposed definition of fatigue, it is described as a symptom that cannot be relieved by the usual strategies of restoring energy, and that impairs, to a variable degree, the individual’s ability to carry out his or her usual daily activities (6). These usual strategies include taking more time to rest and sleep, and reducing or eliminating potential sources of excessive stress. Patients describe fatigue as listlessness, lack of energy, exhaustion, tiredness, early fatigability, sleepiness, a tendency to fall asleep during the day, physical weakness, or a feeling of running on empty (7). Fatigue must be distinguished from muscle weakness, e.g., due to myopathy or a neurological disorder, and from daytime sleepiness, which already leads to the onset of sleep during normal everyday activities. Fatigue lasting longer than six months is called chronic fatigue.
Effects.
Fatigue is associated with emotional, physical, and occupational impairment and with an elevated rate of accidents. It is the most distressing symptom in approximately 60% of cancer patients.
Fatigue is associated with mental, physical, and occupational impairment (e1, e2) as well as with an elevated rate of accidents (e3, e4). Fatigue is subjectively the most bothersome symptom for approximately 60% of cancer patients (e5, e6). Sufferers may describe various qualitative components of fatigue: emotional (loss of interest and motivation), cognitive (lessened mental activity and performance), behavioral (lessened productivity), and physical (e.g., muscle weakness).
Chronic fatigue syndrome (CFS), recently renamed “systemic exertion intolerance disease” (SEID), is a syndrome that has been defined in various ways, without any uniform etiology, and with varying diagnostic criteria. According to most definitions, it involves fatigue for at least 6 months and at least four to six accompanying symptoms, such as increased tiredness after exertion, sleep disturbance, muscle and joint pain, head and neck pain, cognitive impairment, orthostatic disturbances, and marked restriction of everyday activities not attributable to any other specific disease (e7, e8). Moreover, the symptoms tend to worsen with stress. The efficacy of exercise training and cognitive behavior therapy in patients with this syndrome is debated (8). Its prevalence in the population is less than 2% (9, 10), and less than 2% of patients seen in doctors’ practices complaining of fatigue have chronic fatigue syndrome (11). The diagnostic and therapeutic recommendations with regard to this syndrome require an independent discussion and will not be dealt with any further in this article.
Epidemiology
Because of variable study methods, figures on the prevalence of fatigue in European countries other than Germany vary from 22 to 38% (e9, e10, e11); in Germany, they vary from 20% to approximately 60%, depending on the study methods and the age group in question (e12, e13). In three representative cross-sectional studies of women and men aged 18-60 in western Germany, the prevalence of at least moderately severe fatigue was 37.3% in 1975, 20.1% in 1994, and 21.9% in 2013 (12). In 1995, 31% of persons over age 16 who participated in an Allensbach survey said that they sometimes, or often, suffered from fatigue (e14). The prevalence of fatigue rises with age, and women are generally more severely affected (13– 15). Persons suffering from fatigue visit a physician when, from their own point of view, the disturbance cannot be adequately explained, the impairment due to fatigue no longer seems acceptable, or the individual’s compensatory strategies are no longer effective.
Epidemiology.
Because of variable study methods, figures on the prevalence of fatigue in European countries other than Germany vary from 22 to 38%; in Germany, they vary from 20% to approximately 60%, depending on the study methods and the age group in question.
Estimates of the prevalence of fatigue among patients in primary care vary widely because of differences in the mode of data collection (Ractive asking vs. patient-driven), the variable duration of fatigue required for inclusion in the statistics, and the heterogeneous definitions of fatigue itself. It can be concluded, however—despite the high variability among studies—that fatigue is the main reason for consulting a doctor in 2–8% of outpatient consultations. In other studies, the prevalence is as high as 10–20%, when fatigue as a main or secondary reason for the consultation is included in the statistics (16).
Diagnostic evaluation
Fatigue has a wide variety of potential biological, psychological, and social causes, and direct causality cannot always be clearly established. Interactions between these factors are important as well, as are the effects of treatment, the exhaustion of psychosocial compensatory strategies (taking more time to rest and sleep, eliminating potential stressors), and physical inactivity. The following aspects must be considered in the differential diagnosis:
Mental illness
Fatigue can arise in the setting of mental disturbances, above all depression, anxiety disorders, functional somatic conditions such as irritable bowel syndrome and fibromyalgia, and psychosocial stress. Approximately 75% of patients with depression (17) and somatoform disorders (18) suffer from fatigue. Socio-economic factors such as low income, low educational level, and occupational stress play a role as well (19).
Cancer
Fatigue is present, and often very distressing, in 65% of cancer patients (20), nearly all of whom have other clinical evidence of their underlying malignancy (e15). If a patient complains of fatigue without any further clinical evidence of cancer, then an underlying malignancy is a very unlikely cause; in our opinion, there is no indication for a diffuse diagnostic search for such a condition (5, 11, e16).
Anemia, iron deficiency
There is only a weak association between low hemoglobin concentrations and fatigue. Reportedly, iron administration can lessen fatigue in premenopausal women who have a ferritin value below 20 µg/mL and a low normal hemoglobin concentration. However, a Cochrane Review led to no clear conclusions on which to base a recommendation (21).
Differential diagnostic considerations.
Mental illness, cancer, anemia, iron deficiency, endocrine disorders, infections, liver diseases, chronic somatic diseases, sleep disorders, and sleep-related respiratory disorders.
Endocrine disorders
Overt thyroid dysfunction, diabetes mellitus, and the premenstrual syndrome can all cause fatigue. Nonetheless, thyroid hormone administration does not improve fatigue in patients with subclinical hypothyroidism whose TSH values are below 10 mIU/L (e17).
Infectious diseases and liver diseases
Viral respiratory infections, mononucleosis, Giardia infections (22, 23) and other infections (24) are important causes of fatigue. In patients with these conditions, further causative factors are usually present, including treatment side effects, sleep disturbances, anxiety, and depression, if the symptoms persist for many weeks (5). Long-term data are currently lacking with regard to fatigue as a chief complaint in the aftermath of COVID-19 infection, as are relevant studies in patients who had only mild to moderate COVID-19 symptoms and did not need to be hospitalized, as well as in asymptomatic, PCR-positive persons. Patients with hepatitis often suffer from fatigue, as do patients with other serious liver diseases, yet liver disease is a very rare cause of fatigue in patients who have no other evidence of such a disease in their history, physical examination, or laboratory findings.
Chronic somatic diseases
Fatigue is a very common and very distressing symptom of many chronic somatic diseases (5), including congestive heart failure, multiple sclerosis, Parkinson’s disease, residual deficit of stroke, rheumatoid arthritis, sarcoidosis, cancer (many types), chronic renal insufficiency, postoperative states, and nycturia due to prostatic hyperplasia (e18). These diseases, if present, are nearly always known to be so, and they must be considered a likely cause of the patient’s fatigue. Severe fatigue with exhaustion is a risk factor for coronary heart disease (25); in women, it can be a harbinger of myocardial infarction (e19).
Sleep disturbances and sleep-related respiratory disorders
Frequency in primary care, as defined in the DSM-IV.
26.5% of patients in general medical practices have primary insomnia as defined in the DSM-IV (impaired sleep for at least four weeks, with significant social or functional impairment; not due to narcolepsy, a breathing-related or circadian rhythm sleep disorder, a parasomnia, or substance use or abuse).
26.5% of patients seen in general medical practices have primary insomnia as defined in the DSM-IV (impaired sleep of at least four weeks’ duration, with significant social or functional impairment; not due to narcolepsy, a breathing-related or circadian rhythm sleep disorder, a parasomnia, or substance use or abuse) (26). Fatigue and sleep disturbances often have a common cause, such as depression, psychosocial stress, a painful condition, or a somatic disease. Chronic allergic rhinitis can also impair sleep and cause daytime fatigue by impairing nasal breathing (e20). Obstructive sleep apnea is associated with impaired wakefulness, tiredness, depression, accidents (27), and arterial hypertension.
Drugs and psychotropic substances
The ingestion of drugs and/or psychotropic substances may be related to a chief complaint of fatigue. Causative agents include antidepressants and antihistamines, benzodiazepines, antipsychotic drugs, antihypertensive drugs, opiates, antiparkinsonian drugs, interferon, antiviral drugs, cytostatic drugs, and substance abuse disorders, particularly alcoholism (e21).
Environmental influences
Types of environmental influence that have been discussed as possible causes of fatigue, though nearly always without adequate evidence (28), include hydrocarbon compounds, amalgam, sick building syndrome (SBS), multiple chemical sensitivity, and electromagnetic hypersensitivity syndrome. There is, at most, a vague correlation between amalgam and fatigue among persons providing dental care (e22). Studies on SBS provide no data on fatigue in control groups, and potential confounders such as workplace dissatisfaction are difficult to evaluate (e23). Studies of electromagnetic hypersensitivity syndrome are impaired by flawed methods, nocebo effects, and other illnesses in the study subjects (e24).
Other causes
Abdominal surgery in particular can lead to fatigue lasting for weeks or months, presumably by multiple mechanisms (e25). Hydrocarbon poisoning caused 648 deaths in Germany in 2015; milder, nonlethal intoxication can cause fatigue, among other manifestations (e26). The relation between celiac disease and fatigue is unclear. A diagnostic evaluation for celiac disease is indicated only if further manifestations are present (abdominal discomfort, anemia, altered liver function tests). Lack of exercise is a reputed cause of fatigue (15), but it remains unclear how often it is truly the main cause.
Obstructive sleep apnea and rhinitis.
Chronic allergic rhinitis can also impair sleep and cause daytime fatigue by impairing nasal breathing. Obstructive sleep apnea is associated with impaired wakefulness, tiredness, depression, accidents, and arterial hypertension.
The diseases listed in Table 1, most of which have other symptoms, have been linked to fatigue, but their rare occurrence as a cause of fatigue in the primary care setting has not been studied systematically. They should only be considered in case of concrete suspicion; the evaluation not a differential-diagnostic checklist to be worked through in patients with fatigue of undetermined cause. For further information, see the links in eBox 1.
Table 1. Rare causes of fatigue (usually accompanied by other symptoms) (5).
| Type of disease | Rare causes of fatigue |
| Endocrine | ● Addison’s disesase (e27) ● Conn syndrome/Cushing syndrome (e28) ● pituitary deficiency (e29) |
| Metabolic | ● Gilbert’s disease ● hypercalcemia (e30, e31) |
| Infectious | ● tuberculosis ● toxoplasmosis ● brucellosis ● malaria, other tropical diseases ● AIDS, borreliosis/Lyme disease (e32) |
| Inflammatory | ● systemic lupus erythematosus (e33) |
| Cardiac | ● endocarditis |
| Neurological | ● brain tumor (e34) ● multiple sclerosis (e35) ● prior head injury (e36) |
| Psychiatric | ● schizophrenia (e37) |
eBOX 1. Internet adresses (last checked on 20 February 2021).
Patient history questionnaires:
DEGAM guideline on fatigue:
www.awmf.org/uploads/tx_szleitlinien/053–002l_S3_Muedigkeit_2018–06.pdf
Family practice notebook:
Patient information:
Patient information on exercise training (“Bring your life back into swing”):
Progressive muscle relaxation (the Jacobson technique):
Instructions with or without music: www.tk.de/techniker/magazin/life-balance/aktiv-entspannen/progressive-muskelentspannung-zum-download-2021142
Cancer-associated fatigue:
www.esmo.org/guidelines/supportive-and-palliative-care/cancer-related-fatigue
Extensive patient information, advice, and further health information from the IQWIG:
Free medical and scientific search engines and databases:
searching terms: fatigue, tiredness, Müdigkeit (automatic translation):
Cochrane Library: www.cochranelibrary.com
Epistemonikos: www.epistemonikos.org
Google-Scholar: www.scholar.google.de
Livivo: www.livivo.de/app?LANGUAGE=de
PubMed: www.pubmed.ncbi.nlm.nih.gov
Tripdatabase: www.tripdatabase.com
The diagnostic approach
Fatigue can be considered the final common pathway of a multitude of biological, psychological, and social disturbances that are often not clearly distinguishable from one another. A biopsychosocial approach is, therefore, in order, and it should be borne in mind that, frequently, more than one mechanism is at work.
Patients seen in general medical practice with primarily unexplained fatigue have the following conditions in the following frequencies (means and 95% confidence intervals, from a systematic review) (11): anemia, 2.8% [1.6; 4.8]; cancer, 0.6% [0.3; 1.3]; serious somatic disease (including anemia and cancer, because the studies are highly variable in the clinical definitions and methods employed), 4.3% [2.7; 6.7]; depression, 18.5% [16.2; 21.0]. Further relevant conditions include sleep disturbances and sleep-related breathing disturbances, post-infectious states, and substance abuse, particularly alcohol abuse, as has been shown in other studies as well (29, e38).
The diagnostic approach.
Fatigue can be considered the final common pathway of many biological, psychological, and social disturbances that are often not clearly distinguishable from one another. A biopsychosocial approach is, therefore, in order, and often more than one mechanism is at work.
Relevant sleep disorders.
Relevant conditions include sleep disturbances and sleep-related breathing disturbances, post-infectious states, and substance abuse, particularly alcohol abuse, as has been shown in other studies as well.
The recommended basic test battery (table 2) comprises, independently of the age of the patient: history-taking and physical examination focused on potential causes of fatigue, particularly sleep disturbances, major changes in body weight, dysfunction of the cardiac, respiratory, gastrointestinal, urogenital, and central nervous systems, intake of drugs and psychoactive substances, problems in the patient’s social, familial, or occupational setting, chemical exposures or excessive exposure to noise, similar symptoms in family members or co-workers, snoring, falling asleep at the wheel, and (habitual) sleep deficit. Targeted laboratory testing should include blood sugar, complete blood count, erythrocyte sedimentation rate/CRP, transaminases or γ-GT, and TSH. The patient should also fill out a fatigue questionnaire, such as the one found in the accompanying information for patients in the DEGAM guideline on fatigue (e39). In particular, the question about the patient’s own ideas about the cause of fatigue (e40) often yields plausible explanations and important information about the patient’s expectations and fears, which the physician must know in order to establish effective communication with the patient. If the basic evaluation yields evidence of a specific illness, further testing is indicated. In premenopausal women whose basic evaluation is negative, the ferritin concentration should be measured as well.
Table 2. The basic diagnostic evaluation of fatigue (e41)*.
| History | ||
| ● symptom characteristics, differentiation from somnolence ● associated complaints ● fatigue new/unusual ● impairment in everyday life ● the patient’s own conception of the cause and treatment of fatigue ● symptoms of depression and anxiety |
● somatic history ● sleep: duration, quality, changes from personal norm, (habitually) insufficient sleep ● body weight, changes in weight ● cardiac, respiratory, gastrointestinal, urogenital, and central nervous system function ● drugs, psychotropic substances ● post-infectious state, chronic disease |
● social, familial, occupational situations ● exposure to chemicals or noise ● similar symptoms in family members, friends, or coworkers ● snoring, falling asleep at the wheel |
| Physical examination | ||
| ● depending on positive findings in the history | ● if the history does not arouse suspicion of any particular physical illness: abdomen, heart, circulation, airways, skin and mucous membranes, lymph nodes; muscle bulk, strength, and tone; proprioceptive reflexes | |
| Laboratory testing | ||
| ● depending on positive findings in the history and physical examination | ● if there is no evidence of any particular physical illness: fasting blood sugar, complete blood count, erythrocyte sedimentation rate/CRP, transaminases/γ-GT, TSH (creatinine only if there is evidence of renal disease, or in the presence of risk factors such as hypertension, diabetes, nephrotoxic drugs) |
● further laboratory testing only if the history or physical examination arouses suspicion of a particular condition ● ferritin measurement in premenopausal women with normal history, physical examination, and basic laboratory tests |
* These recommendations are also given in the DEGAM guideline.
Many patients presenting with fatigue as their chief complaint suffer from depression or anxiety disorders or have a history of infection. These conditions should be asked about specifically. Screening questions for current depression are: In the last four weeks, have you felt dejected, melancholic, or hopeless? Have you had less interest in, or enjoyment from, activities that you are ordinarily interested in or enjoy (e42)?
Premenopausal women.
In premenopausal women whose basic evaluation is negative, the ferritin concentration should be measured as well.
Analogous screening questions for anxiety disorders are: In the last four weeks, have you suffered from nervous tension / fearfulness / a feeling of being emotionally out of balance? Have you been worried about many different things, or suffered from anxiety attacks (e43)? Positive answers to any of these questions call for further evaluation, and, in depressed patients, the potential risk of suicide must be assessed.
In patients with evidence of sleep disturbances, the following things should be determined: Does the patient have greater difficulty falling asleep, or a worse quality of sleep, than in the normal situation for him or her? Is sleep hygeine inadequate, does the patient have too little time to sleep, or is sleep prevented by situational factors (pain, psychosocial stress, noise, shiftwork)? The suspicion of a sleep-related respiratory disorder (SRRD) is aroused if the patient reports snoring, respiratory pauses in sleep observed by others, and falling asleep at the wheel of a car or any other manifestation of unavoidable, involuntary falling asleep in the daytime. If the patient also has a high STOP-BANG score (number of items from a list of eight: snoring, tiredness, observed apnea, high blood pressure or body-mass index [BMI], age, neck circumference, and gender) (e44), or a high score on the somewhat more practical and probably equally useful GOAL questionnaire (which has only four items: male gender, obesity with a BMI ≥ 30, age ≥ 50 years, loud snoring) (30), evaluation by a specialist in sleep medicine is indicated. A STOP-BANG score of 0–2 implies a low probability of moderate to severe obstructive sleep apnea, while a score of 5–8 implies a high probability. In the intermediate range (3–4 points), further criteria are needed. A GOAL score of 2 or above implies a high probability of sleep apnea.
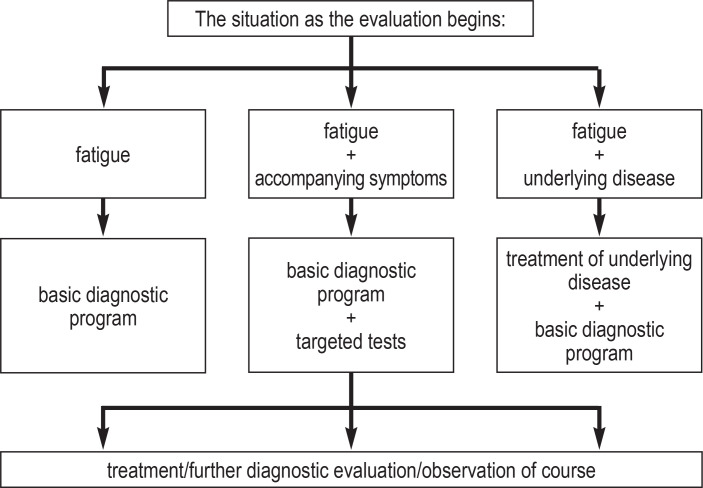
The history and physical examination enable classification into the following three categories (figure 1):
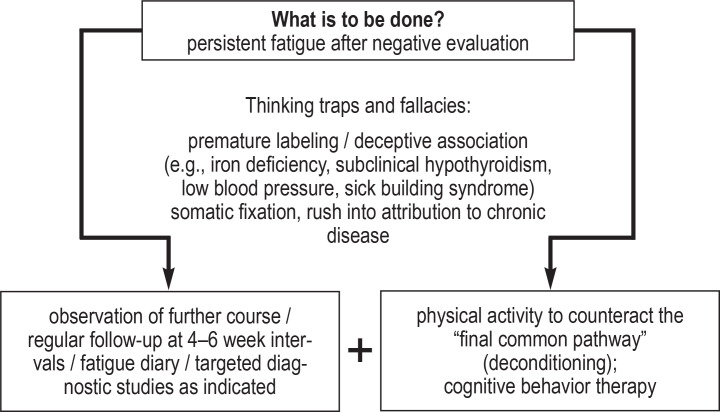
Figure 1.
Thinking traps and fallacies about fatigue as the chief complaint
Sleep-related respiratory disorder.
The suspicion of a sleep-related respiratory disorder (SRRD) is aroused if the patient reports snoring, respiratory pauses in sleep observed by others, and falling asleep at the wheel of a car or any other manifestation of unavoidable, involuntary falling asleep in the daytime.
Fatigue as the chief complaint without any known underlying illness or relevant accompanying manifestations. The history and physical examination should be complemented by a basic laboratory test battery and a patient questionnaire.
Fatigue with the simultaneous presence of further manifestations requiring evaluation. Targeted additional studies are indicated.
Fatigue in the known presence of an underlying disease that might be the reason for fatigue. In addition to the evaluation and treatment of the underlying disease, the basic evaluation program outlined in Table 2 should be used to investigate a potential fatigue-inducing second illness, or the patient’s inability to compensate for the known underlying illness. If drug-induced fatigue is suspected, the drug in question can be switched to another agent after an individualized risk-benefit assessment. Temporary reexposure to the original drug may be helpful to confirm the diagnosis.
When to obtain a specialized sleep study.
A high STOP-BANG score (snoring, tiredness, observed apnea, high blood pressure or BMI, age, neck circumference, and gender) or GOAL score (male gender, obesity with a BMI ≥ 30, age ≥ 50 years, loud snoring) indicates that evaluation by a specialist in sleep medicine is indicated.
If the cause of fatigue remains unknown after initial laboratory testing and (possibly) further technical studies, the strategy from then on is one of expectant observation, with regular follow-up every four to six weeks. A ferritin level should be measured in premenopausal women, but other additional laboratory tests or ancillary studies further on in the patient’s course are indicated only if there are positive findings or specific clinical evidence. The risk of an underlying illness in an early stage is not completely ruled out, but is thought to be no higher in such patients than in the population at large (11, 29, e16), and the low diagnostic yield must be weighed against the stress and risk of diffuse, untargeted diagnostic testing. Current evidence suggests that such testing does not, in general, improve patient outcomes. Rather, it elevates the risk of false-positive findings, overdiagnosis, which can derail the proper care of the patient. The risk of the patient’s focusing or fixating on a putative somatic disease as the cause of fatigue (e45, 31) is also increased if prolonged diagnostic efforts are made—perhaps lasting several weeks—to rule out somatic causes, while psychological matters are not discussed at all. Dreibholz describes somatic fixation as “a cyclic process in which all the patient’s symptoms, signs, problems, and illnesses are considered purely somatic processes by the patient and/or the primary care physician, while the psychosocial aspects of the symptoms or illnesses are overlooked and disregarded, whether consciously or not” (e46) (figure 2).
Figure 2.
Diagnostic pathways for patients with fatigue as the chief complaint
A further risk is that irrelevant changes will be assigned too much importance and labeled the cause of fatigue. Deceptive associations of this kind are most likely in patients who suffer from a known underlying disease, manifest isolated laboratory changes such as mild iron deficiency or subclinical hypothyroidism, or are subject to dubious environmental influences. Conversely, the overall clinical picture must always be borne in mind so that a relevant disease will not be overlooked in the presence of only mild laboratory abnormalities.
Treatment and management
Laboratory tests.
Patients whose initial laboratory tests and ancillary studies do not reveal the cause of their fatigue are treated with a watchful waiting strategy, with follow-up every four to six weeks.
Disease-specific treatment should be given if such a treatment is possible and indicated, and its effect on fatigue should be documented: for example, antidepressant therapy and the treatment of anemia, heart failure, diabetes mellitus, neurological diseases, sleep disturbances, and pain. If fatigue remains unexplained, or if there is evidence of relevant psychosocial stress, the clinical approach should be one of expectant observation for somatic and psychological causes, with regular follow-up at four- to six-week intervals, as appropriate for the individual patient. The physician should counsel the patient empathetically and communicate openly, so that the patient can be motivated to change his or her behavior in order to modify unmanageable (or, in some cases, insufficient) physical and psychosocial challenges. Problem-oriented cognitive behavior therapy is useful in some cases as well. Keeping a symptom diary can be useful (32) as a basis for discussions about symptoms, impairments, and the associated feelings and conceptions. This therapeutic approach accords with the recommendations of the DEGAM guideline on fatigue cited above; the corresponding evidence levels and recommendation strengths can be found in the guideline.
Psychoeducative measures to inform the patient about the disease process and the appropriate way of dealing with it by assuming personal responsibility, thus strengthening patient resources (e47), with the aid of accompanying materials (e39), are useful for dealing with excessive (or insufficient) challenges in everyday life, as well as for patients with sleep disturbances or cancer (33). For patients with many different types of underlying condition or disease, behavior therapy (34) or symptom-oriented activating measures lessen fatigue and improve overall well-being (35, 36). An appropriately adapted program of physical activation lessens fatigue and counteracts physical deconditioning (table 3), as discussed in the Case Illustration (ebox 2).
Table 3. The treatment of diseases that cause fatigue.
| Type of disease and treatment | Results, differences in effect strength*1 [95% confidence intervals] |
| Anxiety, generalized | |
| SSRI and SNRI (e48, 37) | As first-line therapy, vs. placebo: SSRI: g = 0.33 (limits: 0.26–0.39), SNRI: g = 0.36 (limits: 0.29–0.42) (e49); Pp d: SSRI: 3.48 [3.18; 3.78], SNRI: 2.47 [2.09; 2.84]) (e50) |
| CBT (e51, 37) | Strong effect compared to waiting list: d = 1.23 [1.02; 1.45], weak to moderate effect compared to routine treatment and placebo: d = 0.57 [0.20; 0.94] (effect strengths of individual CBT pooled over all anxiety disorders) (e50) |
| Panic disorder | |
| CBT and psychodynamic therapy (e52, 37) | Best long-term treatment outcome of all psychological therapies. Pp individual CBT: d = 1.24 [1.10; 1.39]; Pp psychodynamic therapy: d = 0.97 [0.58; 1.36] (e50) |
| Depression | |
| Antidepressants (38) | More effective than placebo against major depression: odds ratios of 21 antidepressants vs. placebo range from 1.37 [1.16; 1.63] to 2.13 [1.89; 2.41] (e53) |
| Exercise therapy (38) | Only moderate effect: SMD compared to no treatment –0.62 [–0.81; –0.42], no significant effect in high-quality studies: –0.18 [−0.47; 0.11] (e54) |
| Behavior therapy (38) | Similar efficacy to other forms of psychotherapy: response rate of BT vs. all other forms of psychotherapy: risk reduction 0.97 [0.86; 1.09] (e55) |
| Insomnia | |
| Antidepressants (39) | Doxepin pooled with imipramine vs. placebo, for improved sleep quality: SMD −0.39 [−0.56; −0.21) (e56) |
| Antihistamines (39) | Inadequate evidence |
| Antipsychotic drugs (39) | Inadequate evidence |
| Benzodiazepines and benzodiazepine receptor agonists (39)*2 | Improvement of sleep parameters: benzodiazepines: g for TST: 0.64 [0.12; 1.16], for SOL: –0.76 [−1.28; −0.24] benzodiazepine receptor agonists: g for TST: 0.52 [0.33; 0.71], for SOL: −0.46 [−0.61; −0.31] (e57) |
| Melatonin (39) | Generally not recommended because of low efficacy |
| Phytotherapeutic drugs (39) | No improvement to moderate improvement of sleep quality |
| CBT (39)*3 | CBT is recommended as the first line of treatment for adults of any age. CBT vs. placebo: Hedges’ g: 1.07 [0.10; 2.05] (e58) |
| Chronic obstructive pulmonary disease (COPD) | |
| Complex rehabilitation (exercise training and psychological counseling) after a COPD exacerbation (e59) | Good evidence for improvement of fatigue-related quality of life Fatigue domain: mean difference 0.81 [0.16; 1.45] (e60) |
| Congestive heart failure | |
| Exercise training (e61, e62) | Questionable improvement of quality of life |
| Behavior therapy (relaxation, meditation, and guided imagery) (e63, e64) | Potential benefit with regard to quality of life |
| Neurological diseases (residual deficit after stroke, multiple sclerosis, Parkinson’s disease, other) | |
| Exercise training (e65, e66) | May lessen fatigue |
| CBT (e65, e66) | May lessen fatigue |
| Pharmacotherapy (e65, e66) | Individual decision in the absence of convincing evidence |
| Somatoform disorders | |
| Newer-generation antidepressants (30) | Compared to placebo (with very low study quality) moderately effective against somatic symptoms (SMD −0.91, [−1.36; −0.46]), anxiety (SMD −0.88, [−1.81; 0.05]), depression (SMD −0.56, [−0.88; −0.25]) (e67) *4 |
| Cancer | |
| Exercise training (40) | Moderate improvement of CRF: mean weighted effect size (WES) = 0.30 [0.25; 0.36] (e68) |
| CBT (40) | Moderate improvement of CRF: mean weighted effect size (WES) = 0.37 [0.28; 0.47] (e68) |
| Psychological interventions overall (40) | Moderate improvement of CRF: mean weighted effect size (WES) = 0.27 [0.21; 0.33] (e68) |
| Pharmacotherapy (40) | Very little improvement of CRF: mean weighted effect size (WES) = 0.09 [0.00; 0.19] (e68) |
BT, behavior therapy; CRF, cancer-related fatigue; CBT, cognitive behavior therapy; d, Cohen’s d; g, Hedges’ g; SMD, standardized mean difference; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-noradrenaline reuptake inhibitor; TST, total sleep time; SOL, sleep onset latency; WES, weighted effect size; Pp, pre-post
*1 In general, effect strengths (Cohen’s d, Hedges’ g) of > 0.2 are considered weak, > 0.5 as moderate, and > 0.8 as strong. The effect strengths presented here for COPD, neurological diseases and cancer are with respect to fatigue as a symptom of these conditions; the strengths presented for the other diseases are with respect to the overall disease process.
*2 This can be offered if CBT is insufficiently effective or not feasible. Beware of the risk of tolerance and dependence, no information on daytime fatigue
*3 Risk of daytime fatigue and somnolence in sleep restriction therapy
*4 The potential benefit must be weighed against the risk of side effects.
eBOX 2. Case Illustration.
Mr. F., a 32-year-old barber, complains to his family physician of fatigue and exhaustion of approximately ten weeks’ duration. He states that he no longer even wants to go jogging, despite having been an enthusiastic endurance athlete until now. He has hardly ever been ill before, except for minor injuries. He is experiencing considerable stress at the moment, as he will soon be taking an examination for an important professional qualification that will enable him to set up his own barber shop. His history is otherwise positive only for difficulty falling asleep at night. In response to screening questions about anxiety and depression, he reports having suffered from nervous tension in the past four weeks, but no further symptoms. On physical examination, his blood pressure is 118/76 mmHg, height 188 cm, weight 78 kg. The skin, mucous membranes, heart, lungs, abdomen, renal beds, limbs, and basic neurological examination are normal. In the DEGAM patient questionnaire on fatigue, Mr. F. notes that his symptoms are associated with the above-mentioned stresses in his everyday life. Laboratory findings: hemoglobin 14.2 g%, normal complete blood count, ESR 7/14 mm, TSH 2.3 mU/L, ALT 18 U/L, γGT 20 U/L, fasting blood sugar 96 mg%. Assessment and plan: Discussing the findings, the physician and the patient agree that his fatigue and exhaustion are probably due to the stress of his upcoming professional examination and his plan to go into business for himself in the near future. There is no evidence of any serious somatic or psychological disorder. They determine that Mr. F. will seek help with business planning from the Chamber of Industry and Commerce and resume jogging in order to reduce stress and keep fit. He also obtains a CD from his health insurance company with audio instructions on the Jacobson technique of progressive muscle relaxation. He is told to contact the physician in four weeks to report back on his condition, or at any time until then in case any warning symptoms or signs should arise.
Overview
The common causes of fatigue include the following, some of which are both preventable and dangerous: psychosocial problems, depression, anxiety disorders, sleep disorders, and sleep-related respiratory disorders. Patients with an unremarkable history, physical examination, and basic laboratory test battery are highly unlikely to be suffering from anemia, cancer, thyroid dysfunction, or other somatic diseases as the cause of their fatigue.
Aspects that must be considered include known underlying illnesses, drug and substance intake, and health risks. Additional diagnostic studies should only be performed in case of well-founded clinical suspicion. The etiology, course, and optimal treatment of chronic fatigue syndrome are still unclear.
The further management of patients with fatigue as their main symptom is characterized by causally directed as well as symptomatic treatment, empathetic patient counseling, expectant observation, and regularly scheduled follow-up.
Psychoeducation.
Psychoeducative measures to inform the patient about the disease process and how to deal with it by assuming personal responsibility, thus strengthening patient resources, are useful for dealing with excessive (or insufficient) challenges in everyday life.
Further information on CME.
Participation in the CME certification program is possible only via the Internet: cme.aerzteblatt.de.
The submission deadline is 22 August 2022. Submissions by letter, email, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de.
-
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed using the “uniform CME number” (einheitliche Fortbildungsnummer, EFN).
The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in “Meine Daten,” and consent must be given for results to be communicated. The 15-digit EFN can be found on the CME card (8027XXXXXXXXXXX).
Participation is possible at cme.aerzteblatt.de. The submission deadline is 22 August 2022.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
You decide how to plan your continuing medical education on the basis of the frequency and dangerousness of the reasons your patients come to see you. In your primary care practice, you have approximately 50 direct physician-patient contacts every day.
How many times a day, on average, will you see a patient with fatigue/exhaustion as their chief complaint?
zero to one time;
one to four times;
seven times;
ten times;
fifteen times
Question 2
A previously healthy 26-year-old woman studying physical education is working toward the completion of her thesis and expects to be taking her practical and oral final examinations ten weeks from now. She reports having suffered from exhaustion of progressive severity over the past six weeks. In your physical examination, you note very pale conjunctivae.
What serum laboratory test should be obtained in addition to the basic laboratory test battery?
aldosterone; b) cortisol
ferritin; d) LDL cholesterol
tetrahydrocannabinol
Question 3
Mr. F., a 22-year-old bank employee who lost his father in a fatal automobile accident six months ago, reports having suffered from marked fatigue and lack of energy for the past four months. You suspect depression as the main cause.
Which of the following, if the patient states he has suffered from it for several weeks, would tend to confirm your preliminary diagnosis?
marked anxiety
lack of enjoyment
elevated sense of his own worth
altered senses of taste and smell
nervous tension
Question 4
You are pursuing the differential diagnosis of fatigue in your patient Mr. K., a very obese 65-year-old man.
Which of the following is highly consistent with a sleep-related respiratory disorder?
symptom duration more than three months
symptom duration more than six months
falling asleep during everyday activities
high stress on the job
a markedly elevated TSH level
Question 5
Mr. O., a 44-year-old warehouse worker, consults you because of severe fatigue. You suspect obstructive sleep apnea as the cause.
Which of the following symptoms or findings is most consistent with your suspicion?
diminished breathing sounds in sleep
observed respiratory pauses during sleep
a BMI value below 25
a trend to low blood pressure
nycturia at least twice per night
Question 6
Patients with somatic diseases may suffer from fatigue as a direct result of their underlying condition, or else by way of dysfunctional emotional adaptation processes or treatment side effects.
Approximately 65% of patients with which of the following diseases suffer from fatigue and exhaustion?
low blood pressure; b) urinary urge incontinence;
hyperthyroidism; d) cancer;
celiac disease
Question 7
You have not been able to find the cause of longstanding fatigue in your patient Ms. K., a 38-year-old single mother, despite comprehensive history-taking, physical examination, basic laboratory testing, and the use of a patient questionnaire.
Which of the following is a reasonable next step?
measurement of borreliosis antibodies
having the patient keep a symptom diary and following up regularly
laboratory testing for environmental toxins
test administration of levothyroxine
biplanar chest x-ray
Question 8
Ms. S., a 43-year-old kindergarten teacher, complains of fatigue over the past two months, impairing her everyday activities. There is no known underlying disease, and the history and physical examination reveal no evidence of any somatic condition.
Which of the following laboratory tests is a component of the recommended basic battery?
test for borreliosis; b) purine level
melatonin level; d) TSH value
vitamin D level
Question 9
Mr. N. is a 34-year-old software developer who has been suffering from fatigue for four months. You have conducted a diagnostic evaluation according to the basic evaluation program and have come to the conclusion that he has an anxiety disorder.
Which of the following treatments would be appropriate for him?
analytically oriented psychotherapy
trial therapy with methylphenidate
cognitive behavior therapy
having the patient spend 1 hour longer in bed each night
ordering a beta-mimetic drug p.r.n.
Question 10
Long-lasting fatigue carries the risk of physical deconditioning.
What measure do you order to prevent this?
anaerobic interval training three times a week
trace element and dietary supplement administration
an individualized physical activation program
the injection of a cortisone depot preparation once every four weeks
45-minute rest periods around noon every weekday
► Participation is only possible online:
cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Donner-Banzhof has a personal relationship with GSK.
The other authors state that they have no conflict of interest.
References
- 1.Verbraucherzentrale Hamburg Hausarzt. Spezialist für den ganzen Menschen? Eine Studie im Alltag. www.vzhh.de/sites/default/files/medien/251/dokumente/2018-07-16%20M%C3%BCdigkeit-v6.pdf (last accessed on 5 July 2020) [Google Scholar]
- 2.Ely JW, Kaldjian LC, D‘Alessandro DM. Diagnostic errors in primary care: lessons learned. J Am Board Fam Med. 2012;25:87–97. doi: 10.3122/jabfm.2012.01.110174. [DOI] [PubMed] [Google Scholar]
- 3.Morgan S, Henderson KM, Tapley A, et al. Investigation of fatigue by Australian general practice registrars: a cross-sectional study. J Prim Health Care. 2015;7:109–116. [PubMed] [Google Scholar]
- 4.Wübken M, Bühner M, Barth N, Schneider A. Welche Aspekte tragen in der täglichen Routine zur diagnostischen Unsicherheit bei? Z Allg Med. 2015;91:392–398. [Google Scholar]
- 5.Baum E, Donner-Banzhoff N, Maisel P. S3-Leitlinie Müdigkeit der Deutschen Gesellschaft für Allgemeinmedizin, Stand 11/2017. www.awmf.org/uploads/tx_szleitlinien/053-002l_S3_Muedigkeit_2018-06.pdf (last accessed on 25 March 2020) [Google Scholar]
- 6.Mota DDCF, Pimenta CAM. Self-report instruments for fatigue ¬assessment: a systematic review. Res Theory Nurs Pract. 2006;20:49–78. doi: 10.1891/rtnp.20.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Jaime-Lara RB, Koons BC, Matura LA, Hodgson NA, Riegel B. A ¬qualitative meta-synthesis of the experience of fatigue across five chronic conditions. J Pain Symptom Manage. 2020;59:1320–1343. doi: 10.1016/j.jpainsymman.2019.12.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D-Y, Lee J-S, Park S-Y, Kim S-J, Son C-G. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic ¬encephalomyelitis (CFS/ME) J Transl Med. 2020;18 doi: 10.1186/s12967-019-02196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim E-J, Ahn Y-C, Jang E-S, Lee S-W, Lee S-H, Son C-G. Systematic review and meta-analysis of the prevalence of chronic fatigue ¬syndrome/myalgic ¬encephalomyelitis (CFS/ME) J Transl Med. 2020;18 doi: 10.1186/s12967-020-02269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estévez-López F, Mudie K, Wang-Steverding X, et al. Systematic ¬review of the epidemiological burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome across Europe: current evidence and EUROMENE research recommendations for epidemiology. J Clin Med. 2020;9 doi: 10.3390/jcm9051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadje R, Dornieden K, Baum E, et al. The differential diagnosis of ¬tiredness: a systematic review. BMC Fam Pract. www.bmcfampract.biomedcentral.com/articles/10.1186/ s12875-016-0545-5 (last accessed on 10 July 2020) 2016;17 doi: 10.1186/s12875-016-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beutel ME, Klein EM, Henning M, et al. Somatic symptoms in the German General Population from 1975 to 2013. Sci Rep. 2020;10 doi: 10.1038/s41598-020-58602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz R, Krauss O, Hinz A. Fatigue in the general population. ¬Onkologie. 2003;26:140–144. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 14.Watt T, Groenvold M, Bjorner JB, Noerholm V, Rasmussen NA, Bech P. Fatigue in the Danish general population. Influence of ¬sociodemographic factors and ¬disease. J Epidemiol Community ¬Health. 2000;54:827–833. doi: 10.1136/jech.54.11.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engberg I, Segerstedt J, Waller G, Wennberg P, Eliasson M. Fatigue in the general population—associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: the northern Sweden MONICA study 2014. BMC Public Health. 2017;17 doi: 10.1186/s12889-017-4623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadje R. Müdigkeit als Symptom in der Primärversorgung: eine ¬systematische Übersichtsarbeit. [Allgemeinmedizin, Präventive und Rehabilitative Medizin des Fachbereichs Medizin der Philipps - Universität Marburg]: Philipps-Universität Marburg; www.archiv.ub.uni-marburg.de/diss/z2015/0154/ (last accessed on 1 March 2021) 2015 [Google Scholar]
- 17.Tylee A, Gastpar M, Lépine JP, Mendlewicz J. Identification of ¬depressed patient types in the community and their treatment needs: findings from the DEPRES II (Depression Research in European ¬Society II) survey. DEPRES Steering Committee. Int Clin ¬Psychopharmacol. 1999;14:153–165. [PubMed] [Google Scholar]
- 18.Smith GR, Monson RA, Ray DC. Patients with multiple unexplained symptoms. Their characteristics, functional health, and health care ¬utilization. Arch Intern Med. 1986;146:69–72. [PubMed] [Google Scholar]
- 19.Hapke U, Maske UE, Scheidt-Nave C, Bode L, Schlack R, Busch MA. Chronischer Stress bei Erwachsenen in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:749–754. doi: 10.1007/s00103-013-1690-9. [DOI] [PubMed] [Google Scholar]
- 20.Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. 2020;31:713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Miles LF, Litton E, Imberger G, Story D. Intravenous iron therapy for non-anaemic, iron-deficient adults. Cochrane Database Syst Rev. 2019;12 doi: 10.1002/14651858.CD013084.pub2. CD013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnachie E, Schneider A, Mehring M, Enck P. Incidence of irritable bowel syndrome and chronic fatigue following GI infection: a ¬population-level study using routinely collected claims data. Gut. 2018;67:1078–1086. doi: 10.1136/gutjnl-2017-313713. [DOI] [PubMed] [Google Scholar]
- 23.Litleskare S, Rortveit G, Eide GE, et al. Quality of life and its ¬association with irritable bowel syndrome and fatigue ten years after giardiasis. Neurogastroenterol Motil. 2019;31 doi: 10.1111/nmo.13559. e13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barroso J, Leserman J, Harmon JL, Hammill B, Pence BW. Fatigue in HIV-infected people: a three-year observational study. J Pain ¬Symptom Manage. 2015;50:69–79. doi: 10.1016/j.jpainsymman.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frestad D, Prescott E. Vital exhaustion and coronary heart disease risk: a systematic review and meta-analysis. Psychosom Med. 2017;79:260–272. doi: 10.1097/PSY.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 26.Wittchen HU, Krause P, Höfler M, et al. NISAS-2000: Die „Nationwide Insomnia Screening and Awareness Study“. Prävalenz und ¬Verschreibungsverhalten in der allgemeinärztlichen Versorgung. Fortschr Med Orig. 2001;119:9–19. [PubMed] [Google Scholar]
- 27.Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: ¬cross-sectional study (EQUINOX) on sleep-related home, work and car ¬accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23:143–152. doi: 10.1111/jsr.12104. [DOI] [PubMed] [Google Scholar]
- 28.Wiesmüller GA, Hornberg C. Umweltmedizinische Syndrome. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;60:597–604. doi: 10.1007/s00103-017-2546-5. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Wood DR, Mangelsdorff AD, Meier NJ, Powell JB. Chronic fatigue in primary care. Prevalence, patient characteristics, and ¬outcome. JAMA. 1988;260:929–934. [PubMed] [Google Scholar]
- 30.Duarte RL, Magalhães-da-Silveira FJ, Oliveira-E-Sá TS, Silva JA, ¬Mello FC, Gozal D. Obstructive sleep apnea screening with a 4-item ¬instrument, named GOAL questionnaire: development, validation and comparative study with No-Apnea, STOP-Bang, and NoSAS. Nat Sci Sleep. 2020;12:57–67. doi: 10.2147/NSS.S238255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roenneberg C, Hausteiner-Wiehle C, Schäfert R, Sattel H, Henningsen Pea. S3 Leitlinie „Funktionelle Körperbeschwerden“: AWMF-Reg-Nr. 051-001LANGFASSUNG: AWMF; www.awmf.org/uploads/tx_szleitlinien/051-001l_S3_Funktionelle_Koerperbeschwerden_2018-11.pdf (last accessed on 11 February 2021) [Google Scholar]
- 32.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Bennett S, Pigott A, Beller EM, Haines T, Meredith P, Delaney C. Educational interventions for the management of cancer related ¬fatigue in adults. Cochrane Database Syst Rev. 2016;11 doi: 10.1002/14651858.CD008144.pub2. CD008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vibe M, Bjørndal A, Fattah S, Dyrdal GM, Halland E, Tanner Smith EE. Mindfulness based stress reduction (MBSR) for improving health, ¬quality of life and social functioning in adults: a systematic review and meta analysis. Campbell Systematic Reviews. 2017;13:1–264. [Google Scholar]
- 35.Cramp F, Byron-Daniel J. Exercise for the management of cancer-¬related fatigue in adults. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD006145.pub3. CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimes KA, Chalder T. Treatments for chronic fatigue syndrome. Occup Med (Lond) 2005;55:32–39. doi: 10.1093/occmed/kqi015. [DOI] [PubMed] [Google Scholar]
- 37.Bandelow B, Wiltink J, Alpers WG, et al. Deutsche S3-Leitlinie Behandlung von Angststörungen (2019 abgelaufen, z.Zt. Aktualisierung) www.awmf.org/uploads/tx_szleitlinien/051-028l_S3_Angstst%C3%B6rungen_2014-05-abgelaufen.pdf (last accessed on 9 February 2021) 2014 [Google Scholar]
- 38.S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression - Langfassung, 2. Auflage. Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde (DGPPN); Bundesärztekammer (BÄK); Kassenärztliche Bundesvereinigung (KBV); Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) www.awmf.org/uploads/tx_szleitlinien/nvl-005l_S3_Unipolare_Depression_2017-05.pdf (last accessed on 18 February 2021) [Google Scholar]
- 39.Riemann D, Baum E, Cohrs S, et al. S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen Kapitel „Insomnie bei Erwachsenen“, Update 2016. www.awmf.org/uploads/tx_szleitlinien/063-003l_S3_Insomnie-Erwachsene_2018-02-verlaengert.pdf (last accessed on 4 March 2021) 2020 [Google Scholar]
- 40.Palliativmedizin für Patienten mit einer nicht-heilbaren Krebserkrankung: Langversion 2.2 [AWMF-Registernummer: 128/001OL] www.leitlinienprogramm-onkologie.de/leitlinien/palliativmedizin/ (last accessed on 11 February 2021) [Google Scholar]
- E1.Ormel J, VonKorff M, Ustun TB, Pini S, Korten A, Oldehinkel T. Common mental disorders and disability across cultures. Results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA. 1994;272:1741–1748. doi: 10.1001/jama.272.22.1741. [DOI] [PubMed] [Google Scholar]
- E2.Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the US. ¬workforce: prevalence and implications for lost productive work time. J Occup Environ Med. 2007;49:1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- E3.Swaen GMH, van Amelsvoort LGPM, Bültmann U, Kant IJ. Fatigue as a risk factor for being injured in an occupational accident: results from the Maastricht Cohort Study. Occup Environ Med. 2003;60(Suppl 1):i88–i92. doi: 10.1136/oem.60.suppl_1.i88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Smolensky MH, Di Milia L, Ohayon MM, Philip P. Sleep disorders, medical conditions, and road accident risk. Accid Anal Prev. 2011;43:533–548. doi: 10.1016/j.aap.2009.12.004. [DOI] [PubMed] [Google Scholar]
- E5.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(3 Suppl 2):4–12. [PubMed] [Google Scholar]
- E6.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- E7.National Academies Press (US) Beyond Myalgic Encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington (DC) www.ncbi.nlm.nih.gov/books/NBK274235/ (last accessed on 28 October 2020) 2015 [PubMed] [Google Scholar]
- E8.Lim E-J, Son C-G. Review of case definitions for myalgic ¬encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2020;18 doi: 10.1186/s12967-020-02455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Lerdal A, Wahl A, Rustøen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the Fatigue Severity Scale. Scand J Public Health. 2005;33:123–130. doi: 10.1080/14034940410028406. [DOI] [PubMed] [Google Scholar]
- E10.Galland-Decker C, Marques-Vidal P, Vollenweider P. Prevalence and factors associated with fatigue in the Lausanne middle-aged -population: a population-based, cross-sectional survey. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027070. e027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, ¬Wessely SC. Population based study of fatigue and psychological distress. BMJ (Clinical research ed.) 1994;308:763–766. doi: 10.1136/bmj.308.6931.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Beutel ME, Wiltink J, Ghaemi Kerahrodi J, et al. Somatic symptom load in men and women from middle to high age in the Gutenberg Health Study—association with psychosocial and somatic factors. Sci Rep. 2019;9 doi: 10.1038/s41598-019-40709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Hinz A, Ernst J, Glaesmer H, et al. Frequency of somatic symptoms in the general population: normative values for the Patient Health Questionnaire-15 (PHQ-15) J Psychosom Res. 2017;96:27–31. doi: 10.1016/j.jpsychores.2016.12.017. [DOI] [PubMed] [Google Scholar]
- E14.Noelle-Neumann E, Köcher R. Allensbacher Jahrbücher der Demoskopie - Institut für Demoskopie Allensbach (IfD) 1993-1997. 1997;300 [Google Scholar]
- E15.NICE-Guideline Suspected cancer. recognition and referral. www.nice.org.uk/guidance/ng12/resources/suspected-cancer-recognition-and-referral-pdf-1837268071621 (last accessed on 8 ¬November 2020) [Google Scholar]
- E16.Cathébras PJ, Robbins JM, Kirmayer LJ, Hayton BC. Fatigue in ¬primary care: prevalence, psychiatric comorbidity, illness behavior, and outcome. J Gen Intern Med. 1992;7:276–286. doi: 10.1007/BF02598083. [DOI] [PubMed] [Google Scholar]
- E17.Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534–2544. doi: 10.1056/NEJMoa1603825. [DOI] [PubMed] [Google Scholar]
- E18.Tikkinen KAO, Auvinen A, Johnson TM, et al. A systematic ¬evaluation of factors associated with nocturia—the population-based FINNO study. Am J Epidemiol. 2009;170:361–368. doi: 10.1093/aje/kwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Blakeman JR, Booker KJ. Prodromal myocardial infarction ¬symptoms experienced by women. Heart Lung. 2016;45:327–335. doi: 10.1016/j.hrtlng.2016.04.005. [DOI] [PubMed] [Google Scholar]
- E20.Liu J, Zhang X, Zhao Y, Wang Y. The association between allergic rhinitis and sleep: a systematic review and meta-analysis of -observational studies. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228533. e0228533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Okkes IM, Oskam SK, Lamberts H. The probability of specific ¬diagnoses for patients presenting with common symptoms to Dutch family physicians. J Fam Pract. 2002;51:31–36. [PubMed] [Google Scholar]
- E22.Aaseth J, Hilt B, Bjørklund G. Mercury exposure and health impacts in dental personnel. Environ Res. 2018;164:65–69. doi: 10.1016/j.envres.2018.02.019. [DOI] [PubMed] [Google Scholar]
- E23.Sakellaris I, Saraga D, Mandin C, et al. Association of subjective health symptoms with indoor air quality in European office buildings: The OFFICAIR project. Indoor Air. 2021;31:426–439. doi: 10.1111/ina.12749. [DOI] [PubMed] [Google Scholar]
- E24.Schmiedchen K, Driessen S, Oftedal G. Methodological limitations in experimental studies on symptom development in individuals with idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF)—a systematic review. Environ Health. 2019;18 doi: 10.1186/s12940-019-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Rubin GJ, Hardy R, Hotopf M. A systematic review and meta-analysis of the incidence and severity of postoperative fatigue. J Psychosom Res. 2004;57:317–326. doi: 10.1016/S0022-3999(03)00615-9. [DOI] [PubMed] [Google Scholar]
- E26.Eichhorn L, Kieback M, Michaelis D, Kemmerer M, Jüttner B, Tetzlaff K. Behandlung von Kohlenmonoxidvergiftungen in Deutschland: Eine retrospektive Single-Center-Analyse. Anaesthesist. 2019;68:208–217. doi: 10.1007/s00101-019-0544-8. [DOI] [PubMed] [Google Scholar]
- E27.Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab Verfügbar unter: www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116/ (last ¬accessed on 16 February 2021) 2016;101:364–389. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing‘s disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167:311–326. doi: 10.1530/EJE-11-1095. [DOI] [PubMed] [Google Scholar]
- E29.Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla G-K, Ghigo E. Hypopituitarism. Lancet. 2007;369:1461–1470. doi: 10.1016/S0140-6736(07)60673-4. [DOI] [PubMed] [Google Scholar]
- E30.Miedlich S, Koch CA, Paschke R. Primärer Hyperparathyreoidismus: Heute ein meist asymptomatisches Krankheitsbild. Dtsch Arztebl. 2002;99 A:3340–3346. [Google Scholar]
- E31.Zagzag J, Hu MI, Fisher SB, Perrier ND. Hypercalcemia and cancer: differential diagnosis and treatment. CA Cancer J Clin. 2018;68:377–386. doi: 10.3322/caac.21489. [DOI] [PubMed] [Google Scholar]
- E32.Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the prevention, diagnosis and treatment of Lyme Disease. Clin Infect Dis www.academic.oup.com/cid/article/72/1/e1/6010652 (last accessed on 16 February 2021) 72:e1–e48. doi: 10.1093/cid/ciaa1215. [DOI] [PubMed] [Google Scholar]
- E33.Tarazi M, Gaffney RG, Pearson D, Kushner CJ, Werth VP. Fatigue in systemic lupus erythematosus and other autoimmune skin -diseases. Br J Dermatol. 2019;180:1468–1472. doi: 10.1111/bjd.17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Day J, Yust Katz S, Cachia D, et al. Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD011376.pub2. CD011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E35.Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol. 2018;83:1162–1173. doi: 10.1002/ana.25247. [DOI] [PubMed] [Google Scholar]
- E36.Marshall S, Bayley M, Berrigan L, et al. Guideline for concussion/mild traumatic brain injury & prolonged symptoms. www.braininjuryguidelines.org/concussion/fileadmin/media/Concussion_guideline_3rd_edition_final.pdf (last accessed on 16 February 2021) [Google Scholar]
- E37.Reeve S, Sheaves B, Freeman D. Sleep disorders in early ¬psychosis: incidence, severity, and association with clinical ¬symptoms. Schizophrenia Bulletin. 2019;45:287–295. doi: 10.1093/schbul/sby129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Ward MH, DeLisle H, Shores JH, Slocum PC, Foresman BH. Chronic fatigue complaints in primary care: incidence and diagnostic patterns. J Am Osteopath Assoc. 1996;96:34–46. doi: 10.7556/jaoa.1996.96.1.34. [DOI] [PubMed] [Google Scholar]
- E39.Begleitmaterial zur DEGAM-Leitlinie “Müdigkeit“. www.degam.de/zusatz_m%C3%BCdigkeit.html (last accessed on 6 November 2020) [Google Scholar]
- E40.Nijrolder I, Leone SS, van der Horst HE. Explaining fatigue: An examination of patient causal attributions and their (in)congruence with family doctors initial causal attributions. Eur J Gen Pract. 2015;21:164–169. doi: 10.3109/13814788.2015.1055556. [DOI] [PubMed] [Google Scholar]
- E41.Baum E, Dörr C, Donner-Banzhoff N, Maisel P. DEGAM Leitlinie Müdigkeit, Kurzversion, AWMF-Register-Nr. 053-002. www.awmf.org/uploads/tx_szleitlinien/053-002k_S3_Muedigkeit_2018-05.pdf (last accessed on 3 July 2020) [Google Scholar]
- E42.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding ¬instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12:439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E43.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- E44.Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a ¬practical approach to screen for obstructive sleep apnea. Chest www.stopbang.ca/translation/pdf/german.pdf (last accessed on 7 November 2020) 2016;149:631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- E45.Grol RPT, van Eijk JT, editors. Die Prävention somatischer Fixierung: E. Aufgabe für d. Hausarzt. Berlin. Springer. 1985 Patientenorientierte Allgemeinmedizin; Bd. 2. [Google Scholar]
- E46.Dreibholz KJ. Sturm E, Schaefer H, editors. Gefahren der somatischen Fixierung Der kranke Mensch: Gesundheitsgefährdung, Krankheitsbewältigung und Hilfe durch den Hausarzt. Berlin, Heidelberg. Springer. 1986:245–247. (Patientorientierte Allgemeinmedizin; Bd. 3). [Google Scholar]
- E47.Pitschel-Walz G, Bäuml J. Alsleben H., editor. Grundlagen des Konsensuspapiers zur Psychoedukation (2003/2008) Handbuch der Psychoedukation: Für Psychiatrie, Psychotherapie und Psychosomatische Medizin. Stuttgart. Schattauer. 2016;3 [Google Scholar]
- E48.Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393:768–777. doi: 10.1016/S0140-6736(18)31793-8. [DOI] [PubMed] [Google Scholar]
- E49.Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: a meta-analytic review. Expert Opin Pharmacother. 2018;19:883–894. doi: 10.1080/14656566.2018.1472767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E50.Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183–192. doi: 10.1097/YIC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- E51.Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJH. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry. 2016;15:245–258. doi: 10.1002/wps.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E52.Pompoli A, Furukawa TA, Imai H, Tajika A, Efthimiou O, Salanti G. Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta-analysis. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD011004.pub2. CD011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E53.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E54.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD004366.pub6. CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E55.Shinohara K, Honyashiki M, Imai H, et al. Behavioural therapies versus other psychological therapies for depression. Cochrane ¬Database Syst Rev. 2013;10 doi: 10.1002/14651858.CD008696.pub2. CD008696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E56.Everitt H, Baldwin DS, Stuart B, et al. Antidepressants for insomnia in adults. Cochrane Database Syst Rev. 2018;5 doi: 10.1002/14651858.CD010753.pub2. CD010753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E57.Winkler A, Auer C, Doering BK, Rief W. Drug treatment of primary insomnia: a meta-analysis of polysomnographic randomized controlled trials. CNS Drugs. 2014;28:799–816. doi: 10.1007/s40263-014-0198-7. [DOI] [PubMed] [Google Scholar]
- E58.Jiang B, He D, Gao Z. Efficacy and placebo response of multimodal treatments for primary insomnia: a network meta-analysis. Clin -Neuropharmacol. 2019;42:197–202. doi: 10.1097/WNF.0000000000000369. [DOI] [PubMed] [Google Scholar]
- E59.Yang IA, Brown JL, George J, et al. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust. 2017;207:436–442. doi: 10.5694/mja17.00686. [DOI] [PubMed] [Google Scholar]
- E60.Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12 doi: 10.1002/14651858.CD005305.pub4. CD005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E61.Long L, Mordi IR, Bridges C, et al. Exercise based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1 doi: 10.1002/14651858.CD003331.pub5. CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E62.Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz - Langfassung. www.leitlinien.de/mdb/downloads/nvl/herzinsuffizienz/herzinsuffizienz-3aufl-vers2-lang.pdf (last accessed on 16 February 2021) [Google Scholar]
- E63.Kwekkeboom KL, Bratzke LC. A systematic review of relaxation, meditation, and guided imagery strategies for symptom management in heart failure. J Cardiovasc Nurs. 2016;31:457–468. doi: 10.1097/JCN.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E64.Management of chronic heart failure. A national clinical guideline: Healthcare Improvement Scotland. www.sign.ac.uk/our-guidelines/management-of-chronic-heart-failure/ (last accessed on 11 February 2021) [Google Scholar]
- E65.Penner I-K, Paul F. Fatigue as a symptom or comorbidity of ¬neurological diseases. Nat Rev Neurol. 2017;13:662–675. doi: 10.1038/nrneurol.2017.117. [DOI] [PubMed] [Google Scholar]
- E66.Lanctôt KL, Lindsay MP, Smith EE, et al. Canadian stroke best practice recommendations: mood, cognition and fatigue following stroke, 6th edition update 2019. Int J Stroke. 2020;15:668–688. doi: 10.1177/1747493019847334. [DOI] [PubMed] [Google Scholar]
- E67.Kleinstäuber M, Witthöft M, Steffanowski A, van Marwijk H, Hiller W, Lambert MJ. Pharmacological interventions for somatoform -disorders in adults. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD010628.pub2. CD010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E68.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]