Abstract
Patients with oncogene-driven lung cancer have limited therapeutic options after progressing on their targeted tyrosine kinase inhibitor (TKI) therapy. Given the growing role of immune checkpoint inhibitor (ICI) therapy in the treatment of lung cancer, oncogene-driven cancer has warranted further evaluation regarding ICI therapy. However, initial ICI studies have suggested that ICI monotherapy is not only lacking in efficacy, but that it may be less tolerable in oncogene-driven non-small-cell lung cancer (NSCLC). We performed a detailed review of the literature using Pubmed, and present the current and impactful findings here. Studies evaluating the use of concurrent ICI therapy and TKI therapy have also suggested increased toxicity and lack of increased activity in these patients. Larger studies have suggested that the sequence of ICI therapy and TKI, such as utilizing ICI therapy after TKI as opposed to before TKI, may play a role in reducing toxicity (hepatotoxicity, pneumonitis); however, these studies are limited in number. Novel methods of patient selection, including low tumor mutational burden, inflamed phenotyping, and high CD8 + tumor infiltrating lymphocytes, may aid in determining ideal patients to give ICI therapy. Novel therapeutic combinations including the addition of anti-VEGF (vascular endothelial growth factor) therapy or radiotherapy show promising findings for these patients. Given the growing unmet need for therapeutic options in patients with oncogene-driven NSCLC who have failed TKI therapy, further research is warranted.
1. Introduction
There are an estimated 142,670 annual lung cancer deaths in the USA and up to 1.8 million annual lung cancer deaths worldwide despite recent therapeutic advances [1, 2]. Despite our understanding of actionable oncogenic drivers, such as epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements, and the development of multiple generations of targeted tyrosine-kinase inhibitor (TKI) therapies, patients invariably develop resistance [3, 4]. Most of these oncogenic drivers are harbored in non-small-cell lung cancer (NSCLC) of adenocarcinoma histology, as opposed to squamous cell carcinoma [3]. EGFR-driven NSCLC can be found in up to 19% of African-American patients, Caucasian patients, and Hispanic-American patients, and in up to 51% of Asian patients with lung adenocarcinoma [5]. EGFR mutations have a predilection for female and light or never smoking patients. First- and second-generation TKIs have demonstrated therapeutic benefit, but more recently, the third- generation TKI osimertinib has been associated with a clear survival benefit and a higher degree of CNS control over earlier generation EGFR TKIs [5–10]. ALK rearrangements drive lung adenocarcinoma in up to 4–9% of patients, and have been successfully targeted with multiple TKIs including alectinib [11–19]. Occurring in only 1–2% of lung adenocarcinomas, ROS1 mutations in lung adenocarcinoma respond well to crizotinib [20, 21] as well as to ceritinib and lorlatinib [22, 23]. Larotrectinib and entrectinib have both been US Food and Drug Administration (FDA)-approved for NTRK-positive NSCLC [24, 25], while dabrafenib plus trametinib has also been FDA-approved for BRAF V600E-mutated NSCLC [26]. HER2 mutations (such as exon 20 insertions), RET fusions, and MET exon 14 splice mutations have been identified in NSCLC patients with preliminary evidence of a high degree of activity with highly selective targeted agents [27, 28]. Ultimately, there are many agents that are approved and available for oncogene-driven NSCLC, but patients will inevitably develop resistance and require additional therapeutic strategies.
Given the therapeutic benefit of ICI therapy in non-oncogene-driven NSCLC, this strategy was also evaluated in oncogene-driven NSCLC [29]. Specifically, monoclonal antibodies (mAbs) targeting programmed death 1 (PD1) and PD-L1 have demonstrated significant improvement in overall response and survival compared with standard cytotoxic chemotherapy (Fig. 1). Initially, nivolumab was approved for both histologies of pretreated NSCLC. Pembrolizumab was subsequently approved with a companion biomarker for programmed death ligand 1 (PDL1) expression by tumor immunohistochemistry (IHC), also known as a tumor proportion score (TPS), and served as a predictive biomarker for pembrolizumab therapy. It was associated with a survival benefit for the pembrolizumab/chemotherapy combination observed in KEYNOTE-189 and KEYNOTE-407 [30]. Finally, atezolizumab, which targets PDL1, was also approved in the pretreated setting [31–34].
Fig. 1.
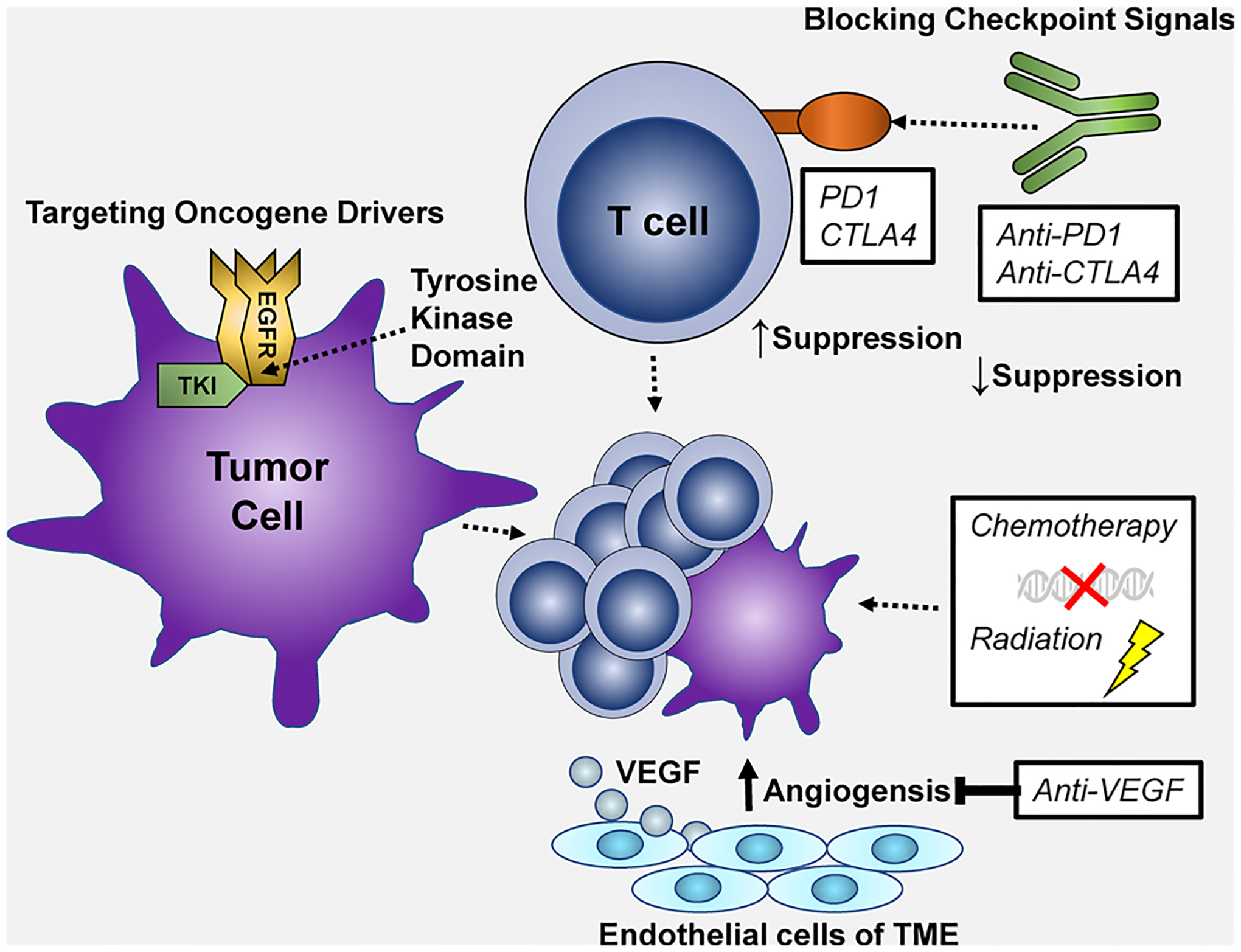
Therapeutic strategies in non-small-cell lung cancer (NSCLC) including: (1) checkpoint signal blockade such as anti-PD1 therapy increasing T-cell activation, (2) targeting oncogene drivers of cancer such as EGFR, ALK, ROS1, BRAF, RET, etc. with tyrosine kinase inhibitor (TKI) therapy, (3) DNA damage and cytotoxic therapy with chemotherapy or radiation therapy, (4) anti-angiogenic therapy such as VEGF blockade
Pembrolizumab monotherapy was later approved in the first-line setting for NSCLC patients with a positive biomarker finding of PDL1 TPS ≥ 50%, and eventually TPS ≥ 1% [35]. The positive findings of KEYNOTE-189 led to the approval of pembrolizumab plus carboplatin and pemetrexed in the first-line setting for non-squamous NSCLC regardless of PD-L1 TPS. This study excluded patients with EGFR mutant or ALK positive disease [36–38]. Atezolizumab in combination with carboplatin and nab-paclitaxel was approved in the first-line setting for NSCLC patients with non-squamous histology based upon the positive findings of IMPower130 [39]. The IMPower150 study included patients with EGFR-mutant or ALK-positive non-squamous NSCLC. This study evaluated atezolizumab in combination with bevacizumab, carboplatin, and paclitaxel and suggested benefit of the combination in patients with oncogene-driven disease; however, the median follow-up was relatively short and estimation of longer-term survival is currently imprecise [40]. In NSCLC of squamous histology, pembrolizumab has been approved in combination with carboplatin and (nab-) paclitaxel based on the KEYNOTE-407 clinical trial [41]. Other markers such as tumor mutational burden (TMB) have also been evaluated, and high TMB (≥ 10 mut/mb) tumors in patients treated with nivolumab in combination with ipilimumab (an ICI targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA4)), derive benefit with regard to progression-free survival (PFS) [42]. The goal of this review was to delineate the efficacy and toxicity of ICI monotherapy and combinatorial approaches in oncogene-driven NSCLC.
2. Immunotherapy Monotherapy
Initial studies of ICI for NSCLC evaluated pretreated patients regardless of mutational status, including CheckMate 017 and CheckMate 057, which evaluated PD1 blockade compared with docetaxel. Both CheckMate 017 in advanced squamous NSCLC and CheckMate 057 in advanced non-squamous NSCLC demonstrated superior overall survival (OS), PFS, 1-year OS, median duration of response (DOR), 1-year PFS, grade 3–4 treatment-related adverse event (trAE) profiles, and overall response rate (ORR) with nivolumab compared with docetaxel [31, 32]. In CheckMate 057, 14% of patients had an EGFR mutation and 4% patients had an ALK translocation. While the hazard ratio (HR) for OS was 0.75 [95% confidence interval (CI) 0.62–0.91] in the overall study population in favor or nivolumab, the HR for OS in the subset of patients with EGFR-mutant NSCLC was 1.18 (95% CI 0.69–2.00), suggesting a trend in survival detriment in patients treated with a single-agent ICI.
The phase II/III study KEYNOTE-010 compared pembrolizumab with docetaxel in patients with pretreated, PDL1-positive (TPS ≥ 1%) advanced NSCLC. These findings led to FDA approval of pembrolizumab with a companion PDL1 diagnostic antibody. Patients who received pembrolizumab had superior OS compared with patients who received docetaxel. In this study, 8% of patients had EGFR-mutant NSCLC and 1% had ALK rearranged NSCLC. Patients with EGFR-mutant tumors were found to have a HR 0.88 (95% CI 0.45–1.70), while patients with EGFR-wild-type malignancies had a more pronounced OS benefit with a HR 0.66 (95% CI 0.55–0.80), suggesting equivocal benefit in patients with EGFR-mutant NSCLC. Median PFS was similar for patients with EGFR-mutant NSCLC and EGFR-wild-type NSCLC. Patients with a TPS ≥ 50% had longer OS with pembrolizumab compared with docetaxel regardless of EGFR mutation status. TrAEs were also less common with pembrolizumab (13% at pembrolizumab 2 mg/kg and 16% at pembrolizumab 10 mg/kg) compared with docetaxel (35%) [43].
The phase III OAK study evaluated atezolizumab compared with docetaxel in pretreated patients with advanced NSCLC, regardless of mutation status. OS favored atezolizumab compared with docetaxel in all patients, and in particular patients with increased tumor PDL1 (TC1/2/3) or immune PDL1 expression (IC1/2/3). OS was similar between squamous and non-squamous histologies (HR 0.73; 95% CI 0.54–0.98) while grade 3–4 trAEs were lower in patients receiving atezolizumab (15%) compared with patients who received docetaxel (43%). Patients in this study were also allowed to have prior TKI therapy for actionable mutations, and use of ICI in the patients with EGFR-positive disease did not improve outcomes compared with docetaxel (HR 1.24; 95% CI 0.71–2.18) [34]. This study and others in later lines of therapy were not powered to evaluate the efficacy or safety of ICI therapy specifically in patients with prior TKI therapy or those with actionable oncogenic drivers. However, a systematic review and meta-analysis in these second- and third-line studies has suggested a trend toward decreased efficacy in patients with actionable mutations [44].
The phase II, open-label, single-arm ATLANTIC trial evaluated durvalumab in patients with pretreated, advanced NSCLC. While the findings of ICI monotherapy were similar to other PD1 blockade agents in patients with non-oncogene driven NSCLC, durvalumab was also evaluated in 111 patients with oncogene-driven NSCLC (further evaluated as having tumors containing at least 25% PDL1-expressing tumor cells, or less than 25% PDL1-expressing tumor cells). The oncogene-driven cohort with at least 25% PDL1 expression demonstrated an overall response rate (ORR) of 12.2% (95% CI 5.7–21.8%) compared with the cohort of patients with oncogene-driven NSCLC and less than 25% PDL1 expression, who demonstrated an ORR of 3.6% (95% CI 0.1–18·3) [45]. Grade 3 or 4 trAEs occurred in 5% of the 111 patients with oncogene-driven NSCLC and was mostly associated with pneumonitis, hepatitis, and immune-related events. This study suggested that PDL1 status may play a role in evaluating which patients with oncogene-driven NSCLC may benefit from later lines of ICI monotherapy, but further evaluation would still be necessary.
In addition, a retrospective analysis of 58 patients with EGFR-mutant or ALK-rearranged advanced NSCLC demonstrated an objective response rate of 3.6% with ICI therapy as opposed to an objective response rate of 23.3% in patients with EGFR/ALK-wild type disease (p = 0.053). A separate retrospective cohort of 68 EGFR-mutant and 27 ALK-positive NSCLC patients demonstrated low PDL1 expression and low CD8 + tumor-infiltrating lymphocytes (TIL) both before and after TKI therapy. These findings suggest that tumors with EGFR mutations or ALK rearrangements favor an immunologically “cold” phenotype with a lack of response to ICI [46] (Table 1).
Table 1.
Selected studies of immune checkpoint inhibitors (ICIs) in EGFR-mutant non-small-cell lung cancer (NSCLC)
| Study/drugs | Size (n) | ORR (%) | mPFS (mos) | mOS (HR/mos) | Grade 3–4 AEs |
|---|---|---|---|---|---|
| ICI monotherapy | |||||
| Gainor et al. CCR 2016 (Retrospective cohort) [46] | 28 | 3.6% | 2.07 | NR | NR |
| Lisberg et al. JTO 2018 (Clinical trial) [51] | 10 | 0.0% | 3.90 | NR | 20% hepatitis 0% pneumonitis** |
| Lee et al. JAMA Oncol 2018 (Meta-analysis) [52] | 271 | NR | NR | HR 1.11 (95% CI 0.80–1.53)* |
NR |
| IMMUNOTARGET Registry (Registry analysis) [47] | 125 | 7.0% | 2.10 | 10.0 mos | 10.8% pneumonitis |
| ICI + TKI | |||||
| Osimertinib + durvalumab (Clinical trial) [57] | 31 | 64.5% | Ongoing | Ongoing | 38.2% pneumonitis |
| Gefitinib + durvalumab (Clinical trial) [59] | 20 | ~ 80% | NR | NR | 20% hepatitis |
| Erlotinib + atezolizumab (Clinical trial) [60] | 28 | 75% | 11.3 | NR | 39% hepatitis |
| Erlotinib + nivolumab (Clinical trail) [61] | 21 | 19% | NR | NR | 19% hepatitis |
| ICI before crizotinib (Clinical trial) [63] | 11 | NR | NR | NR | 45.5% hepatitis |
| IMPower150 | |||||
| Carboplatin + (nab-)paclitaxel + Bevacizumab ± atezolizumab (Clinical trial) [70] | 124 | 70.6% | HR 0.61 (95% CI 0.36–1.03) HR 0.41 (95% CI 0.23–0.75)*** |
HR 0.61 (95%CI 0.29–1.28) HR 0.31 (95%CI 0.11–1.83)*** |
2% hepatitis 1% pneumonitis |
| IMPower130 | |||||
| Carboplatin + nab-paclitaxel ± atezolizumab (Clinical trial) [39] | 44 | NR | HR 0.75 (95% CI 0.36–1.54) |
HR 0.98 (95% CI 0.41–2.31) | 0.4% pneumonitis (Grade 5) 0.4% hepatitis |
| KEYNOTE-789 | |||||
| Carboplatin + pemetrexed ± pembrolizumab (Clinical trial) [71] | Ongoing study in patients with EGFR-mutant TKI-resistant NSCLC | ||||
mos months, NR not reported, ORR overall response rate, mPFS median progression-free survival, mOS median overall survival, HR hazard ratio, CI confidence interval, TKI tyrosine kinase inhibitor
ICI monotherapy versus docetaxel
A grade five pneumonitis event was noted with subsequent TKI therapy
Patients with EGFR-sensitizing mutations (exon 19 deletion and exon 21 L858R)
On a larger scale, the ImmunoTarget registry study retrospectively evaluated 527 advanced NSCLC patients treated with ICI across 25 centers with oncogene-driven cancer (KRAS = 252, EGFR = 110, BRAF = 38, MET = 36, HER2 = 23, ALK = 18, RET = 14, ROS1 = 5, multiple = 31). Best objective response (BOR) was 19% (95% CI 15–22), PFS was 2.8 months (95% CI 2.5–3.1), and OS was 13.3 months (95% CI 9.8–14.8). Response rate (RR) was 26% for patients with KRAS-mutant tumors, 24% for BRAF, 17% for ROS1, 16% for MET, 12% for EGFR, 7% for HER2, 6% for RET, and 0% for ALK [47]. This study suggests that not all mutations are associated with limited ICI efficacy. Notably, MET alterations and mutations such as KRAS or BRAF have a greater association with a positive smoking history (also associated with ICI response) relative to other types of oncogene-addicted NSCLC [48–50].
Prospective clinical trial data include a phase II study evaluating pembrolizumab in ten EGFR-mutant, TPS ≥ 1%, TKI-naïve, advanced NSCLC patients. While 25 patients were planned, enrollment ceased due to a remarkable lack of efficacy. Of the evaluable ten patients, the ORR was 0% and median PFS was 119 days. TrAEs were unremarkable for pembrolizumab, but of note, subsequent TKI therapy in one of the patients led to grade 5 pneumonitis [51].
A meta-analysis evaluated 3025 advanced NSCLC patients across five trials who received ICI monotherapy compared with docetaxel, and found that ICI prolonged OS in patients with EGFR wild-type tumors (HR 0.67; 95% CI 0.60–0.75; P < 0.001), but not in patients with EGFR-mutant tumors (HR 1.11; 95% CI 0.80–1.53; P = 0.54; interaction, P = 0.005). This meta-analysis also demonstrated prolonged overall survival in patients with KRAS mutant tumors (HR 0.65; 95% CI 0.44–0.97; P = 0.03) but not in patients with KRAS wild-type tumors (HR 0.86; 95% CI 0.67–1.11; P = 0.24; interaction, P = 0.24) [52]. In conclusion, despite the remarkable promise shown by ICI monotherapy in non-oncogene-dependent NSCLC in later lines of therapy and now in the first -ine setting, the role of early ICI monotherapy in oncogene-dependent NSCLC appears to be limited.
3. Combination of Tyrosine Kinase Inhibitor (TKI) and Immunotherapy
Early cell-line studies suggest that EGFR-mutant and EML4-ALK-rearranged NSCLC tumors upregulate PDL1. These studies suggest that combination TKI and ICI therapy may overcome inherent resistance to ICI monotherapy [53, 54] and provide the preclinical rationale for combining TKI and ICI in oncogene-addicted NSCLC. The underlying mechanism of overlapping toxicity is poorly understood, but recently EGFR-related TKIs have been shown to enhance tumor cell killing via MHC class I presentation with cytotoxic T lymphocytes; this could be a possible immune-related effect that is further compounded with the known effects of checkpoint blockade therapy [55, 56].
A combination of the third-generation TKI osimertinib and durvalumab was initially evaluated in EGFR-mutant NSCLC patients in a phase Ib setting in the TATTON study. This study demonstrated that 38.2% of the patients had some form of interstitial lung disease (ILD) and 14.7% of the patients had grade 3–4 ILD requiring corticosteroids [57]. While some efficacy was noted, the study was too small to draw meaningful conclusions, and the toxicity was too prohibitive to allow for any further evaluation [57].
A phase I trial of erlotinib or crizotinib in combination with ipilimumab was evaluated in patients with EGFR/ALK-positive disease, and demonstrated excessive grade 3 colitis in four of 11 patients with EGFR-mutated disease. Patients with oncogene-driven NSCLC receiving this combination also developed hypophysitis and pneumonitis leading to early closure of the study [58].
Durvalumab was similarly evaluated in a phase I setting in TKI-naïve EGFR-mutant, advanced NSCLC patients in combination with the first-generation TKI gefinitib. Of the 19 evaluable patients, three patients had to discontinue treatment due to hepatoxicity in the form of elevated AST and/or ALT and one patient had to discontinue due to pneumonitis. Notably, all of the patients who discontinued therapy were in the arm that received the combination therapy after priming with gefitinib for 4 weeks [59]. This study also demonstrated a BOR of ~ 80% [59].
Erlotinib and atezolizumab were also evaluated in a phase Ib setting, demonstrating that this combination was associated with 39% grade 3–4 trAEs including hepatotoxicity, but, interestingly, no pneumonitis [60]. In the 20 TKI-naïve, EGFR-mutant, advanced NSCLC patients, there was an ORR of 75% (95% CI 51–91), CR of 0%, PR of 75%, SD of 15%, DCR (CR + PR + SD ≥ 24 wk) of 90% (95% CI 68–99), median PFS of 11.3 months (95% CI 8.4–not estimable), and median DoR of 9.7 months (95% CI 7.1–not estimable) [60].
Nivolumab was also evaluated in combination with erlotinib in EGFR-mutant, advanced NSCLC patients regardless of prior TKI therapy (however, only one out of the 21 patients was TKI naïve). This study revealed that all 21 patients had trAEs while four out of 21 patients had grade 3–4 trAEs related to elevated AST and/or ALT (three patients) and diarrhea (one patient). None of the patients in this study had pneumonitis of any grade [61]. Of the 21 EGFR-mutant, advanced NSCLC patients, 20 patients were chemotherapy naïve but had progressed on TKI therapy while one patient was TKI naïve. Of the 21 evaluable patients, the ORR was 19%, DOR was NR (60.1–72.3+ weeks), 24-week PFS was 51%, 1-year OS was 73%, PR was 19% (including the one TKI-naïve patient), DOR for these patients were 72.3+, 60.1, 64.6+, and 70+, and SD was 45% (individual time to progression or death was: 9.9+, 15.7, 22.3, 22.7+, 22.7+, 29.4, 35.9, 52.7, and 53 weeks) [61].
To further evaluate the role of ICI and EGFR-TKIs being associated with pneumonitis, a study retrospectively evaluated 5,777 NSCLC patients treated with an EGFR-TKI via the FDA trAE reporting system (FAERS) database from 2015 to 2017. The study demonstrated that of these 5,777 patients, 265 develop pneumonitis (4.59%; 95% CI 4.06–5.16) and of 70 patients treated with both EGFR-TKI and nivolumab, 18 developed pneumonitis (25.7%; 95% CI 16.03–37.6) with an adjusted odds ratio of 4.31 (95% CI 2.37–7.86, p < 0.001). Further analysis of patients treated with and without nivolumab revealed an odds ratio of 5.09 (95% CI 2.87–9.03) for pneumonitis with nivolumab versus 1.22 (95% CI 1.00–1.47) for pneumonitis without nivolumab [62].
A study evaluating 453 patients treated with crizotinib (for disease related to ALK, ROS1, or MET) demonstrated that out of the 11 patients treated with ICI before crizotinib, five (45.5%, 95% CI 14.9–72.2) were associated with a grade 3–4 trAE elevation in AST and/or ALT compared with 34 patients out of 442 (8.1%, 95% CI 5.7–11.0, p < 0.0001) who only received crizotinib therapy [63]. Another study evaluated four out of 26 patients treated with EGFR-TKI therapy who developed pneumonitis. Three of these four patients received osimertinib directly after PD1 blockade, and one patient developed pneumonitis on afatinib before PD1 blockade. However, this study was too small to draw effective conclusions, but warranted further analysis [64]. These studies suggest nivolumab after TKI therapy may be tolerable, but concurrent or sequential use of initial ICI may be associated with increased toxicity [65]. Further studies evaluating combination TKI and ICI therapy in ALK-, ROS1-, or BRAF-positive malignancies are limited [66]. Dabrafenib/trametinib with pembrolizumab in BRAF + melanoma has demonstrated increased antitumor response [67], but similar studies in NSCLC are limited. In conclusion, the combination of TKI and ICI therapy in oncogene-driven NSCLC may not have increased efficacy over TKI therapy alone, and the risk of increased toxicity has limited any further evaluation.
4. Combination of Chemotherapy and Immunotherapy
No clinical trials have specifically evaluated the efficacy of chemotherapy in combination with ICI therapy in oncogene-driven NSCLC outside of subgroup analyses in larger trials. However, evaluating the existing subgroup analyses in larger trials that allowed for the inclusion of oncogene-driven NSCLC is valuable in our understanding of the disease and possible therapeutic strategies (Table 1). IMPower150 is a large phase III prospective study evaluating ICI therapy in combination with standard platinum chemotherapy and anti-VEGF therapy in advanced NSCLC patients. VEGF has been associated with inhibition of T-cell development in tumor-bearing murine models, providing the rationale for the combination of VEGF inhibition with ICI [68]. Notably, there is a growing body of literature suggesting VEGF as a driver of EGFR-mutant disease and resistance [69]. Unlike other large registration studies evaluating chemo-immunotherapy combinations in advanced NSCLC, this study allowed patients with known EGFR-mutant or ALK-rearranged disease, provided they had progression or toxicity on prior TKI therapy. This study demonstrated prolonged PFS in patients treated with atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) (HR 0.61; 95% CI 0.52–0.72) compared with patients treated with BCP [40]. Further analysis of the EGFR-mutant subgroup (n = 124 out of 1,202 enrolled patients) revealed that median OS had not been reached given the short median follow-up (NR; 95% CI 17.0–NR) with ABCP compared with 18.7 months (95% CI 13.4–NR) with BCP (HR 0.61; 95% CI 0.29–1.28]) [70]. A possible OS benefit seems to be driven by the subsets of patients with EGFR sensitizing mutant (exon 19 deletion and exon 21 L858R) disease (n = 58; HR 0.31; 95% CI 0.11–0.83). These findings suggest a benefit with ICI therapy in combination with bevacizumab and chemotherapy in patients with EGFR-mutant NSCLC after progression or intolerance to standard TKI therapy; however, longer-term follow up will be necessary to support these initial findings.
IMPower130 is a large phase III randomized, open-label study evaluating atezolizumab in combination with carboplatin and nab-paclitaxel in the first-line setting for patients with non-squamous NSCLC. Unlike the Keynote-189 clinical trial, which excluded patients with EGFR- and ALK-driven NSCLC, IMPower130 included 44 patients with EGFR- or ALK-driven NSCLC. This study demonstrated a significant improvement in OS (HR 0.79; 95% CI 0.64–0.98) and PFS (HR 0.64; 95% CI 0.54–0.77) with the addition of atezolizumab to chemotherapy compared with chemotherapy alone in the intention-to-treat wild-type population. The OS (HR 0.75; 95% CI 0.36–1.54) and PFS (HR 0.98; 95% CI 0.41–2.31) benefit was similar in the small subset of patients with EGFR-mutant and ALK-rearranged tumors. While the numbers are small, these data suggest that chemoimmunotherapy in the absence of VEGF inhibition may not confer added benefit in oncogene-driven NSCLC [39]. The strategy of chemoimmunotherapy without VEGF inhibition is further being evaluated in KEYNOTE-789, a phase III randomized, double-blind, placebo-controlled study evaluating carboplatin and pemetrexed with or without pembrolizumab in patients with advanced NSCLC and TKI-resistant, EGFR-mutant disease. This study is currently ongoing and results have not been reported at this time [71].
The Phase III PACIFIC trial assessed the efficacy of durvalumab after chemoradiotherapy in stage III NSCLC. Median PFS was 17.2 months for patients treated with durvalumab and 5.6 months for patients treated with placebo (HR 0.51; 95% CI 0.41–0.63). Median OS was significantly increased in favor of durvalumab compared with placebo (HR 0.68; 99.73% CI, 0.47–0.997; P = 0.0025). About 6% of patients in either treatment arm had EGFR-mutant tumors. In these patients, the PFS benefit with durvalumab seemed to be preserved with a HR for all patients of 0.55 (95% CI 0.45–0.68) and a HR in the patients with EGFR-mutant NSCLC of 0.76 (95% CI 0.35–1.64). Unfortunately, the small number of EGFR-mutant patients limited the OS analysis of durvalumab in the EGFR-mutant subgroup [72]. In conclusion, the combination of chemotherapy and immunotherapy in oncogene-driven NSCLC may have a signal for efficacy in later settings, but the current landscape of studies are too limited in scope to be able to properly address this question.
5. Emerging Therapeutic Strategies
Oncogene-addicted tumors are noted to be immunologically “cold” with a phenotype consisting of low PDL1 expression, and low CD8 + TIL [46]. Radiation therapy has demonstrated the capacity to induce tumor mutational burden, increase the release of tumor antigen, and increase CD8 + TIL, leading to a immunologically “hot” tumor [73]. In an effort to induce mutational burden in an existing oncogene-driven malignancy and possibly alleviate the “uninflamed” tumor phenotype, one novel trial will evaluate stereotactic body radiotherapy (SBRT) prior to ICI therapy in advanced NSCLC patients, including patients with oncogene-driven disease (NCT03825510).
Many different strategies have been employed to help identify the ideal patient subpopulation to treat with ICI therapy including PDL1, high CD8 + TIL, high effector T cells, etc. [33, 40]. One study further evaluated the “uninflamed” phenotype within the context of ICI therapy in EGFR-mutant patients. This study conducted 15 pooled analyses and identified that EGFR-mutant patients were associated with decreased PDL1 expression (odds ratio 1.79; 95% CI 1.10–2.93, p = 0.02), lower CD8 + TIL (p = 0.034), decreased TMB, and worse outcomes with PD1/PDL1 blockade (HR 1.09, p = 0.51) compared with EGFR-wt patients (HR 0.73, p < 0.00001) [74]. Another study further evaluated 153 EGFR-mutant patients and demonstrated the TMB was again lower in these patients compared with 1849 EGFR-wt patients (3.77 vs. 6.12 mut/Mb, p < 0.0001). However, within the EGFR-mutant patients, the high TMB patients had a shorter time to treatment discontinuation (TTD) (HR 0.46, p = 0.0008) and OS (HR 0.40, p = 0.0006) compared with patients with low TMB. Further multivariate analysis confirmed that low-TMB, EGFR-mutant patients have increased OS (HR 0.57, p = 0.01) when compared with high TMB, EGFR-mutant patients. TMB also increased after progression on therapy (3.42 vs. 6.56 mut/Mb, p = 0.008) [75]. These findings suggest that high TMB may be a prognostic marker associated with worse disease in oncogene-driven NSCLC, but these studies are still too small to draw meaningful conclusions are this time.
A separate study of ICI (specifically nivolumab) after progression on TKI revealed improved PFS (2.1 months vs. 1.3 months) for T790M-negative patients compared with T790M-positive patients (HR 0.48; 95% CI 0.20–1.24, p = 0.099). Also, patients who responded to ICI had higher CD8 + TIL and increased TMB [76]. However, there are still many subtleties in identifying the ideal patient population as demonstrated by a case report where a 76-year-old never-smoking female developed concurrent SCLC transformation and T790M mutation in separate metastatic sites after progression in TKI therapy [77]. This case illustrates the complex nature of progression of oncogene-driven NSCLC over time.
As of now, there is a paucity of studies demonstrating efficacy of any immunotherapy combinations in other emerging oncogene driver mutations such as HER2-mutant or MET exon 14-mutant NSCLC. However, in patients with KRAS-mutated NSCLC, TMB appears to correlate closely with smoking status [78, 79]. Those mutations with a high fractional allele loss index (FAL) are also associated strongly with increasing pack years [80]. In contrast, single-mutation-associated NSCLC, such as EGFR and ALK, have a lower TMB and poorer response to ICI.
6. Conclusions
The challenges associated with oncogene-driven NSCLC include inevitable acquired resistance. While ICI therapy has been revolutionary for the field of clinical oncology, ICI therapy in oncogene-driven NSCLC has not been as impactful. Early trials demonstrated a lack of efficacy and possible increased risk of toxicity. Further investigation suggested that the sequence of TKI after ICI therapy was associated with increased risk of toxicity such as hepatotoxicity and pneumonitis. Larger phase II/III trials evaluating ICI in the first-line setting began excluding actionable, oncogene-driven NSCLC, unfortunately limiting further investigation. However, the recent findings of the IMPower150 study suggest that there may still be a role for early ICI therapy in patients with actionable, oncogene-driven NSCLC in combination with anti-angiogenic therapy.
Further evaluations of ICI and TKI combinations and in varying sequences are still warranted, along with further dissection into the ideal patient population for therapy such as inflamed, low TMB, high CD8 + TIL or the induction of these findings with possible radiotherapy. Given the increasing prevalence of oncogene-driven NSCLC without sufficient therapeutic options at the time of acquired resistance to TKIs, further research into combinatorial therapy and subpopulation identification is necessary.
Key Points.
Early use of immune checkpoint inhibitor (ICI) therapy alone, or in combination with tyrosine kinase inhibitor (TKI) therapy, is associated with toxicity such as pneumonitis and hepatitis in patients with oncogene-driven non-small-cell lung cancer (NSCLC).
Early use of ICI therapy in patients with oncogene-driven NSCLC is not associated with increased clinical benefit.
The use of anti-VEGF therapy, chemotherapy, and ICI therapy may be associated with response in patients with oncogene-driven NSCLC who have failed TKI therapy.
Acknowledgements
The authors would like to thank the University of Pittsburgh Hillman Cancer Center for their support.
Funding
No external funding was used in the preparation of this manuscript.
Footnotes
Conflict of Interest
Ashwin Somasundaram declares that he has no conflicts of interest that might be relevant to this article. Mark A. Socinski reports honoraria from AstraZeneca, Bristol-Myers Squibb, Celgene, and Genentech; a consultancy/advisory role with Genentech; speakers’ bureau from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech; and research funding from Genentech and Pfizer (institute funding). Liza C. Villaruz reports receiving fees for speaker or advisory board roles for Pfizer.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3.Aisner D, Sholl LM, Berry LD, Haura EB, Ramalingam SS, Glisson BS, et al. Effect of expanded genomic testing in lung adenocarcinoma (LUCA) on survival benefit: the Lung Cancer Mutation Consortium II (LCMC II) experience. Alexandria: American Society of Clinical Oncology; 2016. [Google Scholar]
- 4.Sholl LM, Aisner DL, Varella-Garcia M, Berry LD, Dias-Santagata D, Wistuba II, et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10(5):768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steuer CE, Behera M, Berry L, Kim S, Rossi M, Sica G, et al. Role of race in oncogenic driver prevalence and outcomes in lung adenocarcinoma: results from the lung cancer mutation consortium. Cancer. 2016;122(5):766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376(7):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. [DOI] [PubMed] [Google Scholar]
- 8.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. [DOI] [PubMed] [Google Scholar]
- 11.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–4. [DOI] [PubMed] [Google Scholar]
- 12.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non–small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561. [DOI] [PubMed] [Google Scholar]
- 15.Zou HY, Friboulet L, Kodack DP, Engstrom LD, Li Q, West M, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori M, Ueno Y, Konagai S, Fushiki H, Shimada I, Kondoh Y, et al. The selective anaplastic lymphoma receptor tyrosine kinase inhibitor ASP3026 induces tumor regression and prolongs survival in non–small cell lung cancer model mice. Mol Cancer Ther. 2014;13(2):329–40. [DOI] [PubMed] [Google Scholar]
- 17.Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, et al. Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Can Res. 2011;71(14):4920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28(33):4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama R, Friboulet L, Koike S, Lockerman EL, Khan TM, Gainor JF, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20(22):5686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warth A, Weichert W, Reck M, Reinmuth N. ROS1-translocations in non-small cell lung cancer. Pneumologie. 2015;69(8):477–82. [DOI] [PubMed] [Google Scholar]
- 21.Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12(11):1611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho BC, Drilon AE, Doebele RC, Kim D-W, Lin JJ, Lee J, et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). Alexendria: American Society of Clinical Oncology; 2019. [Google Scholar]
- 24.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drilon A, Siena S, Ou S-HI, Patel M, Ahn MJ, Lee J, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372–001 and STARTRK-1). Cancer Discov. 2017;7(4):400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2019;28:1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai RN, Behera M, Berry LD, Rossi MR, Kris MG, Johnson BE, et al. HER2 mutations in lung adenocarcinomas: a report from the lung cancer mutation consortium. Cancer. 2017;123(21):4099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. [DOI] [PubMed] [Google Scholar]
- 29.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446. [DOI] [PubMed] [Google Scholar]
- 30.Incorvaia L, Fanale D, Badalamenti G, Barraco N, Bono M, Corsini LR, et al. Programmed death ligand 1 (PD-L1) as a predictive biomarker for pembrolizumab therapy in patients with advanced non-small-cell lung cancer (NSCLC). Adv Ther. 2019;36(10):2600–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. [DOI] [PubMed] [Google Scholar]
- 34.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frederickson AM, Arndorfer S, Zhang I, Lorenzi M, Insinga R, Arunachalam A, et al. Pembrolizumab plus chemotherapy for first-line treatment of metastatic nonsquamous non-small-cell lung cancer: a network meta-analysis. Immunotherapy. 2019;11(5):407–28. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 37.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. [DOI] [PubMed] [Google Scholar]
- 38.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 39.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–37. [DOI] [PubMed] [Google Scholar]
- 40.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Ares LG, Luft A, Tafreshi A, Gumus M, Mazieres J, Hermes B, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). Alexendria: American Society of Clinical Oncology; 2018. [Google Scholar]
- 42.Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Cho J, Lee MH, Lim JH. Relative efficacy of checkpoint inhibitors for advanced NSCLC according to programmed death-ligand-1 expression: a systematic review and network meta-analysis. Sci Rep. 2018;8(1):11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapman AM, Sun KY, Ruestow P, Cowan DM, Madl AK. Lung cancer mutation profile of EGFR, ALK, and KRAS: meta-analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–34. [DOI] [PubMed] [Google Scholar]
- 49.Tong JH, Yeung SF, Chan AW, Chung LY, Chau SL, Lung RWM, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–56. [DOI] [PubMed] [Google Scholar]
- 50.Hellmann M, Rizvi N, Wolchok JD, Chan TA. Genomic profile, smoking, and response to anti-PD-1 therapy in non-small cell lung carcinoma. Mol Cell Oncol. 2016;3(1):e1048929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisberg A, Cummings A, Goldman J, Bornazyan K, Reese N, Wang T, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, et al. Induction of PD-L1 expression by the EML4–ALK oncoprotein and downstream signaling pathways in non–small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–21. [DOI] [PubMed] [Google Scholar]
- 54.Hong S, Chen N, Fang W, Zhan J, Liu Q, Kang S, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2016;5(3):e1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30(5):839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lizotte PH, Hong RL, Luster TA, Cavanaugh ME, Taus LJ, Wang S, et al. A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res. 2018;6(12):1511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahn M-J, Yang J, Yu H, Saka H, Ramalingam S, Goto K. Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol. 2016;11(4):S115. [DOI] [PubMed] [Google Scholar]
- 58.Chalmers AW, Patel S, Boucher K, Cannon L, Esplin M, Luckart J, et al. Phase I trial of targeted EGFR or ALK therapy with ipilimumab in metastatic NSCLC with long-term follow-up. Target Oncol. 2019;14(4):417–21. [DOI] [PubMed] [Google Scholar]
- 59.Gibbons DL, Chow L, Kim D-W, Kim S, Yeh T, Song X, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PDL1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11(4):S79. [Google Scholar]
- 60.Ma B, Rudin C, Cervantes A, Dowlati A, Costa D, Schmid P, et al. 441O Preliminary safety and clinical activity of erlotinib plus atezolizumab from a Phase Ib study in advanced NSCLC. Ann Oncol. 2016;27:9. [Google Scholar]
- 61.Rizvi NA, Chow LQM, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. Alexandria: American Society of Clinical Oncology; 2014. [Google Scholar]
- 62.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR–TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin JJ, Chin E, Yeap BY, Ferris LA, Kamesan V, Lennes IT, et al. Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non-small cell lung cancer. J Thorac Oncol. 2019;14(1):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchida T, Kaira K, Yamaguchi O, Mouri A, Shiono A, Miura Y, et al. Different incidence of interstitial lung disease according to different kinds of EGFR-tyrosine kinase inhibitors administered immediately before and/or after anti-PD-1 antibodies in lung cancer. Thorac cancer. 2019;10(4):975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward J, Morgensztern D. Role of immune checkpoint blockers in patients with EGFR mutation. Transl Lung Cancer Res. 2018;7(Suppl 4):S385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortinovis D, Canova S, Abbate MI, Colonese F, Cogliati V, Bidoli P. Challenges in ALK inhibition of ALK-positive non-small-cell lung cancer: from ALK positivity detection to treatment strategies after relapse. Fut Oncol. 2018;14(22):2303–17. [DOI] [PubMed] [Google Scholar]
- 67.Ribas A, Lawrence D, Atkinson V, Agarwal S, Miller WH Jr, Carlino MS, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med. 2019;25(6):936–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–86. [DOI] [PubMed] [Google Scholar]
- 69.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Rev Clin Oncol. 2008;5(9):521. [DOI] [PubMed] [Google Scholar]
- 70.Reck M, Mok TS, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. [DOI] [PubMed] [Google Scholar]
- 71.Riely G, Hui R, Carbone D, Park K, Carrigan M, Xu X, et al. Phase 3 study of pemetrexed-platinum with or without pembrolizumab for TKI-resistant/EGFR-mutated advanced NSCLC: KEYNOTE-789. J Thorac Oncol. 2018;13:10.29258667 [Google Scholar]
- 72.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–50. [DOI] [PubMed] [Google Scholar]
- 73.Simone CB, Heinzerling JH. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl Lung Cancer Res. 2015;4(5):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong Z-Y, Zhang J-T, Liu S-Y, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. 2019;25(3):1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28(7):1532–9. [DOI] [PubMed] [Google Scholar]
- 77.Suda K, Murakami I, Yu H, Kim J, Ellison K, Rivard CJ, et al. Heterogeneity in immune marker expression after acquisition of resistance to EGFR kinase inhibitors: analysis of a case with small cell lung cancer transformation. J Thorac Oncol. 2017;12(6):1015–20. [DOI] [PubMed] [Google Scholar]
- 78.Chae YK, Davis AA, Raparia K, Agte S, Pan A, Mohindra N, et al. Association of tumor mutational burden with DNA repair mutations and response to anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. Clin Lung Cancer. 2019;20(2):88–96. [DOI] [PubMed] [Google Scholar]
- 79.Uguen M, Dewitte JD, Lodde B, Marcorelles P, Uguen A. Asbestos-related lung cancers are rarely associated with ALK, ROS1 and RET rearrangements. Eur Respir J. 2018;51:3. [DOI] [PubMed] [Google Scholar]
- 80.Czarnecka KH, Migdalska-Sek M, Antczak A, Pastuszak-Lewandoska D, Kordiak J, Nawrot E, et al. Allelic imbalance in 1p, 7q, 9p, 11p, 12q and 16q regions in non-small cell lung carcinoma and its clinical association: a pilot study. Mol Biol Rep. 2013;40(12):6671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


