Abstract
Background
Helicobacter pylori (H. pylori) prevalence is lower in patients with inflammatory bowel disease (IBD) than in those without IBD, suggesting that H. pylori plays a protective role in IBD. It has been reported that IBD may occur due to H. pylori eradication; however, it is unclear whether H. pylori eradication increases the incidence of IBD. Moreover, the effect of H. pylori eradication on IBD activity is unclear.
Case presentation
An 11-year-old boy diagnosed with ulcerative colitis (UC) was in clinical remission, with treatment involving 5-aminosalicylic acid. Fecal calprotectin (FC) level had decreased to 33.2 mg/kg, indicating mucosal healing. At age 12, he experienced epigastric pain on an empty stomach, which was relieved with dietary intake. His FC level was elevated without UC symptoms, such as diarrhea and bloody stools. He was diagnosed with H. pylori duodenal ulcer. H. pylori eradication (clarithromycin and amoxicillin for 7 days and a proton-pump inhibitor) led to symptom improvement the day after treatment initiation. However, he developed diarrhea and his FC level remained high despite improvement in duodenal ulcer symptoms and endoscopic findings of H. pylori eradication. Colonoscopy results indicated UC relapse.
Conclusions
H. pylori eradication may worsen UC activity. However, further studies are required as this case report involved only one pediatric patient with increased UC activity after H. pylori eradication.
Keywords: Fecal calprotectin, Helicobacter pylori, Ulcerative colitis
Background
The prevalence of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease, has increased worldwide. [1] Its pathogenesis involves gut microbiota, [2] including Helicobacter pylori (H. pylori). The prevalence of H. pylori is lower in patients with IBD than in those without IBD, which suggests H. pylori plays a protective role in the development of IBD. [3, 4] It has previously been reported that IBD may occur due to H. pylori eradication [5–7]; however, whether H. pylori eradication increases the incidence of IBD as well as the effect of H. pylori eradication on IBD activity remains unclear. We describe a pediatric case of UC, worsened through H. pylori eradication for the treatment of duodenal ulcer.
Case presentation
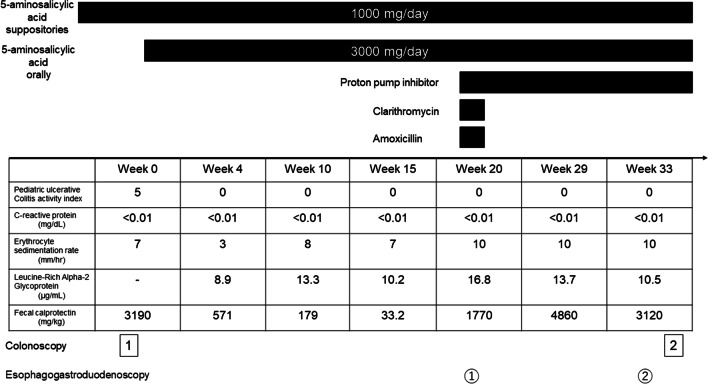
An 11-year-old boy consulted a family doctor for bloody diarrhea. The patient had no specific medical or family history. A colonoscopy from the rectum to the sigmoid colon led to the diagnosis of pediatric UC with a pediatric ulcerative colitis activity index (PUCAI) of 30. He was administered 5-aminosalicylic acid (5-ASA) suppositories (1 g/day) and was referred to our hospital for subsequent treatment. His symptoms promptly improved to a PUCAI of 5. His blood examination results were unremarkable but the fecal calprotectin (FC) level was elevated to 3,190 mg/kg. A complete colonoscopy was performed, which revealed inflammatory findings from the rectum to the transverse colon. Moreover, the cecum and ascending colon showed loss of vascular permeability and adherent purulent mucus (Fig. 1a, b). The patient was prescribed oral 5-ASA (3,000 mg/day). The FC level gradually decreased to 33.2 mg/kg by week 15 (Fig. 2).
Fig. 1.
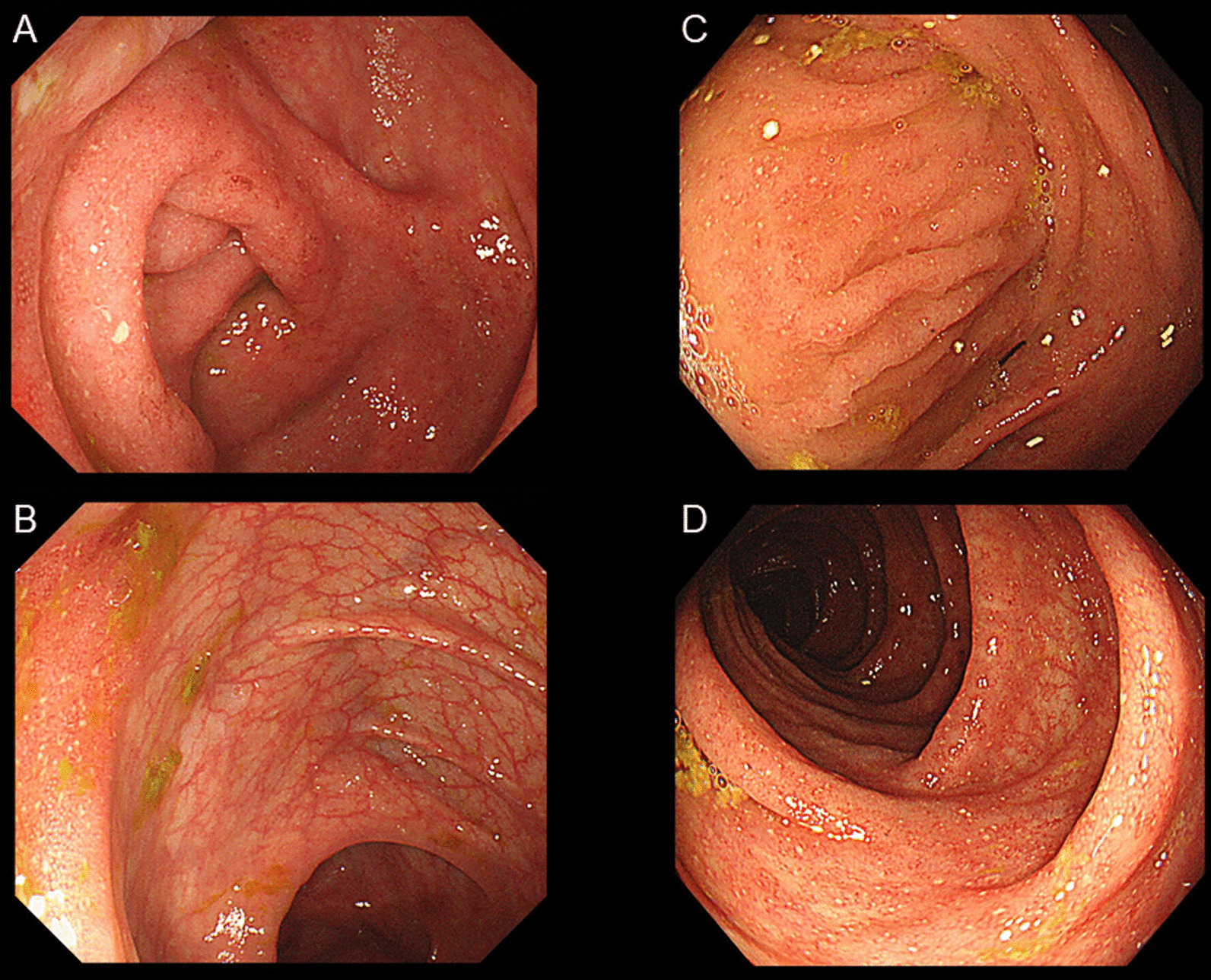
Colonoscopy findings. The cecum (a) and ascending colon (b) at the time of diagnosing ulcerative colitis with slight inflammation from the rectum to the transverse colon. The cecum and ascending colon show loss of vascular permeability and adherent purulent mucus. At the time of ulcerative colitis relapse, the cecum (c) and ascending colon (d) had small aphthae and edematous mucosa
Fig. 2.
A timeline showing the patient’s disease course in terms of pediatric ulcerative colitis activity index values, C-reactive protein, erythrocyte sedimentation rate, leucine-rich alpha-2 glycoprotein, and fecal calprotectin levels at different time points. The timeline and duration of various treatments administered for ulcerative colitis and Helicobacter pylori are indicated. The time points indicating when the colonoscopy and the esophagogastroduodenoscopy were undertaken are also shown
At 12 years old, he complained of epigastric pain on an empty stomach, which was relieved with dietary intake (week 19). He reported no UC symptoms, including diarrhea and bloody stools (PUCAI, 0); however, an elevated FC level was noted (week 20). Esophagogastroduodenoscopy (EGD) findings indicated an A1 ulcer on the lower wall of the duodenal bulb (Fig. 3A). A rapid urease test was positive, and he was diagnosed with a duodenal ulcer due to H. pylori infection. The H. pylori infection was treated using clarithromycin and amoxicillin for 7 days and a proton-pump inhibitor. The patient’s symptoms improved the day after treatment initiation. In week 33, an EGD was performed to evaluate the therapeutic effect. The duodenal ulcer had healed, and scarring was observed (Fig. 3B).
Fig. 3.
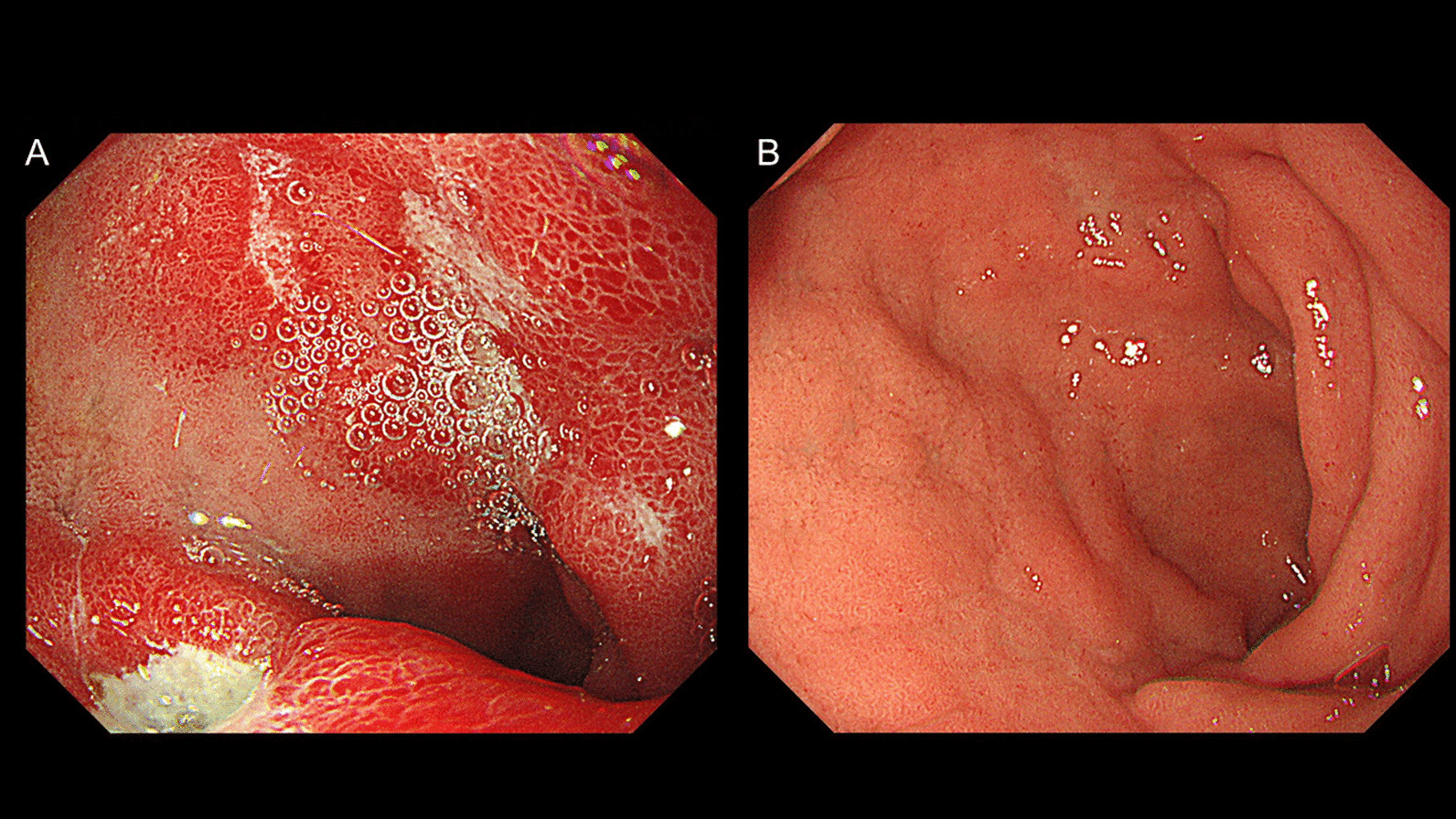
Esophagogastroduodenoscopy. a At the time of duodenal ulcer diagnosis, esophagogastroduodenoscopy findings revealed an A1 ulcer on the lower wall of the duodenal bulb. b Three months after Helicobacter pylori treatment, esophagogastroduodenoscopy findings indicated healing of the duodenal ulcer and scarring
The FC level remained high despite improvement of duodenal ulcer symptoms and endoscopic findings of H. pylori eradication. The patient subsequently developed diarrhea (PUCAI, 10) in week 35 and a UC relapse was considered. Cytomegalovirus antibody, antigenemia, and tuberculosis (T-SPOT assay) tests were negative. Colonoscopy showed small aphthae and edematous mucosa throughout the colon in week 36 (Fig. 1C, D). Histopathological examination revealed UC lesions grades 3–5 based on the Matts classification. The patient was diagnosed with a UC relapse, and the 5-ASA dosage was increased to 4,000 mg/day (100 mg/kg/day).
Discussion and conclusion
H. pylori has been considered protective against immune-mediated diseases, including IBD. However, it has additionally been associated with peptic ulcers and gastric cancer. H. pylori eradication may be more effective in preventing the development of gastric cancer in people of Asian ethnicity than in those of European ethnicity; therefore, most patients of Asian ethnicity actively undergo H. pylori treatment. [8] In Asia, the incidence of IBD has been increasing, [9] and theoretically, active eradication of H. pylori may have a role in this observed increase. Several studies have reported the development of IBD after eradication of H. pylori. [5–7] Furthermore, there is a report that there is an inverse association between H. pylori and UC severity. [10] However, data concerning the effect of H. pylori eradication on IBD activity are limited. A multicenter retrospective cohort study showed that H. pylori eradication did not worsen IBD activity. [11] However, there have been no reports involving pediatric patients with IBD. We report a pediatric patient with worsened UC activity following the eradication of H. pylori.
The mechanism by which H. pylori acts protectively on IBD is unclear; however, there are various reports using mouse models. Zhang et al. reported that H. pylori had a protective action in mice with chronic experimental colitis. They considered that this protective mechanism involved H. pylori colonization increasing regulatory T cells and interleukin (IL)-10 and suppressing IL-17 producing effector T-helper cells. [12] Gravina et al. reported that Hp(2–20) derived from H. pylori accelerates the healing of not only gastric mucosa but also inflamed colonic mucosa in 2-, 4-, 6-trinitrobenzenesulfonic acid-induced colitis, and the effect is considered to be associated with the reduction of inflammatory mediators such as tumor necrosis factor-α in colonic tissue. [13] Chen et al. reported that NLRP12 decreased MCP-1 and MIP-1α expression in intestinal epithelial cells from the analysis of the exomes derived from H. pylori-infected IBD patients, and this report showed NLRP12 has a negative correlation with the disease activity in pediatric IBD patients. [14] Further research on the function of H. pylori in IBD patients is required.
In our patient, endoscopic mucosal healing was not confirmed after the initial 5-ASA treatment. However, an FC level < 250 mg/kg is indicative of endoscopic and histological mucosal healing. [15] Based on these findings, we considered that our patient had achieved remission after the initial treatment. We noted elevation in his FC level following the onset of the duodenal ulcer; however, it was unclear whether this elevated FC level due to the duodenal ulcer or to UC relapse. However, there were no clinical symptoms of UC. Three months after H. pylori eradication, the patient had an increased FC level, and he gradually developed diarrhea. Therefore, the elevated FC level was considered to be due to UC activity. The FC level is the most helpful biomarker of endoscopic disease activity. [16] No other hematological biomarkers, including leucine-rich alpha 2 glycoprotein, are considered helpful in evaluating IBD activity. [17] FC monitoring following H. pylori eradication may help to detect endoscopic and histological relapse prior to clinical relapse. Endoscopic and histological remission in addition to clinical remission should be confirmed in patients with H. pylori-induced peptic ulcer cases.18.
To date, no studies concerning pediatric IBD have evaluated the FC level prior to and post H. pylori eradication. Measuring the FC level is non-invasive and allows early detection of relapse during the asymptomatic phase. Further studies are required to determine the effect of H. pylori eradication in relation to pediatric IBD activity.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- EGD
Esophagogastroduodenoscopy
- FC
Fecal calprotectin
- H.pylori:
Helicobacter pylori
- IBD
Inflammatory bowel disease
- IL
Interleukin
- PUCAI
Pediatric Ulcerative Colitis Activity Index
- UC
Ulcerative colitis
Authors' contributions
YF collected and analyzed the data and drafted and revised the initial manuscript. KT, TT, TS, and SY interpreted all the data and critically revised the manuscript for important intellectual content. All the authors have read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Funding
No funding was received for the work.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approval and consent to participate
Consent to participate is not applicable to this article as it is a case report.
Consent for publication
Written informed consent was obtained from the parents of the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonnenberg A, Genta RM. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:469–476. doi: 10.1111/j.1365-2036.2011.04969.x. [DOI] [PubMed] [Google Scholar]
- 4.Gastano-Rodrigeuez N, Kaakoush NO, Lee WS, Mitchell HM. Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut. 2017;66:235–249. doi: 10.1136/gutjnl-2015-310545. [DOI] [PubMed] [Google Scholar]
- 5.Tursi A. Onset of Crohn’s disease after Helicobacter pylori eradication. Inflamm Bowel Dis. 2006;12:1008–1009. doi: 10.1097/01.mib.0000235100.09231.d7. [DOI] [PubMed] [Google Scholar]
- 6.Jovanovic IR, Milosavjevic TN, Jankovic GP, Micev MM, Dugalic PD, Saranovic D, et al. Clinical onset of the Crohn’s disease after eradication therapy of Helicobacter pylori infection. Does Helicobacter pylori infection interact with natural history of inflammatory bowel diseases? Med Sci Monit. 2001;7:137–41. [PubMed]
- 7.Chiba M, Tsuji T, Takahashi K, Komatsu M, Sugawara T, Ono I. Onset of ulcerative colitis after Helicobacter pylori eradication therapy: A case report. Perm J. 2016;20:e115–e118. doi: 10.7812/TPP/15-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomized controlled trials. BMJ. 2014;348:g3174. [DOI] [PMC free article] [PubMed]
- 9.Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 10.Jin X, Chen YP, Chen SH, Xiang Z. Association between Helicobacter Pylori infection and ulcerative colitis - - a case control study from China. Int J Med Sci. 2013;10:1479–1484. doi: 10.7150/ijms.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinzaki S, Fujii T, Bamba S, Ogawa M, Kobayashi T, Oshita M, et al. Seven days triple therapy for eradication of Helicobacter pylori does not alter the disease activity of patients with inflammatory bowel disease. Intest Res. 2018;16:609–618. doi: 10.5217/ir.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Dai Y, Liu Y, Wu T, Li J, Wang X, et al. Helicobacter pylori colonization protects against chronic experimental colitis by regulating Th17/Treg balance. Inflamm Bowel Dis. 2018;24:1481–1492. doi: 10.1093/ibd/izy107. [DOI] [PubMed] [Google Scholar]
- 13.Gravina AG, Prevete N, Tuccillo C, Musis CD, Romano L, Federico A, et al. Peptide Hp(2–20) accelerates healing of TNBS-induced colitis in the rat. United European Gastroenterol J. 2018;6:1428–1436. doi: 10.1177/2050640618793564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Huang J, Li H, Li P, Xu C. Serum exomes derived from Hp-positive gastritis patients inhibit MCP-1 and MIP-1α expression via NLRP12-Notch signaling pathway in intestinal epithelial cells and improve DSS-induced colitis in mice. Int Immunopharmacol. 2020;88:107012. [DOI] [PubMed]
- 15.Zittan E, Kelly OB, Kirsch R, Milgrom R, Burns J, Nguyen GC, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm Bowel Dis. 2016;22:623–630. doi: 10.1097/MIB.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 16.Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamma Bowel Dis. 2013;19:332–341. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura T, Mitsuyama K, Sakemi R, Takedatsu H, Yoshioka S, Kuwaki K, et al. Evaluation of serum leucine-rich alpha-2 glycoprotein as a new inflammatory biomarker of inflammatory bowel disease. Mediators Inflamm. 2021;8825374. [DOI] [PMC free article] [PubMed]
- 18.Ungaro R, Colombel JF, Lissoos T, Peyrin-Biroulet L. A treat-to-target update in ulcerative colitis: A systematic review. Am J Gastroenterol. 2019;114:874–883. doi: 10.14309/ajg.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.