Abstract
Mitotic checkpoints restrain the onset of mitosis (M) when DNA is incompletely replicated or damaged. These checkpoints are conserved between the fission yeast Schizosaccharomyces pombe and mammals. In both types of organisms, the methylxanthine caffeine overrides the synthesis (S)-M checkpoint that couples mitosis to completion of DNA S phase. The molecular target of caffeine was sought in fission yeast. Caffeine prevented activation of Cds1 and phosphorylation of Chk1, two protein kinases that enforce the S-M checkpoint triggered by hydroxyurea. Caffeine did not inhibit these kinases in vitro but did inhibit Rad3, a kinase that regulates Cds1 and Chk1. In accordance with this finding, caffeine also overrode the G2-M DNA damage checkpoint that requires Rad3 function. Rad3 coprecipitated with Cds1 expressed at endogenous amounts, a finding that supports the hypothesis that Rad3 is involved in direct activation of Cds1.
Mitosis (M) is actively coupled to the completion of DNA synthesis (S) to ensure genome integrity. A pioneering experiment helped establish this fact by demonstrating that caffeine induced mitosis in BHK Syrian hamster fibroblasts arrested in S phase with hydroxyurea (HU), an inhibitor of ribonucleotide reductase (33). These studies were one of the earliest examples of checkpoint override, a concept precisely defined in subsequent genetic investigations performed with the budding yeast Saccharomyces cerevisiae (37). Later studies of the fission yeast Schizosaccharomyces pombe and S. cerevisiae have uncovered checkpoint signal transduction and enforcement mechanisms that are substantially conserved with checkpoint systems in more complex multicellular organisms (11, 29).
Genetic studies of the fission yeast S. pombe have identified a group of seven proteins required for the DNA replication checkpoint, also known as the S-M checkpoint (1, 26). These proteins (Rad1, Rad3, Rad9, Rad17, Rad26, Hus1, and Cut5/Rad4) are believed to be part of a sensor complex that monitors changes of DNA structure and a signal transduction system that transmits the DNA replication arrest signal to the effector kinase Cds1 (8, 17). Indeed, HU treatment leads to dramatic activation of Cds1 (8, 17). Cds1 regulates proteins that control Cdc2, the cyclin-dependent kinase that catalyzes mitotic events. Cds1 is required to increase the abundance of Mik1, a protein kinase that performs inhibitory phosphorylation of Cdc2 on tyrosine-15 (8). Wee1, a second tyrosine-15-directed kinase, might also be regulated by Cds1 (8), although it is not known whether Wee1 and Mik1 are direct substrates of Cds1. There is substantial evidence that Cds1 phosphorylates Cdc25, the tyrosine phosphatase that activates Cdc2 (13, 42). This phosphorylation inhibits Cdc25 activity (13). Cds1 is also important for recovery from an S-M checkpoint arrest, but the nature of these activities is not understood (17).
A second important checkpoint restrains the onset of mitosis in response to DNA damage. In fission yeast, this G2-M DNA damage checkpoint requires the same group of seven sensor and signal transduction proteins that are required for the S-M checkpoint mentioned above, as well as Chk1 and Crb2/Rhp9 (2, 30, 34, 38). Chk1 is the effector kinase of the DNA damage checkpoint (14, 25). Chk1 is hyperphosphorylated in response to DNA damage (35). Chk1 appears to negatively regulate Cdc25 by direct inhibition and by promoting nuclear exclusion of Cdc25 (13, 18). Chk1 appears to have no role in the S-M replication checkpoint, although in the absence of Cds1, HU causes Chk1 phosphorylation and Chk1 prevents the onset of mitosis (9, 17). Cds1 has no ability to enforce the G2-M DNA damage checkpoint.
The DNA structure checkpoints appear to be highly conserved between fission yeast and metazoan species (7, 20, 23, 32). In human cells, for example, it appears that the DNA damage checkpoint leads to inhibition of Cdc25 phosphatase (7). Most checkpoint proteins in fission yeast have presumptive homologs in humans. Notably, Rad3 is similar to human ATM, a kinase that is required for the G2-M DNA damage checkpoint (5). ATM is involved in activation of a Cds1 homolog (Cds1/Chk2) in mammalian cells (7, 20).
Genetic methods are difficult with many metazoan species; thus, compounds that inhibit checkpoint proteins have significant investigative utility. Caffeine, for example, was recently shown to prevent Chk1 phosphorylation in Xenopus oocyte extracts and to preferentially radiosensitize p53-deficient but not ATM-deficient cells (16, 41). The latter observation strengthens the notion that potent checkpoint disrupters may also have anticancer therapeutic potential when used in conjunction with agents that damage DNA or inhibit DNA replication. Thus, it is important to understand how chemicals override checkpoints. Herein, we describe studies aimed at discovering the checkpoint protein that is targeted by caffeine in fission yeast. These studies identify Rad3, the fission yeast kinase related to the human checkpoint protein ATM, as a target of caffeine.
MATERIALS AND METHODS
Strains, plasmids, and general techniques.
The strains used in this study were PR109 (wild type) and strains with genotypes chk1:2HA6HIS:ura4+ cds1::ura4+ (JMB2274), cds1:2HA6HIS:ura4+ (NB2118), mik1:2HA6HIS:ura4+ (OM2183), nmt1:GST-chk1:leu1+ (BF1758), rad3::ura4+ (NR1826), nmt1:3HA:rad3:ura4+ (BF2039), nmt1:3HA:rad3:ura4+ cds1::ura4+ (BM2432), nmt1:3HA:rad3:ura4+ cds1:13myc:kan (BM2591), rad3-ts(A2217V) (PS2358), and rad3-ts(A2217V) cds1::ura4+ (JMB2434). All strains except BF2039 were leu1-32. The chk1::ura4+, cds1::ura4+, and nmt1:GST-chk1+ constructs have been described elsewhere (8, 14, 25). The 3HA (three copies of hemagglutinin) epitope was cloned into pREPrad3 (gift of Antony Carr) after digestion with NdeI to form plasmid pBF132. The nmt1:3HA-rad3+ construct was removed from pBF132 by PstI digest and cloned into the PstI site of pJK210 (15). This construct was linearized with StuI and integrated into OM1603 (leu1-32 ura4-294) at the ura4 locus. The leu1-32 allele was subsequently crossed out of the strain. Growth media and general biochemical and genetic methods for S. pombe have been described elsewhere (21). Cells were grown in EMM2, a medium that induces expression of nmt1, for 18 h prior to caffeine addition. Unless otherwise indicated, yeast cultures were grown at 30°C in YES medium (glucose, yeast extract, amino acid supplements). HU (Sigma) was used at a concentration of 12 mM. Bleomycin (Calbiochem) was used at a concentration of 5 mU/ml.
Caffeine experiments and microscopy.
For each caffeine-induced S-M checkpoint override experiment, cells were grown in YES medium or EMM2 to an optical density at 600 nm (OD600) of ∼0.5 at 30°C and then treated with 12 mM HU for 3 h. Caffeine was added to a final concentration of 10 mM. Approximately 20 × 107 cells were harvested by filtration. Microscopic observation was used to follow cell progression through mitosis (septation index). DNA content was detected by DAPI (4′,6-diamidino-2-phenylindole) (1 mg/ml) staining after ethanol fixation. Cells were photographed with a Nikon Eclipse E800 microscope equipped with a Photometrics Quantix charge-coupled device camera. Images were acquired with IPLab Spectrum software (Signal Analytics Corporation).
Immunoblotting and kinase assay.
For detection of Cds1 with a two-HA, six-histidine tag (Cds1-2HA6HIS) or glutathione S-transferase (GST)–Chk1, cells were lysed in buffer A (50 mM Tris [pH 8], 150 mM NaCl, 5 mM EGTA, 10% glycerol, 0.1% NP-40, 5 μg each of leupeptin, aprotinin, and pepstatin per ml, 1 mM phenylmethylsulfonyl fluoride). The protein concentration was normalized using the OD280 nm reading, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a nitrocellulose membrane. Blots were blocked with 5% milk in TBST (20 mM Tris [pH 7.6], 200 mM NaCl, 0.3% Tween 20). Chk1-2HA6HIS and Mik1-2HA6HIS were precipitated with Ni2+-nitrilotriacetic acid (NTA) beads and revealed with antibodies to HA followed by anti-mouse immunoglobulin G (IgG) antibodies coupled with horseradish peroxidase (HRP) (9). Cds1-2HA6HIS was precipitated with an anti-HA polyclonal antibody (BAbCo) and revealed with a monoclonal antibody to HA (12CA5) followed by anti-mouse IgG antibodies coupled with HRP. GST-Chk1 was purified with glutathione beads and revealed with antibodies to GST followed by anti-rabbit IgG antibodies coupled with HRP. Enhanced chemiluminescence detection (Pierce) was used to visualize proteins. For Rad3 immunoprecipitation experiments, cells were lysed in buffer B (50 mM Tris [pH 8], 120 mM NaCl, 50 mM NaF, 60 mM β-glycerol phosphate, 1 mM Na3VO4, 0.5% NP-40, 5 μg each of leupeptin, aprotinin, and pepstatin per ml, 1 mM phenylmethylsulfonyl fluoride). HA-Rad3 was immunoprecipitated with rabbit polyclonal anti-HA (BAbCo) antibody and revealed with 12CA5. Detection of Cds1-13myc in Rad3 immunoprecipitations was performed with monoclonal Myc antibodies (BAbCo). The Cds1 kinase assay was performed with GST-Wee11-152 or PHAS-I (1 μg; Promega) as the substrate (8). GST-Cdc251-147 was used as the substrate for GST-Chk1 (13). The kinase assay of immunopurified 3HA-Rad3 was performed as described for ATM, with minor modifications, using the 1–91 region of human Cds1 expressed as a GST fusion protein in bacteria (GST-HsCds11-91) as the substrate (3).
RESULTS
Caffeine overrides the S-M checkpoint in fission yeast.
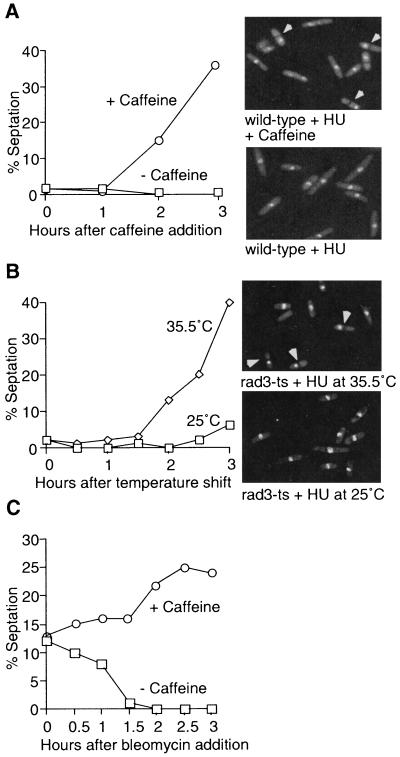
The methylxanthine compound caffeine overrides the cell cycle arrest induced by inhibition of DNA replication in several mammalian cell types. Experiments were designed to test the hypothesis that caffeine targets a checkpoint protein defined in genetic studies with fission yeast. Wild-type cells were incubated for 3 h in 12 mM HU, which imposed a cell cycle arrest with unreplicated DNA. Addition of 10 mM caffeine caused a substantial increase in the percentage of septated cells, indicative of checkpoint override, whereas mock-treated cells remained arrested (Fig. 1A). DNA was unequally segregated in essentially all of the divided cells in the caffeine-treated culture (Fig. 1A). This phenotype is typical of checkpoint mutants treated with HU (12). Indeed, the kinetics of HU checkpoint override was mimicked in an experiment with a strain that contained a temperature-sensitive allele of rad3 (Fig. 1B). Cells with the rad3-ts allele were incubated with 12 mM HU for 3 h at the permissive treatment of 25°C followed by incubation at the restrictive temperature of 35.5°C. Within 3 h at 35.5°C, a large fraction of these cells had divided with DNA unequally segregated to daughter cells (Fig. 1B), yielding a phenotype that was very similar to that observed with wild-type cells treated with caffeine (Fig. 1A). There have been conflicting findings on the effects of caffeine on the S-M checkpoint in fission yeast (22, 36). Our observation that caffeine overrides the S-M checkpoint agrees with the most recent report (36).
FIG. 1.
Caffeine overrides the S-M replication and G2-M DNA damage checkpoints. (A) Caffeine overrides the S-M replication checkpoint. A cds1-2HA6HIS (NB2118) strain was arrested in early S phase with 12 mM HU for 3 h prior addition of 10 mM caffeine for the indicated times. Cell division was monitored by counting septated cells. Three hours after addition of caffeine, cells were stained with DAPI to visualize DNA (right). Arrowheads indicate examples of cells that have divided with unequally segregated DNA. (B) The in vivo effect of caffeine is mimicked by a rad3-ts allele. A rad3-ts strain (PS2358) was grown at 25°C and arrested in early S phase with 12 mM HU for 3 h. Half of the culture was then shifted to the restrictive temperature of 35.5°C, while the other half was kept at 25°C. Septation was monitored for 3 h, and DAPI staining was performed as described above. Arrowheads indicate examples of cells that have divided with unequally segregated DNA. (C) Caffeine overrides the G2-M DNA damage checkpoint. Strain NB2118 was either mock treated or pretreated with 10 mM caffeine 15 min prior to addition of bleomycin (5 mU/ml), a drug that induces DNA damage. Septation was monitored for the next 3 h.
All fission yeast genes that are known to be required for division arrest in HU-treated cells are also essential for the G2-M DNA damage checkpoint arrest. Therefore, an experiment was performed to determine if caffeine overrides the DNA damage checkpoint elicited by bleomycin, a radiometric drug. Treatment with bleomycin alone led to reduction in the septation index, indicative of cells arrested at the G2-M checkpoint (Fig. 1C). Addition of caffeine 15 min before the addition of bleomycin substantially abrogated the checkpoint arrest, as indicated by the increase in septation index (Fig. 1C). Thus, caffeine appeared to target a protein that is required for both the S-M replication checkpoint and G2-M DNA damage checkpoint.
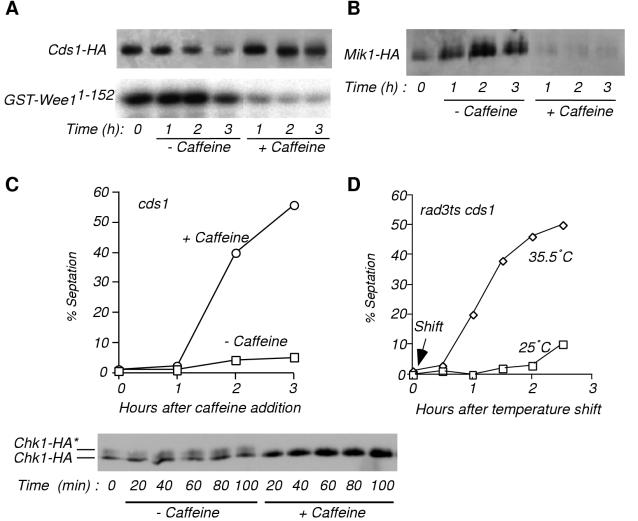
Caffeine abrogates S-M checkpoint-dependent activation of Cds1 and the accumulation of Cdc2 tyrosine kinase Mik1.
Cds1 is a central effector of the S-M checkpoint in fission yeast (8, 17). Cds1 is activated by HU treatment, leading to the maintenance of inhibitory tyrosine phosphorylation of Cdc2. We investigated the effects of caffeine on Cds1 activity. The kinase activity of immunoprecipitated Cds1-2HA6HIS was assayed with GST-Wee11-152 substrate, which consists of GST fused to the N-terminal 152 amino acids of Wee1 (8). Cds1-2HA6HIS activity rapidly decreased in cells treated with caffeine (Fig. 2A). Caffeine also caused the disappearance of Mik1-2HA6HIS (Fig. 2B), an epitope-tagged form of Mik1, which accumulates in HU-arrested cells by a Cds1-dependent process (8). Mik1 is important for maintenance of cell cycle arrest in HU (8, 13, 42); thus, it is likely that Mik1 disappearance contributed to the caffeine-induced failure of the S-M checkpoint.
FIG. 2.
Caffeine causes inactivation of Cds1 and destabilization of Mik1 in vivo and overrides a Chk1-dependent S-M checkpoint. (A) Cds1-2HA6HIS was immunopurified with HA antibody 12CA5 from HU-arrested NB2118 cells either mock treated or treated with 10 mM caffeine for the indicated times. Its kinase activity was assayed with GST-Wee11-152 substrate (bottom). Cds1-2HA6HIS abundance was verified by immunoblot analysis (top). (B) Caffeine treatment results in destabilization of Mik1. A Mik1-2HA6HIS expressing strain (OM2183) was arrested with HU prior addition of 10 mM caffeine for the indicated times. Abundance of Mik1-2HA6HIS was monitored by immunoblot analysis after purification with Ni2+-NTA agarose as described elsewhere (4). (C) Caffeine overrides the S-M checkpoint in a cds1 strain. A cds1 chk1-2HA6HIS strain (JMB2274) was treated with 12 mM HU for 3 h followed by addition of 10 mM caffeine for the indicated times (11). Cell septation was monitored. Chk1 phosphorylation was immediately abrogated by caffeine (bottom). Chk1-2HA6HIS was purified with Ni2+-NTA agarose and analyzed in an immunoblot. Positions of hyperphosphorylated (Chk1-HA*) and hypophosphorylated Chk1-2HA6HIS are indicated. (D) The rad3-ts allele in cds1 cells (JMB2434) mimics the effect of caffeine. Cells were grown at the permissive temperature (25°C) and arrested in early S phase with 12 mM HU for 3 h. Half the culture was then shifted to the restrictive temperature of 35.5°C. Septation was monitored for 2.5 h.
Caffeine abolishes S-M checkpoint in cds1 cells.
The effect of caffeine was investigated in cds1 cells. The S-M checkpoint is intact in cds1 cells due to the activity of Chk1, a structurally dissimilar kinase that inhibits Cdc25 and is essential for the DNA damage checkpoint (8, 17). Chk1 phosphorylation, as detected in immunoblots, signals its involvement in a checkpoint arrest (35). HU does not normally cause Chk1 phosphorylation, nor is Chk1 normally required for the S-M checkpoint, but Chk1 is phosphorylated and essential for the HU-induced checkpoint in cds1 cells. A culture of cds1 chk1-2HA6HIS cells was arrested in early S phase by treatment with HU and then exposed to caffeine or mock treated. Caffeine abrogated the S-M checkpoint in these cells (Fig. 2C). Checkpoint override occurred in >50% of the caffeine-treated cds1 cells, whereas only ∼5% of the mock-treated cds1 cells underwent division. Moreover, phosphorylated Chk1-2HA6HIS rapidly disappeared after addition of caffeine (Fig. 2C). The loss of phosphorylated Chk1-2HA6HIS coincided with an increase of the hypophosphorylated form of the protein, indicative of rapid dephosphorylation of Chk1-2HA6HIS. The checkpoint override effect of caffeine was mimicked in an experiment in which rad3-ts cds1 cells were incubated in HU and then shifted to the restrictive temperature (Fig. 2D). Thus, caffeine overrode the S-M checkpoint regardless of which effector, Cds1 or Chk1, was required to enforce the arrest.
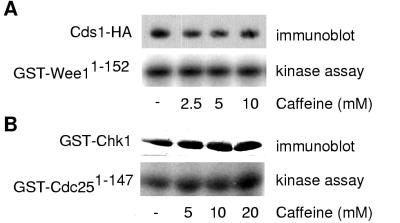
Chk1 and Cds1 kinases are insensitive to caffeine in vitro.
Two possible interpretations emerged from these results. Caffeine might target both Cds1 and Chk1, or it could inhibit one of their common upstream regulators. To distinguish between these possibilities, the ability of caffeine to inhibit Cds1 and Chk1 was tested in vitro. GST-Wee11-152 produced in bacteria was used as a substrate for Cds1-2HA6HIS immunoprecipitated from fission yeast (8). GST-Cdc251-147, which consists of GST fused to the N-terminal 147 amino acids of Cdc25, was used as a substrate for GST-Chk1 expressed in fission yeast (13). Caffeine was ineffective as an inhibitor of either kinase in vitro (Fig. 3). These findings are most simply interpreted to indicate that caffeine targets an upstream regulator shared by Cds1 and Chk1.
FIG. 3.
Cds1 and Chk1 are resistant to inhibition by caffeine in vitro. (A) Cds1-2HA6HIS was immunopurified and tested in a kinase assay with GST-Wee11-152 substrate in the presence of increasing amounts of caffeine (bottom). Cells were treated with 12 mM HU for 3 h prior to harvest. Cds1-2HA6HIS abundance was verified by immunoblot analysis (top). Assays performed with wild-type cells confirmed that phosphorylation was dependent on Cds1-2HA6HIS (Moser et al., unpublished data). (B) GST-Chk1 was purified with glutathione-Sepharose and tested in a kinase assay with GST-Cdc251-147 substrate in the presence of increasing amounts of caffeine (bottom). Cells were exposed to 100 Gy of ionizing radiation prior to harvest. GST-Chk1 abundance was verified by immunoblot analysis (top). Assays performed with wild-type cells confirmed that phosphorylation was dependent on GST-Chk1 (Moser et al., unpublished data).
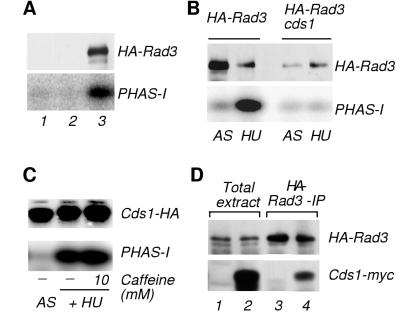
HU-induced Rad3-associated kinase activity is Cds1.
The kinase Rad3 is required for Cds1 activation and Chk1 phosphorylation (8, 17, 35). Therefore, Rad3 was considered a potential target of caffeine. Evaluation of this hypothesis required establishment of an assay for Rad3 kinase activity. The protein PHAS-I was investigated as a suitable Rad3 substrate. PHAS-I is a regulator of translation initiation that is unconnected to checkpoints but is phosphorylated by the Rad3-related kinase ATM in vitro (3). 3HA-Rad3 was expressed from the nmt1 promoter and immunoprecipitated following exposure to HU. Immunoprecipitated 3HA-Rad3 from HU-arrested cells gave rise to a substantial phosphorylation of PHAS-I (Fig. 4A), while no such phosphorylation was observed in HA immunoprecipitates from HU-treated wild-type cells that did not express 3HA-Rad3 or rad3 cells (Fig. 4A). Thus, PHAS-I phosphorylation was dependent on 3HA-Rad3. While only weak phosphorylation of PHAS-I was obtained in immunoprecipitates from asynchronous cells, HU treatment caused an approximately 10-fold increase in PHAS-I phosphorylation (Fig. 4B). Thus, a kinase activity associated with HA-Rad3 was induced in cells arrested by the S-M checkpoint.
FIG. 4.
Phosphorylation of PHAS-I by Rad3 is due to coimmunoprecipitated Cds1. (A) Assay of 3HA-Rad3 expressed in a nmt1:3HA-rad3 strain. Wild-type (PR109), rad3 (NR1826), and 3HA-Rad3 (BF2039) cells (lanes 1 to 3, respectively) were HU arrested prior to immunoprecipitation with antibodies to HA. Kinase assays were performed with PHAS-I substrate (bottom). Immunoblotting with antibodies to HA confirmed the presence of 3HA-Rad3. (B) Phosphorylation of PHAS-I is abolished in a cds1 strain. Wild-type and cds1 cells that contained the nmt1:3HA-rad3 construct (BF2039 and BM2432, respectively) were treated with HU or mock treated (AS [asynchronous]). Immunoprecipitates of 3HA-Rad3 were assayed with PHAS-I substrate in kinase assays (bottom). The amount of 3HA-Rad3 in the assay reaction was determined by immunoblot analysis (top). (C) Cds1 phosphorylates PHAS-I. Cds1-2HA6HIS immunopurified from asynchronous or HU-arrested NB2118 cells was detected by immunoblotting (top). Phosphorylation of PHAS-I by Cds1-2HA6HIS was determined in the absence and presence of 10 mM caffeine in the kinase reaction (bottom). (D) Rad3 and Cds1 interact. 3HA-Rad3 immunoprecipitations were performed from HU-arrested cells expressing 3HA-Rad3 in either wild-type background (BF2039; lanes 1 and 3) or coexpressing 3HA-Rad3 and Cds1-13myc (BM2591; lanes 2 and 4). Expression of 3HA-Rad3 (top) and presence of Cds1-13myc (bottom) in either total cell extracts or 3HA-Rad3 immunoprecipitates were verified by immunoblot analysis.
The potent activation of a Rad3-associated kinase was striking in view of the very modest changes in ATM-associated kinase activity reported in checkpoint-arrested mammalian cells (3). Furthermore, we discovered that Rad3-associated kinase activity was apparently unchanged in cells treated with agents that damage DNA (B. A. Moser, B. J.-M., B. Baber-Furnari, and P. Russell, unpublished data). Cds1 was reported to associate with Rad3 when both proteins are overexpressed from a strong promoter (19). These observations, and the fact that Cds1 is strongly activated in HU-treated cells, suggested that Cds1 might be the Rad3-associated kinase activated in cells arrested at the S-M checkpoint. This proposal was investigated by performing the 3HA-Rad3 kinase assays with a cds1 strain. This investigation showed that the HU stimulation of the Rad3-associated kinase was eliminated in a cds1 strain (Fig. 4B). In fact, the Rad3-associated kinase activity assayed from cds1 cells was not significantly different from the activity measured from negative control cells that expressed untagged Rad3 (Moser et al., unpublished data). Thus, in these assay conditions, the Rad3-associated kinase activity was almost entirely dependent on Cds1.
Immunoprecipitated Cds1 from either mock-treated or HU-arrested cells was tested for its capability to phosphorylate PHAS-I in vitro (Fig. 4C). Cds1-2HA6His from HU-treated cells phosphorylated PHAS-I. In fact, HU caused an approximately 10-fold increase in Cds1 activity, a stimulation that correlated with the HU stimulation of the 3HA-Rad3-associated kinase. This activity of Cds1 was unaffected by addition of 10 mM caffeine to the in vitro kinase assay (Fig. 4C). Association between Cds1 and Rad3 was investigated by immunoblot analysis of 3HA-Rad3 immunoprecipitation complexes. This experiment was performed with a strain that expressed Cds1-13myc from the cds1 genomic locus. Cds1-13myc was readily detected in anti-HA immunoprecipitation complexes from cells that expressed 3HA-Rad3 (Fig. 4D). Taken together, these data argue that the phosphorylation of PHAS-I carried out by 3HA-Rad3 immune complexes was largely if not entirely performed by associated Cds1.
Rad3 kinase activity is inhibited in vitro by caffeine and wortmannin.
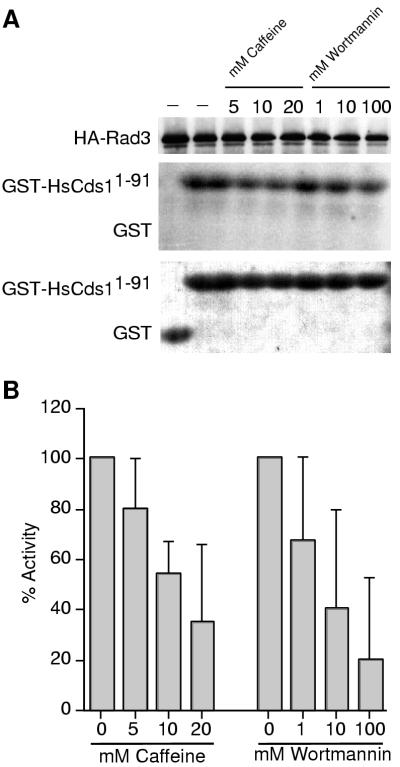
It appeared that PHAS-I was at best a poor substrate of Rad3; therefore, we sought a better Rad3 substrate. Human ATM, a functional analog of Rad3, was recently shown to phosphorylate human Cds1 (HsCds1; also known as HsChk2) (20). In particular, the first 91 amino acids of HsCds1, when fused to GST and produced in bacteria, appeared to be an excellent substrate of ATM. To avoid any Cds1 contamination of Rad3, we used cds1 cells that expressed 3HA-Rad3. 3HA-Rad3 was immunoprecipitated from HU-arrested cells and tested for its capability to phosphorylate GST-HsCds11-91 (Fig. 5A). Substantial phosphorylation of GST-HsCds11-91 was detected with the 3HA-Rad3 immunoprecipitate, whereas unfused GST was not phosphorylated. To confirm the specificity of the assay, we used the ATP analog wortmannin to inhibit Rad3. Wortmannin is a potent inhibitor of phosphatidylinositol 3-like kinases such as ATM, ATR, and DNA-specific protein kinase (6). Wortmannin inhibited the phosphorylation of GST-HsCds11-91 in 3HA-Rad3 immunoprecipitates in a dose-dependent manner (Fig. 5). These findings supported the notion that 3HA-Rad3 was directly responsible for GST-HsCds11-91 phosphorylation. Having established an assay for 3HA-Rad3, we tested whether caffeine inhibited 3HA-Rad3 in vitro. As shown in Fig. 5, caffeine acted in a dose-dependent manner to cause substantial inhibition of GST-HsCds11-91 phosphorylation catalyzed by 3HA-Rad3. These results identified Rad3, or possibly a cofactor essential for Rad3 kinase activity, as a probable checkpoint target of caffeine.
FIG. 5.
Rad3 activity is inhibited by caffeine in vitro. (A) 3HA-Rad3 was immunopurified from HU-arrested cds1 cells (BM2432). Equal amounts of immunoprecipitates were taken to establish 3HA-Rad3 activity in the presence of increasing concentrations of caffeine and wortmannin added to the kinase reaction. GST-HsCds11-91 was used as the substrate. GST served as a control substrate to exclude its phosphorylation by Rad3. The amount of substrate in each assay was verified by Coomassie blue staining (bottom) of the GST/GST-HsCds11-91 autoradiograph (middle). The amount of 3HA-Rad3 in the kinase reaction was verified by HA immunoblotting (top). (B) Quantitative analysis of Rad3 activity. Results shown are the means of multiple independent results from four separate experiments (± standard error of the mean). Statistical significance was determined by two-tailed t test. The following P values were calculated: 0.1054, 0.0005 and 0.0076 for 5, 10, and 20 mM caffeine, respectively; and 0.1667, 0.0344 and 0.0136 for 1, 10, and 100 μM wortmannin, respectively. Findings are regarded as significant if P values are <0.05.
DISCUSSION
Compounds that override checkpoints have significant experimental utility and potential therapeutic applications. Caffeine was the first drug reported to override checkpoints; in fact, the use of caffeine to induce mitosis with unreplicated DNA predates the formal genetic definition of DNA structure checkpoints (33). More recently, caffeine was used to investigate checkpoints in the Xenopus oocyte system and to potentiate the radiation sensitivity of p53-deficient cells (10, 16, 41). These studies demonstrate the continued experimental usefulness of caffeine. Therefore, it is important to understand how caffeine overrides checkpoints. In this report, we have applied the genetic advantages of fission yeast in an attempt to define the molecular target of caffeine that is relevant to checkpoints. Our studies provide strong evidence that caffeine targets Rad3.
Caffeine overrides both S-M DNA replication and G2-M DNA damage checkpoints in S. pombe. Our studies demonstrated that caffeine overrides the S-M checkpoint that is normally enforced by Cds1 and the G2-M DNA damage checkpoint enforced by Chk1. Two general mechanisms of checkpoint override can be envisioned. One mechanism does not interfere with checkpoint signaling. Instead, it causes mitotic initiation in the presence of a checkpoint signal. For example, a compound that inhibited Wee1 and Mik1 would cause mitotic initiation despite an intact S-M replication checkpoint signal (27). The alternative mechanism is to abrogate the checkpoint signal by blocking the activity of a checkpoint protein. Cds1 activation is the S-M checkpoint signal most proximal to the mitotic control proteins. We observed that Cds1 activity is lost very rapidly upon addition of caffeine to HU-treated cells. Likewise, we found that caffeine rapidly induced the disappearance of the phosphorylated form of Chk1 observed in cds1 cells treated with HU. These finding demonstrated that caffeine specifically abrogates checkpoint signal as opposed to modulating the activity of Cdc2 or the proteins that control Cdc2 activity.
Caffeine targets Rad3.
Caffeine abrogates both the S-M and G2-M DNA damage checkpoints. Thus, caffeine must act on protein that is shared by both signaling pathways. Neither Chk1 or Cds1 is normally required for both checkpoints; thus, inhibition of either kinase alone cannot account for the effects of caffeine. It is formally possible that caffeine targets both Cds1 and Chk1, which are collectively required for both checkpoints, but neither protein was inhibited by caffeine in vitro. Thus, caffeine must target an upstream regulator that is shared by Cds1 and Chk1. This conclusion is consistent with studies that established that caffeine inhibits an upstream regulator of Chk1 in Xenopus oocyte extracts (16). An exhaustive genetic search has identified seven such proteins: Cut5/Rad4 and the six checkpoint Rad proteins (Rad1, Rad3, Rad9, Rad17, Rad26, and Hus1). Cut5/Rad4 can probably be excluded as a caffeine target because it is essential for DNA replication, and caffeine treatment did not prevent DNA replication (31). Of the six checkpoint Rad proteins, only Rad3 has an inferred enzymatic function, namely, protein kinase activity (5). The physiological substrate of Rad3 is unknown, but Cds1 is a good candidate because Cds1 activation requires Rad3 in vivo (8, 17). In fact, we observed that an N-terminal portion of human Cds1/Chk2 is an excellent substrate of Rad3. Using this fragment as a substrate, and Rad3 immunoprecipitated from cds1 cells, we established an in vitro protein kinase assay for Rad3. In this assay, we found that caffeine inhibited Rad3 in a concentration range equivalent to that required to override checkpoints in vivo. These findings strongly suggest that Rad3 is targeted by caffeine in vivo. Using a similar assay, Blasina et al. have found that caffeine inhibits human ATM, a presumptive analog of Rad3 (6). Thus, caffeine appears to act by a similar mechanism to override checkpoints in a diverse range of eukaryotic species.
Rad3 associates with Cds1 in vivo.
Our studies showed that Rad3 immunoprecipitates contain a PHAS-I kinase activity. This activity was dependent on Cds1. We demonstrated that Cds1 is associated with Rad3 and can phosphorylate PHAS-I in vitro. A physical interaction between Rad3 and Cds1 was described in a recent study in which both proteins were overexpressed in fission yeast (19). Our experiments differ somewhat in that only Rad3 was overexpressed, thereby increasing the probability that the Rad3-Cds1 physical interaction has physiological significance. Moreover, we found that Cds1 associated with Rad3 is activated by HU treatment and thus is capable of being activated by the checkpoint signal. This observation reinforces the notion that an association between Rad3 and Cds1 is part of the normal checkpoint signaling system. We have also shown that a portion of human Cds1 is an in vitro substrate of Rad3, but we have been unable to activate Cds1 with Rad3 in an in vitro reaction (unpublished data). Thus, while it seems likely that Rad3 and Cds1 associate in a physiologically significant manner in vivo, it remains uncertain whether Cds1 is activated directly by Rad3.
Checkpoint override and cancer.
Rad3 is related functionally and structurally to mammalian ATM, a protein implicated in checkpoint control (5). Therefore, our data provide a mechanistic explanation for the similar effects of caffeine and mutational inactivation of ATM in mammalian cells (4). Furthermore, these data indicate why caffeine radiosensitizes p53-deficient cells, replicating the effect of simultaneous genetic inactivation of p53 and ATM (24, 28, 39–41). Therapeutic application of caffeine is impractical because of its pleiotropic effects, but a biochemical understanding of caffeine-induced checkpoint override improves the framework for discovery of new compounds that specifically target checkpoints.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
Michael N. Boddy and Nick Rhind made helpful comments and suggestions; Antony Carr supplied strains; Alessandra Blasina, Takashi Toda, Chris Norbury, and Clare McGowan discussed results prior to publication. Members of the Scripps Cell Cycle Groups provided support and encouragement.
J.M.B. was supported by INSERM (France). B.A.M. was supported by the Schweizer Krebsliga and the Deutsche Forshungsgemeinschaft. This work was funded by NIH.
ADDENDUM IN PROOF
Since submission of this paper, there have appeared three additional reports of caffeine inhibiting the Rad3-related kinase ATM or ATR: C. A. Hall-Jackson et al. (Oncogene 18:6707–6713, 1999), J. N. Sarkaria et al. (Cancer Res. 59:4375–4382, 1999), and B. B. Zhou et al. (J. Biol. Chem. 275:10342–10348, 2000).
REFERENCES
- 1.al-Khodairy F, Carr A M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.al-Khodairy F, Fotou E, S. S K, Griffiths D J, Lehmann A R, Carr A M. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 4.Bebb D G, Steele P P, Warrington P J, Moffat J A, Glickman B W. Caffeine does not potentiate gamma-radiation induced DNA damage in ataxia telangiectasia lymphoblastoid cells. Mutat Res. 1998;401:27–32. doi: 10.1016/s0027-5107(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 5.Bentley N J, Holtzman D A, Flaggs G, Keegan K S, DeMaggio A, Ford J C, Hoekstra M, Carr A M. The Schizosaccharomyces pombe rad3 checkpoint gene. EMBO J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- 6.Blasina A, Price B D, Turenne G A, McGowan C H. Caffeine inhibits checkpoint kinase ATM. Curr Biol. 1999;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- 7.Blasina A, Van de Weyer I, Laus M C, Luyten W H M L, Parker A E, McGowan C H. A human homolog of the checkpoint kinase Cds1 directly inhibits Cdc25. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 8.Boddy M N, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- 9.Brondello J-M, Boddy M N, Furnari B, Russell P. Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases. Mol Cell Biol. 1999;19:4262–4269. doi: 10.1128/mcb.19.6.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasso M, Newport J W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- 11.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 12.Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- 13.Furnari B, Blasina A, Boddy M N, McGowan C H, Russell P. Cdc25 inhibited in vitro and in vivo by checkpoint kinases Cds1 and Chk1. Mol Biol Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 15.Keeney J B, Boeke J D. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics. 1994;136:849–856. doi: 10.1093/genetics/136.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai A, Guo Z, Emami K H, Wang S X, Dunphy W G. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsay H, Griffiths D, Edwards R, Christensen P, Murray J, Osman F, Walworth N, Carr A. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998;12:382–395. doi: 10.1101/gad.12.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 regulated by DNA damage and 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 19.Martinho R G, Lindsay H D, Flaggs G, DeMaggio A J, Hoekstra M F, Carr A M, Bentley N J. Analysis of Rad3 and Chk1 protein kinases defines different checkpoint responses. EMBO J. 1998;17:7239–7249. doi: 10.1093/emboj/17.24.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 21.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 22.Osman F, McCready S. Differential effects of caffeine on DNA damage and replication cell cycle checkpoints in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1998;260:319–334. doi: 10.1007/s004380050901. [DOI] [PubMed] [Google Scholar]
- 23.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 24.Powell S N, DeFrank J S, Connell P, Eogan M, Preffer F, Dombkowski D, Tang W, Friend S. Differential sensitivity of p53(−) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 1995;55:1643–1648. [PubMed] [Google Scholar]
- 25.Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 26.Rhind N, Russell P. Mitotic DNA damage and replication checkpoints in yeast. Curr Opin Cell Biol. 1998;10:749–758. doi: 10.1016/s0955-0674(98)80118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhind N, Russell P. Tyrosine phosphorylation of Cdc2 required for the replication checkpoint in Schizosaccharomyces pombe. Mol Cell Biol. 1998;18:3782–3787. doi: 10.1128/mcb.18.7.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell K J, Wiens L W, Demers G W, Galloway D A, Plon S E, Groudine M. Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res. 1995;55:1639–1642. [PubMed] [Google Scholar]
- 29.Russell P. Checkpoints on the road to mitosis. Trends Biochem Sci. 1998;24:399–402. doi: 10.1016/s0968-0004(98)01291-2. [DOI] [PubMed] [Google Scholar]
- 30.Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of crb2, a protein with BRCT motif, with cut5 and chk1. Genes Dev. 1997;11:3387–3400. doi: 10.1101/gad.11.24.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saka Y, Yanagida M. Fission yeast cut5+, required for S phase onset and M phase restraint, is identical to the radiation-damage repair gene rad4+ Cell. 1993;74:383–393. doi: 10.1016/0092-8674(93)90428-s. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 33.Schlegel R, Pardee A B. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science. 1986;232:1264–1266. doi: 10.1126/science.2422760. [DOI] [PubMed] [Google Scholar]
- 34.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 35.Walworth N C, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 36.Wang S W, Norbury C, Harris A L, Toda T. Caffeine can override the S-M checkpoint in fission yeast. J Cell Sci. 1999;112:927–937. doi: 10.1242/jcs.112.6.927. [DOI] [PubMed] [Google Scholar]
- 37.Weinert T A, Hartwell L H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. doi: 10.1126/science.3291120. [DOI] [PubMed] [Google Scholar]
- 38.Willson J, Wilson S, Warr N, Watts F Z. Isolation and characterization of the Schizosaccharomyces pombe rhp9 gene: a gene required for the DNA damage checkpoint but not the replication checkpoint. Nucleic Acids Res. 1997;25:2138–2145. doi: 10.1093/nar/25.11.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Yang E M, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol Cell Biol. 1998;18:4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao S L, Akhtar A J, McKenna K A, Bedi G C, Sidransky D, Mabry M, Ravi R, Collector M I, Jones R J, Sharkis S J, Fuchs E J, Bedi A. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat Med. 1996;2:1140–1143. doi: 10.1038/nm1096-1140. [DOI] [PubMed] [Google Scholar]
- 42.Zeng Y, Forbes K C, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]