Abstract
Patients with lung cancer are especially vulnerable to coronavirus disease 2019 (COVID-19) with a greater than sevenfold higher rate of becoming infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) COVID-19, a greater than threefold higher hospitalization rate with high complication rates, and an estimated case fatality rate of more than 30%. The reasons for the increased vulnerability are not known. In addition, beyond the direct impact of the pandemic on morbidity and mortality among patients with lung cancer, COVID-19, with its disruption of patient care, has also resulted in substantial impact on lung cancer screening and treatment/management.COVID-19 vaccines are safe and effective in people with lung cancer. On the basis of the available data, patients with lung cancer should continue their course of cancer treatment and get vaccinated against the SARS-CoV-2 virus. For unknown reasons, some patients with lung cancer mount poor antibody responses to vaccination. Thus, boosting vaccination seems urgently indicated in this subgroup of vulnerable patients with lung cancer. Nevertheless, many unanswered questions regarding vaccination in this population remain, including the magnitude, quality, and duration of antibody response and the role of innate and acquired cellular immunities for clinical protection. Additional important knowledge gaps also remain, including the following: how can we best protect patients with lung cancer from developing COVID-19, including managing care in patient with lung cancer and the home environment of patients with lung cancer; are there clinical/treatment demographics and tumor molecular demographics that affect severity of COVID-19 disease in patients with lung cancer; does anticancer treatment affect antibody production and protection; does SARS-CoV-2 infection affect the development/progression of lung cancer; and are special measures and vaccine strategies needed for patients with lung cancer as viral variants of concern emerge.
Keywords: COVID-19, Lung cancer, SARS-CoV-2, Immunotherapy, Chemotherapy, Vaccine
Introduction
Of the many disparities revealed by the coronavirus disease 2019 (COVID-19) pandemic, one of the immediate medical concern is the apparent increased incidence and severity of COVID-19 among patients with cancer,1 and in particular those with lung cancer (Table 1 ).2 , 3 An Italian study of 59,989 patients receiving cancer treatment, of whom 406 became infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), found that patients with lung cancer had the highest incidence of contracting COVID-19 (n = 91).2 A retrospective case-control study of 73.4 million U.S. patients identified 16,570 patients who contracted COVID-19, of whom 1200 were patients with cancer and 100 were recently diagnosed with having lung cancer.3 Patients recently diagnosed with having lung cancer were at a significantly higher risk of infection with SARS-CoV-2 with an adjusted OR of 7.66 (95% confidence interval [CI]: 7.07–8.29).3
Table 1.
Morbidity and Mortality in Patients With Lung Cancer and SARS-CoV-2 Infection
| Summary of Select Studies on COVID-19 Infection and Outcomes Among Patients With Cancer Data | ||||||
|---|---|---|---|---|---|---|
| Study | Country/Countries | Cancer Types | Number of Patients With COVID-19 | aOR of SARS-CoV-2 Infection (95% CI) | Hospitalization Rate, % | Mortality Rate, % |
| Wang et al.3 | U.S. | All cancer | 1200 | 1.46 (1.42–1.50) | — | — |
| Recent (i.e., past 12 mo) cancer diagnosis | 670 | 7.14 (6.91–7.39) | 48 | 15 | ||
| Recent lung cancer diagnosis | 100 | 7.66 (7.07–8.29) | — | — | ||
| Control (COVID-19, no cancer) | 14,840 | 24 | 5 | |||
| Aschele et al.2 | Italy | All cancer (active anticancer treatment) | 406 | 1.42 (1.29–1.56) | 77 | — |
| Lung cancer (active anticancer treatment) | 91 | — | — | — | ||
| Control (COVID-19, general population) | — | — | — | — | ||
| Grivas et al.42 | 95% in U.S. | All cancer | 4966 | — | 58 | 14 |
| Thoracic | 409 | — | 69 | 26 | ||
| Rivera et al.77 | U.S. | All cancer | 2186 | — | — | 15 |
| Kuderer et al.43 | U.S. | All cancer | 928 | — | 50 | 13 |
| Thoracic | 91 | — | — | — | ||
| Pinato et al.45 | UK, Italy, Spain,and Germany | All cancer | 890 | — | — | 34 |
| Lung | 119 | — | — | — | ||
| Lee et al.44 | UK | All cancer | 800 | — | 88 | 28 |
| Respiratory and intrathoracic organs | 90 | — | — | 36 | ||
| Mehta et al.78 | U.S. | All cancer | 218 | — | — | 28 |
| Lung | 11 | — | — | 55 | ||
| Control (COVID-19, no cancer) | 1090 | — | — | 14 | ||
| Garassino et al.7 | Mostly Italy, Spain, and France | Thoracic cancer | 200 | 76 | 33 | |
| Luo et al.41 | U.S. | Lung cancer | 102 | — | 62 | 25 |
| Tagliamento et al.4 | Global metadata | All cancer | 33,879 | — | — | 25 |
| Lung cancer | 1135 | — | — | 32 | ||
Note: Patients with lung cancer are at higher risk of COVID-19 infection, hospitalization, and mortality.
-
-Wang et al.3: Any death during the study period (August 2019–August 2020), death imported from the Social Security Death Index.
-
-Grivas et al.42: All-cause mortality within 30 days of COVID-19 diagnosis.
-
-Rivera et al.77: 30-Day all-cause mortality.
-
-Kuderer et al.43: All-cause mortality within 30 days of COVID-19 diagnosis.
-
-Pinato et al.45: Patients with SARS-CoV-2 infection and cancer identified February 26 to April 1, 2020, deceased by censoring on May 11, 2020.
-
-Lee et al.44: All-cause mortality during the study period (March 18, 2020–April 26, 2020).
-
-Mehta et al.78: Case fatality rate at the time of analysis.
-
-Garassino et al.7: All-cause mortality; of the 66 patients who died, 52 were due to COVID only, seven due to cancer only, three due to cancer and COVID, one due to complication from cancer therapy, one due to cancer progression and another unstated reason, and two due to unstated reasons.
-
-Luo et al.41: Patients who died during the study period (March 12, 2020–May 6, 2020).
-
-Tagliamento et al.4: Rate of death (i.e., case fatality rate) within the study population.
CI, confidence interval; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UK, United Kingdom; U.S., United States.
In addition to potentially increased susceptibility to SARS-CoV-2 infection, patients with lung cancer seem to have higher mortality rates and more severe outcomes compared with other patients with cancer. A meta-analysis of published studies reported a 32.4% COVID-19 mortality rate, defined as case fatality rate for patients with lung cancer (95% CI: 26.5%–39.6%; n = 1135), compared with a 25.4% (95% CI: 22.9%–28.2%; n = 31,184) mortality rate for the overall cancer cohort.4 A study of the French population found that patients with lung cancer and COVID-19 were at a 3.6-fold higher risk of hospitalization and a 5.7-fold higher risk of death.5 When hospitalized for COVID-19, 62% of patients with lung cancer experienced the most severe form of the disease requiring prolonged intubation and mechanical ventilation.6 Univariate analyses of 200 patients with lung cancer and COVID-19 in the TERAVOLT (Thoracic Cancers International COVID-19 Collaboration) registry observed associations linking increased mortality with age greater than 65 years, chemotherapy treatment, and presence of comorbidities. Nevertheless, in multivariable analyses, only smoking was statistically associated with increased mortality.7 In this international study, most patients succumbed to complications from COVID-19 and not from lung cancer progression.
Collectively, these data suggest that patients with lung cancer are among the most vulnerable populations to COVID-19. There is notable overlap in respiratory symptoms between lung cancer and COVID-19, with theoretically synergistic morbidities. Considering many patients with lung cancer undergo immunosuppressive cancer therapies that may affect the immune response to SARS-CoV-2 infections or vaccines, prioritized protective and therapeutic strategies are strongly warranted.
Biological Context: COVID-19 and Lung Cancer
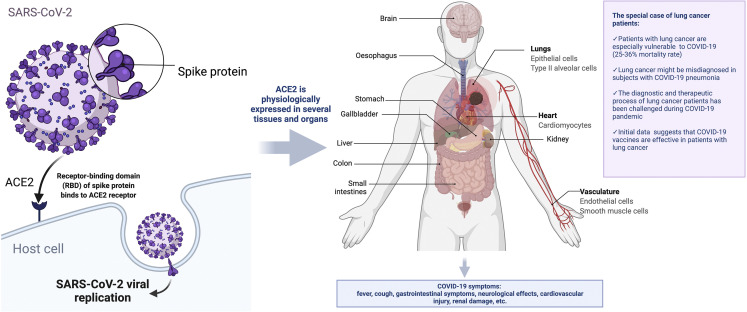
The lungs are the major affected organs of SARS-CoV-2 infection affecting the pulmonary function. Within the lungs, the angiotensin-converting enzyme 2 (ACE2)–expressing (ACE2+) and/or TMPRSS2-expressing (TMPRSS2+) alveolar type II (AT2) cells are the major sites for SARS-CoV-2 infection and replication and contribute to the secretion of cytokines in response to viral infection.8 These eventually lead to one of the most dreaded complications, acute respiratory distress syndrome. In addition, infected lung cells, together with infected cells in the upper respiratory tract, might contribute to the generation of aerosol-containing viruses involved in viral transmission among the population. Investigators exploring the molecular pathogenesis of lung cancer have extensively studied these ACE2+ TMPRSS2+ AT2 cells. ACE2 expression is elevated in tumor and tumor-adjacent normal tissues in patients with cancer, including tumor-adjacent lung tissue in patients with lung cancer, and in lung tissue of individuals who smoke.9 , 10 This might partially explain why patients with lung cancer are potentially at higher risk of severe COVID-19. ACE2 and TMPRSS2 are heterogeneously expressed across different healthy tissues with the highest expression levels in digestive, urinary, and reproductive organs.11 ACE2 expression pattern and level are closely related to the susceptibility and symptoms of COVID-19 (Fig. 1 ).12 Tumor cells are also more susceptible to viral replication owing to defects in innate antiviral immunity associated with transformation.13 It is important to determine whether SARS-CoV-2 replicates in lung cancer cells and whether the potential for replication increases the risk of infection, viral load, ability to mount an antiviral immune response, and/or the development of more severe clinical COVID-19 disease. Likewise, does the histologic type or biological subtypes of lung cancer, other clinical characteristics, or treatments affect on these important issues. Thus, a better understanding of the factors affecting SARS-CoV-2 replication in lung cancer cells is needed to implement strategies to reduce the risk of patients with lung cancer developing severe COVID-19 and to evaluate the efficacy of COVID-19 vaccines in these patients.
Figure 1.
COVID-19 biology and lung cancer (credit: created with BioRender.com). ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Because SARS-CoV-2 infection of the lung results in COVID-19–associated pulmonary changes (as, e.g., lung abnormalities on chest computed tomography scans and changes in pulmonary function), including potential changes in lung epithelial cells, we will also need to study whether there is a correlation between this infection and onset of lung cancer on a population level. There is also a concern that COVID-19–induced inflammation may fuel lung cancer development or its pathophysiology. Patients with severe COVID-19 infections have elevated inflammatory markers (CXCL10, GM-CSF, IL-6), as compared with those presenting moderate disease severity,14 including duration of intubation.15 Tumor-promoting inflammation is one of the hallmarks of cancer16 as inflammation plays a central role in angiogenesis and is critical in metastasis.17, 18, 19 In addition, it has been hypothesized that COVID-19 infection might be associated with respiratory sequelae, including pulmonary fibrosis.20 Idiopathic pulmonary fibrosis is a common comorbidity of lung cancer.21
Clinical Implications of COVID-19 for Patients With Lung Cancer
COVID-19 Implications for Lung Cancer Diagnosis
COVID-19 poses relevant diagnostic challenges in patients with lung cancer. Lung cancer screening declined during the COVID-19 pandemic as a measure to reduce potential virus exposures and enable the reallocation of medical resources.22, 23, 24 A survey of 116 U.S. lung cancer screening programs found that 85% of the programs stopped operating for at least 5 weeks owing to the pandemic.25 Some public health messages (e.g., not seeking medical care for mild respiratory symptoms, a hallmark of initial lung cancer symptoms) exacerbated the problem. Nevertheless, a recent population-based study revealed that lung cancer screening rates in the United States remained stable between 2019 and 2020 and just under 1 in 15 eligible persons were screened. These data reflect the large underutilization of low-dose computed tomography screening before the pandemic, in which only 5% to 6% of adults received screening in 2018.26 A study of U.S. patients found that the weekly number of patients diagnosed with having cancer decreased by 46.4% during the pandemic period (March 1, 2020–April 18, 2020) when compared with the prepandemic period (January 6, 2019–February 29, 2020). A decrease greater than 25% was found in the number of patients diagnosed with having lung cancer, but specific numbers were not disclosed.27 Another study found a sharp decline in screenings for breast (91%) and colorectal (79%) cancers in the United States from March to May of 2020 when compared with 2019, with a near complete recovery by July 2020.28 As a result of such lung cancer-specific screening delays and scheduling restrictions, researchers are projecting a 5% increase in lung cancer deaths in the United Kingdom in the next 5 years.1 These data might be taken with caution, as other additional factors might affect lung cancer deaths, including COVID-19 itself, and future studies need to address this issue. In addition, some centers have found an increase in the proportion of patients diagnosed with having more advanced stages of lung cancer (stages III and IV)29 potentially owing to delays in seeking medical care during the pandemic.30 When screening resumed, another center found a threefold increase in the percent of patients with potentially malignant lung nodules (29% versus 8%, p < 0.01).22 Whether a more rapid progression of lung cancer in patients infected with SARS-CoV-2 may have happened and eventually led to a higher proportion of advanced stages is undetermined but conceivable. A decline in surgical activities was recently reported in a survey of thoracic surgeons in Spain, leading to substantial changes in standard protocols for early stage cancer and in the preoperative workup.31
An additional pandemic-related challenge is concurrent diagnosis of lung cancer and COVID-19. For example, radiographic findings of SARS-CoV-2 infection in lung cancer may mimic lung cancer progression or radiation/drug-induced/immune-related pneumonitis as a complication to anticancer therapies.32 Thus, interpreting lung cancer initial diagnosis or clinical status may in some cases be problematic with the potential of misdiagnosing the radiographic examinations in patients with COVID-19–related pulmonary disease.33 , 34
A significant decline of patient attendance to the hospitals was initially observed, with a slow return to prepandemic numbers only after implementation of infection preventive measures that requested redesign of health care services to accommodate pandemic precautions.35, 36, 37 Nevertheless, inequalities across patient subgroups were reported in terms of returning to expected prepandemic numbers of pathologic cancer diagnoses, as recently reported for lung cancer diagnoses in Northern Ireland.38
Impact of Cancer Therapy on SARS-CoV-2 Infection
In addition to screening delays, patients with lung cancer experienced treatment delays. In a survey of 356 cancer centers across 54 countries, 88% of centers reported challenges in delivering care during the pandemic with 46% of cancer centers reporting greater than 10% of patients missing at least one treatment cycle.39 A study of 165 patients with cancer being treated at a Japanese hospital found that 15 patients (9%) had their treatment delayed owing to COVID-19. Given that Japan was among the best performing countries that are controlling the pandemic, it is conceivable that a greater proportion of global patients with cancer experienced treatment delays.40
Most patients with lung cancer undergo some form of potential immune-modulating therapy (including both immunosuppressive and immunostimulatory interventions), such as immune checkpoint blockade–based immunotherapy, chemotherapy, molecular-targeted therapies, and/or radiotherapy. It is important to understand whether/how the immune response to SARS-CoV-2 is influenced by patients receiving immune checkpoint inhibitors (ICIs) or other immune-modulating treatments, including steroid therapy. A retrospective single-center cohort study did not find any difference in COVID-19 severity in a cohort of patients with lung cancer diagnosed with having COVID-19 between March 12, 2020, and April 13, 2020, and stratified by programmed death-1 (PD-1) blockade exposure.41 These findings were confirmed in the larger registries of TERAVOLT.7
Most studies so far have found no association between cancer therapy and increased mortality among patients with cancer and COVID-19 (Table 2 ). Nevertheless, one study of 4966 patients with cancer, 409 with thoracic cancer, found that recent (past 3 mo) cytotoxic chemotherapy treatments were associated with an increased risk of severe COVID-19 (OR = 1.28, 95% CI: 1.04–1.58) and 30-day mortality (OR = 1.61, 95% CI: 1.15–2.24). The study found no association between noncytotoxic anticancer therapies and COVID-19 outcomes.42 An analysis by the COVID-19 and Cancer Consortium of 928 patients with cancer and COVID-19, 91 with thoracic malignancies and COVID-19, found no association between 30-day all-cause mortality and recent surgery, recent noncytotoxic therapy, or recent cytotoxic therapy.43 A separate analysis of 800 patients with cancer, including 90 with respiratory and intrathoracic cancer, and COVID-19 found no significant effect between recent (i.e., within 4 wk of a positive SARS-CoV-2 test result) treatment of chemotherapy, immunotherapy, hormonal therapy, targeted therapy, or radiotherapy and mortality.44 An additional study of 890 patients with cancer, 119 with lung cancer, and COVID-19 found that receiving active cancer therapies was associated with a lower risk of death for patients with cancer and COVID-19 (multivariable OR = 0.68, 95% CI: 0.48–1.00, p = 0.03).45 The TERAVOLT study found no statistically significant association between steroid treatment (>10 mg of prednisone) and COVID-19 outcomes among 42 patients with lung cancer and COVID-19 treated with steroids.7 A study of 69 patients with lung cancer and COVID-19, 41 with previous PD-1 blockage treatment, found no association between PD-1 blockage treatment and COVID-19 severity once adjusting for smoking history for 5 or more pack years.6 These data are nearly all from unvaccinated patients. Given increasing vaccination rates among patients with lung cancer, an important question today is on how vaccinated patients with lung cancer will be affected by treatment approaches (see the vaccine section).
Table 2.
Impact of Anticancer Treatments on COVID-19 Severity
| Summary of Select Studies Exploring the Impact of Cancer Treatments on COVID-19 Severity | ||||
|---|---|---|---|---|
| Study | Country/Countries | Cancer Types | Number of Patients With COVID-19 | Key Insights |
| Grivas et al.42 | 95% in U.S. | All cancer | 4966 | Recent (past 3 mo) cytotoxic chemotherapy associated with severe COVID-19 (OR = 1.28) and 30-d mortality (OR = 1.61). Noncytotoxic anticancer therapies, including immunotherapy, targeted therapy, and endocrine therapy not associated with severe COVID-19 nor 30-d mortality |
| Thoracic | 409 | |||
| Kuderer et al.47 | U.S. | All cancer | 928 | No association between 30-d all-cause mortality and recent surgery, recent noncytotoxic therapy, or recent cytotoxic systemic therapy |
| Thoracic | 91 | |||
| Pinato et al.45 | UK, Italy, Spain, and Germany | All cancer | 890 | No association between cytotoxic chemotherapy, targeted therapy, or immunotherapy and COVID-19 severity |
| Lee et al.44 | UK | All cancer | 800 | No significant mortality effect for recent (past 4 wk) chemotherapy, immunotherapy, hormonal therapy, targeted therapy, or radiotherapy |
| Respiratory and intrathoracic organs | 90 | |||
| Mehta et al.78 | U.S. | All cancer | 218 | Neither chemotherapy nor radiotherapy associated with |
| Lung | 11 | increased case fatality rate | ||
| Garassino et al.7 | Mostly Italy, Spain, and France | Thoracic cancer | 200 | In multivariable analysis, TKIs, chemotherapy, and immunotherapy, not associated with increased mortality |
| Luo et al.41 | U.S. | Lung cancer | 102 | No observed impact of TKIs or chemotherapy and COVID-19 severity |
| Luo et al.6 | U.S. | Lung cancer | 69 | No significant association between PD-1 blockade and COVID-19 severity |
Note: Active cancer treatments do not worsen COVID-19 outcomes in patients with cancer and COVID-19.
COVID-19, coronavirus disease 2019; PD-1, programmed cell death protein-1; TKI, tyrosine kinase inhibitor; U.S., United States; UK, United Kingdom.
COVID-19 Vaccine Safety and Efficacy in Patients With Lung Cancer
Global SARS-CoV-2 vaccinations are rapidly rolling out. Despite the significant burden on patients with lung cancer, the initial COVID-19 vaccine trials had few, if any, patient with lung cancer enrolled. Only 3.7% of patients enrolled in the Pfizer and BioNTech vaccine trial (BNT162b2) (NCT04368728)46 had cancer listed as a comorbidity.47 Moderna’s vaccine (mRNA-1273) trial (NCT04470427) did not list the number of enrolled patients with cancer. Moderna also excluded study participants who received “immunosuppressive” medication for greater than 14 days in the past 6 months.48 Recent studies found the COVID-19 vaccine to be safe and effective on the basis of more than 900 patients with cancer, including 381 patients with thoracic/head and neck cancer.5 , 49, 50, 51, 52, 53 An additional study explored the short-term safety profile of the BNT162b2 vaccine in 134 patients with cancer under immune checkpoint blockage.54 Early on when vaccines became available, the COVID-Lung Cancer Consortium recommended special vaccination programs for patients with lung cancer.55
Initial data suggest that COVID-19 vaccines are safe in patients with lung cancer.5 , 49, 50, 51, 52, 53, 54 The safety profile of patients with cancer, including those undergoing ICI treatment, is similar to healthy controls. In addition, there was no correlation between previous immune-related adverse events and systemic side effects.54 The most common side effects were mild (e.g., pain at the site of injection). These results suggest that administration of SARS-CoV-2 mRNA vaccines is safe in patients with cancer, including those treated with immunotherapy, similarly to other common vaccines, such as seasonal influenza.56
Initial data suggest that SARS-CoV-2 vaccines are effective in patients with lung cancer (Table 3 ). A prospective study from Israel evaluated 102 patients with solid tumors undergoing active intravenous anticancer treatment and 78 controls who received the second dose of the BNT162b2 vaccine at least 12 days before enrollment. The study revealed that mRNA SARS-CoV-2 vaccines are effective in patients with cancer, after the second dose, but their effectiveness may be reduced when compared with healthy individuals (seroconversion 90% versus 100%). Patients with lung cancer (n = 24) had a 92% seroconversion rate.50 A follow-up analysis found that 87% of patients with cancer were seropositive at a median of 123 days (∼5 mo) after the second dose (versus 100% for health individuals).57 Another study of 154 patients with cancer, 36 with lung cancer, found that 6 months after vaccination, 79% of patients with cancer were seropositive versus 84% of age-matched controls without cancer.58 Chemotherapy treatment was associated with lower rates of seropositivity when compared with biological and immunotherapy treatments (73% versus 90%, p = 0.02).58 The ideal timing for vaccination is still to be defined, although a recent study revealed that most of the initial nonresponders had blood collected for immune analysis 7 to 14 days after their most recent treatment with cytotoxic agents, when a nadir in blood counts and the peak of myelosuppression from traditional chemotherapy agents is observed.59 In a larger study, including 200 patients with cancer who had received full dosing of a Food and Drug Administration–approved SARS-CoV-2 vaccine (mRNA-1273, BNT162b2, or Ad26.COV2.S), patients with solid tumors (including 13% with thoracic or head neck cancers) had a high seroconversion rate (98%) with those receiving ICIs (97%) or hormonal therapies (100%) revealing the highest seroconversion postvaccination. Patients with previous SARS-CoV-2 infection had higher antispike immunoglobulin G titers postvaccination. Highest immunoglobulin G titers were found with the mRNA-1273 vaccine (median of 11,963 arbitrary unit [AU]/mL) followed by the BNT162b2 (5173 AU/mL) vaccine and the single-dose Ad26.COV2.S vaccine (1121 AU/mL).51
Table 3.
Summary of COVID-19 Vaccine Effectiveness in Fully Vaccinated Patients With Cancer
| Summary of COVID-19 Vaccine Effectiveness in Fully Vaccinated Patients With Cancer | |||||||
|---|---|---|---|---|---|---|---|
| Study | Country/Countries | Cancer Types | # of Patients or Control | Vaccine | # of Patients or Control | % Seroconversion | Median Titer Level (AU/mL or U/mL) |
| Gounant et al.61 | France | Thoracic | 269 | Mostly BNT162b2 | 269 | 94 | 4725 |
| Control | 13 | BNT162b2 | 13 | — | 10,594 | ||
| Goshen-Lago et al.49 | Israel | Various | 218 | BNT162b2 | 218 | 86 | — |
| Lung cancer | 43 | BNT162b2 | 43 | 86 | — | ||
| Massarweh et al.50 | Israel | Solid cancers | 102 | BNT162b2 | 102 | 90 | 1931 |
| Lung cancer | 26 | BNT162b2 | 26 | 92 | 1334 | ||
| Control | 78 | BNT162b2 | 78 | 100 | 7160 | ||
| Thakkar et al.51 | U.S. | Various | 200 | BNT162b2 | 115 | 95 | 5173 |
| Thoracic/head and neck | 25 | mRNA-1273 | 62 | 94 | 11,963 | ||
| Ad26.COV2.S | 20 | 85 | 1121 | ||||
| Control | 26 | — | — | — | >15,000 | ||
| Addeo et al.52 | Switzerland, U.S. | Various | 131 | BNT162b2 | 30 | 93 | 1232 |
| Thoracic malignancy | 18 | mRNA-1273 | 93 | 95 | 2500 | ||
| Barriere et al.5 | France | Solid cancers | 42 | BNT162b2 | 42 | 95 | 245 |
| Control | — | BNT162b2 | — | 100 | 2517 | ||
| Monin et al.53 | United Kingdom | Various | 24 | BNT162b2 | 24 | 79 | — |
| Control | 12 | BNT162b2 | 12 | 100 | — | ||
Note: Two-dose mRNA vaccines are highly effective (∼90%) in patients with cancer, though patients with cancer have lower titers than health controls.
#, number; AU, arbitrary unit; COVID-19, coronavirus disease 2019; U.S., United States.
Vaccines could potentially be less effective in patients with lung cancer than in healthy controls. In the Israeli study, patients with lung cancer (n = 24) had lower antibody levels (1334 AU/mL) compared with healthy individuals (7160 AU/mL) and the overall cancer cohort (1931 AU/mL).50 Furthermore, researchers from England found poor vaccine efficacy among patients with cancer after one dose of BNT162b2 (38% seropositivity in patients with solid tumors versus 94% for the healthy controls).53 Similarly, a prospective study evaluating the seroconversion rates and anti–SARS-CoV-2 spike protein antibody titers after the first and second doses of BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in 131 patients with cancer in the United States and Europe revealed that the seroconversion rates and antibody titers were significantly lower after the first vaccine dose compared with those after the second dose in all subgroups, confirming the poor antibody response after the first dose in patients with cancer. Nevertheless, excellent antibody response at 3 weeks after the second doses with mRNA SARS-CoV-2 vaccines was observed (94% seroconversion).52 A pronounced lag in antibody production compared with the rate in noncancer controls was also reported in a recent Israeli study (29% seroconversion after the first dose of BNT162b2 vaccine compared with 84% of the controls, but the seropositive rate reached 86% in the patients after the second dose). This study enrolled 45 patients with lung cancer (19%), 43 of whom received a second dose.49 Similarly, the prospective observational VOICE study has recently evaluated the impact of immunotherapy, chemotherapy, and chemoimmunotherapy on immunogenicity and safety of COVID-19 vaccination in patients treated for a solid tumor, including a significant proportion of patients with lung cancer. The study confirmed that mRNA-1273 vaccine is safe in patients with cancer undergoing active treatments with a high seroconversion rate, not inferior to controls. Nevertheless, a significant minority of the patients does not develop an adequate antibody response (6.9% in the immunotherapy cohort, 16.2% in the chemotherapy cohort, and 11.2% in the chemoimmunotherapy cohort).60 Recently, French investigators reported the results on 306 patients with thoracic cancer from the prospective observational COVIDVAC-OH study investigating SARS-CoV-2 vaccination’s (mainly mRNA-based vaccines) effectiveness (NCT04776005).61 They observed only seven mild COVID-19 cases among 306 vaccinated patients (2.3%), supporting the efficacy of mRNA COVID-19 vaccines in patients with thoracic cancer. Of 269 serologic results available beyond day 14 post-second vaccine dose, 6.3% were still negative (<50 AU/mL), whereas 11% were less than 300 AU/mL (12.5th percentile). Lack of immunization was associated in a multivariate analysis with age and long-term corticosteroid treatment. Interestingly, 30 patients with persistent low antibody titers received a third vaccine dose, resulting in an 88% immunization rate.61 These results support the use of a third dose for patients with lack of immunization after complete two vaccine doses and could contribute to appropriate seroprotection in patients still poorly immunized post-two vaccines.
As novel SARS-CoV-2 virus variants are increasingly reported, the efficacy of vaccines in preventing SARS-CoV-2 infections and COVID-19 is a major source of concerns, especially for the highly transmissible B.1.617.2 (delta) variant. For instance, British researchers found that BNT162b2 (BioNTech) and ChAdOx1 nCoV-19 (AstraZeneca) vaccines are effective against the delta variant, though their effectiveness is reduced. Effectiveness against symptomatic disease after one dose of vaccine was notably lower among persons with the delta variant (30.7%, 95% CI: 25.2–35.7) than among those with the alpha variant (48.7%, 95% CI: 45.5–51.7), with similar results for both vaccines. The second dose of BNT162b2 vaccine was found to be 88% effective against the delta variant (95% CI: 85.3–90.1) versus 93.7% effective against the alpha variant (95% CI: 91.6–95.3). The second dose of ChAdOx1 nCoV-19 vaccine was 67% effective against the delta variant (95% CI: 61.3%–71.8%) versus 74.5% against the alpha variant (95% CI: 68.4%–79.4%).62 Similarly, preliminary data reported from the Israel’s Ministry of Health reveal that the delta variant could negatively affect the capacity of the BioNTech vaccine to reduce infection transmission.63 Data from Canadian researchers on the effectiveness of BNT162b2, mRNA-1273, and ChAdOx1 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes (COVID-19 hospitalization or death) caused by the alpha (B.1.1.7), beta (B.1.351), gamma (P.1), and delta (B.1.617.2) variants of concern (VOCs) were recently reported. The results of this study suggest that even a single dose of these three vaccines provides good to excellent protection against symptomatic infection and severe outcomes caused by the four currently circulating VOCs and that two doses are likely to provide even higher protection.64
Another observational study conducted in Scotland confirmed that ChAdOx1 and BNT162b2 vaccines are effective in reducing the risk of SARS-CoV-2 infection and COVID-19 hospitalization in people with the delta VOC, but these effects on infection seemed to be diminished when compared with those with the alpha VOC.65 Further data, especially in patients with cancer, are urgently needed.
Importantly, vaccine efficacy has been linked to antispike antibody titers both from a global perspective across phase 3 efficacy trials66, 67, 68 and recently from an individual level.69 , 70 The establishment of a threshold antibody titer needed for protection will help to guide patient management because serology tests are widely available and patients with low antibody responses could then receive a booster dose. An absolute correlate of protection will also in general inform policy-making regarding potential booster doses in nonresponders or individuals with suboptimal responses as found in some patients with lung cancer. As a first step to improve immunity in those who do not respond well, the U.S. Centers for Disease Control and Prevention has recently recommended booster doses (a third mRNA vaccine dose) for immunocompromised and immunosuppressed individuals.
Nevertheless, although most of the above-mentioned studies do not specifically apply to lung cancer population(s), many questions have to be answered for this particular patient group. At Mt. Sinai in New York, a Center of Excellence for COVID-19 serologic studies was established through an NCI/SeroNet U-54 grant and in collaboration with UT Southwestern and University of Colorado. The center is conducting a large, prospective, match-controlled study of patients with lung cancer evaluating their antibody and cellular immune responses after SARS-CoV-2 vaccination. Initial findings confirm that most patients with lung cancer mount a good response to vaccination.71 Nevertheless, a subgroup of patients did not mount “adequate” antibody response. The goals of the study are to evaluate the magnitude, quality, and duration of neutralizing antibody response after SARS-CoV-2 infection or vaccination and correlate to cellular immunity in a long-term follow-up program.
COVID-19 Impact on Lung Cancer Research
Lung cancer clinical research was severely affected by the COVID-19 pandemic. Overall clinical trial accrual in May 2020 was 74% compared with the year before.72 Between March and May 2020, the number of patients enrolled in trials for the Alliance for Clinical Trials in Oncology declined by more than 50% and only returned to baseline levels in the fall of 2020.25 A March 2020 survey by the American Society of Clinical Oncology of 32 clinical sites found that 60% of sites halted screening/enrollment for some clinical trials and ceased research only visits.72 The American SWOG cancer research group found an inverse relationship between local prevalence of COVID-19 and clinical trial enrollment.73 Although no specific data are reported for lung cancer clinical trials, the International Association for the Study of Lung Cancer is conducting a global survey study on COVID-19 and impact on clinical trials.74 Results will be presented at the Presidential Symposium at World Conference on Lung Cancer 21.
Future Perspectives
Although at higher risk of development of severe COVID-19 disease, patients with lung cancer should continue their course of cancer treatments. Further lung cancer-specific investigations are warranted to evaluate the impact of specific cancer treatments and impaired COVID-19 outcomes. As part of this, there needs to be increased vigilance in minimizing the risk of viral spread in the lung cancer health care delivery arena by vaccinating medical staff, using masks and social distancing, installing appropriate clinic ventilation, and improving patient access to high-quality cancer care by means of telemedicine.75
Most importantly, given their vulnerability to severe COVID-19, all people living with lung cancer should get vaccinated in countries where vaccines are readily available and vaccine use in patients with cancer should be prioritized in countries where vaccines are scarce. Although the duration of COVID-19 vaccine protection among patients with cancer generally, and lung cancer in particular, is unknown, the vaccines are effective and safe.5 , 49, 50, 51, 52 , 53 , 61 In addition, further investigation is needed to determine which vaccines are most effective in patients with lung cancer. Initial data suggest that the single-dose Ad26.COV2.S vaccine generated lower antibody titers and lower seroconversion in patients with cancer in general when compared with the two-dose mRNA vaccines. Finally, patients with solid tumors receiving anticancer treatment within one year of their first mRNA vaccine dose should get a third booster vaccination where available.76
Several clinically significant questions on COVID-19 and lung cancer remain unanswered (Table 4 ), as follows: (1) Do patients with lung cancer develop the same immune response as “healthy” individuals and does this vary by vaccine type? (2) What is the duration of vaccine protection in patients with lung cancer? (3) Do specific cancer therapies influence the response to SARS-CoV-2 vaccinations? (4) If patients with lung cancer have a lower immunologic response, should a special vaccination program be developed? What would the program look like (e.g., extra “boost” of previous given vaccination? different vaccination platform? routine serologic monitoring?) (5) Will patients with low or no antibody response to SARS-CoV-2 vaccination be particularly vulnerable to new variants? (6) What is required to assure clinical protection against COVID-19 and which immunologic components beyond neutralizing antibody titers need to also be studied, including cellular immunity and particularly T-cell functions? and (7) What are the causes and frequency of “breakthrough” infections in vaccinated patients with lung cancer?
Table 4.
Key Open Questions on COVID-19 and Lung Cancer
|
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
As patients with lung cancer are highly vulnerable to COVID-19 and many lung cancer therapies (e.g., immune checkpoint blockade) might limit the generation of an immune response to the SARS-CoV-2 vaccine, lung cancer-specific clinical and basic-translational studies are needed. An additional question is what did we learn from the pandemic in terms of modifications/mitigation measures for clinical care and clinical trials, which can be applied to future practice? As it seems that we will be dealing with COVID-19 (and a variety of viral VOCs) for the foreseeable future, both continued collection of retrospective data, and planning for prospective studies of mitigation measures taken during COVID 19 pandemic, and clinical pathologic/molecular correlative studies for SARS-CoV-2 infection and development of COVID-19 in patients with lung cancer seem essential to help fill in our current knowledge gaps.
Conclusions
The COVID-19 pandemic reveals that patients with lung cancer are more susceptible and more likely to develop more severe COVID-19 disease after SARS-CoV-2 infection. The clinical impact of newly evolved virus variants is not yet completely known. The biology of lung cancer, hyperinflammatory milieu, and the uniqueness of lung cancer treatments make it important to perform studies specific to lung cancer. Although it is encouraging that the initial available data suggest that COVID-19 vaccines seem to be protective in most patients with lung cancer, further research is needed to understand the mechanisms underlying SARS-CoV-2 infection, COVID-19 vaccination, and immunity in patients with lung cancer to better protect and care for this vulnerable population. Furthermore, although new virus variants are emerging, there will be an ongoing need to continue such studies to understand factors that contribute to clinical protection of patients with lung cancer. Are some subgroups more vulnerable than others and what is the impact of antineoplastic therapies? Further studies on vaccination in patients with lung cancer are needed to determine the role of booster vaccination, and to determine the optimal vaccination programs. The duration of immunologic protection is another key factor that will affect on long-term care of patients with lung cancer. To evaluate antiviral immune protection, it is likely that routine serologic monitoring for antiviral antibody and potential antiviral T-cell activity will be required. Likewise, detailed studies on the relationship between vaccination and the efficacy/toxicity of individual cancer therapies are paramount. Finally, as we consider the critical role that immunogenicity plays in lung cancer pathogenesis and treatment, it is highly likely that fundamental questions surrounding the intersection of COVID-19 and cancer will lead to discoveries and breakthroughs that will positively affect future lung cancer treatment an-=d prevention strategies.
CRediT Authorship Contribution Statement
Christian Rolfo, Fred R. Hirsch, Paul A. Bunn Jr., John D. Minna: Conceptualization.
Christian Rolfo, Noy Meshulami, Alessandro Russo, Amin Benyounes, Rafael Sirera, Fred R. Hirsch: Writing—original draft.
Christian Rolfo, Noy Meshulami, Alessandro Russo, Florian Krammer, Adolfo García-Sastre, Philip C. Mack, Jorge E. Gomez, Nina Bhardwaj, Amin Benyounes, Rafael Sirera, Amy Moore, Nicholas Rohs, Claudia I. Henschke, David Yankelevitz, Jennifer King, Yu Shyr, Paul A. Bunn Jr., John D. Minna, Fred R. Hirsch: Writing—review and editing.
Christian Rolfo, Fred R. Hirsch: Supervision, Project administration.
Acknowledgments
Work in the Krammer laboratory on severe acute respiratory syndrome coronavirus 2. is supported by the National Institute of Allergy and Infectious Diseases Collaborative Influenza Vaccine Innovation Centers contract 75N93019C00051, the Centers of Excellence for Influenza Research and Surveillance (contract #HHSN272201400008C), the JPB Foundation, the Open Philanthropy Project (research grant 2020-215611 [5384]), and anonymous donors. In addition, serology efforts in the Krammer laboratory are supported by the National Cancer Institute SeroNet grant U54CA260560 and by the SeroNet in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract number 75N91019D00024, task order number 75N91021F00001. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosure: Dr. Rolfo reports receiving funding from the Lung Cancer Research Foundation—Pfizer Grant 2019; personal fees for attending advisory board meetings from ArcherDx, Bristol-Myers Squibb, Boston Pharmaceuticals, Inivata, MD Serono, and Novartis; fees for speakers bureau from AstraZeneca, Merck Sharp & Dohme, and Roche; and nonfinancial support from Guardant Health through a research collaboration. Dr. Russo reports receiving personal fees for attending advisory board meetings from AstraZeneca, Merck Sharp & Dohme, and Novartis. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serologic assays and NDV-based SARS-CoV-2 vaccines which list Dr. Krammer as coinventor. Mount Sinai has spun out a company, Kantaro, to market serologic tests for SARS-CoV-2. Dr. Krammer has consulted for Merck and Pfizer (before 2020) and is currently consulting for Pfizer, Third Rock Ventures, Seqirus, and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. Dr. García-Sastre reports receiving funding from the National Institutes of Health, National Cancer Institute (NCI) U54CA260560 and National Institutes of Health, National Institute of Allergy and Infectious Diseases 75N93019R00028; having royalties or licenses from Avimex and Medimmune; receiving consulting fees from 7Hills Pharma, Avimex, Esperovax, Farmak, Applied Biological Laboratories, Pharmamar, and Pfizer; having speakers bureau for Sequirus; having patents planned, issued, or pending for use of NDV as vaccine vector for coronavirus disease 2019; participating at the advisory board for coronavirus disease 2019 vaccines in the New York State; and having stock options in Vivaldi Biosciences, Contrafect, and Pagoda. Dr. Mack reports receiving funding from NCI U54CA260560 grant and speakers bureau from Guardant Health and Amgen. Dr. Gomez reports receiving funding from NCI U54CA260560 grant and personal fees for attending advisory board meetings from Bristol-Myers Squibb. Dr. Bhardwaj is an extramural member of the Parker Institute for Cancer Immunotherapy; receives research funds from Regeneron, Harbor Biomedical, and Dragonfly Therapeutics; and is on the advisory boards of Neon Therapeutics, Novartis, Avidea, Boehringer Ingelheim, Rome Therapeutics, Roswell Park Comprehensive Cancer Center, BreakBio, Carisma Therapeutics, Rubio, CureVac, Genotwin, BioNTech, Gilead and Tempest Therapeutics, and the Cancer Research Institute. Dr. Sirera reports receiving support from Merck Sharp & Dohme for attending meetings, having honoraria, and conducting lectures. Dr. Moore reports receiving unpaid participation in the NTRKers Board of Directors. Dr. Rohs reports receiving institutional grant support from U54 Grant; receiving personal consulting fees from AstraZeneca, Genentech, and BeiGene; having speakers bureau from PER/OncLive; participating on the Mount Sinai Data Safety and Monitoring Committee; and being the founder of the New York Lung Cancer Foundation. Dr. Henschke is a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on computed tomography scans of the chest which are owned by the Cornell Research Foundation (CRF). Since 2009, Dr. Henschke does not accept any financial benefit from these patents, including royalties, and any other proceeds related to the patents or patent applications owned by CRF. Dr. Henschke is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation and receives no compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. Recipients include I-ELCAP, among others. The funding comes from a variety of sources, including philanthropic donations, grants, and contracts with agencies (federal and nonfederal), imaging, and pharmaceutical companies relating to image processing assessments. The various sources of funding exclude any funding from tobacco companies or tobacco-related sources. Dr. Yankelevitz reports receiving consulting fees from AstraZeneca, Pfizer, and Genentech; being a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest, including measurement of nodules, in which some of these, which are owned by CRF, are nonexclusively licensed to General Electric; serving on the medical advisory board of Carestream; and being an equity owner in Accumetra, a privately held technology company committed to improving the science and practice of image-based decision-making. Dr. King reports receiving funding support from NCI Seronet U54 Funding to Mount Sinai School of Medicine (subcontract to GO2 Foundation for Lung Cancer); receiving grants from Bristol-Myers Squibb and Genentech for scientific research projects funding paid to GO2 Foundation for Lung Cancer; having speakers bureau (paid to GO2 Foundation for Lung Cancer) from AstraZeneca, Foundation Medicine, Merck, and Thermo Fisher Scientific; and participating on a data safety monitoring board or advisory board (paid to GO2 Foundation for Lung Cancer) from Boehringer Ingelheim and Guardant. Dr. Shyr reports receiving funding support from the National Institutes of Health (P30CA068485; U24CA163056; U24CA213274; P50CA236733; P50CA098131; U54CA163072); receiving grants or contracts from the National Institutes of Health (P30CA068485; U24CA163056; U24CA213274; P50CA236733; P50CA098131; U54CA163072); having speakers bureau from Roche, AstraZeneca, and Eisai; and participating on a data safety monitoring board or advisory board from Novartis, Pfizer, Janssen (Johnson & Johnson), AstraZeneca, and Roche. Dr. Bunn reports receiving consulting fees from Bristol-Myers Squibb, Ascentage, Merck, CStone, AstraZeneca, Eli Lilly, Ipsen, and Verastem; participating on a data safety monitoring board or advisory board from Merck and Bristol-Myers Squibb; and having leadership role in Verastem. Dr. Minna reports receiving funding support from the National Cancer Institute. Dr. Hirsch reports receiving grant support from NCI U54CA260560; participating in scientific advisory boards for Amgen, AstraZeneca, Bristol-Myers Squibb, Daiichi, Genentech/Roche, Merck, Novartis, OncoCyte, Pfizer, Regeneron, and Sanofi; receiving payment for expert testimony from GLG; and being an investigator in a University of Colorado–owned patent: “EGFR protein expression and EGFR high copy number as predictive biomarker for EGFR directed therapy.” The remaining authors declare no conflict of interest.
References
- 1.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschele C., Negru M.E., Pastorino A., et al. Incidence of SARS-CoV-2 infection among patients undergoing active antitumor treatment in Italy. JAMA Oncol. 2021;7:304–306. doi: 10.1001/jamaoncol.2020.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagliamento M., Agostinetto E., Bruzzone M., et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103365. doi: 10.1016/j.critrevonc.2021.103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrière J., Re D., Peyrade F., Carles M. Current perspectives for SARS-CoV-2 vaccination efficacy improvement in patients with active treatment against cancer. Eur J Cancer. 2021;154:66–72. doi: 10.1016/j.ejca.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J., Rizvi H., Preeshagul I.R., et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues R., Costa de Oliveira S. The impact of angiotensin-converting enzyme 2 (ACE2) expression levels in patients with comorbidities on COVID-19 severity: a comprehensive review. Microorganisms. 2021;9:1692. doi: 10.3390/microorganisms9081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkler T., Ben-David U. Elevated expression of ACE2 in tumor-adjacent normal tissues of cancer patients. Int J Cancer. 2020;147:3264–3266. doi: 10.1002/ijc.33145. [DOI] [PubMed] [Google Scholar]
- 10.Liu A., Zhang X., Li R., et al. Overexpression of the SARS-CoV-2 receptor ACE2 is induced by cigarette smoke in bronchial and alveolar epithelia. J Pathol. 2021;253:17–30. doi: 10.1002/path.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbarayan K., Ulagappan K., Wickenhauser C., Seliger B. Expression and clinical significance of SARS-CoV-2 human targets in neoplastic and non-neoplastic lung tissues. Curr Cancer Drug Targets. 2021;21:428–442. doi: 10.2174/1568009620666201207145019. [DOI] [PubMed] [Google Scholar]
- 12.Jia W., Wang J., Sun B., Zhou J., Shi Y., Zhou Z. The mechanisms and animal models of SARS-CoV-2 infection. Front Cell Dev Biol. 2021;9:578825. doi: 10.3389/fcell.2021.578825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howells A., Marelli G., Lemoine N.R., Wang Y. Oncolytic viruses—interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol. 2017;7:195. doi: 10.3389/fonc.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thwaites R.S., Sanchez Sevilla Uruchurtu A., Siggins M.K., et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blot M., Bour J.B., Quenot J.P., et al. The dysregulated innate immune response in severe COVID-19 pneumonia that could drive poorer outcome. J Transl Med. 2020;18:457. doi: 10.1186/s12967-020-02646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polyak K., Weinberg R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 20.Tanni S.E., Fabro A.T., de Albuquerque A., et al. Pulmonary fibrosis secondary to COVID-19: a narrative review. Expert Rev Respir Med. 2021;15:791–803. doi: 10.1080/17476348.2021.1916472. [DOI] [PubMed] [Google Scholar]
- 21.Yoo H., Jeong B.H., Chung M.J., Lee K.S., Kwon O.J., Chung M.P. Risk factors and clinical characteristics of lung cancer in idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med. 2019;19:149. doi: 10.1186/s12890-019-0905-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Haren R.M., Delman A.M., Turner K.M., et al. Impact of the COVID-19 pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232:600–605. doi: 10.1016/j.jamcollsurg.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzone P.J., Gould M.K., Arenberg D.A., et al. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST expert panel report. Radiol Imaging Cancer. 2020;2 doi: 10.1148/rycan.2020204013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boughey J.C., Snyder R.A., Kantor O., et al. Impact of the COVID-19 pandemic on cancer clinical trials. Ann Surg Oncol. 2021;28:7311–7316. doi: 10.1245/s10434-021-10406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King J., Criswell A., Mohon R., Fine L. FP13.03 the impact of the COVID-19 pandemic on lung cancer screening programs in the United States. J Thorac Oncol. 2021;16(suppl) S969–S969. [Google Scholar]
- 26.Fedewa SA, Bandi P, Smith RA, Silvestri GA, Jemal A. Lung cancer screening rates during the COVID-19 pandemic [e-pub ahead of print]. Chest. https://doi.org/10.1016/j.chest.2021.07.030, accessed October 19, 2021. [DOI] [PMC free article] [PubMed]
- 27.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R.C., Haynes K., Du S., Barron J., Katz A.J. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7:878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.Y., Lee Y.J., Kim T., et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer. 2020;20:1040. doi: 10.1186/s12885-020-07544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantini L., Mentrasti G., La Verde N., et al. 35P Lung cancer diagnosis and continuum of care: how did the COVID-19 outbreak impact? Data from an Italian multicenter study. J Thorac Oncol. 2021;16(suppl 4):S713. [Google Scholar]
- 31.Martínez-Hernández N.J., Caballero Silva U., Cabañero Sánchez A., et al. Effect of COVID-19 on thoracic oncology surgery in Spain: a Spanish Thoracic Surgery Society (SECT) survey. Cancers (Basel) 2021;13:2897. doi: 10.3390/cancers13122897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorospe L., Ayala-Carbonero A.M., Paredes-Rodríguez P., et al. Challenges in management of patients with lung cancer in times of COVID-19: an imaging perspective. Clin Lung Cancer. 2020;21:568–570. doi: 10.1016/j.cllc.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y.J., Yang W.J., Liu D., et al. COVID-19 and early-stage lung cancer both featuring ground-glass opacities: a propensity score-matched study. Transl Lung Cancer Res. 2020;9:1516–1527. doi: 10.21037/tlcr-20-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J., Zhang Y., Gao X.H., Xi E.P. Coronavirus disease 2019 or lung cancer: a differential diagnostic experience and management model from Wuhan. J Thorac Oncol. 2020;15:e141–e142. doi: 10.1016/j.jtho.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter M.A., Murphy J., Cameron D., et al. The impact of COVID-19 on systemic anticancer treatment delivery in Scotland. Br J Cancer. 2021;124:1353–1356. doi: 10.1038/s41416-021-01262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark J.J., Dwyer D., Pinwill N., Clark P., Johnson P., Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22:66–73. doi: 10.1016/S1470-2045(20)30619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iadevaia C., Perrotta F., Mazzeo G., et al. Incidental diagnosis of lung adenocarcinoma following coronavirus OC 43 severe pneumonia. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1313. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton A.C., Donnelly D.W., Loughrey M.B., et al. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID-19 pandemic: a population-based study. Br J Cancer. 2021;125:798–805. doi: 10.1038/s41416-021-01472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jazieh A.R., Akbulut H., Curigliano G., et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020;6:1428–1438. doi: 10.1200/GO.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita K., Ito T., Saito Z., Kanai O., Nakatani K., Mio T. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983–2986. doi: 10.1111/1759-7714.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinato D.J., Zambelli A., Aguilar-Company J., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuderer N.M., Hill J.A., Carpenter P.A., Lyman G.H. Challenges and opportunities for COVID-19 vaccines in patients with cancer. Cancer Invest. 2021;39:205–213. doi: 10.1080/07357907.2021.1885596. [DOI] [PubMed] [Google Scholar]
- 48.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goshen-Lago T., Waldhorn I., Holland R., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination amongst patients with cancer. Cancer Cell. 2021;39:1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Addeo A., Shah P.K., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098.e2. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The Cancer Letter Cancer groups urge CDC to prioritize cancer patients for COVID-19 vaccination. https://cancerletter.com/covid-19-cancer/20210108_2/ Accessed October 19, 2021.
- 56.Rossi G., Pezzuto A., Sini C., et al. Concomitant medications during immune checkpoint blockage in cancer patients: novel insights in this emerging clinical scenario. Crit Rev Oncol Hematol. 2019;142:26–34. doi: 10.1016/j.critrevonc.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Eliakim-Raz N., Massarweh A., Stemmer A., Stemmer S.M. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 2021;7:1716–1718. doi: 10.1001/jamaoncol.2021.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldhorn I., Holland R., Goshen-Lago T., et al. Six month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov. 2021;11:2430–2435. doi: 10.1158/2159-8290.CD-21-1072. [DOI] [PubMed] [Google Scholar]
- 59.Shroff R.T., Chalasani P., Wei R., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oosting S., Van der Veldt A., GeurtsvanKessel C., et al. LBA8 Vaccination against SARS-CoV-2 in patients receiving chemotherapy, immunotherapy, or chemo-immunotherapy for solid tumors. Ann Oncol. 2021;32(suppl):S1337. [Google Scholar]
- 61.Gounant V., Ferré V.M., Soussi G., et al. Efficacy of SARS-CoV-2 vaccine in thoracic cancer patients: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. medRxiv. J Thorac Oncol. 2022;17:239–251. doi: 10.1016/j.jtho.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nature COVID vaccines slash viral spread—but delta is an unknown. https://www.nature.com/articles/d41586-021-02054-z#ref-CR1 Accessed October 19, 2021. [DOI] [PubMed]
- 64.Nasreen S., He S., Chung H., et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv. https://www.medrxiv.org/content/10.1101/2021.06.28.21259420v3 Accessed October 19, 2021.
- 65.Sheikh A., McMenamin J., Taylor B., Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 67.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 68.Earle K.A., Ambrosino D.M., Fiore-Gartland A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gilbert P.B., Montefiori D.C., McDermott A., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. https://www.medrxiv.org/content/10.1101/2021.08.09.21261290v4 Accessed October 19, 2021. [DOI] [PMC free article] [PubMed]
- 70.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez D.R., et al. OA01.01—analysis of lung cancer patients receiving SARS-CoV-2 vaccines revealed a minority subset with poor antibody responses relative to controls. J Thorac Oncol. 2021;16(suppl):S848. [Google Scholar]
- 72.Waterhouse D.M., Harvey R.D., Hurley P., et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology survey. JCO Oncol Pract. 2020;16:417–421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 73.Unger J.M., Blanke C.D., LeBlanc M., Hershman D.L. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smeltzer M., Bunn P., Clark R., et al. PL02.09 International Association for the Study of Lung Cancer (IASLC) study of the impacts of COVID-19 on International Lung Cancer Clinical Trials. J Thorac Oncol. 2020;16(suppl):S847–S848. [Google Scholar]
- 75.Pennell N.A., Dillmon M., Levit L.A., et al. American Society of Clinical Oncology Road to Recovery Report: learning from the COVID-19 experience to improve clinical research and cancer care. J Clin Oncol. 2021;39:155–169. doi: 10.1200/JCO.20.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Comprehensive Cancer Network Information on third mRNA COVID-19 vaccine dose added to NCCN guidance for people with cancer. https://www.nccn.org/home/news/newsdetails?NewsId=2867&fbclid=IwAR1smOyfRqs1UTHid1EbKAyRg4Ug_2tFWXp_FMdSzWEXxhmEraZaP14TPsE Accessed October 19, 2021.
- 77.Rivera D.R., Peters S., Panagiotou O.A., et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discov. 2020;10:1514–1527. doi: 10.1158/2159-8290.CD-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehta V., Goel S., Kabarriti R., et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]