Abstract
Up to 87% of patients hospitalized with coronavirus disease 2019 (COVID-19) experience chronic sequelae following infection. The long-term impact of COVID-19 infection on kidney function is largely unknown at this point in the COVID-19 pandemic. In this review, we highlight the current understanding of the pathophysiology of COVID-19-associated kidney injury and the impact COVID-19 may have on long-term kidney function. COVID-19-induced acute kidney injury may lead to tubular injury, endothelial injury, and glomerular injury. We highlight histopathologic correlates from large kidney biopsy and autopsy series. By conducting a comprehensive review of published literature to date, we summarize the rates of recovery from COVID-19-associated-AKI. Finally, we discuss how certain genetic differences, including APOL1 risk alleles (a risk factor for collapsing glomerulopathy), coupled with systemic healthcare disparities, may lead to a disproportionate burden of post-COVID-19-kidney function decline among racial and ethnic minority groups. We highlight the need for prospective studies to determine the true incidence of chronic kidney disease burden after COVID-19.
Abbreviations: AKI, acute kidney disease; aOR, adjusted odds ratio; ATN, acute tubular necrosis; COVAN, COVID-19-associated-nephropathy; COVID-19, coronavirus disease 2019; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HR, ratio; TMA, thrombotic microangiopathy
INTRODUCTION
As of September 30, 2021, over 233 million patients worldwide have been infected with SARS-CoV-2 virus and over 4.7 million people have died.1 Coronavirus disease 2019 (COVID-19) is a respiratory illness that ranges in severity from asymptomatic or mild upper respiratory symptoms to respiratory failure and death. The acute manifestations of COVID-19 have been well described and severe COVID-19 can cause multi-organ injury and failure.2, 3, 4
Recently, there has been increased coverage in the lay and scientific press about the chronic health consequences of COVID-19 in survivors. Accumulating data suggest that surviving patients may experience a wide array of persistent symptoms referred to as post-acute sequelae of SARS-CoV-2 (PASC) or long COVID. Some studies suggests that symptoms of COVID-19 persist beyond 8-12 weeks in up to 25% to 50% of patients with mild COVID-19 (not requiring hospitalization) and up to 87% of patients hospitalized for COVID-19.5, 6, 7 In a large study of >1300 hospitalized patients with COVID-19 who survived and were discharged home with home health care services, only 40% of patients were independent in all activities of daily living by 30 days.8 Chronic fatigue is the most commonly reported long-term symptom, affecting up to 69% of survivors.9 Long-term symptoms can also affect the lungs, leading to exercise intolerance and chronic cough. Neuropsychiatric long-term effects have been reported in 33% to 61% of patients and include chronic headache, depression, insomnia, and memory and concentration impairment. Persistent cardiometabolic complications include chest pain or tightness.5 Vaccinated individuals are susceptible to breakthrough infection and the effects of long COVID as well; up to 19% of individuals with breakthrough cases have reported having at least one symptom of long COVID (loss of smell, cough, fatigue, weakness, shortness of breath, or myalgia persisting beyond 6 weeks).10
In this review, we examine the current understanding of the pathophysiology of kidney injury in COVID-19 infected patients and its potential long-term effects on the kidney, which may promote chronic kidney disease (CKD) incidence and progression after COVID-19. We explore how acute kidney injury (AKI), as well as chronic and end-stage kidney disease (ESKD), are significant risk factors for mortality from COVID-19. Finally, we explore how racial and ethnic disparities may contribute to CKD risk in COVID-19 survivors.
EPIDEMIOLOGY OF ACUTE KIDNEY INJURY IN HOSPITALIZED PATIENTS WITH COVID-19
AKI is common in patients hospitalized for COVID-19. A recent systematic review and meta-analysis of 54 publications that included 30,639 patients found that the pooled prevalence of AKI was 28% (95% CI 22%–34%) among hospitalized patients and 9% (95% CI 7%–11%) required dialysis for AKI (Stage 3D).11 Stage 3D AKI is even more common among patients with COVID-19 who require intensive care; in a multicenter study in the United States, Gupta et al. found that 637 of 3,099 patients (21%) admitted to intensive care required renal replacement therapy for AKI.12 Well-designed studies prior to COVID-19 estimated that only 5% of patients in intensive care typically require RRT.13 Several studies have compared the risk of AKI and need for dialysis in patients hospitalized with COVID-19 to patients hospitalized with other respiratory infections (i.e. influenza.) and found a substantially higher risk in COVID-19.14, 15, 16 However, over time, the rate of AKI in hospitalized patients with COVID-19 appears to be declining; Charytan et al. determined there was a 32.5% AKI incidence in patients hospitalized in New York City in March 2020, which decreased to 17.2% of hospitalized patients with COVID-19 in August 2020.17 Dellepiane and colleagues showed that AKI rates in a NYC healthcare system continued to fall though fall and winter of 2020.18
As for other conditions, preexisting CKD is an important risk factor for AKI. A prospective cohort study of 701 patients with COVID-19 found that the incidence of AKI was significantly higher in patients with elevated baseline creatinine than in patients with normal baseline creatinine (11.9% vs. 4.0%).19 A study of 3,993 hospitalized patients in New York City found that CKD was an independent predictor of severe AKI (adjusted odds ratio [aOR] 2.8, 95% CI 2.1 to 3.7).20 , 21
Even though AKI is a common adverse sequela of COVID-19, it is widely underrecognized by the public. While most Americans are aware of effects of COVID-19 commonly reported in the media, such as acute respiratory failure, pneumonia, and acute respiratory distress syndrome (58%, 54%, 52%, respectively), a National Kidney Foundation-Harris Poll survey conducted in May 2020, found that only 17% of Americans are aware that COVID-19 can result in AKI. This is not unique to COVID-19 related AKI, as awareness of kidney disease is low in other settings, including among the lay public, healthcare workers, and even among patients who develop AKI and CKD.22, 23, 24, 25 Lack of awareness of the risks of COVID-19-associated kidney disease may hinder efforts to ensure appropriate post-AKI follow-up to diminish the effects of kidney disease on the individual and society.
ACUTE, CHRONIC AND END-STAGE KIDNEY DISEASE AS RISK FACTORS FOR MORTALITY FROM COVID-19
Preexisting CKD and ESKD have been among the most reproducible and robust predictors of severe and critical illness in patients with COVID-19. A prospective cohort study of 701 hospitalized patients with COVID-19 showed that patients with kidney disease had a 2 to 4-fold higher risk for in-hospital death depending on how kidney disease is defined. Proteinuria and hematuria of any degree, elevated baseline blood urea nitrogen, elevated serum creatinine, and AKI greater than stage 2 (≥ 2-fold rise from baseline) were all associated with in-hospital death after adjustment for age, sex, disease severity on admission, comorbidities, and lymphocyte count.19 Chan et al.found a 50% mortality rate among patients with AKI, compared to 8% in patients without AKI.26 Gupta et al. found that critically ill patients requiring renal replacement therapy for AKI had a 55% mortality rate.27 Among critically ill patient who require both mechanical ventilation and dialysis, mortality rates exceed 70%.28
A meta-analysis conducted in 2020 from four studies including 1,389 unique patients found a significant association between CKD and severe COVID-19 [OR 3.03 (95% CI 1.09–8.47), I2 = 0.0%, Cochran's Q, P = 0.84], despite the fact that none of the studies found this association individually.29 A separate meta-analysis with data from 9 studies found that CKD was associated with a greater risk of mortality (unadjusted RR 3.25 [1.13 to 9.28]).30 The OPENSAFELY study analyzed approximately 17 million adults in England from February 1, 2020 to May 6, 2020 and found that eGFR 30-60 and eGFR <30 were associated with a 1.33 and 2.52 increased risk of mortality from COVID-19 in fully adjusted models.31
Logistical factors involved in how patients with CKD and ESKD access care may have additionally placed these patients at higher risk of acquiring COVID-19 during the first-wave of the pandemic. Patients with ESKD were unable to quarantine due to their need for dialysis and frequent health care interactions. According to a midsize national dialysis provider, in clinics with at least 1 case of COVID-19 during the first 3 months of the pandemic in the United States, 5.5% of all patients at those clinics became infected.32 Severity was very high in the dialysis population as well: a retrospective cohort study of 7,533 patients with ESKD receiving dialysis in California found that among their 133 COVID-19 infected patients on dialysis, 57% of required hospitalization.33 Per existing literature, mortality rates in patients with ESKD who were hospitalized for COVID-19 have ranged from 21%–32%.33, 34, 35, 36, 37 The risk of mortality among patients on dialysis who were admitted to the intensive care unit due to COVID-19 exceeds 50%.38
Finally, patients with CKD and ESKD may be less likely to be eligible for COVID-19 treatments or trials. Remdesivir, the only antiviral therapy currently approved for hospitalized patients in the United States, was not initially studied in patients with eGFR < 30 mL/min/1,73m2 due to concerns regarding accumulation of the drug, its active metabolite, and its cyclodextrin carrier.39 However, multiple small case series have suggested remdesivir may be safe in patients with eGFR < 30 mL/min/1,73m2 or on dialysis.40, 41, 42 A post hoc analysis of clinical trial data showed that patients treated with remdesivir had less kidney function decline; 30% of patients receiving placebo experienced a decline in creatinine clearance, whereas only 15% of patients on a 5-day remdesivir course experienced a decline in creatinine clearance.43 Studies summarizing the inclusion and exclusion criteria of clinical trials of therapeutics for COVID-19 have noted that kidney disease is an exclusion criteria in approximately half of trials.44 , 45 Thus, in addition to being at high risk for poor outcomes, there is a lack of adequate evidence to inform treatment decisions in this group of high-risk patients.
There is likely a bidirectional relationship between COVID-19 and CKD: CKD increases the risk of severe COVID-19 and increased severity of COVID-19 leads to increased risk of acute and chronic kidney dysfunction. Patients with a high burden chronic comorbidities and frailty, and patients who are immunosuppressed, are at increased risk of both progressive CKD and severe COVID-19.
INCIDENT AND PROGRESSIVE CKD AS A LONG-TERM COMPLICATION OF COVID-19
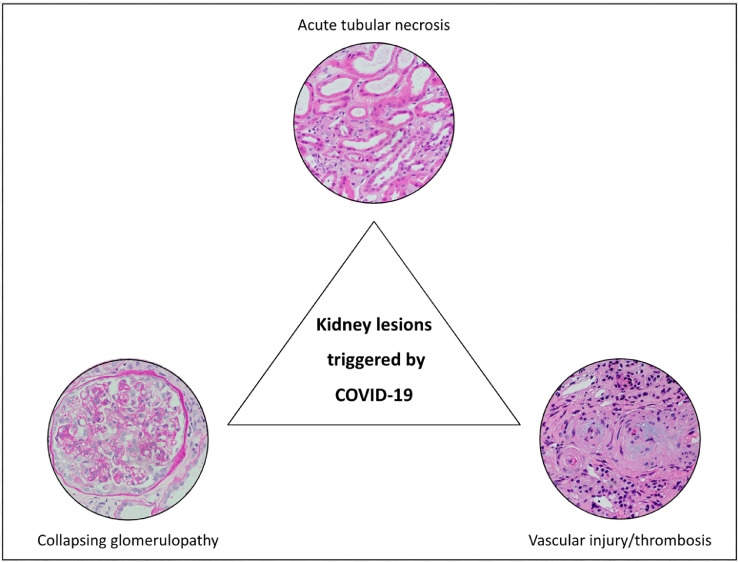
Though the risk of AKI has been well characterized; the longer term sequela of COVID-19 on kidney function is unknown.46 A study that used electronic health records from the Veterans Health Administration to conduct a high-dimensional assessment of long COVID reported increased risk of adverse kidney manifestations, including AKI, CKD, and urinary system infections that occurred even after the first 30 days following diagnosis of COVID-19.47 Potential mechanisms driving CKD progression after COVID-19 can be largely grouped into three categories: unresolved tubular injury, micro or macrovascular injury, or podocytopathy/collapsing glomerulopathy (Fig 1 ).
Fig 1.
Pathologic findings in COVID-19 associated acute kidney injury. Representative histology (hematoxylin and eosin staining) of the three most-common kidney lesions associated with COVID-19, including acute tubular necrosis, collapsing glomerulopathy, and vascular injury and thrombosis.
Unresolved tubular injury contributing to CKD risk
Possible direct viral effects on the kidney from SARS-CoV-2 include endothelial damage from viral entry and complement activation, local inflammation/cytokine release, and collapsing glomerulopathy. The indirect effects of COVID-19 that can lead to AKI include volume depletion, hypotension/shock, rhabdomyolysis, as well as the common causes of in-hospital AKI, such as nephrotoxin exposure and sepsis.46 An early autopsy series of patients who died due to COVID-19 in China found acute tubular injury ranging from mild to severe in all 26 patients studied.48 The first autopsy series in the United States also found that acute tubular injury was the most prominent finding on light microscopy.49 Given the potential for severe AKI, the risk of CKD from unresolved acute tubular necrosis (ATN) may affect a significant number of patients with severe COVID-19 who survive to hospital discharge.
Acute tubular injury is most likely caused by local and systemic response to COVID-19 that can lead to hypotension, activation of the renin–angiotensin system, endothelial injury, activation of coagulation pathways, and mitochondrial injury.49, 50, 51 Local release of cytokines in response to damage-associated and pattern-associated molecular patterns leads to recruitment of inflammatory cells and tissue damage. Neutrophil extracellular traps released by activated neutrophils have an important role in viral clearance, but they also contribute to local inflammation, immunothrombosis, and tissue damage.52 Additionally, there are non-specific hemodynamic alterations that lead to AKI in COVID-19. Dehydration from poor oral intake and gastrointestinal manifestations can activate the renin-angiotensin system and impair renal perfusion. There is also a substantial burden of hospital-acquired AKI; patients with severe COVID-19 are commonly exposed to nephrotoxic medications and those who become critically ill may develop shock, low cardiac output, hypoxia, and hypotension.
Rhabdomyolysis is a rare but reported consequence of COVID-19 that may also lead to acute tubular injury via myoglobin precipitation and free radical release.53 May et al. found that 8 of 240 (3.3%) of native kidney biopsies performed in patients with COVID-19 had myoglobin cast nephropathy, which was significantly elevated compared to the 0.1% incidence of myoglobin cast nephropathy in their biopsy database consisting of 63,575 controls.54 The likelihood of AKI recovery in patients with myoglobin cast nephropathy is unknown.
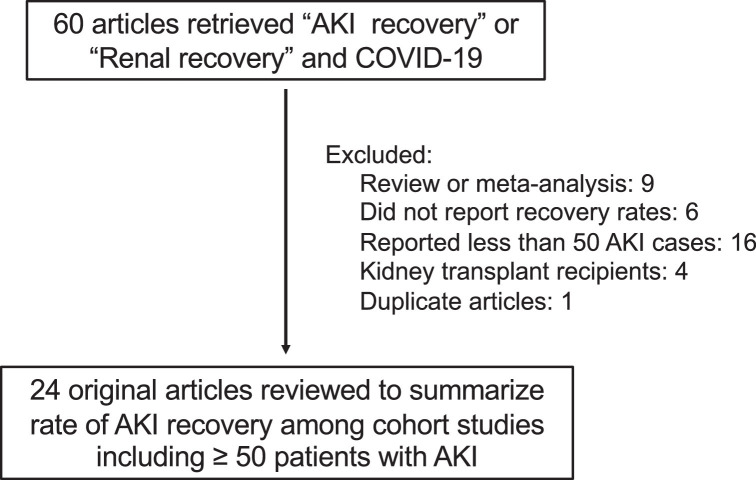
To summarize the available literature reporting AKI recovery in patientst hospitalized with COVID-1, we performed a literature review using the natural library of medicine. Using the search terms “AKI recovery” or “Renal recovery” and “COVID-19″ we identified 60 articles. After the exclusions shown in Fig 2 , we identified 24 unique cohort studies with ≥ 50 cases of hospitalized patients with COVID-19-related AKI that reported AKI recovery rates (Table I ). 12 , 14 , 16 , 17 , 20 , 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 Although “full recovery” is variably defined in these studies, the proportion of surviving patients that experience a full recovery by hospital discharge ranges between 19% to 79%, with the majority of studies reporting that that >50% of survivors experience “kidney recovery” by hospital discharge.16 , 20 , 56 Among those with AKI severe enough to require RRT, most survivors can discontinue RRT prior to hospital discharge, with continued dependence on RRT at hospital discharge ranging from 8% to 34% of surviving patients (Table I).12 , 20 , 55 , 58 , 59 Though only a small number of publications provide post-discharge kidney function follow-up, the available literature suggests that among those surviving 30-90 days, kidney recovery rates are very high, ranging from 75% to 91%.14 , 56 , 57 A prospective, uncontrolled cohort study followed 95 patients with AKI who survived 4 months and found new-onset CKD had developed in only 2 patients (2.1%);69 this suggests that the vast majority of patients with COVID-19 induced AKI who survive will recover kidney function.
Fig 2.
Identification of articles reporting AKI recovery rates in patients with Severe COVID-19
Abbreviations: AKI = acute kidney injury, COVID-19 = coronavirus disease 2019.
Table I.
AKI recovery in COVID-19 associated AKI.
| Reference | Population | Recovery |
|---|---|---|
| Overall AKI | ||
| Birkelo, Kidney Int, 202114 | AKI | 1024 hospitalized veterans with AKI, among those surviving > 90 days, 91% recovered to within 20% BL SCr |
| Ng Am J Kid Dis 202055 | AKI | 3216 patients with AKI, 51% survived, among survivors, 74% with at least partial recovery (< 33% from peak Scr or < stage 1 AKI) |
| Chan J Am Soc Nephrol 202120 | AKI | 1835 patients with AKI, 832 discharged alive, 65% to SCr within ≤0.3mg/dL and within 25% of BL SCr |
| Bowe Clin J Am Soc Nephrol, 202156 | AKI | 1655 hospitalized veterans with AKI, 47% recovered to within 0.3mg/dL of BL SCr at discharge |
| Strohbehn, Kidney Int Rep, 202116 | AKI | 251 patients with AKI, 56% recovered to within 20% of BL SCr by discharge |
| Zhang, BMC Infectious Diseases 202157 | New-onset elevated SCr during COVID-19 hospitalization | 143 patients discharged alive, during a 4-month median follow-up 91% normalized (serum creatinine ≤84 µmol/L [0.95mg/dL] in women, ≤104 µmol/L [1.18 mg/dL] in men with negative urine protein) |
| Nugent, JAMA Open Net, 202160 | AKI | 786 patients with AKI, 182 were discharged alive, 82.4% recovered by discharge |
| Sang, BMC Pulm Med, 202061 | AKI | 92 patients with AKI, 16 (17%) improved by discharge |
| Hittesdorf, Blood Purif, 202162 | AKI in ICU | 76 patients with AKI, 48 were discharged alive, 77% recovered to within 1.5-fold BL by discharge, 82% by 90 days |
| Moledina, Am J Kid Dis, 202163 | AKI | 796 patients with AKI, 462 (58%) recovered to within 1.5-fold BLby discharge, and |
| Teoh, JASN, 202164 | AKI | 66 patients with AKI, complete recovery (within 1.5-fold BL) noted in 87% by 30 days and 92% by 90 days |
| Saggi, Clin Med Insightts Circ Respir Pulm Med, 202065 | AA patients with AKI | 75 patients with AKI, 65% experienced either a 50% increase in eGFR or discontinued RRT by discharge. |
| Charytan, Kidney Int Rep, 202117 | AKI | 1386 patients with AKI, 678 (49%) were discharged alive. 523 (77%) recovered to within 0.3mg/dL above BL SCr |
| Abdallah, Saudi J Kidney Dis Transpl, 202166 | AKI in ICU | 61 patients with AKI, 37 (61%) were discharged alive, 11 had full or partial recovery and 26 remained on RRT |
| Rahimzadeh, Kidney Blood Press Res, 202167 | AKI | 194 patients with AKI, 117 (60%) were discharged alive, only 28% recovered to within 0.3mg/dL of baseline by hospital discharge and 72% did not fully recover by discharge |
| Lumlertgul, Ann Intensive Care, 202168 | AKI in ICU | 240 patients with AKI, 158 (66%) were discharged alive and 82% recovered to within 1.5-fold BL SCr by discharge and 91% recovered by 90 days |
| Sampathkumar, J Assoc Physicians India, 202173 | AKI | Among 52 hospitalized patients with AKI 29 (55%) were discharged alive. AKI recovered in 41% by hospital discharge and 72% followed 4-6 weeks post-discharge |
| Morin, JAMA, 202169 | AKI | Among patients 95 patients with AKI surviving 4 months, only 2 (2.1%) developed new-onset CKD |
| Chaudhri, Kidney Blood Press Res, 202070 | AKI | 63 patients with AKI, 79% had complete recovery (to BL or normal range) by hospital discharge |
| AKI requiring RRT | ||
|---|---|---|
| Birkelo, Kidney Int, 202114 | AKI requiring RRT | 1517 hospitalized veterans with AKI, 12% required acute RRT, 7% remained on RRT at discharge |
| Chen, Kidney Int, 202171 | AKI receiving acute PD | 94 patients. 51 (54%) survived 30 days, 21 recovered off PD, 30 remained on PD. |
| Charytan, Kidney Int Rep, 202117 | AKI requiring RRT | 237 received RRT for AKI, 66 were discharged alive, 41 (62%) discontinued RRT prior to discharge |
| Eriksson, J Crit Care, 202172 | AKI requiring RRT | 82 patients in ICU with AKI requiring continuous RRT, 45 (55%) survived to hospital discharge. Among 42 patients with a post-hospitalization SCr, 31 (74%) recovered to within 1.5-fold BL SCr. |
| Ng, Am J Kid Dis, 202055 | AKI requiring RRT | 638 had AKI requiring RRT, 108 were discharged alive, 36 (33%) of survivors remained on RRT |
| Chan J Am Soc Nephrol, 202120 | AKI requiring RRT | 347 with AKI requiring RRT, 87 were discharged alive, 26 (30%) of survivors remained on RRT |
| Stevens, PLOS One, 202058 | AKI requiring RRT | 115 patients with AKI requiring RRT, 57 were discharged alive, 10 (18%) of survivors remained on RRT |
| Gupta, J Am Soc Neph, 202112 | AKI requiring RRT in ICU | 637 patients with AKI requiring RRT, 216 were discharged alive, 73 (34%) of survivors remained on RRT, 26% had partial AKI recovery, 40% complete AKI recovery (within 0.35mg/dL BL) |
| Stockmann, Kidney Int, 202159 | AKI requiring RRT in ICU | 74 with AKI requiring RRT, 37 were discharged alive, 3 (8%) of survivors remained on RRT, 23 (62%) had full AKI recovery at median of 151 days |
All publications that reported on the outcomes of ≥50 patients with COVID-19-associated-AKI were included in this table. Abbreviations: AKI, acute kidney injury; AA, African American; SCr, serum creatinine; RRT, renal replacement therapy; ICU, intensive care unit; BL, baseline; PD, peritoneal dialysis
However, it is important to note that serum creatinine level is an insensitive marker of kidney damage; and estimates of recovery must take into account that patients often lose muscle mass during the course of critical illness, which may make creatinine-based eGFR estimates less reliable.69 Although in many patients serum creatinine returns to near-normal levels following AKI, the kidneys may not completely recover and studies with longer follow-up are needed.
Finding the appropriate populations to compare rates of AKI recovery and eGFR decline after hospitalization is challenging. A cohort study of 182 hospitalized patients with COVID-19 associated AKI found a greater rate of post-hospitalization eGFR decline compared to 1,430 hospitalized patients with AKI not associated with COVID-19, even after adjustment for comorbidities and AKI severity.74 in contrast, two studies that compared AKI outcomes in patients with COVID-19 to patients hospitalized with influenza found very similar rates of medium to long-term recovery and eGFR decline.14 , 16 Notably, most observational studies of AKI recovery have very high rates of loss to follow-up; thus, to understand the burden of CKD and eGFR decline, prospective studies that include assessment of blood and urine biomarkers are necessary.
Micro or macrovascular injury/endothelial activation contributing to CKD risk
Early autopsy reports suggested that microvascular injury may occur in multiple organs, with prominent endothelial lung injury demonstrated on autopsies.75 Segmental fibrin microthrombi were also observed in the glomeruli of the first kidney autopsy data from China.48 Since then there have been multiple case reports of thrombotic microangiopathy (TMA) identified by kidney biopsy and autopsy in patients with COVID-19.76, 77, 78, 79, 80, 81 However, May et al. reviewed 240 native kidney biopsies and found that only 3.3% had evidence of TMA, which was not significantly elevated compared to the non-COVID-19 infected kidney biopsy control cohort.54
Thrombocytopenia, elevated LDH, and D-dimer are common in COVID-19, and may be a feature of disseminated intravascular coagulation. At the cellular level, it has been postulated that platelet activation may be part of the etiology of the prothrombotic state seen in COVID-19, as SARS-CoV-2 can bind to platelets via angiotensin-converting enzyme 2, activating platelets.82 Severe COVID-19 may lead to cytokine release syndrome, macrophage activation, and release of pathogen-associated and damage-associated molecular pattern molecules that lead to release of tissue factor and activation of coagulation factors.83 Complement activation may also upregulate tissue factor and lead to loss of thrombomodulin which promotes hypercoagulability.53 , 84 Emerging evidence suggest that excessive formation of neutrophil extracellular traps plays an important role in the pathophysiology of endothelial injury and immunothrombosis that characterize severe cases of COVID-19.52 , 85, 86, 87 COVID-19 triggered endothelial dysfunction may exacerbate underlying chronic diseases that are associated with chronic endothelial dysfunction and are major causes of CKD, such as hypertension, diabetes, and atherosclerosis.
Macro-vascular thrombosis can also occur in COVID-19. Pulmonary embolisms, strokes, right ventricular thromboses, aortic thromboses, and kidney-related macrovascular events have all been documented in patients with COVID-19.88 Renal artery thrombosis and renal vein thrombosis have both been documented, including cases with associated renal infarction; however, these are likely rare events.88, 89, 90, 91, 92
Collapsing glomerulopathy and podocytopathy contributing to CKD risk
Collapsing glomerulopathy is a well-documented complication of viral infections, most commonly human immunodeficiency virus infection (HIV), Epstein-Barr virus, cytomegalovirus, and parvovirus B19.93 Recently, it has been reported that SARS-CoV-2 infection may be an additional “viral hit” that can cause collapsing glomerulopathy.94
Collapsing glomerulopathy is characterized by segmental or global glomerular tuft collapse with hypertrophy and hyperplasia of the overlying podocytes.95, 96, 97, 98 Collapsing glomerulopathy is associated with high-risk APOL1 genetic variants (G1/G1, G2/G2, or G1/G2). The vast majority of cases of documented COVID-19 associated collapsing glomerulopathy have been reported in patients of West African or African-American descent. It is estimated that 10% to 15% of African-American individuals have two high risk APOL1 alleles, suggesting that a large proportion of the U.S. black population may be at risk for collapsing glomerulopathy.99 May et al. reported that of 44 of 48 (92%) of patients with COVID-19-associated collapsing glomerulopathy had high-risk APOL1 genotypes.54
Cases of COVID-19 associated collapsing glomerulopathy typically present with AKI, heavy proteinuria, and hypoalbuminemia.95 , 100, 101, 102, 103, 104, 105 Acute tubular injury is also found in the vast majority of cases of COVID-19 associated collapsing glomerulopathy.54 COVID-19 associated collapsing glomerulopathy can occur in patients with mild respiratory symptoms suggesting that, unlike COVID-associated ATN or rhabdomyolysis, the risk of COVID-19 associated collapsing glomerulopathy is not directly correlated to the severity of respiratory symptoms related to COVID-19.95
Some patients have presented with minimal change disease or focal segmental glomerulosclerosis without collapsing features on biopsy, suggesting that there may be a spectrum of podocytopathy affecting patients with COVID-19. May et al. performed APOL1 genotyping on black or Hispanic patients who developed other forms of focal segmental glomerulosclerosis or minimal change disease and found that 8 of 11 (72.7%) had high risk APOL1 genotypes, suggesting there may be a spectrum of APOL1 related kidney disease triggered by COVID-19.54 It is likely that cases of COVID-19 associated collapsing glomerulopathy and podocytopathy reported in the literature represent a severe phenotype of this disease. Prospective studies are needed to determine the incidence of proteinuria after COVID-19. Furthermore, because collapsing glomerulopathy may occur in patients with mild COVID-19, even patients who do not require medical attention may still be at risk of developing overt or subclinical collapsing glomerulopathy that may only be detected by incidental lab testing months or years after infection. This is a particularly important consideration given the high prevalence of high risk APOL1 genotypes among African-Americans.99
The exact mechanism by which SARS-CoV-2 infection leads to collapsing glomerulopathy has not been elucidated. Antiviral pathways, particularly interferon gamma upregulation, may be important inducers of kidney disease in individuals with the high-risk APOL1 genotype.53 , 94 , 106 There has been conflicting evidence as to whether viral particles and RNA from SARS-CoV-2 are directly deposited in the kidney.107 Evidence for and against direct viral infection was well summarized recently by Hassler and colleagues.108 In their review of studies to date, the presence of SARS-CoV-2 was suggested by at least one of the methods used (immunohistochemistry, RT-PCR, or in situ hybridization) in kidneys from 102 of 235 patients who underwent biopsy (43%).108 The largest series of kidney biopsies by May et al. was unable to confirm SARS-CoV-2 RNA by in situ hybridization.54 There is remaining uncertainty, with a need for more kidney biopsy data, particularly earlier in the disease course. However, even without direct invasion, viral-induced changes in the microenvironment surrounding the podocytes (increased cytokine production) can trigger collapsing glomerulopathy.98 APOL1 risk alleles may also play a mechanistic role—viral infections stimulate host interferon production which stimulates APOL1 gene expression, potentially exacerbating the deleterious effects of APOL1 polymorphism on kidney function and leading to collapsing glomerulopathy.109
May et al. sought to determine if COVID-19 associated collapsing glomerulopathy was enriched in patients with COVID-19 by conducting a multi-center study that compared 240 native kidney biopsies obtained in COVID-19 infected patients to a 5-year U.S. kidney pathology database as a control.54 They found that collapsing glomerulopathy occurred in 25.8% of the COVID-19 cases compared to 1.8% in the overall 5-year database and 28% of patients with HIV. This study highlights the importance of benchmarking against control patients. It is also possible that some kidney pathologies develop randomly and concurrently with COVID-19, which is challenging to know when relying on case reports and case series that may suffer from publication bias. Prospective studies with carefully matched controls will be needed to estimate the risk of new-onset proteinuria and glomerular disease after COVID-19.
RACIAL AND ETHNIC DISPARITIES AND THE RISK OF CKD AFTER COVID-19
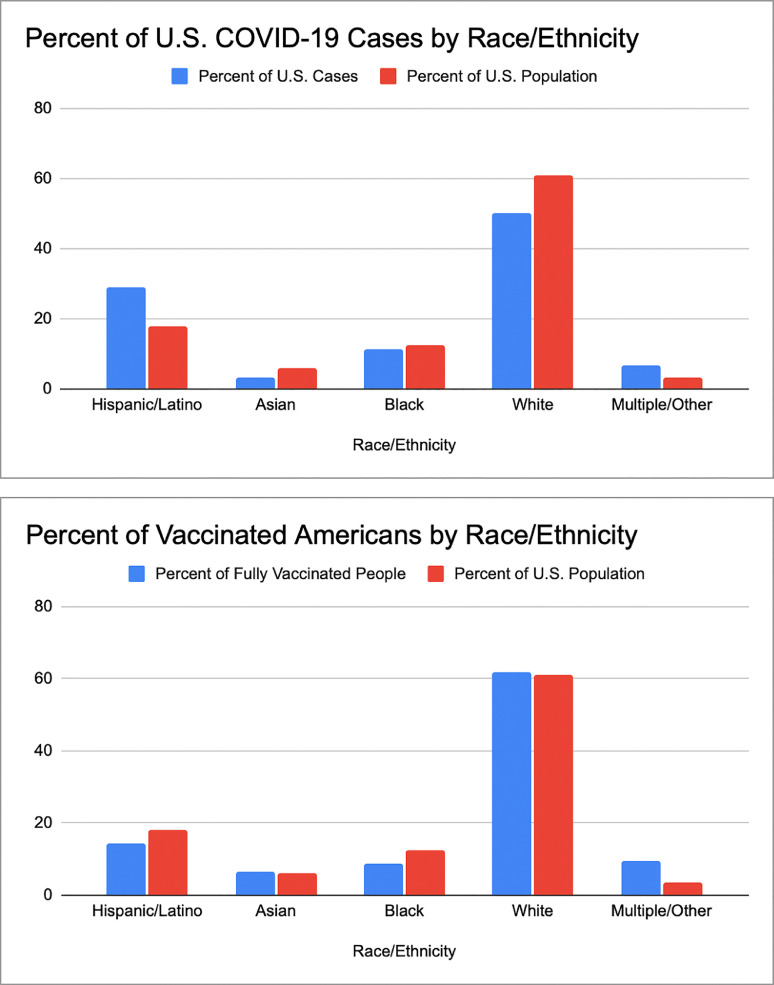
COVID-19 has disproportionately affected racial and ethnic minority populations in terms of both overall infection and disease mortality rates. Black and Latinx patients are more likely than white patients to test positive for COVID-19.110 As of June 2021, the CDC reported that 28.8% of COVID-19 cases in the U.S. occurred in Hispanic or Latinx individuals, despite only representing 18.5% of the population. Fig 3A shows the breakdown of COVID-19 cases in the U.S. as well as the breakdown of the overall U.S. population.111 Among hospitalized patients, Black patients were less likely than white patients to become severely ill or die; however, Black patients had a higher out-of-hospital COVID-19 mortality rate, and the overall mortality rate in Black Americans is over twice the COVID-19 mortality rate in white Americans.110 , 112 Sickle cell disease, which primarily affects people of African descent, may also be a risk factor for hospitalization and death from COVID-19.112 , 113 COVID-19 associated kidney disease already appears to be affecting blacks disproportionately; in the study by May et al. black patients were disproportionately impacted, making up 44.6% of the 240 native COVID-19 kidney biopsies, compared to 15.4% of patients in the U.S. biopsy database that served as a control.
Fig 3A.
Rate of COVID-19 infection among racial and ethnic groups in the U.S. population. The percentage of patients infected with COVID-19 broken down by self-reported racial and ethnic groups. The multiple/other group includes Native Americans, Pacific Islanders/Hawaiians, and other/multiple. The number of COVID-19 infected individuals is disproportionately high in Hispanic populations when compared to the percentage of the United States population this group makes up, according to data from the CDC accessed June 20, 2021.111. Fig 3B Rate of COVID-19 vaccination among racial and ethnic groups in the U.S. population. The percentage of COVID-19 vaccinated patients broken down by self-reported racial and ethnic groups. The multiple/other group includes Native Americans, Pacific Islanders/Hawaiians, and other/multiple. The COVID-19 vaccination rate is disproportionately low in Black and Hispanic groups when compared to the percentage of the United States population these groups make up, according to data from the CDC accessed June 20, 2021.111
Disparities in post-hospital care may also affect the risk of CKD. It is recommended that patients with AKI (including COVID-19-associated AKI) receive post-hospital care with a nephrologist to ensure resolution of AKI, optimize blood pressure, and minimize exposure to potential nephrotoxins. There are well-documented disparities in post-hospitalization care in minority populations, thus disparities in access to subspecialty care and post-hospitalization follow-up may contribute to CKD burden faced by members of racial and ethnic minority groups affected by COVID-19.114 The majority of Medicaid recipients are black or Latinx; Medicaid recipients are overall less likely to be transferred to a long-term care or skilled nursing facilities after being in the intensive care unit.114 Black and Latinx patients are more likely to be discharged home, with black patients less likely than white patients to see improvements in their daily life after critical illness.115, 116, 117 Black and Latinx patients who are discharged to a skilled nursing facility often go to facilities with higher readmission rates and lower rates of successful discharge into the community.118 To minimize the risk of CKD after COVID-19-related AKI, it is critical to ensure patients have access to post-acute care and nephrology care when clinically indicated.
Disparities also manifest in differential rates of COVID-19 vaccination. As of June 20, 2021, the CDC reported that out of 92 million vaccinated people in the United States, 8.7% were black and 14.1% were Latinx despite these groups making up 12.5% and 18.5% of the U.S. population, respectively (Fig 3B).111
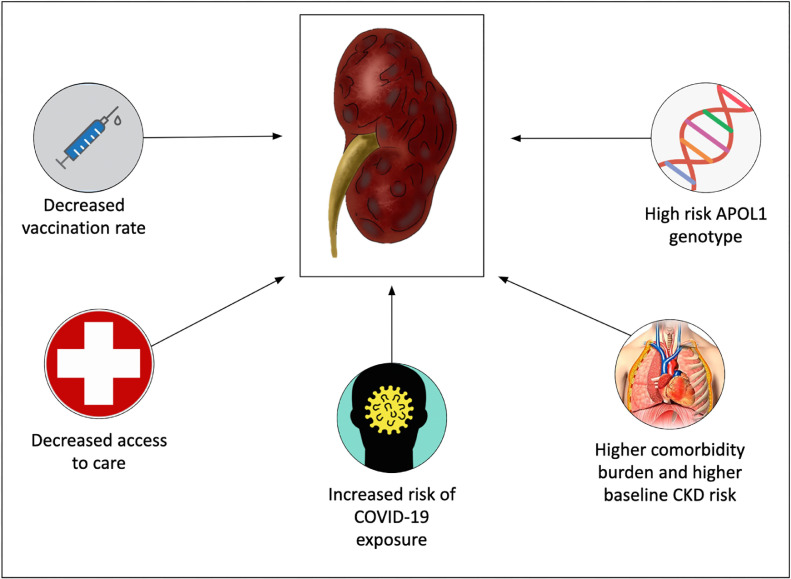
Clinicians and the public should be aware that the confluence of biological, social, and economic factors, as well as systemic and institutional racism, may lead to an increased burden of CKD in patients from racial and ethnic minority populations who develop COVID-19 (Fig 4 ).
Figure 4.
Factors contributing to increased risk of adverse kidney outcomes among racial and ethnic minorities. Several factors increase the risk of deleterious kidney outcomes in racial and ethnic minorities. These include decreased COVID-19 vaccination rate, decreased access to care, increased risk of COVID-19 exposure, higher comorbidity burden and baseline CKD risk, and increased prevalence of the high risk APOL1 genotype in these populations. Abbreviations: CKD = chronic kidney disease.
CONCLUSION AND FUTURE DIRECTIONS
Kidney injury is an important and common adverse outcome of COVID-19. Both AKI and CKD are associated with severe COVID-19 and risk of death. COVID-19 may lead to CKD in survivors via unresolved acute tubular injury that occurs in patients with severe disease; as a result of podocytopathy, which has been strongly linked to high risk APOL1 genotypes; or by causing endothelial or vascular injury, which promotes CKD progression. Although several studies suggest that AKI rates in COVID-19 may be higher than matched controls and recovery by hospital discharge may be lower than matched controls, medium to long-term recovery of AKI seems to be high among patients who are followed after hospital discharge. The true burden of CKD after COVID-19 has not yet been accurately ascertained, and we are limited in estimating CKD risk from observational datasets due to the high rates of loss to follow-up. Prospective studies that longitudinally measure kidney function and proteinuria are needed. Hopefully, this will be addressed through the Researching COVID to Enhance Recovery (RECOVER) cohort studies and other planned prospective studies.119
Research data
Not applicable
Acknowledgements
All authors have read the journal's policy on disclosure of potential conflicts of interest and have disclosed any financial or personal relationship with organizations that could potentially be perceived as influencing the described research. The authors acknowledge Dr. Ivy Rosales and Dr. Xavier Vela Parada for assistance with Fig. 1 and 4, respectively.
Funding: MES was funded by NIH RO3 DK128533
Disclosure:MES has been awarded research funding to her institution from Gilead in the area of COVID-19 and kidney disease.
REFERENCES
- 1.COVID-19 Map . Center for Systems Science and Engineering at Johns Hopkins University; 2021. COVID-19 Dashboard Web site.https://coronavirus.jhu.edu/map.html Published 2021. Accessed 07/31/2021. [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Int Med. 2020;180:1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers. Ann Clin Trans Neurol. 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325:2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles KH, McDonald M, Barron Y, Kennedy E, O'Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174:316–325. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wostyn P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. New Eng J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silver SA, Beaubien-Souligny W, Shah PS, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med. 2021;3:83–98. doi: 10.1016/j.xkme.2020.11.008. e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Coca SG, Chan L, et al. AKI treated with renal replacement therapy in critically Ill patients with COVID-19. J Am Soc Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 14.Birkelo BC, Parr SK, Perkins AM, et al. Comparison of COVID-19 versus influenza on the incidence, features, and recovery from acute kidney injury in hospitalized United States Veterans. Kidney Int. 2021;100:894–905. doi: 10.1016/j.kint.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. Bmj. 2020;371 doi: 10.1136/bmj.m4677. m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strohbehn IA, Zhao S, Seethapathy H, et al. Acute kidney injury incidence, recovery, and long-term kidney outcomes among hospitalized patients with COVID-19 and influenza. Kidney Int Rep. [DOI] [PMC free article] [PubMed]
- 17.Charytan DM, Parnia S, Khatri M, et al. Decreasing Incidence of acute kidney injury in patients with COVID-19 Critical Illness in New York City. Kidney Int Rep. 2021;6:916–927. doi: 10.1016/j.ekir.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dellepiane S, Vaid A, Jaladanki SK, et al. Acute kidney injury in patients hospitalized with COVID-19 in New York City: temporal trends from march 2020 to april 2021. Kidney Med. 2021;3:877–879. doi: 10.1016/j.xkme.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16:747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siew ED, Parr SK, Wild MG, et al. Kidney disease awareness and knowledge among survivors ofacute kidney injury. Am J Nephrol. 2019;49:449–459. doi: 10.1159/000499862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macedo E, Hemmila U, Sharma SK, et al. Recognition and management of community-acquired acute kidney injury in low-resource settings in the ISN 0by25 trial: A multi-country feasibility study. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu CD, McCulloch CE, Banerjee T, et al. CKD awareness among us adults by future risk of kidney failure. Am J Kidney Dis. 2020;76:174–183. doi: 10.1053/j.ajkd.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 26.Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with covid-19. j am soc nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Coca SG, Chan L, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CM, Weiner DE, Aweh G, et al. COVID-19 Among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77:748–756. doi: 10.1053/j.ajkd.2021.01.003. e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim JJ, Huang CW, Selevan DC, Chung J, Rutkowski MP, Zhou H. COVID-19 and survival in maintenance dialysis. Kidney Med. 2021;3:132–135. doi: 10.1016/j.xkme.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couchoud C, Bayer F, Ayav C, et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020;98:1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and covid-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77:190–203. doi: 10.1053/j.ajkd.2020.09.003. e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamsick ML, Gandhi RG, Bidell MR, et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31:1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakare S, Gandhi C, Modi T, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep. 2021;6:206–210. doi: 10.1016/j.ekir.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estiverne C, Strohbehn IA, Mithani Z, et al. Remdesivir in patients with estimated GFR <30 ml/min per 1.73 m(2) or on renal replacement therapy. Kidney Int Rep. 2021;6:835–838. doi: 10.1016/j.ekir.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettit NN, Pisano J, Nguyen CT, et al. Remdesivir use in the setting of severe renal impairment: a theoretical concern or real risk? Clin Infect Dis. 2020;6:206–210. doi: 10.1093/cid/ciaa1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. Jama. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chewcharat A, Chang YT, Sise ME, Bhattacharyya RP, Murray MB, Nigwekar SU. Phase-3 Randomized Controlled Trials on Exclusion of Participants With Kidney Disease in COVID-19. Kidney Int Rep. 2021;6:196–199. doi: 10.1016/j.ekir.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Major R, Selvaskandan H, Makkeyah YM, Hull K, Kuverji A, Graham-Brown M. The exclusion of patients with CKD in prospectively registered interventional trials for COVID-19-a rapid review of international registry data. J Am Soc Nephrol. 2020;31:2250–2252. doi: 10.1681/ASN.2020060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat Rev Nephrol. 2020;16:747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 48.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santoriello D, Khairallah P, Bomback AS, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Legrand M, Bell S, Forni L, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17:751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golmai P, Larsen CP, DeVita MV, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31:1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ackermann M, Anders HJ, Bilyy R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021;17:751–764. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legrand M, Bell S, Forni L, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;77:204–215.e1. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May RM, Cassol C, Hannoudi A, et al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID-19) Kidney Int. 2021;100:1303–1315. doi: 10.1016/j.kint.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng JH, Hirsch JS, Hazzan A, et al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77:204–215. doi: 10.1053/j.ajkd.2020.09.002. e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized us veterans with COVID-19. Clin J Am Society Nephrol. 2021;16:14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang N-H, Cheng Y-C, Luo R, Zhang C-X, Ge S-W, Xu G. Recovery of new-onset kidney disease in COVID-19 patients discharged from hospital. BMC Infect Dis. 2021;21:397. doi: 10.1186/s12879-021-06105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens JS, King KL, SY Robbins-Juarez, et al. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLOS ONE. 2021;15:e0244131. doi: 10.1371/journal.pone.0244131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stockmann H, Hardenberg J-HB, Aigner A, et al. High rates of long-term renal recovery in survivors of coronavirus disease 2019-associated acute kidney injury requiring kidney replacement therapy. Kidney Int. 2021;99:1021–1022. doi: 10.1016/j.kint.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4:e211095. doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sang L, Chen S, Zheng X, et al. The incidence, risk factors and prognosis of acute kidney injury in severe and critically ill patients with COVID-19 in mainland China: a retrospective study. BMC Pulm Med. 2020;20:290. doi: 10.1186/s12890-020-01305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hittesdorf E, Panzer O, Wang D, et al. Mortality and renal outcomes of patients with severe COVID-19 treated in a provisional intensive care unit. J Crit Care. 2021;62:172–175. doi: 10.1016/j.jcrc.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moledina DG, Belliveau O, Yamamoto Y, et al. Variation in best practice measures in patients with severe hospital-acquired acute kidney injury: a multicenter study. Am J Kidney Dis. 2021;77:547–549. doi: 10.1053/j.ajkd.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teoh JY, Yip TC, Lui GC, et al. Risks of AKI and major adverse clinical outcomes in patients with severe acute respiratory syndrome or coronavirus disease 2019. J Am Soc Nephrol. 2021;32:961–971. doi: 10.1681/ASN.2020071097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saggi SJ, Nath S, Culas R, et al. Early experience with methylprednisolone on SARS-CoV-2 infection in the african american population, a retrospective analysis. Clin Med Insights Circ Respir Pulm Med. 2020;4 doi: 10.1177/1179548420980699. 1179548420980699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdallah E, Al Helal B, Asad R, et al. Incidence and outcomes of acute kidney injury in critically Ill patients with coronavirus disease 2019. Saudi J Kidney Dis Transpl. 2021;32:84–91. doi: 10.4103/1319-2442.318551. [DOI] [PubMed] [Google Scholar]
- 67.Rahimzadeh H, Kazemian S, Rahbar M, et al. The risk factors and clinical outcomes associated with acute kidney injury in patients with COVID-19: data from a large cohort in Iran. Kidney Blood Press Res. 2021;46:620–628. doi: 10.1159/000517581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lumlertgul N, Pirondini L, Cooney E, et al. Acute kidney injury prevalence, progression and long-term outcomes in critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11:123. doi: 10.1186/s13613-021-00914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Writing Committee for the CSG, Morin L, Savale L, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaudhri I, Moffitt R, Taub E, et al. Association of proteinuria and hematuria with acute kidney injury and mortality in hospitalized patients with COVID-19. Kidney Blood Press Res. 2020;45:1018–1032. doi: 10.1159/000511946. [DOI] [PubMed] [Google Scholar]
- 71.Chen W, Caplin N, El Shamy O, et al. Use of peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City: a multicenter observational study. Kidney Int. 2021;100:2–5. doi: 10.1016/j.kint.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eriksson KE, Campoccia-Jalde F, Rysz S, Rimes-Stigare C. Continuous renal replacement therapy in intensive care patients with COVID-19; survival and renal recovery. J Crit Care. 2021;64:125–130. doi: 10.1016/j.jcrc.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sampathkumar Hanumaiah H, Rajiv A, et al. Incidence, risk factors and outcome of COVID-19 associated AKI- a study from South India. J Associat Physic Ind. 2021 69. [PubMed] [Google Scholar]
- 74.Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Network Open. 2021;4:e211095. doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parra-Medina R, Herrera S, Mejia J. Systematic Review of Microthrombi in COVID-19 Autopsies. Acta Haematol. 2021;144:476–483. doi: 10.1159/000515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma P, Uppal NN, Wanchoo R, et al. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akilesh S, Nast CC, Yamashita M, et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77:82–93. doi: 10.1053/j.ajkd.2020.10.001. e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elsoukkary SS, Mostyka M, Dillard A, et al. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. 2021;88:56–68. doi: 10.1159/000511325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferlicot S, Jamme M, Gaillard F, et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab042. epub only. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jhaveri KD, Meir LR, Flores Chang BS, et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98:509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salvatore SP, Borczuk AC, Seshan SV. Renal pathology of 34 consecutive COVID autopsies: A single-institution experience. J Am Society Nephrol. 2020;31:299. [Google Scholar]
- 82.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 84.Lo MW, Kemper C, Woodruff TM. COVID-19: Complement, coagulation, and collateral damage. J Immunol. 2020;205:1488–1495. doi: 10.4049/jimmunol.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veras FP, Pontelli MC, Silva CM, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mui LW, Lau JF, Lee HK. Thromboembolic complications of COVID-19. Emer Radiol. 2021;28:423–429. doi: 10.1007/s10140-020-01868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mukherjee A, Ghosh R, Furment MM. Case Report: COVID-19 Associated Renal Infarction and Ascending Aortic Thrombosis. Am J Trop Med Hyg. 2020;103:1989–1992. doi: 10.4269/ajtmh.20-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acharya S, Anwar S, Siddiqui FS, Shabih S, Manchandani U, Dalezman S. Renal artery thrombosis in COVID-19. IDCases. 2020;22:e00968. doi: 10.1016/j.idcr.2020.e00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Philipponnet C, Aniort J, Chabrot P, Souweine B, Heng AE. Renal artery thrombosis induced by COVID-19. Clin Kidney J. 2020;13:713. doi: 10.1093/ckj/sfaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El Shamy O, Munoz-Casablanca N, Coca S, Sharma S, Lookstein R, Uribarri J. Bilateral renal artery thrombosis in a patient with COVID-19. Kidney Med. 2021;3:116–119. doi: 10.1016/j.xkme.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chandra P, Kopp JB. Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J. 2013;6:1–5. doi: 10.1093/ckj/sft002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Friedman DJ, Pollak MR. APOL1 Nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol. 2021;16:294–303. doi: 10.2215/CJN.15161219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferlicot S, Jamme M, Gaillard F, et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury and/or proteinuria. Nephrol Dial Transplan. 2021;36:1253–1262. doi: 10.1093/ndt/gfab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 97.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 98.Izzedine H, Brocheriou I, Arzouk N, et al. COVID-19-associated collapsing glomerulopathy: a report of two cases and literature review. Intern Med J. 2020;50:1551–1558. doi: 10.1111/imj.15041. [DOI] [PubMed] [Google Scholar]
- 99.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kissling S, Rotman S, Gerber C, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peleg Y, Kudose S, D'Agati V, et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gaillard F, Ismael S, Sannier A, et al. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98:241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Couturier A, Ferlicot S, Chevalier K, et al. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J. 2020;13:347–353. doi: 10.1093/ckj/sfaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nasr SH, Alexander MP, Cornell LD, et al. Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis. 2021;77:465–468. doi: 10.1053/j.ajkd.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015;87:332–342. doi: 10.1038/ki.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID-19 and the kidney: what we think we know so far and what we don't. J Nephrol. 2020;33:1213–1218. doi: 10.1007/s40620-020-00789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassler L, Reyes F, Sparks MA, Welling P, Batlle D. Evidence for and against direct kidney infection by SARS-CoV-2 in patients with COVID-19. Clin J Am Soc Nephrol. 2021;16:1755–1765. doi: 10.2215/CJN.04560421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27:365–376. doi: 10.1053/j.ackd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Network Open. 2020;3:e2026881. doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demographic trends of COVID-19 cases and deaths in the US reported to CDC. Centers Dis Control Prevent. https://covid.cdc.gov/covid-data-tracker/#demographics. Published 2021. Accessed June 20, 2021.
- 112.Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Centers Dis Control Prevent. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Published 2021. Updated 07/16/2021. Accessed June 20, 2021.
- 113.Panepinto JA, Brandow A, Mucalo L, et al. Coronavirus disease among persons with sickle cell disease, United States, march 20-may 21, 2020. Emerg Infect Dis. 2020;26:2473–2476. doi: 10.3201/eid2610.202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flash MJE, Johnson SF, Tiako MJN, Tan-McGrory A, Betancourt JR. Disparities in post-intensive care syndrome during the COVID-19 pandemic: challenges and solutions. NEJM Catalyst. 2020 doi: 10.1056/CAT.20.0568. [DOI] [Google Scholar]
- 115.Lane-Fall MB, Iwashyna TJ, Cooke CR, Benson NM, Kahn JM. Insurance and racial differences in long-term acute care utilization after critical illness. Crit Care Med. 2012;40:1143–1149. doi: 10.1097/CCM.0b013e318237706b. [DOI] [PubMed] [Google Scholar]
- 116.DiMeglio M, Dubensky J, Schadt S, Potdar R, Laudanski K. Factors underlying racial disparities in sepsis management. Healthcare (Basel) 2018;6:133. doi: 10.3390/healthcare6040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chase JD, Huang L, Russell D, et al. Racial/ethnic disparities in disability outcomes among post-acute home care patients. J Aging Health. 2018;30:1406–1426. doi: 10.1177/0898264317717851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rivera-Hernandez M, Rahman M, Mukamel DB, Mor V, Trivedi AN. Quality of post-acute care in skilled nursing facilities that disproportionately serve black and hispanic patients. J Gerontol A Biol Sci Med Sci. 2019;74:689–697. doi: 10.1093/gerona/gly089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.RECOVER. Researching COVID to enhance recovery. https://recovercovid.org/. Published 2021. Accessed June 16, 2021.