Abstract
Background:
Patients at high-risk for lung cancer and qualified for CT lung cancer screening (CTLS) are at risk for numerous cardio-pulmonary comorbidities. We sought to examine if qualitatively assessed coronary artery calcifications (CAC) on CTLS exams could identify patients at increased risk for non-cardiovascular events such as all cause, COPD and pneumonia related hospitalization and to verify previously reported associations between CAC and mortality and cardiovascular events.
Study Design and Methods:
Patients (n=4673) from Lahey Hospital and Medical Center who underwent CTLS from January 12, 2012 through September 30, 2017 were included with clinical follow-up through September 30, 2019. CTLS exams were qualitatively scored for the presence and severity of CAC at the time of exam interpretation using a four point scale: none, mild, moderate, and marked. Multivariable Cox regression models were used to evaluate the association between CT qualitative CAC and all-cause, COPD-related, and pneumonia-related hospital admissions.
Results:
3631 (78%) of individuals undergoing CTLS had some degree of CAC on their baseline exam: 1308 (28.0%), 1128 (24.1%), and 1,195 (25.6%) had mild, moderate and marked coronary calcification, respectively. Marked CAC was associated with all-cause hospital admission and pneumonia related admissions HR 1.48; 95% CI 1.23–1.78 and HR 2.19; 95% 1.30–3.71, respectively. Mild, moderate and marked CAC were associated with COPD-related admission HR 2.30; 95% CI 1.31–4.03, HR 2.17; 95% CI 1.20–3.91 and HR 2.27; 95% CI 1.24–4.15.
Conclusion:
Qualitative CAC on CTLS exams identifies individuals at elevated risk for all cause, pneumonia and COPD-related hospital admissions.
INTRODUCTION
Coronary artery calcification (CAC) is a well-documented predictor of cardiovascular events, cardiovascular mortality and all-cause mortality.(1–3) Qualitative scoring of CAC in a computed tomographic lung cancer screening (CTLS) population has been shown to be comparable to Agatston CAC scoring and has been shown to be associated with cardiovascular events.(1–3) There are limited studies examining the risk of non-cardiovascular events, such as chronic obstructive pulmonary disease (COPD) and pneumonia related hospitalizations, in CLTS populations.(4–6)
In the United States, 14.5 million patients are estimated to be eligible for CTLS(7, 8). Patients who qualify for CTLS are high-risk patients often suffering from numerous cardio-pulmonary comorbidities making this group an ideal study population.(9) Using a large clinical CTLS cohort, we sought to examine the following primary and secondary endpoints. Primary endpoint: Utility of qualitatively assessed CAC to identify patients at increased risk for non-cardiovascular events such as all cause and COPD and pneumonia related hospitalization. Secondary endpoints: Confirm previously reports association between coronary calcification and mortality and cardiovascular events. Determine if there is a clinical opportunity to improve quality metrics: active smoking, blood pressure control and lipid management in patients with CAC identified on baseline CTLS exams.
METHODS
Subjects
This is a retrospective, single-center study of all patients, from Lahey Hospital and Medical Center (LHMC), Burlington, MA, who underwent CTLS from January 1, 2012 through September 30, 2017.(10, 11) All patients met the National Comprehensive Cancer Network (NCCN) Guidelines® Lung Cancer Screening Version 1.2012 high-risk criteria for lung cancer, Briefly, individuals eligible for lung cancer screening can be classified into NCCN group 1 and 2. This includes group 1) patients aged 55–74 years with ≥ 30 pack-year smoking history and smoking cessation <15 years or group 2) age ≥ 50 years and ≥ 20 pack year smoking history and 1 additional risk factor (other than 2nd hand smoke). All groups were pooled for the statistical analysis. All patients were asymptomatic and had a physician order for CTLS, were free of lung cancer for ≥ 5 years, and had no known metastatic disease, as previously described.(10–12)
Clinical Variables
Multiple clinical variables were collected prospectively as part of the CTLS program and stored in a centralized data repository. Additional clinical variables not already available in this data repository, including, last date of follow up, body mass index (BMI), race, mortality, cause of death, blood pressure, total cholesterol, low density lipoprotein (LDL), clinical scoring of emphysema and CAC were collected retrospectively by automated and manual review of the electronic medical record (EMR) and stored utilizing a custom-designed database (FileMaker ProVersion 11; Filemaker Inc, Santa Clara, California). Clinical follow-up data was obtained through September 30th 2019. Hospital admissions were collected using Lahey administrative coding data. Principal admission diagnoses of COPD, pneumonia, congestive heart failure (CHF), acute myocardial infarction (AMI), and stroke were characterized based on diagnosis codes per 2018 Center for Medicare and Medicaid Services (CMS) condition-specific measures.(13)
Qualitative Emphysema and Coronary Calcium
Staff radiologists specifically trained in the interpretation of lung screening exams prospectively scored the degree of emphysema and coronary calcium qualitatively on all CTLS exams at the time of exam interpretation, using standardized ordinal categories of “none”, “mild”, “moderate”, and “marked”, as previously described.(1, 10)
CT scans
All CTLS examinations were performed on ≥64-row multidetector CT scanners (LightSpeed VCT and Discovery VCT [GE Medical Systems, Milwaukee, Wisconsin]; Somatom Definition [Siemens AG, Erlangen, Germany]; iCT [Philips Medical Systems, Andover, Massachusetts]) at 100 kV and 30 to 100 mA, depending on the scanner and the availability of iterative reconstruction software. Axial images were obtained at 1.25- to 1.5-mm thickness with 50% overlap and reconstructed with both soft tissue and lung kernels. Axial maximum-intensity projections (16 × 2.5 mm) and coronal and sagittal multiplanar reformatted images were reconstructed and used for interpretation.
Statistical Analysis
Cox proportional hazards regression was utilized to evaluate associations with primary and secondary outcomes of time to first all cause hospitalization, COPD, pneumonia, all-cause mortality, CHF, AMI and stroke related admissions. Variables known to be associated with risk of mortality all cause admission, COPD, pneumonia: age, sex, race, BMI, current smoking status, pack years of smoking, and emphysema severity were included in multivariable Cox proportional hazards models for each of the outcomes.(10, 14) In addition, NCCN group was included in the multivariable model to adjust for inherent confounding based on the baseline differences between these groups. CHF, AMI and stroke admission were adjusted for age, sex, race and pack years of smoking so not to overfit the models. Kaplan-Meier plots were generated to visualize the associations between observed baseline CAC and mortality; first all cause hospitalization; and first COPD, pneumonia, CHF, AMI and stroke related admissions. Chi-squared tests were used for inter group comparisons for differences between radiologist scoring of CAC and emphysema. Sensitivity analyses were performed adjusting for radiologist, and separately, the subset of patients who had Lahey PCPs. A subset analysis of quality metrics including blood pressure, total cholesterol and total LDL were extracted from the EMR were performed utilizing patient with in network PCP’s. Data was limited to in network PCP’s as our EMR does not have access to patients with out of network primary care physician’s routine blood pressure and lipid profiles. Data was expressed and total and % of patients meeting the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) recommendation of a minimum goal BP <140/90(15) and the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III recommendation for treatment of total cholesterol >200 and LDL ≥130 mg/dL in patients with 2 or more cardiovascular risk factors.(16) To adjust for multiplicity Bonferroni correction was utilized for our three primary outcomes and p-value significance was set at <0.017. Otherwise, significance levels were set at a p-value < 0.05. All statistical analyses were performed using STATA14.1 software.
RESULTS
A total of 4,673 individuals underwent CTLS during the study period. The mean age was 62.4 ± 6.2 years, 2543 (54.4%) were male, 4,405 (94.3%) were white, 3,657 (78.3%) were NCCN Group 1, 2310 (49.4%) were former smokers with an average year quit of 10.0 ± 8.4, 2,666 (57.0%) had emphysema and 3,631 (77.7%) had evidence of CAC. Of these, 1308 (28.0%), 1128 (24.1%), and 1,195 (25.6%) had mild, moderate and marked coronary calcification, respectively. Table 1.
Table 1.
Demographics of CTLS Cohort.
| LHMC | |
|---|---|
| (N=4,673) | |
| Age | 62.4 ± 6.2 |
| Sex: | |
| Male | 2543 (54.4%) |
| Race: | |
| White Race | 4405 (94.3%) |
| Asian | 41 (0.9%) |
| African American | 18 (0.4%) |
| Other | 209 (4.5%) |
| BMI | 29.2 ± 6.0 |
| In Network PCP | 3225 (69.0%) |
| Smoking: | |
| Current | 2363 (50.6%) |
| Pack Years | 48.6 ± 21.8 |
| Years Quit | 10.0 ± 8.4 |
| Years Follow Up | 3.97 ± 2.11 |
| Screening Group: | |
| NCCN Group 1 | 3657 (78.3%) |
| Emphysema (Yes) | 2665 (57.0%) |
| Mild | 1839 (39.4%) |
| Moderate | 594 (12.7%) |
| Marked | 178 (3.8%) |
| Not scored | 54 (1.2%) |
| CAC (Yes) | 3632 (77.7%) |
| Mild | 1308 (28.0%) |
| Moderate | 1128 (24.1%) |
| Marked | 1195 (25.6%) |
| Not scored | 0 (0%) |
There was a similar distribution of CAC scoring among radiologists with three radiologists reading the vast majority 3915 (83.8%) of the scans. Radiologists reading less than 100 scans each were grouped together for this comparison as they read a combined total of only 143 (3.06%) scans, see Supplemental Table 1. There was a statistically significant difference between radiologist scoring of moderate and marked, P = <0.001 but there was no statistical difference between radiologists for none and mild CAC, P > 0.05.
There was a similar distribution of emphysema scoring among radiologists, as previously described.(10)
Primary Endpoints:
Qualitative CAC and All-cause hospital admission
There were 2,829 all-cause hospital admissions, of which 1,248 (26.7%) patients were admitted to the hospital at least once. The one year and two year hospital admission rates were 387 (8.3%) and 679 (14.5%), respectively.
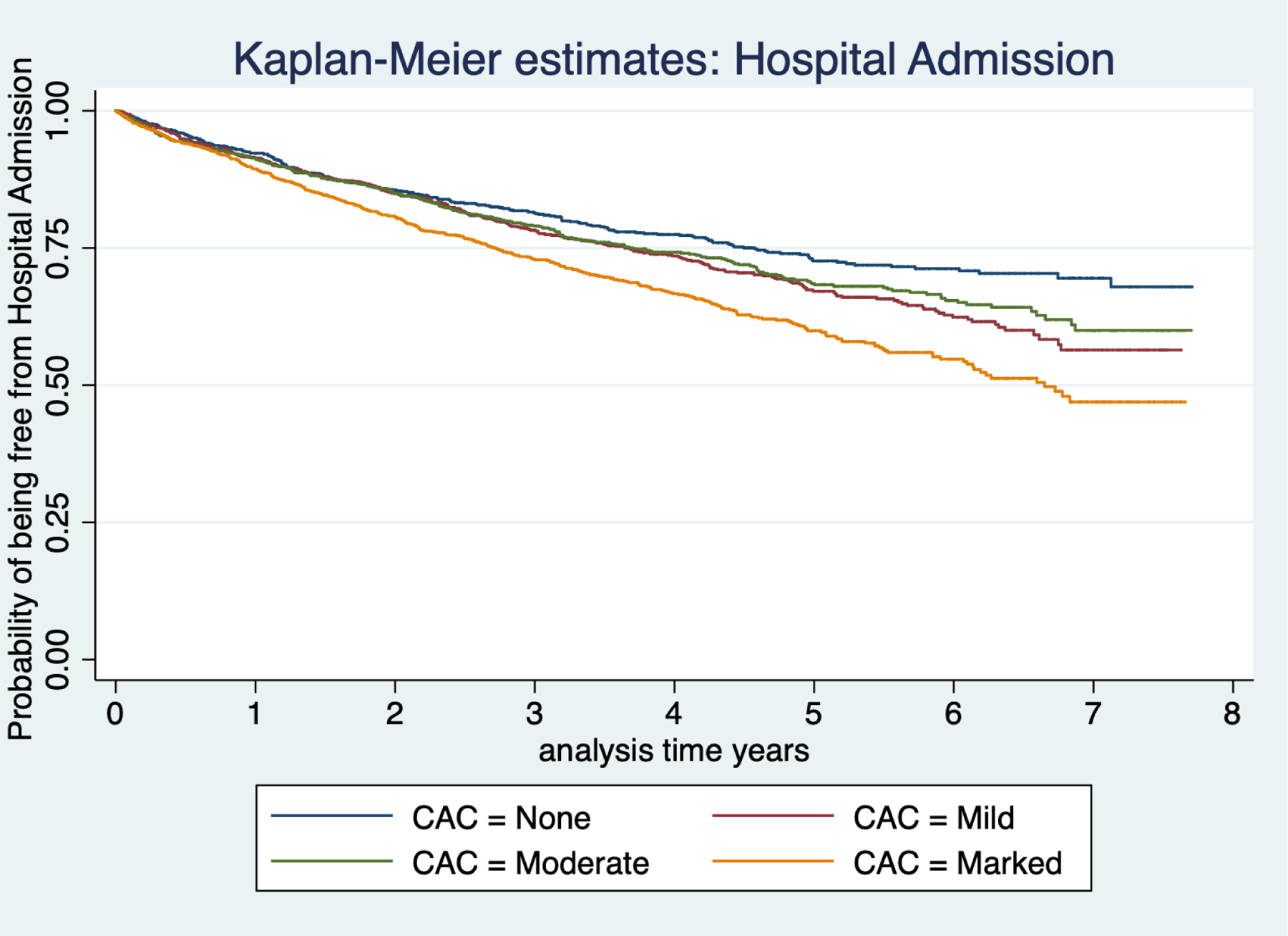
On multivariable analysis the presence of marked CAC was associated with an increased risk for all-cause hospital admission, HR 1.48; 95% CI 1.23–1.78. Figure 1, shows the unadjusted Kaplan-Meier survival plot for extent of CAC and the probability of being free from all-cause admission over time, Table 2.
Figure 1:
Kaplan-Meier Plot for Coronary artery calcification extent and all cause hospital admission
Table 2:
multivariable analyses for model all cause, COPD and pneumonia admissions.
| Multivariable | All-cause hospital admission | COPD | Pneumonia | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CAC Extent | 1.12 (1.06–1.18) | <0.001 | 1.19 (1.01–1.41) | 0.036 | 1.26 (1.08–1.48) | 0.004 |
| None | Reference | Reference | Reference | |||
| Mild | 1.21 (1.02–1.44) | 0.032 | 2.30 1.31–4.03) | 0.004 | 1.48 (0.88–2.47) | 0.140 |
| Moderate | 1.11 (0.92–1.34) | 0.263 | 2.17 (1.20–3.91) | 0.010 | 1.54 (0.90–2.64) | 0.111 |
| Marked | 1.48 (1.23–1.78) | <0.001 | 2.27 (1.24–4.15) | 0.008 | 2.19 (1.30–3.71) | 0.003 |
adjusted for age, sex, race, BMI, current smoking status, pack years of smoking, emphysema severity and NCCN group.
Qualitative CAC and COPD admission
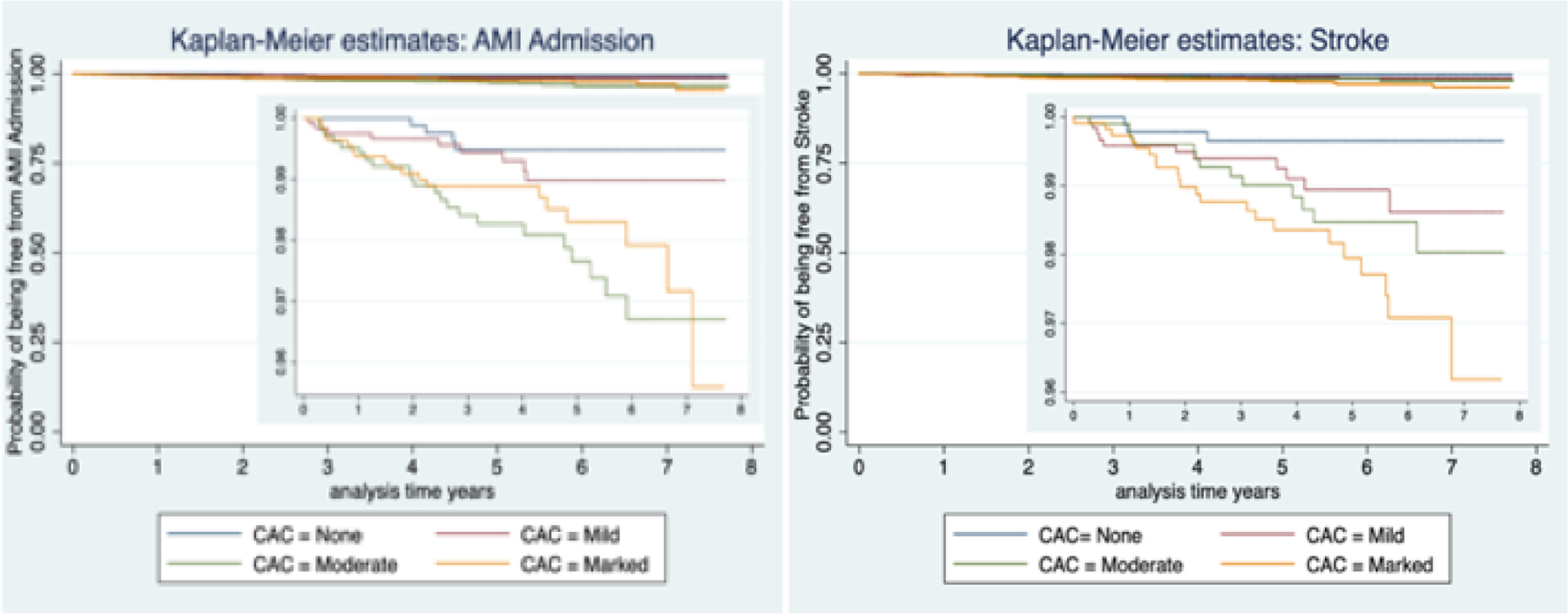
Of the 4,673 participants, 150 (3.21%) were admitted for COPD at least once. On multivariable analysis mild, moderate and marked CAC (compared to no CAC) was associated with an increased risk of COPD admission , HR 2.30; 95% CI 1.31–4.03, HR 2.17; 95% CI 1.20–3.91 and HR 2.27; 95% CI 1.24–4.15, respectively, Table 2. Figure 2, shows a Kaplan-Meier survival plot for CAC severity and the probability of being free from COPD admission over time.
Figure 2:
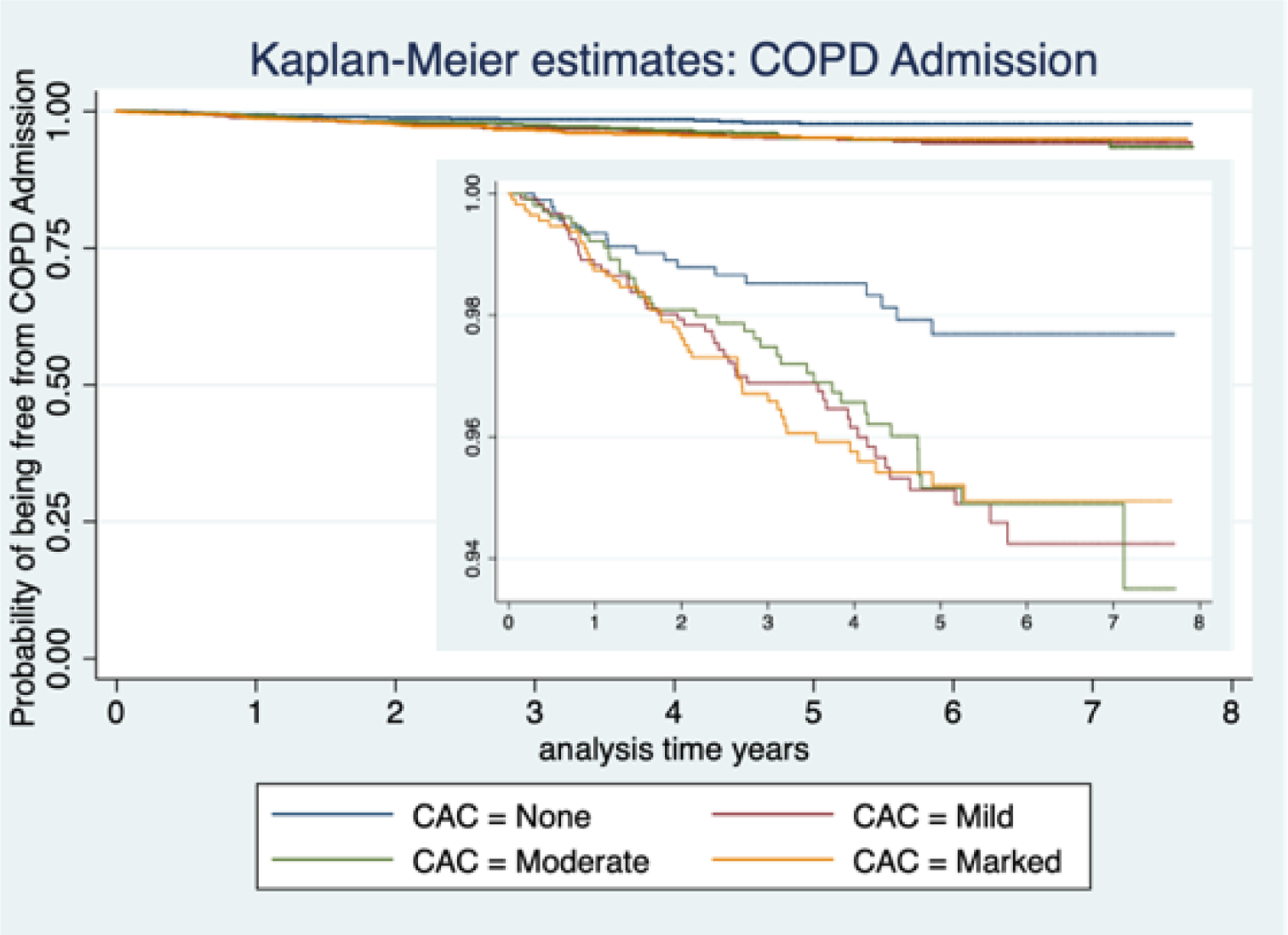
Kaplan-Meier Plot for Coronary artery calcification extent and COPD related hospital admission
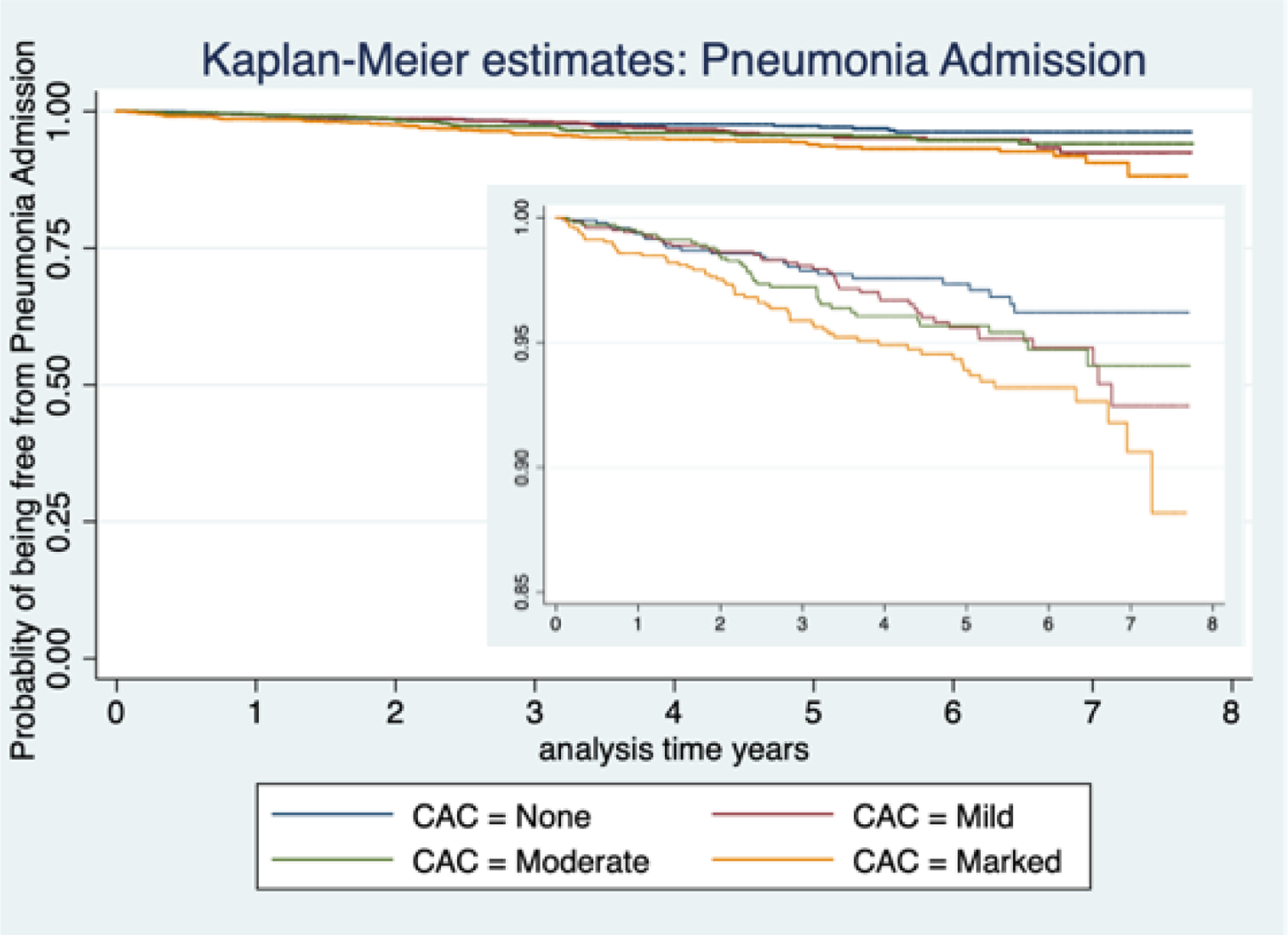
Qualitative CAC and Pneumonia admission
A total of 169 (3.61%) participants were admitted for pneumonia at least once. On multivariable analysis marked CAC was associated with an increased risk of pneumonia admission, HR 2.19; 95% 1.30–3.71, Table 2, Figure 3 shows unadjusted Kaplan-Meier survival plot for CAC and the probability of being free from pneumonia admission over time.
Figure 3:
Kaplan-Meier Plot for Coronary artery calcification extent and pneumonia related hospital admission
Secondary Endpoints:
Qualitative CAC and Mortality
There were 273 (5.8%) deaths, of which 119 (43.6%) were other/unknown etiology, 70 (25.6%) secondary to cancer, 47 (17.2%) secondary to pulmonary etiology and 47 (17.2%) secondary to cardiovascular etiology.
On multivariable analysis the presence of marked CAC compared to no CAC was associated with an increased risk for mortality, HR 2.48; 95% CI 1.60–3.85, Table 3. Figure 4, shows unadjusted Kaplan-Meier survival plot for extent of CAC and the probability of being free from mortality over time.
Table 3:
Cox Multivariable analyses for all-cause mortality, CHF, AMI and Stroke admission.
| Multivariable | All-cause mortality | CHF | AMI | Stroke | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CAC Extent | 1.36 (1.20–1.55) | <0.001 | 1.27 (1.00–1.62) | 0.049 | 1.49 (1.12–1.99) | 0.007 | 1.56 (1.15–2.12) | 0.005 |
| None | Reference | Reference | Reference | Reference | ||||
| Mild | 1.33 ( 0.84–2.10) | 0.218 | 1.38 ( 0.61–3.13) | 0.438 | 1.78 ( 0.55–5.85) | 0.338 | 2.85 ( 0.79–10.33) | 0.110 |
| Moderate | 1.49 (0.95–2.36) | 0.084 | 1.46 (0.63–3.36) | 0.377 | 4.81 (1.61–14.34) | 0.005 | 3.62 (1.00–13.03) | 0.049 |
| Marked | 2.48 (1.60–3.85) | <0.001 | 2.16 (0.96–4.86) | 0.062 | 3.66 (1.17–11.52) | 0.026 | 5.45 (1.53–19.39) | 0.009 |
All-cause mortality adjusted for age, sex, race, BMI, current smoking status, pack years of smoking, emphysema severity and NCCN group. CHF, AMI and stroke adjusted for age, sex, race and pack years of smoking.
Figure 4:
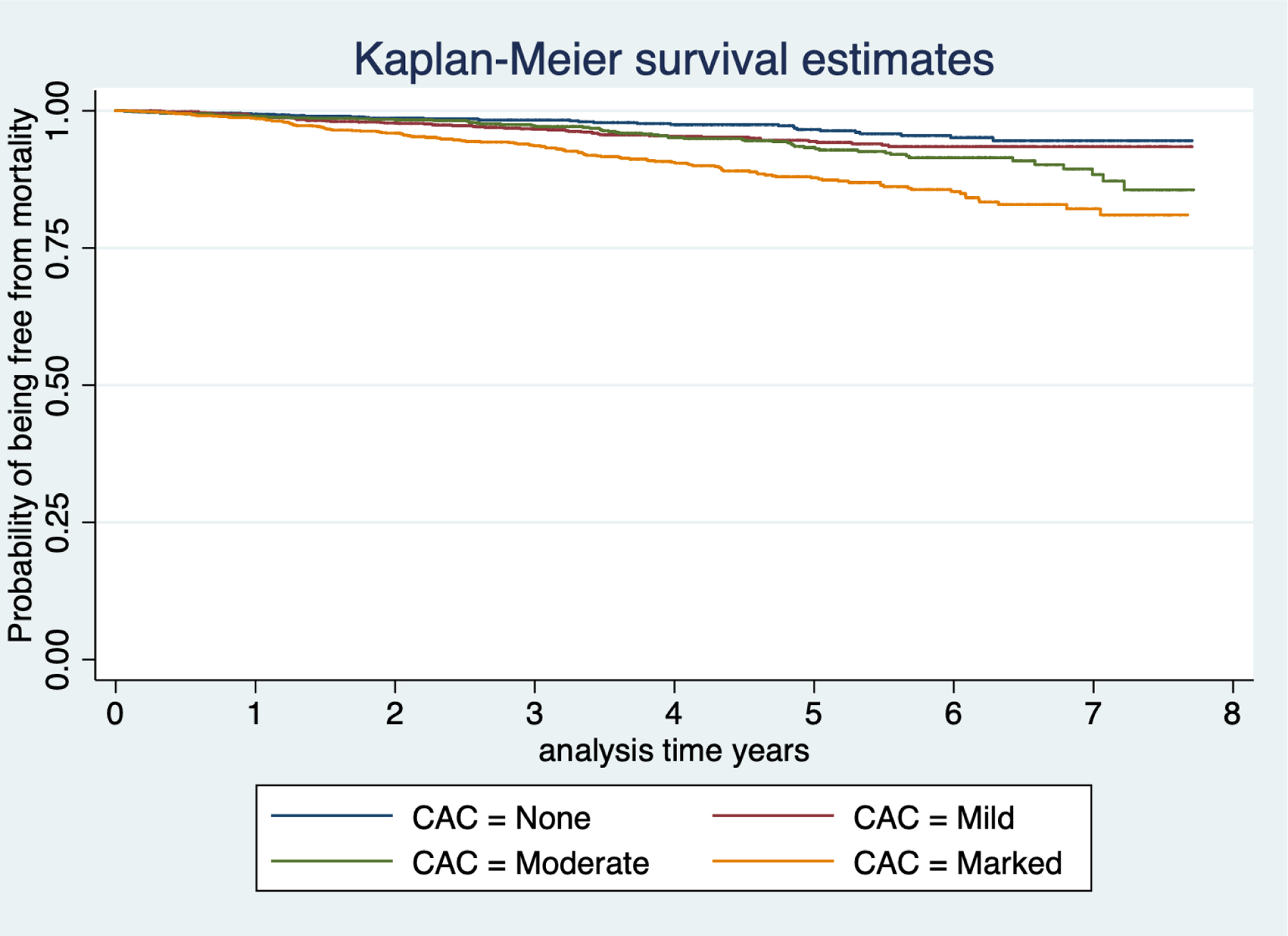
Kaplan-Meier Plot for Coronary artery calcification extent and all-cause mortality
Qualitative CAC and Cardiovascular admissions
A total of 73 (1.6%), 53 (1.1%) and 49 (1.0%) patients were admitted for CHF, AMI and stroke at least once.
On multivariable analysis moderate and marked were associated with increased risk of AMI admission, HR 4.81; 95% CI 1.61–14.34 and HR 3.66; 95% CI 1.17–11.52, respectively, Table 3. Figure 5 shows a Kaplan-Meier survival plot for CAC and the probability of being free from AMI admission over time.
Figure 5:
Kaplan-Meier Plot for Coronary artery calcification extent and acute myocardial infarction and stroke related hospital admission
On multivariable analysis moderated and marked CAC were associated with an increased risk for stroke, HR 3.62; 95% CI 1.00–13.03 and HR 5.45; 95% CI 1.53–19.39, Table 3. Figure 5, shows the Kaplan-Meier survival plot for CAC and the probability of being free from stroke admission over time.
Quality Metrics:
In a subset of patients with an in-network primary care physician, approximately 50% of patients with CAC identified on their baseline CTLS exams were actively smoking. Of these patients with mild, moderate and marked CAC on baseline CTLS exams, 58.6%, 60.3% and 76% of the patients had total cholesterol levels < 200 mg/dL, respectively. Approximately 80% of the patients with CAC on baseline exams had systolic blood pressures less than 140, Table 4. In a subset of active smokers with moderate CAC, 73.1% had a total cholesterol level <130mg/dL and 40.4% <100mg/dL. Of those with marked CAC, 85% had LDL cholesterol levels less than 130 mg/dL; and 59.6% had a total cholesterol < 100 mg/dL, Table 5.
Table 4.
Active smoking, total cholesterol<200, LDL <130. HDL>40 and systolic BP <140 in patients with in network primary care physicians.
| N = 3,226 (69%) | No CAC | Mild CAC | Moderate CAC | Marked CAC |
|---|---|---|---|---|
| Active smoking | 370 (53.7%) | 500 (54.4%) | 418 (54.0%) | 406 (48.2%) |
| Total Cholesterol <200 | 342 (55.4%) | 476 (58.6%) | 420 (60.3%) | 578 (76.0%) |
| Systolic BP < 140 | 591 (86.4%) | 730 (79.9) | 615 (80.3%) | 641 (76.5%) |
Table 5.
LDL control in active smokers with moderate and marked CAC on baseline CTLS exam with in network primary care physicians.
| Active smoking n 1,695 (52.5%) | Moderate CAC n 371 (21.9%) | Marked CAC n 359 (21.2%) |
|---|---|---|
| LDL <130 | 271 (73.1%) | 305 (85.0%) |
| LDL <100 | 150 (40.4%) | 214 (59.6%) |
DISCUSSION
The association between CAC and the risk for COPD and pneumonia admissions has not been well defined.(1–4) The association between COPD and coronary artery disease (CAD) is well documented and is thought to be due to inflammation.(17–19) Mota et al. demonstrated that the presence of COPD was associated with an increase in CAC independent of established risk factors for CAD.(6) In patients with COPD, CAC correlates with degree of functional impairment and mortality.(20) We have previously demonstrated that qualitatively assessed emphysema is associated with risk for COPD hospitalization in two independent CTLS cohorts.(10) Our current study demonstrates that qualitative assessments of CAC are also associated with an increased risk for COPD and pneumonia admission independent of emphysema severity.
Our results confirm the previously reported association between CT CAC and risk of mortality and cardiovascular events.(1–3) The JNC 7 recommends a minimum goal BP <140/90 with lower treatment thresholds for patients with diabetes or chronic kidney disease.(15) The National Cholesterol Education Program (NCEP Adult Treatment Panel (ATP) III recommends treatment of LDL ≥130 mg/dL in patients with 2 or more cardiovascular risk factors.(16) Our data suggests there are opportunities to improve known cardiovascular risk factors such as smoking, lipid profiles and blood pressure management in our CTLS population. Fentanes et al demonstrated that preventative cardiology clinics focused on cardiovascular disease risk-management improved lipid and blood pressure management and treatment compliance. (21) In addition, clinic patients were more likely to be reclassified to higher risk categories with the use of CAC scores.(21)
As a single center study our results may not be generalizable other patient populations. In addition our study sample was predominantly white and lacked minority representation, which is a function of the location of our facility in a particular metropolitan suburb with relatively homogenous ethnicity. This lack of diversity may underestimate the effects of certain social determinants of health on CTLS outcomes. Our hospitalization data was only collected for those patients admitted within our hospital network. Hence, data about admissions of patients to hospitals outside our health systems may not have been captured. This may result in an underestimation of the true rates of hospitalization. However, in our sensitivity analysis excluding patients without in network primary care physicians the hazard ratios were similar (data not shown). Hospital admissions data were collected using Lahey administrative coding data to provide a principal diagnosis for each hospitalization thus the validity of each diagnosis may not be as rigorous as a detailed chart review. However, these metrics are the basis for how hospital systems are assessed and penalized for 30 day readmission metrics based on 2018 Center for Medicare and Medicaid Services (CMS) condition-specific measures.(13)
An additional limitation is that we did not account for the competing risk of death in our hospital admission models.
We also included both NCCN group 1 and group 2 in our study design. NCCN group 2 patients are younger with lower tobacco exposure. When the analysis was run separately for NCCN group 2 there was no significant difference in outcomes in our group 2 patients which had a significantly lower number of events (data not shown). Since our cohort is predominately NCCN group 1 these results are only generalizable to NCCN group 1 patients.
A limitation of our study is that it is standard practice for our radiologists to clinically score CAC and this data was utilized for our analysis. Our standard clinical scoring did not allow us to assess inter-rater reliability between radiologists for the scoring of degrees of CAC. We did include scoring radiologist in the multivariable models and the results did not change (data not shown). Future studies utilizing automated quantitative methods to measure CAC may help address this limitation.
Future study is needed to see if quantitative CAC scores improves risk stratification. We did not have baseline hypertension treatment and diabetes status in our cohort and were unable to calculate an atherosclerotic cardiovascular disease risk score. A better characterization of cardiovascular risk profiles in CTLS populations would allow for a more accurate estimation of the potential for risk factor modification in this high risk population.
Patients who qualify for CTLS are at high-risk for numerous cardio-pulmonary comorbidities. The ability to identify patients at high risk for cardiovascular and pulmonary hospitalization, would enable health care institutions to implement programs that provide targeted clinical care for this subgroup of higher-risk patients. Interventions, such as COPD screening through pulmonary function testing (PFTs), intensive smoking cessation programs, vaccination against pneumococcal pneumonia, referral to a pulmonary specialist and dedicated clinics for cardiovascular risk reduction can potentially be implemented in CTLS populations and referral guided by qualitative or quantitative CAC and emphysema scores. These interventions have all been shown to reduce hospitalizations and improve outcomes in patients with COPD and cardiovascular risk factors.(10, 22–28)
As previously reported CAC and emphysema are common incidental findings on CTLS exams and have the potential to be both a qualitative and quantitative biomarker to help discriminate patients at risk for adverse events.(10, 29, 30) Further study is needed to determine if qualitative and quantitative CAC scoring can be incorporated in risk prediction models for cardiovascular and pulmonary related hospitalizations in CTLS populations.
Supplementary Material
Acknowledgments:
The Authors would like to thank Adam Medina, John Lemmerman, Dr. Michael Cundiff, MD, and Brittney Wilson, PA for their assistance with the data collection for this manuscript.
Funding:
This work was supported by the following National Institutes of Health grants:
Lee Gazourian, MD was supported by a grant from the Robert E. Wise Institute at Lahey Hospital and Medical Center, as well as an institutional 2018 Physician Research Stipend and Clinical and Translational Science Institute (CTSI) support.
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR002544. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Giulia S. Rizzo was supported by the 2016 Tufts University School of Medicine Summer Research Fellowship. Cristina F. Stefanescu was supported by the 2016 Tufts University School of Medicine Summer Research Fellowship. Ava M. Sanayei was supported by the 2017 Harold Williams Summer Research Fellowship. William P. Long was supported by the 2017 The Aid for Cancer Research Fellowship. William B. Thedinger was supported by the 2017 Tufts University Post Bac Research Fellowship and 2018 and 2019 Rescue Lung, Rescue Life Summer Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES:
- 1.Malcolm KB, Dinwoodey DL, Cundiff MC, Regis SM, Kitts AKB, Wald C, Lynch ML, Al-Husami W, McKee AB, McKee BJ. Qualitative coronary artery calcium assessment on CT lung screening exam helps predict first cardiac events. J Thorac Dis 2018; 10: 2740–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, DeMello S, Desjardins SS, Munden RF, Team NS. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: A Comparison of Three Scoring Methods. Radiology 2015; 276: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XR, Zhang JJ, Xu XX, Wu YG. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail 2019; 41: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrales-Medina VF, Dwivedi G, Taljaard M, Petrcich W, Lima JA, Yende S, Kronmal RA, Chirinos JA. Coronary artery calcium before and after hospitalization with pneumonia: The MESA study. PLoS One 2018; 13: e0191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaisl T, Schlatzer C, Schwarz EI, Possner M, Stehli J, Sievi NA, Clarenbach CF, Dey D, Slomka PJ, Kaufmann PA, Kohler M. Coronary artery calcification, epicardial fat burden, and cardiovascular events in chronic obstructive pulmonary disease. PLoS One 2015; 10: e0126613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mota IL, Sousa ACS, Almeida MLD, de Melo EV, Ferreira EJP, Neto JB, Matos CJO, Telino C, Souto MJS, Oliveira JLM. Coronary lesions in patients with COPD (Global Initiative for Obstructive Lung Disease stages I-III) and suspected or confirmed coronary arterial disease. Int J Chron Obstruct Pulmon Dis 2018; 13: 1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson LM, Rivera MP, Basch E. Broadened Eligibility for Lung Cancer Screening: Challenges and Uncertainty for Implementation and Equity. JAMA 2021; 325: 939–941. [DOI] [PubMed] [Google Scholar]
- 8.Force UPST. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021; 325: 962–970. [DOI] [PubMed] [Google Scholar]
- 9.Mets OM, de Jong PA, Prokop M. Computed tomographic screening for lung cancer: an opportunity to evaluate other diseases. JAMA 2012; 308: 1433–1434. [DOI] [PubMed] [Google Scholar]
- 10.Gazourian L, Thedinger WB, Regis SM, Pagura EJ, Price LL, Gawlik M, Stefanescu CF, Lamb C, Rieger-Christ KM, Singh H, Casasola M, Walker AR, Rupal A, Patel AS, Come CE, Sanayei AM, Long WP, Rizzo GS, McKee AB, Washko GR, San Jose Estepar R, Wald C, McKee BJ, Thomson CC, Liesching TN. Qualitative emphysema and risk of COPD hospitalization in a multicenter CT lung cancer screening cohort study. Respir Med 2020; 176: 106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazourian L, Durgana CS, Huntley D, Rizzo GS, Thedinger WB, Regis SM, Price LL, Pagura EJ, Lamb C, Rieger-Christ K, Thomson CC, Stefanescu CF, Sanayei A, Long WP, McKee AB, Washko GR, Estepar RSJ, Wald C, Liesching TN, McKee BJ. Quantitative Pectoralis Muscle Area is Associated with the Development of Lung Cancer in a Large Lung Cancer Screening Cohort. Lung 2020; 198: 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, Klippenstein D, Lackner RP, Leard L, Leung AN, Massion PP, Meyers BF, Munden RF, Otterson GA, Peairs K, Pipavath S, Pratt-Pozo C, Reddy C, Reid ME, Rotter AJ, Schabath MB, Sequist LV, Tong BC, Travis WD, Unger M, Yang SC. Lung cancer screening. J Natl Compr Canc Netw 2012; 10: 240–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evaluation YNHHSC-CfOR. 2018 Condition-Specific Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standrdidized Readmission Measures. 2018.
- 14.Luben R, Hayat S, Wareham N, Khaw KT. Predicting admissions and time spent in hospital over a decade in a population-based record linkage study: the EPIC-Norfolk cohort. BMJ Open 2016; 6: e009461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Roccella EJ, National Heart L, Blood Institute Joint National Committee on Prevention DE, Treatment of High Blood P, National High Blood Pressure Education Program Coordinating C. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr., Stone NJ, National Heart L, Blood I, American College of Cardiology F, American Heart A. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110: 227–239. [DOI] [PubMed] [Google Scholar]
- 17.Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991–1999. COPD 2005; 2: 35–41. [DOI] [PubMed] [Google Scholar]
- 18.Pinto-Plata VM, Mullerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, Celli BR. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006; 61: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E Jr., She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006; 16: 63–70. [DOI] [PubMed] [Google Scholar]
- 20.Williams MC, Murchison JT, Edwards LD, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Vestbo J, Wouters E, Yates JC, van Beek EJ, Newby DE, MacNee W, Evaluation of CLtIPSEi. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax 2014; 69: 718–723. [DOI] [PubMed] [Google Scholar]
- 21.Fentanes E, Vande Hei AG, Holuby RS, Suarez N, Slim Y, Slim JN, Slim AM, Thomas D. Treatment in a preventive cardiology clinic utilizing advanced practice providers effectively closes atherosclerotic cardiovascular disease risk-management gaps among a primary-prevention population compared with a propensity-matched primary-care cohort: A team-based care model and its impact on lipid and blood pressure management. Clinical Cardiology 2018; 41: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavish R, Levy A, Dekel OK, Karp E, Maimon N. The Association Between Hospital Readmission and Pulmonologist Follow-up Visits in Patients With COPD. Chest 2015; 148: 375–381. [DOI] [PubMed] [Google Scholar]
- 23.Deepak JA, Ng X, Feliciano J, Mao L, Davidoff AJ. Pulmonologist involvement, stage-specific treatment, and survival in adults with non-small cell lung cancer and chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015; 12: 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershon A, Mecredy G, Croxford R, To T, Stanbrook MB, Aaron SD. Outcomes of patients with chronic obstructive pulmonary disease diagnosed with or without pulmonary function testing. CMAJ 2017; 189: E530–E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Sin DD. Improved patient outcome with smoking cessation: when is it too late? Int J Chron Obstruct Pulmon Dis 2011; 6: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters JA, Tang JN, Poole P, Wood-Baker R. Pneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017; 1: CD001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueira Goncalves JM, Garcia Bello MA, Bethencourt Martin N, Diaz Perez D, Perez-Mendez LI. Impact of 13-valent pneumococcal conjugate polysaccharide vaccination on severe exacerbations in patients with chronic obstructive pulmonary disease and established cardiovascular disease. Eur J Intern Med 2019; 63: e14–e16. [DOI] [PubMed] [Google Scholar]
- 28.Fentanes E, Vande Hei AG, Holuby RS, Suarez N, Slim Y, Slim JN, Slim AM, Thomas D. Treatment in a preventive cardiology clinic utilizing advanced practice providers effectively closes atherosclerotic cardiovascular disease risk-management gaps among a primary-prevention population compared with a propensity-matched primary-care cohort: A team-based care model and its impact on lipid and blood pressure management. Clin Cardiol 2018; 41: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulshine JL. One Screening for Ischemic Heart Disease, Lung Cancer, and Chronic Obstructive Pulmonary Disease: A Systems Biology Bridge for Tobacco and Radiation Exposure. Am J Public Health 2018; 108: 1294–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulshine JL. Status of lung cancer screening. J Thorac Dis 2017; 9: 4311–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


