Abstract
Background
Patients with obstructive sleep apnea (OSA) most commonly receive positive airway pressure therapy (PAP) as primary treatment, which is highly effective when used consistently. Little is known about the preferences for and relevance of attributes of OSA treatments, especially of non-PAP alternatives. The aim of this study was to evaluate treatment preferences and willingness to pay (WTP) among patients with and without previous experience of OSA therapies.
Methods
A discrete choice experiment and a structured survey were applied to patients presenting for overnight polysomnography at a tertiary sleep center. Medical variables were obtained from hospital case records.
Results
Over a period of 4 months, 241 subjects were enrolled and answered the questionnaire (61.8% with an existing diagnosis, 38.2% with a new diagnosis). The most preferred treatment among all patients was PAP therapy (51.1%), followed by mandibular advancement devices (18.1%), hypoglossal nerve stimulation (17.2%), and medication (13.7%). Approval for the different treatments varied by gender as well as by OSA therapy experience. The importance of attributes of OSA treatment varied too, with low rates of treatment-related side effects being equally important, independent of the preferred therapy. The most often stated monthly WTP for optimal sleep was € 50, with increasing age leading to lower WTP values.
Conclusion
Preferences for OSA therapies vary among patients and patient subgroups. PAP therapy is the most preferred treatment, though non-PAP interventions receive high approval ratings too, particularly in treatment-naïve patients. The importance of treatment attributes varies as well, depending on the choice of preferred treatment.
Keywords: Sleep apnea syndrome, Continuous positive airway pressure, Hypoglossal nerve stimulation, Mandibular advancement device, Drug therapy
Abstract
Hintergrund
Patienten mit obstruktiver Schlafapnoe (OSA) werden standardmäßig mit PAP-Therapie („positive airway pressure“) versorgt, welche bei kontinuierlicher Anwendung die Krankheitslast effektiv reduziert. Bisher ist zu Patientenpräferenzen und Bedeutung einzelner Therapiemerkmale bei der Behandlung der OSA wenig bekannt, insbesondere hinsichtlich alternativer Nicht-PAP-Therapien. Studienziel war die Ermittlung von Therapiepräferenzen und der Zahlungsbereitschaft bei Patienten mit OSA mit und ohne Therapieerfahrung.
Material und Methoden
Ein diskretes Entscheidungsexperiment sowie ein strukturierter Fragebogen wurden entwickelt und Patienten vorgelegt, die sich in einem Zentrum der Tertiärversorgung zur Polysomnographie vorstellten. Medizinische Daten wurden den elektronischen Patientenakten entnommen.
Ergebnisse
Insgesamt wurden 241 Patienten in die Studie eingeschlossen (61,8 % mit und 38,2 % ohne Therapieerfahrung). Die häufigste präferierte Therapie war die PAP-Beatmung (51,1 %), gefolgt von der Behandlung mit der Unterkieferprotrusionsschiene (18,1 %), der Hypoglossusnervstimulation (17,2 %) sowie der medikamentösen Therapie (13,7 %). Die Zustimmung zu den einzelnen Behandlungsformen unterschied sich erheblich nach Geschlecht sowie Erfahrungen mit OSA-Therapie. Die Bedeutung einzelner Behandlungsmerkmale war ebenfalls unterschiedlich, dabei waren unabhängig von der bevorzugten Behandlung geringe Raten des Auftretens therapiebezogener Nebenwirkungen gleichermaßen wichtig. Die am häufigsten genannte Zahlungsbereitschaft für guten Schlaf lag bei € 50 pro Monat, mit niedrigeren Werten bei höherem Lebensalter.
Schlussfolgerung
Die Präferenzen von Patienten und Patientenuntergruppen in Bezug auf verschiedene OSA-Therapien unterscheiden sich erheblich. PAP-Beatmung wird am häufigsten bevorzugt, jedoch gibt es auch hohe Zustimmungswerte für Nicht-PAP-Verfahren, insbesondere bei Patienten ohne Therapieerfahrung. Ebenso variiert die Bedeutung der einzelnen Behandlungsmerkmale in Abhängigkeit von der präferierten Primärtherapie.
Schlüsselwörter: Schlafapnoesyndrom, Überdrucktherapie, Hypoglossusnerv-Stimulation, Unterkieferprotrusionsschiene, Medikamentöse Therapie
Obstructive sleep apnea (OSA) is one of the most common chronic conditions in adults. The disease is characterized by cessation of airflow to the lungs, which is caused by collapse of upper airway soft tissues during sleep when muscles relax [1]. This can lead to a partial or complete halt of gas exchange in the lungs and subsequent phases of hypoxemia and hypercapnia. Affected patients often suffer from interrupted sleep due to arousals following apneic or hypopneic events. The prevalence in the general population is estimated at between 23 and 46%, depending on severity level [2, 3]. Though symptoms can vary, daytime sleepiness and reduced daytime functioning are often reported and usually lead to confirmation of an OSA diagnosis [4, 5]. Due to repeated phases of desaturation, patients with OSA frequently suffer from chronic inflammation, which increases the risk of cardiovascular and metabolic diseases [6]. Given the high prevalence, changing clinical presentation, and inconsistent evidence on treatment benefits, the decision regarding which patients require treatment is the subject of ongoing discussion.
Primary treatment for OSA is positive airway pressure therapy (PAP), which is applied via a nasal or facial mask and prevents obstructions by splinting the airway. PAP therapy is highly effective in reducing OSA severity and improving symptoms as well as OSA-related comorbidities such as hypertension [7, 8]. Mandibular advancement devices (MAD), which increase the posterior airway space, have been introduced as an alternative treatment that can be equivalent to PAP therapy in patients with mild or moderate OSA and without significant obesity [9]; however, superior adherence compared to PAP therapy is of offset by slightly lower efficacy. With nightly stimulation of the hypoglossal nerve (HNS), another alternative treatment has been introduced into clinical routine in recent years for patients who cannot adhere to PAP treatment. HNS has been demonstrated to be comparable to PAP therapy in reducing disease severity and improving symptoms of OSA [10, 11]. In addition, different pharmacological agents are being evaluated for use in OSA, though none have yet received market approval [12]. Beside the abovementioned treatments, different surgical interventions, including palate or tongue-base surgery, and positional therapy are used in clinical practice [9, 13].
Though many different treatment modalities exist, PAP therapy is by far the most commonly used treatment and patients in most circumstances are not free to choose alternatives, given financial constraints or limited availability. Therefore, evidence on patients’ preferences for OSA treatment methods is scarce. The scope of this study was to investigate treatment preferences using stated-preference techniques.
Materials and methods
Experimental design
The questionnaire was developed using two main elements: a discrete choice experiment (DCE) to estimate the importance of treatment attributes and a structured choice survey to record preferences for different OSA therapies. Relevant attributes of OSA treatments used for the discrete choice experiment were identified from a structured literature review and validated in a quantitative pre-study with 78 participants. The results have been reported previously [14]. To evaluate preferences for concrete OSA treatment, the following three different therapies were selected, which were theoretically available to patients in Germany when the experiment was conducted: positive airway pressure therapy, treatment with a mandibular advancement device, and hypoglossal nerve stimulation. As pharmaceutical therapies for OSA are currently under development and may potentially be available in the future, this treatment modality was added as fourth option. Participants were then asked to choose their preferred primary treatment. In addition, a four-level Likert scale was used to estimate general approval for treatment with the four therapies.
Willingness to pay (WTP) was assessed using a bipolar scale with nine levels, which represent different monetary values that patients would be willing to spend out of their personal budget to obtain optimal sleep.
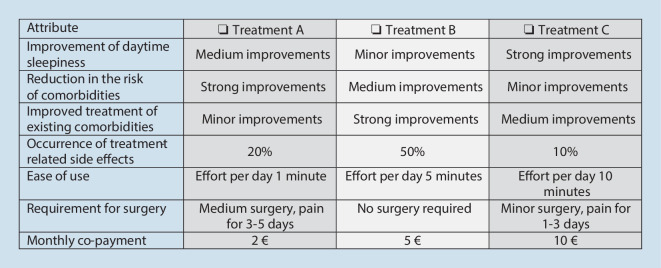
The DCE used seven attributes of OSA treatment, each with three levels, which were presented in four randomly allocated choice tasks per patient. The attributes were “Improvement of daytime sleepiness,” “Reduction in the risk of OSA-related comorbidities,” “Improved treatment of existing comorbidities,” “Occurrence of treatment-related side effects,” “Ease of use,” “Requirement for surgery,” and “Monthly co-payment.” In each choice task, participants were asked to choose one out of three alternatives, which could require a trade-off between increased benefits in one attribute and fewer benefits in another attribute (Fig. 1). To maximize information gain from the DCE, a forced-choice approach was selected, which meant that a no-treatment option was not displayed.
Fig. 1.
Discrete choice experiment: example choice task
For both elements of the experiment, graphical elements were used where applicable, and the survey language was adjusted to patient level.
Enrollment
Patients presenting at the study center for an overnight polysomnography and suspected or confirmed diagnosis of OSA were recruited for the study after consultation with a sleep physician and provision of written informed consent. Medical information on each participant was obtained from patient records. The study was approved by the local ethics committee (Ethics Commission University Hospital Essen, Germany: 20-9315-BO).
Statistical analysis
Data management and descriptive analysis was performed with SPSS software (IBM Corp., Armonk, NY, USA, version 26.0). Multivariate analysis was performed using R with RStudio (https://www.r-project.org, version 3.6.3 and https://www.rstudio.com, version 1.1.463). P-values of < 0.05 were considered statistically significant and recent guidelines for the analyses of choice-based experiments were applied [15, 16]. Depending on data distribution and the scale of variables, Mann–Whitney U, Kruskal–Wallis, and Chi2-tests were used.
Since previous or recent experience with certain treatments—positive or negative—will naturally impact decision-making, subgroup analyses were performed using this criterion.
Results
Sample characteristics
Over a period of 4 months, 241 patients provided informed consent and participated in the study. The majority were of male gender (69.3 vs. 30.7%) and the mean age of the cohort was 57.2 ± 13.8 years (Table 1). More than half of all participants had previous experience with OSA therapies (61.8%), while the rest were treatment naïve (38.2%). The mean Respiratory Disturbance Index (RDI) was 15.5 ± 18.6 events/h (range: 0–101.1 events/h) and patients were mostly asymptomatic with a mean Epworth Sleepiness Scale (ESS) score of 8.5 ± 5.0 (range: 0–23 points). Participants had 2.0 ± 1.5 comorbidities on average, the most common being arterial hypertension (58.9%) followed by diabetes mellitus type II (14.5%) and coronary artery disease (11.2%). Almost one fifth of the cohort (19.5%) had coexisting sleep-related disorders, such as restless legs syndrome, periodic limb movements, or insomnia.
Table 1.
Sample characteristics
| All patients, N = 241 | Previous therapy experience, n = 149 | No previous therapy experience, n = 92 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | Mean | ± SD | % | Mean | ± SD | % | Mean | ± SD | p-value | |
| Gender | – | – | – | – | – | – | – | – | – | 0.052 |
| Female | 30.7 | – | – | 26.2 | – | – | 38.0 | – | – | – |
| Male | 69.3 | – | – | 73.8 | – | – | 62.0 | – | – | – |
| Age (years) | – | 57.2 | 13.8 | – | 61.3 | 12.4 | – | 51.6 | 14.0 | < 0.001 |
| Body mass index (kg/m2) | – | 31.4 | 6.2 | – | 32.1 | 6.5 | – | 30.7 | 6.2 | 0.224 |
| Respiratory disturbance index | – | 15.5 | 18.6 | – | 14.1 | 19.0 | – | 22.5 | 16.7 | < 0.001 |
| Epworth Sleepiness Scale | – | 8.1 | 5.0 | – | 8.1 | 5.1 | – | 9.1 | 5.0 | 0.145 |
| Comorbidities | – | – | – | – | – | – | – | – | – | – |
| Arterial hypertension | 59.9 | – | – | 62.1 | – | – | 56.5 | – | – | 0.397 |
| Diabetes mellitus type II | 14.8 | – | – | 19.3 | – | – | 7.6 | – | – | 0.014 |
| Coronary artery disease | 11.4 | – | – | 15.2 | – | – | 5.4 | – | – | 0.022 |
| s/p myocardial infarction | 4.2 | – | – | 4.8 | – | – | 3.3 | – | – | 0.560 |
| s/p stroke | 3.4 | – | – | 4.8 | – | – | 1.1 | – | – | 0.121 |
| Current OSA treatment | – | – | – | – | – | – | – | – | – | < 0.001 |
| None | 50.7 | – | – | 20.1 | – | – | 100.0 | – | – | – |
| PAP therapy | 47.7 | – | – | 77.2 | – | – | 0.0 | – | – | – |
| Other | 1.6 | – | – | 2.7 | – | – | 0.0 | – | – | – |
N = 241
SD standard deviation, OSA obstructive sleep apnea, PAP positive airway pressure therapy, s/p status post
Upon study participation, the majority of patients had not been treated for OSA (50.7%). The most used therapy was PAP ventilation (96.6%), followed by MAD (2.5%) and positional therapy (0.8%).
Treatment preferences
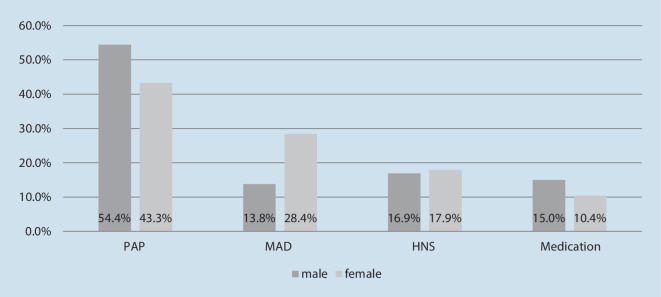
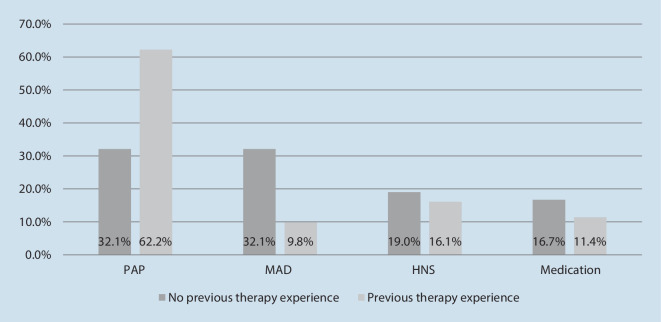
If asked which therapy one would choose if all displayed options were freely available, most patients in the study cohort opted for PAP therapy (51.1%), followed by MAD (18.1%), HNS therapy (17.2%), and medication (13.7%). Though not statistically significant (p = 0.059), gender differences were observed, with a higher preference for MAD among female patients and a higher preference for PAP therapy in male patients (Fig. 2). Statistically significant differences in preferences were found between patients with previous therapy experience vs. those who were treatment naïve (p =0.001, Fig. 3). In the subgroup of participants without previous therapy exposure, MAD and PAP therapy were equally preferred (32.1% and 32.1%), followed by HNS (19.3%). Among treatment-experienced patients, PAP therapy was the treatment of choice by far (62.2%), followed by HNS (16.1%) and medication (11.9%). In this subgroup, MAD was the least preferred treatment method, with 9.8% (Fig. 4).
Fig. 2.
Preferred OSA treatment by gender (%). OSA obstructive sleep apnea, PAP positive airway pressure therapy, MAD mandibular advancement device, HNS hypoglossal nerve stimulation
Fig. 3.
Preferred OSA treatment by therapy experience (%). OSA obstructive sleep apnea, PAP positive airway pressure therapy, MAD mandibular advancement device, HNS hypoglossal nerve stimulation
Fig. 4.
Preferences for use of OSA treatments by therapy experience on a four-level Likert scale: 1 = I cannot imagine using this treatment; 4 = I can very well imagine using this treatment; mean ± standard error. OSA obstructive sleep apnea, PAP positive airway pressure therapy, MAD mandibular advancement device, HNS hypoglossal nerve stimulation
General approval ratings for treatment with the four therapeutic concepts confirmed the findings, with highest approval for PAP therapy, followed by MAD, HNS, and drug therapy. Approval varied by gender, though this was not statistically significant for any of the four therapies. Significant differences were, however, found between treatment-experienced patients and those who were treatment naïve, and were in line with the differences reported above. Intention to use PAP therapy was greater in participants who had used treatments before (p < 0.001) and approval for MAD was higher in patients without treatment experience (p = 0.021). For HNS and medication, no significant differences in approval ratings were found (p = 0.052 and p = 0.848, respectively).
Relative importance of OSA treatment attributes
A random effects logit model was used to estimate the importance of treatment attributes. For analysis of the DCE, data were dummy coded by encoding the most positive level of the attribute with “1” and the remaining levels with “0.” First, coefficients of all attribute levels in the DCE were computed. All coefficients lead in the expected direction, which confirmed the model assumptions set a priori. Thereafter, level differences for the attributes were defined by calculating the difference between the positive and the negative poles. Finally, the resulting level differences were weighted and adjusted on a scale from 0 (least relevant) to 10 (most relevant) and grouped by preferred treatment. While the most important attribute of OSA treatment was “Improvement of daytime sleepiness” in patients who preferred MAD, “Reduction in the risk of OSA-related comorbidities” was most relevant in patients who chose HNS or drug therapy (Fig. 5). Among patients who preferred PAP therapy, “Requirement for surgery” was most important, with a strong disutility for invasive surgical procedures (coefficient −2.536, Table 2). The least importance treatment attributes were “Ease of use” and “Monthly co-payment” across all subgroups (Table 3). “Occurrence of treatment-related side effects” was equally important, independent of the preferred therapy, with significant contributions to choice decisions and high coefficients among all participants.
Fig. 5.
Relative importance of treatment attributes by preferred OSA treatment. SE standard error, OSA obstructive sleep apnea, PAP positive airway pressure therapy, MAD mandibular advancement device, HNS hypoglossal nerve stimulation
Table 2.
Coefficients for attribute levels according to subgroups by preferred OSA therapy
| Attribute and levels | Preferred choice PAP therapy | Preferred choice MAD therapy | Preferred choice HNS therapy | Preferred choice medication |
|---|---|---|---|---|
| 1. Improvement of daytime sleepiness | ||||
| Strong improvement | 1.142 | 4.787 | 1.775 | 2.122 |
| Medium improvement | 0.433 | 3.177 | 0.588 | 1.760 |
| Small improvement | −1.574 | −7.964 | −2.362 | −3.882 |
| 2. Reduction in the risk of comorbidities | ||||
| Strong reduction | 0.998 | 3.312 | 3.783 | 3.082 |
| Medium reduction | 0.642 | 1.452 | 2.489 | 2.861 |
| Small reduction | −1.640 | −4.764 | −6.272 | −5.943 |
| 3. Improved treatment of existing comorbidities | ||||
| Strong improvement | 0.804 | 1.775 | 2.425 | 1.786 |
| Medium improvement | 0.083 | 0.599 | 1.942 | 1.446 |
| Small improvement | −0.886 | −2.374 | −4.367 | −3.232 |
| 4. Occurrence of treatment-related side effects | ||||
| 10% of patients | 1.247 | 2.743 | 3.183 | 1.766 |
| 20% of patients | 0.717 | 1.635 | 2.502 | 1.167 |
| 50% of patients | −1.956 | −4.377 | −5.685 | −2.933 |
| 5. Ease of use | ||||
| Effort per day 1 min | 0.059 | 0.059 | 0.205 | −0.030 |
| Effort per day 5 min | −0.366 | −0.366 | −0.028 | −0.090 |
| Effort per day 10 min | −0.425 | −0.425 | −0.233 | −0.119 |
| 6. Requirement for surgery | ||||
| No surgery required | 1.885 | 3.015 | 0.666 | 0.873 |
| Minor surgery, pain for 1–3 days | 0.651 | 0.964 | 0.769 | −0.040 |
| Medium surgery, pain for 3–5 days | −2.536 | −3.978 | −1.435 | −0.873 |
| 7. Co-payment | ||||
| 2 € per month | 0.014 | 1.180 | 1.072 | 0.347 |
| 5 € per month | 0.035 | 0.668 | 0.147 | −1.233 |
| 10 € per month | −0.049 | 0.512 | 0.925 | −1.580 |
Positive values represent utility, negative values represent disutility
OSA obstructive sleep apnea, PAP positive airway pressure therapy, MAD mandibular advancement device, HNS hypoglossal nerve stimulation
Table 3.
Subgroup analyses of relative importance, separated by preferred OSA treatment
| Attribute | PAP therapy | MAD therapy | HNS therapy | Medication | ||||
|---|---|---|---|---|---|---|---|---|
| Level diff | Rank | Level diff | Rank | Level diff | Rank | Level diff | Rank | |
| 1. Improvement of daytime sleepiness | 2.57 | 4 | 11.27 | 1 | 6.14 | 4 | 6.96 | 2 |
| 2. Reduction in the risk of comorbidities | 2.63 | 3 | 8.07 | 2 | 10.05 | 1 | 9.02 | 1 |
| 3. Improved treatment of existing comorbidities | 1.68 | 5 | 4.14 | 5 | 6.79 | 3 | 4.67 | 4 |
| 4. Occurrence of treatment-related side effects | 3.21 | 2 | 7.11 | 3 | 8.86 | 2 | 4.69 | 3 |
| 5. Ease of use | 0.79 | 6 | 0.48 | 7 | 0.43 | 7 | 0.14 | 7 |
| 6. Requirement for surgery | 4.42 | 1 | 6.99 | 4 | 2.10 | 5 | 1.70 | 6 |
| 7. Co-payment | 0.06 | 7 | 1.69 | 6 | 1.99 | 6 | 1.92 | 5 |
OSA obstructive sleep apnea, PAP positive airway pressure therapy, MAD mandibular advancement device, HNS hypoglossal nerve stimulation
Willingness to pay
The majority of participants stated a monthly WTP of € 50 to achieve the full benefits of optimal sleep. WTP varied highly though, with more than half of the cohort willing to spend € 50 or more and 16.2% willing to spend more than € 100 per month (Fig. 6). Female patients reported a slightly higher WTP compared to male patients, though the differences were not statistically significant (p = 0.123). Differences between participants with previous treatment experience compared to those without previous experience were not found (p = 0.662), though slightly more patients were willing to spend € 50 or beyond per month in the latter group (treatment-naïve patients: 51.3%; patients with previous treatment experience: 47.8%).
Fig. 6.
Monthly willingness to pay in euro
Spearman’s rho correlation coefficient was used to assess interactions between WTP and medical and demographic variables (ESS, Respiratory Disturbance Index, number of comorbidities present, body mass index, and age). No significant correlation was found for any of the variables, though a trend was identified for age, which negatively correlated with WTP (rs = −0.132, p = 0.056).
Discussion
Though patient engagement and patient-centered care are becoming more relevant in respiratory sleep medicine, little is known about the treatment preferences of affected patients so far. This study adds evidence that diverse preferences exist with regards to the relevance of attributes of OSA treatment, but also for specific therapies. In this cohort, PAP was the most preferred treatment, with more than half of the sample reporting this as their primary choice. However, close to one fifth of participants would like to be treated with either MAD (18.1%) or HNS therapy (17.2%), and slightly fewer would prefer medication for OSA treatment. Subgroup analyses revealed relevant differences in treatment preferences not only by gender, but also by disease history. For example, female patients preferred MAD, while male patients more often opted for PAP ventilation. Among patients with a new OSA diagnosis and without PAP experience, preferences were more diverse, with MAD being the most commonly chosen treatment. On the other hand, patients with treatment experience, who had mostly used PAP therapy recently, preferred this treatment. An explanation could be that patients realize that PAP can be tolerated well once they try it. However, the cohort with treatment experience was 9 years older on average (61.2 ± 12.4 vs. 52.3 ± 14.2), which could also lead to different preferences.
Treatment preferences reported from this cohort diverge slightly from previous data, as reported by Campbell et al. and Almeida et al. [17, 18]. In both studies, preferences for PAP therapy were significantly lower. This could be explained by either diverse preferences due to a different cultural background or by a rather high number of patients with an established diagnosis and PAP experience in the current sample. In addition, participants in the present cohort did not receive a detailed explanation of the therapies presented. This might have influenced their decision to opt for a treatment with which they are more familiar. As 16.2% (MAD), 16.6% (medications), and 19.5% (HNS) of patients did not provide a response when asked if they could imagine using a certain treatment, insufficient knowledge on these therapies could have influenced the decisions as well.
The majority of patients in the sample stated a WTP for optimal sleep of € 50 per month, which represented 0.82% of the monthly disposable income in Germany at the time the study was conducted [19]. However, more than half of the participants were willing to spend € 50 or more per month and more than a quarter could afford € 100 or more. Given the high relevance of improving daytime sleepiness that participants stated in the DCE, higher values were expected a priori. It is important to have in mind, however, that the results were not corrected for disposable household incomes. It may be that € 50 or more additional spending is simply not affordable for some patients, even though they would be willing to pay more. On the other hand, an increase in monthly co-payment did not contribute significantly to any decision-making in the DCE part of the study. This may be due to the comparably well-funded German health care system, which provides many healthcare services for free or asks only for small co-payments, which, in addition, are limited to a certain amount per year. It is also important to mention that the study did not elicit the WTP for specific OSA therapies. Due to reimbursement limitations for some treatments, patients might be more willing to pay for therapies that are not covered by health insurance.
In this study, age was the only factor that was correlated with WTP, with increasing age resulting in slightly lower WTP values. All other variables, such as as RDI, ESS, or the number of comorbidities, did not influence the stated WTP.
The results from this study underline the need for differentiated treatment of OSA and highlight the demand for non-PAP therapies such as MAD or HNS from a patient perspective. Given that even more OSA interventions or devices, such as positional therapy or other surgical interventions, are available in many healthcare systems, it is likely that preferences are even more diverse than reported in this study. From the perspectives of individual providers, but also at a decision-maker level, this is important information, as many healthcare systems limit their portfolio of reimbursed OSA therapies to few, mostly PAP-based treatments. Increasing numbers of patients receive a diagnosis of OSA at a younger age, resulting in longer disease journeys due to the chronic nature of the disease. However, it is likely that preferences for treatments will change when side effects or complications of a treatment are experienced, as occurs regularly with PAP therapy [20–22]. Having a broader portfolio of interventions, medical devices, and pharmaceuticals available will aid continuous treatment of patients along their disease journey.
In addition to the emerging evidence on phenotyping using pathophysiological traits of OSA, information on patient preferences can help to identify an optimal treatment for each patient, which not only provides ideal treatment of the underlying pathomechanism, but also satisfies the patient’s individual treatment goals [23].
Limitations
This study recruited participants from one tertiary sleep center that serves a dedicated geography in western Germany, which may limit the generalizability of the results. By recruiting a larger cohort of OSA patients who are likely to be representative of many sleep centers, it was sought to overcome this limitation. Another potential limitation is the timing of the study, which was conducted during the SARS-CoV‑2 pandemic, which could have influenced the composition of the study sample and thus the responses obtained.
Secondly, patients did not receive a priori explanation of the different treatment concepts. It is likely that not all participants had the same knowledge of each of the therapies presented, which naturally influences their choice decision.
A general limitation of stated-preference methods is that participants might act differently to that which they stated in an experiment. Though logistically difficult to conduct, a combination of stated- and revealed-preference analyses would improve the understanding of patient preferences. Nevertheless, stated-preference methods such as DCE have demonstrated high external validity in the healthcare context [24].
Conclusion
Preferences for treatments differ significantly among patients with OSA. Gender as well as disease history and previous treatment experiences lead to different response profiles. The attributes of OSA treatment are of varying relevance, depending on treatment preference. PAP therapy was the preferred intervention in this sample, especially in patients with an existing diagnosis and previous treatment experience. The study shows that non-PAP therapies, such as MAD or HNS, are also well accepted from a patient perspective, which is important information given the high rates of non-adherence to PAP ventilation. The findings from this study can help to tailor optimal therapies in individual patients and support the development of patient-centered OSA care.
Acknowledgments
Acknowledgements
The authors would like to thank the staff at the Sleep lab at Ruhrlandklinik Essen (University hospital Essen, West German Lung Center, Essen, Germany) for their support in conducting the study.
Funding
This study received no funding.
Author Contribution
MB and CS conceived the original idea and planned the study. MB and SD carried out the study and analyzed the data. MB wrote the manuscript with support from SD, CT, and CS. CT and CS supervised the project.
Declarations
Conflict of interest
M. Braun is an employee of Inspire Medical Systems Inc., Golden Valley, MN, USA. S. Dietz-Terjung, C. Taube, and C. Schoebel declare that they have no competing interests.
The study was approved by the Ethics Commission of the University Duisburg-Essen (20-9315-BO) and written informed consent was obtained from all participants at time of enrollment.
Footnotes

Scan QR code & read article online
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fietze I, Laharnar N, Obst A, Ewert R, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J Sleep Res. 2018 doi: 10.1111/jsr.12770. [DOI] [PubMed] [Google Scholar]
- 4.Appleton S, Gill T, Taylor A, McEvoy D, et al. Influence of gender on associations of obstructive sleep apnea symptoms with chronic conditions and quality of life. Int J Environ Res Public Health. 2018;15:930. doi: 10.3390/ijerph15050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, et al. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47:194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 6.Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22:6. doi: 10.1007/s11886-020-1257-y. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn E, Schwarz EI, Bratton DJ, Rossi VA, et al. Effects of CPAP and mandibular advancement devices on health-related quality of life in OSA: a systematic review and meta-analysis. Chest. 2017;151:786–794. doi: 10.1016/j.chest.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Pengo MF, Soranna D, Giontella A, Perger E, et al. Obstructive sleep apnoea treatment and blood pressure: which phenotypes predict a response? A systematic review and meta-analysis. Eur Respir J. 2020;5:1901945. doi: 10.1183/13993003.01945-2019. [DOI] [PubMed] [Google Scholar]
- 9.Mayer G, Arzt M, Braumann B, Ficker JH, et al. German S3 guideline nonrestorative sleep/sleep disorders, chapter ‘sleep-related breathing disorders in adults,’ short version: German sleep society. Somnologie. 2017;21:290–301. doi: 10.1007/s11818-017-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strollo PJJ, Soose RJ, Maurer JT, de Vries N, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 11.Walia HK, Thompson NR, Strohl KP, Faulx MD, et al. Upper airway stimulation vs positive airway pressure impact on BP and sleepiness symptoms in OSA. Chest. 2019;157:173–183. doi: 10.1016/j.chest.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Taranto-Montemurro L, Messineo L, Wellman A. Targeting endotypic traits with medications for the pharmacological treatment of obstructive sleep apnea. A review of the current literature. J Clin Med. 2019;8:1846. doi: 10.3390/jcm8111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verse T, Dreher A, Heiser C, Herzog M, et al. ENT-specific therapy of obstructive sleep apnoea in adults: A revised version of the previously published German S2e guideline. Sleep Breath. 2016;20:1301–1311. doi: 10.1007/s11325-016-1353-9. [DOI] [PubMed] [Google Scholar]
- 14.Braun M, Dietz-Terjung S, Taube C, Schoebel C. Understanding preferences in chronic diseases—Importance of treatment attributes differs among patients with obstructive sleep apnea. J Sleep Disord Manag. 2021;7:033. doi: 10.23937/2572-4053.1510033. [DOI] [Google Scholar]
- 15.Bridges JFP, Hauber AB, Marshall D, Lloyd A, et al. Conjoint analysis applications in health—A checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Hauber AB, Gonzàles JM, Groothuis-Oudshoorn CGM, Prior T, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19:300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Campbell T, Pengo MF, Steier J. Patients’ preference of established and emerging treatment options for obstructive sleep apnoea. J Thorac Dis. 2015;7:938–942. doi: 10.3978/j.issn.2072-1439.2015.04.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida FR, Mulgrew A, Ayas N, Tsuda H, et al. Mandibular advancement splint as short-term alternative treatment in patients with obstructive sleep apnea already effectively treated with continuous positive airway pressure. J Clin Sleep Med. 2013;9:319–324. doi: 10.5664/jcsm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.German Federal Statistical Office DESTATIS (2021) Einkommen, Einnahmen und Ausgaben. Statistisches Bundesamt. https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Einkommen-Konsum-Lebensbedingungen/Einkommen-Einnahmen-Ausgaben/_inhalt.html. Accessed 12 May 2021
- 20.Schoch OD, Baty F, Niedermann J, Rüdiger JJ, et al. Baseline predictors of adherence to positive airway pressure therapy for sleep apnea: a 10-year single-center observational cohort study. Respiration. 2014;87:121–128. doi: 10.1159/000354186. [DOI] [PubMed] [Google Scholar]
- 21.Galetke W, Puzzo L, Priegnitz C, Anduleit N, et al. Long-term therapy with continuous positive airway pressure in obstructive sleep apnea: adherence, side effects and predictors of withdrawal—A ‘real-life’ study. Respiration. 2011;82:155–161. doi: 10.1159/000322838. [DOI] [PubMed] [Google Scholar]
- 22.Ritter J, Geißler K, Schneider G, Guntinas-Lichius O. Einfluss einer strukturierten Nachsorge auf die Therapietreue bei OSAS-Patienten unter CPAP-Therapie. Laryngol Rhinol Otol. 2018;97:615–623. doi: 10.1055/a-0640-9198. [DOI] [PubMed] [Google Scholar]
- 23.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea—New pathways for targeted therapy. Sleep Med Rev. 2016;37:45–59. doi: 10.1016/j.smrv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Quaife M, Terris-Prestholt F, Di Tanna GL, Vickerman P. How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur J Health Econ. 2018;19:1053–1066. doi: 10.1007/s10198-018-0954-6. [DOI] [PubMed] [Google Scholar]