Abstract
Background
Intraoperative anti-A/B immunoadsorption (ABO-IA) was recently introduced for ABO-incompatible heart transplantation. Here we report the first case series of patients transplanted with ABO-IA, and compare outcomes with those undergoing plasma exchange facilitated ABO-incompatible heart transplantation (ABO-PE).
Methods
Data were retrospectively analysed on all ABO-incompatible heart transplants undertaken at a single centre between January 1, 2000 and June 1, 2020. Data included all routine laboratory tests, demographics and pre-operative characteristics, intraoperative details and post-operative outcomes. Primary outcome measures were volume of blood product transfusions, maximum post-transplant isohaemagglutinin titres, occurrence of rejection and graft survival. Secondary outcome measures were length of intensive care and hospital stay. Demographic and survival data were also obtained for ABO-compatible transplants during the same time period for comparison.
Results
Thirty-seven patients underwent ABO-incompatible heart transplantation, with 27 (73%) using ABO-PE and 10 (27%) using ABO-IA. ABO-IA patients were significantly older than ABO-PE patients (p < 0.001) and the total volume of blood products transfused during the hospital admission was significantly lower (164 [126-212] ml/kg vs 323 [268-379] ml/kg, p < 0.001). No significant differences were noted between methods in either pre or post-transplant maximum isohaemagglutinin titres, incidence of rejection, length of intensive care or total hospital stay. Survival comparison showed no significant difference between antibody reduction methods, or indeed ABO-compatible transplants (p = 0.6).
Conclusions
This novel technique appears to allow a significantly older population than typical to undergo ABO-incompatible heart transplantation, as well as significantly reducing blood product utilization. Furthermore, intraoperative anti-A/B immunoadsorption does not demonstrate increased early post-transplant isohaemagglutinin accumulation or rates of rejection compared to ABO-PE. Early survival is equivalent between ABO-IA, ABO-PE and ABO-compatible heart transplantation.
KEYWORDS: immunoadsorption, cardiopulmonary bypass, heart transplantation, pediatrics
Introduction
ABO-incompatible heart transplantation (ABOi) has been used for over two decades in our institution, and is considered a routine option for infants that have acceptably low isohaemagglutinin titres.1 Despite a preconceived premise that there would be a high risk of hyperacute rejection, the procedure has demonstrated comparable graft survival rates to those in similarly aged ABO-compatible recipients with no evidence of increased rates of rejection.2, 3, 4, 5 This success is related to the relative immaturity of the infant immune system, imparting a tolerance to A/B antigens, and the prevention of isohaemagglutinin accumulation following transplantation, achieved through plasma exchange in the immediate pre-transplant period, minimizing the potential for hyperacute rejection.6, 7, 8 The original protocol utilized a plasma exchange process (ABO-PE), undertaken prior to the initiation of cardiopulmonary bypass (CPB), with a two to three-fold volume exchange capable of reducing pre-transplant isohaemagglutinin titres to below a maximum threshold of 1:2.5 For practical reasons such a procedure was generally only undertaken in smaller children due to the large volumes of blood products required. Despite the apparent success of this procedure, it exposed the recipient to multiple donor sources, increasing the risk of transfusion related morbidity, as well as a period of haemodynamic instability.8
The concept of intraoperative isohaemagglutinin removal via immunoadsorption (ABO-IA) was introduced to address these issues.9 The incorporation of an anti-A/B immunoadsorption column directly into the CPB circuit allowed the patient to be fully supported on CPB, whilst removing anti-A/B isohaemagglutinins prior to the reperfusion of the donor organ, without the requirement of large volume plasma exchange.10 This promising first clinical demonstration published alongside the original description of the ABO-IA methodology, led to further questions addressed in this study.11 The study aims to determine if the ABO-IA process (1) enabled larger patients to undergo ABOi than traditionally accepted and (2) significantly reduced the total transfusion burden observed in the transplant admission. Furthermore, (3) whether patients were more likely to display isohaemagglutinin accumulation and therefore increased episodes of rejection, and (4) whether survival was comparable to ABO-PE and ABO-compatible (ABO-C) transplant patients that do not require isohaemagglutinin removal.
Materials and methods
Study design and participants
Retrospective analysis of routinely collected hospital data fulfilling the ethical guidelines of the Helsinki Declaration and approved by the Institutional Review Board as part of a wider study on Antibody Immunoadsorption for Transplantation (19HL02). All clinical data were collated in a research platform within the hospital's governance structure and de-identified prior to analysis. Data from all primary isolated heart transplants carried out at the Institution between January 1, 2000 and 1, June 2020 were included. All ABOi heart transplants prior to the introduction of the ABO-IA technique in 2015 were carried out using ABO-PE. All subsequent ABOi transplants were ABO-IA. Individual consent was not required since only routinely collected de-identified hospital data were evaluated within the secure digital research environment as part of an existing research database approval (17/LO/0008).
Data collection
Routine clinical information was extracted from the Institution's Electronic Health Record system using a custom structured query language script. These data included patient demographic information, laboratory results and blood product transfusion requirements, admission and hospital stay information, intraoperative and CPB data, intensive care requirements and outcomes. Data for ABO-C transplantations were limited to demographic data for survival and cohort comparison only.
ABOi protocols
Isohaemagglutinin titres were quantified by reverse blood typing using a neutral (no anti-human globulin) gel card technique (NaCl, Enzyme Test and Cold Agglutinins, Bio-Rad Laboratories Ltd, Hemel Hempstead, UK). Briefly, the cards are incubated at room temperature for 15 minutes and then centrifuged at 3000rpm for 10 minutes. The resulting reactions are then assessed and the last positive reaction is assigned as the titre. Titres were repeated periodically while on the waiting list and immediately once a potential donor was identified. A dilutional ratio less than 1:32 was required for consideration of ABOi heart transplantation. Following transplantation anti-donor blood group titres were repeated regularly for the first six months with increasing intervals (within the first 72 hours, weekly for two weeks, monthly for three months and then yearly). Anti-third-party blood group titres were not tested in the post-transplant period.
ABO-PE
The process for ABO-PE facilitated heart transplantation has been previously described.8 Briefly, the CPB circuit is primed with fresh frozen plasma (FFP) of the donor blood type, or group AB, and packed red blood cells (PRBC) of the recipient blood type in a 1:1 ratio to give a total prime volume three times greater than the recipient's circulating volume. Following pre-bypass ultrafiltration of the prime to achieve biochemical compatibility, the recipient's circulating volume is drained into a cell saver at the same rate as the CPB prime is returned to the patient (approximately 10ml/kg/min) over approximately 30 minutes. Isohaemagglutinin titres are measured following completion of the plasma exchange. CPB is then initiated and the transplant occurs in the standard manner.
ABO-IA
The process for ABO-IA facilitated heart transplantation has been previously described.9,10 Briefly, a plasma separator and anti-A/B immunoadsorption column are placed into the CPB circuit. Once CPB is initiated, plasma is pumped from the plasma separator to the column. Isohaemagglutinin depleted plasma is then returned to the circulating volume via the venous reservoir. Treatment duration is based upon pre-transplant titre and therefore number of plasma volumes required to treat. This is then carried out within the normal timeframe for a heart transplant. For example, a titre of 1:32 would require a minimum of 4 plasma volume passes through the immunoadsorption column (to reach required maximum threshold of 1:2) prior to reperfusion of the donor organ. Isohaemagglutinin titres are measured following each plasma volume treated. Transplantation is undertaken using standard surgical methods.
Immunosuppressive treatment
Immunosuppression was achieved as previously described.8 Briefly, all ABOi patients received basiliximab at a dose of 10 mg following the PET and 15 mg/kg methylprednisolone immediately prior to donor organ reperfusion and then 10 mg/kg, 2 mg/kg and 2 mg/kg on postoperative days one, two and three, respectively, before switching to prednisolone. Once oral medication was tolerated, tacrolimus (0.05 mg/kg twice daily from postoperative day two to a target plasma concentration of 10-15 ng/ml) and mycophenolate mofetil (600 mg/m2, twice daily) and prednisolone (1 mg/kg daily, weaning to 0.2 mg/kg daily over four weeks) were used for immunosuppressive maintenance.
Outcome data
Data on blood product transfusions was available at the unique unit code level, but individual unit volumes were not. To standardise across all patients, a unit of PRBC was assumed to be 250ml, unless specified as a pediatric volume pack, in which case the volume was assumed to be 50ml. FFP was assumed to be 200ml/unit unless specified as a pediatric volume pack (50ml), whilst cryoprecipitate and platelet volumes were calculated to be 10ml/kg (standard protocol dosage). Differences excluding the volume used in the plasma exchange were investigated by substituting this volume with a standard 250ml PRBC pack (equivalent to that used in the ABO-IA group). Only blood product transfusions occurring during the transplant procedure, and in the postoperative course were included in the analysis. Isohaemagglutinin accumulation was defined as the maximum recorded isohaemagglutinin titre, recorded in the post-transplant period (defined as “early post-transplant” if occurring within 3 months of the transplant and “late post-transplant” thereafter). Similarly, maximum preoperative isohaemagglutinin titres were used as a gauge of pre-transplant immune system maturity, rather than the immediate pre-transplant titre. Episodes of rejection were defined either as clinically suspected and treated for rejection even in the absence of pathology, or based upon endocardial biopsy histopathology, in accordance with International Society for Heart and Lung Transplantation 2013 Working Formulation.12 A pathologic antibody mediated rejection (pAMR) grade of >2 or 1 where there were also clinical manifestations, or ACR grade >1R were used to define significant rejection. Data on the production of post-transplant donor-specific antibodies (DSA) were also collected. Clinical outcomes were as of December 1, 2020. Survival time was defined as the time from primary transplantation to either death, or date of re-transplantation, whilst intensive care stay and hospital stay were defined as time from the date of transplantation to date of discharge from ward or hospital respectively.
Power calculation
Previous data suggest a mean ± SD total blood transfusion volume for pediatric patients undergoing cardiac surgery of 271 ± 112 ml/kg.13 The volume required for the ABO-PE plasma exchange is 201 ± 74 ml/kg, therefore we estimated an ABO-PE patient would receive approximately 400 ± 150 ml/kg of transfused blood products throughout the entire transplant stay.8 An ABO-IA patient conversely would be expected to have similar volumes to those described for other pediatric patients (i.e., a reduction of 200 ml/kg compared to an ABO-PE patient). Therefore a total of 37 patients was determined by an a priori power calculation (with an allocation ratio of 2.7; ηABO-PE = 27, ηABO-IA = 10), to detect a mean 200ml/kg reduction in blood product transfusion (effect size [d] of 0.97) with a power (1-β) of 0.8 and α = 0.05.
The incidence of rejection (both cellular and antibody-mediated) following ABO-PE has been estimated at approximately 30%.14 To demonstrate equivalence of the methods in the occurrence of rejection, the power was calculated with an a priori 2-sample non-inferiority test. Given estimated proportions of rejection equal in both groups (30%) for a non-inferiority margin (δ) of 20%, 37 patients were required (with a sampling ratio, κ, of 2.7; ηABO-PE = 27, ηABO-IA = 10) for a power (1–β) of 0.8 and α = 0.1.
Statistics
Continuous variables were described by their median and interquartile range. Categorical variables were described by counts and percentages. Isohaemagglutinin titres were transformed using binary logarithm prior to analysis. Proportional testing of groups was undertaken using a 3-sample (Chi-squared) test for equality of proportions. Analysis of differences between methodologies was undertaken using 2-tailed t-tests or Wilcoxon-Mann-Whitney tests according to the variables’ distribution. Comparisons between transplantation methods was undertaken using either Kruskal-Wallis or one-way ANOVA testing dependent upon variable distribution. Post-hoc Dunn Test or Tukey's honestly significant difference with Bonferroni adjustment for pairwise multiple comparisons was used to identify significant groups. Time to event analyses were undertaken using Kaplan-Meier estimates and tested using the logrank test with Peto-Peto weighting (to detect early survival differences). To provide equivalence in follow-up data, patients were censored at 5 years post-transplantation in Kaplan Meier estimates in the ABO-PE and ABO-C groups. The effect of age at transplantation on survival was undertaken using a Cox proportional hazards regression analysis and tested using a likelihood ratio test. Linear mixed effects regression models with a random effect to account for within-patient measurements were fitted to analyse isohaemagglutinin titres. Pre and post-transplant time points (early and late) and method of isohaemagglutinin reduction (without interaction term) were used as fixed effects. p-values were obtained by likelihood ratio tests of the patient only (null model) with the random effect against the model with the time point and time point with the addition of isohaemagglutinin reduction technique. Statistical tests were conducted assuming a 0.05 significance level. All analyses were performed in the R language and environment for statistical computing, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographics
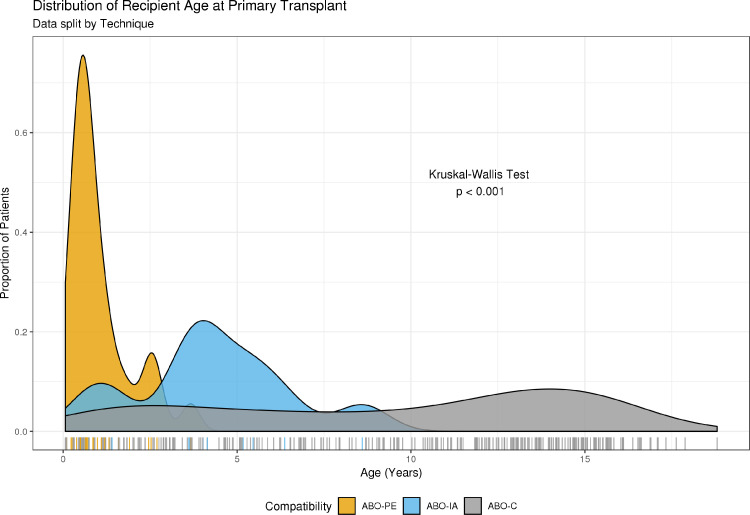
Between January 1, 2000 and June 1, 2020, 37 ABO-incompatible heart transplants were undertaken. Of these, 27 (73%) were ABO-PE and 10 (27%) were the first ten patients who underwent ABO-PE using immunoadsorption at our institution (ABO-IA). Pre-transplant details for the ABO-IA transplants are given in Table 1. The most common ABO-IA diagnosis was Restrictive Cardiomyopathy which occurred in five patients (50%). In Patient 8 (donor's blood type, AB; recipient's blood type, O), Restrictive Cardiomyopathy occurred in the presence of severe mitral valve stenosis and left ventricular dysfunction, for which they had previously undergone mitral valve replacement. Three patients (30%) had Dilated Cardiomyopathy. Patient 3 (donor's blood type, A; recipient's blood type, O) had hypertrophic cardiomyopathy in the presence of an unidentified underlying metabolic disease. Patient 9 (donor's blood type, B; recipient's blood type, O) had hypoplastic left heart syndrome for which they had undergone a Norwood procedure and subsequent bidirectional Glenn anastomosis, but due to high Glenn/pulmonary artery pressures was not suitable for Fontan completion. For seven patients (70%), transplantation involved re-sternotomy due to previous surgical repair of a congenital defect, or implantation of a mechanical support device. By comparison, 23 ABO-PE patients (85.2%) had heart failure due to Dilated Cardiomyopathy with the remaining 4 patients (14.8%) requiring transplantation for congenital heart disease. Ten ABO-PE patients (37%) required transplantation via re-sternotomy (9 patients having required pre-transplant VAD support; 33.3%). Patients undergoing ABO-IA were significantly older than patients in the ABO-PE group (p < 0.001). These differences were also reflected in the weights of patients (p < 0.001). During the same period, 301 ABO-compatible (ABO-C) heart transplants were undertaken. Patients undergoing ABO-C transplantation were significantly older (10.7 [4.7-14.1] years) than both ABO-PE and ABO-IA groups (p <0.001 and p = 0.027 respectively; Figure 1). Patient demographics and clinical characteristics are given in Table 2.
Table 1.
ABO-Incompatible Immunoadsorption Case Series
| ABO-IA patient preoperative data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. |
Weight (kg) |
Age (years) |
Diagnosis | Recipient's blood group |
Donor's blood group |
Ischemic time |
CPB time |
Isohaemagglutinin Titre Anti-A Anti-B |
Pre-transplant support |
|
| 1 | 15.6 | 3.5 | Familial DCM | B | A | 255 | 303 | 1:8 | ECMO | |
| 2 | 12 | 2.1 | RCM | B | A | 193 | 128 | 1:8 | VAD | |
| 3 | 5.8 | 0.7 | HCM/Metabolic | O | A | 237 | 101 | 1:2 | 1:16 | VAD |
| 4 | 10.8 | 4 | RCM | O | B | 111 | 194 | 1:32 | 1:16 | VAD |
| 5 | 11.5 | 4.2 | DCM | A | B | 296 | 219 | 1:4 | VAD | |
| 6 | 18 | 5.3 | RCM | A | AB | 226 | 224 | 1:8 | ECMO/VAD | |
| 7 | 9.2 | 1.4 | Familial DCM | O | A | 111 | 122 | 1:8 | 1:1 | |
| 8 | 16.6 | 6 | RCM/CHD | O | AB | 339 | 255 | 1:16 | 1:4 | |
| 9 | 14.7 | 5 | CHD | O | B | 154 | 246 | 1:128 | 1:8 | VAD |
| 10 | 27 | 8 | RCM | O | B | 276 | 176 | 1:128 | 1:16 | |
Abbreviations: DCM, Dilated Cardiomyopathy; RCM, Restrictive Cardiomyopathy; CHD, Congenital heart disease; ECMO, Extracorporeal Membrane Oxygenation; VAD, Ventricular Assistance Device.
Figure 1.
Comparison of Age at Transplantation. Age of 338 isolated cardiac transplants undertaken at Great Ormond Street Hospital during the period 2000 to 2020. ABO-C, Compatible Heart Transplant; ABO-PE, Incompatible Heart Transplantation with Plasma Exchange; ABO-IA, Incompatible Heart Transplantation with Immunoadsorption. Dunn tests revealed significant differences between ABO-PE and ABO-AI (p < 0.001) as well as between ABO-C and ABO-IA and ABO-C and ABO-PE (p = 0.027 and <0.001 respectively).
Table 2.
Patient Clinical Characteristics and Demographic Data
| Patient demographics | ||||
|---|---|---|---|---|
| ABO-C | ABO-PE | ABO-IA | p | |
| Sex | ||||
| Male | 146 (48.5) | 9 (33.3) | 3 (30) | 0.18 |
| Female | 155 (51.5) | 18 (66.7) | 7 (70) | 0.18 |
| Ethnicity | ||||
| White | 221 (73.4) | 20 (74.1) | 5 (50) | 0.26 |
| BAME | 75 (24.9) | 7 (25.9) | 4 (40) | 0.56 |
| Unrecorded/Refused | 5 (1.7) | 0 | 1 (10) | 0.11 |
| Age at Transplant (years) | 10.7 [4.7-14.1] | 0.7 [0.5-1.3] | 4.1 [3.6-5.4] | <0.001 |
| Weight at Transplantation (kg) | 26.8 [14.2-44] | 7.5 [5.6-10] | 13.4 [11-16.4] | <0.001 |
| Pre-transplant location data | 301 (100) | 27 (100) | 10 (100) | |
| Hospital ward/HDU | 176 (58.5) | 16 (59.3) | 4 (40) | 0.5 |
| ICU | 77 (25.6) | 6 (22.2) | 5 (50) | 0.2 |
| Home | 48 (15.9) | 5 (18.5) | 1 (10) | 0.82 |
| Pre-transplant mechanical support | ||||
| None | 211 (70) | 17 (63) | 4 (40) | 0.1 |
| VAD (LVAD/BiVAD) | 81 (27) | 9 (33.3) | 6 (60) | 0.06 |
| ECMO | 35 (11.6)a | 2 (7.4)b | 3 (30)c | 0.16 |
Abbreviations: ABO-C, Compatible Heart Transplant; ABO-PE, Incompatible Heart Transplantation with Plasma Exchange; ABO-IA, Incompatible Heart Transplantation with Immunoadsorption; BAME, Black, Asian and Minority Ethnicity; HDU, High Dependency Unit; ICU, Intensive CareUnit; VAD, Ventricular Assistance Device; LVAD, Left Ventricular Assistance Device; BiVAD, Biventricular Assistance Device; ECMO, Extracorporeal Membrane Oxygenation.
Continuous variables are presented as median [interquartile range] whilst categorical data are presented as count (percentage).
26 patients were converted to LVAD/BiVAD.
1 patient was converted to LVAD/BiVAD.
two patients were converted to LVAD/BiVAD.
Operative characteristics
There was no difference in proportions of patients receiving pre-transplant mechanical support (although a trend was seen towards higher usage in the ABO-IA group, χ2 = 5.56, p = 0.06) or ECMO (χ2 = 3.68, p = 0.16). There were also no differences in pre-transplant hospitalization requirement between groups, whether on a hospital ward (χ2 = 1.48, p = 0.5) or in intensive care (χ2 = 3.32, p = 0.2). Organ ischemic times in the ABO-IA group (232 [164-271] minutes) were not significantly different from the ABO-PE group (258 [234-294] minutes, p = 0.17). ABO-IA procedures had a significantly increased CPB time (206 [140-240] minutes vs 133 [112-162] minutes, p = 0.02; Table 3). However, it should be noted that the length of CPB reported in the ABO-PE group did not include the 30 minutes required for the plasma exchange itself prior to initiation of full CPB.
Table 3.
Incompatible Heart Transplantation Operative Characteristics and Outcomes
| ABO-incompatible transplant details and outcomes | |||
|---|---|---|---|
| ABO-PE | ABO-IA | p | |
| No. of patients | 27 (73) | 10 (27) | |
| No. requiring re-sternotomy | 10 (37) | 7 (70) | 0.16 |
| Cardiopulmonary Bypass Duration (minutes) | 133 [112-162] | 206 [140-240] | 0.02 |
| Organ Ischemic Duration (minutes) | 258 [234-294] | 232 [164-271] | 0.17 |
| Volume of Blood Products Transfused (ml/kg) | |||
| Total | 323 [268-379] | 164 [126-212] | <0.001 |
| Packed Red Blood Cells | 163 [130-210] | 83.3 [24.1-116] | 0.004 |
| Fresh Frozen Plasma | 128 [107-158] | 36.1 [33.3-37] | <0.001 |
| Platelets | 10 [10-20] | 20 [10-20] | 0.63 |
| Cyroprecipitate | 20 [20-40] | 30 [20-45] | 0.62 |
| Volume of Blood Products Transfused (excluding PE; ml/kg) | |||
| Packed Red Blood Cells | 160 [131-209] | 83.3 [24.1-116] | 0.003 |
| Fresh Frozen Plasma | 128 [108-155] | 36.1 [33.3-37] | <0.001 |
| Follow-Up Time (years) | 11.4 [9.2-14.8] | 3.3 [1.2-4.3] | <0.001 |
| Time to Hospital Discharge (days) | 20.5 [17.5-32.1] | 19.7 [17.6-64.6] | 0.69 |
| Time to Intensive Care Discharge (days) | 10 [8-22] | 9.5 [9-26.8] | 0.53 |
| No. requiring re-transplantation | 1 (3.7) | 0 | 1 |
| No. deceased | 5 (18.5) | 0 | 1 |
| Survival Time (Non-survivors) | 3.2 [3.1-11.1] | ||
Abbreviations: ABO-PE, Incompatible Heart Transplantation with Plasma Exchange; ABO-IA, Incompatible Heart Transplantation with Immunoadsorption.
Operative characteristics and outcomes following ABOi transplantation. Continuous variables are presented as median [interquartile range] whilst categorical data are presented as count (percentage). Cardiopulmonary bypass times in the ABO-PE group do not include the plasma exchange process (typically 30 minutes). Times to Intensive Care and Hospital discharge are from the date of transplantation to discharge.
Outcomes
Blood product transfusion requirements
Full transfusion data were available for 35 patients (94.6%; ABO-PE = 25, ABO-IA = 10). Total volumes of blood products transfused during the transplant admission were significantly lower in the ABO-IA group than the ABO-PE group (164 [126-212] ml/kg vs 323 [268-379] ml/kg, p <0.001). Sub analysis of the product type by group comparison with Bonferroni correction demonstrated no significant difference in volumes of cryoprecipitate (p = 0.62) or platelets (p = 0.63) between groups but significant differences in PRBC (p = 0.004) and FFP (p < 0.001; Table 3). Excluding the volume required for plasma exchange from the analysis, showed significantly lower PRBC and FFP volumes transfused in the ABO-IA group (p = 0.003 and p < 0.001, respectively).
Isohaemagglutinin Titres
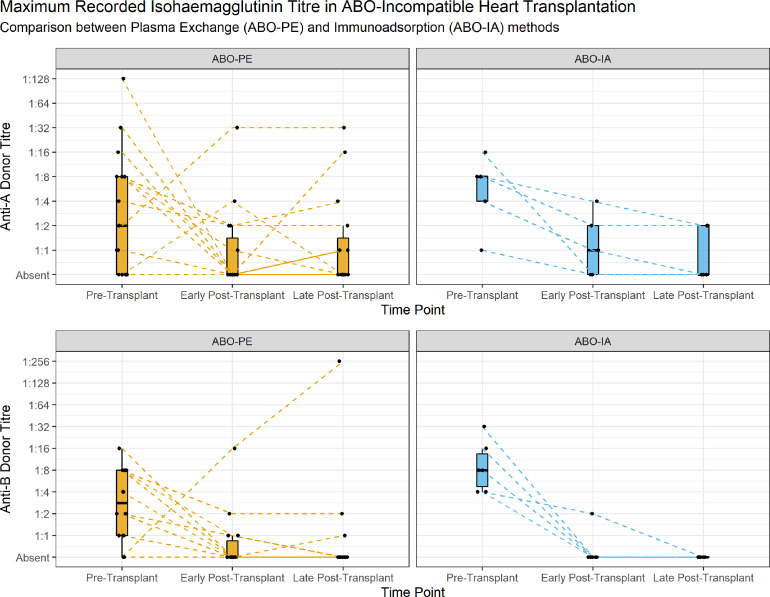
Pre and post-transplant isohaemagglutinin titres were available for all 37 patients undergoing ABO-incompatible transplantation. Analysis of maximum recorded isohaemagglutinin titres in the pre-transplant period showed no significant difference in anti-A titres, but significantly higher anti-B titres in the ABO-IA group compared to the ABO-PE group (1:16[1:8-1:16] vs 1:4[1:2-1:16], p = 0.35 and 1:16[1:8-1:32] vs 1:8[1:2-1:16], p = 0.048 respectively; Figure 2). Early maximum post-transplant titres were not significantly different in either anti-A or anti-B titres between groups (1:2[0-1:4] vs 1:1[0-1:4], p = 0.47 and 1:1[0-1:1] vs 1:1[0-1:1], p = 0.7 respectively), and neither were the late maximum post-transplant titres (anti-A 1:1[0-1:4] vs 1:1[0-1:4], p = 0.47; anti-B 0[0-1:1] vs 0[0-1:1], p = 0.86). Random effects analysis of repeated isohaemagglutinins titres demonstrated significantly lower post-transplant titres (2.02 ± 0.1 titres, χ2 = 250.92, p < 0.001) but that the addition of isohaemagglutinin reduction technique as a fixed effect did not significantly alter this (0.42 ± 0.33 titres, χ2 = 1.36, p = 0.24).
Figure 2.
Comparison of Maximum Recorded Anti-Donor Blood Group Isohaemagglutinin Titre. Titres were transformed using binary logarithm prior to analysis and back transformed for graphical representation. Post-transplant period defined as early if within 3 months of the date of ABOi transplantation, or late thereafter. Each line represents individual patient's titre course, faceted by antibody group; blood O group recipients receiving a donor AB organ have a line for both antibody titre.
Rejection
Histopathological examination records were available for all patients. No ABO-IA patients displayed clinical manifestations of rejection, with 1 patient (10%) developing DSA in the post-transplant period. Nine ABO-PE patients (33.3%) developed DSA in the post-transplant period. Four ABO-PE patients (14.8%) exhibited significant rejection episodes, of which 2 had developed DSA. There were no differences in the rate of DSA production between groups (p = 0.32). One patient displayed early acute cellular rejection (occurring 6 weeks post-transplant) and one early pAMR grade 2 (H+/I+; occurring 2 weeks post-transplant) which resolved after 6 months. The median time to an early rejection episode was 6 weeks. Three ABO-IA patients (30%) and six ABO-PE patients (22.2%) exhibited an early (< 3 months) pAMR grade of 1 with immunohistochemical staining (χ2 = 0.003, p = 0.95).
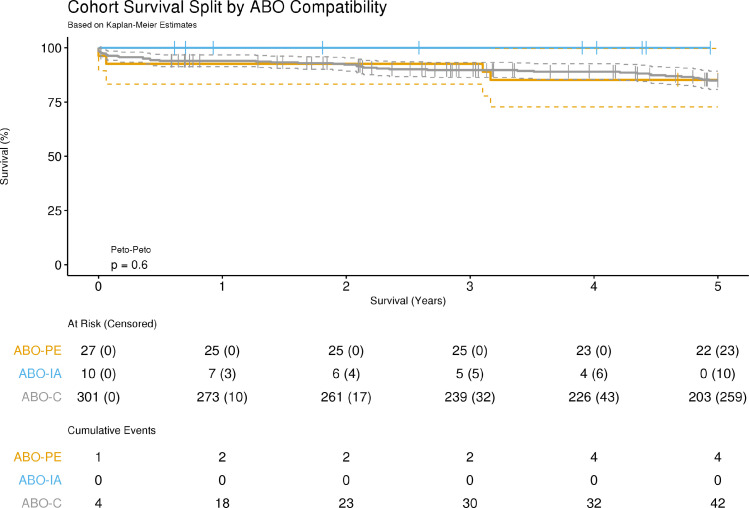
Survival
Five ABO-PE patients (18.5%) died with a median survival time of 3.2 [3.1-11.1] years with one dying of AMR. Seventy-two ABO-C patients (19.8%) died with a median survival time of 4.5 [0.9-8.2] years. Kaplan Meier analysis of early graft survival demonstrated no significant difference in mortality rates between groups (p = 0.6; Figure 3). Cox proportional hazards regression showed a significant effect of age at transplantation on survival, with increasing age being associated with reduced survival time (hazard ratio 1.07, 95%CI = 1.3 to 1.12; p = 0.001). No ABO-IA patients required re-transplantation. One ABO-PE patient (due to cardiac allograft vasculopathy) and three ABO-C patients required re-transplantation. There were no deaths in the ABO-IA group. Length of stay analysis showed no significant differences between groups in either postoperative intensive care unit or hospital ward stay (p = 0.69 and p = 0.53 respectively).
Figure 3.
Survival Analysis following Cardiac Transplantation. Survival analysis of 338 isolated cardiac transplants undertaken at Great Ormond Street Hospital during the period 2000 to 2020. Cumulative events were defined as recipient death or re-transplantation within 5 years of heart transplantation. All ABOi heart transplants prior to 2015 were undertaken using ABO-PE, and from 2015 -2020 all were ABO-IA. p-value represents logrank with Peto-Peto weighting. ABO-C, Compatible Heart Transplant; ABO-PE, Incompatible Heart Transplantation with Plasma Exchange; ABO-IA, Incompatible Heart Transplantation with Immunoadsorption.
Discussion
The use of ABOi heart transplantation has been widely adopted since its inception in pediatric patients, and has helped alleviate the competition for organs in smaller patients.3 Despite comparable survival outcomes with those recipients of compatible heart transplants, the use of a conservative three-fold plasma exchange process to minimise any mediators of acute rejection that had evaded detection places the recipient at increased risk of transfusion related morbidity.9 The plasma exchange process also limits ABOi to smaller patients due to practical considerations, as well as increasing pressures on allogeneic blood product usage.15 For this reason we introduced the routine use of isohaemagglutinin immunoadsorption during the transplant procedure.9 This early study has demonstrated that the use of immunoadsorption may enable larger patients, than have previously been considered, to be offered the option of ABOi heart transplantation. The avoidance of the plasma exchange process also reduced blood product utilization during the transplant admission by 50% in this cohort without increasing length of intensive care or hospital stay, isohaemagglutinin accumulation, incidence of rejection episodes or displaying inferior patient survival.
Increasing recipient pool
A key theoretical benefit of ABO-IA was that it could open up ABOi transplants to older, larger patients on the premise that fewer blood products would be needed, and that there is evidence that even in older patients, isohaemagglutinin titres can remain low.16 Compared to patients that have undergone ABO-PE at our institution, patients undergoing ABO-IA were significantly older (p <0.001). The oldest patient that underwent this procedure was 8 years old and 27kg, twice the age of our eldest ABO-PE patient and 12kg heavier. It should be noted however, that the underlying diagnosis of the patients treated was different from those that had undergone ABO-PE. The increased length of CPB observed in the ABO-IA group, corresponding to the higher frequency of re-sternotomy, reflect these altered patient demographic and subsequent pre-transplant requirements.
Reducing blood product transfusion
We attempted to ascertain the impact of switching techniques to immunoadsorption on the transfusion requirements of children undergoing ABO-incompatible transplantation throughout their hospital stay. Data were obtained on the number of blood products transfused to patients during and following their transplant procedure, and indexed to body weight. Whilst the exact volumes were unrecorded, we used the average volumes of each product type, based upon clinical experience, to estimate the volume of products. The data demonstrate that total transfusion volume was significantly lower in the ABO-IA group (p = 0.001), representing a reduction of approximately 50%. Given that the ABO-PE patients received a 1:1 mix of PRBC and FFP during the plasma exchange (of three-fold the patient's circulating volume), that there are significant differences in these products is unsurprising. To elucidate whether this difference was explained by the plasma exchange itself, the volume of the exchange transfusion was excluded in a sub-analysis. The data demonstrated significant reductions in both PRBC and FFP volumes transfused postoperatively in the ABO-IA group (p = 0.003 and p < 0.001, respectively). However, it is important to note that we did not examine the effects of age on coagulation parameters, and therefore cannot rule out any age-related effects on coagulation cascade maturity. Further work is required to determine the effects the introduction of the immunoadsorption process might have on the incidence of transfusion related morbidity.
Absence of isohaemagglutinin accumulation or rejection
A key safety consideration with ABO-IA is whether recipients would exhibit an accumulation in isohaemagglutinin titres or an increased incidence of rejection.11 Whilst the United Network for Organ Sharing (UNOS) policy currently allows for children between 1 and 2 years of age to be listed for ABO-PE if titres are ≤1:16, this is often taken as a point measure immediately prior to the transplant operation. We observed a high level of variability over time in isohaemagglutinin titres of individual patients. In order to account for individual variability, we used a random effects model for the analysis of isohaemagglutinin titres to determine the overall difference between ABO-PE and ABO-IA taking into account repeated measures. The random effects model revealed a significant time effect (pre-transplant vs post-transplant) when compared to the individual patient as a control (p < 0.001). The addition of the isohaemagglutinin reduction technique did not present a significant difference compared to the time point model (p = 0.24). Long term follow up and increased sample size are required to determine if this effect is seen in the longer term.
We did not observe a significant difference in the maximum titres between the two groups (or when separated into anti-A and anti-B donor isohaemagglutinins) in the early or late post-transplant period, although there were significantly higher preoperative anti-B titres in the ABO-IA group (p = 0.048). The lack of difference in the post-transplant period suggests that the ABO-IA process is not associated with an increased accumulation in isohaemagglutinins compared to ABO-PE. This is further supported by a lack of histopathological evidence of AMR from endocardial biopsies in ABO-IA patients, suggesting that the use of intraoperative immunoadsorption does not increase the incidence of rejection in the short term. Whilst the follow-up for the ABO-IA was shorter than the ABO-PE group, it did however encompass the timeframe in which three quarters of the ABO-PE rejection episodes occurred. Longer-term follow-up is required to determine the effectiveness of immunoadsorption in maintaining a low incidence of AMR.
Equivalent survival
The equivalence of survival in ABOi vs ABO-C heart transplantation has been well documented.4 Furthermore it has been demonstrated that ABOi transplantation reduces the waiting-list risk of death without increasing the risk of death post-transplant.2,4 Comparison of early patient survival between ABO-incompatible methods and compatible heart transplants showed no significant difference between groups (p =0.6), with no mortality in the ABO-IA group. Due to the difference in population age between the two methodologies, and to ensure that the younger ABO-PE patients were not affected detrimentally, a Cox regression model with age as the independent variable was used to assess any age-related effect on survival time across all transplant methods. Whilst the effect was small, there was a significant effect of age on survival time, with an increasing age at transplant associated with a decreased survival time (p = 0.001). This observation is supported by recent analysis of the International Thoracic Organ Transplant Registry from the International Society for Heart and Lung Transplantation.17 This study suggests that ABO-IA has equivalent survival to both ABO-PE and ABO-C transplants.
Limitations
This study has a number of limitations. Firstly, it is a retrospective analysis over a 20-year period and as such may include patients treated with heterogeneous protocols and follow up periods. Whilst the methodology of isohaemagglutinin titre reduction was confined to the methods described here, we did not include the impact of differing drug regimens or intensive care practices over time, although immunosuppression protocols for ABOi patients described here have not altered since the first ABOi transplant at our institution in 2001. Secondly, blood product transfusion volumes for each individual unit were estimated as these data were not available, and an assumption was made that the total volume was administered to the patient. It is not possible to be certain that this is accurate and therefore the volumes stated should be interpreted with caution. However, as assumptions were the same in both groups, and volumes are reported indexed to body weight, these limitations should have equivalence in both groups. Finally, the sample sizes described here are small and of a single centre.
Conclusions
These data report the first case series of ABOi patients using intraoperative immunoadsorption for removal of ABO antibodies. We have demonstrated that a significant reduction in blood product usage with the immunoadsorption method can be achieved compared to the established ABO-PE method. The technique has shown the potential to expand the recipient pool for incompatible transplants. Within the shorter time available to follow up the ABO-IA patients, we have demonstrated an equivalence of outcomes in terms of survival, intensive care and hospital stay, reduction in isohaemagglutinin titres and rates of histopathological reported rejection compared to patients undergoing ABO-PE. Further work across multiple centres is required to corroborate these findings and to assess long-term outcomes of this novel technique.
Authors’ contribution
The corresponding author (RI), JB, and MF confirm that they had full access to all the data in the study and had final responsibility for the decision to submit for publication. RI, RC, AR and MF conceived the study. JB and RI performed the analyses. All authors contributed to the critical appraisal and writing of the manuscript and approved the final submission.
Disclosure statement
The authors declare no conflicts of interests.
The authors would like to thank Moona Malik, Rochelle Wilson and Alice Hayward for their Perfusion input and feedback, and Sophie Henwood and Sarah-Jane Mead-Regan from the Transplant team for their assistance and dedication to the patients described here. Also, Mohsin Shah, William Bryant, Lydia Briggs and Anastasia Spiridou for advising on data analysis. Finally, to the patients themselves for providing a constant reminder to keep pushing boundaries.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: British Heart Foundation Research Fellowship to RI (FS/19/52/34563).
The study sponsor / funders had no role or influence in study design, in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
References
- 1.West LJ. ABO-incompatible hearts for infant transplantation. Curr Opin Organ Transplant. 2011;16:548–554. doi: 10.1097/MOT.0b013e32834a97a5. [DOI] [PubMed] [Google Scholar]
- 2.West LJ, Karamlou T, Dipchand AI, Pollock-BarZiv SM, Coles JG, McCrindle BW. Impact on outcomes after listing and transplantation, of a strategy to accept ABO blood group-incompatible donor hearts for neonates and infants. J Thorac Cardiovasc Surg. 2006;131:455–461. doi: 10.1016/j.jtcvs.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 3.Patel ND, Weiss ES, Scheel J, Cameron DE, Vricella LA. ABO-incompatible heart transplantation in infants: analysis of the United network for organ sharing database. J Heart Lung Transplant. 2008;27:1085–1089. doi: 10.1016/j.healun.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Saczkowski R, Dacey C, Bernier P-L. Does ABO-incompatible and ABO-compatible neonatal heart transplant have equivalent survival? Interact Cardiovasc Thorac Surg. 2010;10:1026–1033. doi: 10.1510/icvts.2009.229757. [DOI] [PubMed] [Google Scholar]
- 5.West LJ, Pollock-Barziv SM, Dipchand AI, et al. ABO-incompatible heart transplantation in infants. N Engl J Med. 2001;344:793–800. doi: 10.1056/NEJM200103153441102. [DOI] [PubMed] [Google Scholar]
- 6.Urschel S, Ryan LA, Larsen IM, et al. C3d plasma levels and CD21 expressing B-cells in children after ABO-incompatible heart transplantation: Alterations associated with blood group tolerance. J Heart Lung Transplant. 2014;33:1149–1156. doi: 10.1016/j.healun.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Foreman C, Gruenwald C, West L. ABO-incompatible heart transplantation: a perfusion strategy. Perfusion. 2004;19:69–72. doi: 10.1191/0267659104pf708oa. [DOI] [PubMed] [Google Scholar]
- 8.Issitt RW, Crook RM, Cross NT, et al. Incompatible ABO-plasma exchange and its impact on patient selection in paediatric cardiac transplantation. Perfusion. 2012;27:480–485. doi: 10.1177/0267659112453076. [DOI] [PubMed] [Google Scholar]
- 9.Robertson A, Issitt R, Crook R, et al. A novel method for ABO-incompatible heart transplantation. J Heart Lung Transplant. 2018;37:451–457. doi: 10.1016/j.healun.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Issitt R, Crook R, Shaw M, Robertson A. The Great Ormond Street Hospital immunoadsorption method for ABO-incompatible heart transplantation: a practical technique. Perfusion. 2020;36:34–37. doi: 10.1177/0267659120926895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson KE, Canter C. Can we push the boundaries of ABO-incompatible pediatric heart transplantation? J Heart Lung Transplant. 2018;37:433–434. doi: 10.1016/j.healun.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2013;32:1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Durandy Y. Blood transfusion in pediatric cardiac surgery. Artif Organs. 2010;34:1057–1061. doi: 10.1111/j.1525-1594.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 14.Irving C, Gennery A, Kirk R. Pushing the boundaries: the current status of ABO-incompatible cardiac transplantation. J Heart Lung Transplant. 2012;31:791–796. doi: 10.1016/j.healun.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 16.Bucin D, Johansson S, Malm T, et al. Heart transplantation across the antibodies against HLA and ABO. Transpl Int. 2006;19:239–244. doi: 10.1111/j.1432-2277.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh TP, Hsich E, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 23rd pediatric heart transplantation report—2020; focus on deceased donor characteristics. J Heart Lung Transplant. 2020;39:1028–1037. doi: 10.1016/j.healun.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]