Abstract
The development of venous intimal hyperplasia (IH) has been historically associated with failure of arteriovenous fistulas (AVFs) used for hemodialysis. This long-standing assumption, made on the basis of histologic observations, has been recently challenged by clinical studies indicating that the size of the intima by itself is not enough to explain stenosis or AVF maturation failure. Irrespective of this lack of association, IH is present in most native veins and fistulas, is prominent in many patients, and suggests a role in the vein that may not be reflected by its dimensions. Therefore, the contribution of IH to AVF dysfunction remains controversial. Using only clinical data and avoiding extrapolations from animal models, we critically discuss the biologic significance of IH in vein remodeling, vascular access function, and the response of the venous wall to repeated trauma in patients receiving hemodialysis. We address questions and pose new ones such as the following: What are the factors that contribute to IH in preaccess veins and AVFs? Do cellular phenotypes and composition of the intima influence AVF function? Are there protective roles of the venous intima? This review explores these possibilities, with hopes of rekindling a critical discussion about venous IH that goes beyond thickness and AVF outcomes.
Keywords: hemodynamics and vascular regulation, arteriovenous fistula, hyperplasia, intimal hyperplasia, outcomes, pre-access vein, stenosis, vascular access
Introduction
Intimal hyperplasia (IH) is a buildup of myofibroblast-like cells (neointimal cells) and extracellular matrix (ECM) within the tunica intima, the innermost layer of the vein (1). In preaccess veins of patients with CKD, IH manifests as an idiopathic and benign histologic feature that does not compromise blood flow (2–6). After arteriovenous fistula (AVF) creation, expansion of the intima may remain innocuous or aggravate inward remodeling, leading to stenosis and access failure (3). The actual contribution of IH in relation to other transformation processes in the wall after AVF creation remains uncertain (7). Importantly, once stenosis becomes a pathology, it is not easily treatable, with frequent recurrences after endovascular procedures (8–14). The lack of more effective therapies against stenosis reflects our simplistic view of the processes of intimal expansion and wall remodeling, and our unawareness of the characteristics that distinguish between benign and occlusive IH.
This review critically discusses what we know about IH in human veins and AVFs, what we are missing, and how this knowledge may influence IH-targeted therapies to improve AVF outcomes. We discuss the historical assumption that IH alone causes stenosis and AVF failure and extend our debate beyond IH size, the focus of published research in this area. Finally, we highlight the necessity for innovation, state-of-the-art omics, and single-cell technology to clarify the actual role of IH in venous remodeling.
Preexisting IH
IH in the Preaccess Vein: More Common than Previously Thought
The cephalic and basilic veins are the preferred choices for AVF creation (15). These are medium-sized veins with the three vascular layers well defined (tunica intima, tunica media, and tunica externa or adventitia) and diameters between 1 and 5 mm (Figures 1 and 2) (16,17). The intima is the innermost layer of the vessel and is demarcated by a thin or discontinuous internal elastic lamina on the medial side, and a continuous endothelial line that separates it from the lumen. Thin folds of collagen-rich connective tissue, covered by endothelium, extend from the intima and form the valves at regular intervals along the vein.
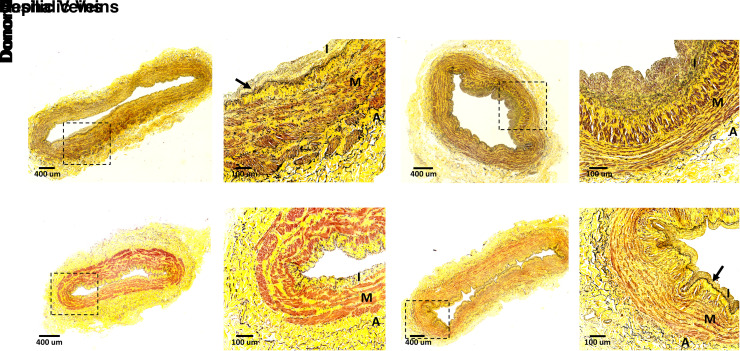
Figure 1.
Minimal to moderate intimal hyperplasia in trauma donors with normal renal function. (A–D) Cross-sections of the (A and B) cephalic and (C and D) basilic veins from a Hispanic 56-year-old male without history of hypertension, diabetes, or coronary artery disease. (E and F) Cephalic and (G and H) basilic veins from a Hispanic 55-year-old male with history of controlled hypertension (<5 years). Sections were stained with Movat pentachrome stain, with cells showing in brown/red, collagen in yellow, and elastin in black. Boxed areas in (A), (C), (E), and (G) are magnified in (B), (D), (F), and (H), respectively. Arrows in (B) and (H) identify the internal elastic lamina. A, adventitia; I, intima; M, media.
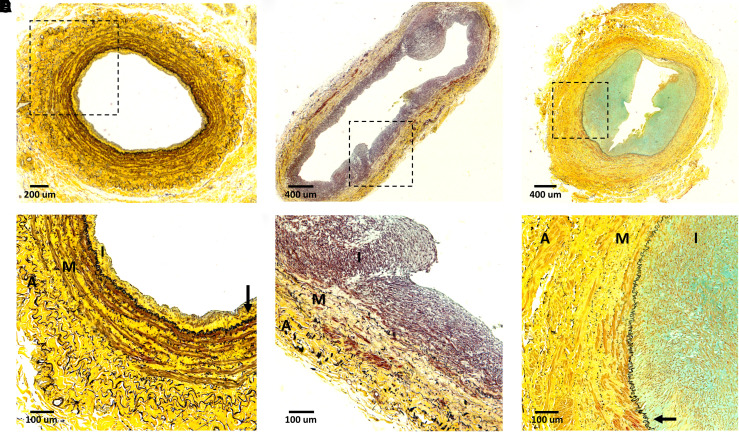
Figure 2.
Variability in intimal hyperplasia in preaccess basilic veins from patients with ESKD. (A–C) Cross-sections of basilic veins collected during first-stage surgery of a two-stage brachiobasilic arteriovenous fistula (AVF). Patient in (A) is a 42-year-old Black female with history of hypertension; patient in (B) is a 68-year-old Black female with hypertension and diabetes; and patient in (C) is a 65-year-old Hispanic female positive for hypertension, diabetes, and coronary artery disease. All three veins matured successfully after AVF creation. Sections were stained with Movat pentachrome stain, with cells showing in brown/red, collagen in yellow, and elastin in black. Boxed areas in (A) to (C) are magnified in (D) to (F), respectively. Arrows in (E) and (F) identify the internal elastic lamina. A, adventitia; I, intima; M, media.
The size of the intima layer in preaccess veins ranges from almost inexistent to thick and rich in intimal cells and ECM, with this latter scenario being the norm rather than the exception (2–6,18–24). Almost 20 years ago, Wali et al. (25,26) observed generalized IH in cephalic veins from 20 patients with renal failure. In more recent and larger patient cohorts (N=57–129), maximal intimal thickness (the longest distance between the media and the lumen) ranged from 1 to 660 µm in forearm and upper-arm preaccess veins (Table 1), with higher values in the latter (2,4,6). The Hemodialysis Fistula Maturation (HFM) Study confirmed the high prevalence of IH in 365 preaccess veins and quantified it as the percentage of luminal occlusion (Table 1) (5). Of the patients, 22% presented moderate IH (21%–40% luminal occlusion), whereas 35% portrayed severe IH (>41% occlusion). In agreement with Allon et al. (4), the percentage of luminal occlusion was lower in cephalic veins (mean 31%) than in upper-arm vessels (mean 40%). Martinez et al. (3) expressed IH as intima/media area ratio (I/M ratio) in 110 upper-arm veins to account for morphometric changes in the media, such as atrophy or hypertrophy (18,20,25,26). The median I/M ratio in this cohort was 0.32, in line with previous reports of cephalic and other preaccess veins (Table 1) (18,19,24).
Table 1.
Intimal morphometry and associations with arteriovenous fistula outcomes in the hemodialysis population
| Study | N a | Vein | Parameter(s) | Measurementsb | Association(s) |
| Preexisting intimal hyperplasia | |||||
| Feinfeld et al. (23) | 15 | Brachial (n=15) | Ave. I thickness | 6.0±0.9 µm | N/A |
| Lee et al. (18) | 12 | Cephalic (n=6), axillary (n=3), antecubital (n=1), basilic (n=1), brachial (n=1) | Ave. IM thickness | 0.34±0.12 mm | Significant association with maturation failure (P=0.03, n=7) |
| Max. IM thickness | 1.16±0.30 mm | N/A | |||
| I/M area ratio | 0.24±0.07 | N/A | |||
| % Luminal occlusion | 47%±9% | Lack of association with maturation failure (P=0.09, n=7) | |||
| Wasse et al. (24) | 10 | Cephalic, basilic | Ave. I thickness | 0.066±0.019 mm | N/A |
| Max. I thickness | 0.166±0.042 mm | N/A | |||
| Mean I/M thickness ratio | 0.26±0.07 | N/A | |||
| Max. I/M thickness ratio | 0.69±0.19 | N/A | |||
| Intimal area | 0.27±0.08 mm2 | N/A | |||
| I/M area ratio | 0.24±0.06 | N/A | |||
| Allon et al. (4) | 113 | Upper arm (65%), forearm (35%) | Max. I thickness | 0.022 (0.013–0.045) mm | Lack of association with postoperative stenosis (P=0.49) |
| Lee et al. (21) | 29 | N/A | Mean I/M thickness ratio | 0.43±0.07 | N/A |
| Max. I/M thickness ratio | 0.86±0.07 | N/A | |||
| Lazich et al. (19) | 18 | Cephalic (n=18) | Max. I thickness | 0.052–0.81 mm | N/A |
| Intimal area | 0.16–7.70 mm2 | N/A | |||
| I/M area ratio | 0.07–1.80 | N/A | |||
| Mean I/M thickness ratio | 0.07–1.99 | N/A | |||
| Max. I/M thickness ratio | 0.14–2.44 | N/A | |||
| % Luminal stenosis | 45%–96% | N/A | |||
| Tabbara et al. (2) | 57 | Basilic (n=54), brachial (n=3) | Max. I thickness | 0.18 (0.10–0.20) mm | Lack of association with primary unassisted patency (P=0.2, n=52) |
| I/M area ratio | N/A | Lack of association with primary unassisted patency (P=0.2, n=52) | |||
| HFM Study (5,52)c | 365 | Cephalic (69%), basilic (29%), brachial (2%) | % Luminal occlusion | 28%±27% (cephalic), 40%±30% (basilic), 21%±23% (brachial) | Lack of association with postoperative stenosis at 1 day (P=0.49), 2 weeks (P=0.91), or 6 weeks (P=0.07); lack of association with unassisted (P=0.07) or overall maturation failure (P=0.11) |
| Martinez et al. (3) | 110 | Basilic (n=104), brachial (n=4), cephalic (n=2) | I/M area ratio | 0.32 (0.22–0.52) | Lack of association with maturation failure (P=0.7) |
| Allon et al. (6) | 129 | Upper arm (65%), forearm (35%) | Max. I thickness | 0.037±0.040 mm | N/A |
| Postoperative intimal hyperplasia | |||||
| Roy-Chaudhury et al. (41) | 4 | Cephalic (n=4), all early failures | Mean I/M thickness ratio | 3.12±0.43 | N/A |
| Max. I/M thickness ratio | 7.77±1.49 | N/A | |||
| I/M area ratio | 1.67±0.10 | N/A | |||
| % Luminal stenosis | 86%±3% | N/A | |||
| Lee et al. (21) | 20 | Cephalic (n=15), basilic (n=5); all stenotic segments | Mean I/M thickness ratio | 3.84±0.55 | N/A |
| Max. I/M thickness ratio | 7.78±0.88 | N/A | |||
| Tabbara et al. (2) | 79 | Basilic (n=74), brachial (n=5) | Max. I thickness | 0.62 (0.38–0.86) mm | Lack of association with maturation failure (P=0.3); lack of association with primary unassisted patency (P=0.6) |
| I/M area ratio | N/A | Lack of association with maturation failure (P=0.4); lack of association with primary unassisted patency (P=0.8) | |||
| Duque et al. (71)d | 14 | Basilic (n=12), brachial (n=2); all AVFs had stenotic and nonstenotic segments | I area | 3.33 (1.94–4.86) mm2 in nonstenotic, 3.33 (2.29–5.16) mm2 in stenotic | Lack of association with focal stenosis (P=0.26) |
| Min. I thickness | 0.09 (0.05–0.31) mm in nonstenotic, 0.11 (0.05–0.43) mm in stenotic | Lack of association with focal stenosis (P=0.18) | |||
| Max. I thickness | 0.75 (0.54–1.08) mm in nonstenotic, 0.98 (0.78–1.20) mm in stenotic | Lack of association with focal stenosis (P=0.22) | |||
| Min. IM thickness | 0.37 (0.17–0.70) mm in nonstenotic, 0.30 (0.23–0.88) mm in stenotic | Lack of association with focal stenosis (P=0.22) | |||
| Max. IM thickness | 1.14 (0.84–1.38) mm in nonstenotic, 1.38 (1.30–1.57) mm in stenotic | Lack of association with focal stenosis (P=0.13) | |||
| I/M area ratio | 0.97 (0.63–1.18) in nonstenotic, 1.00 (0.70–1.20) in stenotic | Lack of association with focal stenosis (P=0.73) | |||
| Martinez et al. (3) | 115 | Basilic (n=97), brachial (n=14), cephalic (n=4) | I/M area ratio | 0.77 (0.48–1.30) | Lack of association with maturation failure by itself (P=0.09, n=115), but significant association in AVFs with high medial fibrosis (P=0.04, n=58) |
Ave., average; I, intima; N/A, not reported or studied; IM, intima plus media; max., maximum; I/M, intima/media; HFM, Hemodialysis Fistula Maturation; AVF, arteriovenous fistula; min., minimum.
Number of veins analyzed after study exclusions.
Values presented as mean±SEM (SD in the HFM Study [5,52] and Allon et al. [6]), median (interquartile range), or range in Lazich et al. (19).
Pairwise comparison of stenotic and adjacent nonstenotic segments in upper-arm AVFs.
Despite its common occurrence in preaccess veins, whether IH development is influenced by CKD remains unknown because experimental animal models do not develop spontaneous IH. Various studies support an increase in IH in the setting of CKD (6,21,25–27), but the number of non-CKD upper-extremity veins is low (three to 15 individuals), which makes it difficult to draw a definite conclusion on this issue. The I/M ratio of the great saphenous vein was also found to be significantly higher in patients with CKD compared with controls, and in those with ESKD versus CKD stages 1 and 2 (27), but it is not clear whether these groups were matched with respect to age and baseline characteristics. It is tempting to speculate that IH increases during the course of renal dysfunction secondary to volume/flow overload (anemia, sodium, and water retention) and other poorly defined clinical factors. However, frequent IH was observed in cephalic and saphenous veins from elderly patients with normal renal function (28), suggesting uremia is not the only vascular insult causing intimal thickening. Synergistic insults may include endothelial dysfunction in CKD and vascular injury related to venipuncture or catheterization. On the other hand, the presence of a thick intima in basilic veins (2,3), as in the superficial cephalic vein (4–6,19), suggests that mechanisms other than venipuncture-related trauma promote IH. Single-cell sequencing and spatial proteomics may help identify differences in cell and ECM composition (if any) between the CKD and non-CKD preaccess intima. This may, in turn, uncover common and disease-relevant origins of IH.
Composition of the Preaccess Intima: Identifying Knowledge Gaps
Three types of cells predominate in the intima of preaccess veins: endothelial cells (ECs), smooth muscle cells (SMCs), and myofibroblasts/fibroblasts (1,2,5,18–20,29). ECs line the luminal side of the intima, whereas SMCs and myofibroblasts, embedded in ECM, populate the core of the layer. ECs play an essential role in preventing thrombosis, but their contribution to controlling IH has not been fully elucidated. Although there is wide support for the inhibitory effect of EC-derived nitric oxide in intimal cell proliferation and migration (30), there is also evidence for other endothelial paracrine factor(s) that stimulate venous IH (31). The overall effect of the endothelium on IH is likely dependent on flow and pathophysiologic conditions. Along these lines, profound changes in EC and SMC morphology (25,26,32) and function (33–39) have been detected in patients with CKD.
Using a combination of contractile (smooth muscle myosin heavy chain [SM-MHC], desmin, h-caldesmon, calponin), synthetic (vimentin), and pan SMC markers (α-smooth muscle actin [αSMA]), various groups have observed a mixture of SMC and/or myofibroblast phenotypes in the intima of preaccess veins (2,18–20,29,40,41), whose functions remain uncertain. Contractile SMCs are typically associated with low proliferation and migration rates, and low secretion of ECM (42). The opposite behavior is characteristic of “synthetic” or “myofibroblastic” SMCs that have lost expression of contractile markers. Serum from patients with dialysis favors the synthetic transformation of cultured human SMCs by promoting epigenetic downregulation of contractile gene expression (27). Interestingly, despite the high number of synthetic SMCs in preaccess veins, they show minimal staining of the proliferation and metabolic markers Ki-67 and phosphoglucomutase 1, respectively, suggesting that intimal cells are relatively quiescent before access creation (5,18). The HFM Study also reported rare apoptotic cells by cleaved caspase 3 expression in <10% of analyzed intimas (four of 48) (5). How expression of contractile markers in CKD veins relates to venoconstriction or dilation is unclear. A thick intima likely serves as a barrier for the diffusion of both circulatory vasoactive factors and EC-derived molecules that regulate medial SMC contraction or dilation. This may be an advantageous adaptation to reduce vasoconstrictive responses. On the other hand, studies in saphenous veins proposed that intimal thickness >120 µm is associated with impaired endothelium-dependent vasodilation (43). If there is a signaling cascade of soluble factors from ECs or the lumen that is amplified by intimal myofibroblasts or SMCs remains to be discovered.
In contrast with the abundance of inflammatory cells in the arterial intima with disease, the number of immune cells in preaccess veins is minimal. Approximately 50% of analyzed veins in the HFM Study (25 of 48) showed only one CD68-expressing macrophage in the intima, with approximately 7.7 cells in the whole section (5). Martinez et al. (44) also reported low numbers of CD68+ macrophages (about 40 cells per cross-section) in 45 basilic veins, mostly located at the edge between the media and the adventitia. In terms of T cells, Lee et al. (18) observed minimal CD3+ staining in the intima. Despite the low levels of immune infiltration in preaccess veins, a transcriptomic analysis uncovered expression of myeloid-related inflammatory genes in intimal and medial SMCs and myofibroblasts, suggesting a key role of resident cells in vascular inflammation (44). Five genes (CSF3R, FPR1, S100A8, S100A9, and VNN2) were associated with AVF maturation failure, and expression of S100A8 and S100A9 had a weak correlation with postoperative IH (44). Wasse et al. (24) also found expression of TNF-α, TGF-β, and IL-6 in the intima of preaccess veins. Immunohistochemistry analyses demonstrate that not all cells in the intima and media are positive for these proteins (24,44), demonstrating again a heterogeneity of SMC and myofibroblast phenotypes with potential implications for the inflammatory status of the vessel.
The ECM composition of the intima is an important aspect of remodeling, and perhaps the most neglected characteristic of this layer. The HFM Study observed significant interpatient variability by histology in the amount and distribution of collagen and proteoglycans in the intima (5). Intimal expansion in earlier reports of 20 cephalic veins was also characterized by marked deposition of fragmented collagen fibers and dispersed elastin (25,26). Intimal calcification was observed in 2% of the patients in the HFM Study and 15% in Wali et al. (5,25). It is important to note that the proportion and configuration of the ECM and the types of ECM proteins in the intima may play a role in cell proliferation and migration (45–48), vein stiffness (49,50), and/or compressibility of this layer under high flow conditions (51). Accumulation of collagen is associated with fibrosis, whereas high proteoglycan content may confer resistance to compression and act as a reservoir of cytokines and growth factors that influence cell survival and proliferation (49,51). Future proteomic studies are needed for a more accurate characterization of the intimal ECM.
Does Preexisting IH Increase the Risk of AVF Failure?
The initial idea that preexisting IH potentially led to stenosis and poor AVF outcomes has been recently challenged in several independent studies (2–4,52). Allon et al. (4) studied the association between maximal intimal thickness in the preaccess vein and postoperative AVF stenosis. Of the 113 patients included in the analysis, 50% developed a hemodynamically significant stenosis. However, there was no association between IH and the presence of postoperative stenosis (Table 1). This lack of association remained true when analyzed by type of AVF and location of the stenosis (4). The results of this study were confirmed by the HFM Study in 365 individuals (52). The development of stenosis was evaluated by ultrasound at 1 day, 2 weeks, and 6 weeks after AVF creation. Preexisting IH (percentage of luminal occlusion) was not associated with AVF stenosis at any of these time points, nor with the internal diameter of the vessel (52).
The relationship between preexisting IH and maturation failure was analyzed by the HFM Study and Martinez et al. (3,52). The HFM Study found a significant association between the preexisting percentage of luminal occlusion and venous blood flow rate at 6 weeks after access creation (Table 1). However, the association with unassisted or overall maturation failure did not reach statistical significance (52). Preexisting I/M ratio also failed to predict nonmaturation in 110 patients in the study by Martinez et al. (3). Lastly, Tabbara et al. (2) analyzed the association between preexisting IH and primary unassisted patency in 52 upper-arm fistulas. Neither maximal intimal thickness nor I/M area ratio predicted loss of primary patency. Although there seems to be no association between preexisting intimal morphometry and AVF failure, additional studies are needed to assess the effects of intimal cell and ECM composition on postoperative outcomes.
Postoperative IH
IH after AVF Creation: Selective Activation of Preexisting Cells?
The transformation of the vein after AVF creation remains one of the least understood processes in vascular biology. Current knowledge emphasizes the role of ECs in sensing arterial shear stress to release vasodilators that potentially lead to maturation (53,54). However, the endothelium is almost certainly severely damaged by surgical trauma, secondary to the common use of dilators and saline flushing to expand venous size before anastomosis. This suggests that intimal and medial cells likely play a protagonist’s role as mechanosensors of hemodynamic changes and vascular trauma. The best evidence we have about postoperative remodeling of the intima is from two-stage AVFs, which allow us to collect a biopsy of the remodeled vein (now a fistula) during transposition surgery.
In upper-arm fistulas, maximal intimal thickness increased approximately four-fold with respect to the preaccess vein, with values ranging from 0.1 to 2.0 mm in 79 patients who underwent two-stage AVF creation (Table 1) (2). This increase was not associated with the waiting time between AVF creation and transposition surgeries, or with the thickness of the intima in the preaccess vessel. The lack of relationship between preexisting and postoperative IH agrees with a selective activation of cells in the wall and different responses to surgical or hemodynamic injury between patients. Moreover, the absence of correlation between IH and the time between AVF creation and transposition surgeries suggests that most intimal expansion occurs early during maturation. Medial atrophy is frequently seen in AVFs (Figure 3), possibly as a result of cell death or migration of SMCs into the intima. This SMC loss is either replaced by ECM (fibrosis) or results in thinning of the media. A median I/M area ratio of 0.77 was reported in 115 two-stage AVF cross-sections, significantly higher than in native veins (Table 1) (3). In agreement with maximal intimal thickness, I/M ratio also demonstrated a lack of correlation between preexisting and postoperative values in pairwise analyses.
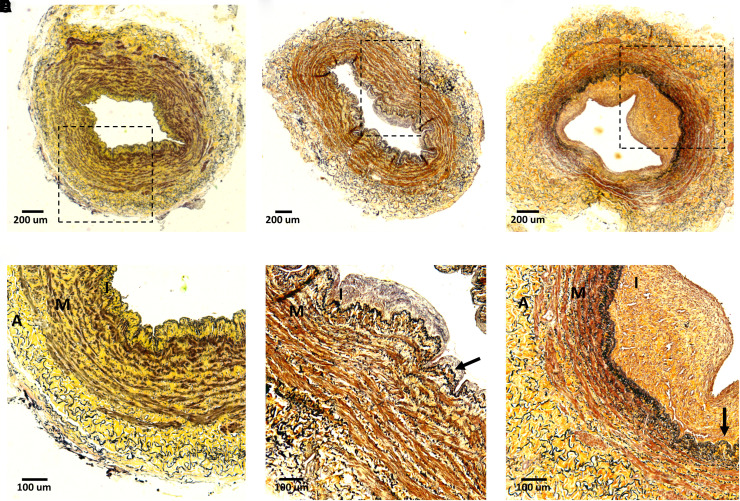
Figure 3.
Heterogeneity of venous remodeling in AVFs from patients with ESKD. (A–C) Cross-sections of juxta-anastomotic AVF segments from two-stage brachiobasilic fistulas collected during second-stage surgery (77–91 days after AVF creation). Patient in (A) is a 38-year-old Hispanic male with history of hypertension, patient in (B) is an 80-year-old Black female with hypertension and diabetes, and patient in (C) is a 40-year-old Black male positive for hypertension and coronary artery disease. All three AVFs failed to mature and underwent a salvage procedure or creation of a new fistula. Sections were stained with Movat pentachrome stain, with cells showing in brown/red, collagen in yellow, proteoglycans in blue, and elastin in black. Boxed areas in (A) to (C) are magnified in (D) to (F), respectively. Arrows in (D) and (F) identify the internal elastic lamina. A, adventitia; I, intima; M, media.
In contrast to native veins (5), IH in most upper-arm AVFs is eccentric (Figure 3). Unequal hemodynamic forces along the length of the vein are thought to explain this morphometry. This has been recently imaged in mice (55), although confirmatory studies in large animal models and humans are needed. It has been proposed that low wall shear stress, pulsatile stretch, and flow turbulence causes injury and elicits proliferation of intimal cells (56–60). However, it is possible that such eccentric appearance may be explained by pockets of increased cell proliferation, migration, and/or ECM deposition; and that these pockets are determined, in turn, by the phenotypes of the preexisting intimal cells.
At the cellular level, the postoperative remodeling process seems to favor the intimal expansion and/or survival of myofibroblasts and synthetic SMCs in the AVF wall. Tabbara et al. (2) observed that the intima of upper-arm AVFs collected at the time of transposition was mostly made up of synthetic SMCs (positive for αSMA, negative for SM-MHC). Other studies also reported a majority of myofibroblasts (αSMA+, vimentin+, desmin−) (40,41,61) and reduced expression of SM-MHC and calponin (29) in the intima of stenotic samples collected at the time of AVF revision. In all studies, medial SMCs retained contractile protein expression (2,29,40,41). The proportions and phenotypes of AVF intimal cells that are actively proliferating remain unknown. High intimal levels of the proliferation marker proliferating cell nuclear antigen (PCNA) were observed in stenotic areas of resected AVFs (41,62), but were contradicted by the more accurate marker Ki-67 (63). High PCNA and cyclin dependent kinase 2 (CDK2) levels in the study by de Graaf et al. (62) were also accompanied by significantly lower expression of the cell cycle regulator p21Waf1. Future single-cell tracing experiments will help define whether the increase in synthetic SMCs and myofibroblasts in the AVF intima is due to the postoperative dedifferentiation and expansion of contractile SMCs in the wall, or proliferation of a preexisting synthetic population. This information will be instrumental for the design of targeted therapies.
The role of immune cell infiltration in postoperative IH and AVF dysfunction is not clear at the moment. Increased macrophage and T-cell infiltration was seen in 15 stenotic AVF sections compared with preaccess veins (64). In contrast, a comparison of 13 nonthrombosed stenotic samples and 23 thrombosed specimens revealed that immune cell infiltration was in fact characteristic of the latter (65). Similar to the localization of proinflammatory proteins in intimal SMCs and myofibroblasts in preaccess veins (44), various studies have also demonstrated elevated inflammatory and oxidative markers in resident intimal cells of resected AVF specimens (64,66). It is important to note that most of the information about immune cell infiltration after AVF creation comes from extrapolation from animal models (67–70), where it is possible to obtain AVF samples early after surgery. Whether inflammation from infiltrated or resident cells plays a role in human AVF maturation or dysfunction will require the analysis of early human AVF samples (within 2 weeks of surgery), including nonstenotic segments. This may be possible through a multicenter collection of veins from steal syndrome and stenotic accesses that require early surgical revision.
As in preaccess veins, the ECM composition of the AVF intima has been barely studied. Martinez et al. (3) observed various levels and patterns of ECM deposition in the intima of two-stage transposition fistulas, although a comparative analysis of the samples was not presented. These patterns included intimas that were mostly cellular (low in ECM), with widespread ECM distribution, or with separate areas for cells and ECM deposition. Such interpatient variability in composition is likely relevant to the occlusive character of the intima, the response of the vein to cannulation injury, and the efficacy of endovascular treatments.
Postoperative IH and AVF Outcomes: Let Us Update the Theory
The most important question about IH is whether growth of this layer after AVF creation underlies access failure. Various case reports of AVFs that failed observed the presence of moderate to severe IH, but lacked a comparative group of functional fistulas (21,29,41,61). Despite this limitation, these observations reinforced the assumption that a thicker intima was responsible for AVF failure. The analysis of postoperative samples from two-stage upper-arm AVFs has challenged this idea (2,3,71).
Tabbara et al. (2) found a lack of association between postoperative IH (measured as maximal intimal thickness and I/M ratio) and maturation failure in a cohort of 79 individuals (Table 1). These analyses were not adjusted for any other clinical characteristics. Martinez et al. (3) also failed to find an association between I/M ratio and maturation failure in 115 individuals after adjusting for sex effects. Interestingly, the same study demonstrated that postoperative medial fibrosis was significantly associated with failure. Furthermore, IH was associated with failure only in those AVFs with medial fibrosis over the median value, and not in the other half of the accesses (Table 1) (3). Given that high medial fibrosis can adversely influence the biomechanical properties and distensibility of AVFs, this study proposed that, under highly fibrotic wall conditions, high IH is occlusive, but not when the vessel is able to compensate through other biomechanical mechanisms. This underscores the importance of understanding AVF remodeling as a whole and the mechanistic relationships between IH and other wall remodeling processes.
Pairwise comparisons of adjacent stenotic and nonstenotic segments from 14 two-stage upper-arm AVFs further confirmed that IH does not define the true luminal area of the access (71). In this report, there were no significant differences in intima size between both segments (Table 1). Lastly, no significant association has been found between maximal intimal thickness or I/M ratio and primary unassisted patency (2). The above postoperative data on maturation failure, focal stenosis, and primary patency are limited to upper-arm AVFs due to practical limitations. It is possible that postoperative IH has a larger effect on the outcomes of forearm fistulas.
Research Models, Current Challenges, and Pending Questions
Role of Animal Models in the Study of IH
Animals are essential to address basic science questions such as the origin and differentiation of intimal cells, temporal remodeling of the wall, and the effects of local and circulatory stimuli or treatments (29,67,68,72–79). Research mice and rats have the added advantage of allowing genetic manipulation (gene knockins and knockouts, cell labeling, etc.) and inclusion of high numbers of animals. Swine and sheep are often used as translational models to test pharmacologic and endovascular interventions, primarily in arteriovenous grafts (80–83). Arguably, the best animal model for AVF functional studies is the one in sheep, due to the superficial location of peripheral veins, which allows for not only AVF creation but also for potential cannulation (73).
Most animal models develop a certain form of venous IH within 2–6 weeks after AVF creation (29,67,68,72,74–79). However, they have important limitations for the study of the occlusive role of IH in AVF remodeling and failure. In the case of small animals, there are profound differences in preexisting vein morphology (very thin walls and subendothelial space) and hemodynamic characteristics (low blood flow) with respect to humans (29,67,68, 76,78,79). In addition, most models lack an underlying long-term CKD component and a human-equivalent definition of failure. These limitations underscore the necessity of expanding tissue biobanks of human AVFs to all possible forms to promote retro-translational studies, where human findings could be further dissected at the mechanistic level in animal models. Excellent reviews of AVF and CKD animal models have been included in the References (84–87).
Current Challenges in Treating AVF Stenosis: A Call for Mechanism-based Approaches
The idea that IH was the main cause of stenosis in AVFs motivated the use of therapies that treat restenosis in coronary circulation to salvage dysfunctional accesses. As a result, percutaneous transluminal angioplasty (PTA) became the first-line treatment for postoperative stenoses (15). Angioplasty mechanically stretches the vein and compresses the intima, but may cause significant injury to the vessel. Although efficacious in the short term, PTA frequently requires reintervention within 1 or 2 years after the first angioplasty procedure, either due to regrowing of occlusive IH and/or fibrotic scarring of the AVF wall (8–14). Stent placement is the last line of treatment for recurrent and high recoil stenoses due to concerns of vein depletion, stent migration or fracture, and intrastent thrombosis (88,89). Stent grafts are favored for in-stent restenosis (15,89), but are prone to “edge stenosis,” which occurs close to both ends of the stent and migrates toward the center (90,91).
In an attempt to improve postprocedure patency, antiproliferative drugs (mainly paclitaxel) are delivered to the AVF wall by means of drug-eluting balloons (DEB) or stents. Multiple individual studies have shown patency and/or reintervention benefits of DEB versus conventional angioplasty (12–14,92–95). However, meta-analysis studies have yielded variable conclusions, and patency rates >6 months still have much room for improvement (96–99). The observed variability in efficacy with antiproliferative agents and failure to significantly extend long-term patency may indicate insufficient delivery or retention of the drug (100–102), low sensitivity of cells to treatment (103), or a mismatch between the therapeutic effects of the drug and mechanisms of restenosis in AVFs. In vitro data suggest that paclitaxel targets all three presumed processes of restenosis (proliferation, migration, and ECM production) (104,105). However, it is not clear which of these cellular mechanisms are actually targeted in AVFs in vivo. Of note, the effect of paclitaxel in SMCs is cytostatic and not cytotoxic (106,107). Therefore, any stenotic mechanisms that remain unaffected may continue happening or possibly worsen after DEB treatment.
Antistenotic treatment modalities to improve venous remodeling during maturation include perivascular delivery of sirolimus (108), allogeneic ECs (109,110), or pancreatic elastase (111,112), and devices (VasQ, Optiflow) that support the ideal angle of arteriovenous anastomosis (113–117). The latest results on the sirolimus implants are pending (108). VasQ resulted in high maturation rates in single-arm and retrospective studies (117–119), but mixed results in short-term primary or secondary patency compared with the control arm (114,118,119). The rest are currently not recommended by the Kidney Disease Outcomes Quality Initiative guidelines due to lack of phase 3 studies (Optiflow) or significant benefits in AVF outcomes (ECs, elastase) (15). Far infrared radiation and external pneumatic compression (Fist Assist) have shown promising results in AVF maturation parameters (120–123) and secondary patency after PTA in specific patient demographics (124), but require further validation in a broader hemodialysis population. These clinical trials illustrate the desire to innovate in the search for preventive and postoperative AVF treatments. However, until we understand how human AVF cells respond to flow disturbances, repeated cannulations, and endovascular trauma at the molecular level, it is likely that any successes will come after significant trial and error.
What to Look at Beyond Intimal Thickness
Why have we failed to find an association between intima size measurements and AVF outcomes? The answer may be methodologic and/or biologic in nature. From the methodologic point of view, there are many limitations to the way we measure intima size. Two-dimensional and static histologic assessment of IH misses the actual size of the lumen under circulation. In addition, none of the measurements considers the potential compressibility of the intima or distensibility of the vessel. At least one clinical study has reported a lack of association between maximal intimal thickness by histology and internal diameter of the vein (4), illustrating the limitations of two-dimensional morphometry measurements in determining luminal area.
From the biologic point of view, intima size only represents a partial measurement of inward remodeling. Looking for associations between intima size and AVF outcomes ignores other macro processes of the wall, such as inward remodeling of the media, outward remodeling of the wall, and changes in the ECM. Importantly, we still do not understand what drives any of these processes. Is it cell death, changes on SMC phenotypes, or SMC- or immune cell-derived inflammation? Are IH and medial fibrosis mechanistically related? From the biomechanical point of view, how compressible is the intima? What characteristics make it more or less compressible? Does vein distensibility change after AVF creation? A better understanding of the role of SMCs and myofibroblasts in intimal expansion and wall remodeling, and where in the range of their phenotypic transformation they become problematic, will require detailed phenotypic analyses and single-cell omics techniques in clinically relevant human samples.
Lastly, the complexity of AVF remodeling lies in identifying an optimal level of IH and fibrosis that maintains vein integrity under extreme hemodynamic conditions and frequent cannulations, but without causing stenosis. Thus, can we envision protective roles for the intima? Does it prevent excessive immune cell infiltration? Does it protect medial SMCs from the oxidative stress of high oxygen pressures? Do synthetic cells confer regenerative capacity for wall healing after cannulation? We must consider all of these possibilities if we truly want to optimize maturation and prevent restenosis after endovascular treatments.
Disclosures
L. H. Salman reports receiving research funding from Albany Medical Center, Roche funds, and Transonics Inc.; having other interests in/relationships with the American Society of Diagnostic and Interventional Nephrology, American Society of Nephrology, Phraxis (data safety monitoring board), and Renal Physician Association; and having a patent application for the use of 4-methylumbelliferone in diabetic kidney disease (the application is pending review). R. I. Vazquez-Padron reports receiving research funding from the National Institutes of Health, and serving as a scientific advisor for, or member of, Scientific Reports. All remaining authors have nothing to disclose.
Funding
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK098511 (to L. H. Salman and R. I. Vazquez-Padron) and R01-DK121227 (to R. I. Vazquez-Padron); National Heart, Lung, and Blood Institute grant K08-HL151747 (to L. Martinez); and the US Department of Veterans Affairs Merit Award IBX004658 (to R. I. Vazquez-Padron).
Author Contributions
J. C. Duque was responsible for data curation; J. C. Duque, L. Martinez, M. Tabbara, and R. I. Vazquez-Padron were responsible for methodology; J. C. Duque, L. Martinez, and R. I. Vazquez-Padron were responsible for formal analysis; L. Martinez was responsible for visualization; L. Martinez, L. H. Salman, M. Tabbara, and R. I. Vazquez-Padron conceptualized the study; L. Martinez and R. I. Vazquez-Padron were responsible for funding acquisition, provided supervision, and wrote the original draft; and all authors were responsible for investigation and reviewed and edited the manuscript.
References
- 1.Dilley RJ, McGeachie JK, Prendergast FJ: A review of the histologic changes in vein-to-artery grafts, with particular reference to intimal hyperplasia. Arch Surg 123: 691–696, 1988. 10.1001/archsurg.1988.01400300033004 [DOI] [PubMed] [Google Scholar]
- 2.Tabbara M, Duque JC, Martinez L, Escobar LA, Wu W, Pan Y, Fernandez N, Velazquez OC, Jaimes EA, Salman LH, Vazquez-Padron RI: Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis 68: 455–464, 2016. 10.1053/j.ajkd.2016.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez L, Duque JC, Tabbara M, Paez A, Selman G, Hernandez DR, Sundberg CA, Tey JCS, Shiu YT, Cheung AK, Allon M, Velazquez OC, Salman LH, Vazquez-Padron RI: Fibrotic venous remodeling and nonmaturation of arteriovenous fistulas. J Am Soc Nephrol 29: 1030–1040, 2018. 10.1681/ASN.2017050559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Litovsky S: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013. 10.2215/CJN.02740313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpers CE, Imrey PB, Hudkins KL, Wietecha TA, Radeva M, Allon M, Cheung AK, Dember LM, Roy-Chaudhury P, Shiu YT, Terry CM, Farber A, Beck GJ, Feldman HI, Kusek JW, Himmelfarb J; Hemodialysis Fistula Maturation Study Group: Histopathology of veins obtained at hemodialysis arteriovenous fistula creation surgery. J Am Soc Nephrol 28: 3076–3088, 2017. 10.1681/ASN.2016050598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allon M, Litovsky SH, Tey JCS, Sundberg CA, Zhang Y, Chen Z, Fang Y, Cheung AK, Shiu YT: Abnormalities of vascular histology and collagen fiber configuration in patients with advanced chronic kidney disease. J Vasc Access 20: 31–40, 2019. 10.1177/1129729818773305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothuizen TC, Wong C, Quax PH, van Zonneveld AJ, Rabelink TJ, Rotmans JI: Arteriovenous access failure: More than just intimal hyperplasia? Nephrol Dial Transplant 28: 1085–1092, 2013. 10.1093/ndt/gft068 [DOI] [PubMed] [Google Scholar]
- 8.Wu CC, Wen SC, Yang CW, Pu SY, Tsai KC, Chen JW: Plasma ADMA predicts restenosis of arteriovenous fistula. J Am Soc Nephrol 20: 213–222, 2009. 10.1681/ASN.2008050476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark TW, Hirsch DA, Jindal KJ, Veugelers PJ, LeBlanc J: Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol 13: 51–59, 2002. 10.1016/S1051-0443(07)60009-8 [DOI] [PubMed] [Google Scholar]
- 10.Romann A, Beaulieu MC, Rhéaume P, Clement J, Sidhu R, Kiaii M: Risk factors associated with arteriovenous fistula failure after first radiologic intervention. J Vasc Access 17: 167–174, 2016. 10.5301/jva.5000459 [DOI] [PubMed] [Google Scholar]
- 11.Malka KT, Flahive J, Csizinscky A, Aiello F, Simons JP, Schanzer A, Messina LM, Robinson WP: Results of repeated percutaneous interventions on failing arteriovenous fistulas and grafts and factors affecting outcomes. J Vasc Surg 63: 772–777, 2016. 10.1016/j.jvs.2015.09.031 [DOI] [PubMed] [Google Scholar]
- 12.Troisi N, Frosini P, Romano E, Guidotti A, Chisci E, Michelagnoli S: Freeway paclitaxel-releasing balloons to treat recurrent stenosis of arteriovenous fistula in hemodialysis patients. Minerva Cardioangiol 66: 233–237, 2018. 10.23736/S0026-4725.17.04534-0 [DOI] [PubMed] [Google Scholar]
- 13.Yin Y, Shi Y, Cui T, Li H, Chen J, Zhang L, Yu Z, Li H, Yan Y, Wu K, Jin Q: Efficacy and safety of paclitaxel-coated balloon angioplasty for dysfunctional arteriovenous fistulas: A multicenter randomized controlled trial [published online ahead of print January 5, 2021]. Am J Kidney Dis 10.1053/j.ajkd.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 14.Trerotola SO, Saad TF, Roy-Chaudhury P; Lutonix AV Clinical Trial Investigators: The Lutonix AV Randomized Trial of paclitaxel-coated balloons in arteriovenous fistula stenosis: 2-Year results and subgroup analysis. J Vasc Interv Radiol 31: 1–14.e5, 2020. 10.1016/j.jvir.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 15.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, Graham J, Moist LM, Rajan DK, Roberts C, Vachharajani TJ, Valentini RP; National Kidney Foundation: KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update [published correction appears in Am J Kidney Dis 77: 551, 2021]. Am J Kidney Dis 75[Suppl 2]: S1–S164, 2020. Available at: 10.1053/j.ajkd.2019.12.001. Accessed May 20, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Spivack DE, Kelly P, Gaughan JP, van Bemmelen PS: Mapping of superficial extremity veins: Normal diameters and trends in a vascular patient-population. Ultrasound Med Biol 38: 190–194, 2012. 10.1016/j.ultrasmedbio.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 17.van Bemmelen PS, Kelly P, Blebea J: Improvement in the visualization of superficial arm veins being evaluated for access and bypass. J Vasc Surg 42: 957–962, 2005. 10.1016/j.jvs.2005.06.021 [DOI] [PubMed] [Google Scholar]
- 18.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 26: 2264–2270, 2011. 10.1093/ndt/gfq733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazich I, Chang A, Watson S, Dhar P, Madhurapantula RS, Hammes M: Morphometric and histological parameters in veins of diabetic patients undergoing brachiocephalic fistula placement. Hemodial Int 19: 490–498, 2015. 10.1111/hdi.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oprea A, Molnar A, Encică S, VlăduŢiu DŞ, Scridon GT, Săcui DM, Săsărman VA, Mircea PA: Effect of the veins histopathological characteristics and preexisting medical conditions on arteriovenous fistula maturation and primary patency in patients with end-stage renal disease: An observational, prospective study. Rom J Morphol Embryol 58: 871–880, 2017 [PubMed] [Google Scholar]
- 21.Lee T, Somarathna M, Hura A, Wang Y, Campos B, Arend L, Munda R, Roy-Chaudhury P: Natural history of venous morphologic changes in dialysis access stenosis. J Vasc Access 15: 298–305, 2014. 10.5301/jva.5000212 [DOI] [PubMed] [Google Scholar]
- 22.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011. 10.1053/j.ajkd.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinfeld DA, Batista R, Mir R, Babich D: Changes in venous histology in chronic hemodialysis patients. Am J Kidney Dis 34: 702–705, 1999. 10.1016/S0272-6386(99)70396-3 [DOI] [PubMed] [Google Scholar]
- 24.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A: Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access 13: 168–174, 2012. 10.5301/jva.5000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wali MA, Eid RA, Dewan M, Al-Homrany MA: Intimal changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. J Smooth Muscle Res 39: 95–105, 2003. 10.1540/jsmr.39.95 [DOI] [PubMed] [Google Scholar]
- 26.Wali MA, Eid RA, Dewan M, Al-Homrany MA: Pre-existing histopathological changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Ann Thorac Cardiovasc Surg 12: 341–348, 2006 [PubMed] [Google Scholar]
- 27.Monroy MA, Fang J, Li S, Ferrer L, Birkenbach MP, Lee IJ, Wang H, Yang XF, Choi ET: Chronic kidney disease alters vascular smooth muscle cell phenotype. Front Biosci 20: 784–795, 2015. 10.2741/4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies AH, Magee TR, Baird RN, Sheffield E, Horrocks M: Pre-bypass morphological changes in vein grafts. Eur J Vasc Surg 7: 642–647, 1993. 10.1016/S0950-821X(05)80710-8 [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Jourd’heuil FL, Xue M, Conti D, Lopez-Soler RI, Ginnan R, Asif A, Singer HA, Jourd’heuil D, Long X: Dual function for mature vascular smooth muscle cells during arteriovenous fistula remodeling. J Am Heart Assoc 6: e004891, 2017. 10.1161/JAHA.116.004891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahanchi SS, Tsihlis ND, Kibbe MR: The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg 45: A64–A73, 2007. 10.1016/j.jvs.2007.02.027 [DOI] [PubMed] [Google Scholar]
- 31.Allen KE, Varty K, Jones L, Sayers RD, Bell PR, London NJ: Human venous endothelium can promote intimal hyperplasia in a paracrine manner. J Vasc Surg 19: 577–584, 1994. 10.1016/S0741-5214(94)70029-X [DOI] [PubMed] [Google Scholar]
- 32.Wali MA, Eid RA, Al-Homrany MA: Smooth muscle changes in the cephalic vein of renal failure patients before use as an arteriovenous fistula (AVF). J Smooth Muscle Res 38: 75–85, 2002. 10.1540/jsmr.38.75 [DOI] [PubMed] [Google Scholar]
- 33.Bolton CH, Downs LG, Victory JG, Dwight JF, Tomson CR, Mackness MI, Pinkney JH: Endothelial dysfunction in chronic renal failure: Roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant 16: 1189–1197, 2001. 10.1093/ndt/16.6.1189 [DOI] [PubMed] [Google Scholar]
- 34.Małyszko J, Małyszko JS, Myśliwiec M: Endothelial cell injury markers in chronic renal failure on conservative treatment and continuous ambulatory peritoneal dialysis. Kidney Blood Press Res 27: 71–77, 2004. 10.1159/000075810 [DOI] [PubMed] [Google Scholar]
- 35.Kuczmarski JM, Darocki MD, DuPont JJ, Sikes RA, Cooper CR, Farquhar WB, Edwards DG: Effect of moderate-to-severe chronic kidney disease on flow-mediated dilation and progenitor cells. Exp Biol Med (Maywood) 236: 1085–1092, 2011. 10.1258/ebm.2011.011008 [DOI] [PubMed] [Google Scholar]
- 36.Costa-Hong V, Bortolotto LA, Jorgetti V, Consolim-Colombo F, Krieger EM, Lima JJ: Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol 92: 381–386, 398–403, 413–418, 2009. 10.1590/s0066-782x2009000500013 [DOI] [PubMed] [Google Scholar]
- 37.Han L, Zhang Y, Zhang M, Guo L, Wang J, Zeng F, Xu D, Yin Z, Xu Y, Wang D, Zhou H: Interleukin-1β-induced senescence promotes osteoblastic transition of vascular smooth muscle cells. Kidney Blood Press Res 45: 314–330, 2020. 10.1159/000504298 [DOI] [PubMed] [Google Scholar]
- 38.Iwamoto Y, Maruhashi T, Kajikawa M, Oda N, Kishimoto S, Matsui S, Hashimoto H, Aibara Y, Yusoff FM, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Higashi Y: Chronic kidney disease is associated with vascular smooth muscle dysfunction but not with endothelial dysfunction. Int J Cardiol 254: 284–290, 2018. 10.1016/j.ijcard.2017.10.122 [DOI] [PubMed] [Google Scholar]
- 39.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC: Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease--a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 216: 446–451, 2011. 10.1016/j.atherosclerosis.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 40.Lee T, Wang Y, Arend L, Cornea V, Campos B, Munda R, Roy-Chaudhury P: Comparative analysis of cellular phenotypes within the neointima from vein segments collected prior to vascular access surgery and stenotic arteriovenous dialysis accesses. Semin Dial 27: 303–309, 2014. 10.1111/sdi.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R: Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 50: 782–790, 2007. 10.1053/j.ajkd.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 42.Rensen SS, Doevendans PA, van Eys GJ: Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15: 100–108, 2007. 10.1007/BF03085963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li FD, Sexton KW, Hocking KM, Osgood MJ, Eagle S, Cheung-Flynn J, Brophy CM, Komalavilas P: Intimal thickness associated with endothelial dysfunction in human vein grafts. J Surg Res 180: e55–e62, 2013. 10.1016/j.jss.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez L, Tabbara M, Duque JC, Selman G, Falcon NS, Paez A, Griswold AJ, Ramos-Echazabal G, Hernandez DR, Velazquez OC, Salman LH, Vazquez-Padron RI: Transcriptomics of human arteriovenous fistula failure: Genes associated with nonmaturation. Am J Kidney Dis 74: 73–81, 2019. 10.1053/j.ajkd.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson PR, Yamamura S, Kent KC: Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J Vasc Surg 24: 25–33, 1996. 10.1016/S0741-5214(96)70141-6 [DOI] [PubMed] [Google Scholar]
- 46.Hollenbeck ST, Itoh H, Louie O, Faries PL, Liu B, Kent KC: Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the alpha2beta1 integrin and PDGFbeta receptor. Biochem Biophys Res Commun 325: 328–337, 2004. 10.1016/j.bbrc.2004.10.031 [DOI] [PubMed] [Google Scholar]
- 47.Raines EW: The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol 81: 173–182, 2000. 10.1046/j.1365-2613.2000.00155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raines EW, Koyama H, Carragher NO: The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann N Y Acad Sci 902: 39–51, discussion 51–52, 2000. 10.1111/j.1749-6632.2000.tb06299.x [DOI] [PubMed] [Google Scholar]
- 49.Xu J, Shi GP: Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta 1842: 2106–2119, 2014. 10.1016/j.bbadis.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang R, Raykin J, Li H, Gleason RL Jr, Brewster LP: Differential mechanical response and microstructural organization between non-human primate femoral and carotid arteries. Biomech Model Mechanobiol 13: 1041–1051, 2014. 10.1007/s10237-014-0553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandhi NS, Mancera RL: The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des 72: 455–482, 2008. 10.1111/j.1747-0285.2008.00741.x [DOI] [PubMed] [Google Scholar]
- 52.Cheung AK, Imrey PB, Alpers CE, Robbin ML, Radeva M, Larive B, Shiu YT, Allon M, Dember LM, Greene T, Himmelfarb J, Roy-Chaudhury P, Terry CM, Vazquez MA, Kusek JW, Feldman HI; Hemodialysis Fistula Maturation Study Group: Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol 28: 3005–3013, 2017. 10.1681/ASN.2016121355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remuzzi A, Bozzetto M: Biological and physical factors involved in the maturation of arteriovenous fistula for hemodialysis. Cardiovasc Eng Technol 8: 273–279, 2017. 10.1007/s13239-017-0323-0 [DOI] [PubMed] [Google Scholar]
- 54.Asif A, Roy-Chaudhury P, Beathard GA: Early arteriovenous fistula failure: A logical proposal for when and how to intervene. Clin J Am Soc Nephrol 1: 332–339, 2006. 10.2215/CJN.00850805 [DOI] [PubMed] [Google Scholar]
- 55.Pike D, Shiu YT, Cho YF, Le H, Somarathna M, Isayeva T, Guo L, Symons JD, Kevil CG, Totenhagen J, Lee T: The effect of endothelial nitric oxide synthase on the hemodynamics and wall mechanics in murine arteriovenous fistulas [published correction appears in Sci Rep 9: 15555, 2019]. Sci Rep 9: 4299, 2019. 10.1038/s41598-019-40683-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Predel HG, Yang Z, von Segesser L, Turina M, Bühler FR, Lüscher TF: Implications of pulsatile stretch on growth of saphenous vein and mammary artery smooth muscle. Lancet 340: 878–879, 1992. 10.1016/0140-6736(92)93287-W [DOI] [PubMed] [Google Scholar]
- 57.Paszkowiak JJ, Dardik A: Arterial wall shear stress: Observations from the bench to the bedside. Vasc Endovascular Surg 37: 47–57, 2003. 10.1177/153857440303700107 [DOI] [PubMed] [Google Scholar]
- 58.Corpataux JM, Haesler E, Silacci P, Ris HB, Hayoz D: Low-pressure environment and remodelling of the forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial Transplant 17: 1057–1062, 2002. 10.1093/ndt/17.6.1057 [DOI] [PubMed] [Google Scholar]
- 59.Shiu YT, Rotmans JI, Geelhoed WJ, Pike DB, Lee T: Arteriovenous conduits for hemodialysis: How to better modulate the pathophysiological vascular response to optimize vascular access durability. Am J Physiol Renal Physiol 316: F794–F806, 2019. 10.1152/ajprenal.00440.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartlett M, Diaz-Zuccarini V, Tsui J: Computer assisted Doppler waveform analysis and ultrasound derived turbulence intensity ratios can predict early hyperplasia development in newly created vascular access fistula: Pilot study, methodology and analysis. JRSM Cardiovasc Dis 10: 20480040211000185, 2021. 10.1177/20480040211000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L: Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant 24: 2786–2791, 2009. 10.1093/ndt/gfn708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Graaf R, Dammers R, Vainas T, Hoeks AP, Tordoir JH: Detection of cell-cycle regulators in failed arteriovenous fistulas for haemodialysis. Nephrol Dial Transplant 18: 814–818, 2003. 10.1093/ndt/gfg033 [DOI] [PubMed] [Google Scholar]
- 63.Hofstra L, Tordoir JH, Kitslaar PJ, Hoeks AP, Daemen MJ: Enhanced cellular proliferation in intact stenotic lesions derived from human arteriovenous fistulas and peripheral bypass grafts. Does it correlate with flow parameters? Circulation 94: 1283–1290, 1996. 10.1161/01.CIR.94.6.1283 [DOI] [PubMed] [Google Scholar]
- 64.Stracke S, Konner K, Köstlin I, Friedl R, Jehle PM, Hombach V, Keller F, Waltenberger J: Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int 61: 1011–1019, 2002. 10.1046/j.1523-1755.2002.00191.x [DOI] [PubMed] [Google Scholar]
- 65.Chang CJ, Ko YS, Ko PJ, Hsu LA, Chen CF, Yang CW, Hsu TS, Pang JH: Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int 68: 1312–1319, 2005. 10.1111/j.1523-1755.2005.00529.x [DOI] [PubMed] [Google Scholar]
- 66.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 37: 970–980, 2001. 10.1016/S0272-6386(05)80013-7 [DOI] [PubMed] [Google Scholar]
- 67.Wong CY, de Vries MR, Wang Y, van der Vorst JR, Vahrmeijer AL, van Zonneveld AJ, Roy-Chaudhury P, Rabelink TJ, Quax PH, Rotmans JI: Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure. J Vasc Surg 59: 192–201.e1, 2014. 10.1016/j.jvs.2013.02.242 [DOI] [PubMed] [Google Scholar]
- 68.Duque JC, Martinez L, Mesa A, Wei Y, Tabbara M, Salman LH, Vazquez-Padron RI: CD4(+) lymphocytes improve venous blood flow in experimental arteriovenous fistulae. Surgery 158: 529–536, 2015. 10.1016/j.surg.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo X, Fereydooni A, Isaji T, Gorecka J, Liu S, Hu H, Ono S, Alozie M, Lee SR, Taniguchi R, Yatsula B, Nassiri N, Zhang L, Dardik A: Inhibition of the Akt1-mTORC1 axis alters venous remodeling to improve arteriovenous fistula patency [published correction appears in Sci Rep 10: 8301, 2020]. Sci Rep 9: 11046, 2019. 10.1038/s41598-019-47542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy-Chaudhury P, Khan R, Campos B, Wang Y, Kurian M, Lee T, Arend L, Munda R: Pathogenetic role for early focal macrophage infiltration in a pig model of arteriovenous fistula (AVF) stenosis. J Vasc Access 15: 25–28, 2014. 10.5301/jva.5000151 [DOI] [PubMed] [Google Scholar]
- 71.Duque JC, Tabbara M, Martinez L, Paez A, Selman G, Salman LH, Velazquez OC, Vazquez-Padron RI: Similar degree of intimal hyperplasia in surgically detected stenotic and nonstenotic arteriovenous fistula segments: A preliminary report. Surgery 163: 866–869, 2018. 10.1016/j.surg.2017.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P: Venous stenosis in a pig arteriovenous fistula model--anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant 23: 525–533, 2008. 10.1093/ndt/gfm547 [DOI] [PubMed] [Google Scholar]
- 73.Florescu MC, Foster KW, Sacks AR, Lof J, Stolze EA, Fry GM, Bumgardner DP, Tysinger T, Kuchta MJ, Runge HJ, Hadley WB, Morris MC: Sheep model of hemodialysis arteriovenous fistula using superficial veins. Semin Dial 28: 687–691, 2015. 10.1111/sdi.12407 [DOI] [PubMed] [Google Scholar]
- 74.Krishnamoorthy MK, Banerjee RK, Wang Y, Zhang J, Sinha Roy A, Khoury SF, Arend LJ, Rudich S, Roy-Chaudhury P: Hemodynamic wall shear stress profiles influence the magnitude and pattern of stenosis in a pig AV fistula. Kidney Int 74: 1410–1419, 2008. 10.1038/ki.2008.379 [DOI] [PubMed] [Google Scholar]
- 75.Chan JS, Campos B, Wang Y, Mistry M, Lee T, Munda R, Arend L, Roy-Chaudhury P: Proliferation patterns in a pig model of AV fistula stenosis: Can we translate biology into novel therapies? Semin Dial 27: 626–632, 2014. 10.1111/sdi.12240 [DOI] [PubMed] [Google Scholar]
- 76.Manning E, Skartsis N, Orta AM, Velazquez OC, Liu ZJ, Asif A, Salman LH, Vazquez-Padron RI: A new arteriovenous fistula model to study the development of neointimal hyperplasia. J Vasc Res 49: 123–131, 2012. 10.1159/000332327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skartsis N, Manning E, Wei Y, Velazquez OC, Liu ZJ, Goldschmidt-Clermont PJ, Salman LH, Asif A, Vazquez-Padron RI: Origin of neointimal cells in arteriovenous fistulae: Bone marrow, artery, or the vein itself? Semin Dial 24: 242–248, 2011. 10.1111/j.1525-139X.2011.00870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall MR, Yamamoto K, Protack CD, Tsuneki M, Kuwahara G, Assi R, Brownson KE, Bai H, Madri JA, Dardik A: Temporal regulation of venous extracellular matrix components during arteriovenous fistula maturation. J Vasc Access 16: 93–106, 2015. 10.5301/jva.5000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan CY, Chen YS, Ma MC, Chen CF: Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats [published correction appears in J Vasc Surg 45: 1293, 2007]. J Vasc Surg 45: 804–811, 2007. 10.1016/j.jvs.2006.12.063 [DOI] [PubMed] [Google Scholar]
- 80.Kohler TR, Toleikis PM, Gravett DM, Avelar RL: Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable vascular wrap paclitaxel-eluting mesh. J Vasc Surg 45: 1029–1037, discussion 1037–1038, 2007. 10.1016/j.jvs.2007.01.057 [DOI] [PubMed] [Google Scholar]
- 81.Terry CM, Li L, Li H, Zhuplatov I, Blumenthal DK, Kim SE, Owen SC, Kholmovski EG, Fowers KD, Rathi R, Cheung AK: In vivo evaluation of the delivery and efficacy of a sirolimus-laden polymer gel for inhibition of hyperplasia in a porcine model of arteriovenous hemodialysis graft stenosis. J Control Release 160: 459–467, 2012. 10.1016/j.jconrel.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rotmans JI, Pattynama PM, Verhagen HJ, Hino I, Velema E, Pasterkamp G, Stroes ES: Sirolimus-eluting stents to abolish intimal hyperplasia and improve flow in porcine arteriovenous grafts: A 4-week follow-up study. Circulation 111: 1537–1542, 2005. 10.1161/01.CIR.0000159332.18585.B5 [DOI] [PubMed] [Google Scholar]
- 83.Kelly B, Melhem M, Zhang J, Kasting G, Li J, Krishnamoorthy M, Heffelfinger S, Rudich S, Desai P, Roy-Chaudhury P: Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model. Nephrol Dial Transplant 21: 2425–2431, 2006. 10.1093/ndt/gfl250 [DOI] [PubMed] [Google Scholar]
- 84.Rotmans JI: Animal models for studying pathophysiology of hemodialysis access. Open Urol Nephrol J 7: 14–21, 2014. 10.2174/1874303X01407010014 [DOI] [Google Scholar]
- 85.Kokozidou M, Katsargyris A, Verhoeven ELG, Schulze-Tanzil G: Vascular access animal models used in research. Ann Anat 225: 65–75, 2019. 10.1016/j.aanat.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 86.Bao YW, Yuan Y, Chen JH, Lin WQ: Kidney disease models: Tools to identify mechanisms and potential therapeutic targets. Zool Res 39: 72–86, 2018. 10.24272/j.issn.2095-8137.2017.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang HC, Zuo Y, Fogo AB: Models of chronic kidney disease. Drug Discov Today Dis Models 7: 13–19, 2010. 10.1016/j.ddmod.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacRae JM, Dipchand C, Oliver M, Moist L, Lok C, Clark E, Hiremath S, Kappel J, Kiaii M, Luscombe R, Miller LM; Canadian Society of Nephrology Vascular Access Work Group: Arteriovenous access failure, stenosis, and thrombosis. Can J Kidney Health Dis 3: 2054358116669126, 2016. 10.1177/2054358116669126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ginsburg M, Lorenz JM, Zivin SP, Zangan S, Martinez D: A practical review of the use of stents for the maintenance of hemodialysis access. Semin Intervent Radiol 32: 217–224, 2015. 10.1055/s-0035-1549844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dolmatch BL, Tio FO, Li XD, Dong YH: Patency and tissue response related to two types of polytetrafluoroethylene-covered stents in the dog. J Vasc Interv Radiol 7: 641–649, 1996. 10.1016/S1051-0443(96)70822-9 [DOI] [PubMed] [Google Scholar]
- 91.Jones RG, Willis AP, Tullett K, Riley PL: Results of stent graft placement to treat cephalic arch stenosis in hemodialysis patients with dysfunctional brachiocephalic arteriovenous fistulas. J Vasc Interv Radiol 28: 1417–1421, 2017. 10.1016/j.jvir.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 92.Swinnen JJ, Hitos K, Kairaitis L, Gruenewald S, Larcos G, Farlow D, Huber D, Cassorla G, Leo C, Villalba LM, Allen R, Niknam F, Burgess D: Multicentre, randomised, blinded, control trial of drug-eluting balloon vs sham in recurrent native dialysis fistula stenoses. J Vasc Access 20: 260–269, 2019. 10.1177/1129729818801556 [DOI] [PubMed] [Google Scholar]
- 93.Karnabatidis D, Kitrou PM, Ponce P, Chong TT, Pietura R, Pegis JD, Ko PJ, Lin CH; Lutonix AV Global Registry Investigators: A multicenter global registry of paclitaxel drug-coated balloon in dysfunctional arteriovenous fistulae and grafts: 6-Month results. J Vasc Interv Radiol 32: 360–368.e2, 2021. 10.1016/j.jvir.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 94.Kocaaslan C, Oztekin A, Bademci MS, Denli Yalvac ES, Bulut N, Aydin E: A retrospective comparison analysis of results of drug-coated balloon versus plain balloon angioplasty in treatment of juxta-anastomotic de novo stenosis of radiocephalic arteriovenous fistulas. J Vasc Access 21: 596–601, 2020. 10.1177/1129729819893205 [DOI] [PubMed] [Google Scholar]
- 95.Lookstein RA, Haruguchi H, Ouriel K, Weinberg I, Lei L, Cihlar S, Holden A; IN.PACT AV Access Investigators: Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med 383: 733–742, 2020. 10.1056/NEJMoa1914617 [DOI] [PubMed] [Google Scholar]
- 96.Liao MT, Chen MK, Hsieh MY, Yeh NL, Chien KL, Lin CC, Wu CC, Chie WC: Drug-coated balloon versus conventional balloon angioplasty of hemodialysis arteriovenous fistula or graft: A systematic review and meta-analysis of randomized controlled trials [published correction appears in PLoS One 15: e0233923, 2020]. PLoS One 15: e0231463, 2020. 10.1371/journal.pone.0231463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rokoszak V, Syed MH, Salata K, Greco E, de Mestral C, Hussain MA, Aljabri B, Verma S, Al-Omran M: A systematic review and meta-analysis of plain versus drug-eluting balloon angioplasty in the treatment of juxta-anastomotic hemodialysis arteriovenous fistula stenosis. J Vasc Surg 71: 1046–1054.e1, 2020. 10.1016/j.jvs.2019.07.075 [DOI] [PubMed] [Google Scholar]
- 98.Yan Wee IJ, Yap HY, Hsien Ts’ung LT, Lee Qingwei S, Tan CS, Tang TY, Chong TT: A systematic review and meta-analysis of drug-coated balloon versus conventional balloon angioplasty for dialysis access stenosis. J Vasc Surg 70: 970–979.e3, 2019. 10.1016/j.jvs.2019.01.082 [DOI] [PubMed] [Google Scholar]
- 99.Abdul Salim S, Tran H, Thongprayoon C, Fülöp T, Cheungpasitporn W: Comparison of drug-coated balloon angioplasty versus conventional angioplasty for arteriovenous fistula stenosis: Systematic review and meta-analysis. J Vasc Access 21: 357–365, 2020. 10.1177/1129729819878612 [DOI] [PubMed] [Google Scholar]
- 100.Granada JF, Stenoien M, Buszman PP, Tellez A, Langanki D, Kaluza GL, Leon MB, Gray W, Jaff MR, Schwartz RS: Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: Impact on neointimal proliferation and healing. Open Heart 1: e000117, 2014. 10.1136/openhrt-2014-000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Speck U, Häckel A, Schellenberger E, Kamann S, Löchel M, Clever YP, Peters D, Scheller B, Trog S, Bettink S: Drug distribution and basic pharmacology of paclitaxel/resveratrol-coated balloon catheters. Cardiovasc Intervent Radiol 41: 1599–1610, 2018. 10.1007/s00270-018-2018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy GJ, Johnson TW, Chamberlain MH, Rizvi SI, Wyatt M, George SJ, Angelini GD, Karsch KR, Oberhoff M, Newby AC: Short- and long-term effects of cytochalasin D, paclitaxel and rapamycin on wall thickening in experimental porcine vein grafts. Cardiovasc Res 73: 607–617, 2007. 10.1016/j.cardiores.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 103.Yang H-M, Cho H-J, Park K-W, Cho H-J, Kang H-J, Koo B-K, Sohn D-W, Oh B-H, Park Y-B, Kim H-S: Paclitaxel itself induces drug-resistance to paclitaxel in vascular smooth muscle cells and reduces its effect of suppression of neointimal hyperplasia after angioplasty. J Am Coll Cardiol 57: E2053, 2011. 10.1016/S0735-1097(11)62053-6 [DOI] [Google Scholar]
- 104.Wiskirchen J, Schöber W, Schart N, Kehlbach R, Wersebe A, Tepe G, Claussen CD, Duda SH: The effects of paclitaxel on the three phases of restenosis: Smooth muscle cell proliferation, migration, and matrix formation: An in vitro study. Invest Radiol 39: 565–571, 2004. 10.1097/01.rli.0000133815.22434.55 [DOI] [PubMed] [Google Scholar]
- 105.Sollott SJ, Cheng L, Pauly RR, Jenkins GM, Monticone RE, Kuzuya M, Froehlich JP, Crow MT, Lakatta EG, Rowinsky EK: Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest 95: 1869–1876, 1995. 10.1172/JCI117867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blagosklonny MV, Demidenko ZN, Giovino M, Szynal C, Donskoy E, Herrmann RA, Barry JJ, Whalen AM: Cytostatic activity of paclitaxel in coronary artery smooth muscle cells is mediated through transient mitotic arrest followed by permanent post-mitotic arrest: Comparison with cancer cells. Cell Cycle 5: 1574–1579, 2006. 10.4161/cc.5.14.3113 [DOI] [PubMed] [Google Scholar]
- 107.Blagosklonny MV, Darzynkiewicz Z, Halicka HD, Pozarowski P, Demidenko ZN, Barry JJ, Kamath KR, Herrmann RA: Paclitaxel induces primary and postmitotic G1 arrest in human arterial smooth muscle cells. Cell Cycle 3: 1050–1056, 2004. 10.4161/cc.3.8.986 [DOI] [PubMed] [Google Scholar]
- 108.DeVita MV, Khine SK, Shivarov H: Novel approaches to arteriovenous access creation, maturation, suitability, and durability for dialysis. Kidney Int Rep 5: 769-778, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Conte MS, Nugent HM, Gaccione P, Guleria I, Roy-Chaudhury P, Lawson JH: Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: The V-HEALTH study. J Vasc Surg 50: 1359–68.e1, 2009. 10.1016/j.jvs.2009.07.108 [DOI] [PubMed] [Google Scholar]
- 110.Conte MS, Nugent HM, Gaccione P, Roy-Chaudhury P, Lawson JH: Influence of diabetes and perivascular allogeneic endothelial cell implants on arteriovenous fistula remodeling. J Vasc Surg 54: 1383–1389, 2011. 10.1016/j.jvs.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 111.Hye RJ, Peden EK, O’Connor TP, Browne BJ, Dixon BS, Schanzer AS, Jensik SC, Dember LM, Jaff MR, Burke SK: Human type I pancreatic elastase treatment of arteriovenous fistulas in patients with chronic kidney disease. J Vasc Surg 60: 454–461.e1, 2014. 10.1016/j.jvs.2014.02.037 [DOI] [PubMed] [Google Scholar]
- 112.Peden EK, Leeser DB, Dixon BS, El-Khatib MT, Roy-Chaudhury P, Lawson JH, Menard MT, Dember LM, Glickman MH, Gustafson PN, Blair AT, Magill M, Franano FN, Burke SK: A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. J Vasc Access 14: 143–151, 2013. 10.5301/jva.5000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chemla E, Velazquez CC, D’Abate F, Ramachandran V, Maytham G: Arteriovenous fistula construction with the VasQ™ external support device: A pilot study. J Vasc Access 17: 243–248, 2016. 10.5301/jva.5000527 [DOI] [PubMed] [Google Scholar]
- 114.Palumbo R, Dominijanni S, Centi A, D’Urso G, Tatangelo P, Floccari F, Smedile G, Niscola P, Londrino F, Di Daniele N: Hemodynamic impact of VASQ device in vascular access creation [published online ahead of print December 21, 2020]. J Vasc Access 10.1177/1129729820983153 [DOI] [PubMed] [Google Scholar]
- 115.Chemla E, Tavakoli A, Nikam M, Mitra S, Malete T, Evans J, Roy-Chaudhury P: Arteriovenous fistula creation using the Optiflow™ vascular anastomotic connector: The OPEN (Optiflow PatEncy and MaturatioN) study. J Vasc Access 15: 38–44, 2014. 10.5301/jva.5000169 [DOI] [PubMed] [Google Scholar]
- 116.Nikam M, Chemla ES, Evans J, Summers A, Brenchley P, Tavakoli A, Roy-Chaudhury P, Mitra S: Prospective controlled pilot study of arteriovenous fistula placement using the novel Optiflow device. J Vasc Surg 61: 1020–1025, 2015. 10.1016/j.jvs.2014.11.082 [DOI] [PubMed] [Google Scholar]
- 117.Leonardi G, Campagna M, Pellicanò V, Guarena C, Bergamo D, Lavacca A, Fop F, Biancone L: Implanted blood vessel external support device (VasQ™) for creation of hemodialysis arteriovenous fistula: A single-center experience [published online ahead of print November 12, 2020]. J Vasc Access 10.1177/1129729820971533 [DOI] [PubMed] [Google Scholar]
- 118.Shahverdyan R, Meyer T, Matoussevitch V: Patency and functionality of radiocephalic arteriovenous fistulas with an external support device (VasQ™): Real-world single-center experience. J Vasc Access 22: 166–172, 2021. 10.1177/1129729820904599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benedetto F, Spinelli D, Derone G, Cutrupi A, Barillà D, Pipitò N: Initial single-center experience with a new external support device for the creation of the forearm native arteriovenous fistula for hemodialysis [printed online ahead of print March 16, 2021]. J Vasc Access 10.1177/11297298211002570 [DOI] [PubMed] [Google Scholar]
- 120.Wan Q, Yang S, Li L, Chu F: Effects of far infrared therapy on arteriovenous fistulas in hemodialysis patients: A meta-analysis. Ren Fail 39: 613–622, 2017. 10.1080/0886022X.2017.1361835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hadimeri U, Wärme A, Stegmayr B: A single treatment, using Far Infrared light improves blood flow conditions in arteriovenous fistula. Clin Hemorheol Microcirc 66: 211–217, 2017. 10.3233/CH-170254 [DOI] [PubMed] [Google Scholar]
- 122.Desai S, Mitra A, Arkans E, Singh TM: Early application of an intermittent pneumatic compression device is safe and results in proximal arteriovenous fistula enlargement. J Vasc Access 20: 24–30, 2019. 10.1177/1129729818773295 [DOI] [PubMed] [Google Scholar]
- 123.Sullivan B, Desai S, Singh TM, Mitra A: Early application of an intermittent pneumatic compression device assists dilation of radiocephalic fistulas. J Vasc Access 20: 146–152, 2019. 10.1177/1129729818787717 [DOI] [PubMed] [Google Scholar]
- 124.Io H, Nakata J, Aoyama R, Inoshita H, Nakano T, Ishizaka M, Fukui M, Tomino Y, Suzuki Y: Far-infrared therapy for secondary vascular access patency of hemodialysis patients. Ren Replace Ther 5: 31, 2019. 10.1186/s41100-019-0224-9 [DOI] [Google Scholar]