Abstract
Research in the development of ophthalmic drug formulations and innovative technologies over the past few decades has been directed at improving the penetration of medications delivered to the eye. Currently, approximately 90% of all ophthalmic drug formulations (e.g. liposomes, micelles) are applied as eye drops. The major challenge of topical eye drops is low bioavailability, need for frequent instillation due to the short half-life, poor drug solubility, and potential side effects. Recent research has been focused on improving topical drug delivery devices by increasing ocular residence time, overcoming physiological and anatomical barriers, and developing medical devices and drug formulations to increase the duration of action of the active drugs. Researchers have developed innovative technologies and formulations ranging from sub-micron to macroscopic size such as prodrugs, enhancers, mucus-penetrating particles (MPPs), therapeutic contact lenses, and collagen corneal shields. Another approach towards the development of effective topical drug delivery is embedding therapeutic formulations in microdevices designed for sustained release of the active drugs. The goal is to optimize the delivery of ophthalmic medications by achieving high drug concentration with prolonged duration of action that is convenient for patients to administer.
Keywords: Ophthalmic drug-delivery, Formulations, Ocular, Cornea-conjunctiva barrier
1. Introduction
The use of topical drug delivery approach can be traced back to ancient Egypt, where the juice of compressed liver was applied to the ocular surface to treat night blindness (Wolf, 1996) (Maumenee, 1993). While it is difficult to identify the first ‘modern’ eye medication, ophthalmology as a science can be traced as a specialty in Europe back to the 17th and 18th centuries (Grossniklaus, 2015). The knowledge of corneal anatomy was greatly increased through the work of Sir William Bowman using a microscope in 1847 (Moffatt et al., 2005). In addition, Seefelder and Wolfrum studied the development of the cornea and the anterior chamber in 1906 (Moffatt et al., 2005) (Stocker, 1953). In the period from 1953 to 1960 s, Stocker, Teng and other scientists made additional insight into topical ocular drug delivery using electron microscopy, where their studies on the structure and different layers of the normal cornea coincided with the major development of technologies and therapeutic drugs (Crawford et al., 2013) (Teng, 1962). Finally, Patton and colleagues demonstrated that the permeation of topical drugs was limited by a passive process of the epithelium and that drugs can absorb into the eye via non-corneal routes across the conjunctiva and sclera (Patton and Robinson, 1976) (Doane et al., 1978) (Ahmed et al., 1987). While eye drop is the major method of delivering medications to the cornea and anterior segment of the eye, much effort has been devoted to evaluating alternative drug delivery devices for the eye (Ralph et al., 1975) (Hoyng and van Beek, 2000) (Michels and Maumenee, 1975) (Hussain and Truelove, 1976). This led to the development of therapeutic contact lenses (TCL) in the 1970 s, which yielded the first approved by FDA in 1972. This was a contact lens soaked in 3% hydrogen peroxide to treat for soft lens disinfection (Phillips and Speedwell, 2018). In 1984, FDA approved collagen corneal shields developed by Fyodorov (Fig. 1) (Friedberg et al., 1991).
Fig. 1.
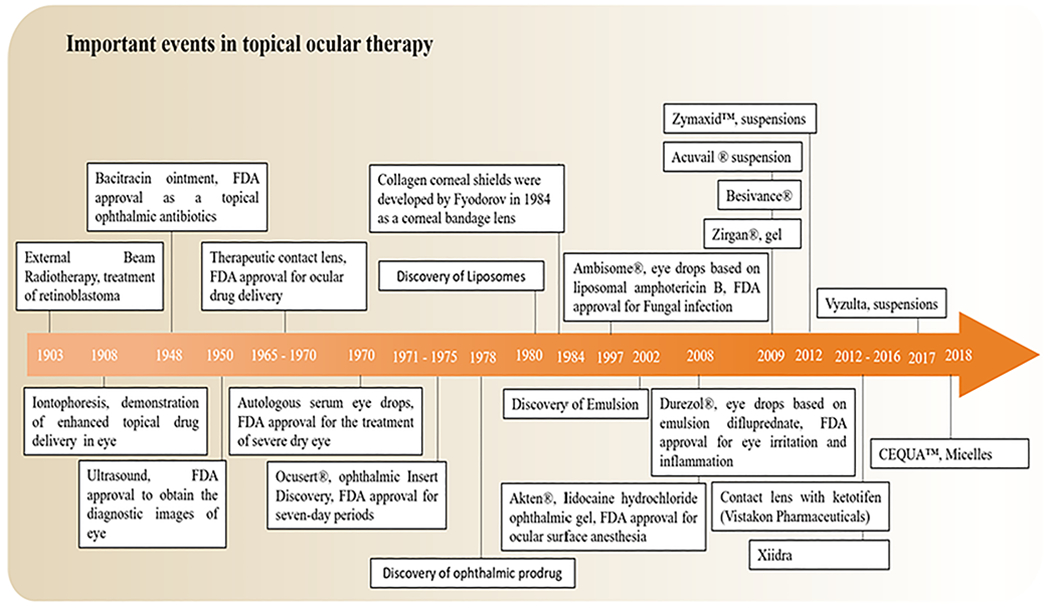
Historical timeline of major developments in the field of topical ocular drug delivery systems.
Currently, there are numerous ophthalmic medications, including anti-microbial agents, anti-inflammatory agents, glaucoma medications, cycloplegic agents, among others (Gote et al., 2019). These ocular medications consist of different formulations, including solutions, suspensions, emulsions, gels, and ointments. Contact lenses have been useful in developing new applications for existing topical ophthalmic drugs and for the avoidance of the first-pass metabolism. They can also reduce side effects; for example, a contact lens-based drug delivery system for ketotifen, an anti-histamine, recently shown to provide allergy relief, while avoiding the potential adverse effects of preservatives commonly found in ophthalmic preparations (Soluri et al., 2012). Similarly, an Econazole-eluting contact lens designed to treat fungal ocular infections was shown to provide extended antifungal activity and achieve fungicidal levels within the cornea without the need for frequent drop instillation and thus, improving compliance and treatment efficacy (Ciolino et al., 2011). Likewise, a corneal collagen shield (72-hour) presoaked in ciprofloxacin (0.3%) exhibited fewer adverse effects than conventional eye drops (Willoughby et al., 2002). Using contact lenses (CLs) for drug delivery can provide sustained high concentration in the anterior chamber postoperatively. Whether for healing purposes or drug delivery, it is likely that therapeutic applications of CLs will continue to expand.
2. Barriers, major routes, and challenges to topical ocular drug absorption
During the past two decades, translational research has increased our ability to understand the major routes, and also physiological barriers of the ocular surface and cornea. A better understanding of the ocular surface anatomy and drug delivery routes will enable us to ensure better development platforms for effective topical drug delivery systems for the anterior segment diseases, whose final end point may be preclinical or clinical testing.
2.1. The barriers of the cornea
The limitations of anterior segment ocular drug delivery are governed largely by the static barrier (corneal epithelium, epithelial tight junctions, corneal stroma, corneal endothelium, blood aqueous) and dynamic barrier (tear dilution and conjunctival) of human ocular anatomy. The cornea is a transparent structure and structural barrier covering the front of the eye. The barrier function of the cornea leads to less drug absorption and a very low permeability to the penetration of foreign molecules. In this section, we discuss the key physiological barriers on the ocular surface to the drug delivery and explore the limitations and challenges of drug delivery of a molecule to cross the physiological barriers.
2.1.1. Tear film
The tear film is the first barrier for topical drug penetration that a topically administered drug meet (Sitharaman, 2016) (Morrison and Khutoryanskiy, 2014). The medication is quickly diluted by tears and removed from the ocular surface through blinking and turnover of the tears (Shirasaki, 2008) (Janoria, 2007) (Agarwal et al., 2016). A large portion of the topical drug is washed away from the corneal surface in 2–3 min or less following application, significantly reducing drug contact time with the corneal surface (Molokhia et al., 2013) (Fig. 2). In addition, reflex tearing stimulated by drug instillation reduces drug bioavailability through dilution (Agarwal et al., 2016). The tear film is 6–7 μm thick and composed of three layers: lipid, aqueous, and mucin (Morrison and Khutoryanskiy, 2014) (Xu et al., 2018). The lipid layer is the outermost layer of the tear film and prevents evaporative loss of the aqueous layer, and maintains surface tension, which stabilizes the tear film and preventing it from overflowing onto the cheek (Agarwal et al., 2013). The aqueous layer makes up 90% of the total tear volume (Gan et al., 2013) and is composed of 98% of the tear film thickness with dissolved oxygen and nutrients contains water, electrolytes, and a large variety of proteins, peptides and glycoproteins (Ohashi et al., 2006) (Sariri and Ghafoori, 2008) (Ali and Byrne, 2008). The volume of the tear film is 7.0–30.0 μL in human with an average tear turnover rate of 1.2 ml/min (0.5–2.2 ml/min) (Ali and Byrne, 2008) (Mishima, 1966) (Ghate and Edelhauser, 2008), therefore smaller volumes of topical formulations might require higher drug concentration to achieve effective therapeutic drug level at the target site. The mucin layer is the deepest layer of the tear film and located adjacent to the underlying epithelial cells of the cornea and conjunctiva (Ohashi et al., 2006) (Argüeso and Gipson, 2001) (Mandal et al., 2017). The mucin layer plays a vital role in stabilizing the tear film by reducing the surface tension between the aqueous layer and the hydrophobic membranes of the corneal and conjunctival epithelial cells (Bron et al., 2004) (Cwiklik, 2016) (Rolando and Zierhut, 2001). Thus, allowing the aqueous layer to firmly adhere to the corneal epithelium. The non-specific binding of drugs with tear enzymes (e.g., lysozyme), mucin layer, and proteins (e.g., albumin) makes drugs unable to reach the cornea, anterior chamber, and therefore is quickly cleared with each blink (Wels et al., 2021).
Fig. 2.
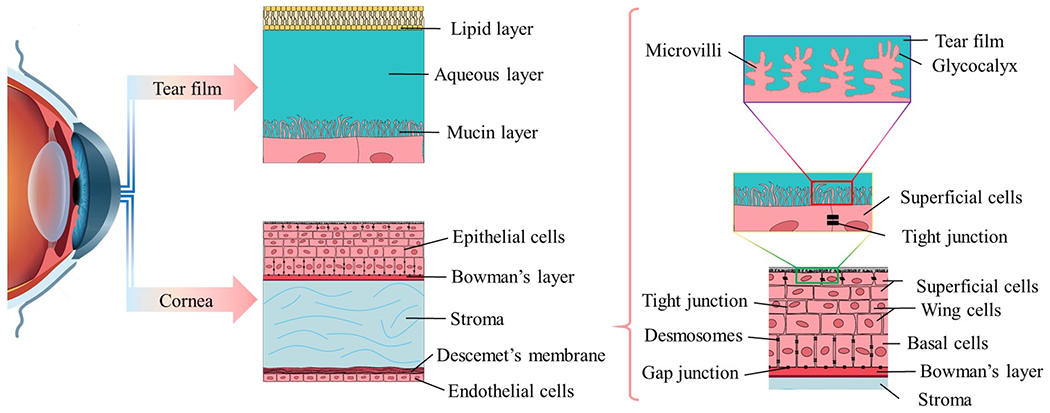
Cross-sectional view of tear film: a high tear turnover rate and gel-like mucin layer make a tear film as a major barrier in topical ocular drug delivery before drug penetration into cornea and corneal barrier.
2.1.2. Corneal barrier
The adult human cornea is composed of three cell layers: the lipophilic epithelium, the hydrophilic stroma, and the lipophilic endothelium (in order from anterior to posterior). There are also two interfaces: the Bowman layer and Descemet’s membrane (Fig. 2) (Zavala et al., 2013) (Soh et al., 2020). The limitations of topical ocular drug delivery are also governed by the cornea anatomy (Fig. 2). The cornea is approximately 11.7 mm in diameter, 0.5–0.7 mm thick, and has a mean surface area of 1.3 cm2, representing around 7% of the total surface area of an globe (Mcdevitt, 2012). The outer most layer of the cornea consists of a uniform 5–7 lipid-rich cell layer with embedded desmosomes that is highly resistant to drug penetration (Lu et al., 2001) (Sridhar, 2018). Similar to skin, the cornea is directly exposed to the outside environment and is therefore subject to interacting with a multitude of pathogens, allergens and environmental stressors. Unlike the skin, however, the corneal epithelium is non-keratinized and more permeable (Forrester et al., 2016) (Stella, et al., 2007). The corneal epithelium is held together and maintains its barrier function by four types of tight intercellular junctions distributed at different depths of the stratified epithelia. The foremost apical layer of the epithelium contains tight junctions (zonula occludens), which consist of focal connections between neighboring cells and acts as a barrier to the diffusion of drugs through the paracellular channel by sealing the intercellular space. The other intercellular junctions include desmosomes, which are abundant in the wing cell layers, adherent junctions, which are distributed throughout the different epithelial layers, and hemidesmosomes, which anchor the basal epithelial cells to the basal lamina. Collectively, these intercellular junctions provide structural integrity to the corneal epithelium, as well as an anchoring function by linking the cytoskeletons of adjoining cells and maintaining adhesion to the underlying basal lamina. It is this elaborate junctional complex network distributed throughout the layers of the corneal epithelium that forms the formidable resistance barrier to drug absorption (Lu et al., 2001) (Mantelli et al., 2013) (Hou, 2018) (Downie et al., 2021).
The Bowman layer is an 8–12 μm thick layer located between the epithelium and the stroma. It consists of acellular condensation of type I and type III collagen fibrils, which makes it resistant to trauma. The Bowman layer becomes continuous with the most anterior part of the corneal stroma, which can be distinguished from Bowman layer due to a different orientation of the collagen fibers. The collagen fibers are randomly interwoven to form a dense sheet (Wels et al., 2021) (Soh et al., 2020) (Addo, 2016). This layer is considered as a less constrained filter layer for the passage of drugs or particles to the stroma (Holowka et al., 2014). The stroma represents about 90% of the thickness of the cornea, consists of hydrated type I collagen that greatly contributes to its rigidity and transparency (Wels et al., 2021) (Holowka et al., 2014). In contrast to permeability of the epithelium and endothelium for nonpolar and lipophilic drugs, the hydrophilic nature of corneal stroma offers minimal or low resistance to the diffusion of highly hydrophilic drugs (Molokhia et al., 2013) (Ramsay et al., 2018). The Descemet’s membrane, which is the base of the stroma, functions as a protective membrane for the endothelium, like the Bowman’s membrane to epithelium with the exception of the type IV collagen sheets (Wels et al., 2021). It provides support for the monolayer of corneal endothelial cells (Soh et al., 2020). While Descemet’s membrane does not serve as a barrier to the diffusion of molecules due to the larger pore sizes, macromolecules, and particles administered directly into the stroma could be prevented from reaching the endothelium (Wels et al., 2021) (Fannon et al., 2012). The corneal endothelium is a monolayer of cells that provides a leaky barrier for the transportation of water and solute from stroma to the anterior chamber by both an active mechanism (the activity of Na+/K+; ATPase pumps) and a passive mechanism (the barrier function of endothelial intercellular tight junctions) (Soh et al., 2020) (Shah et al., 2008) (Tuft and Coster, 1990). These transfer mechanisms and movements counter a natural tendency for the stroma to swell, thus maintaining corneal transparency and the exchange of nutrients (Wels et al., 2021). Due to the transportation of water and solutes and monolayer structure of endothelium cells, it acts as a leaky lipophilic layer without a significant barrier for the ocular drug absorption (Prausnitz and Noonan, 1998).
2.2. Major routes for drug delivery to the anterior segment of the eye
Upon topical instillation onto the ocular surface, a drug can gain entry into the eye by passing through either the cornea or the conjunctiva or sclera (HUGHES et al., 2005) (Kang-Mieler et al., 2014). The cornea plays a predominant role for drug entry into the eye. The cornea constitutes a compact surface barrier designed to prevent the entry of foreign molecules and particles. It is this innate barrier function of the cornea that makes topical drug delivery to the eye a significant challenge.
The alternative pathway for drugs to enter the eye following topical instillation is the non-corneal route consisting of the conjunctiva and sclera (Tsai et al., 2018). The conjunctiva has approximately 17 times the surface area compared to cornea (Watsky et al., 1988). The permeability of the conjunctiva for hydrophilic drugs is also 17-fold greater than the corneal epithelium. Consequently, hydrophilic drugs and macromolecules preferentially absorb through conjunctiva (Prausnitz and Noonan, 1998) (Zhang et al., 2004). Drug diffusion across the sclera occurs through perivascular spaces and between scleral fibrils to eventually reach the choroid, and finally reaching the retina.
“Corneal and Conjunctival” ocular routes are two main strategies for topical ocular drug delivery. In “Corneal” ocular route, once the drug is applied to cornea, it diffuse across the cornea, the anterior chamber, through the pupil and into posterior chamber, then passes through zonules (suspensory body) and into the vitreous, and finally passes through retina and retinal pigment epithelium (RPE) layer to enter the systemic circulation via the choroidal vasculature (Gaudana et al., 2010) (Weng et al., 2017). Theoretically, the drug can be distributed to cornea, iris, trabecular meshwork in the angle, lens, retina, RPE and choroid along the way, although most drugs do not reach therapeutic level in the vitreous cavity or retina (Fig. 3.). A drug can gain access to the trabecular meshwork in the angle in contrast, in “Conjunctival” ocular route, a drug is absorbed by conjunctiva and passes through the sclera, choroid, retina, and vitreous body. It also goes directly to systemic circulation, mostly at the level of the choroid (Xu et al., 2018; Weng et al., 2017). Most of the drug exits the eye through the scleral outflow and goes to systemic circulation (Fig. 2.) and very little drug, if any, reaches the RPE (Xu et al., 2018).
Fig. 3.
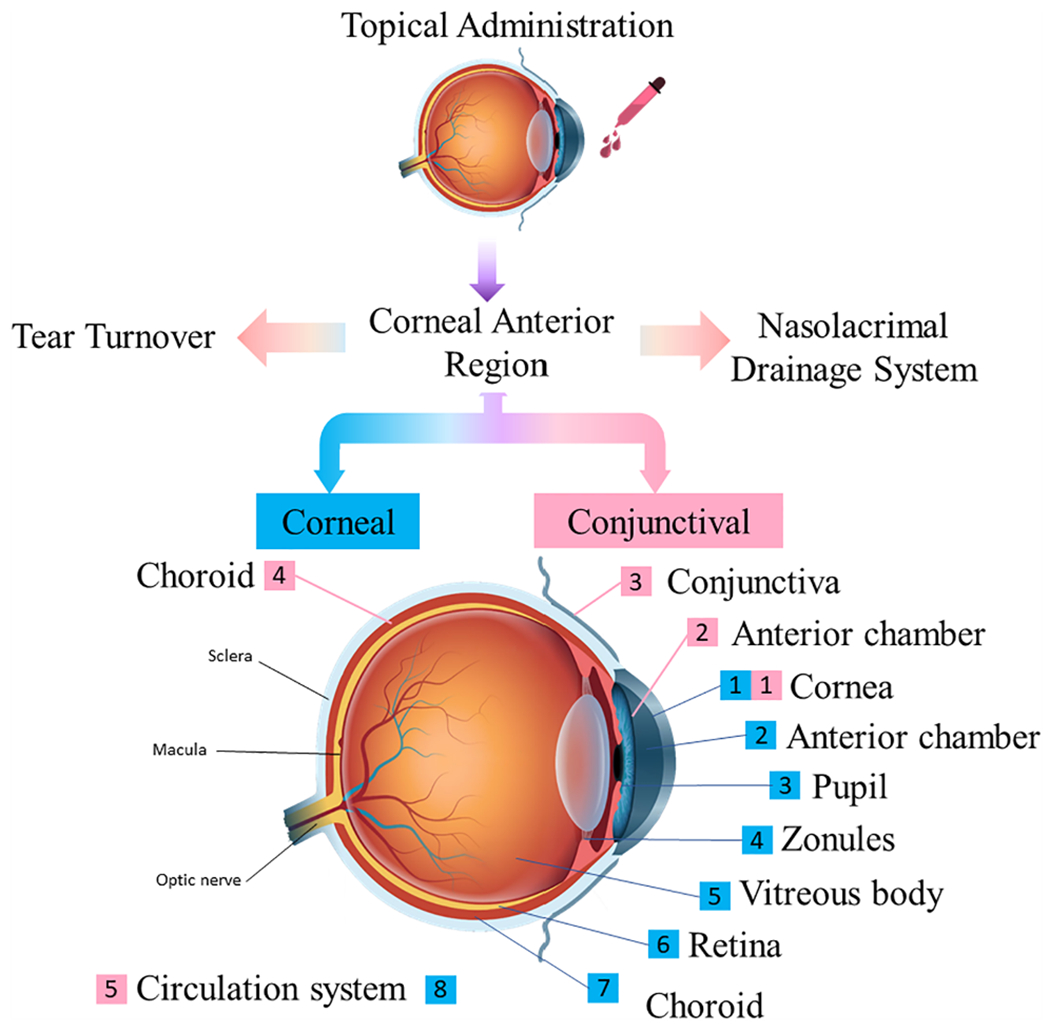
Corneal and Conjunctival routes explored for drug delivery to the anterior segment of the eye.
2.3. Drug delivery challenges to the cornea and conjunctiva
The transport of fluids and solutes across the ocular surface is largely limited by the tear film barrier and corneal barrier (Gan et al., 2013). The first barriers for the topically applied formulation are precorneal factors, such as a continuous high tear turnover rate, blinking, and draining of tears through the nasolacrimal duct system, which rapidly removes instilled compounds from the eye (Shirasaki, 2008) (Agarwal et al., 2016). In addition, the corneal epithelial apical cells produce cell surface membrane associated gel forming mucins, which are concentrated on the tips of the apical cells’ microvilli. These gel forming mucins secreted by the apical cells form a dense glycocalyx at the epithelial-tear film interface providing a protective cell surface barrier preventing the penetrance of extracellular molecules and other environmental particulates, as well as facilitating the clearance of allergens, pathogens and debris from the ocular surface (Gan et al., 2013) (Kambhampati and Kannan, 2013). After transportation of the solute and fluids across the tear film and mucin barriers, tight intercellular junctions between adjacent outer superficial epithelial cells and a lipophilic epithelium form another critical obstacle to drug absorption (Baba et al., 2011). A lipophilic epithelium and a hydrophilic stroma with opposite characteristics are crucial barriers to most lipophilic and hydrophilic drugs.
Like other bilayer lipid systems, nonlipophilic soluble molecules are believed to diffuse in a very long and a tortuous path through tight junctions and around cells (Zhang et al., 2004). These molecules travel along the narrow intercellular spaces in order to reach the other side of the epithelium. This is most likely the reason for poor permeation through the corneal epithelium for hydrophilic drugs and macromolecules (Gaudana et al., 2010) (Willoughby et al., 2010) (Hegde et al., 2013). The permeability of endothelium is about 2.7 times more than the permeability of epithelium (Zhang et al., 2004).
In light of the fact that hydrophilic solutes have lower corneal permeability than hydrophobic solutes, investigation into the transportation of topical hydrophilic solutes across the cornea has been of limited success. However, the recent introduction of mucus penetrating particles (MPP) and penetration enhancing agents has generated substantial interest into the drug delivery of hydrophilic solutes through the cornea as well as, genetically engineered therapeutic proteins, DNA, and RNA, which also possess poor corneal permeability. Accordingly, mathematical models to describe hydrophilic drug transport in corneal epithelium, stroma, and endothelium are becoming increasingly important. Such models have been used to delineate passive transdermal transport of hydrophilic solutes (Prausnitz and Noonan, 1998) (Zhang et al., 2004). The topical transport of hydrophilic solutes into the eye can also occur through the non-corneal barrier routes of the conjunctiva and sclera.
Despite the advances in ophthalmic drug formulations, the delivery of therapeutic agents is still severely limited by the diffusion of drug across the cornea and conjunctival barriers. Drug diffusion across these barriers via the transcellular pathway is regulated by several key drug parameters, including drug lipophilicity, drug pKa which determines the proportion of drug in its preferentially absorbed form at a given pH, drug molecular weight (<500 Da), and drug molecular size (i.e., << 10 Å). In addition, the charge and degree of ionization of drug molecules also affect the passage of topical ocular drugs through the cornea (Shirasaki, 2008) (Prausnitz and Noonan, 1998) (Lee, 1990). Of all the physiochemical drug properties that determine corneal penetration, lipophilicity is the most well studied and perhaps the best understood.
Another area active research has been in the development of mathematical models that describe and predict the relationship between the corneal permeability and drug transport, especially for hydrophobic drugs (Prausnitz and Noonan, 1998) (Zhang et al., 2004) (Huang and Schoenwald, 1983). These models can be categorized based on the series combination of the resistance to transport of the epithelium, stroma, and endothelium (Eq. (1)) (Edwards and Prausnitz, 2001), or a quantitative multiple regression analyses related to cornea permeability (P) for various physicochemical factors (Eq. (2)). One equation, developed by Huang, Schoenwald, and Lach (Huang and Schoenwald, 1983), correlates cornea permeability (PT) of a drug in aqueous solution to solute molecular mass (MW), degree of ionization (DI) and octanol–water partition coefficient (PC) by Eq. (2):
| (1) |
where the subscripts “epi” and “endo” refer to the epithelium and endothelium, respectively.
| (2) |
Other methods for estimation of corneal permeability include quantitative structure –permeability relationships (QSPRs) (Chen and Yang, 2006), as well as, newer techniques including the combination of net atomic charges with molecular volume (Fu and Liang, 2002). The majority of models propose a single equation to explain the cornea permeability of all molecules, which implicitly assumes that all solutes have the same permeation pathways. The shortcomings of this assumption are clearly illustrated in the fact that hydrophobic drugs easily cross the lipophilic corneal epithelia, while hydrophilic drugs enable diffusion through the transcellular pathway, without altering leaky tight junctions (Gan et al., 2013).
2.4. Corneal and external diseases
In a last few decades, many approaches have been utilized to enhance drug delivery for the treatment of anterior segment eye diseases (Patel, 2013). Corneal diseases can adversely impact vision and are a major cause of blindness, second only to cataracts (Wels et al., 2021) (Shah et al., 2008). Corneal and external diseases can involve the eyelids, conjunctiva, cornea, anterior chamber of the eye, iris, and lens (Holland et al., 2013). Common corneal and external diseases include, but not limited to dry eye disease, keratitis, anterior uveitis, glaucoma and many others (Molokhia et al., 2013) (Wels et al., 2021) (Holland et al., 2013) (Bennett et al., 2014) (Tamhane et al., 2019) (Stapleton and Carnt, 2012). Owing to limitations of space, we cannot include an extended discussion of every anterior segment disease in this review. Here, we briefly highlight the common diseases for which targeted drug delivery to the anterior segment may be useful.
Dry Eye Disease (DED) is a condition effecting the ocular surface, resulting in pathological changes to the conjunctival and cornea epithelium with disruption of corneal epithelial barrier function and loss of mucus-secreting goblet cells (Holland et al., 2013) (Shimazaki, 2018). DED causes a variety of symptoms including ocular irritation, foreign body sensation, blurred vision, and light sensitivity (Marshall and Roach, 2016). DED can lead to ocular surface inflammation which can result in decreased tear production, therefore setting up a vicious cycle of disease (ChenZhuo et al., 2002). Treatment typically ranges from the use of artificial tears to topical anti-inflammatory and immunomodulatory agents (Calonge, 2001).
Keratitis refers to the inflammation of the cornea, frequently caused by infections (Ansari et al., 2013). The treatment of infectious keratitis most commonly requires antibacterial, antifungal, or antiviral therapy, depending on the etiology. All commonly prescribed topical antibiotics constitute best treatment for bacterial keratitis (Austin et al., 2017). While bacterial keratitis is usually associated with contact lens wear and pre-existing ocular diseases, fungal keratitis is more likely caused by ocular trauma (Wong, et al., 1997). Fungal keratitis usually have worse clinical outcomes between all keratitis that caused by organisms because the infection is less responsive to treatment and diagnosis (Ansari et al., 2013) (Austin et al., 2017) (Ansari et al., 2013). Treatment can be challenging due to lack of effective commercially available ophthalmic anti-fungal medications (Forrester, et al., 2020) (Thomas and Geraldine, 2007). It causes severe damage to the cornea, and penetrates deeper stromal layers, Descemet’s membrane, and into the anterior chamber or sclera, which leads to blindness and loss of eye (Ansari et al., 2013) (Brown, 2020). Fungal keratitis appears to have a five-to six-fold higher risk of perforation than in bacterial keratitis, and often result in the need for urgent therapeutic penetrating keratoplasty, or in severe cases, even evisceration or enucleation (Wong, et al., 1997) (Brown, 2020). Since the 1960′s, topical Natamycin is the most effective medication for the treatment of fungal keratitis (Ansari et al., 2013). However, 12–38% of fungal keratitis cases will progress despite medical treatment, and requires surgical intervention such as penetrating keratoplasty (Selver, 2015) (Rogers et al., 2013).
Anterior uveitis is the intraocular inflammatory eye disease involving the iris, ciliary body, or both (iridocyclitis) in the anterior segment and surrounding ocular tissue of the eye (Neti et al., 2021). Anterior uveitis typically causes photophobia, redness, acute onset of pain and blurred vision (Agrawal et al., 2010) (Schaal and Kaplan, 2008). The initial treatment of anterior uveitis consists of topical corticosteroids (Harthan, 2016), which has good penetration into the anterior chamber. However, there are significant potential serious side effects with topical steroids, including elevated intraocular pressure leading to glaucoma, cataract, and higher risk of eye infection. When topical steroids do not control the inflammation, systemic immunotherapy is required, such as, high-dose prednisone (e.g., 1 mg/kg/day) (Thorne and Jabs, 2015). Repeated episodes of intraocular inflammation within the eye can lead to tissue damage as well as glaucoma, cataract, cystoid macula edema and irreversible loss of vision (Neti et al., 2021) (Martin et al., 2002).
Glaucoma is an optic neuropathy characterized by a structural change of retinal ganglion cells and optic nerve axons. It leads to visual field defect and blindness (Kühn et al., 2021) (Weinreb et al., 2014) (De Moraes et al., 2017). Due to the asymptomatic progression, the diagnosis is frequently delayed and advanced with substantial neural damage result in visual disability (Rotchford et al., 2003) (Hennis et al., 2007). Ocular hypotensive drops are the first-line treatment of glaucoma. Laser trabeculoplasty and surgical intervention may also be used to slow down the progression of the disease (Weinreb et al., 2014). The main aim of glaucoma therapies and treatments (medical, laser or surgery) is to lower intraocular pressure (IOP) and the preservation of visual function (Migdal, 2000) (Boland et al., 2013).The currently available typically applied eye drops used in the treatment of glaucoma may not be effective due to poor compliance (Quigley, 2019). Sustained delivery of drugs is actively being studied to improve consistent reduction of IOP. These include contact lenses-releasing glaucoma medications, injectables such as biodegradable micro- and nanoparticles, and surgically implanted systems (Lavik et al., 2011). Therefore, successful treatment strategies for sustained delivery of glaucoma medication that improves patient compliance and adherence to the cornea has the potential to prevent disease progression.
2.5. Hypothetical plot of topical ocular drug concentration in tear film
In order for topical medications to be effective, it requires the patients to be compliant with the dosing schedule. Therefore, many patients do not achieve the full therapeutic effect of the medication due to poor compliance. Thus, many technologies are developing nanoparticles and contact lenses delivery approaches that can achieve high drug concentrations that is constant and over and extended period of time. Fig. 4 highlights these effects on the drug concentration profile of topically applied drugs in the anterior segment of the eye.
Fig. 4.
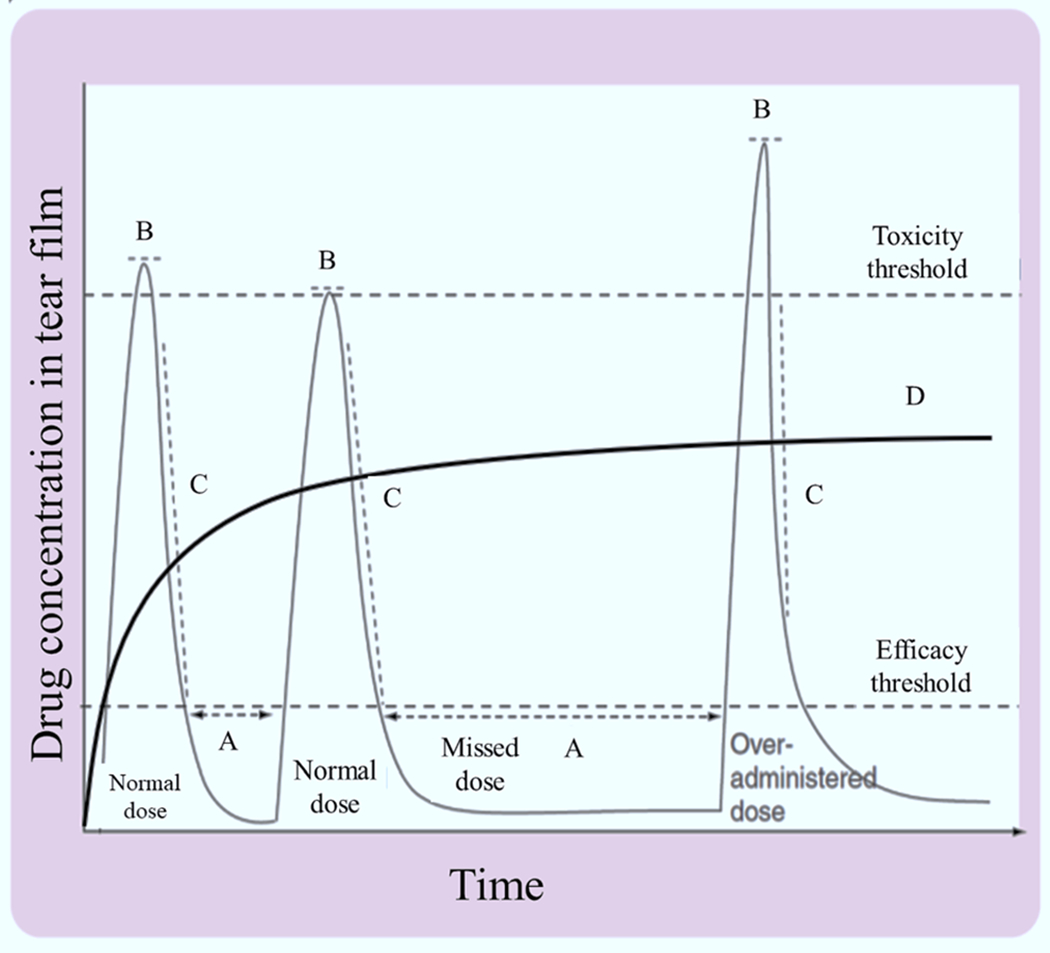
Hypothetical potential pharmacokinetic profile of topically applied drug concentration in the tear film over time. (A) missed dose, the eye tissue without therapeutic levels of drug over time, (B) toxicity threshold, over administration of drug to its maximum level considered therapeutic, (C) the excessive volume of drug solution increases the drainage rate of instilled volume, and (D) controlled release drug delivery profile with a plateau level of drug distribution of topically applied formulations. Adapted, with permission, from (Ali and Byrne, 2008).
3. Materials for “Corneal and Conjunctival” drug delivery
The optimal sizes of materials to enable topical ocular delivery, ranging from sub-micron to macroscopic systems, continue to be developed. This section outlines several different classes of various pharmaceutical agents (Fig. 5) that facilitate cornea permeability, exploring mechanisms of action, experimental considerations, major strengths and limitations, and the challenges ahead.
Fig. 5.
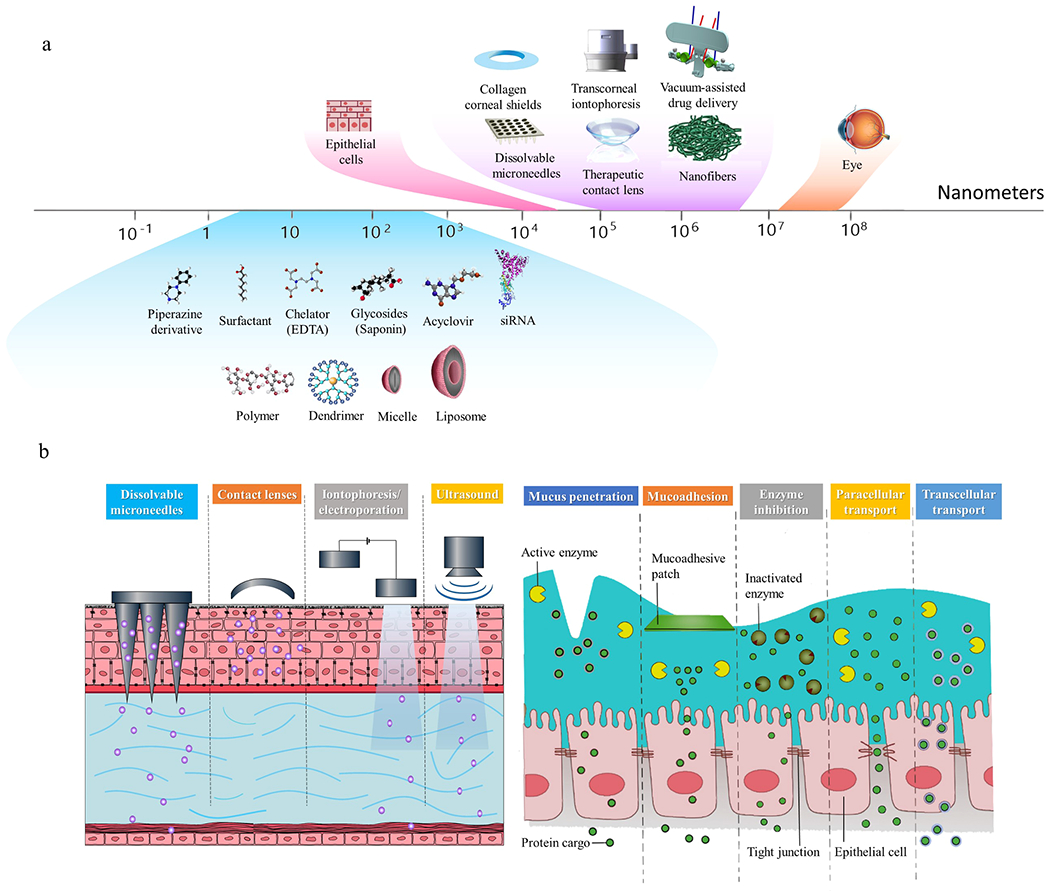
The feature sizes of materials a) ranging from sub-micron to macroscopic systems as a function of length b) materials of vastly different sizes, ranging from nanometers to centimeters to enable topical ocular delivery of drugs.
3.1. Sub-micron systems
Although numerous studies have attempted to develop sub-micron systems for topical ocular drug delivery; developing novel formulations and particles are required to circumvent current strategies and challenges with regards to in clinical trials, commercialization of ocular formulations, dosing administration, diffusion and retention of the drugs. Herein, we highlight the predominant classes of nano-formulations introduced for anterior segment ocular drug delivery.
3.1.1. Viscosity and permeation enhancers
Because the corneal epithelium and tear film provide formidable barriers for topical formulations, a wide range of viscosity and permeation enhancers have been studied to improve the permeation across the epithelial lining and increase the precorneal residence time of drug. Increased viscosity provides certain advantages, including reduction of drop size, and increased bioavailability and permeability of conventional ophthalmic drugs. Extensive research during the last two decades has revealed the formulation of several different classes of permeation enhancers, including fatty acids (Caprylic acid), surfactants (Palmitoyl carnitine), chelating agents (Ethylenediaminetetraacetic acid, EDTA), preservatives (Benzalkonium chloride, BAC), glycosides (Saponin), and bile salts (D) (Kaur and Smitha, 2002). Penetration enhancers facilitate cornea permeability through affecting both the cell membranes and the tight junctions by the following mechanisms: (i) destabilizing the tear film and the mucous layer at the cornea surface, (ii) increased partitioning due to changes in the arrangements and solubility of lipid bilayers of cornea epithelial cells, and (iii) fluidizing the epithelial cell membranes by loosening and disrupting tight junctions, and (iv) enhancing drug solubility (Kaur and Smitha, 2002) (Moiseev et al., 2019). Polyethylene glycol, digitonin, and ethers are examples of permeation enhancers that increase drug solubility. Chelators are believed to enhance drug permeation through the tight junctions, while surfactants act mainly through the cell membranes or via the transcellular pathway (Vyas et al., 2011).
Viscosity enhancers are compounds developed by natural and synthetic hydrophilic polymers to absorb water and form viscoelastic gels to improve precorneal residence time of the drug (Gote et al., 2019) (Irimia et al., 2018). Carbomers (weakly crosslinked polyacrylic acids), derivatives of cellulose (Carboxymethylcellulose), anionic polysaccharides (Hyaluronic acid), polyvinyl alcohol, and polyvinyl pyrrolidone are compounds with viscosity-enhancing properties (Ali and Byrne, 2008) (Wadhwa, 2009) (Wilson et al., 1998). Despite considerable research in the area of enhancer vehicles, only few have proven to be of any therapeutic utility. Potent viscosity enhancers significantly interfere with vision (Ali and Byrne, 2008), while the permeation enhancers can cause irritation and damage to the corneal and conjunctival epithelia (Gote et al., 2019). In recent years novel chemical penetration enhancers have been developed with demonstrable therapeutic efficacy. For example, Azone™ has established safety profiles for enhanced transport of therapeutics (Newton et al., 1988). Cyclodextrins, similarly, represent a new ‘specialty’ class of corneal penetration enhancers that promote tissue absorption of dexamethasone administered via eye drops without irritation and are being evaluated for clinical applications (Loftsson and Stefánsson, 2002) (Shimazaki, 2018). Large molecules such as cyclodextrins are unable to permeate the intact lipophilic membranes but able to disrupt the tear film, alter mucin, take-up the cholesterol and phospholipids in cell-membrane lipid bilayers, and transit the drug into the cornea (Morrison et al., 2013) (Loftssona and Järvinen, 1999) (Calonge, 2001) (Ansari et al., 2013). Some cyclodextrins are currently used in commercial ocular formulations e.g. Vitaseptol eye drops (Novartis) (Moiseev et al., 2019).
3.1.2. Colloidal nanocarriers
Colloidal nanocarriers such as dendrimers, liposomes, emulsions, micelles, and deformable vesicles have been used to enhance penetration of therapeutic agents into the eye. The key challenges for hydrophobic drugs include poor absorption in the tear film and therefore is quickly cleared with each blink. Mucus-penetrating particles (MMPs) increase the drug’s retention time on the mucin layer of the tear film which is in the direct contact with corneal epithelium (Fig. 6) (Langer and Chen, 2014). These applications are under development by engineering the size and surface characteristics of the particles.
Fig. 6.
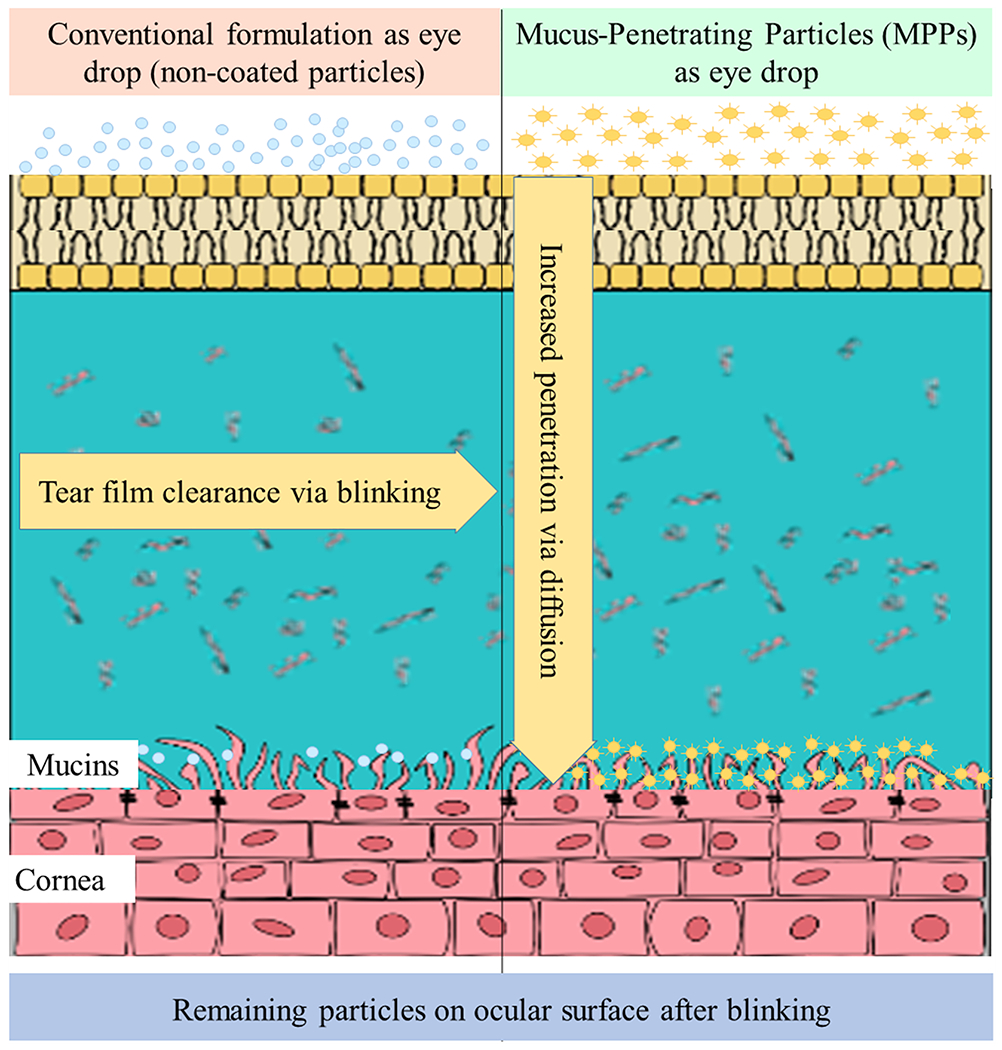
Mechanism of action of mucus penetrating particles (MPP) compared with conventional topical eye drops (Langer and Chen, 2014). Traditional suspension eye drops rapidly clear via blinking on the tear film. MPPs particles diffuse through the tear film into the membrane-bound mucin and ocular surface to reach target tissue. Image Courtesy of Kala Pharmaceuticals.
Liposome-based mucus-penetrating particles (MPP) are colloidal nanovehicles formed by lipid bilayers surrounding an internal aqueous core with typical size from 10 nm to several micrometers (Pattni et al., 2015) (Brown et al., 2019). These structures have the capability of entrapping either hydrophobic compounds into the bilayer membrane of liposomes or hydrophilic water-soluble molecules into the liposome interior (Bruun and Hille, 2019). The lipophilic nature of corneal epithelium prevented the diffusion of hydrophilic drugs by the corneal epithelial cells, whereas lipophilic drugs may easily diffuse across lipophilic cornea by a transcellular mechanism (Molokhia et al., 2013) (Zhang et al., 2004). Therefore, liposomes are considered highly efficient vehicles for delivering topical drug molecules across the cornea. Similarly, mucoadhesive chitosan-coated liposomes improve the penetration of topical administration of hydrophilic drugs by allowing the drugs to pass through the tight junctions, increasing the precorneal retention time, and slowing down the drug metabolism to the ocular surface (Irimia et al., 2018).
Liposomes have also been used as chemical enhancers that not only protect the drugs from the external environment, but also are biocompatible, biodegradable with high drug encapsulation yield, slow drug release and non-toxic properties (Brown et al., 2019) (Nisini, 2018). However, the major disadvantages of liposomal topical ophthalmic delivery systems are low bioavailability, instability of the lipid aggregated on the mucin surface, short half-life due to the tear turnover, and poor solubility of drugs (Meisner and Mezei, 1995) (Agrawal et al., 2010). The lipophilic nature of liposomes enables them to penetrate the corneal epithelium via transcellular route with four different mechanisms, including adsorption, lipid exchange, fusion, and endocytosis (Meisner and Mezei, 1995) (Schaeffer and Krohn, 1982).
The most important mechanisms of liposomal drug delivery via the transcellular pathway are: (i) absorption of liposome to cell membrane and then disruption, cellular uptake, passive diffusion and transportation of highly concentrated drug at the epithelial membrane or (ii) lipid exchange and destabilization of liposomal bilayer with consequential intracellular release of drug molecules (Agarwal et al., 2016) (Mishra et al., 2011) or (iii) endocytosis, which is the engulfment and internalization of adsorb liposomes into the cell, formation of endosomes, enzymatic degradation of lipids and finally, delivery of entrapped drugs into the cytoplasm (Guo et al., 2015) (Lee et al., 1985). The presence of liposomes on the cornea epithelial cells causes the fusion of lipid bilayers with corneal lipoidal cell membrane and allows for direct delivery of liposomal contents into the cytoplasm. The mechanisms of endocytosis or fusion of liposome membranes for the ophthalmic system are not fully elucidated although it is thought to increase the transcellular pathway through the corneal epithelial cells.
Nanomicelles.
Nanomicelles can be defined as colloidal constructs consisting of amphiphilic molecules/monomers that can self-assemble in an aqueous medium. They generally have two parts, a small hydrophobic interior/core that can hold and interact with hydrophobic drugs/agents, and a long hydrophilic tail that helps the complex to surround with aqueous phase and enhances solubility. Due to these characteristics nanomicelles can be used for both therapeutics and diagnostics, by encapsulation of hydrophobic drugs as well as imaging agents, respectively (Trinh et al., 2017) (Vaishya et al., 2014) (Vadlapudi and Mitra, 2013) (Xu, 2020). The unique structure of micelles avoids the direct contact of topically applied hydrophobic drugs in the hydrophilic portion of the cornea, the stroma, which constitutes 85–90% of the cornea (LEE and ROBINSON, 1986). In addition to penetration through the cornea, small size of micelles enables them to diffuse through the conjunctival-scleral pathway after topical application to reach the posterior segment of the eye (Di Tommaso, 2012). Micelles can be prepared from surfactant (ionic, nonionic, zwitterionic) or block copolymers (Cholkar et al., 2012) (Vadlapudi, 2015). In general, surfactant micelles are smaller in size, tend to absorb onto surfaces or interfaces with rapid onset of action (Cholkar et al., 2012). In contrast, polymeric micelles offer significant advantages in providing reduced side effects, extended circulation time, and sustained drug release with relatively low toxicity (Torchilin, 2001) (Jones and Leroux, 1999). It is believed that polymeric micelles are more stable than surfactant nanomicelles and are more promising for nucleic acids and gene delivery.
The use of hydrogel containing cross-linked micelles have recently gained significant interest due to the potential improvement of higher ocular biodistribution and enhanced drug stability (Mandal et al., 2017). This has led to the emergence of a new field called ‘micelles laden hydrogels.’ However, despite the improvement in biodistribution, topical delivery of drugs is influenced by nasolacrimal drainage of solution, non-productive absorption by the conjunctiva, blinking, and tear turnover (Ali and Byrne, 2008). Plutonic F127-pluronic F68 micellar formulations of itraconazole incorporated in Carbopol 934P hydrogels demonstrated enhanced ex vivo transcornal penetration compared with sustained drug Itral® (itraconazole) eye drops and a pure drug suspension release (Jaiswal et al., 2015). α-cyclodextrin (α-CD) hydrogel has also been developed based on methoxy poly(ethylene glycol) block polymer micelles for topical ocular delivery of diclofenac (Zhang et al., 2016). These micelles laden hydrogels showed low cytotoxicity, irritation, with controlled drug delivery for long-acting applications (Mandal et al., 2017).
3.1.3. Dendrimers
Dendrimers are “tree-like” nanostructured polymers with nanosized dimensions (1–100 nm) (Yavuz et al., 2013). The structure of dendrimer consists of a central core with a series of “radically concentric layers” of repeated branches called “generations” which define its shape (Caminade et al., 2005). Hydrophilic drugs can be attached to the dendrimer surface by covalent conjugation or electrostatic interaction, whereas lipophilic drugs can be entrapped through simple encapsulation in the internal cavity of the dendrimer and provide sustained release (Vadlapudi, 2015). Corneal permeability of dendrimer is dependent on the molecular weight, size, and method of formulation preparation. Smaller dendrimers have higher permeability than larger dendrimers since they can easily pass between the epithelial cells (Kaminskas et al., 2011) (Venuganti and Perumal, 2009).
Dendrimers’ well-defined terminal functional groups, precisely controllable nanosized range, and tailorable and monodisperse structure make them suitable carriers for topical ocular drugs. Although dendrimers can cause temporary blurred vision, they offer significant advantages with a decreased drug dosing frequency, prolonged residence time, and improved bioavailability. Vandamme et al. studied dendrimer with amine, carboxylate and hydroxyl surface groups to increase residence time in the eye and found that residence time increases with carboxylic and hydroxyl surface groups (Vandamme and Brobeck, 2005). Dendrimers made of polyamidoamine (PAMAM) with carboxylic and hydroxyl functional groups improve drug delivery by loosening epithelial tight junction via paracellular transport and/or transcellular pathways across corneal epithelial barriers (Souza et al., 2015).
3.1.4. Prodrugs
Prodrugs comprise chemically modified agents in the molecular radius size range of 25–100 nm (Cheetham et al., 2017). Prodrugs are bioreversible modification of the active drugs by chemically attaching a side-chain group (Rautio et al., 2008). After transcellular penetration through the corneal epithelium cells, prodrugs are converted into the active parent drugs by enzymatic or chemical reactions or by a combination of the two with their desired pharmacological effect (Ali and Byrne, 2008) (Wadhwa, 2009). Prodrugs enhance drug solubility, retain a long shelf-life, improve chemical and metabolic stability, and therefore improve the passive drug absorption (Lallemand et al., 2005) (Dey et al., 2003). For example, acyl diester prodrugs such as acyclovir (ACV) and prodrug of ganciclovir (GCV), was confirmed to enhance drug transportation across corneal epithelial cells (Macha et al., 2004) (Tirucherai et al., 2002) (Hughes and Mitra, 1993). Since direct GCV administration has low corneal permeability, GCV was esterified to the corresponding diacetate, dipropionate and dibutyrate derivatives to achieve sustained therapeutic concentrations and increase corneal permeability 2-fold compared with the direct GCV administration. Although, these prodrugs improve the permeability across the cornea, they lacked adequate aqueous solubility restricting their formulation in 1 – 3% eye drops (Hughes and Mitra, 1993). ILEVRO® (nepafenac ophthalmic suspension), nonsubstituted amide prodrug of amfenac, was approved by the FDA in 2005 for treatment of pain and inflammation following cataract surgery dosing. It was designed to improve the corneal permeability and tissue distribution profile and therefore, only required once daily dosing (Ke, 2000). Nepafenac ophthalmic suspension enables the 4- to 30-fold higher corneal permeability than conventional Nonsteridal Anti-Inflammatory Drugs (NSAIDS) such as diclofenac, bromfenac and ketorolac (Lindstrom and Kim, 2006). Notably, these amide prodrugs and their derivatives require around approximate 15 min of hydrolase activity, which cause greater duration of action than ester derivatives of other (NSAIDS) following topical administration.
Although the ophthalmic use of prodrugs was first proposed in the late 1970 s for enhancing ocular bioavailability (Yellepeddi and Palakurthi, 2016), strategies such as transporter/receptor viability and expression by chemical modification did not become widely available until a decade ago (Dey et al., 2003). While lipophilic prodrugs penetrate the epithelial cell membranes through the diffusion mechanism, transporter/receptor targeted prodrugs enable transcellular pathway penetration via active transport by transporter recognition. Novel technologies have been developed through the addition of lipid raft to ligand in targeted prodrug to facilitate enhanced interactions into the binding domain of membrane proteins (transporter or receptor). This novel strategy has been used to synergize the prodrug permeability through the combination of lipid and transporter/receptor combination (Dey et al., 2003) (Patel, 2013).
3.2. Macroscopic systems
The purpose of macroscopic devices for topical ocular drug delivery is to prolong and improve drug entry for topical administration. These approaches include topically applied patches such as therapeutic contact lenses and collagen shields. In addition, physical methods including iontophoresis and electroporation are being adapted for long-lasting drug delivery and disrupt and bypass the cornea barrier (Huang et al., 2018).
3.2.1. Hydrogel-based therapeutic contact lenses
Therapeutic contact lenses (TCLs) are an upcoming technology for long-lasting ocular drug delivery. While eye drops have less than 5% corneal bioavailability (Gote et al., 2019), the use of TCLs enhance theoretical corneal bioavailability up to 50%, which significantly provide enough drugs to penetrate into the cornea and the posterior chamber (Xu et al., 2018) (Jung et al., 2013). Hydrogel polymers are ideal for a variety of reasons. The cross-linked structure of hydrogels, achieved through the hydrophilic polymer chains, retains their three-dimensional structure, remains them insoluble, and possesses a higher water content (Guzman-Aranguez et al., 2013) (Xinming et al., 2008). TCLs made of hydrogels can enable a tunable controlled release profile for extended periods of time, within the therapeutic window and carry drug concentrations with minimal risk of drug toxicity (Xu et al., 2018) (Carvalho et al., 2015) (Phan et al., 2013). TCLs are the current state-of-the-art technology for ocular delivery. Different strategies have been developed with the use of colloids, prodrugs, and nanoparticles to serve as depots and carriers for many ophthalmic drugs, including small molecules and biologies (Alvarez-Lorenzo et al., 2019). Despite the excellent enhancement of drug penetration into the cornea with these strategies, about 5% is absorbed in a short time of drug presence before being washed away by the tear film. Toward this end, TCLs serve as precorneal drug reservoirs and delivery systems for the compressed and expanded tear film that formed between the inner surface of the lens and the cornea called post-lens tear film (Fig. 7) (Janoria, 2007) (Guzman-Aranguez et al., 2013). The fluid in the post-lens tear film is protected from the remaining tear fluid. This device is an innovative technology compared with eye drops because the mixing time of drugs in the post-lens tear film is about 30 min, which greatly enhances the bioavailability and penetration of the medication across the cornea compared to the typical two minute exposure for typical eye drops (Guzman-Aranguez et al., 2013) (Maulvi et al., 2016). The major challenges for successful commercialization of TCLs include the potential for corneal toxicity, risk of infection, oxygen diffusion, and continuous and constant drug release.
Fig. 7.
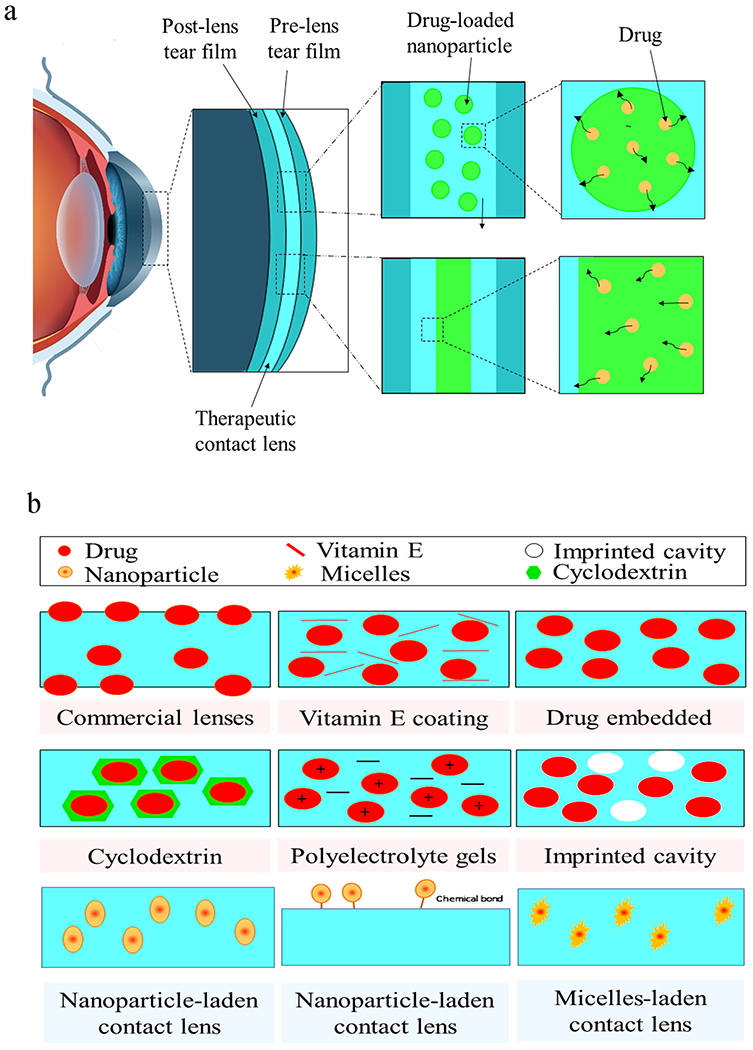
Schematics of a) drug diffusion from drug-eluting hydrogel therapeutic contact lens and b) strategies to control drug release from contact lens. Adapted, with permission, from (Choi and Kim, 2018).
Different techniques have been developed to increase the bioavailability and sustained release of the desired drugs. These strategies include (i) soaking method through “soak and release” model, (ii) colloidal particle-laden CLs, (iii) molecular imprinting through “lock and key” model, and d) ion ligands or microemulsion-loaded gels (Fig. 7. a) (Guzman-Aranguez et al., 2013) (Carvalho et al., 2015) (Alvarez-Lorenzo et al., 2019) (Carvalho et al., 2015; Gupta and Aqil, 2012). The main objectives of these techniques are to enhance the duration of drug presence in TCLs, improve the ocular surface bioavailability, allow sustained drug release and increase the retention and permeation of therapeutics. However, there were a number of challenges in the clinical trials, including transparency, mechanical properties, oxygen permeability, ion permeability, lack of stability during processing, and water content of the TCLs (Xu et al., 2018; Jung et al., 2013; Maulvi et al., 2016) (Guzman-Aranguez et al., 2013). The simplest and most cost-effective method involves soaking the commercially available contact lenses into a drug solution and subsequently releasing them into the post-lens lacrimal fluid (Xu et al., 2018; Guzman-Aranguez et al., 2013). It has been demonstrated that the “soak and release” method achieved a higher bioavailability for a few hours of sustained drug release, which is much better than eye drops (Carvalho et al., 2015). Molecular diffusion is the main driving force for the drug release from contact lens to the post-lens tear film, dispersion in the tear fluid and subsequently absorption by the cornea (Xu et al., 2018) (Carvalho et al., 2015). Fig. 7.b demonstrated the strategies used to achieve sustained release of drugs by different techniques. Further modification of the TCLs has the potential to improve upon the desired effects of drug delivery to the eye.
Initially the therapeutic agents in the particle-laden contact-lenses are entrapped in microemulsions, or colloids such as liposomes, and micelles. For example, liposome-laden CLs containing lidocaine entrapped in 1,2-dimyristoylsn-glycero-3-phosphocholine (DMPC) lipid in pHEMA is used for postoperative pain control following corneal surgery. The results demonstrated transparent hydrogels with an initial 30% burst release of lidocaine over the first few hours followed by near zero-order release for approximately 8 days (Gulsen et al., 2005). However, there is concern of cornea toxicity with prolonged exposure to topical anesthetics. Peng et al. recently studied the transportation of topical anesthetics including lidocaine in nanosized vitamin E loaded silicone hydrogel CLs. This resulted in extended delivery of an anesthetic can alleviate postoperative pain following corneal surgery such as the photorefractive keratectomy (PRK) procedure for vision correction (Peng et al., 2012).
To enhance drug uptake and achieve sustained release of drugs, molecularly imprinted polymers (MIPs) were created in the fabrication of TCLs (White and Byrne, 2010) (Ali et al., 2007) (Tieppo et al., 2012). The bioinspired contact lenses are designed to selectively engineered binding sites inside the TCLs for drug molecules that mimic the natural receptors (Alvarez-Lorenzo et al., 2019). For example, molecular imprinted TCLs recently demonstrated that drug uptake and the overall binding affinity of timolol are 4.9-fold and 12.3-times higher, respectively, than the nonimprinted gels (Hiratani and Alvarez-Lorenzo, 2002). Therefore, MIPs offer a significant advantage over standard TCLs with the ability to provide controlled release of medication onto the surface of the cornea.
3.2.2. Fibers
Fibers can be formed continuously from polymeric solutions through single uniaxial or coaxial nozzles, followed by the application of a high voltage electric field (Zamani et al., 2013) (Kenawy et al., 2009) (Mofidfar et al., 2019). Electrospun drug-eluting fibers drive from an extruded solution, containing drug and polymers, through a syringe needle (Mofidfar and Prausnitz, 2019) (Ahadian et al., 2020). By contrast, extruded fibers from forced assembly polymer force, rather than electrostatic force in electrospinning techniques, render a novel attractive method for the fabrication of drug-releasing polymer fibers (Ren et al., 2019) (Mofidfar et al., 2017). Using these approaches, random or aligned polymeric nanofibers can be prepared via inexpensive and scalable processes (Brown et al., 2019) (Kitto et al., 2019) (Radacsi et al., 2018). Large surface area to volume ratio, high drug encapsulation efficiency, and flexibility in surface functionality make fibers a promising alternative to drug saline eye drops (Tawfik et al., 2020). Different fiber shapes and sizes, with diameters in the range of a few hundred nanometers to micrometers, can be produced by changing the applied voltage, flow rate, viscosity and conductivity of the polymeric solution (Sharma et al., 2014) (Shahriar et al., 2019). Electrospinning technique was used to fabricate fast dissolving dendrimer nanofibers (DNF) as a topical delivery vehicle for the glaucoma drug brimonidine tartrate (BT) (Lancina, 2017). The fabricated DNF matrix demonstrated extended and improved efficacy with daily dosing over a 3-week test period during in vivo experiments. Therefore, this technique has potential for the fabrication of low cost and scalable patches for applications in ocular drug delivery.
3.2.3. Collagen corneal shields
An alternative to TCLs is the use of collagen shields to achieve sustained release and enhanced bioavailability of ocular drugs to the eye. Collagen shields are soluble discs - manufactured from porcine scleral tissue or bovine corium (dermis) collagen, and have been used to promote corneal epithelial healing (KUWANO et al., 1997). Collagen shields are used for drug delivery by presoaking in a pharmacological agent with adjunctive topical treatment (Willoughby et al., 2002). Collagen corneal shields can deliver drugs to the eye up to several days while avoiding the need for continuous medication (Ali and Byrne, 2008) (Vasantha et al., 1988). The disadvantages of these collagen shields are that they have to be inserted by a health care provider and that they are not fully transparent and thus compromise vision (Vasantha et al., 1988). Bucolo et al. showed the in vitro and in vivo study of Hyalobend®, hyaluronic acid derivative corneal shields, impregnated with methyl prednisone for the treatment of inflammation (BUCOLOet al., 1996). The results demonstrated that collagen shields are able to maintain effective levels of methyl prednisone in the rabbit aqueous humor for up to 48 h.
3.2.4. Transcorneal iontophoresis
Transcorneal iontophoresis is a noninvasive method that enables targeted topical therapy of medication to the cornea. Iontophoresis delivers small molecules such as dexamethasone and macromolecules such as albumin, and bevacizumab to the cornea or sclera through an electric field (Molokhia et al., 2013) (Eljarrat-Binstock, 2005) (Gratieri et al., 2017). However, topical application of corneal iontophoresis is rarely used today in the development of novel drugs. In addition to medications and macromolecules, iontophoresis can be used to deliver nucleic acids for gene therapy (Myles et al., 2005). The mechanism of delivery by iontophoresis is by opening the tight junctions without significantly affecting the barrier properties of the eye (Ali and Byrne, 2008).
Iontophoresis can enhance permeability and transport across cornea by electrophoresis (direct electric field interaction), electro-osmosis (convective solvent flow, i.e., directional liquid flow due to applied potential across a charged membrane), and electroporation (field-induced tissue alteration and pore induction) (Bejjani, 2007). Ideally, for transcorneal iontophoresis, the drug is dissolved in water and is instilled into a cylindrical eye cup placed over the limbs to cover the entire cornea surface (Callegan, 1995). Transcorneal iontophoresis may be amenable to topical ocular drug delivery of hydrophilic drugs across the cornea. For example, a related technology using an ocular applicator with dexamethasone phosphate has been studied in a cornea graft rejection clinical trial (Kompella et al., 2010). The safety and efficacy of enhanced-fluence pulsed-light iontophoresis cross-linking (EF-CXL) is also recently used in patients with progressive keratoconus during epithelium-on corneal crosslinking with riboflavin (Mazzotta, 2020) (Liao, 2019). The results showed that iontophoretic transepithelial corneal cross-linking (I-ON CXL) slows down keratoconus progression in 50% of pediatric patients’ eyes in 3 years after treatment (Buzzonetti, 2019). However, the main limitations for transcorneal iontophoresis include the inconvenience of having to setup the device, potential discomfort to patients, and inadequate sustained delivery. The safety of prolonged and frequent application of iontophoretic drug delivery have yet to be established. Therefore, the long-term goal of transcorneal iontophoresis is to make this approach more user-friendly with disposable component in a system designed for home use without professional training.
3.2.5. Vacuum-assisted drug delivery
Vacuum-assisted transepithelial corneal cross-linking has been developed to facilitate topical drug delivery through the intact corneal epithelium. A vacuum pump that induces negative pressure has been used to create a sealed reservoir over the cornea and accelerate diffusion without significant change or adverse effects in intraocular pressure (IOP). Lombardo, Abbate, and Manche reported the successful transepithelial riboflavin deposition with the vacuum-mediated device in 2017. Since then, vacuum-assisted drug delivery has been developed for transepithelial riboflavin deposition at concentration levels comparable to the control tissues with epithelium removed (Lombardo, 2017) (https://clinicaltrials.gov, 2018). In addition to existing ultrasound or iontophoresis devices that are used for topical ocular drug delivery, vacuum-assisted drug delivery systems are under development for drug delivery to the cornea.
UV irradiation in the range of 12 mW/cm2, applied in approximately less than 10 min combined with vacuum-assisted riboflavin delivery, is likely to be safe and desirable due to minimal discomfort, faster vision recovery and shorter the period of treatment without compromising the efficacy of the procedure. In addition to being safe, medications using vacuum-assisted drug delivery can be administered painlessly and noninvasively into the eye through an intact corneal epithelium.
3.2.6. Intracorneal microneedles
Microneedle technology is comprised of a plurality of micrometer-scale needles embedded in a macroscopic substrate (Brown et al., 2019) (Prausnitz et al., 2004). Solid microneedles (MNs) are a minimally invasive route for localized ocular drug delivery and a promising technology developed to deliver various therapeutic compounds (e.g. vaccination, local anesthesia, treatment of diabetes and obesity) into the skin (Than, 2018) (Lee et al., 2008). This technology has the potential to provide the unmet medical need for localized, long-lasting and efficient topical ocular therapy with good patient compliance. Microneedle-based treatment of a corneal ulcer is of special interest, in part by targeting delivery of the antimicrobial drug precisely to the corneal depth where microorganism invasion has occurred (Ansari et al., 2013). Microneedles can deliver topical ocular therapy to the eye with direct injection of therapeutics into cornea with minimal perception of pain (Bhatnagar, 2018). The high turnover rate of mucus in tear film and epithelial lining in the cornea enables the rapid restoration of disrupted epithelial cells by the microneedles.
Potential intracorneal microneedles have been developed using metals or polymers to deliver drugs into the eye while avoiding complications associated with intraocular injection and systemic administration (Thakur Singh, 2017). The first studies have been initiated for in vivo drug release of fluorescein and pilocarpine surface-coated microneedles via intracorneal routes (Jiang, 2007). The results showed that the fluorescein-coated microneedles increase fluorescein concentrations 60 times higher than topical application without microneedles with no inflammatory responses, and thereby improving the bioavailability of fluorescein. Moreover, biphasic drug release kinetics of detachable microneedles provide synergistic therapeutic outcome through a quick release of diclofenac followed by a sustained release of an anti-angiogenic monoclonal antibody, resulted in improved treatment of corneal neovascularization (NV) (Than, 2018). Therefore, the use of microneedles for intraocular drug delivery is a new approach aimed at providing effective home-based treatment for many eye diseases that ensures an optimal patient compliance.
4. Conclusion and future plans
Topical eye drops have traditionally been the main treatment modality for many ocular diseases. Since the cornea and conjunctival barriers are poorly soluble to medications, the application of ophthalmic drugs requires frequent doses in order to overcome the short half-life and poor drug dolubility. This may result in potential drug side effects, and poor drug solubility. Over the last decade, new formulations and technologies have advanced rapidly toward innovative long-acting topical ocular drug delivery systems. Despite the advances in ophthalmic drug delivery, there remain challenges with regards to dosing administration, diffusion and retention of the drugs. New designs are needed to achieve a long-acting topical ocular drug delivery method. Recent developments have moved toward the use of nanoparticles and gene therapy where drug concentration and release period could be controlled more effectively. However, overcoming the barrier of corneal permeability coninues to be challenging.
The development of long-acting topical ocular drug delivery systems in lieu of eyedrops is essential to the efficiency of the ocular therapy. The goal is to increase the drug’s ocular residence time through innovative solutions that overcome the corneal static and dynamic barriers. The two methods for drug delivery are either through the cornea or directly conjunctivaand sclera. Different size materials can deliver a drug by either of these ocular routes. Extensive research is conducted on nanocarrier materials including viscosity and permeation enhancers. In addtion, colloidal nanocarriers such as MPPs and nanomicelles can facilitate the transfer of hydrophobic drugs into the eye. Alternatively prodrugs can enhance the drug solubility without the need for a carrier.
On the macroscopic scale, biomedical devices or materials can be embedded with the drug and then applied to the eye for direct delivery. TCLs allow for the controlled release of the drug without the need for reapplication as opposed to eye drops. To eliminate the hassle of removing the contact lens, dissolvable collagen shields provide a more effortless alternative. Intracorneal microneedles carrying the drug allow for a minimally invasive but longer lasting localized ocular therapy.
Despite extensive innovation in the novel ocular topical therapy field, the vast majority of these ideas are still in the development stage and more research is required in order to be commercially acceptable. Furthermore, the most common method to delivering drugs into the eye remains topical eye drops despite the many of its drawbacks. Developing techniques involving both the through and to ocular routes would allow different hydrophobicities, sizes, and formulations of drugs to reach the cornea, anterior chamber, and perhaps even the retina. Advances in nanoscale and macroscale therapies permit variations in therapies depending on patient preference. Some patients may prefer to undergo a one time minimally invasive microneedle application while others may choose to apply eye drops multiple times a day. Just as important, the cost effectiveness of each therapy must be taken into consideration. In addition, combinations of nanoscale and macroscale therapy, such as viscosity and permeation enhancers in addition to TCLs or collagen shields might be most effective in minimizing drug pentration to the eye.
The aforementioned ocular drug delivery systems highlight the potential for drug formulations embedded in ophthalmic devices that provide increased delivery of the drug for longer-lasting ocular therapy, while overcoming the eye’s various barriers. It thereby decreases the need for frequent doses in order to maintain therapeutic drug level, resulting in more effective and convenient ocular therapy.
Acknowledgments:
C. N. T. would like to acknowledge funding from the National Eye Institute (P30-EY026877) and a grant from Research to Prevent Blindness. E. M. would like to acknowledge the support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035). Authors gratefully acknowledge support was provided by Stanford University and University of Southern California.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Addo RT (Ed.), 2016. Ocular Drug Delivery: Advances, Challenges and Applications. Springer International Publishing, Cham. [Google Scholar]
- Agarwal R, Iezhitsa I, Agarwal P, Abdul Nasir NA, Razali N, Alyautdin R, Ismail NM, 2016. Liposomes in topical ophthalmic drug delivery: an update. Drug Delivery 23 (4), 1075–1091. [DOI] [PubMed] [Google Scholar]
- Agrawal RupeshV, Murthy S, Sangwan V, Biswas J, 2010. Current approach in diagnosis and management of anterior uveitis. Indian J. Ophthalmol 58 (1), 11. 10.4103/0301-4738.58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahadian S, Finbloom JA, Mofidfar M, Diltemiz SE, Nasrollahi F, Davoodi E, Hosseini V, Mylonaki I, Sangabathuni S, Montazerian H, Fetah K, Nasiri R, Dokmeci MR, Stevens MM, Desai TA, Khademhosseini A, 2020. Micro and nanoscale technologies in oral drug delivery. Adv. Drug Deliv. Rev 157, 37–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I, Gokhale RD, Shah MV, Patton TF, 1987. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. J. Pharm. Sci 76 (8), 583–586. [DOI] [PubMed] [Google Scholar]
- Ali M, Byrne ME, 2008. Challenges and solutions in topical ocular drug-delivery systems. Expert review of clinical pharmacology 1 (1), 145–161. [DOI] [PubMed] [Google Scholar]
- Ali M, Horikawa S, Venkatesh S, Saha J, Hong JW, Byrne ME, 2007. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J. Control. Release 124 (3), 154–162. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C, Anguiano-Igea S, Varela-García A, Vivero-Lopez M, Concheiro A, 2019. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 84, 49–62. [DOI] [PubMed] [Google Scholar]
- Ansari Z, Miller D, Galor A, 2013. Current thoughts in fungal keratitis: diagnosis and treatment. Current fungal infection reports 7 (3), 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argüeso P, Gipson IK, 2001. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp. Eye Res 73 (3), 281–289. [DOI] [PubMed] [Google Scholar]
- Austin A, Lietman T, Rose-Nussbaumer J, 2017. Update on the management of infectious keratitis. Ophthalmology 124 (11), 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Tanaka Y, Kubota A, Kasai H, Yokokura S, Nakanishi H, Nishida K, 2011. A method for enhancing the ocular penetration of eye drops using nanoparticles of hydrolyzable dye. J. Control. Release 153 (3), 278–287. [DOI] [PubMed] [Google Scholar]
- Bejjani RA, et al. , 2007. Electrically assisted ocular gene therapy. Surv. Ophthalmol 52 (2), 196–208. [DOI] [PubMed] [Google Scholar]
- Bennett JE, Dolin R, and Blaser MJ, Mandell, douglas, and bennett’s principles and practice of infectious diseases: 2-volume set. Vol. 2. 2014: Elsevier Health Sciences. [Google Scholar]
- Bhatnagar S, et al. , 2018. Corneal delivery of besifloxacin using rapidly dissolving polymeric microneedles. Drug delivery and translational research 8 (3), 473–483. [DOI] [PubMed] [Google Scholar]
- Boland MV, Ervin AM, Friedman DS, Jampel HD, Hawkins BS, Vollenweider D, Chelladurai Y, Ward D, Suarez-Cuervo C, Robinson KA, 2013. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the US Preventive Services Task Force. Ann. Intern. Med 158 (4), 271. 10.7326/0003-4819-158-4-201302190-00008. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW, 2004. Functional aspects of the tear film lipid layer. Exp. Eye Res 78 (3), 347–360. [DOI] [PubMed] [Google Scholar]
- Brown L, et al. , 2020. The global incidence and diagnosis of fungal keratitis. Lancet. Infect. Dis [DOI] [PubMed] [Google Scholar]
- Brown TD, Whitehead KA, Mitragotri S, 2019. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater 1–22. [Google Scholar]
- Bruun K, Hille C, 2019. Study on intracellular delivery of liposome encapsulated quantum dots using advanced fluorescence microscopy. Sci. Rep 9 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Mangiafico S, Spadaro A, 1996. Methylprednisolone delivery by Hyalobend® corneal shields and its effects on rabbit ocular inflammation. Journal of ocular pharmacology and therapeutics 12 (2), 141–149. [DOI] [PubMed] [Google Scholar]
- Buzzonetti L, et al. , 2019. Iontophoretic transepithelial collagen cross-linking versus epithelium-off collagen cross-linking in pediatric patients: 3-year follow-up. Cornea 38 (7), 859–863. [DOI] [PubMed] [Google Scholar]
- Callegan MC, et al. , 1995. Ocular drug delivery: a comparison of transcorneal iontophoresis to corneal collagen shields. Int. J. Pharm 123 (2), 173–179. [Google Scholar]
- Calonge M, 2001. The treatment of dry eye. Surv. Ophthalmol 45, S227–S239. [DOI] [PubMed] [Google Scholar]
- Caminade A, Laurent R, Majoral J, 2005. Characterization of dendrimers. Adv. Drag Deliv. Rev 57 (15), 2130–2146. [DOI] [PubMed] [Google Scholar]
- Carvalho IM, Marques CS, Oliveira RS, Coelho PB, Costa PC, Ferreira DC, 2015. Sustained drag release by contact lenses for glaucoma treatment—a review. J. Control. Release 202, 76–82. [DOI] [PubMed] [Google Scholar]
- Cheetham AG, Chakroun RW, Ma W, Cui H, 2017. Self-assembling prodrags. Chem. Soc. Rev 46 (21), 6638–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C, YANG J, 2006. MI-QSAR models for prediction of corneal permeability of organic compounds1. Acta Pharmacol. Sin 27 (2), 193–204. [DOI] [PubMed] [Google Scholar]
- ChenZhuo L, et al. , Different concentrations of amino acids in tears of normal and human dry eyes, in Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3. 2002, Springer, p. 617–621. [DOI] [PubMed] [Google Scholar]
- Choi SW, Kim J, 2018. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: a review. Materials 11 (7), 1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholkar K, Patel A, Dutt Vadlapudi A, K. Mitra A, 2012. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drag delivery. Recent patents on nanomedicine 2 (2), 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino JB, Hudson SP, Mobbs AN, Hoare TR, Iwata NG, Fink GR, Kohane DS, 2011. A prototype antifungal contact lens. Invest. Ophthalmol. Vis. Sci 52 (9), 6286. 10.1167/iovs.10-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AZ, Patel DV, McGhee CN, 2013. A brief history of corneal transplantation: From ancient to modern. Oman journal of ophthalmology 6 (Suppl 1), S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiklik L, 2016. Tear film lipid layer: A molecular level view. Biochimica et Biophysica Acta (BBA)-Biomembranes 1858 (10), 2421–2430. [DOI] [PubMed] [Google Scholar]
- De Moraes CG, Liebmann JM, Levin LA, 2017. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Progress in retinal and eye research 56, 107–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Anand BS, Patel J, Mitra AK, 2003. Transporters/receptors in the anterior chamber: pathways to explore ocular drug delivery strategies. Expert Opin. Biol. Ther 3 (1), 23–44. [DOI] [PubMed] [Google Scholar]
- Di Tommaso C, et al. , 2012. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur. J. Pharm. Biopharm 81 (2), 257–264. [DOI] [PubMed] [Google Scholar]
- Doane MG, Jensen AD, Dohlman CH, 1978. Penetration routes of topically applied eye medications. Am. J. Ophthalmol 85 (3), 383–386. [DOI] [PubMed] [Google Scholar]
- Downie LE, Bandlitz S, Bergmanson JPG, Craig JP, Dutta D, Maldonado-Codina C, Ngo W, Siddireddy JS, Wolffsohn JS, 2021. CLEAR-anatomy and physiology of the anterior eye. Contact Lens and Anterior Eye 44 (2), 132–156. [DOI] [PubMed] [Google Scholar]
- Edwards A, Prausnitz MR, 2001. Predicted permeability of the cornea to topical drugs. Pharm. Res 18 (11), 1497–1508. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, et al. , 2005. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drag loaded hydrogel. J. Control. Release 106 (3), 386–390. [DOI] [PubMed] [Google Scholar]
- Fannon M, Forsten-Williams K, Zhao B, Bach E, Parekh PP, Chu CL, Goerges-Wildt AL, Buczek-Thomas JA, Nugent MA, 2012. Facilitated diffusion of VEGF165 through descemet’s membrane with sucrose octasulfate. J. Cell. Physiol 227 (11), 3693–3700. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E, 2016. In: The Eye. Elsevier, pp. 1–102.e2. 10.1016/B978-0-7020-5554-6.00001-0. [DOI] [Google Scholar]
- Forrester JV, et al. , The eye e-book: basic sciences in practice. 2020: Elsevier Health Sciences. [Google Scholar]
- Friedberg ML, Pleyer U, Mondino BJ, 1991. Device Drug Delivery to the Eye: Collagen Shield’s, Iontophoresis, and Pumps. Ophthalmology 98 (5), 725–732. [DOI] [PubMed] [Google Scholar]
- Fu XC, Liang WQ, 2002. A simple model for the prediction of corneal permeability. Int. J. Pharm 232 (1-2), 193–197. [DOI] [PubMed] [Google Scholar]
- Gan L.i., Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, Liu J, Gan Y, 2013. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drag Discovery Today 18 (5-6), 290–297. [DOI] [PubMed] [Google Scholar]
- Gaudana R, Ananthula HK, Parenky A, Mitra AK, 2010. Ocular drug delivery. The AAPS journal 12 (3), 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghate D, Edelhauser HF, 2008. Barriers to glaucoma drug delivery. J. Glaucoma 17 (2), 147–156. [DOI] [PubMed] [Google Scholar]
- Gote V, Sikder S, Sicotte J, Pal D, 2019. Ocular drag delivery: present innovations and future challenges. J. Pharmacol. Exp. Ther 370 (3), 602–624. [DOI] [PubMed] [Google Scholar]
- Gratieri T, Santer V, Kalia YN, 2017. Basic principles and current status of transcorneal and transscleral iontophoresis. Expert opinion on drag delivery 14 (9), 1091–1102. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, 2015. Ophthalmic pathology: history, accomplishments, challenges, and goals. Ophthalmology 122 (8), 1539–1542. [DOI] [PubMed] [Google Scholar]
- Gulsen D, Li C-C, Chauhan A, 2005. Dispersion of DMPC liposomes in contact lenses for ophthalmic drag delivery. Curr. Eye Res 30 (12), 1071–1080. [DOI] [PubMed] [Google Scholar]
- Guo C, Cui F, Li M, Li F, Wu X, 2015. Enhanced corneal permeation of coumarin-6 using nanoliposomes containing dipotassium glycyrrhizinate: in vitro mechanism and in vivo permeation evaluation. RSC Adv. 5 (92), 75636–75647. [Google Scholar]
- Gupta H, Aqil M, 2012. Contact lenses in ocular therapeutics. Drug Discovery Today 17 (9–10), 522–527. [DOI] [PubMed] [Google Scholar]
- Guzman-Aranguez A, Colligris B, Pintor J, 2013. Contact lenses: promising devices for ocular drug delivery. J. Ocul. Pharmacol. Ther 29 (2), 189–199. [DOI] [PubMed] [Google Scholar]
- Harthan JS, et al. , 2016. Diagnosis and treatment of anterior uveitis: optometric management. Clinical optometry 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde RR, Verma A, Ghosh A, 2013. Microemulsion: new insights into the ocular drug delivery. ISRN pharmaceutics 2013, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennis A, Wu S-Y, Nemesure B, Honkanen R, Leske MC, 2007. Awareness of incident open-angle glaucoma in a population study: the Barbados Eye Studies. Ophthalmology 114 (10), 1816–1821. [DOI] [PubMed] [Google Scholar]
- Hiratani H, Alvarez-Lorenzo C, 2002. Timolol uptake and release by imprinted soft contact lenses made of N, N-diethylacrylamide and methacrylic acid. J. Control. Release 83 (2), 223–230. [DOI] [PubMed] [Google Scholar]
- Holland EJ, Mannis MJ, and Lee WB, Ocular surface disease: cornea, conjunctiva and tear film: expert consult-online and print. 2013: Elsevier Health Sciences. [Google Scholar]
- Holowka EP, Bhatia SK, 2014. In: Drug Delivery. Springer New York, New York, NY, pp. 63–116. 10.1007/978-1-4939-1998-7_3. [DOI] [Google Scholar]
- Hou J, The paracellular channel: biology, physiology, and disease. 2018: Academic Press. [Google Scholar]
- Hoyng PFJ, van Beek LM, 2000. Pharmacological therapy for glaucoma. Drags 59 (3), 411–434. [DOI] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/show/NCT03442751?term=epithelium+on+corneal+cross+linking&draw=2&rank=2.
- Huang D.i., Chen Y-S, Rupenthal ID, 2018. Overcoming ocular drug deli very barriers through the use of physical forces. Adv. Drug Deliv. Rev 126, 96–112. [DOI] [PubMed] [Google Scholar]
- Huang H-S, Schoenwald RD, 1983. Corneal penetration behavior of β-blocking agents I: Physicochemical factors. J. Pharm. Sci 72 (11), 1266–1272. [DOI] [PubMed] [Google Scholar]
- Hughes Patrick M., Mitra Ashim.K., 1993. Effect of acylation on the ocular disposition of acyclovir II: Corneal permeability and anti-HSV 1 activity of 2′-esters in rabbit epithelial keratitis. J. Ocul. Pharmacol. Ther 9 (4), 299–309. [DOI] [PubMed] [Google Scholar]
- Hughes P, Olejnik O, Changlin J, Wilson C, 2005. Topical and systemic drag delivery to the posterior segments. Adv. Drag Deliv. Rev 57 (14), 2010–2032. [DOI] [PubMed] [Google Scholar]
- Hussain A, Traelove JE, 1976. Prodrag approaches to enhancement of physicochemical properties of drags IV: novel epinephrine prodrag. J. Pharm. Sci 65 (10), 1510–1512. [DOI] [PubMed] [Google Scholar]
- Irimia T, Ghica M, Popa L, Anuťa V, Arsene A-L, Dinu-Pîrvu C-E, 2018. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polymers 10 (11), 1221. 10.3390/polym10111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, Kumar M, Pathak K, 2015. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf., B 130, 23–30. [DOI] [PubMed] [Google Scholar]
- Janoria KG, et al. , 2007. Recent patents and advances in ophthalmic drug delivery. Recent Pat. Drag Delivery Formulation 1 (2), 161–170. [DOI] [PubMed] [Google Scholar]
- Jiang J, et al. , 2007. Coated microneedles for drug delivery to the eye. Invest. Ophthalmol. Vis. Sci 48 (9), 4038–4043. [DOI] [PubMed] [Google Scholar]
- Jones M-C, Leroux J-C, 1999. Polymeric micelles–a new generation of colloidal drag carriers. Eur. J. Pharm. Biopharm 48 (2), 101–111. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Abou-Jaoude M, Carbia BE, Plummer C, Chauhan A, 2013. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 165 (1), 82–89. [DOI] [PubMed] [Google Scholar]
- Kambhampati SP, Kannan RM, 2013. Dendrimer nanoparticles for ocular drug delivery. J. Ocul. Pharmacol. Ther 29 (2), 151–165. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Boyd BJ, Porter CJH, 2011. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine 6 (6), 1063–1084. [DOI] [PubMed] [Google Scholar]
- Kang-Mieler JJ, Osswald CR, Mieler WF, 2014. Advances in ocular drag delivery: emphasis on the posterior segment. Expert opinion on drag delivery 11 (10), 1647–1660. [DOI] [PubMed] [Google Scholar]
- Kaur IP, Smitha R, 2002. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drag delivery. Drag Dev. Ind. Pharm 28 (4), 353–369. [DOI] [PubMed] [Google Scholar]
- Ke T-L, et al. , 2000. Nepafenac, a unique nonsteroidal prodrag with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 24 (4), 371–384. [DOI] [PubMed] [Google Scholar]
- Kenawy E-R, et al. , Processing of polymer nanofibers through electrospinning as drag delivery systems, in Nano materials: Risks and Benefits. 2009, Springer, p. 247–263. [Google Scholar]
- Kitto T, et al. , Processing and patterning of conducting polymers for flexible, stretchable, and biomedical electronics, in Handbook of Organic Materials for Electronic and Photonic Devices. 2019, Elsevier, p. 817–842. [Google Scholar]
- Kompella UB, Kadam RS, Lee VH, 2010. Recent advances in ophthalmic drag delivery. Therapeutic delivery 1 (3), 435–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn T, Rohrmann S, Karavasiloglou N, Friedman DS, Cassidy A, Bärnighausen T, Schuster AK, Nickels S, 2021. Glaucoma and mortality risk: findings from a prospective population-based study. Sci. Rep 11 (1) 10.1038/S41598-021-91194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M, Horibe Y, Kawashima Y, 1997. Effect of collagen cross-linking in collagen corneal shields on ocular drug delivery. Journal of ocular pharmacology and therapeutics 13 (1), 31–40. [DOI] [PubMed] [Google Scholar]
- Lallemand F, Perottet P, Felt-Baeyens O, Kloeti W, Philippoz F, Marfurt J, Besseghir K, Gurny R, 2005. A water-soluble prodrug of cyclosporine A for ocular application: a stability study. Eur. J. Pharm. Sci 26 (1), 124–129. [DOI] [PubMed] [Google Scholar]
- Lancina MG III, et al. , 2017. Fast dissolving dendrimer nanofiber mats as alternative to eye drops for more efficient antiglaucoma drug delivery. ACS Biomater. Sci. Eng 3 (8), 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Chen H, 2014. Breakthrough Nanotechnology Enhances Delivery to the Front and Back of the Eye. ONdrugDelivery Magazine 48, 16–18. [Google Scholar]
- Lavik E, Kuehn MH, Kwon YH, 2011. Novel drug delivery systems for glaucoma. Eye 25 (5), 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VHL, 1990. Mechanisms and facilitation of corneal drug penetration. J. Control. Release 11 (1-3), 79–90. [Google Scholar]
- Lee JW, Park J-H, Prausnitz MR, 2008. Dissolving microneedles for transdermal drug delivery. Biomaterials 29 (13), 2113–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Vincent.H.L., Robinson Joseph.R., 1986. Topical ocular drug delivery: recent developments and future challenges. J. Ocul. Pharmacol. Ther 2 (1), 67–108. [DOI] [PubMed] [Google Scholar]
- Lee VHL, Urrea PT, Smith RE, Schanzlin DJ, 1985. Ocular drug bioavailability from topically applied liposomes. Surv. Ophthalmol 29 (5), 335–348. [DOI] [PubMed] [Google Scholar]
- Liao K, et al. , 2019. Clinical and microstructural changes with different iontophoresis-assisted corneal cross-linking methods for keratoconus. International journal of ophthalmology 12 (2), 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom R, Kim T, 2006. Ocular permeation and inhibition of retinal inflammation: an examination of data and expert opinion on the clinical utility of nepafenac. Curr. Med. Res. Opin 22 (2), 397–404. [DOI] [PubMed] [Google Scholar]
- Loftsson T and Stefánsson E, Cyclodextrins in eye drop formulations: enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmologica Scandinavica, 2002. 80 (2): p. 144–150. [DOI] [PubMed] [Google Scholar]
- Loftssona T, Järvinen T, 1999. Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev 36 (1), 59–79. [DOI] [PubMed] [Google Scholar]
- Lombardo G, et al. , 2017. Real time, non invasive, assessment of transepithelial delivery of riboflavin in the human cornea. Invest. Ophthalmol. Vis. Sci 58 (8), 3518. [Google Scholar]
- Lu L, Reinach PS, Kao W-Y, 2001. Corneal epithelial wound healing. Exp. Biol. Med 226 (7), 653–664. [DOI] [PubMed] [Google Scholar]
- Macha S, Duwuri S, Mitra AK, 2004. Ocular disposition of novel lipophilic diester prodrugs of ganciclovir following intravitreal administration using microdialysis. Curr. Eye Res 28 (2), 77–84. [DOI] [PubMed] [Google Scholar]
- Mandal A, Bisht R, Rupenthal ID, Mitra AK, 2017. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J. Control. Release 248, 96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Mauris J, and Argüeso P, The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Current opinion in allergy and clinical immunology, 2013. 13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LL and Roach JM, Treatment of dry eye disease. The Consultant Pharmacist®, 2016. 31(2): p. 96–106. [DOI] [PubMed] [Google Scholar]
- Martin TM, Smith JR, Rosenbaum JT, 2002. Anterior uveitis: current concepts of pathogenesis and interactions with the spondyloarthropathies. Curr. Opin. Rheumatol 14 (4), 337–341. [DOI] [PubMed] [Google Scholar]
- Maulvi FA, Soni TG, Shah DO, 2016. A review on therapeutic contact lenses for ocular drug delivery. Drug Delivery 23 (8), 3017–3026. [DOI] [PubMed] [Google Scholar]
- Maumenee AE, 1993. The history of vitamin A and its ophthalmic implications: a personal viewpoint. Arch. Ophthalmol 111 (4), 547–550. [DOI] [PubMed] [Google Scholar]
- Mazzotta C, et al. , 2020. Iontophoresis corneal cross-linking with enhanced fluence and pulsed UV-A light: 3-year clinical results. J. Refract. Surg 36 (5), 286–292. [DOI] [PubMed] [Google Scholar]
- Mcdevitt D, Cell biology of the eye. 2012: Elsevier. [Google Scholar]
- Meisner D, Mezei M, 1995. Liposome ocular delivery systems. Adv. Drug Deliv. Rev 16 (1), 75–93. [Google Scholar]
- Michels RG, Maumenee AE, 1975. Cystoid macular edema associated with topically applied epinephrine in aphakic eyes. Am. J. Ophthalmol 80 (3), 379–388. [DOI] [PubMed] [Google Scholar]
- Migdal C, 2000. Glaucoma medical treatment: philosophy, principles and practice. Eye 14 (3), 515–518. [DOI] [PubMed] [Google Scholar]
- Mishima S, et al. , 1966. Determination of tear volume and tear flow. Invest. Ophthalmol. Vis. Sci 5 (3), 264–276. [PubMed] [Google Scholar]
- Mishra GP, Bagui M, Tamboli V, Mitra AK, 2011. Recent applications of liposomes in ophthalmic drug delivery. Journal of drug delivery 2011, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt SL, Cartwright VA, Stumpf TH, 2005. Centennial review of corneal transplantation. Clinical & experimental ophthalmology 33 (6), 642–657. [DOI] [PubMed] [Google Scholar]
- Mofidfar M, Wang J, Long L, Hager CL, Vareechon C, Pearlman E, Baer E, Ghannoum M, Wnek GE, 2017. Polymeric nanofiber/antifungal formulations using a novel co-extrusion approach. AAPS PharmSciTech 18 (6), 1917–1924. [DOI] [PubMed] [Google Scholar]
- Mofidfar M, O’Farrell L, Prausnitz MR, 2019. Pharmaceutical jewelry: Earring patch for transdermal delivery of contraceptive hormone. J. Control. Release 301, 140–145. [DOI] [PubMed] [Google Scholar]
- Mofidfar M and Prausnitz MR, 2019. Electrospun transdermal patch for contraceptive hormone delivery. Current Drug Delivery 16 (6), 577 - 583. [DOI] [PubMed] [Google Scholar]
- Moiseev Morrison, Steele Khutoryanskiy, 2019. Penetration enhancers in ocular drug delivery. Pharmaceutics 11 (7), 321. 10.3390/pharmaceutics11070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokhia SA, Thomas SC, Garff KJ, Mandell KJ, Wirostko BM, 2013. Anterior eye segment drug delivery systems: current treatments and future challenges. J. Ocul. Pharmacol. Ther 29 (2), 92–105. [DOI] [PubMed] [Google Scholar]
- Morrison PWJ, Connon CJ, Khutoryanskiy VV, 2013. Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol. Pharm 10 (2), 756–762. [DOI] [PubMed] [Google Scholar]
- Morrison PWJ, Khutoryanskiy VV, 2014. Advances in ophthalmic drug delivery. Therapeutic delivery 5 (12), 1297–1315. [DOI] [PubMed] [Google Scholar]
- Myles ME, Neumann DM, Hill JM, 2005. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv. Drug Deliv. Rev 57 (14), 2063–2079. [DOI] [PubMed] [Google Scholar]
- Neti N, Pimsri A, Boonsopon S, Tesavibul N, Choopong P, 2021. Triggering factors associated with a new episode of recurrent acute anterior uveitis. Sci. Rep 11 (1) 10.1038/s41598-021-91701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C, Gebhardt BM, Kaufman HE, 1988. Topically applied cyclosporine in azone prolongs corneal allograft survival. Invest. Ophthalmol. Vis. Sci 29 (2), 208–215. [PubMed] [Google Scholar]
- Nisini R, et al. , 2018. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol 9, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Dogru M, Tsubota K, 2006. Laboratory findings in tear fluid analysis. Clin. Chim. Acta 369 (1), 17–28. [DOI] [PubMed] [Google Scholar]
- Patel A, et al. , 2013. Ocular drug delivery systems: An overview. World journal of pharmacology 2 (2), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni BS, Chupin VV, Torchilin VP, 2015. New developments in liposomal drug delivery. Chem. Rev 115 (19), 10938–10966. [DOI] [PubMed] [Google Scholar]
- Patton TF, Robinson JR, 1976. Quantitative precorneal disposition of topically applied pilocarpine nitrate in rabbit eyes. J. Pharm. Sci 65 (9), 1295–1301. [DOI] [PubMed] [Google Scholar]
- Peng C-C, Burke MT, Chauhan A, 2012. Transport of topical anesthetics in vitamin E loaded silicone hydrogel contact lenses. Langmuir 28 (2), 1478–1487. [DOI] [PubMed] [Google Scholar]
- Phan C-M, Subbaraman LN, Jones L, 2013. In vitro uptake and release of natamycin from conventional and silicone hydrogel contact lens materials. Eye & contact lens 39 (2), 162–168. [DOI] [PubMed] [Google Scholar]
- Phillips AJ and Speedwell L, Contact lenses e-book. 2018: Elsevier Health Sciences. [Google Scholar]
- Prausnitz MR, Mitragotri S, Langer R, 2004. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discovery 3 (2), 115–124. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Noonan JS, 1998. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J. Pharm. Sci 87 (12), 1479–1488. [DOI] [PubMed] [Google Scholar]
- Quigley HA, 2019. 21st century glaucoma care. Eye 33 (2), 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radacsi N, Campos FD, Chisholm CRI, Giapis KP, 2018. Spontaneous formation of nanoparticles on electrospun nanofibres. Nat. Commun 9 (1) 10.1038/S41467-018-07243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RA, Doane MG, Dohlman CH, 1975. Clinical experience with a mobile ocular perfusion pump. Arch. Ophthalmol 93 (10), 1039–1043. [DOI] [PubMed] [Google Scholar]
- Ramsay E, del Amo EM, Toropainen E, Tengvall-Unadike U, Ranta V-P, Urtti A, Ruponen M, 2018. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci 119, 83–89. [DOI] [PubMed] [Google Scholar]
- Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, Savolainen J, 2008. Prodrugs: design and clinical applications. Nat. Rev. Drug Discovery 7 (3), 255–270. [DOI] [PubMed] [Google Scholar]
- Ren Y, Mei L, Zhou L, Guo G, 2019. Recent perspectives in hot melt extrusion-based polymeric formulations for drug delivery: Applications and innovations. Aaps Pharmscitech 20 (3). 10.1208/s12249-019-1300-8. [DOI] [PubMed] [Google Scholar]
- Rogers GM, et al. , Outcomes of treatment of fungal keratitis at the University of Iowa Hospitals and Clinics: a 10-year retrospective analysis. Cornea, 2013. 32(8): p. 1131–1136. [DOI] [PubMed] [Google Scholar]
- Rolando M, Zierhut M, 2001. The ocular surface and tear film and their dysfunction in dry eye disease. Surv. Ophthalmol 45, S203–S210. [DOI] [PubMed] [Google Scholar]
- Rotchford AP, Kirwan JF, Muller MA, Johnson GJ, Roux P, 2003. Temba glaucoma study: a population-based cross-sectional survey in urban South Africa. Ophthalmology 110 (2), 376–382. [DOI] [PubMed] [Google Scholar]
- Sariri R, Ghafoori H, 2008. Tear proteins in health, disease, and contact lens wear. Biochemistry (Moscow) 73 (4), 381–392. [DOI] [PubMed] [Google Scholar]
- Schaal S and Kaplan H, Ocular Inflammation in Anterior Segment, in Ocular Therapeutics. 2008, Elsevier, p. 275–300. [Google Scholar]
- Schaeffer HE, Krohn DL, 1982. Liposomes in topical drug delivery. Invest. Ophthalmol. Vis. Sci 22 (2), 220–227. [PubMed] [Google Scholar]
- Selver OB, et al. , 2015. Therapeutic corneal transplant for fungal keratitis refractory to medical therapy. Exp Clin Transplant 13, 355–359. [DOI] [PubMed] [Google Scholar]
- Shah A, Brugnano J, Sun S, Vase A, Orwin E, 2008. The development of a tissue-engineered cornea: biomaterials and culture methods. Pediatr. Res 63 (5), 535–544. [DOI] [PubMed] [Google Scholar]
- Shahriar S, Mondal J, Hasan M, Revuri V, Lee D, Lee Y-K, 2019. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 9 (4), 532. 10.3390/nano9040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Singh H, Joshi M, Sharma A, Garg T, Goyal AK, Rath G, 2014. Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst 31 (3), 187–217. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, 2018. Definition and diagnostic criteria of dry eye disease: historical overview and future directions. Invest. Ophthalmol. Vis. Sci 59 (14), DES7. 10.1167/iovs.17-23475. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y, 2008. Molecular design for enhancement of ocular penetration. J. Pharm. Sci 97 (7), 2462–2496. [DOI] [PubMed] [Google Scholar]
- Sitharaman B 2016. Nanobiomaterials handbook. CRC Press. [Google Scholar]
- Soh YQ, Kocaba V, Weiss JS, Jurkunas UV, Kinoshita S, Aldave AJ, Mehta JS, 2020. Corneal dystrophies. Nat. Rev. Dis. Primers 6 (1). 10.1038/S41572-020-0178-9. [DOI] [PubMed] [Google Scholar]
- Soluri A, Hui A, Jones L, 2012. Delivery of ketotifen fumarate by commercial contact lens materials. Optom. Vis. Sci 89 (8), 1140–1149. [DOI] [PubMed] [Google Scholar]
- Souza JG, Dias K, Silva SAM, de Rezende LCD, Rocha EM, Emery FS, Lopez RFV, 2015. Transcorneal iontophoresis of dendrimers: PAMAM corneal penetration and dexamethasone delivery. J. Control. Release 200, 115–124. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, 2018. Anatomy of cornea and ocular surface. Indian J. Ophthalmol 66 (2), 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton F, Carat N, 2012. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye 26 (2), 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella V, et al. , Prodrags: challenges and rewards. 2007: Springer Science & Business Media. [Google Scholar]
- Stocker FW, 1953. The endothelium of the cornea and its clinical implications. Trans. Am. Ophthalmol. Soc 51, 669. [PMC free article] [PubMed] [Google Scholar]
- Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath V, 2019. Review of biomarkers in ocular matrices: challenges and opportunities. Pharm. Res 36 (3) 10.1007/s11095-019-2569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik EA, Craig DQ, Barker SA, 2020. Dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int. J. Pharm 581, 119296. [DOI] [PubMed] [Google Scholar]
- Teng C, 1962. Fine structure of the human cornea: epithelium and stroma. Am. J. Ophthalmol 54 (6), 969–1002. [PubMed] [Google Scholar]
- Thakur Singh RR, et al. , 2017. Minimally invasive microneedles for ocular drug delivery. Expert opinion on drag delivery 14 (4), 525–537. [DOI] [PubMed] [Google Scholar]
- Than A, et al. , 2018. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drag delivery. Nat. Commun 9 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PA, Geraldine P, 2007. Infectious keratitis. Current opinion in infectious diseases 20 (2), 129–141. [DOI] [PubMed] [Google Scholar]
- Thorne JE, Jabs DA, 2015. In: Rheumatology. Elsevier, pp. 260–266. 10.1016/B978-0-323-09138-1.00034-6. [DOI] [Google Scholar]
- Tieppo A, White CJ, Paine AC, Voyles ML, McBride MK, Byrne ME, 2012. Sustained in vivo release from imprinted therapeutic contact lenses. J. Control. Release 157 (3), 391–397. [DOI] [PubMed] [Google Scholar]
- Tiracherai GS, Dias C, Mitra AK, 2002. Corneal permeation of ganciclovir: mechanism of ganciclovir permeation enhancement by acyl ester prodrag design. J. Ocul. Pharmacol. Ther 18 (6), 535–548. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, 2001. Structure and design of polymeric surfactant-based drag delivery systems. J. Control. Release 73 (2-3), 137–172. [DOI] [PubMed] [Google Scholar]
- Trinh HM, Joseph M, Cholkar K, Mitra R, Mitra AK, 2017. In: Emerging Nanotechnologies for Diagnostics, Drag Delivery and Medical Devices. Elsevier, pp. 45–58. 10.1016/B978-0-323-42978-8.00003-6. [DOI] [Google Scholar]
- Tsai C-H, Wang P-Y, Lin IC, Huang H.u., Liu G-S, Tseng C-L, 2018. Ocular drag delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci 19 (9), 2830. 10.3390/ijms19092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuft SJ, Coster DJ, 1990. The corneal endothelium. Eye 4 (3), 389–424. [DOI] [PubMed] [Google Scholar]
- Vadlapudi AD, Mitra AK, 2013. Nanomicelles: an emerging platform for drug delivery to the eye. Therapeutic delivery 4 (1), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlapudi A et al. , 2015.Ocular drag delivery. Drug Deliv. Jones Bartlett Learn. Burlingt. Ma USA. 1: p. 219–263. [Google Scholar]
- Vaishya RD, Khurana V, Patel S, Mitra AK, 2014. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 6 (5), 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme TF, Brobeck L, 2005. Poly (amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 102 (1), 23–38. [DOI] [PubMed] [Google Scholar]
- Vasantha V, Sehgal P, Rao KP, 1988. Collagen ophthalmic inserts for pilocarpine drug delivery system. Int. J. Pharm 47 (1–3), 95–102. [Google Scholar]
- Venuganti VVK, Perumal OP, 2009. Poly (amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci 98 (7), 2345–2356. [DOI] [PubMed] [Google Scholar]
- Vyas SP, Paliwal R, Paliwal SR, 2011. Ocular delivery of peptides and proteins. In: Peptide and Protein Delivery. Elsevier, pp. 87–103. [Google Scholar]
- Wadhwa S, et al. , 2009. Nanocarriers in ocular drag delivery: an update review. Curr. Pharm. Des 15 (23), 2724–2750. [DOI] [PubMed] [Google Scholar]
- Watsky MA, Jablonski MM, Edelhauser HF, 1988. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr. Eye Res 7 (5), 483–486. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The pathophysiology and treatment of glaucoma: a review. JAMA 311 (18), 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wels M, Roels D, Raemdonck K, De Smedt SC, Sauvage F, 2021. Challenges and strategies for the delivery of biologies to the cornea. J. Control. Release 333, 560–578. [DOI] [PubMed] [Google Scholar]
- Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z, 2017. Nanotechnology-based strategies for treatment of ocular disease. Acta pharmaceutica sinica B 7 (3), 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CJ, Byrne ME, 2010. Molecularly imprinted therapeutic contact lenses. Expert opinion on drag delivery 7 (6), 765–780. [DOI] [PubMed] [Google Scholar]
- Willoughby CE, Batterbury M, Kaye SB, 2002. Collagen corneal shields. Surv. Ophthalmol 47 (2), 174–182. [DOI] [PubMed] [Google Scholar]
- Willoughby CE, et al. , Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function–a review. Clinical & Experimental Ophthalmology, 2010. 38: p. 2–11.20447093 [Google Scholar]
- Wilson CG, Zhu YP, Frier M, Rao LS, Gilchrist P, Perkins AC, 1998. Ocular contact time of a carbomer gel (GelTears) in humans. Br. J. Ophthalmol 82 (10), 1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, 1996. A history of vitamin A and retinoids. FASEB J. 10 (9), 1102–1107. [DOI] [PubMed] [Google Scholar]
- Wong T-Y, et al. , Risk factors and clinical outcomes between fungal and bacterial keratitis: a comparative study. The CLAO journal: official publication of the Contact Lens Association of Ophthalmologists, Inc, 1997. 23(4): p. 275–281. [PubMed] [Google Scholar]
- Xinming L.i., Yingde C, Lloyd AW, Mikhalovsky SV, Sandeman SR, Howel CA, Liewen L, 2008. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens and Anterior Eye 31 (2), 57–64. [DOI] [PubMed] [Google Scholar]
- Xu X, et al. , 2020. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym 227, 115356. [DOI] [PubMed] [Google Scholar]
- Xu J, Xue Y, Hu G, Lin T, Gou J, Yin T, He H, Zhang Y.u., Tang X, 2018. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 281, 97–118. [DOI] [PubMed] [Google Scholar]
- Yavuz B, Bozdaǧ Pehlivan S, Ünlü N, 2013. Dendrimeric systems and their applications in ocular drug delivery. The Scientific World Journal 2013, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellepeddi VK, Palakurthi S, 2016. Recent advances in topical ocular drug delivery. J. Ocul. Pharmacol. Ther 32 (2), 67–82. [DOI] [PubMed] [Google Scholar]
- Zamani M, Prabhakaran MP, Ramakrishna S, 2013. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed 8, 2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala J, López Jaime GR, Rodríguez Barrientos CA, Valdez-Garcia J, 2013. Corneal endothelium: developmental strategies for regeneration. Eye 27 (5), 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, He Z, Liang R, Ma Y.i., Huang W, Jiang R, Shi S, Chen H, Li X, 2016. Fabrication of a micellar supramolecular hydrogel for ocular drug delivery. Biomacromolecules 17 (3), 798–807. [DOI] [PubMed] [Google Scholar]
- Zhang W, Prausnitz MR, Edwards A, 2004. Model of transient drug diffusion across cornea. J. Control. Release 99 (2), 241–258. [DOI] [PubMed] [Google Scholar]


