Strongyloidiasis is a parasitic infection caused by the intestinal nematode Strongyloides stercoralis. It is a chronic and asymptomatic parasite in most immunocompetent individuals. In immunocompromised patients, especially those undergoing corticotherapy, the disease can progress to advanced stages, such as hyperinfection syndrome and disseminated disease (1). Corticosteroids are metabolized in the liver to chemical compounds structurally similar to ecdysone and are used by helminths for oviposition and larval ecdysis stimulation, promoting an accelerated autoinfection cycle (2).
The coronavirus disease (COVID-19) pandemic remains a health challenge for society worldwide. The advancing knowledge of the physiopathology suggests that a rapid replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may lead to an exacerbated immune response involving many cytokines (cytokine storm), triggering a severe inflammatory response in the lungs. Using corticosteroids and anticoagulants in such cases has produced beneficial results (3- 5), with dexamethasone and methylprednisolone being used most frequently (6-7).
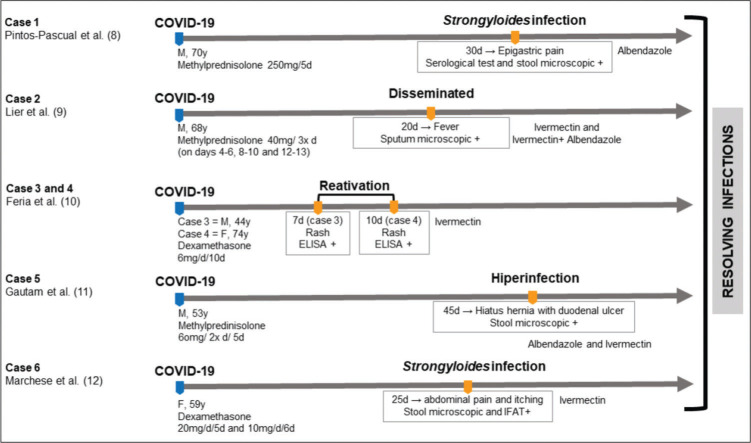
The impact of the COVID-19 pandemic on S. stercoralis infection remains to be elucidated. However, there are few reports of severe strongyloidiasis in patients with COVID-19 after undergoing treatment with corticosteroids (8- 12); currently, only six cases of coinfection with S. stercoralis and COVID-19 have been reported (Figure 1). Clinical data of these patients are incipient or partial; however, generally, the majority of the reported cases tended to be in male individuals (66.7%), with an average age of 61.3 years. These individuals were admitted to the hospital for COVID-19 treatment, were released from the hospital, and returned after 22.8 days on average with skin signs and symptoms, such as dermatitis, followed by respiratory and intestinal manifestations. The diagnosis of S. stercoralis infection was confirmed by parasitic stool examination in one case (16.7%), enzyme-linked immunosorbent assay in two (33.3%), microscopic stool and serological methods in two (33.3%), and microscopic sputum in one (16.7%).
Figure 1. Timeline of the main events related to SARS-CoV-2 and S. stercoralis coinfections.
Different therapeutic regimens (Figure 1) were used to treat SARS-CoV-2 infection with methylprednisolone and dexamethasone. In parallel with corticosteroids, antiviral drugs were also used. For S. stercoralis infection, ivermectin was used in 50% of the patients, albendazole in 16.7%, and the combination of both in 33.3%. All patients survived COVID-19 and strongyloidiasis. It is important to highlight that 83.3% of the described cases occurred in non-endemic areas (1,13) for S. stercoralis infection, possibly indicating a link to immigration flows.
Oliveira (6) reported the importance of the identification of S. stercoralis infection using diagnostic methods for parasite detection, especially cultures, or even serological screening. Once the helminth parasite is detected, anti-helminthic treatment is recommended before corticosteroid therapy is administered. De Wilton et al. (5) proposed an algorithm for screening the risk of S. stercoralis exposure in patients samples with COVID-19 at a hospital in England. In general, all patients who had travelled or immigrated from endemic areas and those with a high risk of S. stercoralis infection underwent a diagnostic test and treatment with ivermectin. Although England is not considered an endemic area for S. stercoralis infection, there is a concern about the development of severe forms of this parasitosis when associated with corticosteroid therapy, especially in immigrants.
Unlike in the countries that reported cases of coinfection, the reality of strongyloidiasis in Brazil is distinct, with an estimated occurrence varying between 5.5% and 20% based on the microscopic parasitic examination of stool. When other diagnostic methods are analyzed, these numbers may be significantly higher since directly detecting larval forms is not needed (13,14). Pandemic situations, such as COVID-19, bring awareness to the scientific community worldwide to the need for studies on other non-viral infectious agents for the prevention of fatal consequences of possible associations. In this scenario, the possibility of asymptomatic infection by S. stercoralis becoming symptomatic and severe with the use of corticosteroids is evident.
There is a need to apply and to implement more sensitive and specific diagnostic techniques for strongyloidiasis that could be used in immunocompetent and immunocompromised individuals (5,6). The public health systems need to consider the construction and validation of a diagnostic protocol. Many diagnostic methods have been reported in the literature (15) based on the direct detection of the parasite by agar plate culture or molecular methods, or even the indirect detection of antibodies, antigens, or immune complexes; however, none of these methods are available in the Brazilian national health system, only for research. Therefore, strongyloidiasis continues to be considered one of the most neglected parasitic diseases worldwide. The rapid identification of positive cases could minimize the occurrence of severe cases resulting from coinfection; in Brazil, this would demonstrate the real situation of the condition in the country since it has high incidence rates of this important parasitosis.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Toledo R, Muãoz-Antoli C, Esteban JG. Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol. 2015;88:165–241. doi: 10.1016/bs.apar.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Marty FM, Lowry CM, Rodriguez M, Milner DA, Pieciak WS, Sinha A, et al. Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clin Infect Dis. 2005;41(1):e5–e8. doi: 10.1086/430827. [DOI] [PubMed] [Google Scholar]
- 3.Formiga FR, Leblanc R, de Souza Rebouças J, Farias LP, de Oliveira RN, Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID-19. J Control Release. 2021;329:758–61. doi: 10.1016/j.jconrel.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilli M, Olliaro P, Gobbi F, Bisoffi Z, Bartoloni A, Zammarchi L. Neglected tropical diseases in non-endemic countries in the era of COVID-19 pandemic: the greath forgotten. J Travel Med. 2021;28(1):taaa179. doi: 10.1093/jtm/taaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wilton A, Nabarro LE, Godbole GS, Chiodini PL, Boyd A, Woods K. Risk of Strongyloides Hyperinfection Syndrome when prescribing dexamethasone in severe COVID-19. Travel Med Infect Dis. 2021;40:101981. doi: 10.1016/j.tmaid.2021.101981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivera MJ. Dexamethasone and COVID-19: Strategies in Low- and Middle-Income Countries to Tackle Steroid-Related Strongyloides Hyperinfection. Am J Trop Med Hyg. 2021;104(5):1611–2. doi: 10.4269/ajtmh.20-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stauffer WM, Alpern JD, Walker PF. COVID-19 and Dexamethasone: A Potential Strategy to Avoid Steroid-Related Strongyloides Hyperinfection. JAMA. 2020;324(7):623–4. doi: 10.1001/jama.2020.13170. [DOI] [PubMed] [Google Scholar]
- 8.Pintos-Pascual I, López-Dosil M, Castillo-Núãez C, Múãez-Rubio E. Eosinophilia and abdominal pain after severe pneumonia due to COVID 19. Enferm Infecc Microbiol Clin (Engl Ed) 2020 doi: 10.1016/j.eimce.2021.08.007. S0213-005X(20)30332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lier AJ, Tuan JJ, Davis MW, Paulson N, McManus D, Campbell S, et al. Case Report: Disseminated Strongyloidiasis in a Patient with COVID-19. Am J Trop Med Hyg. 2020;103(4):1590–2. doi: 10.4269/ajtmh.20-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feria L, Torrado M, Anton-Vazquez V. Reactivation of Strongyloides stercoralis in patients with SARS-CoV-2 pneumonia receiving dexamethasone. Med Clin (Barc) 2021;27 doi: 10.1016/j.medcli.2021.05.004. S0025-7753(21)00292-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautam D, Gupta A, Meher A, Siddiqui F, Singhai A. Corticosteroids in Covid-19 pandemic have the potential to unearth hidden burden of strongyloidiasis. IDCases. 2021;25:e01192. doi: 10.1016/j.idcr.2021.e01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchese V, Crosato V, Gulletta M, Castelnuovo F, Cristini G, Matteelli A, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2021;49(3):539–42. doi: 10.1007/s15010-020-01522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138(11):1331–40. doi: 10.1017/S003118201100120X. [DOI] [PubMed] [Google Scholar]
- 14.Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muãoz J, Gobbi F, et al. Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect. 2015;143(3):452–60. doi: 10.1017/S0950268814001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa IN, Bosqui LR, Corral MA, Costa-Cruz JM, Gryschek RCB, de Paula FM. Diagnosis of human strongyloidiasis: Application in clinical practice. Acta Trop. 2021;223:106081. doi: 10.1016/j.actatropica.2021.106081. [DOI] [PubMed] [Google Scholar]