Abstract
OBJECTIVE:
This pilot study assessed feasibility of video-enhanced care management for complex older veterans with suspected mild cognitive impairment (CI) and their care partners, compared with telephone delivery.
DESIGN:
Pilot randomized controlled trial.
SETTING:
Durham Veterans Affairs Health Care System.
PARTICIPANTS:
Participants were enrolled as dyads, consisting of veterans aged 65 years or older with complex medical conditions (Care Assessment Need score ≥90) and suspected mild CI (education-adjusted Modified Telephone Interview for Cognitive Status score 20–31) and their care partners.
INTERVENTION:
The 12-week care management intervention consisted of monthly calls from a study nurse covering medication management, cardiovascular disease risk reduction, physical activity, and sleep behaviors, delivered via video compared with telephone.
MEASUREMENTS:
Dyads completed baseline and follow-up assessments to assess feasibility, acceptability, and usability.
RESULTS:
Forty veterans (mean (standard deviation (SD)) age = 72.4 (6.1) years; 100% male; 37.5% Black) and their care partners (mean (SD) age = 64.7 (10.8) years) were enrolled and randomized to telephone or video-enhanced care management. About a third of veteran participants indicated familiarity with relevant technology (regular tablet use and/or experience with videoconferencing); 53.6% of internet users were comfortable or very comfortable using the internet. Overall, 43 (71.7%) care management calls were completed in the video arm and 52 (86.7%) were completed in the telephone arm. Usability of the video telehealth platform was rated higher for participants already familiar with technology used to deliver the intervention (mean (SD) System Usability Scale scores: 65.0 (17.0) vs 55.6 (19.6)). Veterans, care partners, and study nurses reported greater engagement, communication, and interaction in the video arm.
CONCLUSION:
Video-delivered care management calls were feasible and preferred over telephone for some complex older adults with mild CI and their care partners. Future research should focus on understanding how to assess and incorporate patient and family preferences related to uptake and maintenance of video telehealth interventions.
Keywords: cognitive impairment, virtual care, medical complexity, older adults
INTRODUCTION
Unrecognized cognitive impairment (CI) is common, especially among older adults with chronic medical illness.1,2 CI that does not meet the threshold of dementia affects approximately 22% of adults older than 70 years in the United States3 and yet the diagnosis of CI is often missed in primary care clinics.4,5 This may be due, in part, to the high prevalence of coexisting conditions that compete for a providerʼs time and attention. Although universal screening for CI is not recommended, a targeted case-finding approach in groups with higher-than-average risk is widely cited as a good strategy.6 Older adults with medical complexity certainly constitute such a high-risk group.
Older patients with multiple chronic conditions and the added burden of CI often find themselves in the perfect storm of complexity, simultaneously experiencing escalated healthcare utilization and demands for self-management despite reduced capacity.7 In a study in the Veterans Health Administration (VHA), patients who screened positive for CI had twice the number of hospitalizations and their mean length of stay was nearly four times as long as those who screened negative.8 CI is known to be associated with higher healthcare costs, increased severity of comorbid illnesses, potentially avoidable hospitalizations, and long-term institutional care.9,10 Older adults with CI are seen frequently by healthcare providers of various disciplines and in multiple settings, such as hospitals, emergency departments (EDs), specialty clinics, and home health. The need to receive, process, and integrate information from multiple sources is especially difficult for patients with CI. This highlights the importance of care management, which is intended to help patients and care partners more effectively manage the full spectrum of health conditions, better coordinate care across primary and specialty visits, and avoid unnecessary ED and hospital use.
Care management intended to help with coordination of care and chronic disease self-management is often delivered over the telephone.11–13 VHA has made major investments in expanding telehealth capabilities to include a video platform that enables patients to interact with providers in virtual medical rooms using the camera on a smartphone, computer, or tablet.14,15 Although increasing numbers of older adults use the internet and are open to technology-enhanced interventions,16,17 it is uncertain whether the advantages of video visits, such as the ability to see nonverbal cues and view the living environment, negate the disadvantages, such as the need to master new technology to participate.11,12,18–21 Thus, the primary objectives of this study were to test the feasibility, acceptability, usability, and perceived value of a 14-week video-delivered nurse care management program for medically complex older veterans with CI and their care partners compared with a similar telephone-based program.
METHODS
The Institutional Review Board of the Durham VA Health Care System (HCS) approved this study. The study was registered at ClinicalTrials.gov (NCT02962687).
Design and Setting
This two-group pilot randomized controlled trial was conducted at the Durham VA HCS from May 2017 to February 2018. Following verbal informed consent and baseline data collection, participants were randomized (1:1) in blocks of two to receive a care management intervention via telephone or through video visits on tablets. Randomization was stratified by whether patients were “experienced with relevant technology,” as indicated by use of a tablet and/or experience using videoconference platforms.
Participants and Recruitment
Patients were eligible if they received primary care from a Durham VA HCS-affiliated primary care clinic, were aged 65 years or older, and were medically complex, defined as having a Care Assessment Need (CAN) score of 90 or greater. The CAN score (v. 2.0) used for this study was calibrated to predict risk of hospitalization at 1 year.22,23 A score of 90 or above corresponds to high event probability compared with other VA patients. Patients were excluded if they had a previous diagnosis of CI or lacked decision-making capacity because our focus was on patients whose CI had not been previously recognized. Other exclusion criteria included no available care partner, serious mental illness, high suicide risk, active substance abuse, current hospitalization or residence in a nursing home, hospice eligible, unable to communicate on the telephone, or no email address or willingness to obtain one. Email was required to access the video telehealth platform. Potentially eligible subjects were screened with the modified version of the Telephone Interview for Cognitive Status (TICS-m), a 50-point cognitive status measure designed to be administered over the telephone.24–26 Veterans with an education-adjusted TICS-m score between 20 and 31, a range previously correlated with mild CI, were offered enrollment.27 Patients identified their care partner of choice to participate jointly and be included on calls with the study nurse. Care partners had to be 18 years or older and willing and able to provide informed consent for study participation.
Care Management Program
Intervention content was designed to incorporate key features of effective care management programs.28 Given our focus on individuals with CI, we also incorporated three evidence-based strategies for promoting cognitive health endorsed by the Institute of Medicine29: (1) managing medications and health conditions that could affect cognition; (2) reducing cardiovascular risk factors, including hypertension, diabetes mellitus, and smoking; and (3) improving physical activity and sleep behaviors. In this feasibility pilot, we limited the care management intervention to a total of three scheduled calls with study nurses, both of whom had experience with telephone-delivered interventions.
Arm 1: Telephone
For patients randomized to the telephone-based care management arm, all study contacts occurred over the telephone. Scheduled calls with the study nurse began 2 to 3 weeks after randomization (to reflect the time needed for a practice call in the video arm) and occurred monthly thereafter.
Arm 2: Video
Patients randomized to the video-enhanced care management arm participated in a practice call with study staff after randomization and then received three monthly contacts with study nurses delivered on the same schedule and focused on the same key components as the telephone-based program. The primary difference in the video-enhanced arm was that the study nurse communicated with the patient and care partner using video visits in secure virtual meeting rooms using VA Video Connect (VVC). Participants who had their own tablet were able to utilize it for the study; others were loaned an iPad for use during the study. Participants received a user guide created by the study team that provided instructions for using VVC.
Measures
Feasibility and acceptability were assessed by examining numbers of eligible patients, enrollment and retention rates, and adherence to nurse telephone or video calls. We used a simplified language version of the System Usability Scale to measure usability of video visits via tablet.30 Pilot clinical outcomes were collected to test administration of measures that might be useful in a future randomized controlled trial of a care management intervention in patients with CI, including physical activity,31 social support,32 depression,33 anxiety,34 pain,35,36 physical function,35,36 social roles,35,36 and sleep.37 We assessed VA ED visits and hospitalizations using administrative data. Perceived value associated with each delivery method was explored through interviews with participants after the 14-week assessment and study staff.
Data Analysis
We used descriptive statistics to assess participant characteristics and outcome measures. Due to small sample size and because it was not the purpose of this pilot study, we did not conduct statistical hypothesis testing. Directed content analysis was used to assess perceived value based on detailed notes from in-depth interviews. Three study team members separately coded the notes based on codes derived a priori from interview guide concepts and from review of note text. Team members met to review coding and to sort and summarize the coded data according to a thematic framework. A matrix was used to identify themes and subthemes, and to explain the range of perceived value and satisfaction with the intervention.
RESULTS
Feasibility and Acceptability
Enrollment and Retention
Among all individuals who met initial eligibility criteria, we selected a simple random sample of 500 patients and mailed them letters regarding the study (Figure 1). Twenty-nine requested not to be contacted further, and 86 were ineligible after further medical record review. Of 344 veterans screened over the telephone, 194 were eligible for enrollment. At least one member of 154 dyads declined to participate (usually the veteran). The most common reasons for refusal included: not interested, too busy, too ill, did not return telephone calls, and impaired hearing/speech. Overall, 40 dyads (20.6%) were enrolled and randomized, and 31 dyads (77.5%) completed the 14-week assessment. Characteristics of veteran participants and care partners are described in Tables 1 and 2, respectively.
Figure 1.
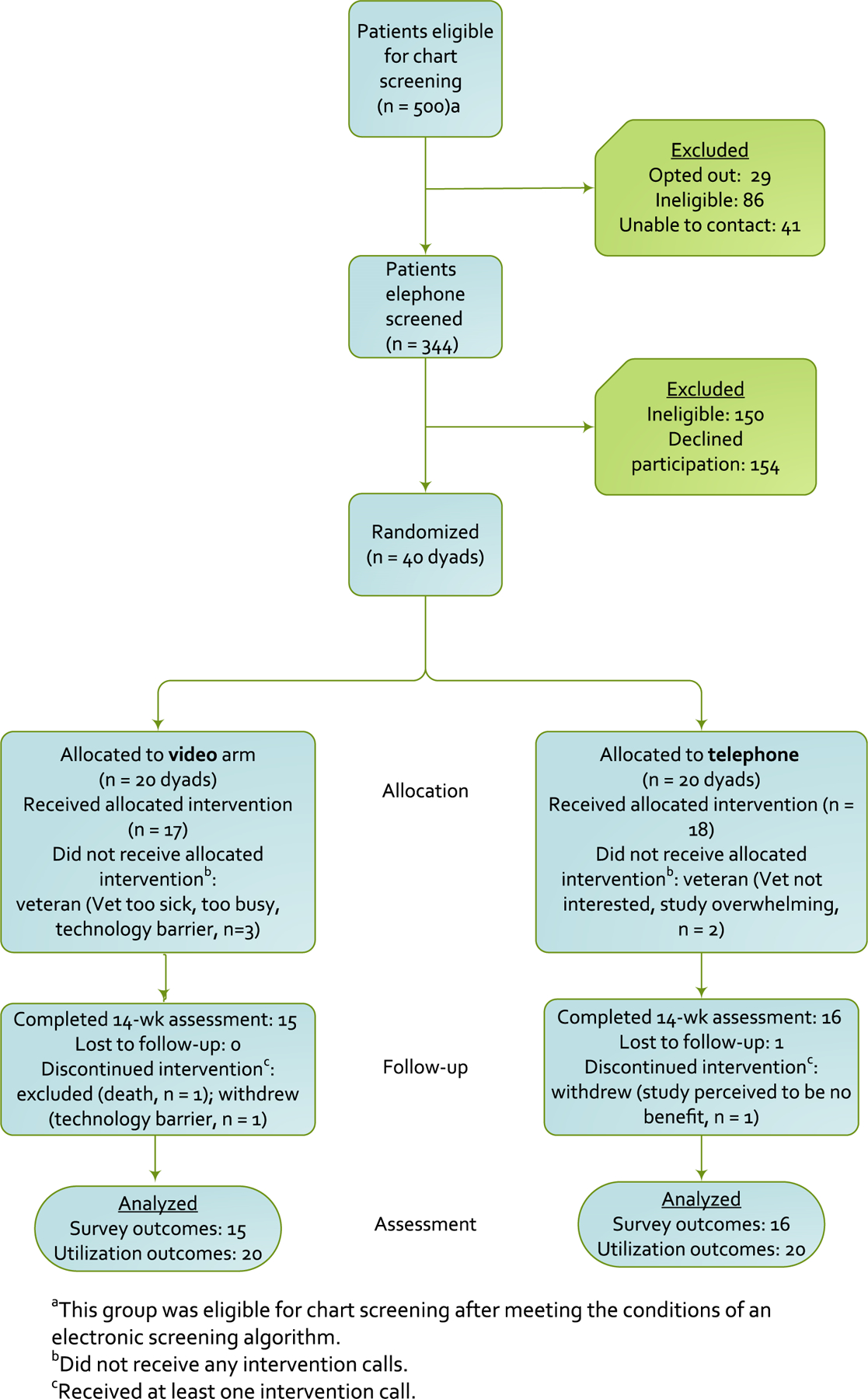
Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram
Table 1.
Baseline Veteran Characteristics
| Characteristic | Total sample (N = 40) | Telephone intervention (n = 20) | Video intervention (n = 20) |
|---|---|---|---|
| Age, mean (SD), y | 72.4 (6.1) | 74.6 (7.1) | 70.2 (3.8) |
| Male, No. (%) | 40 (100.0) | 20 (100.0) | 20 (100.0) |
| Black or African American race, No. (%)a | 15 (37.5) | 7 (35.0) | 8 (40.0) |
| Married or living together, No. (%) | 31 (77.5) | 15 (75.0) | 16 (80.0) |
| High school education or less, No. (%) | 14 (35.0) | 9 (45.0) | 5 (25.0) |
| Inadequate income, No. (%)b | 6 (15.0) | 4 (20.0) | 2 (10.0) |
| Private insurance, No. (%)c | 7 (17.5) | 4 (20.0) | 3 (15.0) |
| Medicaid, No. (%) | 7 (17.5) | 4 (20.0) | 3 (15.0) |
| Household size, mean (SD) | 2.3 (1.5) | 2.3 (1.6) | 2.4 (1.4) |
| Distance to nearest VA hospital or clinic, No. (%) | |||
| 0–20 miles | 13 (32.5) | 9 (45.0) | 4 (20.0) |
| 21–40 miles | 15 (37.5) | 5 (25.0) | 10 (50.0) |
| ≥41 miles | 12 (30.0) | 6 (30.0) | 6 (30.0) |
| Receive health care outside the VA, No. (%) | 10 (25.0) | 4 (20.0) | 6 (30.0) |
| Out-of-pocket healthcare expenses in last 12 mo, median (Q1–Q3), $ | 88 (0–600) | 500 (15–1,350) | 0 (0–150) |
| Fair/poor self-rated health, No. (%) | 26 (65.0) | 11 (55.0) | 15 (75.0) |
| TICS-m score, education adjusted, mean (SD) | 27.6 (2.6) | 27.4 (2.6) | 27.8 (2.6) |
| Inadequate health literacy, No. (%)d | 26 (65.0) | 11 (55.0) | 15 (75.0) |
| Familiar with relevant technology, No. (%)e | 14 (35.0) | 7 (35.0) | 7 (35.0) |
| Used internet in the past year, No. (%) | 28 (70.0) | 13 (65.0) | 15 (75.0) |
| Comfortable/very comfortable with internet, No. (%)f | 15 (53.6) | 8 (61.5) | 7 (46.7) |
Note: Percentages may not sum to 100% due to rounding.
Abbreviations: Q1, quartile 1; Q3, quartile 3; SD, standard deviation; TICS-m, Modified Telephone Interview for Cognitive Status.
Includes those who selected another race in addition to Black or African American.
Defined as an answer of “You have money to pay the bills, but only because you have to cut back on things” or “You are having a difficult time paying the bills, no matter what you do” to the question “Without giving exact dollars, how would you describe your householdʼs financial situation right now?”
Private, nongovernmental health insurance.
Defined as an answer of “not at all,”“a little bit,” or “somewhat” to “How confident are you in filling out medical forms by yourself?”
Stratification variable; defined as experience with videoconference (e.g., Skype, FaceTime) and/or regularly accesses the internet with an iPad or tablet.
Participants were asked this question if they had indicated “yes” to using the internet in the past year.
Table 2.
Baseline Care Partner Characteristics
| Characteristic | Total sample (N = 40) | Telephone intervention (n = 20) | Video intervention (n = 20) |
|---|---|---|---|
| Age, mean (SD), y | 64.7 (10.8) | 68.9 (7.7) | 60.6 (12.0) |
| Female, No. (%) | 38 (95.0) | 19 (95.0) | 19 (95.0) |
| Black or African American race, No. (%)a | 11 (27.5) | 3 (15.0) | 8 (40.0) |
| Living with veteran or within walking distance, No. (%) | 38 (95.0) | 18 (90.0) | 20 (100.0) |
| Caregiver is the veteran’s…, No. (%) | |||
| Spouse or significant other | 36 (90.0) | 18 (90.0) | 18 (90.0) |
| Other | 4 (10.0) | 2 (10.0) | 2 (10.0) |
| Care partner helps care for veteran due to health problems, No. (%) | 39 (97.5) | 19 (95.0) | 20 (100.0) |
| Other care for veteran in his/her home, No. (%) | |||
| No other help needed | 26 (65.0) | 12 (60.0) | 14 (70.0) |
| Other family member or friend | 9 (22.5) | 5 (25.0) | 4 (20.0) |
| Needs help but does not have it | 3 (7.5) | 2 (10.0) | 1 (5.0) |
| Paid care provider | 2 (5.0) | 1 (5.0) | 1 (5.0) |
| Used internet in the past year, No. (%) | 32 (80.0) | 13 (65.0) | 19 (95.0) |
| Comfortable/very comfortable with internet, No. (%)b | 27 (84.4) | 11 (84.6) | 16 (84.2) |
| Regularly accesses internet with a tablet or iPad, No. (%)b | 16 (50.0) | 4 (30.8) | 12 (63.2) |
| Used videoconference (e.g., Skype, FaceTime) before, No. (%) | 23 (57.5) | 10 (50.0) | 13 (65.0) |
| Likely/very likely to see healthcare provider using video, No. (%) | 26 (65.0) | 14 (70.0) | 12 (60.0) |
Note: Percentages may not sum to 100% due to rounding.
Abbreviation: SD, standard deviation.
Includes those who selected another race in addition to Black or African American.
Participants were asked this question if they had indicated “yes” to using the internet in the past year.
Intervention Delivery
Of 35 veteran participants who engaged with the intervention, 97.1% had a care partner participate in at least one call. Of 60 total possible intervention calls per arm, 46 (76.7%) were completed in the video arm and 53 (88.3%) were completed in the telephone arm. Of the 46 completed calls in the video arm, 52.2% were conducted by video only, as intended; 13.0% were completed by video after a telephone call to trouble-shoot connectivity or other issues, and 34.8% were completed by telephone only due to problems with the video call platform or patient preference. Study nurses conducted six additional unplanned calls for acute care follow-up and accompanied patients to five inperson primary care provider visits.
Usability of Video Calls
Overall mean (standard deviation (SD) System Usability Scale (SUS) score among video participants was 59.3 (18.6). Usability of the video telehealth platform was rated higher for participants already familiar with technology (mean (SD) SUS scores: 65.0 (17.0) vs 55.6 (19.6)). At the conclusion of the study, 35.5% of video arm participants who completed the follow-up assessment reported they were likely (score of 4 or 5) to communicate with a healthcare provider by video if that option was offered in the future, citing convenience/access, feeling comfortable/familiar with technology, and appreciating the learning opportunity. Those who indicated they were unlikely (55%; score of 1 or 2) to use video in the future with a healthcare provider reported they were not comfortable with technology and/or preferred in-person visits.
Perceived Value
A subset (n = 16 participants (10 patients and 3 caregivers in the video arm, 3 patients in the telephone arm)) completed in-depth interviews regarding their experience with the intervention. We identified four themes from qualitative data obtained from participants: perceived value of the delivery mode, usefulness for managing chronic conditions, satisfaction with the care management intervention, and usefulness of care partner participation. Theme summaries and example quotes are provided in Supplementary Table S1.
Study staff reported several areas of perceived value of the video-enhanced intervention. The first benefit was patient engagement and communication. Video was novel and fun for some patients, more interactive than telephone, and nurses felt they got to know video patients better. Technology and audio issues were commonly reported. Staff noted that care partners often were more positive about the video component than the patient participant. Study nurses reported the video was useful for some indications, such as viewing a drawer of medications or observing a patient doing shoulder exercises, but did not find it useful for viewing living space due to the concerns about the patient falling while walking and holding the iPad.
Clinical Outcomes and Utilization
Pilot clinical measures are reported for the whole sample because we had no a priori expectation that they would differ based on arm (Table 3). Regarding feasibility of collecting these measures, individual item missingness was low (<10%) for all items except the Physical Activity Scale for the Elderly, which was not administered to all patients due to a programming error. Among Patient-Reported Outcomes Measurement Information System (PROMIS)-29 domain items, baseline scores were lower (worse) for physical function and satisfaction with social role than U.S. general population norms. Pain interference scores were higher (worse) than general population normative values.38,39 Study participants, on average, reported clinically significant poor sleep quality (as indicated by Pittsburgh Sleep Quality Index (PSQI) total score >5).37 Overall, seven individuals experienced a total of four VA hospitalizations and five VA ED visits during the study observation period.
Table 3.
Veteran Baseline and 14-Week Pilot Clinical Measures
| Measure | Baseline (n = 40) | 14 wk (n = 31) |
|---|---|---|
| Physical Activity Scale for the Elderly, mean (SD) | 83.3 (72.3)a | 92.0 (70.5)b |
| Patient Health Questionnaire-9, mean (SD) | 4.8 (4.6) | 5.5 (4.8) |
| Generalized Anxiety Disorder-7, mean (SD) | 4.2 (3.8) | 4.2 (3.6) |
| Modified Medical Outcomes Study-Social Support, mean (SD) | 77.8 (21.9) | 71.5 (25.6)b |
| PROMIS-29, Pain Interference T score, mean (SD) | 57.9 (8.6) | 59.4 (9.3)b |
| PROMIS-29, Physical Function T score, mean (SD) | 36.8 (8.7) | 37.8 (9.0) |
| PROMIS-29, Social Roles T score, mean (SD) | 42.5 (8.4) | 43.7 (8.5)b |
| Pittsburgh Sleep Quality Index, mean (SD) | 9.1 (2.8) | 8.5 (3.3) |
| Modified Telephone Interview for Cognitive Status, education adjusted, mean (SD) | 27.6 (2.6) | 28.4 (3.5)b |
n = 29.
n = 30.
Abbreviations: SD, standard deviation.
DISCUSSION
Nurse care management delivered by video visits was feasible and acceptable to most medically complex veterans with suspected CI and their care partners. In response to COVID-19, the United States has seen a rapid and massive expansion in use of telehealth and an early observation and concern has been that the most complex or sickest patients may be the least likely to participate.18 Our findings are important because we demonstrated that medically complex individuals with the added burden of CI can be engaged in video visits successfully, with the systematic involvement of a care partner. Our study also adds to the existing literature on use of video telehealth platforms among older adults with CI40,41 by focusing on video visits delivered in the home, rather than a clinical setting.
A recent review of in-home video telehealth for dementia management noted the lack of systematic inquiries comparing in-home video telehealth with traditional visit formats.42 Our study addresses this need by examining video visits to deliver a care management intervention, compared with telephone, which is the typical delivery mode. Given the goals and design of the study, we cannot make inferences about the effectiveness of the care management program; however, the summary scores on clinical measures that we piloted suggest several areas of high need in this population. For example, overall self-reported sleep quality was low and comparable to previous studies focused on older veterans.43,44 Lower (better) PSQI scores at follow-up indicate a trend toward improvement in sleep quality. A longer intervention period is likely needed to detect improvements related to positive behavior change and improved disease control.
Video visits, when successful, offered some benefits over telephone in delivering a care management program. Benefits over telephone reported by both patients and study nurses included relationship building and enhanced communication, and patient engagement. Both patients and study nurses felt more connected to one another using video rather than telephone, and patients appreciated the personal touch and being able to see the provider. Video visits were embraced by older adults who valued convenience, were comfortable with technology, and were excited by learning new things. Video visits were also challenging in some circumstances, and overall quantitative usability scores fell in the “marginally acceptable” range. Frequent changes and updates in the telehealth platform during the study period frustrated both patients and providers. A recent systematic review found that providersʼ attitudes may be related to their previous experience with a telehealth platform, and acceptance may increase with use.45 Although most participants in video calls reported some positive aspects about them, less than half indicated they would want to see their healthcare provider by video in the future, and this did not necessarily correlate with experiencing technical problems with the platform. Similarly, in a nationally representative survey of VA patients who received VA-issued tablets for video visits, only 32% indicated that they would prefer to conduct their future VA appointments by video despite strong satisfaction ratings for tablets overall.21 Our findings highlight the importance of considering the heterogeneous needs and preferences of both older adults and their caregivers in selecting use cases for video telehealth and optimizing its benefits.46–48
Rates of technology use by older adults have quadrupled in the past 5 years,16,17 and about a third of older adults now own tablets.16,17 Some technology barriers can be overcome by providing access to equipment. A recent VA initiative successfully reached rural and chronically ill veterans, with four in five recipients using a videoconference enabled tablet.14 Providing access to devices is an important step for mitigating the risk of technology-based interventions to inadvertently exacerbate disparities in health care and must be paired with appropriate training for using both the device and telehealth platform. In our sample of participants with CI, not unexpectedly, we found that adopting more than one new technology at a time was especially difficult for participants (e.g., learning how to use both the iPad and the video visit software). Support from a care partner, most often a family member, was a key element in successful engagement with the technology. Conducting a test call with the patient before the “real” call was worth-while, and it was helpful to have a back-up in place if the video platform did not work (e.g., telephone). A user guide is highly recommended, including basic instructions and information on who to contact for problems and troubleshooting.
There are limitations to our study, most importantly the impact of evolving technology and usage policies. At the beginning of the pilot, VVC was in its early stages of development and few facilities were using the new technology. Over the course of the study, VVC improved; problems with the scheduling system that resulted in crossovers from video to telephone for some participants during the study have since been resolved. The current scheduling system for VVC (Virtual Care Manager) allows providers to send veterans a link to the secure virtual medical room at the time of the call. This feature would have reduced the number of technical challenges for veterans who had trouble locating an appointment link in their email inbox and allow for greater scheduling flexibility. We only administered the SUS to participants in the video arm; therefore, we do not have responses from individuals who participated by telephone to use as context in interpreting these findings. Also, we conducted more interviews with patients and care partners in the video arm and those who agreed to participate may have had stronger preferences about the delivery mode to which they were assigned. Other limitations included small sample size and setting in a single VA HCS, which reduces generalizability.
CONCLUSION
A video-enhanced care management program for older adults with suspected CI and medical complexity was feasible and offered some potential benefits over telephone. Providing devices (iPads) and technical support and coenrollment of a care partner helped overcome some barriers to use, but not all participants were interested in engaging with the video component. In planning for a future trial, we plan to (1) actively involve patients and family members to solicit preferences for mode of delivery and incorporate them into the study design; (2) focus intervention content on medication management, physical activity promotion, and healthy sleep as key areas for potential benefit; and (3) explore the extent to which video and/or telephone-delivered care management can be integrated with other technologies to support either everyday activities or those designed to help manage health (e.g., medication adherence devices).
Supplementary Material
Supplementary Table S1: Summary of Themes and Example Quotes from the Patient/Care Partner Interviews.
ACKNOWLEDGMENTS
Financial Disclosure: This work was supported by the U.S. Department of Veterans Affairs, Health Services Research, and Development Service (PPO 15–425; HX002030–01A1) and by the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13–410) at the Durham VA Health Care System.
Sponsor’s Role: The funding agency had no role in the design or conduct of the study; collection, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Footnotes
Portions of this work were presented at the 2018 Annual Scientific Meeting of the American Geriatrics Society in Orlando, FL.
Conflict of Interest: None.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Holsinger T, Plassman BL, Stechuchak KM, Burke JR, Coffman CJ, Williams JW Jr. Screening for cognitive impairment: comparing the performance of four instruments in primary care. J Am Geriatr Soc 2012;60(6): 1027–1036. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins LA, Kilian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung 2012;41(6):572–582. [DOI] [PubMed] [Google Scholar]
- 3.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008;148(6): 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med 2000;160(19):2964–2968. [DOI] [PubMed] [Google Scholar]
- 5.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayeddiagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord 2009;23(4):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kansagara D, Freeman M. A Systematic Evidence Review of the Signs and Symptoms of Dementia and Brief Cognitive Tests Available in VA Washington, DC: Department of Veterans Affairs; 2010. [PubMed] [Google Scholar]
- 7.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol 2012;65(10):1041–1051. [DOI] [PubMed] [Google Scholar]
- 8.Wray LO, Wade M, Beehler GP, Hershey LA, Vair CL. A program to improve detection of undiagnosed dementia in primary care and its association with healthcare utilization. Am J Geriatr Psychiatry 2014;22(11):1282–1291. [DOI] [PubMed] [Google Scholar]
- 9.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA 2012;307(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willink A, Davis K, Schoen C. Risks for nursing home placement and Medicaid entry among older Medicare beneficiaries with physical or cognitive impairment. Issue Brief (Commonw Fund) 2016;37:1–14. [PubMed] [Google Scholar]
- 11.Guo Y, Albright D. The effectiveness of telehealth on self-management for older adults with a chronic condition: a comprehensive narrative review of the literature. J Telemed Telecare 2018;24(6):392–403. [DOI] [PubMed] [Google Scholar]
- 12.Rush KL, Hatt L, Janke R, Burton L, Ferrier M, Tetrault M. The efficacy of telehealth delivered educational approaches for patients with chronic diseases: a systematic review. Patient Educ Couns 2018;101(8):1310–1321. [DOI] [PubMed] [Google Scholar]
- 13.Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res 2017;19(5):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zulman DM, Wong EP, Slightam C, et al. Making connections: nationwide implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open 2019;2(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darkins A. The growth of telehealth services in the veterans health administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health 2014;20(9):761–768. [DOI] [PubMed] [Google Scholar]
- 16.Pew Research Center. Older Adults and Technology Use April 2014. https://www.pewresearch.org/internet/2014/04/03/older-adults-and-technology-use/. Accessed January 20, 2020. [Google Scholar]
- 17.Pew Research Center. Tech Adoption Climbs Among Older Adults May 2017. https://www.pewresearch.org/internet/2017/05/17/tech-adoption-climbs-among-older-adults/. Accessed January 5, 2020. [Google Scholar]
- 18.Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc 2020;27:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gionfriddo MR, Branda ME, Fernandez C, et al. Comparison of audio vs.audio + video for the rating of shared decision making in oncology using the observer OPTION(5) instrument: an exploratory analysis. BMC Health Serv Res 2018;18(1):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams K, Herman R, Bontempo D. Comparing audio and video data for rating communication. West J Nurs Res 2013;35(8):1060–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slightam C, Gregory AJ, Hu J, et al. Patient perceptions of video visits using veterans affairs telehealth tablets: survey study. J Med Internet Res 2020;22 (4):e15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the veterans health administration. Med Care 2013;51(4):368–373. [DOI] [PubMed] [Google Scholar]
- 23.Chokshi DA, Schectman G, Agarwal M. Patient-centered innovation: the VA approach. Healthc (Amst) 2013;1(3–4):72–75. [DOI] [PubMed] [Google Scholar]
- 24.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using the telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1993;6(2):103–110. [Google Scholar]
- 25.Plassman BL, Newman TT, Welsh KA, Helms M, Breitner JCS. Properties of the telephone interview for cognitive status: application in epidemiological and longitudinal studies. Neuropsychiatry Neuropsychol Behav Neurol 1994;7:235–241. [Google Scholar]
- 26.Cook SE, Marsiske M, McCoy KJ. The use of the modified telephone interview for cognitive status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol 2009;22(2):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopman DS, Roberts RO, Geda YE, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology 2010;34(1): 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RS, Peikes D, Peterson G, Schore J, Razafindrakoto CM. Six features of Medicare coordinated care demonstration programs that cut hospital admissions of high-risk patients. Health Aff 2012;31(6): 1156–1166. [DOI] [PubMed] [Google Scholar]
- 29.Blazer DG, Yaffe K, Karlawish J. Cognitive aging: a report from the Institute of Medicine. JAMA 2015;313(21):2121–2122. [DOI] [PubMed] [Google Scholar]
- 30.Brooke J. SUS: a quick and dirty usability scale. In: Jordan PW, Thomas B, Weerdmeester BA, McClelland AL, ed. Usability Evaluation in Industry London: Taylor & Francis; 1996. [Google Scholar]
- 31.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993; 46(2):153–162. [DOI] [PubMed] [Google Scholar]
- 32.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 33.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care 2004;42(12):1194–1201. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166(10): 1092–1097. [DOI] [PubMed] [Google Scholar]
- 35.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol 2016;73: 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 38.Sauro J. 5 Ways to interpret at SUS score. MeasuringU Web site https://measuringu.com/interpret-sus-score/. Published 2018. Accessed January 6, 2020, 2020. [Google Scholar]
- 39.LaVela SL, Etingen B, Miskevics S, Cella D. Use of PROMIS-29(R) in US veterans: diagnostic concordance and domain comparisons with the general population. J Gen Intern Med 2019;34(8):1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang S, Gomez-Orozco CA, van Zuilen MH, Levis S. Providing dementia consultations to veterans using clinical video telehealth: results from a clinical demonstration project. Telemed J E Health 2018;24(3):203–209. [DOI] [PubMed] [Google Scholar]
- 41.Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel) 2018;3(3):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gately ME, Trudeau SA, Moo LR. In-home video telehealth for dementia management: implications for rehabilitation. Curr Geriatr Rep 2019;8(3): 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alessi C, Martin JL, Fiorentino L, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc 2016;64(9):1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol 2018;41(2):145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connolly S, Miller CJ, LIndsay JA, Bauer MS. A systematic review of providersʼ attitudes toward telemental health via videoconferencing. Clin Psychol Sci Pract 2020;27(2):e12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robillard JM, Cleland I, Hoey J, Nugent C. Ethical adoption: a new imperative in the development of technology for dementia. Alzheimers Dement 2018;14(9):1104–1113. [DOI] [PubMed] [Google Scholar]
- 47.Meiland F, Innes A, Mountain G, et al. Technologies to support community-dwelling persons with dementia: a position paper on issues regarding development, usability, effectiveness and cost-effectiveness, deployment, and ethics. JMIR Rehabil Assist Technol 2017;4(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vick JB, Amjad H, Smith KC, et al. “Let him speak”: a descriptive qualitative study of the roles and behaviors of family companions in primary care visits among older adults with cognitive impairment. Int J Geriatr Psychiatry 2018;33(1):e103–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Summary of Themes and Example Quotes from the Patient/Care Partner Interviews.


