ABSTRACT
Background:
Endothelial and microvascular dysfunction may be a key pathogenic feature of severe COVID-19. The aim of this study was to investigate endothelial-dependent and endothelial-independent skin microvascular reactivity in patients with critical COVID-19.
Methods:
Twelve patients with COVID-19 treated with non-invasive or invasive mechanical ventilation were included in the study. We investigated skin microvascular reactivity on 2 separate days during hospitalization (study day 1 and 2) and at least 3 months after disease onset (study day 3). Twelve controls with no confirmed or suspected COVID-19 infection during 2020 were also examined. Skin perfusion was investigated through Laser Speckle Contrast Imaging before and after iontophoresis of acetylcholine (ACh) and sodium nitroprusside (SNP) to determine the endothelial-dependent and the endothelial-independent vasodilation, respectively.
Results:
Compared to controls, patients with critical COVID-19 had higher basal skin perfusion and reduced responses to endothelial-dependent (ACh, P = 0.002) and endothelial-independent (SNP, P = 0.01) vasodilator drugs on study day 1. In addition, the ACh/SNP ratio was significantly reduced in patients (0.50 ± 0.36 vs. 0.91 ± 0.49 in controls, P = 0.02). Three months after disease onset, surviving patients tended to have reduced ACh-mediated vasodilation compared to controls (P = 0.08).
Conclusions:
This small-sized pilot study demonstrates that critical COVID-19 infection is associated with microvascular impairment and, in particular, a markedly reduced endothelial function. Our results also suggest that microvascular function may not be fully recovered 3 months after disease onset.
Keywords: COVID-19, endothelial dysfunction, microangiopathy, microcirculation, skin microvascular function
INTRODUCTION
SARS-CoV-2 infects the host cells via the transmembrane angiotensin-converting enzyme 2 (ACE2) receptor, expressed in various cells and tissues, including vascular endothelial cells (1, 2). The infected endothelium induces inflammation and coagulation, through expression and release of various adhesion molecules and cytokines. Furthermore, a disturbed endothelial function of the microvasculature may be a major cause of organ failure in patients with severe COVID-19 (3).
Investigations of skin microvascular reactivity have been described as suitable for assessment of generalized microvascular function (4, 5). In this pilot study, we hypothesized that critical COVID-19 is associated with reduced endothelial-dependent skin microvascular reactivity.
To investigate this, we measured skin microvascular reactivity in critically ill patients with confirmed COVID-19 bedside through assessment of skin perfusion using Laser Speckle Contrast Imaging (LSCI) and transdermal application of the vasoactive drugs. Analyses were performed during hospitalization and at least 3 months after disease onset in surviving patients. The results were compared between patients and controls, in order to investigate the impact of critical COVID-19 on microvascular function in acute (primary aim) and post-infectious phase (secondary aim).
PATIENTS AND METHODS
Study design
We performed a prospective pilot study with assessment of skin microvascular function on 3 separate days:
Study day 1: inclusion at the Intermediate Care Unit (IMCU) or the Intensive Care Unit (ICU).
Study day 2: during hospitalization.
Study day 3: follow-up after discharge, at least 90 days after disease onset.
Subjects
The study included 12 patients with a critical COVID-19 infection at the IMCU or the ICU at Danderyd University Hospital between 15th May and 17th June 2020. Patients eligible for the study were adults at any age, with a confirmed COVID-19 infection and severe respiratory failure in need for invasive/non-invasive ventilation or high flow nasal oxygen support. Patients were included by convenience sampling. Data on demographics, co-morbidities, medical treatments, laboratory results, radiographical findings, complications, and hospital discharge was obtained from the patients and electronic health records.
Twelve control subjects were included into the study. The control group consisted of healthy individuals and patients with co-morbidities recruited from an out-patient clinic at the hospital. We intended to include controls that matched the patient group with regard to background factors such as age, sex, and relevant co-morbidities. Exclusion criteria for the control group was confirmed or suspected COVID-19 infection during 2020.
COVID-19 infection was defined by the detection of SARS-CoV-2 in a respiratory specimen by a polymerase chain reaction assay. Overweight and obesity were defined as body mass index (BMI) ≥ 25.0 and 30.0 kg/m2, respectively. Chronic kidney disease (CKD) was defined as CKD stage 3 or higher with reduced estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2. No antivirals, corticosteroids, or chloroquine derivatives were in use for the treatment of COVID-19 during the study period.
Skin microvascular reactivity
Skin microvascular reactivity was investigated through iontophoresis, which is a non-invasive method for drug application across the skin using a small electric current. Acetylcholine (ACh, Sigma–Aldrich AB, Stockholm, Sweden) and sodium nitroprusside (SNP, Hospira, Inc., Lake Forest, IL), diluted in sodium chloride solutions to final concentrations of 2%, were used to investigate endothelium-dependent and endothelium-independent microvascular reactivity, respectively. Two electrode chambers (LI611 Drug Delivery Electrode Imaging; Perimed, Järfälla, Sweden) were attached to the volar side of the left forearm at 5 cm apart, avoiding hair, broken skin, or visible veins. The proximal electrode was filled with ACh, while the distal electrode was filled with SNP. ACh and SNP iontophoreses were simultaneously performed using a battery-powered iontophoresis controller (LI 760 PeriIont System, Perimed, Järfälla, Sweden) which provided a single current application of 0.02 mA for 200 s. The forearm was stabilized with a vacuum fixation cushion to avoid recording artefacts. Skin perfusion was recorded with one image per second before, during and up to 11 min after iontophoresis through laser speckle contrast imaging (LSCI, PeriCam PSI NR; Perimed, Järfälla, Sweden) (6).
Data was processed through dedicated software (PIMSoft, Perimed, Järfälla, Sweden). The entire electrode chamber (1.5 cm2) was used as region of interest (ROI), i.e. the skin area of interest. Time of interest (TOI), defined as the duration in seconds over which data are averaged, was set to 60 s for assessment of baseline values, 20 s for peak values and 5 s for the other measurement points. The results are expressed in perfusion units (PU).
Skin temperature
Skin temperature of the volar side of left forearm was recorded with an electronic thermistor (Exacon, Copenhagen, Denmark).
Statistical analysis
Categorical and continuous variables were presented as number of patients (n), means ± standard deviations (SD), or medians with interquartile range (IQR). Independent t tests, Mann–Whitney U test, or Fisher's exact test were used to evaluate differences between groups, as appropriate. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed in Statistica, TIBCO Software Inc., version 13.
A sample size calculation estimated 22 participants in each group to adequately detect a large effect size (Cohen's d > 0.8) in a two-tailed independent t test with significance level 0.05 and power of 80%. Due to a markedly reduced incidence of critical COVID-19 cases during the summer, we were only able to include 12 patients into the study. However, our data showed a considerably higher effect size between patients and controls than expected. Post-hoc sample size analyses of our results showed that 5 individuals were sufficient to show significant differences in ΔACh-mediated responses between COVID-19 patients and controls.
Ethical consideration
The study protocol was approved by the Ethical Review Authority in Sweden.
RESULTS
The characteristics of patients and controls are presented in Table 1. There were no significant differences in age, sex distribution, BMI, or co-morbidities between patients and controls. None of the patients or controls were current smokers. Nine patients and eight controls were overweight (BMI > 25 kg/m2), while obesity was found in six patients (all survivors) and one control subject. Eleven patients (92%) and six controls (50%) had co-morbidities and cardiovascular risk factors. Eight patients were in good physical condition (regular sport activities and/or long daily walks) prior to disease onset, while the remaining four were less physically active (occasional walks).
Table 1.
Characteristics of study patients and controls.
| Controls (n = 12) | All patients (n = 12) | Survivors (n = 8) | Non-survivors (n = 4) | P patients/controls | P patient groups | |
| Age (years) | 60 (55–67) | 66 (54–70) | 63 (52–71) | 68 (59–70) | 0.39 | 0.73 |
| Sex (male/female) | 7/5 | 11/1 | 7/1 | 4/0 | 0.17 | 1.0 |
| BMI (kg/m2) | 25.8 (23.4–29.1) | 30.1 (25.0–36.1) | 32.6 (27.6–36.6) | 26.3 (22.6–26.5) | 0.10 | 0.13 |
| Co-morbidity (n) | ||||||
| Obesity | 1 | 6 | 6 | 0 | 0.07 | 0.06 |
| Hypertension | 6 | 8 | 6 | 2 | 0.68 | 0.55 |
| Diabetes mellitus | 0 | 4 | 3 | 1 | 0.09 | 1.0 |
| CKD | 0 | 0 | 0 | 0 | – | – |
| IHD | 1 | 2 | 2 | 0 | 1.0 | 0.52 |
| Heart failure | 0 | 2 | 2 | 0 | 0.48 | 0.52 |
| COPD | 0 | 1 | 1 | 0 | 1.0 | 1.0 |
| Asthma | 1 | 0 | 0 | 0 | 1.0 | – |
| No co-morbidities | 6 | 1 | 1 | 0 | 0.07 | 1.0 |
| Blood chemistry | ||||||
| Peak CRP (mg/L) | – | 347 (330–370) | 341 (308–354) | 370 (350–460) | – | 0.08 |
| Peak creatinine (μmol/L) | – | 114 (99–219) | 108 (98–297) | 157 (99–219) | – | 0.68 |
| Peak Troponin I (ng/L) | – | 35 (11–89) | 17 (10–74) | 64 (61–89) | – | 0.26 |
Data is presented in medians and interquartile range or in numbers (n). BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; IHD, ischemic heart disease.
Brief clinical status of the patients on study day 1 to 3 is presented in Table 2. At the time of inclusion (study day 1), 10 patients were treated with non-invasive ventilation while two were treated with invasive ventilation. The patients had been treated with non-invasive or invasive ventilation for 1.5 days (median, IQR 0.5–3.0) at the time of inclusion. The second examination on study day 2 was performed at a later stage of the hospital course, 8.5 days (median, IQR 6–21) after study day 1. Three of the four fatal cases were due to progression of respiratory failure despite mechanical ventilation, while one patient died from cardiac arrest during the acute COVID-19 phase. One patient (survivor) developed septic shock and systemic circulatory failure due to secondary bacterial infection, while blood cultures were negative in the remaining 11 patients. We found no significant differences in any baseline characteristics between survivors and non-survivors.
Table 2.
Clinical status of patients on study day 1–3.
| Study day 1 (n = 12) During hospitalization | Study day 2 (n = 10) During hospitalisation | Study day 3 (n = 7) Post-COVID phase | |
| Respiratory status | |||
| Respiratory rate (rate per minute) | 30 (25–31) | 24 (22–28) | – |
| Arterial saturation (%) | 91 (90–92) | 95 (93–97) | – |
| No respiratory support (n) | 0 | 4 | 7 |
| Oxygen supply only (n) | 0 | 4 | 0 |
| Non-invasive ventilation (n) | 10 | 0 | 0 |
| Invasive ventilation (n) | 2 | 2 | 0 |
| Circulatory status | |||
| Systolic blood pressure (mm Hg) | 126 (120–133) | 129 (110–146) | 109 (108–121) |
| Diastolic blood pressure (mm Hg) | 70 (62–83) | 70 (60–83) | 83 (73–85) |
| Heart rate (beats per minute) | 74 (56–83) | 75 (62–88) | 65 (73–85) |
| Vasoactive drugs (n) | 1 | 2 | 0 |
| Body temperature (°C) | 37.7 (36.8–38.1) | 36.9 (36.4–37.2) | – |
| C-reactive protein level (mg/L) | 180 (115–311) | 26 (16–185) | – |
Data is presented in medians and interquartile range or in numbers (n).
Microcirculation analyses were performed in seven of the eight surviving patients during the post-infectious phase. One patient was lost to follow-up due to other engagements. Median time from disease onset until time of follow-up was 114 days (range 96–144 days). At the time of follow-up, all surviving patients had been discharged to their homes and were no longer in need for oxygen supply. Five patients experienced a slightly or markedly reduced physical condition compared to their physical status prior to COVID-19, while two patients reported restored activity level. No patients had started any new medical treatments.
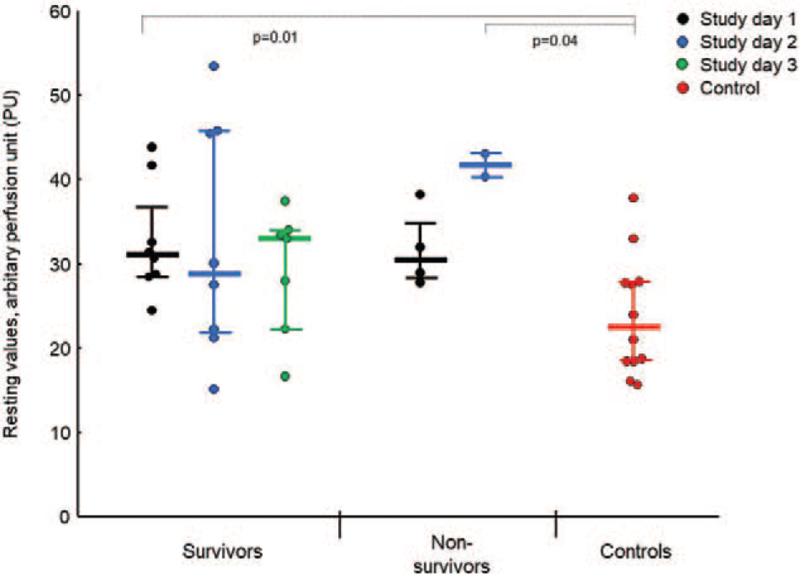
Basal skin perfusion levels in surviving and non-surviving patients examined on separate days and in controls, respectively, are presented in Figure 1. Overall, the patients had significantly increased basal skin perfusion on study day 1 (32 ± 6 PU) and a tendency toward increased basal skin perfusion on study day 2 (35 ± 13 PU), compared to controls (24 ± 7 PU, P = 0.006 for study day 1 and P = 0.06 for study day 2). Also, basal skin perfusion differences between patients and controls on study day 1 remained significant when two patients treated with sedation and intravenous vasoactive drugs were excluded from analyses (data not shown). Skin perfusion at rest was not correlated to body temperature and did not differ between patients with and without fever (data not shown). We found no differences in room temperature or in forearm skin temperature on investigated arm between patients and controls on study day 1 to 3, respectively (data not shown).
Fig. 1.
Mean basal skin perfusion in patients with critical COVID-19 and controls. Mean basal values (absolute values, medians and interquartile range) of forearm skin perfusion in patients with critical COVID-19 on study day 1 (black circles, n = 12), study day 2 (blue circles, n = 10), and study day 3 (the post-COVID-19 phase, green circles, n = 7); and in control subjects (red circles, n = 12). Basal skin perfusion on study day 1 was significantly increased among the patients in total (survivors and non-survivors, P = 0.006) and among the survivors (P = 0.01) compared to controls. We found no significant differences in basal skin perfusion between survivors and non-survivors. During the post-COVID phase (study day 3), basal skin perfusion was not significantly different in survivors compared to controls (P = 0.16).
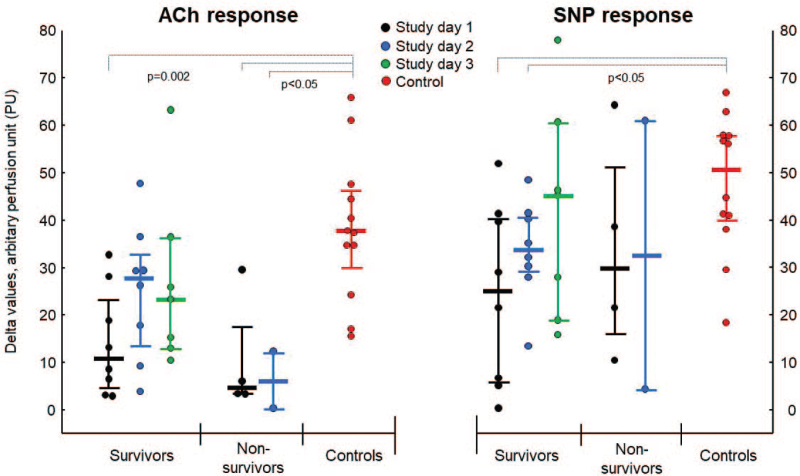
Delta values in ACh-mediated (endothelial-dependent) vasodilation was significantly reduced in the patients (survivors and non-survivors combined) on study day 1 and 2 (7 [3–23] and 22 [9–29] PU, respectively), compared to controls (38 [30–47] PU, P < 0.05 for both), equivalent to a reduction of vasodilatory capacity by 81% and 43% in COVID patients on study day 1 and 2, respectively. In the post-COVID phase (study day 3), patients still showed a reduced ACh-mediated vasodilation by 39% compared to controls, although this difference was no longer significant (23 [13–36] vs. 38 [30–47] PU, P = 0.08). Subgroup analyses showed that both surviving and non-surviving patients had significantly reduced ACh-induced vasodilation on study day 1 compared to controls (see Fig. 2, left panel). During study day 2 and the post-COVID phase, ACh-mediated vasodilation still tended to be reduced in survivors compared to controls (P = 0.08 for both).
Fig. 2.
Changes in skin microvascular reactivity in response to acetylcholine and sodium nitroprusside iontophoresis during the course of critical COVID-19. Delta values (absolute values, medians, and interquartile range) of forearm skin microcirculation flux before and after iontophoresis of acetylcholine (ACh, left panel) and sodium nitroprusside (SNP, right panel) in patients with critical COVID-19 on: study day 1 (black circles, n = 12); study day 2 (blue circles, n = 10); study day 3, the post-COVID phase (green circles, n = 7), and in control subjects (red circles, n = 12). ACh-mediated vasodilation was significantly reduced in patients on study day 1 compared to controls (P = 0.002 for survivors and P = 0.01 for non-survivors). Survivors had reduced SNP-mediated responses on study day 1 and 2 compared to controls (P < 0.05 for both days).
SNP-mediated (endothelial-independent) vasodilation was significantly reduced in patients in total on study day 1 (P = 0.01) and study day 2 (P = 0.04), compared to controls, and median SNP-mediated vasodilation was reduced by 51% and 34%, on study day 1 and 2, respectively. Subgroup analyses of survivor and non-survivors showed significant differences in SNP-mediated responses between survivors and controls on study day 1 and 2 (P < 0.05 for both, see right panel of Fig. 2). Neither ACh- nor SNP-mediated vasodilation was correlated to body temperature, skin temperature, mean arterial blood pressure, ratio oxygen saturation/FiO2, or days since disease onset or (data not shown).
ACh/SNP ratio of the delta levels on study day 1 was significantly reduced in patients compared to controls (0.50 ± 0.36 vs. 0.91 ± 0.49, P = 0.02). Among surviving patients, ACh/SNP ratios increased from 0.47 to 0.83 (medians) from study day 1 to study day 2, while corresponding values in non-survivors decreased from 0.36 to 0.17.
DISCUSSION
Skin microcirculation has a unique role in thermoregulation where variations in skin blood flow are strictly regulated through separate populations of sympathetic adrenergic vasoconstrictor and sympathetic cholinergic vasodilator nerves (7). Normally, at rest and thermoneutral conditions, cutaneous blood flow is mainly influenced by vasoconstrictors and skin microcirculation is low. During exercise or increased body heat, stimulation of vasodilator sympathetic nerves and release of vasodilatory factors from local endothelial cells lead to increased skin blood flow. Skin microvascular function is determined by measurement of skin blood flow at rest and following a physiological and/or pharmacological provocation in order to assess the vasodilatation capacity. Iontophoresis of ACh induces a local endothelial-dependent vasodilation in skin microcirculation, while SNP is a NO donor that directly relaxes vascular smooth muscle cells without involvement of endothelial cells (5). A reduction in vascular response to ACh with no concurrent reduction in SNP response is thus indicative of endothelial dysfunction.
In this pilot study, we investigated skin perfusion and microvascular reactivity in 12 patients with critical COVID-19. The patients were examined during the acute and the post-infectious phase of the disease. Compared to controls, the patients had an increased basal skin perfusion during the acute disease phase (study day 1). The increased skin perfusion was not found to be associated with body temperature, room temperature, or ongoing treatments with vasoactive substances. Thus, it seems that critical COVID infection causes a dysregulated skin perfusion during the acute disease phase. Whether this microvascular dysregulation is explained by an altered sympathetic function and/or increased release of vasodilatory factors from inflamed microvascular endothelial cells and/or other factors remains to be elucidated. In the present study, we found no significant difference in basal skin perfusion between survivors and controls during the post-infectious phase. However, this could be due to the small sample size on study day 3, and a possible long-standing impact on microvascular regulation following critical COVID-19 needs to be investigated in a larger cohort.
Interestingly, skin temperature of the investigated forearm did not differ between patients and controls despite the significant differences in basal skin perfusion between the groups. Since capillaries in the dermal papillae regulate the skin and core body temperature, this may indicate that increased skin perfusion during critical COVID-19 is on a subcapillary level and that most blood surpasses the cutaneous capillaries through arteriovenous shunts. Indeed, reduced capillary red blood cell velocity and a decreased portion of perfused microvessels have been demonstrated in patients with severe COVID-19 through sublingual video microscopy (8, 9). Rovas et al. showed that while vascular density of larger vessels (>10 μm in diameter) in the sublingual microcirculation of patients with severe COVID-19 was similar to that of controls, there was a significant reduction in capillary density (4–10 μm in diameter) among the patients (8). Similar microvascular shunting mechanisms in the pulmonary microcirculation cannot be excluded in patients with severe COVID-19, and could in part explain the progression to respiratory failure (10).
Parallel with the increased basal skin perfusion, patients with critical COVID-19 had significantly reduced endothelial-dependent (ACh-mediated) and endothelial-independent (SNP-mediated) vasoreactivity during acute disease phase (study day 1). Impaired microvascular reactivity in the skin with reduced responses to both ACh and SNP indicate that critical COVID-19 is associated with a systemic endothelial cell dysfunction and disturbance in the NO-dependent vasodilation pathway in the microvasculature. SNP mediates its effect on the vascular smooth muscle cells by activating the NO-sensitive guanylyl cyclase within the cells, which leads enhanced levels of intracellular cyclic guanosine monophosphate (cGMP) and muscle cell relaxation. The widespread inflammatory response and increased oxidative stress during critical COVID-19 may lead to reduced NO bioavailability and/or reduced responsiveness in the NO-sensitive signaling cascade in the vascular smooth vascular cells. Impairment of the microvascular endothelial cell dysfunction seems however to be more pronounced since patients had significantly reduced ACh/SNP vasodilation ratio on study day 1 compared to controls. Altogether, our results of increased basal skin perfusion and reduced skin vasodilation capacity indicate that the pronounced proinflammatory state induced by SARS-CoV-2 virus is associated with systemic microvascular dysregulation and endothelial malfunction. Accordingly, a previous study has demonstrated increased baseline skin microvascular perfusion and reduced skin endothelium-dependent microvascular reactivity, assessed by single-point laser Doppler fluxmetry, in patients with COVID-19 compared to healthy controls (11). Moreover, COVID-19 related endothelial cell activation and endothelitis in vascular beds of various organs have been demonstrated in post-mortem studies (1, 12). It has also been shown that levels of circulating markers of endothelial dysfunction and damage are increased in patients with severe COVID-19 and correlate with disease severity (8, 13). Whether endothelial cell damage and dysfunction is restored after the acute disease phase needs to be investigated. In the present study, we found a non-significant trend (P = 0.08) of reduced endothelial-dependent vasodilation compared to controls during the post-infectious phase, despite the low number of post-COVID cases (n = 7). Possible prolonged endothelial dysfunction and damage in the microvasculature following a severe or critical COVID-19 infection may contribute to the long-term symptoms experienced by numerous COVID-19 survivors.
The main limitation of this study is the small sample size. As the number of infected patients in our region decreased markedly at the end of the study period, we were unable to include additional patients. However, despite the small sample size, we were able to detect significant differences in basal skin perfusion and endothelial-dependent and endothelial-independent vasoreactivity between patients and controls during the acute phase of a critical COVID-19 infection. The non-significant differences between patients and controls regarding basal skin perfusion on day 2 and 3, and microvascular reactivity on study day 3, may be explained by type II errors due to the low sample size. Nevertheless, this hypothesis-generating pilot study rendered results that deserve attention, while awaiting data from larger studies investigating the effects of COVID-19 on the microcirculation.
We conclude that patients with critical COVID-19 and respiratory failure have a microvascular dysregulation and endothelial cell dysfunction.
Footnotes
This study was funded through a grant from The Swedish Medical Association.
The authors report no conflicts of interest.
REFERENCES
- 1.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, et al. Extrapulmonary manifestations of COVID-19. Nat Med 26:1017–1032, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105:370–372, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 19:47–64, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Humeau-Heurtier A, Abraham P, Durand S, Mahé G. Excellent inter- and intra-observer reproducibility of microvascular tests using laser speckle contrast imaging. Clin Hemorheol Microcirc 58:439–446, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 78:603–612, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, Kühn J, Braune S, Göbel U, Thölking G, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis 24:145–157, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edul VSK, Eguillor JFC, Ferrara G, Estenssoro E, Siles DSP, Cesio CE, Dubin A. Microcirculation alterations in severe COVID-19 pneumonia. J Crit Care 61:73–75, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res 21:198, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabioni L, De Lorenzo A, Lamas C, Muccillo F, Castro-Faria-Neto HC, Estato V, Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res 134:104119, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagashima S, Mendes MC, Camargo Martins AP, Borges NH, Godoy TM, Miggiolaro AFRDS, da Silva Dezidério F, Machado-Souza C, de Noronha L. Endothelial dysfunction and thrombosis in patients with COVID-19 – brief report. Arterioscler Thromb Vasc Biol 40:2404–2407, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 8:e575–e582, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]