Abstract
The cyclic AMP (cAMP)-responsive factor CREB promotes cellular gene expression, following its phosphorylation at Ser133, via recruitment of the coactivator paralogs CREB-binding protein (CBP) and p300. CBP and p300, in turn, appear to mediate target gene induction via their association with RNA polymerase II complexes and via intrinsic histone acetyltransferase activities that mobilize promoter-bound nucleosomes. In addition to cAMP, a wide variety of stimuli, including hypoxia, UV irradiation, and growth factor addition, induce Ser133 phosphorylation with stoichiometry and kinetics comparable to those induced by cAMP. Yet a number of these signals are incapable of promoting target gene activation via CREB phosphorylation per se, suggesting the presence of additional regulatory events either at the level of CREB-CBP complex formation or in the subsequent recruitment of the transcriptional apparatus. Here we characterize a Tyr134Phe CREB mutant that behaves as a constitutive activator in vivo. Like protein kinase A (PKA)-stimulated wild-type CREB, the Tyr134Phe polypeptide was found to stimulate target gene expression via the Ser133-dependent recruitment of CBP and p300. Biochemical studies reveal that mutation of Tyr134 to Phe lowers the Km for PKA phosphorylation and thereby induces high levels of constitutive Ser133 phosphorylation in vivo. Consistent with its constitutive activity, Tyr134Phe CREB strongly promoted differentiation of PC12 cells in concert with suboptimal doses of nerve growth factor. Taken together, these results demonstrate that Ser133 phosphorylation is sufficient for cellular gene activation and that additional signal-dependent modifications of CBP or p300 are not required for recruitment of the transcriptional apparatus to the promoter.
A number of growth factors have been shown to induce cellular responses via the reversible phosphorylation of specific transcription factors. Phosphorylation, in turn, has been found to activate these factors by a number of mechanisms including nuclear import, DNA binding, and coactivator recruitment (23). Cyclic AMP (cAMP) stimulates cellular gene expression, for example, via the protein kinase A (PKA)-mediated phosphorylation of CREB at Ser133 (20), and this modification enhances the transactivation potential of CREB by promoting the recruitment of the coactivator paralogs CREB-binding protein (CBP) and p300 (4, 28). Biochemical reconstitution studies suggest that CBP and p300 mediate target gene activation, in turn, via their association with RNA polymerase II complexes (14, 25, 31, 32). In vitro transcription studies with purified basal transcription factors reveal that Ser133 phosphorylation of CREB is sufficient to recruit CBP-RNA polymerase II complexes and to induce transcription from a cAMP-responsive promoter (33).
The solution structure of the CREB-CBP complex, which was determined by using relevant interaction domains referred to as KID and KIX, respectively, reveals that the phosphorylated KID domain of CREB undergoes a random coil-to-helix transition upon binding to the KIX domain of CBP (38). This transition in KID stabilizes complex formation by promoting hydrophobic interactions with residues lining a shallow hydrophobic groove in KIX (38). Phospho-Ser133 also forms direct contacts with residues in KIX that account for approximately half of the free energy of complex formation (35, 38). The direct participation of Ser133 and flanking amino acids in coactivator recruitment illustrates a dual role for these residues in substrate recognition and recruitment of the transcriptional apparatus.
In addition to cAMP, a variety of stimuli, including hypoxia (6), UV irradiation (24), and growth factor addition (11, 19, 37, 39), have been shown to promote Ser133 phosphorylation of CREB with stoichiometry and kinetics comparable to those induced by cAMP. But many of these pathways appear to be incapable of stimulating target gene expression via CREB phosphorylation per se (9), suggesting the presence of additional regulatory events either at the level of CREB-CBP complex formation or in the subsequent recruitment of the transcriptional apparatus.
In artificial recruitment assays with a GAL4-CBP chimera, PKA was found to potentiate CBP activity directly (15, 28). Calcium-dependent induction of cellular genes via phospho-Ser133 CREB was also reported to require a nuclear calcium signal that modulates CBP transcriptional activity (12, 22). Ras-dependent growth factors were apparently incapable of stimulating phospho-Ser133 CREB activity, for example, due to a block in the nuclear calcium signal (12).
In contrast with the results of the above studies, recombinant Ser133-phosphorylated CREB appears competent to induce transcription from a CRE-luciferase reporter plasmid following its microinjection into mouse embryo fibroblasts, supporting the notion that Ser133 phosphorylation is indeed sufficient for target gene induction (2). The apparent discrepancy between these studies prompted us to search for mutant CREB polypeptides that associate with CBP constitutively in vivo. Here we report a gain-of-function CREB polypeptide that interacts constitutively with CBP, due to a mutation that promotes Ser133 phosphorylation under basal conditions. Our results demonstrate that Ser133 phosphorylation is sufficient for association with CBP and p300 and for recruitment of the transcriptional apparatus to the promoter.
MATERIALS AND METHODS
Yeast two-hybrid assays and mammalian transfections.
Yeast assays were performed with wild-type or Tyr134Phe GAL4-KID cDNAs inserted into pAS2.1 (Clontech). The Tyr134Phe substitution was generated by PCR mutagenesis and verified by manual sequencing. A CBP (amino acids [aa] 226 to 1830)-GAL4 activation domain protein was expressed from the yeast parent vector pACT2. Yeast GC1954 cells were cotransformed with pAS2.1 KID or pAS2.1 Tyr134Phe KID mutant, and pACT2-CBP vectors were cotransformed by lithium chloride-mediated transformation. β-Galactosidase assays were performed according to standard protocols. The somatostatin chloramphenicol acetyltransferase (CAT) reporter construct (see Fig. 1D) contained 1.4 kb of promoter sequence. The mitogen-activated protein (MAP) kinase phosphatase reporter construct contained 1.6 kb of promoter sequence (27) and was a generous gift of J. Dixon (University of Michigan). The c-fos reporter gene (FC4-CAT) contained 400 bases of upstream promoter sequence (7). Transient-transfection assays were performed with 293T cells as previously reported (18), using a cotransfected cytomegalovirus (CMV)–β-galactosidase vector as the internal control.
FIG. 1.
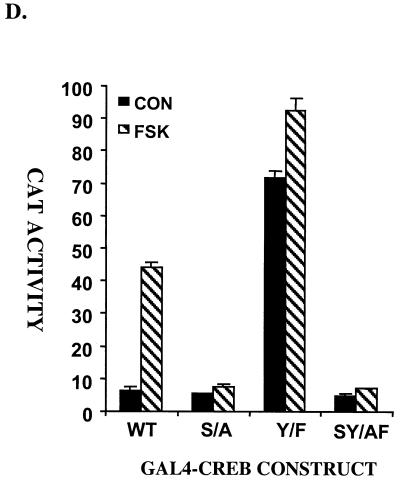
A gain-of-function Tyr134Phe CREB polypeptide stimulates target gene expression in a Ser133-dependent manner. (A) Yeast two-hybrid assay of pAS2.1 KID (WT) and pAS2.1 Tyr134Phe KID (Y/F) constructs containing the GAL4 DNA binding domain fused to aa 100 to 160 of CREB. Yeast GC1954 cells were cotransformed with a pACT2-CBP expression vector encoding CBP (aa 226 to 1830) fused to the GAL4 activation domain. β-Galactosidase activity derived from cells containing the integrated GAL4 reporter is shown. (B) Amino acid sequence alignment of KID domains in mammalian CREB family members (CREB, ATF1, and CREM) and CREB homologues from Aplysia (Aplys), Hydra, Drosophila (Droso), and Caenorhabditis elegans (c. ele). The conserved Tyr134 is in bold. The positions of αA and αB helices in KID are shown. (C) (Left) CAT activity derived from CRE-CAT reporter cotransfected with wild-type (WT) or Tyr134Phe mutant CREB (Y/F) expression plasmid in 293T cells. (Right) Activity of somatostatin (SMT), MAP kinase phosphatase (MKP) and c-fos CAT reporter plasmids following cotransfection with wild-type or Tyr134Phe CREB expression plasmids into PC12 cells. (D) Luciferase activity derived from 293T cells transfected with wild-type or Tyr134Phe mutant GAL4-CREB expression plasmids containing the GAL4 DNA-binding domain (aa 1 to 147) fused to the CREB transactivation domain (aa 1 to 283). The effect of a PKA phosphoacceptor site (Ser/Ala133) mutation (S/A) on activity of wild-type (W/T) and Tyr134Phe mutant (SY/AF) polypeptides is also shown. CON, control; FSK, forskolin treated.
Fluorescence polarization and phosphorylation assays.
Synthetic wild-type and Tyr134Phe mutant KID peptides (aa 119 to 147) were phosphorylated in vitro with recombinant PKA (generous gift of S. Taylor, University of California, San Diego), N-terminally fluoresceinated, and evaluated by fluorescence polarization assay as previously described (35). Quantitative phosphorylation assays of CREB peptides were performed using increasing concentrations of synthetic CREB peptide (generous gift of J. Rivier, Salk Institute). Levels of 32P incorporation were measured on P-81 paper after extensive washing with 10% trichloroacetic acid. Phosphatase assays on 32P-labeled PKA-phosphorylated CREB peptides were performed with purified PP-1 catalytic subunit (generous gift of M. Bollen) under standard conditions.
Phosphotryptic mapping.
293T cells were transfected with GAL4-KID (8) and GAL Y134F-KID expression vectors containing the GAL4 DNA binding domain fused to aa 100 to 160 of CREB. After 24 h, transfected cells were incubated with phosphate-free Dulbecco's modified Eagle medium containing 10% dialyzed serum and 1 mCi of [32P]orthophosphate/ml. After a 4-h incubation, the cells were treated with vehicle or forskolin (10 μM) for 25 min, washed with cold phosphate-buffered saline, and harvested in sodium dodecyl sulfate (SDS) lysis buffer (0.5% SDS, 50 mM Tris [pH 8.0], 1 mM EDTA) containing a cocktail of phosphatase inhibitors (100 mM sodium fluoride, 1 mM sodium vanadate, 25 mM β-glycerophosphate, and 2 mM sodium pyrophosphate). After boiling for 10 min, the lysate was cooled to 4°C and precleared with 50 μl of protein A/G agarose. The supernatants were diluted 1:5 with cold radioimmunoprecipitation assay buffer containing phosphatase and protease (1 mM phenylmethylsulfonyl fluoride, 5 μM leupeptin, 10 μM pepstatin, 1 mM dithiothreitol) inhibitors and were immunoprecipitated for 2 to 4 h using anti-GAL4 DNA binding domain antibody (Santa Cruz) and anti-CREB antibody (244). The immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis, and tryptic phosphopeptide maps were developed as previously described (21).
Differentiation of PC12 cells.
PC12 cells (106) in 100-mm plates were cotransfected with CMV-green fluorescent protein expression plasmid plus either wild-type or Tyr134Phe CREB expression constructs by calcium phosphate precipitation. One day after transfection, cells were stimulated with nerve growth factor (NGF) (10 or 100 ng/ml) in 1% horse serum plus Dulbecco's modified Eagle medium. After an additional 3 days, transfected cells containing neurites at least two cell diameters long were counted under the fluorescent microscope. Results were repeated and confirmed in three independent experiments.
RESULTS
In the course of yeast two-hybrid screening experiments to identify mutations in the KID domain of CREB that alter its binding to the KIX domain of CBP, we discovered a mutant Tyr134Phe KID peptide with activity 10-fold higher than that of wild-type KID (Fig. 1A). Notably, Tyr134 is absolutely conserved amongst CREB homologues, suggesting an important function for this residue in transcriptional regulation (Fig. 1b).
To test whether mutation of Tyr134 to Phe stimulates CREB activity in the context of the full-length protein, we performed transient-transfection assays in 293T cells. When cotransfected with a CRE reporter plasmid, Tyr134Phe CREB was eight times more active than wild-type CREB in unstimulated cells (Fig. 1C, left). Tyr134Phe CREB was also more active than wild-type CREB on several CRE-containing promoters in PC12 cells (Fig. 1C). Somatostatin, MAP kinase phosphatase-1, and c-fos reporter genes were all induced under basal conditions, although the level of induction by Tyr134Phe compared to wild-type CREB appeared to be highest on promoters containing simple CRE sites (somatostatin) versus multiple promoter-bound activators (MAP kinase phosphatase and c-fos) (Fig. 1C).
To rule out potential regulatory contributions from other mammalian CRE-binding proteins, we examined the activity of the Tyr134Phe CREB polypeptide in the context of a GAL4-CREB (1 to 283) construct lacking the basic region-leucine zipper domain. The Tyr134Phe mutant GAL4-CREB construct showed 10-fold higher activity than its wild-type counterpart under basal conditions (Fig. 1D). Addition of cAMP agonist further potentiated GAL4-Tyr134Phe CREB activity, although the induction of Tyr134Phe was lower than for wild-type GAL4-CREB (Fig. 1D). Illustrating the importance of the PKA phosphoacceptor site for the constitutive activity of Tyr134Phe CREB, mutation of Ser133 to alanine completely disrupted the activity of both wild-type and mutant (Tyr134Phe) constructs. These results demonstrate that transcriptional induction via Tyr134Phe CREB is Ser133 dependent (Fig. 1D).
To determine whether Tyr134Phe CREB stimulates transcription via CBP recruitment, we performed mammalian two-hybrid studies using the relevant interaction domains in CREB (KID) and CBP (KIX). In mammalian two-hybrid assays, mutant Tyr134Phe GAL4-KID polypeptide was 10- to 20-fold more active than wild-type GAL4-KID under basal conditions (Fig. 2A). Following cotransfection with a KIX-VP16 expression vector, the activity of Tyr134Phe mutant GAL4-KID but not wild-type GAL4-KID was enhanced fivefold, demonstrating that the Tyr134Phe mutation promotes a constitutive interaction between the KID and KIX domains (Fig. 2A).
FIG. 2.
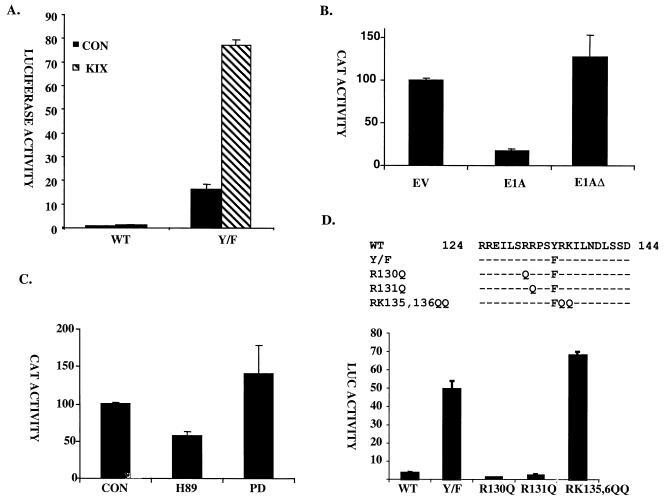
Mutation of Tyr134 to Phe enhances CREB activity via a PKA- and CBP-dependent mechanism. (A) Mammalian two-hybrid assay comparing activity of wild-type (WT) and Tyr134Phe (Y/F) mutant GAL4-KID constructs following cotransfection with empty vector (CON) or KIX-VP16 expression plasmid in 293T cells. (B) GAL4 reporter activity derived from 293T cells following cotransfection with Tyr134Phe GAL4-KID construct plus wild-type E1A or CBP interaction-defective mutant E1AΔ vector lacking aa 15 to 35 (5). (C) Effect of protein kinase inhibitors on Tyr134Phe GAL4-CREB activity. 293T cells were transfected with GAL4-Tyr134Phe CREB expression vector plus GAL4-luciferase reporter. After 24 h, transfected cells were treated with PKA-specific (H89, 10 μM) or MAP kinase-specific (PD98059, 20 μM) inhibitors for 6 h. CON, control. (D) Importance of basic residues flanking phospho-Ser133 acceptor site for target gene activation via GAL4-Tyr134Phe CREB. The graph shows results of a transient-transfection assay of 293T cells transfected with wild-type and mutant GAL4-CREB expression vector plus a GAL4-luciferase (LUC) reporter. The sequences of wild-type and mutant CREB proteins are shown above.
The adenovirus E1A oncoprotein has been shown to inhibit target gene activation via CBP, in part by blocking its association with RNA polymerase II complexes (3, 30, 33). As a further test of Tyr134Phe activity, we performed transfection assays with wild-type E1A or a CBP interaction-defective E1A construct (E1AΔ) (5). Wild-type E1A reduced the basal activity of Tyr134Phe CREB about 10-fold, but E1AΔ had no effect, supporting the notion that Tyr134Phe CREB stimulates target gene expression via a CBP-dependent pathway (Fig. 2B).
To determine whether the constitutive activity of Tyr134Phe CREB is PKA dependent, we employed H89, a selective pharmacological inhibitor of PKA. 293T cells were transfected with a GAL4-Tyr134Phe CREB expression vector; after 24 h, the transfected cells were treated with H89 (10 μM) or vehicle for 6 h and were then assayed for luciferase activity derived from a cotransfected GAL4-luciferase reporter construct (Fig. 2C). Basal Tyr134Phe GAL4-CREB activity was blocked about 50% by H89 (Fig. 2C). In contrast, treatment with the MAP kinase-specific inhibitor PD98059 (20 μM) actually enhanced reporter activity somewhat, revealing that Tyr134Phe activity is dependent on a specific subset of cellular kinases (Fig. 2C).
Substrate recognition by PKA relies primarily on arginine residues located at −2 and −3 relative to the serine phosphoacceptor site (43). Supporting a role for PKA in target gene activation via Tyr134Phe CREB, mutagenesis of −3 (Arg130) or −2 (Arg131) arginines in GAL4-Tyr134Phe CREB to glutamine blocked reporter induction (Fig. 2D). In contrast, mutation of Arg135 or Lys136 to glutamine in the context of Tyr134Phe CREB potentiated Tyr134 Phe CREB activity about 40% (Fig. 2D). In this regard, basic residues C terminal to the PKA phosphoacceptor site have been shown to function as negative determinants of PKA phosphorylation (43), and mutagenesis of these residues to glutamine would be predicted to enhance substrate recognition by this kinase. Taken together, these results indicate that Tyr134Phe CREB polypeptide induces cellular gene expression via a CBP-dependent mechanism that requires basal PKA activity.
The importance of Ser133 and PKA activity for target gene induction via Tyr134Phe CREB prompted us to examine Ser133 phosphorylation on the mutant polypeptide in vivo. Although phospho-CREB levels are typically evaluated by Western blot assay with phosphoSer133 CREB antiserum, the importance of Tyr134 for recognition by this antibody precluded its use in comparative phosphorylation experiments (not shown). Consequently, we performed two-dimensional phosphotryptic mapping studies on immunoprecipitates of wild-type and Tyr134Phe mutant GAL4-KID proteins prepared from metabolically labeled 293T cells with a nondiscriminating CREB antiserum (244). Western blot analysis of 293T cell lysates, using either anti-GAL4 (Fig. 3A) or 244 antiserum (data not shown), revealed that wild-type and Tyr134Phe mutant GAL4-KID polypeptides are comparably expressed in transfected cells. Ser133 phosphorylation in unstimulated cells was four times higher for the Tyr134Phe than for wild-type polypeptide (Fig. 3B, compare spots A). In contrast, phosphorylation of an unrelated phosphoacceptor site on KID was comparable in both wild-type GAL4-KID polypeptides, demonstrating that Ser133 phosphorylation is specifically enhanced in the mutant CREB polypeptide (Fig. 3B, compare spots B). Consistent with their activation by cAMP agonist, both wild-type and mutant proteins were inducibly phosphorylated in response to forskolin treatment (10 μM, 25 min), although the level of Ser133 phosphorylation was two times higher in the mutant Tyr134Phe polypeptide (Fig. 3B). These results demonstrate that the higher activity of the Tyr134Phe CREB polypeptide is indeed correlated with enhanced Ser133 phosphorylation in unstimulated cells.
FIG. 3.
Dominant active Tyr134Phe CREB polypeptide is constitutively phosphorylated at Ser133 in vivo. (A) Western blot assay of 293T cells transfected with wild-type and Tyr134Phe mutant GAL4-KID expression vectors. Immunoblotting of whole extracts from transfected 293T cells was performed with anti-GAL4 antiserum (α-GAL). Extracts from control and forskolin (FSK)-treated (10 μM, 25 min) cells are indicated. (B) Two-dimensional tryptic mapping of immunoprecipitated 32P-labeled wild-type and Tyr134Phe mutant GAL4-KID polypeptides following treatment of transfected 293T cells with forskolin (10 μM) or vehicle for 25 min. The phosphoSer133 tryptic peptide is labeled A, and the unrelated tryptic peptide in KID is labeled B. The minor spot over peptide A corresponds to a partially digested fragment that also contains Ser133.
We considered several scenarios to explain the altered activity of the Tyr134Phe CREB protein; enhanced phosphorylation by PKA or other cellular kinase, reduced dephosphorylation by the Ser/Thr phosphatase PP-1, or higher affinity of the Ser133-phosphorylated Tyr134Phe polypeptide for the KIX domain of CBP. To test these models, we prepared synthetic peptides for wild-type and Tyr134Phe CREB, extending from aa 119 to 147 and containing the minimal KIX interaction domain. The affinity of mutant and wild-type CREB peptides for the KIX domain was evaluated using a fluorescence polarization assay. Following quantitative Ser133 phosphorylation by PKA and N-terminal fluorescein addition, wild-type and Tyr134Phe peptides were incubated with increasing concentrations of purified KIX peptide. The estimated Kd values for complex formation with KIX were 516 nM for wild-type CREB and 730 nM for the Tyr134Phe mutant (Fig. 4A). The comparable binding affinity of these peptides for KIX by fluorescence anisotropy assay is predicted by the nuclear magnetic resonance structure of the KID-KIX complex, which shows that the Tyr134 hydroxyl group is directed towards the solvent and is therefore unavailable for binding to residues in KIX or KID. By providing similar hydrophobic contacts. Phe is therefore competent to substitute for Tyr134.
FIG. 4.
Mutation of Tyr134 to Phe enhances the Km of PKA for CREB. (A) PKA-phosphorylated wild-type and Tyr134Phe CREB peptides exhibit similar affinities for KIX in vitro. A fluorescence anisotropy assay of mutant and wild-type fluorescein-tagged phosphoSer133 KID (aa 119 to 147) peptides was performed in the presence of increasing concentrations of KIX (aa 588 to 672). Apparent Kd values were 516 ± 88 nM for KID and 730 ± 112 nM for Tyr134Phe KID. (B) Wild-type and Tyr134Phe mutant CREB peptides are comparably dephosphorylated by the Ser/Thr phosphatase PP-1. Dephosphorylation of 32P-labeled phosphoSer133 CREB peptides by purified PP-1 catalytic subunit is indicated. The graph shows trichloroacetic acid-precipitable counts from 32P-labeled peptide phosphorylated in vitro with PKA. (C) Double-reciprocal plot showing relative Ser133 phosphorylation of Tyr134Phe mutant (Y/F) and wild-type KID (WT) peptides (aa 119 to 147) by PKA in vitro. The estimated Km values for PKA were 8.9 μM for wild-type KID and 1.7 μM for Tyr134Phe KID.
The importance of the Ser-Thr phosphatase PP-1 in attenuating CREB activity in vivo (21) prompted us to test whether mutation of Tyr134 to Phe enhances CREB activity by reducing PP-1-mediated dephosphorylation at Ser133. Following incubation with purified catalytic subunit of PP-1, 32P-labeled wild-type and Tyr134Phe mutant phosphoSer133 CREB peptides were comparably dephosphorylated over the course of a 1-h incubation, indicating that PP-1 binds with similar affinity to wild-type and mutant CREB proteins. Thus, reduced dephosphorylation at Ser133 is unlikely to account for the constitutive activity of the Tyr134Phe CREB construct (Fig. 4B).
To test whether mutation of Tyr134 to Phe alters the Km of CREB for PKA, we performed phosphorylation experiments using synthetic wild-type or Tyr134Phe mutant peptides plus recombinant PKA. Remarkably, the Km for PKA, estimated by double-reciprocal plot, was fivefold lower for the mutant Tyr134Phe peptide (Km = 1.7 μM) than for the wild-type CREB peptide (Km = 8.9 μM) (Fig. 3C). This result is in agreement with the crystal structure of PKA, showing that residues at the P+1 position fit into a hydrophobic pocket within the catalytic cleft (26).
NGF has been reported to stimulate differentiation of PC12 cells, in part via the MAP kinase-dependent phosphorylation of Elk-1 and via the pp90RSK-mediated phosphorylation of CREB at Ser133 (40). The importance of CREB for induction of cellular genes by NGF (1) prompted us to evaluate the ability of Tyr134Phe CREB to promote differentiation of PC12 cells in conjunction with a suboptimal NGF stimulus. Towards this end, we transfected PC12 cells with expression vectors encoding constitutively active Tyr134Phe or wild-type CREB polypeptides. The transfected cells were treated with subthreshold doses of NGF (10 ng/ml) or vehicle (dimethyl sulfoxide). After 3 days, PC12 cells transfected with empty vector alone showed no change in cell morphology either in the control or with low-dose NGF treatment (Fig. 5). High-dose (100-ng/ml)-NGF-treated PC12 cells showed abundant neurite extension as expected (data not shown). Consistent with the requirement for induction of Elk-1 activity, expression of Tyr134Phe CREB alone had no effect on the morphology of untreated PC12 cells, despite high levels of transcriptional activity by CRE reporter assay (Fig. 5 and data not shown). Following treatment with low-dose NGF (10 ng/ml), however, Tyr134Phe strongly induced neurite formation compared with wild-type-CREB-transfected cells (Fig. 5). These results demonstrate the ability of Tyr134Phe CREB to stimulate cellular gene expression in concert with growth factor signals.
FIG. 5.
Tyr134Phe CREB promotes differentiation of PC12 cells in cells exposed to subthreshold doses of NGF. (Left) Photomicrographs of PC12 cells transfected with either wild-type (WT) or Tyr134Phe mutant CREB constructs and treated with low-dose NGF for 2 days. Transfected cells were visualized by fluorescence derived from a cotransfected CMV-green fluorescent protein expression vector. (Right) Bar graph showing the percentage of cells (% Dif. Cells) with neurite extensions at least two cell diameters in length. Transfection with wild-type or Tyr134Phe mutant (Y/F) is indicated. Cells were either maintained in control medium or supplemented with 10 mg of NGF/ml. Values are from three independent experiments in which at least 200 cells were counted per assay point. Error bars are shown.
DISCUSSION
A number of signaling pathways have been found to promote Ser133 phosphorylation of CREB without concomitant induction of cellular genes. In this regard, recruitment of the transcriptional apparatus has been proposed to require the subsequent calcium- or cAMP-mediated induction of CBP transcriptional activity, perhaps via a phosphorylation-dependent mechanism (12).
In this report, we characterized a novel gain-of-function CREB mutant that promotes cellular gene expression in unstimulated cells, due to constitutive phosphorylation of the mutant polypeptide at Ser133. Biochemical reconstitution experiments indicate that mutation of Tyr134 to Phe lowers the Km of CREB for PKA, and studies with the pharmacological PKA inhibitor H89 implicate the involvement of basal PKA activity in this process. The crystal structure of the PKA catalytic subunit reveals that the +1 pocket preferentially recognizes bulky hydrophobic residues (26), and affinity selection assays with a randomized peptide library demonstrate that Leu and Phe are highly preferred by PKA at the +1 site (41). Preference for a Phe at +1 by PKA is also suggested by the yeast ADR1 transcriptional activator, which contains a high-affinity PKA recognition site (RRASF; Km = 0.23 μM) at Ser230 (13, 16). PKC family members also favor Phe at this position (34), suggesting the potential involvement of additional cellular kinases in phosphorylating the Tyr134Phe mutant CREB protein in vivo. However, as apparent evidence against a role for PKC in this process, down-regulation of PKC by prolonged (16-h) exposure to tetradecanocyl phorbol acetate had no effect on Tyr134Phe CREB activity in a transient-transfection assay (K. Du and M. Montminy, unpublished observations). Moreover, PKC family members often require basic residues downstream of the phosphoacceptor site (34), but mutagenesis of Arg135 and Lys136 actually potentiated Tyr134Phe CREB activity in 293T cells. These results support the notion that Tyr134Phe CREB is phosphorylated by PKA under basal conditions.
Tyr134 is absolutely conserved amongst CREB homologues, and our results suggest that this residue functions importantly as part of a rheostat mechanism that maintains low levels of Ser133 phosphorylation under basal conditions. In addition to kinase recognition, Tyr134 also functions importantly in complex formation with CBP. The solution structure of the KID-KIX complex reveals that Tyr134 forms hydrophobic contacts with residues in the shallow hydrophobic groove of KIX, as well as with hydrophobic residues in KID (Ile 128 and Leu138) (38). Indeed, mutagenesis of Tyr134 to Ala blocks association of phosphoSer133 CREB with KIX in vitro, demonstrating the dual role of this site in interfacing with the signaling machinery and in promoting recruitment of the transcriptional apparatus (35). Mutation of Tyr134 to Phe appears to be well tolerated by the complex; no change in binding affinity of the mutant Tyr134Phe KID peptide for KIX was observed relative to that of wild-type KID.
A number of studies have indicated that target gene activation in response to calcium and cAMP additionally requires signal-dependent phosphorylation of CBP (12). Although our data do not exclude the possibility that signal-dependent phosphorylation of CBP enhances target gene activation in response to calcium and cAMP, they indicate that Ser133 phosphorylation of CREB is sufficient for target gene activation without modification of other components in the transcriptional apparatus. These results are supported by in vitro transcription studies showing that CBP-polymerase II complexes are efficiently recruited to the promoter following Ser133 phosphorylation of CREB by PKA (33) and by cellular studies showing that phosphoSer133 CREB is transcriptionally active following microinjection into rat embryo fibroblasts (2). Moreover, CREB-myb and CREB-sterol response element binding protein fusion proteins containing sequences that permit constitutive binding to the KIX domain also show constitutive target gene activation via CBP (10, 36). Taken together, these observations argue that recruitment of CBP or p300 to the promoter is sufficient for induction of cellular genes.
The identification of a gain-of-function mutant in CREB that operates through the same mechanism as wild-type CREB should be of general interest in characterizing growth factor responses in various cell types. In this study, for example, we found that Tyr134Phe CREB cooperated with a suboptimal dose of NGF to promote differentiation of PC12 cells. CREB has also been shown to promote proliferation of certain endocrine cells, in part by stimulating the expression of cell cycle-dependent genes (17, 42, 44). Mutations in cytoplasmic proteins that regulate cAMP production have been shown, for example, to promote somatotroph proliferation and tumor progression in acromegalic patients (29). Based on the ability of a single amino acid substitution to dramatically induce CREB activity, it will be of interest to determine whether mutation of this site occurs naturally within a subset of endocrine tumors.
ACKNOWLEDGMENTS
We thank Jean Rivier for synthesis of CREB peptides, Ishwar Radhakrishnan and Peter Wright for advice on CREB-CBP structure, and Rusty Gage for help with microscopy. We also thank J. Dixon for the gift of map kinase phosphatase reporter plasmid.
This work was supported by NIH grant RO1-37828. H.A. is supported by JSPS postdoctoral fellowships for research abroad (FY1999).
Hiroshi Asahara and Ulupi Jhala contributed equally to this work.
REFERENCES
- 1.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D D, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts A, Arias J, Hagiwara M, Montminy M, Feramisco J. Recombinant cyclic AMP response element binding protein (CREB) phosphorylated on ser-133 is transcriptionally active upon its introduction into fibroblast nuclei. J Biol Chem. 1994;269:7623–7630. [PubMed] [Google Scholar]
- 3.Arany Z, Newsome D, Oldread E, Livingston D, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:8184. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 4.Arias J, Alberts A, Brindle P, Claret F, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–228. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 5.Asahara H, Dutta S, Kao H-Y, Evans R M, Montminy M. Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner. Mol Cell Biol. 1999;19:8219–8225. doi: 10.1128/mcb.19.12.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beitner-Johnson D M D. Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism. J Biol Chem. 1998;273:19834–19839. doi: 10.1074/jbc.273.31.19834. [DOI] [PubMed] [Google Scholar]
- 7.Bertherat J, Chanson P, Montminy M. The cyclic adenosine 3′,5′-adenosine-responsive factor CREB is constitutively activated in human somatotroph adenomas. Mol Endocrinol. 1995;9:777–783. doi: 10.1210/mend.9.7.7476961. [DOI] [PubMed] [Google Scholar]
- 8.Brindle P, Linke S, Montminy M. Protein-kinase-A dependent activator in transcription factor CREB reveals new role for CREM repressors. Nature. 1993;364:821–824. doi: 10.1038/364821a0. [DOI] [PubMed] [Google Scholar]
- 9.Brindle P, Nakajima T, Montminy M. Multiple PK-A regulated events are required for transcriptional induction by cAMP. Proc Natl Acad Sci USA. 1995;92:10521–10525. doi: 10.1073/pnas.92.23.10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardinaux J-R, Notis J C, Zhang Q, Vo N, Craig J C, Fass D M, Brennan R G, Goodman R H. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol Cell Biol. 2000;20:1546–1552. doi: 10.1128/mcb.20.5.1546-1552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesare D D, Jackquot S, Hanauer A, Sassone-Corsi P. Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc Natl Acad Sci USA. 1998;95:12202–12207. doi: 10.1073/pnas.95.21.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chawla S, Hardingham G E, Quinn D R, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1999;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 13.Cherry J R, Johnson T R, Dollard C, Shuster J R, Denis C L. Cyclic AMP-dependent protein kinase phosphorylates and inactivates the yeast transcriptional activator ADR1. Cell. 1989;56:409–419. doi: 10.1016/0092-8674(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Orphanides G, Sun X, Yang X-J, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 16.Denis C L, Kemp B E, Zoller M J. Substrate specificities for yeast and mammalian cAMP-dependent protein kinases are similar but not identical. J Biol Chem. 1991;266:17932–17935. [PubMed] [Google Scholar]
- 17.Desdouets C, Matesic G, Molina C A, Foulkes N S, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB*. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 19.Ginty D, Bonni A, Greenberg M. Nerve growth factor activates a Ras dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- 22.Hardingham G E, Chawla S, Cruzalegui F H, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- 23.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 24.Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf H J, Herrlich P. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 1997;16:1009–1022. doi: 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kee B, Arias J, Montminy M. Adaptor mediated recruitment of RNA polymerase II to a signal dependent activator. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 26.Knighton D R, Zheng J H, Eyck L F T, Ashford V A, Xuong N H, Taylor S S, Sowadski J M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 27.Kwak S P, Hakes D J, Artell K J, Dixon J E. Isolation and characterization of a human dual specificity protein-tyrosine phosphatase gene. J Biol Chem. 1994;269:3596–3604. [PubMed] [Google Scholar]
- 28.Kwok R, Lundblad J, Chrivia J, Richards J, Bachinger H, Brennan R, Roberts S, Green M, Goodman R. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 29.Landis C A, Masters S B, Spada A, Pace A M, Bourne H R, Vallar L. GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 30.Lunblad J, Kwok R, Laurance M, Harter M, Goodman R. Adenoviral E1A associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 31.McKenna N, Nawaz Z, Tsai S, Tsai M, O'Malley B. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA. 1998;95:11697–11702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima T, Uchida C, Anderson S, Lee C, Hurwitz J, Parvin J, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima T, Uchida C, Anderson S, Parvin J, Montminy M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa K, Toker A, Johannes F J, Songyang Z, Cantley L C. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;252:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 35.Parker D, Jhala U, Radhakrishnan I, Yaffe M, Reyes C, Shulman A, Cantley L, Wright P, Montminy M. Analysis of an activator: coactivator complex reveals an essential role for secondary structure in transcriptional activation. Mol Cell. 1998;2:353–359. doi: 10.1016/s1097-2765(00)80279-8. [DOI] [PubMed] [Google Scholar]
- 36.Parker D, Rivera M, Zor T, Henrion-Caude A, Radhakrishnan I, Kumar A, Shapiro L H, Wright P E, Montminy M, Brindle P K. Role of secondary structure in discrimination between constitutive and inducible activators. Mol Cell Biol. 1999;19:5601–5607. doi: 10.1128/mcb.19.8.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugazhenthi S, Boras T, O'Connor D, Meintzer M K, Heidenreich K A, Reusch J E. Insulin-like growth factor I-mediated activation of the transcription factor cAMP response element-binding protein in PC12 cells. Involvement of p38 mitogen-activated protein kinase-mediated pathway. J Biol Chem. 1999;274:2829–2837. doi: 10.1074/jbc.274.5.2829. [DOI] [PubMed] [Google Scholar]
- 38.Radhakrishnan I, Perez-Alvarado G C, Parker D, Dyson H J, Montminy M, Wright P E. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator-coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- 39.Seternes O M, Moens U, Johansen B. A dominant role for the Raf-MEK pathway in forskolin, 12-O-tetradecanoyl-phorbol acetate, and platelet-derived growth factor-induced CREB (cAMP-responsive element-binding protein) activation, uncoupled from serine 133 phosphorylation in NIH 3T3 cells. Mol Endocrinol. 1999;13:1071–1083. doi: 10.1210/mend.13.7.0293. [DOI] [PubMed] [Google Scholar]
- 40.Shaywitz A J, Greenberg M E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 41.Songyang Z, Blechner S, Hoagland N, Hoekstra M F, Piwnica-Worms H, Cantley L C. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 42.Struthers R S, Vale W W, Arias C, Sawchenko P E, Montminy M R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature. 1991;350:622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- 43.Walsh D A, Patten S M V. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J. 1994;8:1227–1236. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- 44.Woloshin P, Walton K, Rehfuss R, Goodman R, Cone R. 3′,5′ cyclic adenosine monophosphate-regulated enhancer binding (CREB) activity is required for normal growth and differentiated phenotype in the FRTL5 thyroid follicular cell line. Mol Endocrinol. 1992;6:1725–1733. doi: 10.1210/mend.6.10.1333055. [DOI] [PubMed] [Google Scholar]