Abstract
Angelman syndrome (AS), a genetic disorder that primarily affects the nervous system, is characterized by delayed development, intellectual disability, severe speech impairment, and problems with movement and balance (ataxia). Most affected children also have recurrent seizures (epilepsy). No existing therapies are capable of comprehensively treating the deficits in AS; hence, there is an urgent need to identify new treatments. Here we show that insulin-like growth factor 2 (IGF-2) and mannose-6-phosphate (M6P), ligands of two independent binding sites of the cation-independent M6P/IGF-2 receptor (CIM6P/IGF-2R), reverse most major deficits of AS modeled in mice. Subcutaneous injection of IGF-2 or M6P in mice modeling AS restored cognitive impairments as assessed by measurements of contextual and recognition memories, motor deficits assessed by rotarod and hindlimb clasping, and working memory/flexibility measured by Y-maze. IGF-2 also corrected deficits in marble burying and significantly attenuated acoustically induced seizures. An observational battery of tests confirmed that neither ligand changed basic functions including physical characteristics, general behavioral responses, and sensory reflexes, indicating that they are relatively safe. Our data provide strong preclinical evidence that targeting CIM6P/IGF-2R is a promising approach for developing novel therapeutics for AS.
Keywords: Angelman syndrome, Ube3a, insulin-like growth factor 2, mannose-6-phosphate, insulin-like growth factor 2 receptor, cation-independent mannose-6-phosphate receptor, memory, motor response, mouse model, audiogenic seizure
Lay Summary:
There is no effective treatment for the neurodevelopmental disorder Angelman syndrome (AS). Using a validated AS mouse model, the Ube3am−/p+, in this study we show that systemic administration of ligands of the cation independent mannose-6-phosphate receptor, also known as insulin-like growth factor 2 receptor (CIM6P/IGF-2R) reverses cognitive impairment, motor deficits, as well as seizures associated with AS. Thus, ligands that activate the CIM6P/IGF-2R may represent novel, potential therapeutic targets for AS.
Introduction
Angelman syndrome (AS) is a genetic neurodevelopmental disorder characterized by developmental delay, motor dysfunction, lack of speech, sleep disturbances, and highly penetrant EEG abnormalities and seizures [Buiting, Williams, & Horsthemke, 2016; Mabb, Judson, Zylka, & Philpot, 2011; Williams et al., 2006]. The prevalence of AS is estimated to be approximately one in 12,000–20,000 people in the general population. Most cases of AS result from deletions or abnormal expression of the 15q11–q13 chromosome region, in which neurons are paternally imprinted [Williams et al., 1990]. The common gene associated with these genetic defects is Ube3a, which encodes the E6-associated protein (E6-AP), an enzyme that functions as both an E3 ligase and a transcriptional factor [Nawaz et al., 1999].
No effective treatment is currently available for AS [Tan & Bird, 2016; Wheeler, Sacco, & Cabo, 2017]. To manage the multiple severe symptoms, AS patients need a multidisciplinary team of healthcare professionals throughout life that includes physicians who prescribe drugs to treat seizures and sleep problems, physical therapists to help with movement and balance problems, and communication therapists and behavioral therapists for developmental deficits [Tan & Bird, 2016]. Hence, novel therapies are urgently needed. One line of ongoing translational research is seeking to replace the missing Ube3a gene or reactivate the imprinted and therefore silenced paternal Ube3a allele in the central nervous system (CNS) [Daily et al., 2011; Huang et al., 2012; Meng et al., 2013]. However, alternative, non-genetic therapies capable of comprehensively addressing AS deficits could have enormous advantages.
Most AS deficits seem to involve alterations in mechanisms of synaptic plasticity due to the activity-dependent regulation of Ube3a expression, which has been linked to synaptogenesis and synaptic plasticity [Bird, 2014; Scheiffele & Beg, 2010; Sell & Margolis, 2015]. An important alteration associated with both synaptic plasticity and neural function deficits in AS is neuronal dysregulation of protein metabolism homeostasis (proteostasis), a problem shared by other neurodevelopmental diseases including multiple disorders on the autism spectrum [Ebrahimi-Fakhari & Sahin, 2015; Louros & Osterweil, 2016]. Although much remains to be understood about the mechanisms altered in the CNS of AS patients, it is clear that therapeutics aimed at restoring healthy synaptic and neuronal functions would be clinically valuable.
We and others have identified the polypeptide insulin-like growth factor 2 (IGF-2) as a critical mechanism of synaptic plasticity and memory processes in mice and rats [Chen et al., 2011; Agis-Balboa et al., 2011; Alberini & Chen, 2012; Stern, Kohtz, Pollonini, & Alberini, 2014; Stern, Chen, & Alberini, 2014]. Our studies showed that administration of recombinant IGF-2, either intracerebrally or systemically (subcutaneously, s.c.), significantly enhances memory retention and persistence [Chen et al., 2011; Stern, Kohtz, et al., 2014, Stern, Chen, & Alberini, 2014]. In BTBR T+ Itpr3tf/J (BTBR) mice, a model that reproduces most of the core behavioral phenotypes of autism spectrum disorder (ASD), a s.c. injection of IGF-2 reverses cognitive and social impairments, as well as repetitive behaviors, and significantly ameliorates the underlying deficits of the AMPK–mTOR–S6K pathway and increased protein synthesis [Steinmetz, Stern, Kohtz, Descalzi, & Alberini, 2018]. IGF-2 injected into the hippocampus reverses aging-related memory loss in rats [Steinmetz, Johnson, Iannitelli, Pollonini, & Alberini, 2016]. Furthermore, IGF-2 hippocampal over-expression or intraventricular injection rescues memory impairments, amyloid plaque load, and cholinergic dys-functions in multiple mouse models of Alzheimer’s disease [Mellott, Pender, Burke, Langley, & Blusztajn, 2014; Pascual-Lucas et al., 2014]. Finally, IGF-2 is neuro-protective in models of neuronal oxidative damage [Castilla-Cortázar et al., 2011; Martín-Montañez et al., 2017]. Where assessed, these positive effects of IGF-2 were found to be mediated by its high-affinity receptor, the IGF-2 receptor (IGF-2R), also known as cation-independent mannose-6-phosphate receptor (CIM6PR), and not by the IGF-1 receptor to which IGF-2 can bind with lower affinity [Chen et al., 2011; Stern, Kohtz, et al., 2014; Stern, Chen, & Alberini, 2014; Steinmetz et al., 2018]. CIM6P/IGF-2R is a single transmembrane protein that belongs to the IGF/insulin system but acts distinctively from the other receptors of this system, that is, IGF-1R and insulin receptors, by regulating endosomal protein trafficking and lysosomal targeting [Alberini & Chen, 2012; Ghosh, Dahms, & Kornfeld, 2003; Wang, MacDonald, Thinakaran, & Kar, 2017].
Given the beneficial effects of IGF-2 selectively via the CIM6P/IGF-2R on plasticity, memory, and in disease models, including deficits typical of autism spectrum disorder, we sought to specifically assess the CIM6P/IGF-2R as a target for potential therapeutic effects in AS. Hence, we determined whether two ligands, binding distinct sites on the CIM6P/IGF-2R, could affect AS phenotypes. We assessed the effects of a s.c. injection of IGF-2 or M6P in a mouse model lacking a functional maternal allele of Ube3a (Ube3am−/p+ mice; also referred to as AS mice) [Jiang et al., 1998]. These mice have been widely employed for preclinical studies of AS because they recapitulate the genetics of human patients and exhibit many clinical manifestations of the disease, including cognitive impairments, motor deficits, repetitive behaviors, EEG abnormalities, and circuit hyperexcitability [Berry, Leitner, Clarke, Einfeld, & Einfeld, 2005; Chamberlain & Lalande, 2010; Dindot, Antalffy, Bhattacharjee, & Beaudet, 2007; Jiang et al., 1998; Sonzogni et al., 2018; F. Sun et al., 2015]. Thus, the Ube3am−/p+ mice represent a clinically relevant rodent model of AS [Rotaru, Mientjes, & Elgersma, 2020].
Materials and Methods
Mice
All studies were performed on adult male and female wild-type (WT) and Ube3am−/p+ mice, aged 8–12 weeks. All studies, except for audiogenic seizures, were carried out in the genetic background C57BL/6J. The audiogenic seizure studies were conducted on a colony of Ube3am−/p+ mice in the 129S2 background (Jackson labs stock #004477). In all experiments, except for the spontaneous alternation task, as detailed in the Results section, both males and females were combined and analyzed as a single group because statistical analyzes of separate sex groups yielded no significant difference (unpaired two-tailed Student’s t-test, P > 0.05). To establish the Angelman syndrome mouse colonies, male mice carrying a paternally imprinted Ube3a knockout mutation on a C57BL/6J background (Jackson labs www.jax.org Stock # 016590) were paired with C57BL/6J female mice (F0). The obtained maternal heterozygotes were bred with paternal wild-type (WT) mice (F1) to obtain Ube3am−/p+ and WT littermates (F2). Mice from an F2 generation were used for all the experiments of this study. WT male and female littermates were used as controls. Housing of mice and all procedures were conducted at NYU animal facilities. Mice were group-housed on a 12:12 light/dark cycle with ad libitum access to food and water and were genotyped from tail biopsies when they were 4 weeks old using real time PCR with specific probes at Transnetyx (Cordova, TN, USA). Equal genotype ratios of male and female mice were used for experiments, and mice were randomly assigned to experimental groups. All experiments were carried out during the light cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee of the New York University and were performed in accordance with guidelines of the U.S. National Institutes of Health.
Protein Extracts and Western Blot Analysis
Mice were euthanized by decapitation, and their dorsal hippocampi were rapidly dissected. Tissue was homogenized in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 10% glycerol, 50 mM Tris, pH 8.0) supplemented with 0.5 mM PMSF, 2 mM DTT, 1 mM EGTA, 1 μM microcystin LR, 10 mM NaF, 1 mM Na3VO4, benzamidine, protease inhibitor cocktail, and phosphatase inhibitor cocktails (Sigma, St. Louis, MO, USA).
Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Twenty micrograms of total protein extract/sample were resolved on denaturing 4–20% gradient SDS-PAGE gels (Bio-Rad) and transferred to Immobilon-FL PVDF membranes (Bio-Rad). The membranes were dried and then blocked with either 5% milk in Tris-buffered saline (TBS, pH 7.4; for pro-IGF-2 and IGF-2 antibody staining) or Odyssey® Blocking Buffer (Li-Cor, Lincoln, NE, USA; for CIM6P/IGF-2R antibody staining) for 1 hr at room temperature. Membranes were then incubated overnight at 4 °C in primary antibody diluted in either TBS with 0.5% milk for pro-IGF-2 and IGF-2 antibody staining or TBS with 0.1% Tween 20 (TBST), pH 7.4; for CIM6P/IGF-2R antibody staining.
The following primary antibodies were used: rabbit anti-IGF-2 (1:500, Abcam, Cambridge, UK, #ab9574), rabbit anti-IGF-2R (1:1000, Abcam, #ab124767), and mouse anti-actin (1:10000, Santa Cruz Biotechnology, Dallas, TX, USA, #sc-47778,). The membranes were washed three times in TBST for 10 min and incubated in secondary antibodies for 1 hr at room temperature. The following secondary antibodies were used: anti-rabbit IRDye800CW and anti-mouse IRDye680 (1:10000, Li-Cor, Lincoln, NE, USA). After three additional 10 min TBST washes, membranes were scanned on the Li-Cor Odyssey imager. Data were quantified using pixel intensities with the Odyssey software (Li-Cor). Intensities were normalized against the corresponding actin band intensity (loading control) and expressed as percentages relative to the control group.
Ligands
Recombinant mouse IGF-2 (R&D Systems, 792-MG-050) was dissolved in 0.1% BSA–PBS (pH 7.4) and injected subcutaneously (s.c.) at 30 μg/kg in a total volume of 0.3 mL [Steinmetz et al., 2018; Stern, Kohtz, et al., 2014]. M6P (Sigma) was dissolved in PBS (pH 7.4) on the day of the experiment and administered s.c. at the indicated doses. Specifically, based on the results of a dose response curve (25, 250, 850, and 1500 μg/kg), 850 μg/kg was then used for all experiments. Mice were injected with IGF-2 or respective vehicle or M6P or respective vehicle 20 min before training (CFC and nOR), 20 min before test (spontaneous alternation, marble burying, open field, tail suspension), 20 min before the first test (Rotarod), or 20 min before induction of audiogenic seizures.
Experimental Design for IGF-2 and M6P Injection and Behavioral Tasks
Experiments with IGF-2 or M6P with their respective vehicle control injections were carried out in separate experiments. In all experiments, mice were handled for 2–3 min/day for 5 days before their first behavioral procedure. Mice were used for one behavioral assessment only, except for experiments with M6P in which batteries of two or three behaviors were carried out (M6P experiments of Figs. 2A, D and 4A–C). Mice exposed to these behavioral batteries were randomly assigned to receive treatment with either M6P or vehicle at the first session. In subsequent behaviors, each carried out 7–10 days later to allow for drug washout, the mice received a counterbalanced treatment (see description in Fig. S1). Each data point in each graph represents the value obtained from one mouse. Validation of data was ensured by repetitions of experiments conducted in different days and on different groups of mice (n = 3–4/group per experiment), obtained from different littermates, and performed by different experimenters. Four groups (WT injected with Veh, WT injected with ligand, AS injected with Veh, and AS injected with ligand) were always included in each experiment repetition. All experiments and analyzes were performed blind to genotype and treatment.
Figure 2.
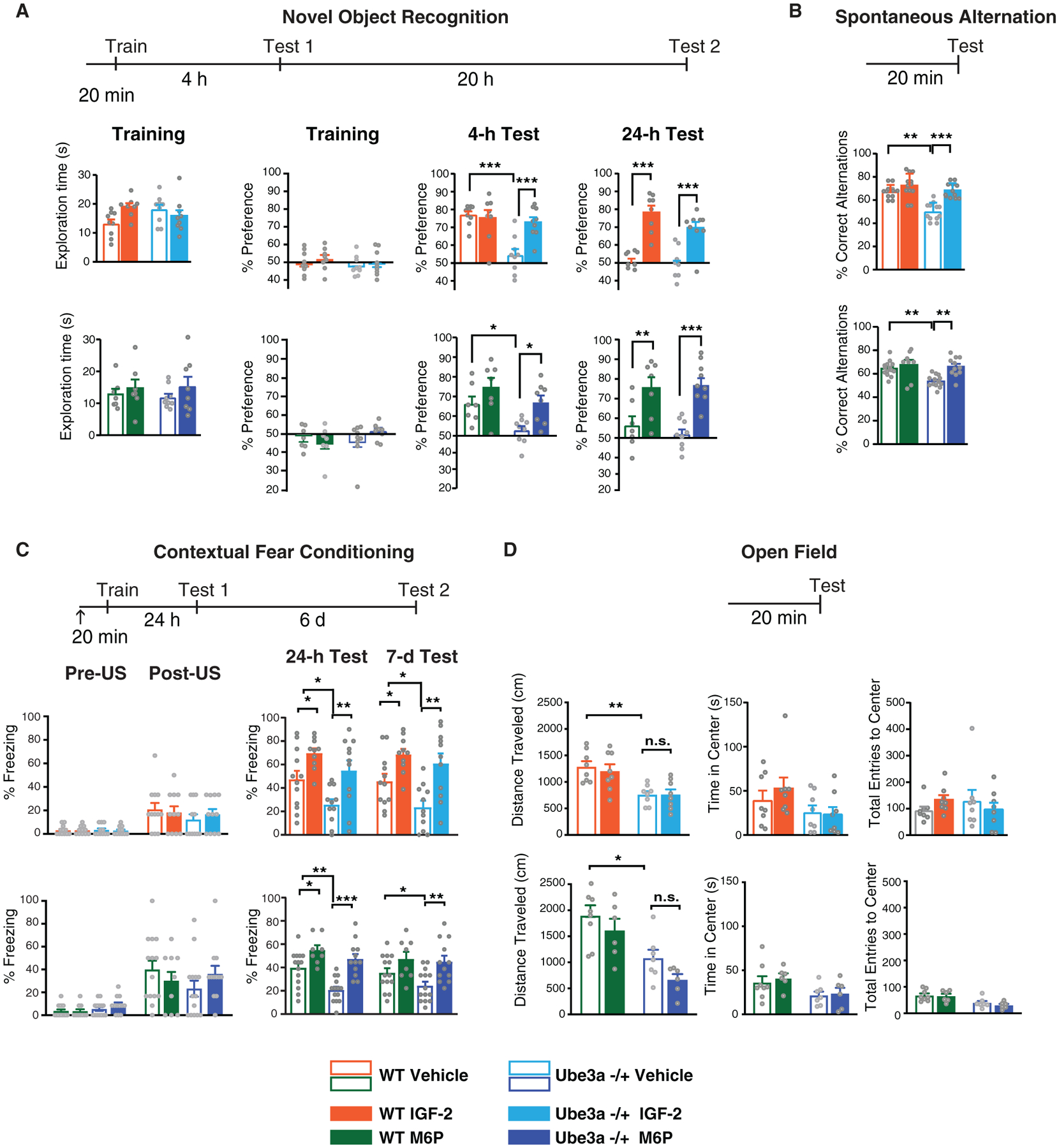
Subcutaneous injection of IGF-2 or M6P reverses deficits in memory retention and flexibility in Ube3am−/p+ mice. (A–D) Experimental schedules are shown above graphs. S.c. injection of IGF-2, M6P, or Vehicle (↑) was administered 20 min before commencement of behavior. (A) Novel object recognition. From left to right: exploration time of both objects (expressed in seconds) and percent of time exploring both objects (% preference) during training, percent of time spent exploring the novel object in the nOR test by WT and Ube3am−/p+ mice tested at 4 hr and 24 hr after training. (B) Spontaneous alternation. Percent of correct alternations in a Y-maze test of WT and Ube3am−/p+ mice. (C) Contextual fear conditioning. Percent of time spent freezing (% freezing) in contextual fear conditioning (CFC) in WT and Ube3am−/p+ mice at training (pre-unconditioned stimulus [pre-US, footshock] and post-US), 24 hr (Test 1), and 7 days (Test 2) after training. (D) Open field behavior. From left to right: distance traveled (in cm), time spent in the center of the open field expressed in seconds, and total number of entries into the center of the arena expressed of WT and Ube3am−/p+ mice. All data are expressed as mean ± s.e.m; n = 6–14 per group. Two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc tests: *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significantly different.
Figure 4.
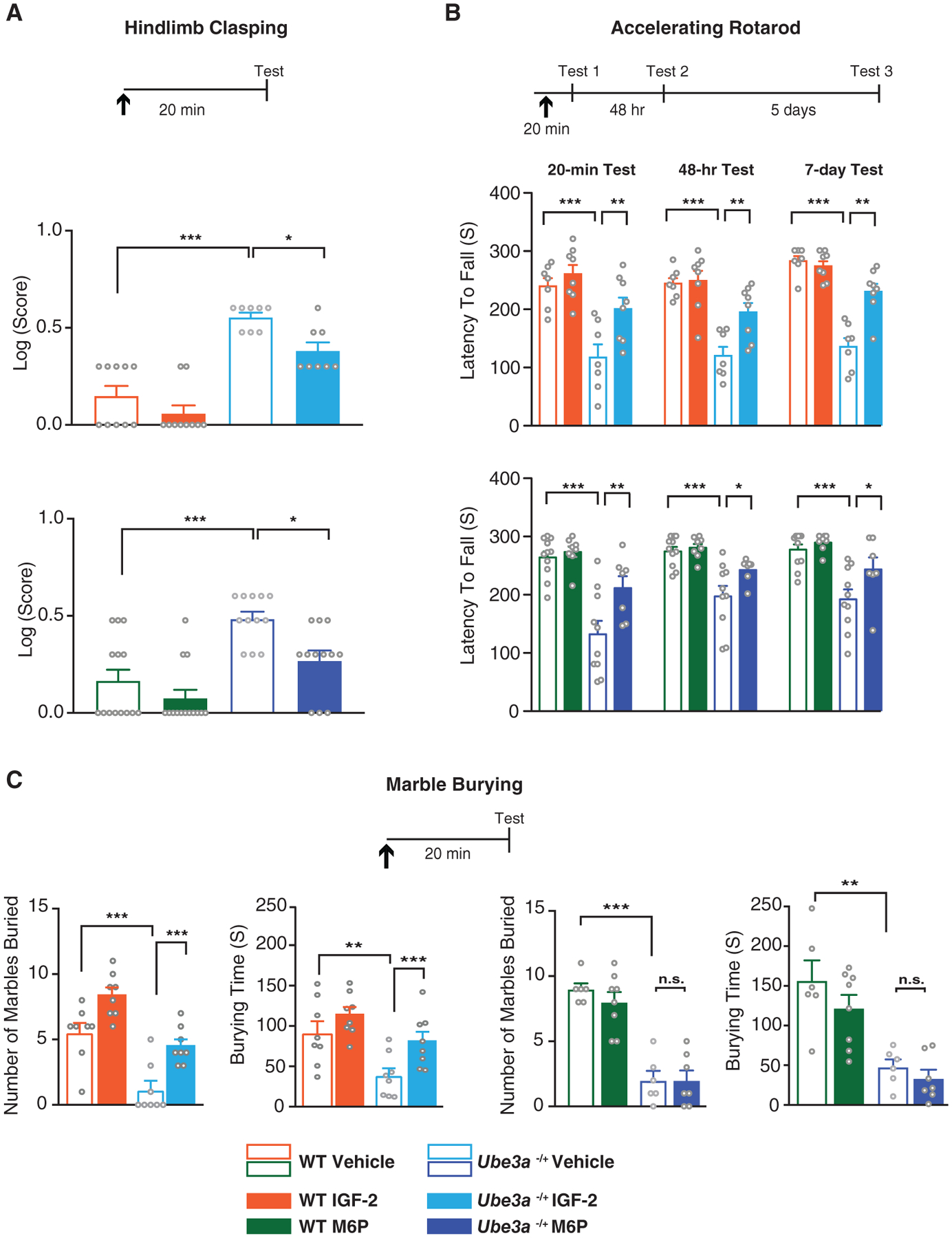
Subcutaneous injection of IGF-2 or M6P reverses motor deficits and marble burying impairment of Ube3am−/p+ mice. (A–C) Experimental schedules are shown above graphs. S.c. injection of IGF-2, M6P, or respective vehicles (↑) was administered 20 min before commencement of behavior. (A) Hindlimb clasping behavior. Log transformation of hindlimb clasping scores of WT and Ube3am−/p+ mice. (B) Accelerating rotarod. Latency in seconds of falling off a rotating rod in WT and Ube3am−/p+ mice tested 20 min, 48 hr, and 7 days following injection. (C) Marble burying. Number of marbles buried, and time spent burying marbles (in seconds) by WT and Ube3am−/p+ mice 20 min after injection. All data are expressed as means ± s.e.m.; n = 6–14 per group. Two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significantly different.
Novel Object Recognition (nOR)
nOR was performed as previously described [Steinmetz et al., 2018; Stern, Kohtz, et al., 2014]. One day before training, mice were placed in a clean square novel arena, free of bedding, for 5 min to allow habituation. The following day, mice were trained in the same arena, now containing two identical objects (Mega Bloks 120), and allowed explore freely for 3 min. Memory tests were performed at 4 hr and again at 24 hr after training: mice were returned to the same arena, but this time one of the objects was replaced by a novel object, and the time spent exploring both objects was scored. Each session was video-recorded and analyzed off-line by an observer blind to treatment and genotype. Time spent interacting/sniffing each object over 5 min was recorded in seconds, and memory retention was expressed as the percentage index exploration preference for the novel object 100 × (novel object/novel object + old object in seconds) over 5 min.
Spontaneous Alternation
The spontaneous alternation test was performed as previously described [Steinmetz et al., 2018; Stern, Kohtz, et al., 2014] on a three-arm Y-maze consisting of three black polycarbonate arms (7.62 × 12.7 × 38.1 cm3). Mice were allowed to freely explore the arms from the center of the maze for 10 min while being video-recorded for off-line analysis. An experimenter blind to treatment and genotype scored the number of entries into each arm and the total number of arm entries. Spontaneous alternation was defined as successive entries into each of the three arms on overlapping triplet sets (e.g., ABC, BCA, CAB, etc.). Percentage alternation was defined as the ratio of actual alternations (total alternations) to possible alternations (total arm entries − 2) × 100.
Contextual Fear Conditioning (CFC)
CFC was carried out as previously described [Stern, Kohtz, et al., 2014; Steinmetz et al., 2018]. Mice were trained to associate a context with a foot shock. The conditioning chamber consisted of a rectangular Perspex box (30.5 × 24.1 × 21.0 cm3) with a metal grid floor (Model ENV-008 Med Associates, St Albans, Vermont, USA) through which foot shocks were delivered via a constant current scrambler circuit. Twenty minutes after a s.c. IGF-2, M6P, or vehicle injection, the mouse was placed into the CFC chamber; after 2 min, the mouse received an un-signaled 2-sec foot-shock of 0.7 mA. After 1 additional minute in the chamber, the mouse was returned to its home cage. At testing, the mouse was placed back in the conditioning chamber and allowed to freely explore it for 3 min in the absence of a foot-shock. This session was video-recorded for offline analysis. Freezing, defined as lack of movement except for heartbeat and respiration, was recorded every 10th second by two independent experimenters blind to genotype and treatment conditions. Percent freezing was calculated as total number of freezing bouts divided by the total number of bouts assessed for each mouse × 100 [Schrick et al., 2007; Steinmetz et al., 2018; Stern, Kohtz, et al., 2014].
Open Field
To test locomotor activity and anxiety, the open field test was performed [Steinmetz et al., 2018]. The light intensity of the box during open field testing was maintained at 195 lx. Mice were allowed to freely explore an open-field arena (43.2 cm × 43.2 cm × 30.5 cm; Med Associates; ENV-515), designated into 16 quadrants, for 5 min. Activity was analyzed with Ethovision-XT (Noldus Information Technology). Activity was measured by scoring the number of times that the mice moved from one square to another. Also, the numbers of entries in the four center quadrants, as well as the time spent (in seconds) in the four center quadrants, were measured.
Hindlimb Clasping
Hindlimb clasping was used to test motor function. It was assessed as previously described [Ciarlone, Wang, Rogawski, & Weeber, 2017] in WT and Ube3am−/p+ mice. Mice were suspended from their tail 10 cm above their home cage. Scores were assigned based on the clasping behavior observed: 0 if the hind limbs were consistently splayed outward, away from the abdomen; 1 if one hind limb was retracted to the abdomen; 2 if both hind limbs were partially retracted; and 3 if both hindlimbs were completed retracted to the abdomen. Two observers blind to the treatment and genotype of the mice performed these tests.
Rotarod
The rotarod test was used to evaluate balance and motor coordination on an accelerating rotating rod (rotarod) (Model 47700, Ugo Basile, Gemonio, Italy). Mice were placed on the rotarod; revolution per minute (rpm) were initially set at 4 rpm and progressively increased to a maximum of 40 rpm over 5 min. The latency of falling during these 5 min was measured in seconds. Three trials per day were carried out, and the average latency was calculated. Trials on each day were separated by intervals of 5–10 min. Mice were first tested 20 min after receiving s.c. injection of IGF-2, M6P, or vehicle, and then again 48 hr and 7 days later.
Marble Burying
Marble burying was performed as previously described [Steinmetz et al., 2018]. Empty home cages were filled with 5 cm of bedding, on top of which 12 glass marbles of 1.3 cm diameter were placed equidistantly in four rows of three marbles each. The mice were given access to the marbles for 10 min under a red light, during which the fraction of time spent burying them was measured. Sessions were video-recorded, and the time spent burying was quantified by two independent observers blind to strain and injection. Digging was defined as coordinated movements of fore or hind limbs that displace the substrate. Total number of marbles buried (>75% marble covered by bedding material) was determined at the end of the testing session.
Audiogenic Seizures
An independent line of Ube3am−/p+ mice in the 129S2 background was established for these experiments. To induce audiogenic seizures, we used a slight modification of a standard protocol [Sonzogni et al., 2018]. Specifically, Ube3am−/p+ mice were habituated to the testing environment (polycarbonate cage) for 2 min. Audiogenic seizures were induced by vigorously scraping scissors across the metal grating of the cage lid (which creates approximately a 100-dB sound) approximately 25 cm above the test subject for 60 sec [Jiang et al. 1998; Silva-Santos et al. 2015; Sonzogni et al., 2018]. The stimulus lasted 60 sec or terminated earlier if a tonic–clonic seizure was observed. Manifestation of seizures generally occurred in two stages. First, mice exhibited wild involuntary seizure-like movements, also known as “wild running.” In most cases, wild running progresses into a more severe tonic–clonic seizure with extension of extremities. We measured seizure phenotypes by quantifying the presence of wild running and tonic–clonic convulsions on video-recordings. Scoring was performed by experimenters blinded to genotype and treatment. The response of each mouse was scored on the basis of the most severe phenomenon seen (no seizure response = 0; wild running and jumping = 1; tonic–clonic seizure = 2), and a mean seizure severity score was calculated.
Observational Battery
The observational battery was carried out as previously described [Stern, Kohtz, et al., 2014] at designated time points after injection (30 min, 24 hr, and 7 days). Body temperature was taken with a digital rectal probe, and physical characteristics were recorded. Each mouse was then observed in an empty cage for 1 min, and general behavioral observations were recorded. Sensorimotor reflexes and simple motor responses were then tested in the order described in Table 1. Observers blind to experimental procedures scored all experiments.
Table 1.
A s.c. IGF-2 or M6P Injection Does Not Produce Adverse Effects in Either WT or Ube3am−/p+ mice
| 30-min test | ||||||
|---|---|---|---|---|---|---|
| WT Veh (n = 8) | AS Veh (n = 7) | WT IGF-2 (n = 6) | AS IGF-2 (n = 6) | WT M6P (n = 6) | AS M6P (n = 6) | |
| Physical characteristics | ||||||
| Weight (males) | 25.0 ± 0.55 | 29.0 ± 0.58 | 25.3 ± 0.33 | 29.3 ± 0.81 | 25.3 ± 0.88 | 29.0 ± 0.58 |
| Weight (females) | 19.3 ± 0.67 | 22.0 ± 1.15 | 20.7 ± 0.81 | 22.3 ± 0.33 | 21.3 ± 0.88 | 22.7 ± 0.33 |
| Temp | 36.7 ± 0.05 | 36.7 ± 0.03 | 36.8 ± 0.05 | 36.7 ± 0.03 | 36.8 ± 0.06 | 36.7 ± 0.03 |
| Whiskers (% with) | 100 | 100 | 100 | 100 | 100 | 100 |
| Bald patches (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| Palpebral closure (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| Exothalmos (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| General behavioral observations (% of subject displaying response) | ||||||
| Wild running | 0 | 0 | 0 | 0 | 0 | 0 |
| Freezing | 0 | 0 | 0 | 0 | 0 | 0 |
| Sniffing | 100 | 100 | 100 | 100 | 100 | 100 |
| Licking | 0 | 0 | 0 | 0 | 0 | 0 |
| Rearing | 100 | 100 | 100 | 100 | 100 | 100 |
| Jumping | 0 | 0 | 0 | 0 | 0 | 0 |
| Defecation | 25 | 29 | 33 | 33 | 17 | 33 |
| Urination | 0 | 0 | 0 | 0 | 17 | 0 |
| Sensorimotor reflexes (% of subjects showing normal response) | ||||||
| Righting | 100 | 100 | 100 | 100 | 100 | 100 |
| Whiskers | 100 | 100 | 100 | 100 | 100 | 100 |
| Eye-blink | 100 | 100 | 100 | 100 | 100 | 100 |
| Ear-twitch | 100 | 100 | 100 | 100 | 100 | 100 |
| 24-hr test | ||||||
| Physical characteristics | ||||||
| Weight (males) | 25.6 ± 0.75 | 29.2 ± 0.48 | 26.3 ± 0.88 | 29.7 ± 1.1 | 26.7 ± 1.3 | 29.7 ± 0.33 |
| Weight (females) | 20.0 ± 0.58 | 22.0 ± 1.15 | 21.3 ± 0.82 | 22.7 ± 0.67 | 21.0 ± 0.58 | 22.7 ± 0.33 |
| Temp | 36.7 ± 0.05 | 36.7 ± 0.03 | 36.8 ± 0.04 | 36.8 ± 0.07 | 36.7 ± 0.03 | 36.7 ± 0.03 |
| Whiskers (% with) | 100 | 100 | 100 | 100 | 100 | 100 |
| Bald patches (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| Palpebral closure (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| Exothalmos (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| General behavioral observations (% of subject displaying response) | ||||||
| Wild running | 0 | 0 | 0 | 0 | 0 | 0 |
| Freezing | 0 | 0 | 0 | 0 | 0 | 0 |
| Sniffing | 100 | 100 | 100 | 100 | 100 | 100 |
| Licking | 0 | 0 | 0 | 0 | 0 | 0 |
| Rearing | 100 | 100 | 100 | 100 | 100 | 100 |
| Jumping | 0 | 0 | 0 | 0 | 0 | 0 |
| Defecation | 0 | 0 | 17 | 0 | 17 | 33 |
| Urination | 0 | 14 | 0 | 0 | 0 | 0 |
| Sensorimotor reflexes (% of subjects showing normal response) | ||||||
| Righting | 100 | 100 | 100 | 100 | 100 | 100 |
| Whiskers | 100 | 100 | 100 | 100 | 100 | 100 |
| Eye-blink | 100 | 100 | 100 | 100 | 100 | 100 |
| Ear-twitch | 100 | 100 | 100 | 100 | 100 | 100 |
| 7-day test | ||||||
| Physical characteristics | ||||||
| Weight (males) | 25.6 ± 0.75 | 29.2 ± 0.48 | 26.3 ± 0.88 | 29.3 ± 1.08 | 27.3 ± 0.67 | 29.7 ± 0.33 |
| Weight (females) | 20.0 ± 0.85 | 22.7 ± 1.2 | 21.7 ± 0.82 | 23.0 ± 0.58 | 21.3 ± 0.88 | 23.0 ± 0 |
| Temp | 36.8± 0.04 | 36.7 ± 0.02 | 36.7 ± 0.04 | 36.7 ± 0.02 | 36.7 ± 0.03 | 36.7 ± 0.1 |
| Whiskers (% with) | 100 | 100 | 100 | 100 | 100 | 100 |
| Bald patches (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| Palpebral closure (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| Exothalmos (% with) | 0 | 0 | 0 | 0 | 0 | 0 |
| General behavioral observations (% of subject displaying response) | ||||||
| Wild running | 0 | 0 | 0 | 0 | 0 | 0 |
| Freezing | 0 | 0 | 0 | 0 | 0 | 0 |
| Sniffing | 100 | 100 | 100 | 100 | 100 | 100 |
| Licking | 0 | 0 | 0 | 0 | 0 | 0 |
| Rearing | 100 | 100 | 100 | 100 | 100 | 100 |
| Jumping | 0 | 0 | 0 | 0 | 0 | 0 |
| Defecation | 13 | 14 | 33 | 33 | 33 | 17 |
| Urination | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensorimotor reflexes (% of subjects showing normal response) | ||||||
| Righting | 100 | 100 | 100 | 100 | 100 | 100 |
| Whiskers | 100 | 100 | 100 | 100 | 100 | 100 |
| Eye-blink | 100 | 100 | 100 | 100 | 100 | 100 |
| Ear-twitch | 100 | 100 | 100 | 100 | 100 | 100 |
Observational battery of general physical characteristics and motor and sensory responses of WT and Ube3am−/p+ (AS) mice injected with IGF-2, M6P, or Vehicle (Veh) tested 30 min, 24 hr and 7 days after injection.
Statistics
All statistical analyzes were performed using GraphPad Prism software (GraphPad Software Inc.). Two-group comparisons were analyzed using unpaired t-test; multi-group comparisons were analyzed using two-way ANOVA with Tukey’s post-hoc test. We used Kruskal–Wallis to compare three or more groups on a dependent variable that is measured on an ordinal level. This statistical test has been used in previous publications with the same type of data sets [Forcelli, Kalikhman, & Gale, 2013; Gu et al., 2019]. The chi-square test was used to compare the percent of seizures between two groups. P < 0.05 was considered significant. In cases where our analysis did not show significant interaction effect but had significant main effects (differences between groups or treatment), the post-hoc analysis was performed on the main effects. This is a common approach when comparing treatments at different timepoints [Wei, Carroll, Harden, & Wu, 2012]. See Table S1 for detailed description of statistical analyzes used for each experiment.
Results
Change in pro-IGF-2 but Not Mature IGF-2 or CIM6P/IGF-2R in Ube3am−/p+ Mice
To begin characterizing the biochemistry of the CIM6P/IGF-2R and its ligand IGF-2 in brain regions important for brain plasticity and memory functions in AS, we carried out western blot analysis on dorsal hippocampus protein extracts. We determined the levels of hippocampal CIM6P/IGF-2R (Fig. 1A), pro-IGF-2 (approximately 22 kDa) and mature form of IGF-2 (approximately 14 kDa) in wild-type (WT) and Ube3am−/p+ mice. The CIM6P/IGF-2R is a 300-kDa single transmembrane protein that in its large extracytoplasmic region has distinct binding sites for IGF-2 and M6P-conjugated proteins. A major class of M6P-conjugated proteins is lysosomal enzymes, which is transported through the action of the CIM6P receptor from the Golgi complex and the cell surface to lysosomes [Wang et al., 2017]. IGF-2 is generally first synthesized as a pro-hormone/precursor hormone (pro-IGF-2), which is subsequently processed and finally appears as a mature IGF-2 protein [Qiu, Jiang, Bell, Tsang, & Gruslin, 2007]. M6P is conjugated to proteins in the cis-Golgi and serves as a cell compartment targeting signal [Coutinho, Prata, & Alves, 2012]. Western blot analyzes revealed that the hippocampal levels of CIM6P/IGF-2R and mature 14 kDa form of IGF-2 were similar in WT and Ube3am−/p+ mice, whereas the approximately 22 kDa form of pro-IGF-2 was significantly accumulated in Ube3am−/p+ mice relative to WT [Fig. 1B, C].
Figure 1.
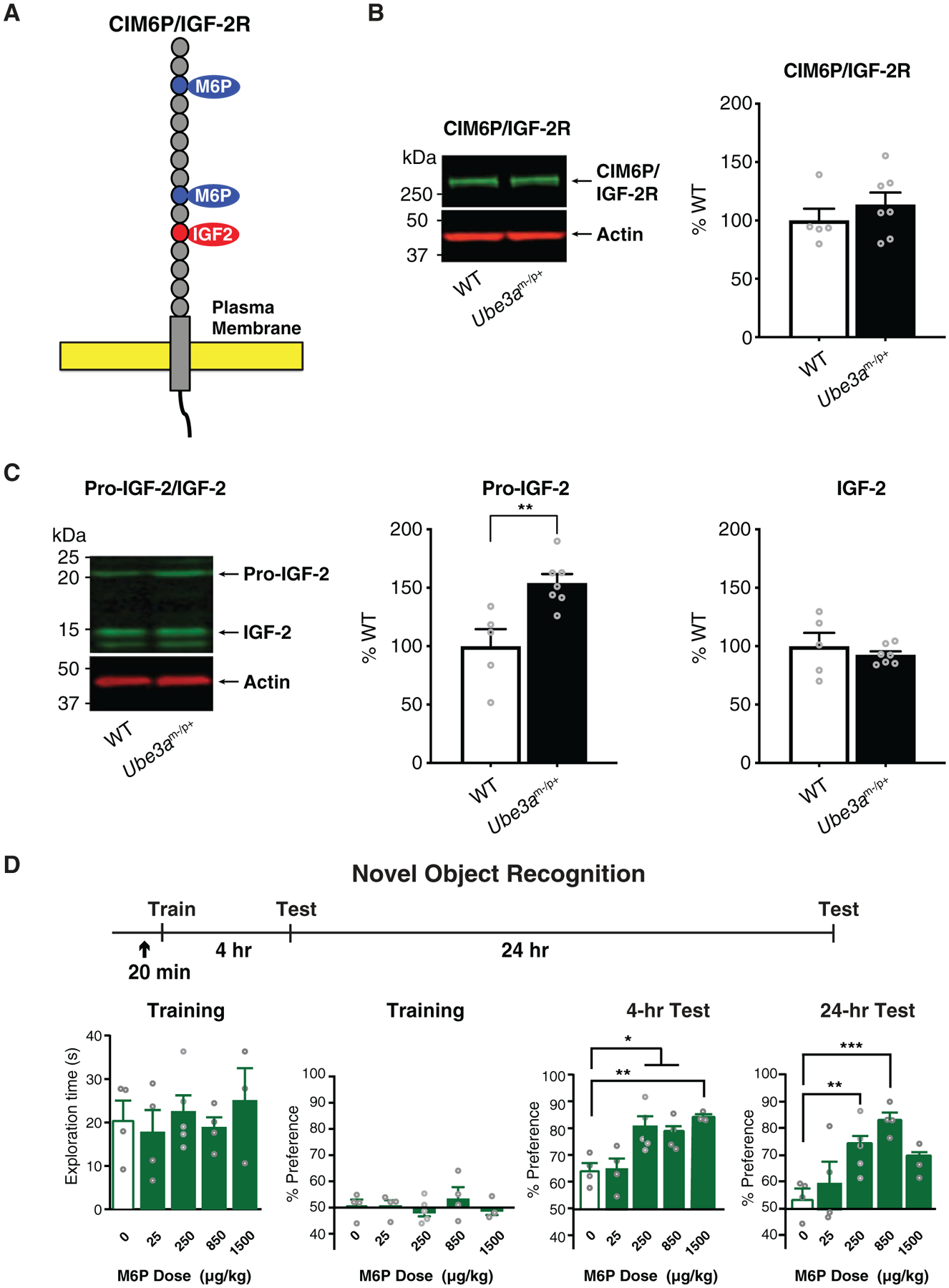
Hippocampal levels of CIM6P/IGF-2 receptor and mature IGF-2 are unaltered, while pro-IGF-2 is increased in Ube3am−/p+ mice; dose–response of M6P injection on object recognition memory in WT mice. (A) Schematic diagram of the cation-independent M6P/IGF-2 receptor (CIM6P/IGF-2R) with the binding sites for IGF-2 and M6P. (B) Western blot analysis comparing dorsal hippocampus levels of CIM6P/IGF-2R in WT and Ube3am−/p+ mice; n = 5–7 per group. (C) Western blot analysis comparing dorsal hippocampus pro-IGF-2 and mature IGF-2 levels in WT and Ube3am−/p+ mice; n = 5–7 per group. (D) Dose response curve of an s.c. injection of M6P in WT mice. Experimental schedule is shown above graphs. In all experiments, mice received a s.c. injection of either vehicle or M6P (↑) 20 min before nOR training and were tested for memory retention 4 hr and 24 hr later. Graphs from left to right show exploration time of both objects expressed in seconds during training, percent preference of objects during training, and percent preference for a novel object relative to a familiar object during nOR testing at 4 hr and 24 hr after training; n = 3–5 per group. Data are expressed as means ± s.e.m. Unpaired t-tests or two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc tests were used. *P < 0.05, **P < 0.01.
We then focused on assessing the effects of IGF-2R ligands on numerous behavioral responses that reproduce AS symptoms; these included cognitive and motor deficits, repetitive behaviors, and seizure. We also tested the ligands’ effects on an observational behavioral battery. Behaviors, numerical values, number of subjects (n) per group, and detailed statistical analyzes of all behavioral experiments are shown in Table S1.
Subcutaneous Injection of IGF-2 or M6P Reverses Deficits in Memory Retention and Flexibility in Ube3am−/p+ Mice
We assessed the effects of an acute s.c. injection of IGF-2 or M6P, two ligands that bind to distinct sites of the CIM6P/IGF-2R on various assays commonly employed to assess memories in adult Ube3am−/p+ mice and their WT littermate controls.
The IGF-2 dose used in all experiments was previously established as the effective dose for memory enhancement in mice (30 μg/kg) [Stern, Kohtz, et al., 2014]. This dose was also found to rescue the behavioral and molecular deficits in the ASD model BTBR mice [Steinmetz et al., 2018]. The effective dose of M6P used in all experiments was identified by a dose–response assessment conducted on the task nOR in WT mice, in which 25, 250, 850, or 1500 μg/kg M6P was injected 20 min before training. M6P at 250, 850 and 1500 μg/kg, but not at 25 μg/kg, significantly enhanced nOR relative to vehicle at 4 hr after training (Fig. 1D). The retention of mice receiving 250 or 850 μg/kg of M6P remained significantly elevated 24 hr after training; hence, 850 μg/kg M6P, which produced the largest effects, was used for all subsequent experiments. As depicted in Fig. 1D, an inverted U-shaped dose response curve was found with M6P, as, in fact, the highest dose (1500 μg/kg) produced less retention enhancement compared to 850 μg/kg at the 24-hr test. This type of curve is typical of pharmacological treatment in learning and memory and is thought to be relate to modulatory actions on memory processes [Baldi & Bucherelli, 2005]. In fact, an inverted U-shaped curve had been found in previous studies also with IGF-2 [Stern, Kohtz, et al., 2014].
Thus, the effective doses of 30 μg/kg IGF-2 or 850 μg/kg M6P, previously indicated by dose–response curve assessments, were used in all experiments. Injection of relative vehicle solutions served as a negative controls. The injection was performed 20 min prior to the commencement of behavior. Males and female mice were included in all experiments, as detailed in the Methods section.
Both IGF-2 and M6P fully reversed recognition deficits in Ube3am−/p+ mice as assessed by the non-aversive nOR task (Fig. 2A). Ube3am−/p+ mice injected with vehicle had no retention of the previously encountered object 4 hr after training, thus showing a significant deficit compared to WT controls. Injection of IGF-2 or M6P fully restored nOR retention to the level of the WT controls (Fig. 2A). Furthermore, while both WT and Ube3am−/p+ mice injected with vehicle lacked nOR retention 24 hr after training, injection of IGF-2 or M6P significantly enhanced the retention at this timepoint (Fig. 2A). These data indicated that both ligands reverse behavioral deficits commonly employed to measure memory functions in AS mice and enhance memory retention in both AS and WT mice with long-lasting effects. In this experiment, as in all the subsequent ones, with the exception of Y-maze retention in WT mice, the effects were similar in both sexes, therefore the data of males and females have been grouped. Data divided according to sex are shown in Table S2.
Next, we measured spontaneous alternation in a Y-maze, a paradigm that is commonly employed to measure active retrograde working memory. In the Y-maze, mice generally explore the least recently visited arm and thus tend to alternate their visits between the three arms. Many brain regions, including the hippocampus, septum, basal forebrain, and prefrontal cortex, play important roles in this task [Lalonde, 2002]. Compared with vehicle-injected WT mice, vehicle-injected Ube3am−/p+ mice exhibited a significant deficit in spontaneous alternation. Injection of IGF-2 or M6P did not change the spontaneous alternation of WT mice, but significantly reversed the deficit in the Ube3am−/p+ mice (Fig. 2B). The effects were similar in both Ube3am−/p+ female and male mice. However, while both IGF-2 and M6P significantly enhanced spontaneous alternation in WT male mice they had no effect in WT females (Fig. 3). This was the only sex-related differential effect found in our experiments.
Figure 3.
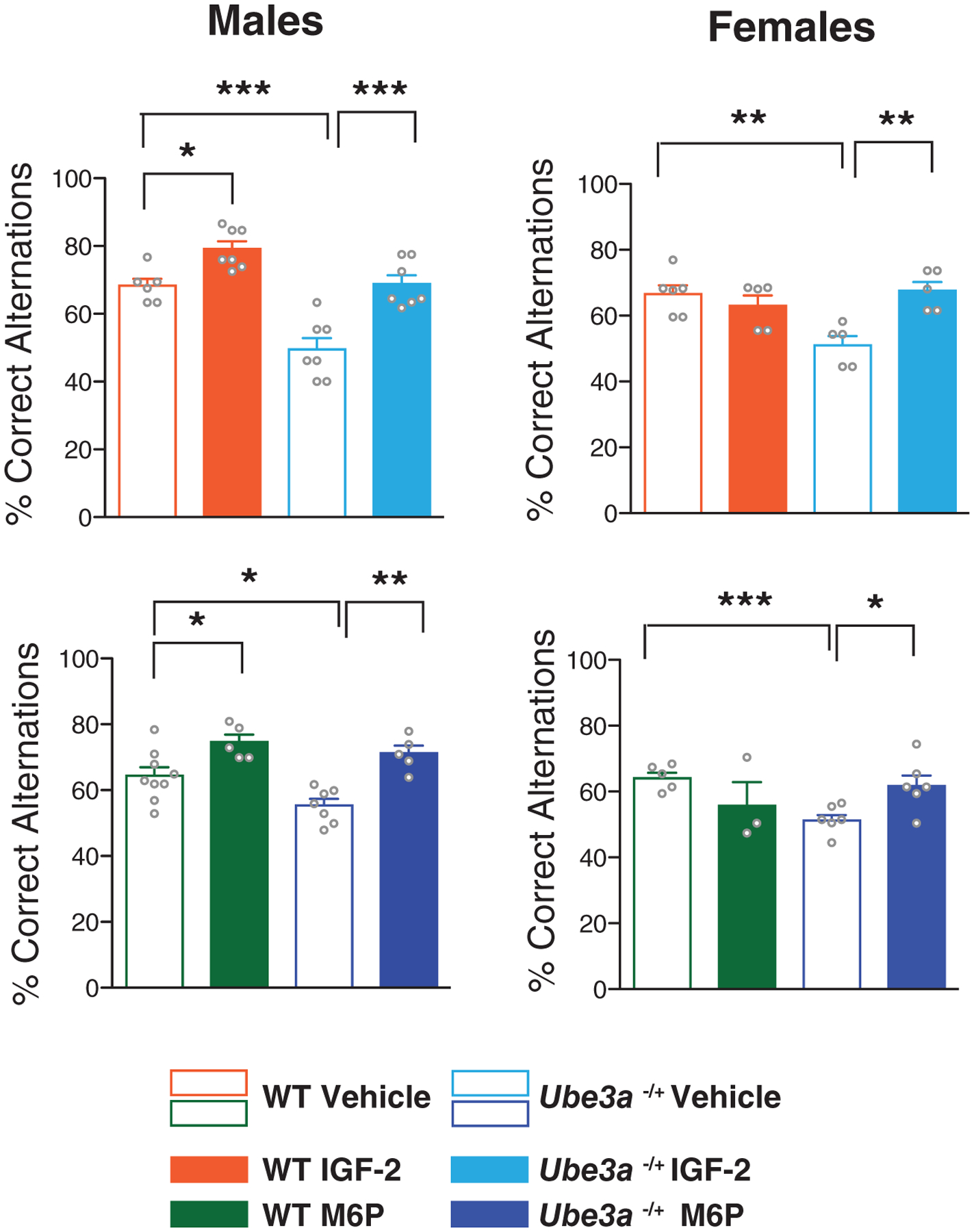
Subcutaneous injection of IGF-2 or M6P enhances Y-maze behavioral flexibility in WT males but not females. Percent of correct alternations in a Y-maze spontaneous alternation test in WT and Ube3am−/p+ male mice (left panel) and female mice (right panel). All data are expressed as means ± s.e.m.; n = 3–9 per group. Two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc tests. *P < 0.05, **P < 0.01, ***P < 0.001.
The mice were then subjected to the aversive memory task contextual fear conditioning (CFC; Fig. 2C), which involves processing information about context experiences through the hippocampus-dependent memory system [Fanselow, 2010; Maren, Phan, & Liberzon, 2013]. Relative to WT control mice, Ube3am−/p+ mice had significant CFC impairment at 24 hr and 7 days after training. IGF-2 or M6P treatment restored their 24 hr and 7 days retention to the levels observed in WT mice (Fig. 2C). Furthermore, IGF-2 significantly improved CFC retention in WT mice both at 24 hr and 7 day after CFC training, consistent with previous findings [Stern, Kohtz, et al., 2014], and M6P had a similar effect (Fig. 2C).
To determine whether the changes in these behaviors could be due to differences in the rate of locomotion or anxiety responses, we tested the effects of IGF-2 or M6P on open-field behavior [Seibenhener & Wooten, 2015; Steinmetz et al., 2018] using an independent cohort of mice. Similar to what was found in previous studies [Mandel-Brehm, Salogiannis, Dhamne, Rotenberg, & Greenberg, 2015; Sonzogni et al., 2018], we detected a significant decrease in the distance traveled in Ube3am−/p+ mice relative to WT mice. No changes were found between Ube3am−/p+ and WT mice in time spent in the center or total number of center entries. Neither IGF-2 nor M6P injection changed distance traveled, time spent in the center of the open field arena or total number of entries into the center in either WT or Ube3am−/p+ mice (Fig. 2D). These data indicated that the changes observed in the nOR, CFC, and spontaneous alternation were not due to problems with anxiety or locomotion responses.
Together, these results provided evidence that two distinct ligands of CIM6P/IGF-2R reverse multiple memory and cognitive deficits of AS mice implying that the effects are mediated by this receptor.
Subcutaneous Injection of IGF-2 or M6P Reverse Motor Deficits of Ube3am−/p+ Mice; IGF-2 Also Reverses Marble Burying Impairment
AS is associated with severe motor impairments that affect patients’ daily lives. As with the cognitive impairments of AS, no effective treatments are available to ameliorate AS-associated motor deficits. These impairments can be assessed in Ube3am−/p+ mice as increased clasping behavior and diminished performance on the accelerating rotarod [Ciarlone et al., 2017; Mandel-Brehm et al., 2015; Sonzogni et al., 2018].
In agreement with this previous literature, we found that WT mice had very low levels of clasping behavior, whereas Ube3am−/p+ mice exhibited significantly elevated clasping, indicative of ataxia. IGF-2 or M6P injection 20 min before the test significantly decreased the clasping behavior of the Ube3am−/p+ mice relative to vehicle injection but did not change the levels of clasping in WT controls (Fig. 4A).
The mice also underwent rotarod testing, which measures motor performance. Relative to WT controls, Ube3am−/p+ mice exhibited a significant, robust decrease in the latency of falling from the rotating rod. IGF-2 or M6P treatment significantly reversed this decrease in fall latency 20 min after injection. The rescue of this motor deficit persisted when the mice were tested 48 hr and 7 days later (Fig. 4B). As indicated in the Methods section, IGF-2 and M6P treatments were carried out in independent experiments performed at different times. The effect of IGF-2 was tested using independent groups of mice for each behavioral assessment. The effect of M6P was also tested on single behaviors except for hindlimb clasping and rotarod behavior, which were tested in sequence and using counterbalanced treatments, as detailed in the Figure S1A.
A separate cohort of mice was evaluated with the marble burying test, which is generally used to assess repetitive and perseverative behaviors, as well as anxiety responses [Angoa-Pérez, Kane, Briggs, Francescutti, & Kuhn, 2013]. As for the motor tasks, the effect of M6P on marble burying was tested in a battery of behaviors (Fig. S1B), whereas IGF-2 effect was tested on independent groups of mice that underwent only marble burying.
In agreement with previous studies [Gu et al., 2019; Sonzogni et al., 2018], Ube3am−/p+ mice had a significant impairment in marble-burying behavior relative to WT littermates: they spent much less time burying marbles and buried significantly fewer marbles. IGF-2 injection 20 min before the marble-burying test fully reversed this impairment. In contrast, M6P injection had no effect on the marble-burying deficits of Ube3am−/p+ mice (Fig. 4C).
Hence, as with cognitive impairments described above, motor deficits of AS mice were also significantly reversed by two distinct ligands of CIM6P/IGF-2R, underscoring the broad effectiveness of the CIM6P/IGF-2R ligands as potential treatments for AS. However only IGF-2 appeared to reverse marble burying impairment of the Ube3am−/p+ mice.
IGF-2, but Not M6P, Attenuates Audiogenic Seizures of Ube3am−/p+ Mice
A total of 80–95% of individuals with AS have seizures [Fiumara, Pittalà, Cocuzza, & Sorge, 2010; Thibert, Larson, Hsieh, Raby, & Thiele, 2013]. Consistent with this, Ube3am−/p+ mice are highly susceptible to audiogenic seizures, a phenotype that is specifically observed in mice of the 129S2 background [Born et al., 2017]. We therefore generated a colony of Ube3am−/p+ mice in the 129S2 background to test whether IGF-2 or M6P affected audiogenic-induced seizures. A very robust seizure phenotype was detected in 100% of these animals injected with vehicle, as assessed using protocols of audiogenic seizures previously established [Mandel-Brehm et al., 2015; Sonzogni et al., 2018]. A s.c. injection of IGF-2 20 min prior to the audiogenic stimulus significantly decreased the frequency and severity of seizures relative to vehicle alone: wild running was reduced by approximately 44% and tonic–clonic seizures decreased by approximately 67% (Fig. 5A, C, D). In contrast, injection of M6P had no significant effects (Fig. 5B–D).
Figure 5.
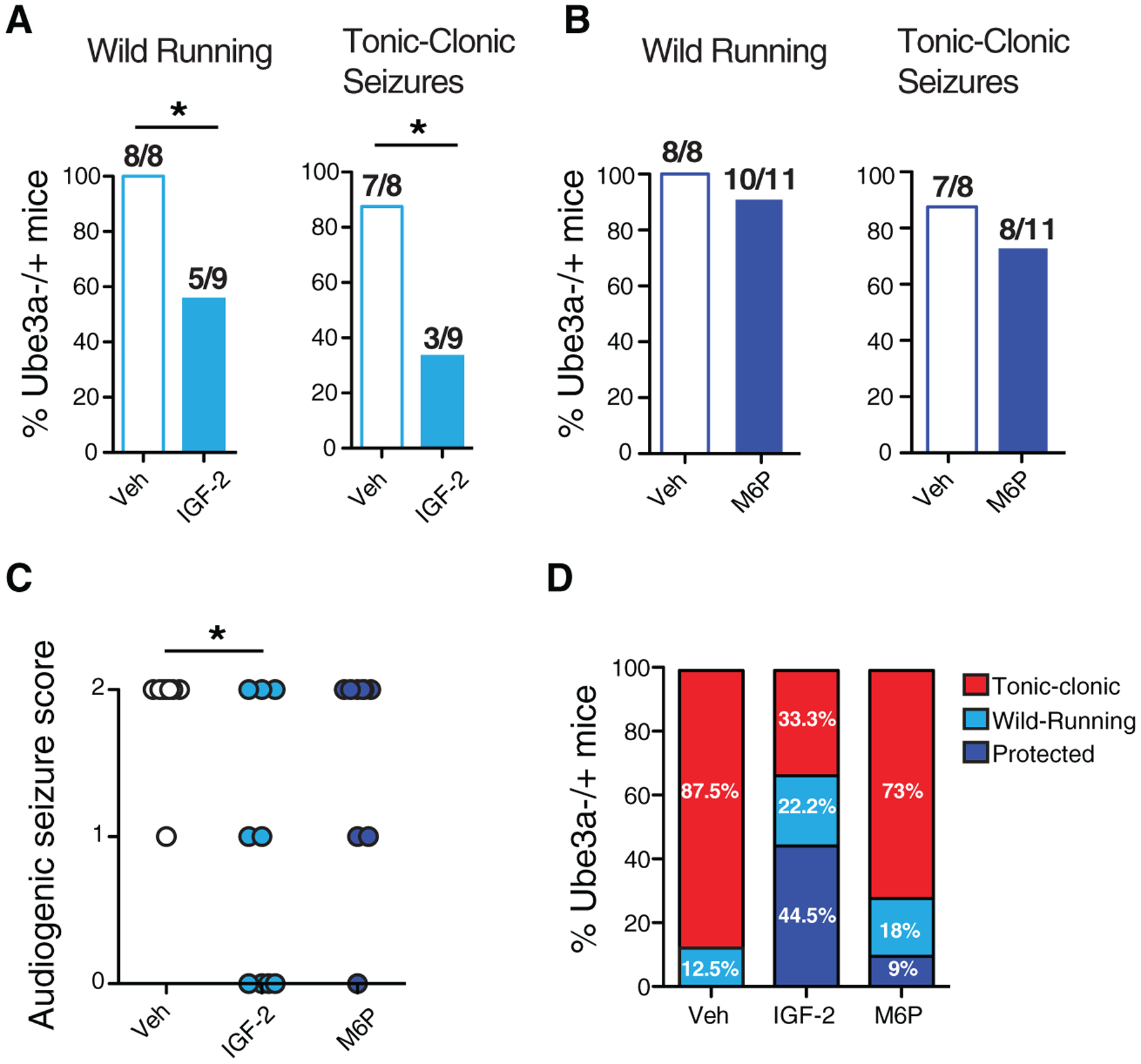
IGF-2, but not M6P, attenuates audiogenic seizures in Ube3am−/p+ mice. S.c. injection of IGF-2, M6P, or respective vehicles was administered 20 min before audiogenic seizure induction. (A) Percentage of Ube3am−/p+ mice injected with vehicle or IGF-2 exhibiting wild running and/or tonic–clonic seizures following an audiogenic stimulus. Chi-square test, *P < 0.05. (B) Percent of Ube3am−/p+ mice injected with vehicle or M6P exhibiting wild running and/or tonic–clonic seizures following an audiogenic stimulus. (C) Severity score of audiogenic seizures; 0 = no seizure response; 1 = wild running; 2 = tonic–clonic seizure. Kruskal–Wallis test with Dunn’s multiple comparisons, *P < 0.05. (D) Graph summarizing the percentage of wild running, tonic–clonic or protection from both wild running and tonic–clonic seizure in Ube3am−/p+ mice with vehicle, IGF-2, or M6P treatment; n = 8–11 per group.
These data showed that IGF-2, in addition to reversing cognitive and motor deficits of AS mice, also protects them from audiogenic seizures indicating that ligands to the CIM6P/IGF-2R have a comprehensive therapeutic effect toward deficits of AS.
Acute IGF-2 or M6P Treatment Does Not Produce Adverse Effects in Ube3am−/p+ Mice
To determine whether IGF-2 or M6P causes adverse effects, we assessed the safety of s.c. injection of each drug using a standard observational battery of tests that included physical, behavioral, and sensorimotor evaluations [Brown, Jones, & Forbes, 2009]. As shown in Table 1, no differences were observed between WT and Ube3am−/p+ mice treated with vehicle, IGF-2, or M6P mice at 30 min, 24 hr, or 7 days after injection. We concluded that acute systemic treatment with IGF-2 or M6P has no major aversive effect.
Discussion
Currently, there is no available therapy that comprehensively treat the major core deficits of AS. Here, we provided extensive behavioral evidence, based on multiple assessments that included several cognitive, motor and repetitive behaviors, and neurological responses, that two distinct ligands of CIM6P/IGF-2R are effective in significantly reversing most of the major deficits of AS modeled in mice. In agreement with the efficacy of its ligands, we found that the levels of CIM6P/IGF-2R were not altered in the dorsal hippocampus of AS mice compared to normal mice. However, AS mice had a significant increase in the level of pro-IGF-2 but not of mature IGF-2 suggesting that IGF-2 synthesis, processing, transport, and/or degradation are altered in the AS mouse model. Future studies are needed to understand these alterations associated to AS.
Our behavioral assessments revealed that an acute systemic (s.c.) injection of either IGF-2 or M6P significantly, and often fully, reversed the typical impairments of AS mouse models considered to reproduce cognitive deficits, assessed by working memory in Y-maze, and long-term recognition and contextual memories, assessed by nOR and contextual fear conditioning. Both treatments also reversed motor deficits, assessed by clasping behavior and rotarod test. Where multiple tests were conducted, including nOR, contextual fear conditioning, and rotarod, the beneficial effect of the treatments persisted for days. At the doses employed throughout our studies that had beneficial effects in all behaviors tested, that is, 30 μg/kg for IGF-2 and 850 μg/kg for M6P, only IGF-2 protected against marble burying impairment and susceptibility of audiogenic seizures. The only behavior that was found unaffected by either IGF-2 or M6P treatment was the open field behavior, which was assessed using distance traveled and center crossing, a measure believed to reflect anxiety. Similarly to what was reported in previous studies [Born et al., 2017; Gu et al., 2019; Huang et al., 2013; Mandel-Brehm et al., 2015; Sonzogni et al., 2018], we found that AS mice have deficits in the total distance traveled during open field but not in the time spent in the center or number of entries in the open field arena. In fact, only a trend toward less time spent in the center of the open field for AS relative to WT was observed. Nevertheless, neither IGF-2 nor M6P changed any of these measures in either WT or AS mice, a result that is similar to what we found with IGF-2 in the mouse model of ASD BTBR [Steinmetz et al., 2018]. This outcome suggests that activating the CIM6P/IGF-2R does not change anxiety type of behaviors, is in line with our previous data showing that IGF-2 does not change amygdala-dependent behavioral responses and has no effects when injected into the amygdala [Stern, Chen, & Alberini, 2014].
Given the similarities of the effects produced by IGF-2 and M6P, which bind to distinct sites in the CIM6P/IGF-2R, we conclude that this receptor is a potential target for developing effective therapies that may treat multiple impairments of AS.
The beneficial effects of the CIM6P/IGF-2R ligands on the variety of AS deficits indicate that they target multiple brain functions in a variety of brain regions.
Except for approaches that aim at unsilencing the paternal allele, therefore restoring the genetic defect, which could in principle repair all deficits, but are still underdeveloped and debated [Tsagkaris et al., 2020], to our knowledge, comprehensive alternative therapeutic agents that broadly ameliorate cognitive, motor and neurological impairments have been lacking [Jamal et al., 2017; Meng et al., 2013; Sun et al., 2016; Yang, 2019]. Our findings suggest that it may be possible to design drug targets against the CIM6P/IGF-2R that can broadly treat AS symptoms and could be readily developed for clinical testing.
The mechanisms of action underlying the therapeutic effects of CIM6P/IGF-2R ligands in mice are not known and future studies are needed to identify them. Here, on the basis of previous findings showing that IGF-2 repairs alterations of mTOR and active mRNA translation in an autism mouse model and on preliminary data in progress in the rat brain [Steinmetz et al., 2018], we speculate that CIM6P/IGF-2R regulates activity- and experience-dependent homeostasis of protein metabolism, also known as proteostasis.
In general, the functions of CIM6P/IGF-2R in the brain remain to be understood. Thus far, this receptor has been mostly characterized in development, where it emerged as critical for embryonic growth, or in cell lines, or CIM6P/IGF-2R knockout mice, where its role was found to be essential for endosomal trafficking and lysosomal targeting [Wang et al., 2017; Wang, Fung, Barlow, & Wagner, 1994]. Given its involvement in targeting lysosomal enzymes and functions, we hypothesize that CIM6P/IGF-2R is engaged in regulating protein metabolism, and in particular protein degradation via lysosomal targeting and its crosstalk with proteasome-mediated protein degradation.
Our experiments tested the effect of only a single dose of each ligand. Although s.c. treatments with either IGF-2 or M6P at the doses used were highly efficacious, we anticipate that the potential utilization of CIM6P/IGF-2R ligands in clinical setting will require prolonged or chronic treatments; hence, future studies should focus also on assessing the efficacy of multiple doses of CIM6P/IGF-2R ligands. Given the lasting effects of a single s.c. injection, we expect that chronic administration of either IGF-2 or M6P will have sustained positive effects in rescuing the memory and motor deficits associated with AS as well as the protection from repetitive behavior and seizures. Chronic administration experiments are needed to substantiate the potential of CIM6P/IGF-2R activation as a therapy for AS. In addition, future efforts should evaluate the effects of CIM6P/IGF-2R ligands on AS models at early ages, to determine whether such treatments could delay the appearance of symptoms.
The two responses that showed to be differentially affected by IGF-2 and M6P were the recovery from marble burying impairment and the protection from audiogenic seizures, where only IGF-2 showed a rescuing effect. As we only tested the doses of IGF-2 and M6P that reversed cognitive and motor deficits in AS mice, it remains to be determined whether other doses of M6P may have a positive effect. However, based on the present data, we cannot exclude the possibility that the two ligands produce differential effects, which may result from differences in their binding and biological properties. For example, the effects of IGF-2 but not of M6P may occur via another type of receptor. In fact, it is known that IGF-2 can bind with lower affinity to the IGF-1 receptor and insulin receptors [Alberini & Chen, 2012; Brown et al., 2009]. Furthermore, the CIM6P/IGF-2R may have distinct binding efficiency or expression pattern in different cell types and/or cellular and subcellular compartments, which may contribute to distinct functions in the brain. Future studies are needed to better understand the CIM6P/IGF-2R mechanisms of action in response to the two ligands, as well as whether/how the two ligands also target some differential functions.
Finally, although the numbers in each group divided according to sex are small and more experiments are needed to make final conclusions, our data indicate that both female and male AS mice are equally showing AS typical behavioral and neurological phenotypes and either IGF-2 or M6P treatment significantly reverse their deficits. The only significant difference we observed in males vs. females was that IGF-2 or M6P treatment enhanced the working memory test Y-maze spontaneous alternation in normal (WT) males but not females. The implications of this difference selective for WT mice in Y-maze spontaneous alternation enhancement remain to be understood. Our findings showing no difference in the Y-maze performance between male and female WT mice are in line with previous literature [Typlt et al., 2013]. Hence, future studies shall expand on investigating the effects of IGF-2 and M6P on sex-related IGF-2 or M6P out-comes on other tasks in order to dissect whether the two ligands differentially affect certain brain circuits. In sum, the differential effect on Y-maze enhancement needs further studies to be fully understood.
In conclusion, CIM6P/IGF-2R ligands may represent a valuable alternative approach to genetic intervention in developing novel treatments for AS. In addition, given the fact that s.c. IGF-2 injection also reverses cognitive impairments, social deficits, and repetitive behaviors in the ASD mouse model BTBR, we suggest that ligands of CIM6P/IGF-2R may have therapeutic effects also in several conditions of the ASD.
Supplementary Material
Table S2. Summary of All Behavioral Data Divided by Sex. Red Values Indicate the Groups That Have Significant Differences Between Sex.
Table S1. Summary of All Behavioral Data and Statistical Analyzes.
Figure S1. Behavioral Batteries. (A) Behavioral Battery 1 consisted of novel Object Recognition (nOR), followed by Tail Suspension (TS), followed by Rotarod (RR) with 7 days intervals between each task and with a M6P or Vehicle (VH) s.c. injection given 20 min before each task and counterbalanced at the subsequent task. (B) Behavioral Battery 2 consisted of Open Field (OF) followed, 7–10 days later by Marble Burying. A s.c. injection of M6P or Vehicle (VH) was given 20 min before each behavior and was counterbalanced at the subsequent behavior.
Acknowledgments
The authors thank Dr. Eric Klann (NYU) for helping us to set up the initial Angelman Syndrome (AS) mouse colony. This work was supported by the Foundation for Angelman Syndrome Therapeutics (FAST), NIH grant MH065635 to C.M.A., NIH grant T32MH019524 and T32AG052909 to E.C., a Canadian Institutes of Health Research Fellowship to G.D., and F31MH116585 to A.K.
Footnotes
Conflict of Interest
C.M.A. and E.C. are inventors of patents filed by NYU on the use of IGF-2 receptor agonist ligands for treatment of Angelman syndrome and autism. C.M.A. is a founder of a company seeking to develop compounds covered by the patent. The other authors declare no conflict of interest.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Agis-Balboa RC, Diaz DA, Wittnam J, Govindarajan N, Blom K, Burkhardt S, … Fischer A (2011). A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. The EMBO Journal, 30, 4071–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, & Chen DY (2012). Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends in Neurosciences, 35(5), 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, & Kuhn DM (2013). Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. Journal of Visualized Experiments, 82, 50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, & Bucherelli C (2005). The inverted “u-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity in Biology, Toxicology and Medicine, 3(1), 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RJ, Leitner RP, Clarke AR, Einfeld SL, & Einfeld SL (2005). Behavioral aspects of Angelman syndrome: A case control study. American Journal of Medical Genetics Part A, 132A(1), 8–12. [DOI] [PubMed] [Google Scholar]
- Bird LM (2014). Angelman syndrome: Review of clinical and molecular aspects. The Application of Clinical Genetics, 7, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born HA, Dao AT, Levine AT, Lee WL, Mehta NM, Mehra S, … Anderson AE (2017). Strain-dependence of the Angelman Syndrome phenotypes in Ube3a maternal deficiency mice. Scientific Reports, 17(1), 8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Jones EY, & Forbes BE (2009). Keeping IGF-II under control: Lessons from the IGF-II–IGF2R crystal structure. Trends in Biochemical Sciences, 34(12), 612–619. [DOI] [PubMed] [Google Scholar]
- Buiting K, Williams C, & Horsthemke B (2016). Angelman syndrome — insights into a rare neurogenetic disorder. Nature Reviews Neurology, 12(10), 584–593. [DOI] [PubMed] [Google Scholar]
- Castilla-Cortázar I, García-Fernández M, Delgado G, Puche JE, Sierra I, Barhoum R, & González-Barón S (2011). Hepatoprotection and neuroprotection induced by low doses of IGF-II in aging rats. Journal of Translational Medicine, 9(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SJ, & Lalande M (2010). Neurodevelopmental disorders involving genomic imprinting at human chromosome 15q11–q13. Neurobiology of Disease, 39(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, … Alberini CM (2011). A critical role for IGF-II in memory consolidation and enhancement. Nature, 469(7331), 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlone SL, Wang X, Rogawski MA, & Weeber EJ (2017). Effects of the synthetic neurosteroid ganaxolone on seizure activity and behavioral deficits in an Angelman syndrome mouse model. Neuropharmacology, 116, 142–150. [DOI] [PubMed] [Google Scholar]
- Coutinho MF, Prata MJ, & Alves S (2012). Mannose-6-phosphate pathway: A review on its role in lysosomal function and dysfunction. Molecular Genetics and Metabolism, 105, 542–550. [DOI] [PubMed] [Google Scholar]
- Daily JL, Nash K, Jinwal U, Golde T, Rogers J, Peters MM, … Weeber EJ (2011). Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS One, 6(12), e27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindot SV, Antalffy BA, Bhattacharjee MB, & Beaudet AL (2007). The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Human Molecular Genetics, 17(1), 111–118. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, & Sahin M (2015). Autism and the synapse. Current Opinion in Neurology, 28(2), 91–102. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2010). From contextual fear to a dynamic view of memory systems. Trends in Cognitive Sciences, 14(1), 7–15. 10.1016/j.tics.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumara A, Pittalà A, Cocuzza M, & Sorge G (2010). Epilepsy in patients with Angelman syndrome. Italian Journal of Pediatrics, 36(1), 31. 10.1186/1824-7288-36-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Kalikhman D, & Gale K (2013). Delayed effect of craniotomy on experimental seizures in rats. PLoS One, 8, e81401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM, & Kornfeld S (2003). Mannose 6-phosphate receptors: New twists in the tale. Nature Reviews Molecular Cell Biology, 4(3), 202–213. [DOI] [PubMed] [Google Scholar]
- Gu B, Zhu M, Glass MR, Rougié M, Nikolova VD, Moy SS, … Philpot BD (2019). Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice. Journal of Clinical Investigation, 129(12), 5462–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Allen JA, Mabb AM, King IF, Miriyala J, Taylor-Blake B, … Philpot BD (2012). Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature, 481(7380), 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Burns AJ, Nonneman RJ, Baker LK, Riddick NV, Nikolova VD, … Moy SS (2013). Behavioral deficits in an Angelman syndrome model: Effects of genetic background and age. Behavioural Brain Research, 243, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal I, Kumar V, Vatsa N, Shekhar S, Singh BK, Sharma A, & Jana NR (2017). Rescue of altered HDAC activity recovers behavioural abnormalities in a mouse model of Angelman syndrome. Neurobiology of Disease, 105, 99–108. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, … Beaudet AL (1998). Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron, 21(4), 799–811. [DOI] [PubMed] [Google Scholar]
- Lalonde R (2002). The neurobiological basis of spontaneous alternation. Neuroscience and Biobehavioral Reviews, 26(1), 91–104. [DOI] [PubMed] [Google Scholar]
- Louros SR, & Osterweil EK (2016). Perturbed proteostasis in autism spectrum disorders. Journal of Neurochemistry, 139 (6), 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabb AM, Judson MC, Zylka MJ, & Philpot BD (2011). Angelman syndrome: Insights into genomic imprinting and neurodevelopmental phenotypes. Trends in Neurosciences, 34(6), 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel-Brehm C, Salogiannis J, Dhamne SC, Rotenberg A, & Greenberg ME (2015). Seizure-like activity in a juvenile Angelman syndrome mouse model is attenuated by reducing Arc expression. Proceedings of the National Academy of Sciences, 112(16), 5129–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews. Neuroscience, 14(6), 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Montañez E, Millon C, Boraldi F, Garcia-Guirado F, Pedraza C, Lara E, … Garcia-Fernandez M (2017). IGF-II promotes neuroprotection and neuroplasticity recovery in a long-lasting model of oxidative damage induced by glucocorticoids. Redox Biology, 13, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Pender SM, Burke RM, Langley EA, & Blusztajn JK (2014). IGF2 ameliorates amyloidosis, increases cholinergic marker expression and raises BMP9 and neurotrophin levels in the hippocampus of the APPswePS1dE9 Alzheimer’s disease model mice. PLoS One, 9 (4), e94287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Person RE, Huang W, Zhu PJ, Costa-Mattioli M, & Beaudet AL (2013). Truncation of Ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. PLoS Genetics, 9(12), e1004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, & O’Malley BW (1999). The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Molecular and Cellular Biology, 19(2), 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Lucas M, Viana da Silva S, Di Scala M, Garcia-Barroso C, González-Aseguinolaza G, Mulle C, … Garcia-Osta A (2014). Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Molecular Medicine, 6(10), 1246–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q, Jiang JY, Bell M, Tsang BK, & Gruslin A (2007). Activation of endoproteolytic processing of insulin-like growth factor-II in fetal, early postnatal, and pregnant rats and persistence of circulating levels in postnatal life. Endocrinology, 148, 4803–4811. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Mientjes EJ, & Elgersma Y (2020). Angelman syndrome: From mouse models to therapy. Neuroscience, S0306–4522(20), 30103–30102. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, & Beg AA (2010). Angelman syndrome connections. Nature, 468(7326), 907–908. [DOI] [PubMed] [Google Scholar]
- Schrick C, Fischer A, Srivastava DP, Tronson NC, Penzes P, & Radulovic J (2007). N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron, 55(5), 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener ML, & Wooten MC (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments, 96, e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell GL, & Margolis SS (2015). From UBE3A to Angelman syndrome: A substrate perspective. Frontiers in Neuroscience, 9, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santos S, van Woerden GM, Bruinsma CF, Mientjes E, Jolfaei MA, Distel B, … Elgersma Y (2015). Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. Journal of Clinical Investigation, 125(5), 2069–2076. 10.1172/jci80554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonzogni M, Wallaard I, Santos SS, Kingma J, du Mee D, van Woerden GM, & Elgersma Y (2018). A behavioral test battery for mouse models of Angelman syndrome: A powerful tool for testing drugs and novel Ube3a mutants. Molecular Autism, 9(1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Johnson SA, Iannitelli DE, Pollonini G, & Alberini CM (2016). Insulin-like growth factor 2 rescues aging-related memory loss in rats. Neurobiology of Aging, 44, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz AB, Stern SA, Kohtz AS, Descalzi G, & Alberini CM (2018). Insulin-like growth factor II targets the mTOR pathway to reverse autism-like phenotypes in mice. The Journal of Neuroscience, 38(4), 1015–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SA, Chen DJ, & Alberini CM (2014). The effect of insulin and insulin-like growth factors on hippocampus- and amygdala-dependent long-term memory formation. Learning and Memory, 21(10), 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern SA, Kohtz AS, Pollonini G, & Alberini CM (2014). Enhancement of memories by systemic administration of insulin-like growth factor II. Neuropsychopharmacology, 39 (9), 2179–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Oristaglio J, Levy SE, Hakonarson H, Sullivan N, Fontanarosa J, & Schoelles KM (2015). Genetic testing for developmental disabilities, intellectual disability, and autism spectrum disorder. Washington, DC: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Sun J, Liu Y, Tran J, O’Neal P, Baudry M, & Bi X (2016). mTORC1–S6K1 inhibition or mTORC2 activation improves hippocampal synaptic plasticity and learning in Angelman syndrome mice. Cellular and Molecular Life Sciences, 73(22), 4303–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W-H, & Bird LM (2016). Angelman syndrome: Current and emerging therapies in 2016. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 172(4), 384–401. [DOI] [PubMed] [Google Scholar]
- Thibert RL, Larson AM, Hsieh DT, Raby AR, & Thiele EA (2013). Neurologic manifestations of Angelman syndrome. Pediatric Neurology, 48(4), 271–279. [DOI] [PubMed] [Google Scholar]
- Tsagkaris C, Papakosta V, Miranda AV, Zacharopoulou L, Danilchenko V, Matiashova L, & Dhar A (2020). Gene therapy for Angelman syndrome: Contemporary approaches and future endeavors. Current Gene Therapy, 19, 359–366. [DOI] [PubMed] [Google Scholar]
- Typlt M, Mirkowski M, Azzopardi E, Ruettiger L, Ruth P, & Schmid S (2013). Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One, 8, e81270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, MacDonald RG, Thinakaran G, & Kar S (2017). Insulin-like growth factor-II/cation-independent mannose 6-phosphate receptor in neurodegenerative diseases. Molecular Neurobiology, 54(4), 2636–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-Q, Fung MR, Barlow DP, & Wagner EF (1994). Regulation of embryonic growth and lysosomal targeting by the imprintedIgf2/Mpr gene. Nature, 372(6505), 464–467. [DOI] [PubMed] [Google Scholar]
- Wei J, Carroll RJ, Harden KK, & Wu G (2012). Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids, 42, 2031–2035. 10.1007/s00726-011-0924-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AC, Sacco P, & Cabo R (2017). Unmet clinical needs and burden in Angelman syndrome: A review of the literature. Orphanet Journal of Rare Disease, 12, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CA, Zori RT, Stone JW, Gray BA, Cantu ES, & Ostrer H (1990). Maternal origin of 15q11–13 deletions in Angelman syndrome suggests a role for genomic imprinting. American Journal of Medical Genetics, 35(3), 350–353. [DOI] [PubMed] [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J, Knoll JH, Kyllerman M, Laan LA, … Wagstaff J (2006). Angelman syndrome 2005: Updated consensus for diagnostic criteria. American Journal of Medical Genetics. Part A, 140(5), 413–418. [DOI] [PubMed] [Google Scholar]
- Yang X (2019). Towards an understanding of Angelman syndrome in mice studies. Journal of Neuroscience Research, 98(6), 1162–1173. 10.1002/jnr.24576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Summary of All Behavioral Data Divided by Sex. Red Values Indicate the Groups That Have Significant Differences Between Sex.
Table S1. Summary of All Behavioral Data and Statistical Analyzes.
Figure S1. Behavioral Batteries. (A) Behavioral Battery 1 consisted of novel Object Recognition (nOR), followed by Tail Suspension (TS), followed by Rotarod (RR) with 7 days intervals between each task and with a M6P or Vehicle (VH) s.c. injection given 20 min before each task and counterbalanced at the subsequent task. (B) Behavioral Battery 2 consisted of Open Field (OF) followed, 7–10 days later by Marble Burying. A s.c. injection of M6P or Vehicle (VH) was given 20 min before each behavior and was counterbalanced at the subsequent behavior.


