ABSTRACT
The vaginal microbiome composition in humans is categorized based upon the degree to which one of four species of Lactobacillus is dominant (Lactobacillus crispatus, community state type I [CST I], Lactobacillus gasseri, CST II, Lactobacillus iners, CST III, and Lactobacillus jensenii, CST V). Women with a vaginal microbiome not dominated by one of the four Lactobacillus species tend to have a more diverse microbiome, CST IV. CSTs I, II, III, and V are common in North America and Europe and are associated with lower incidences of some pathogens, such as human immunodeficiency virus (HIV), human papillomavirus (HPV), and Gardnerella vaginalis. As a result, therapeutic interventions to change the composition of the vaginal microbiomes are under development. However, Homo sapiens is the only mammalian species which has high frequencies of Lactobacillus-dominated vaginal microbiomes. Here, we treated female nonhuman primates (NHPs) with regimens of metronidazole and high levels of L. crispatus to determine how well these animals could be colonized with L. crispatus, how this influenced the immunological milieu, and how Lactobacillus treatment influenced or was influenced by the endogenous vaginal microbiome. We find that NHPs can transiently be colonized with L. crispatus, that beta diversity and not the number of doses of L. crispatus or pretreatment with metronidazole predicts subsequent L. crispatus colonization, that L. crispatus does not alter the local immunological milieu, and that the vaginal microbiome composition was resilient, normalizing by 4 weeks after our manipulations. Overall, this study suggests these animals are not amenable to long-term L. crispatus colonization.
IMPORTANCE NHPs have proven to be invaluable animal models for the study of many human infectious diseases. The use of NHPs to study the effect of the microbiome on disease transmission and susceptibility is limited due to differences between the native microbiomes of humans and NHPs. In particular, Lactobacillus dominance of the vaginal microbiome is unique to humans and remains an important risk factor in reproductive health. By assessing the extent to which NHPs can be colonized with exogenously applied L. crispatus to resemble a human vaginal microbiome and examining the effects on the vaginal microenvironment, we highlight the utility of NHPs in analysis of vaginal microbiome manipulations in the context of human disease.
KEYWORDS: Lactobacillus crispatus, vaginal microbiome, nonhuman primate
INTRODUCTION
The composition of the human vaginal microbiome is categorized into community state types (CSTs) based on the abundance of particular Lactobacillus species (1, 2). Lactobacillus-dominant vaginal microbiomes are of low alpha diversity and are dominated by one of four particular species: Lactobacillus crispatus (CST I), Lactobacillus gasseri (CST II), Lactobacillus iners (CST III), or Lactobacillus jensenii (CST V). Women with vaginal microbiomes comprised of more diverse bacteria are classified into a separate CST (CST IV). CST IV has higher proportions of anaerobic bacteria, including Gardnerella vaginalis, Prevotella spp., Mobiluncus spp., and several Clostridium species. Due to high species and within-species diversity, this CST can be further divided into several distinct CSTs by some analyses (2).
CSTs have been implicated in numerous diseases. Women with the polymicrobial CST IV are at higher risk of developing bacterial vaginosis (BV) than women with CST I (1, 3). Additionally, epidemiological data indicate that women with Lactobacillus-deficient vaginal microbiomes acquire some sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), at higher rates than those with Lactobacillus-dominant CSTs (4). The mechanisms by which Lactobacillus may protect against vaginal dysbiosis and infection are both host and microbe directed. Lactobacilli may promote vaginal health either directly, through beneficial effects of Lactobacillus-derived metabolites on epithelial cells, or indirectly, by inhibiting the growth or virulence of pathogenic species (5). Lactobacilli have been categorized as probiotics in multiple microbiome compartments, including the gastrointestinal tract, oral mucosa, and female genital tract (FGT), and vaginally derived lactobacilli have been shown to inhibit the growth of pathogenic bacteria like G. vaginalis, Atopobium vaginae, and other anaerobic vaginitis-causing bacteria through production of bacteriocin and lactic acid (5). Relative to Lactobacillus-dominant CSTs, the Lactobacillus-deficient CST IV is associated with elevated levels of proinflammatory cytokines in the vaginal milieu and increased HIV target cell numbers in the FGT relative to these parameters for L. crispatus-dominated CST I (4). Furthermore, it has been shown that members of the polymicrobial CST IV metabolize tenofovir, an antiretroviral drug that can be formulated as a microbicide in a topical gel and used to prevent HIV acquisition (6).
Given these trends, there have been efforts to develop probiotic therapeutics to treat recurrent BV and establish Lactobacillus dominance in the FGT. The traditional standard of care for BV is a 5-day course of intravaginal metronidazole gel, though there is a high rate of BV recurrence with this therapy. Recently, a phase II-b clinical trial for an intravaginal live biotherapeutic containing Lactobacillus crispatus strain CTV-05 (Lactin-V; Osel, Inc.) showed significant reduction in risk of BV recurrence at 24 weeks in women treated with a metronidazole-probiotic combination relative to the reduction with a metronidazole and placebo control (7).
Animal models are useful for conducting controlled, longitudinal, invasive experiments with pathogen challenge. Indeed, pigtail macaques (PTM) have been considered for comparison of adolescent and adult vaginal microbiomes and their influence on simian immunodeficiency virus (SIV) susceptibility (8). However, Lactobacillus dominance of the FGT does not occur naturally in other mammalian species, including nonhuman primates (NHPs) (9–11). Given the importance of a Lactobacillus-dominant microbiome for female health, there has been interest in understanding the mechanisms surrounding the establishment of Lactobacillus nondominance and in developing a Lactobacillus-dominant NHP model. Cervicovaginal lavage samples from PTM have been used to study the effect of antiviral compounds on native Lactobacillus spp. (12). Furthermore, FGTs of rhesus macaques (RM) have been experimentally colonized with L. jensenii transformed to express antiviral compounds and then challenged with intravaginal simian-human immunodeficiency virus (SHIV) (13–16). Although Lactobacillus colonization in NHPs has shown some efficacy, the use of NHPs to study the female vaginal microbiome is complicated given that some NHP species, including RM, have evolved to menstruate seasonally (17, 18), whereas others, such as African green monkeys (AGM), menstruate year-round (19). Although PTM cycle more regularly, it remains unclear what effect these differing menstrual patterns may have on long-term colonization (20, 21). While RM and AGM have proven to be invaluable animal models in the study of HIV transmission and disease progression, the prospect of establishing a human-typical microbiome in these NHPs in the context of SIV infection remains understudied.
Here, we assess the degree to which hosts that are susceptible and nonsusceptible to progressive SIV infection, RM and AGM, respectively (22), could be colonized by L. crispatus strain MV-3A-US, whether L. crispatus colonization influenced the immunological milieu or pH, and whether the composition of the vaginal microbiome was dramatically altered by attempts at L. crispatus colonization. Our results show that both RM and AGM are resistant to long-term vaginal colonization by L. crispatus and the benefits that would be imparted by said colonization. These findings suggest that NHP models of vaginal microbiome manipulations and susceptibility to SIV acquisition would be difficult to use to robustly and reliably unravel the therapeutic efficacy of Lactobacillus-based interventions.
RESULTS
Study design and steady-state vaginal microbiome analysis in NHPs.
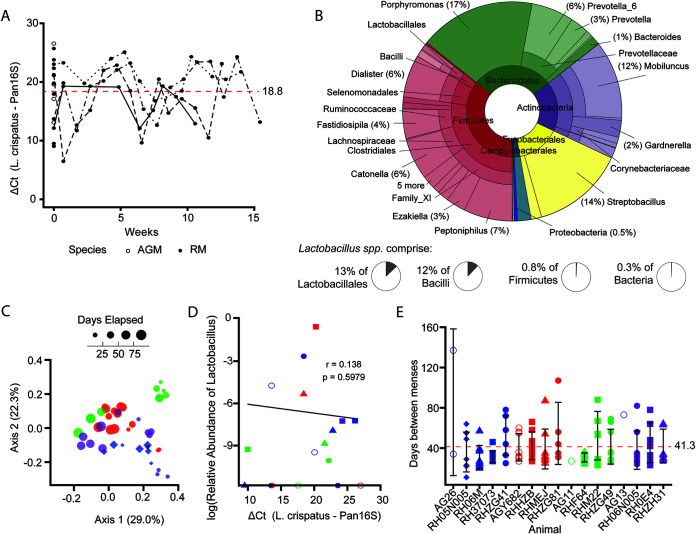
To assess native levels of L. crispatus in NHPs, vaginal swab samples were collected weekly from four animals for the duration of three menstrual cycles prior to any metronidazole or Lactobacillus administration. DNA was extracted and analyzed via quantitative PCR (qPCR) using primers targeting either L. crispatus DNA or total bacterial DNA (Pan16S). The relative levels of L. crispatus were determined using the change in cycle threshold (ΔCT) between the two amplicons. While L. crispatus levels fluctuated throughout the monitoring period, the average ΔCT (L. crispatus, Pan16S) remained high (mean ± standard deviation, 18.8 ± 4.7), indicating stable but very low L. crispatus levels, approximately 1:106 of all bacteria. These low levels of L. crispatus were not influenced by the animals’ menstrual cycles (Fig. 1A). Thus, none of the animals in the study would be assigned to CST I.
FIG 1.
Vaginal microbiomes of NHPs at baseline have low levels of Lactobacillus. (A) Relative levels of L. crispatus in vaginal swab samples of NHPs longitudinally through three menstrual cycles prior to any treatment (RH05N005, RHZG81, RHZG49, and RH06N005) and at one baseline time point for all animals, measured by qPCR. Dashed red line represents the mean ΔCT for all data points shown. (B) Taxonomic composition of NHP vaginal microbiome determined by 16S amplicon sequencing at baseline averaged across all animals. Higher-order taxonomic classifications are collapsed where only one genus is represented. (C) Principal-component analysis of weighted UniFrac distances of vaginal microbiota of four RM longitudinally through three menstrual cycles. Symbol size by elapsed time. (D) Correlation of log relative abundances of Lactobacillus detected by 16S amplicon sequencing and relative levels of L. crispatus detected by qPCR. Correlation coefficient and P value based on Spearman’s rank-order correlation. (E) Days between observed menses for NHPs. Dashed red line represents the mean. Error bars represent standard deviations. Colors correspond to assigned treatment groups (A, blue; B, red; C, green; D, purple), and shapes represent individuals as listed in Table 1. Open points represent AGM. Closed points represent RM.
To understand if any of the animals in our cohort were heavily colonized with other species of Lactobacillus and to determine which bacterial taxa were frequent, we performed 16S sequencing of DNA isolated from vaginal swabs of the animals obtained prior to therapeutic interventions (Fig. 1B). The vaginal microbiomes of the NHPs in this study were predominantly composed of members of Firmicutes (37%) and Bacteroidales (30%) (Fig. 1B). Actinobacteria (17%) and Fusobacteria (13%) were also present at high abundances. Additionally, Campylobacterales were present at low levels (2%), and very low abundances of Treponema (0.2%), Synergistaceae (0.1%), and Proteobacteria (0.5%) were detected. Mobiluncus and Gardnerella, members of Actinobacteria associated with BV in humans, made up 15% and 1% of the RM vaginal microbiome, respectively. Importantly, Lactobacillus spp. make up only 0.1% of total bacteria in the RM vagina (0.4% of Firmicutes, 8% of Bacilli, and 9% of Lactobacillales) (Fig. 1B). Thus, none of the NHPs in our cohort were naturally colonized with any species of Lactobacillus, and their vaginal microbiomes would be best characterized as CST IV.
To assess the stability of the vaginal microbiome, we conducted weighted UniFrac analysis paired with principle component analysis of the total vaginal microbiome for the four individuals monitored through three menstrual cycles. The vaginal microbiome did not appear to shift significantly throughout the monitoring period within individuals, but there were substantial differences between some individuals (Fig. 1C).
To validate our qPCR assay for quantifying the relative levels of L. crispatus and to assess whether the Lactobacillus present in the 16S-quantified microbiome was L. crispatus or another Lactobacillus species, we compared the relative abundance of Lactobacillus as measured by 16S sequencing to the relative level of L. crispatus detected by qPCR. Before metronidazole or Lactobacillus administration, there was a weak yet significant correlation between the level of L. crispatus and the log relative abundance of Lactobacillus (Fig. 1D), indicating that although L. crispatus is present in the vaginal microbiome of NHPs, there are likely other Lactobacillus species present.
The unpredictability of menstrual cycle periodicity can complicate the experimental timing of intravaginal SIV challenge studies (23, 24). Hormonal drugs like medroxyprogesterone can be administered to artificially synchronize menstrual cycles of study animals. However, such hormonal treatment thins epithelial barriers in the female reproductive tract and seems to decrease levels of Lactobacillus (25, 26). Though the NHP menstrual cycles observed in this study were less consistent than those of most women (26 to 34 days [27]), the majority of animals included in the study cycled every 20 to 60 days (Fig. 1D), with a mean of 41.3 ± 20.0 days. The AGM menstruated more sporadically during the monitoring period, likely due to their advanced age (Table 1). Thus, the seasonal menstrual cycling of RM seems to be less pronounced after the animals are put into climate-controlled environments, in agreement with documented observations (17, 18). To minimize potential effects of menstruation on the durability of L. crispatus colonization, we administered metronidazole (if applicable) and L. crispatus treatments at 8 and 13 days, respectively, after the onset of menses for each animal, thus allowing 1 week for menstruation to cease before treatment and 2 to 3 weeks between treatment and the next menses.
TABLE 1.
Summary of study cohort group assignments and animal weights and ages
| Group | Treatment | Animal | Common namea | Wt (kg) | Age (yr) | Symbol, colorb |
|---|---|---|---|---|---|---|
| A | Metronidazole, 5 doses L. crispatus | AG26c | AGM | 2.9 | 14 | ○, blue |
| RH37073 | RM | 9.9 | 14 | ■, blue | ||
| RH06M | RM | 5.5 | 7 | ▴, blue | ||
| RHZG41 | RM | 6 | 12 | ●, blue | ||
| RH05N005 | RM | 8.5 | 15 | ♦, blue | ||
| B | Metronidazole, 1 dose L. crispatus | AGY682d | AGM | 4.6 | 17 | ○, red |
| RHHZB | RM | 4.7 | 5 | ■, red | ||
| RHMEJ | RM | 7.4 | 9 | ▴, red | ||
| RHZG81 | 8.5 | 12 | ●, red | |||
| C | 5 doses L. crispatus | AG11c | AGM | 2.5 | 18 | ○, green |
| RHM22 | RM | 6.3 | 8 | ■, green | ||
| RHF64 | RM | 7.9 | 14 | ▴, green | ||
| RHZG49 | RM | 9 | 12 | ●, green | ||
| D | 1 dose L. crispatus | AG1c | AGM | 2.5 | 18 | ○, purple |
| RH0E4 | RM | 4.9 | 7 | ■, purple | ||
| RHZH31 | RM | 10.7 | 11 | ▴, purple | ||
| RH06N005 | RM | 6.4 | 14 | ●, purple |
AGM, African green monkey; RM, rhesus macaque (Macaca mulatta).
The symbols and colors are those used in the figures and described in the legend to Fig. 1.
Chlorocebus pygerythrus.
Chlorocebus sabaeus.
NHPs can be transiently colonized with Lactobacillus crispatus.
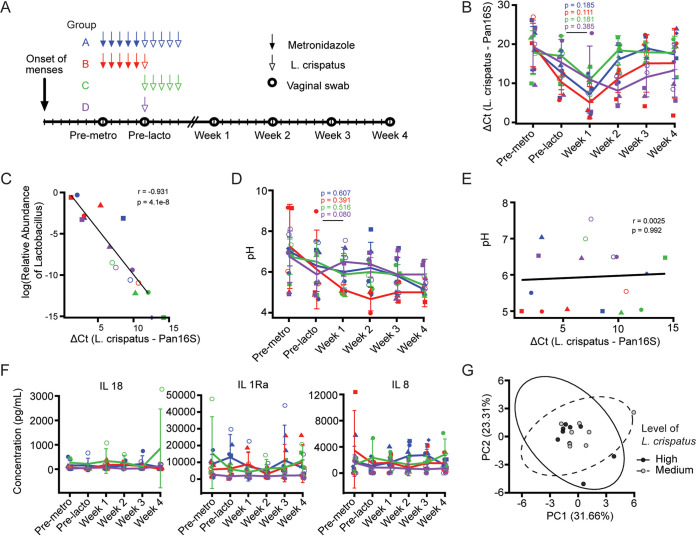
We sought next to determine whether therapeutic interventions could lead to the NHP vaginal microbiome becoming L. crispatus dominant. Thirteen female RM and four female AGM were randomized into one of four treatment groups (Table 1). Therapeutic interventions were initiated 1 week after observed menstruation, and Lactobacillus colonization attempts were performed 2 weeks after menstruation (Fig. 2A). Group A received pretreatment with vaginal metronidazole, as this therapy is a common treatment for BV and common among women with vaginal microbiomes in CST IV. These animals were then treated for 5 consecutive days with 1 × 1011 CFU of L. crispatus. Animals in group B received pretreatment with metronidazole, followed by only one dose of 1 × 1011 CFU of L. crispatus. Animals in group C received 5 consecutive doses of 1 × 1011 CFU of L. crispatus without pretreatment with metronidazole, and group D received only one dose of 1 × 1011 CFU of L. crispatus (Fig. 2A).
FIG 2.
NHPs can be transiently colonized with L. crispatus without altering pH or inflammation. (A) Study design. Animals were assigned to group A, B, C, or D receiving 1 or 5 doses of L. crispatus with or without metronidazole pretreatment. Lactobacillus dose was administered on the 13th day after observed menstruation. Vaginal swabs were collected immediately before each treatment and weekly for 4 weeks beginning 7 days after the final Lactobacillus dose. (B) Levels of L. crispatus DNA relative to total bacterial DNA present in vaginal swabs longitudinally after treatment. (C) Correlation of log relative abundances of Lactobacillus detected by 16S amplicon sequencing and relative levels of L. crispatus detected by qPCR. (D) Longitudinal vaginal pH values. (E) Correlation of vaginal pH values and relative levels of L. crispatus across all animals and time points sampled. (F) Longitudinal concentrations of inflammatory cytokines in vaginal swabs measured by cytokine bead array. (G) Principal-component analysis based on weighted UniFrac analysis of concentrations of 15 analytes measured by cytokine bead array, stratified by relative levels of L. crispatus. Ellipse represents 99% confidence interval (CI) of centroid. (B, D, F) Lines represent group mean values, error bars represent standard deviations. Colors and shapes are as in Fig. 1 and Table 1. Differences between time points were determined by paired Wilcox rank sum test. (C, E) Correlations were determined by Spearman’s rank-order correlation test.
We measured the levels of L. crispatus relative to the total amount of bacteria captured longitudinally in the animals in each treatment group, using a species-specific quantitative PCR for L. crispatus and a qPCR for DNA encoding 16S ribosomal DNA, respectively (Fig. 2B). While metronidazole treatment itself led to a statistically significant increase in the relative abundance of endogenous L. crispatus, indicated by the lower ΔCT, high numbers of exogenously applied live L. crispatus were incapable of significantly and routinely increasing the relative abundance of L. crispatus beyond 1 week (Fig. 2B). While five total animals became fairly dominantly colonized by L. crispatus 1 week after administration, they were not in the same treatment groups, and only two animals maintained fairly durable colonization with L. crispatus. One of these was assigned to group B, while the other was assigned to group D (Fig. 2B). These data demonstrate that we were unsuccessful at consistent and durable alteration of the vaginal microbiome in NHPs from a CST IV to a CST I. However, the correlation between the relative abundance of L. crispatus detected by qPCR using species-specific primers and the relative abundance of Lactobacillus as determined by 16S amplicon sequencing was substantially stronger after administration of L. crispatus than before treatment (Fig. 1C and 2C). This correlation held if only samples from rhesus macaques were used (data not shown).
With the high level of lactic acid production by Lactobacillus, we next sought to examine how or whether our attempts at Lactobacillus colonization led to any alteration in the vaginal pH. Even though metronidazole alone led to an increase in the relative abundance of L. crispatus, there was no significant reduction in pH after metronidazole treatment (Fig. 2D). Indeed, no pH reduction was observed at any time point, in any group of animals. Moreover, the pH approached 4—often observed in women with Lactobacillus-dominated CSTs (2)—in only 1 animal in group B and in the second week of treatment (Fig. 2D). As the animal with the most-sustained L. crispatus colonization (RHHZB) had the lowest pH at 4 weeks after treatment, we wondered if pH might be related to the level of L. crispatus colonization. However, when we compared pH values to relative abundances of L. crispatus as measured by qPCR, no significant association was observed (Fig. 2E). Thus, our attempts at colonization with L. crispatus did not dramatically decrease vaginal pH.
Given the proinflammatory immunological milieu associated with CST IV, we measured how cytokine levels were influenced by our therapeutic interventions. We used cytokine bead arrays to measure 23 individual analytes in our longitudinal vaginal swab samples; of these, 15 were routinely detectable (Fig. 2F). Of the 15 detectable cytokines, the proinflammatory markers interleukin-18 (IL-18), IL-1 receptor alpha, and IL-8 were present at the highest levels. None of these levels were altered in any group of animals when longitudinal samples were studied (Fig. 2F). Given that the degree to which animals were colonized with L. crispatus was not consistently different among the treatment groups, we separated the animals into terciles based upon the relative abundances of L. crispatus and performed principal-component analysis (PCA) of all analyte data from our cytokine bead array analysis to understand whether L. crispatus levels influenced the immunological milieu (Fig. 2G). From this analysis, it is clear that the analytes we measured were not influenced by the relative abundance of L. crispatus.
L. crispatus colonization does not alter the vaginal microbiome composition.
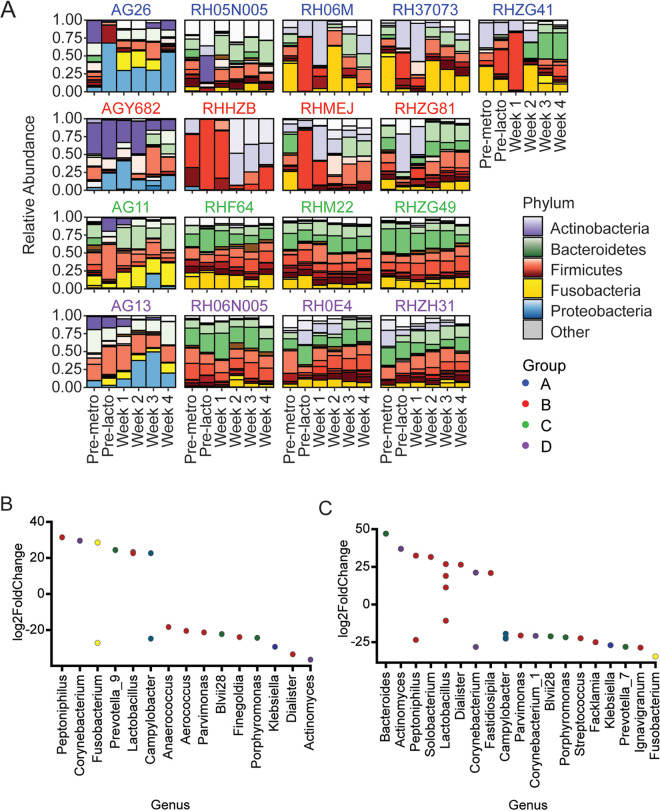
We next conducted 16S amplicon sequencing of DNA extracted from vaginal swab samples to determine the microbiome composition to the genus level (Fig. 3). In animals treated with metronidazole (groups A and B), Bacteroidetes and Fusobacteria were substantially reduced by the antibiotic treatment, while Firmicutes and Actinobacteria increased in relative abundance (Fig. 3A). The increase in the relative abundance of Firmicutes after metronidazole treatment was not sustained after Lactobacillus administration, indicating that endogenous L. crispatus did not substantially recolonize the vaginal environment after metronidazole treatment. In animals receiving no antibiotic pretreatment (groups C and D), the administration of Lactobacillus did not appear to drastically affect the composition of the microbiome, with the levels of bacterial phyla remaining fairly constant longitudinally. Thus, Lactobacillus treatment alone is insufficient to significantly alter the vaginal microbiome from CST IV-like to CST I in NHPs.
FIG 3.
NHPs were not durably colonized with L. crispatus, although microbiome composition was altered. (A) Relative abundances of bacterial taxa longitudinally for individual animals before and after treatment, measured by 16S sequencing. Primary taxon color is by phylum, shading is by family. (B and C) Significantly differentially abundant taxa at week 4 relative to pretreatment for animals pretreated with metronidazole (groups A and B) (B) and for animals without metronidazole pretreatment (groups C and D) (C). Significance level was Padjusted = 0.05 with Benjamini-Hochberg correction for multiple comparisons.
The composition of vaginal microbiota differed substantially at the phylum level between the two NHP species we included in our analyses (Fig. 3A). At baseline, AGM vaginal microbiomes contained higher levels of Actinobacteria and Proteobacteria than RM, with a lower abundance of Bacteroidetes. While Actinobacteria, Bacteroidetes, Firmicutes, and Fusobacteria were present in both AGM and RM, the predominant bacterial genera in AGM and RM appeared to be of different taxonomic families. Additionally, the abundance of Proteobacteria in AGM increased after treatment with metronidazole and/or Lactobacillus, while Proteobacteria were present only in trace amounts in RM.
To investigate changes in the vaginal microbiome at the genus level, we assessed taxa that were differentially abundant pretreatment relative to 4 weeks after Lactobacillus treatment in animals with (Fig. 3B) and without (Fig. 3C) metronidazole pretreatment. In group A and B animals that received metronidazole pretreatment, members of Firmicutes were more abundant 4 weeks after Lactobacillus treatment than at baseline relative to their levels in animals in groups C and D that did not receive metronidazole. Indeed, three ASVs that were more abundant after treatment in groups A and B were assigned to the genus Lactobacillus. Moreover, members of Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria, and even some Firmicutes were reduced in the week 4 microbiome relative to the baseline in both metronidazole-treated and untreated animals (Fig. 3B and C). Thus, even though the NHPs did not develop CST I, their vaginal microbiomes were influenced by our treatment modalities.
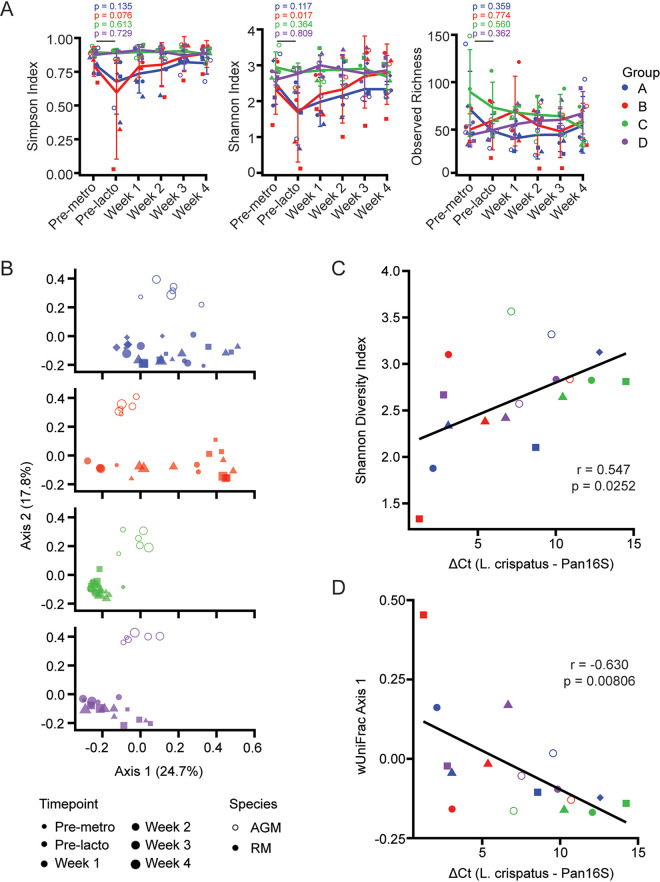
In humans, vaginal CSTs dominated by Lactobacillus spp. tend to have lower overall diversity than Lactobacillus-deficient CSTs (1). Therefore, it was of interest to determine whether L. crispatus treatment and transient colonization by L. crispatus affected alpha diversity in the NHP FGT (Fig. 4A). Longitudinally, alpha diversity decreased after metronidazole treatment in groups A and B by both the Simpson and Shannon diversity indices, rebounding 1 to 2 weeks after Lactobacillus administration (Fig. 4A). Lactobacillus treatment alone in groups C and D did not significantly affect alpha diversity by either index (Fig. 4A).
FIG 4.
Microbiome composition is altered by L. crispatus and predicts degree of colonization. (A) Alpha diversities of vaginal microbiota longitudinally assessed by Simpson, Shannon, and Observed diversity indices. Error bars represent standard deviations. (B) Principal-component analysis of weighted UniFrac distances of vaginal microbiota faceted by treatment group. Time points are distinguished by size. (C) Correlation of Shannon diversity index value before treatment with the peak level of L. crispatus attained per individual. (D) Correlation of position on weighted UniFrac axis 1 before treatment with the peak level of L. crispatus attained per individual. Colors and shapes are as in Fig. 1 and Table 1. Differences between time points were determined by paired Wilcox rank sum test. Correlations were determined by Spearman’s rank-order correlation test.
To further assess whether Lactobacillus colonization altered the microbial profile and identify variation in vaginal microbiomes, we conducted a weighted UniFrac distance analysis paired with principal-component analysis (Fig. 4B). As already observed (Fig. 3A), the NHP species differences between AGM and RM accounted for most of the variation across the vaginal microbiomes we studied. The vaginal microbiome of RM in groups A and B receiving metronidazole pretreatment showed temporal separation between samples obtained premetronidazole and for 2 weeks postmetronidazole treatment, with samples obtained at later time points clustering nearer to baseline samples. The microbial profiles of animals in groups C and D did not separate longitudinally, indicating that Lactobacillus treatment did not significantly alter the vaginal microbial composition, consistent with our other analyses.
Because the success of L. crispatus colonization was variable among individuals and not dependent on treatment group (Fig. 2B), we wondered whether features of the microbiome at baseline were predictive of the degree to which an animal would be able to be colonized with L. crispatus. We therefore explored the relationship between the alpha and beta diversities of the vaginal microbiome at baseline and the maximum level of L. crispatus colonization achieved. Peak L. crispatus levels occurred at week 1 for all animals except RH06N005 and RHZH31, for which L. crispatus peaked at week 2. The Shannon diversity index of the baseline microbiome correlated significantly with the minimum ΔCT measured, though the strength of the correlation was weaker for the Simpson diversity index (Fig. 4C and data not shown). The position on axis 1 of the weighted UniFrac principal-component analysis (PCA) of the baseline microbiome was also significantly correlated with the ΔCT at peak L. crispatus abundance (Fig. 4D), even if only rhesus macaques were used to perform the analysis (data not shown). These data indicate that the composition of the vaginal microbiome at baseline may contribute to the ability of L. crispatus to colonize the FGT in NHPs, more so than which treatment modality was employed.
Finally, we sought to determine whether prior exposure to L. crispatus might influence subsequent attempts at L. crispatus colonization. RHZG41, initially in group A, achieved a transient but significant colonization with L. crispatus and was chosen for a second attempt at colonization. Seven months after the initial colonization, this animal was recolonized with the same treatment regimen (Fig. 5). L. crispatus was detected at low levels at baseline immediately before colonization, as well as before recolonization (Fig. 5). While L. crispatus accounted for the vast majority of bacterial DNA from vaginal swabs at week 2 in the first colonization, the relative abundance of L. crispatus at the same time point during recolonization was approximately 3 orders of magnitude lower. Additionally, the relative abundance of L. crispatus at week 3 was lower in the recolonization attempt than in the first colonization attempt (Fig. 5). Thus, any individual animal’s proclivity to L. crispatus colonization is not necessarily consistent across multiple colonization attempts.
FIG 5.
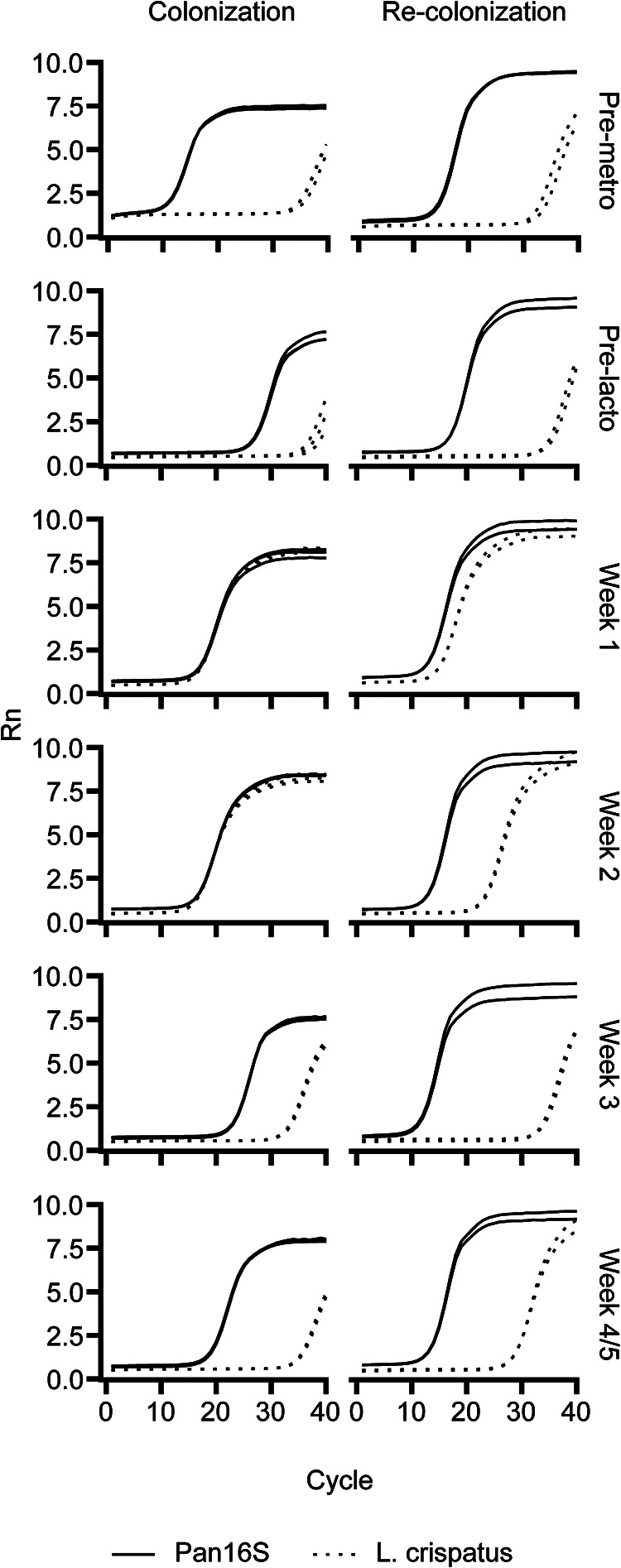
Real-time PCR amplification curves of vagina swab samples from two colonization attempts for RHZG41. Solid line, Pan16S primers. Dashed line, L. crispatus-specific primers.
DISCUSSION
Here, we attempted to change the vaginal microbiome of NHPs from CST IV to CST I with high doses of L. crispatus with or without metronidazole pretreatment. The majority of the animals we treated did not achieve CST I, and the ability to achieve CST I depended on the composition of the vaginal microbiome prior to treatment more than on the individual treatment modalities we employed. While we found some transient colonization of the vaginal microbiome with L. crispatus in NHPs, we found no decrease in vaginal inflammation or pH. Overall, these data suggest that neither RM nor AGM are amendable to long-term Lactobacillus dominance in the vaginal microbiome.
CSTs I, II, III, and V, dominated by L. crispatus, L. gasseri, L. iners, and L. jensenii, respectively, have only been observed in humans. Though there are reports of significant levels of Lactobacillus in some NHP cohorts (15), the vaginal microbiomes of NHPs do not approach the levels of Lactobacillus dominance observed in humans and more frequently resemble the high-diversity Lactobacillus-deficient CST IV (9, 10). Moreover, the proportions of women with Lactobacillus-dominated CSTs vary both with geography and ethnicity, with CSTs I, II, III, and V found in nearly 90% of North American white women and only 60% of Black and Hispanic North American women (1). In a large prospective cohort study of South African women, only 40% of participants had Lactobacillus-dominated vaginal microbiomes (4). High-diversity Lactobacillus-deficient communities are associated with increased inflammation in the FGT and with an increased incidence of BV (4, 28). Furthermore, CST IV has been linked to an increased rate of susceptibility to HIV acquisition, likely explained by the inflammatory milieu associated with CST IV microbes (4). Lactobacillus-dominated CSTs, especially the L. crispatus-dominated CST I, reduce vaginal inflammation and provide protection against STIs, including HIV, through decreased pH and secretion of bacteriocins (5).
Given the adverse health outcomes associated with CST IV and the protective effects of Lactobacillus species, especially L. crispatus, there have been efforts to shift the vaginal microbiota of women from CST IV to CST I by using a combination of antibiotics and exogenously applied probiotics or live biotherapeutics (29). Typically, metronidazole antibiotic gel is used in the treatment of BV because of its targeted effects on anaerobes (30). However, BV recurrence is common among antibiotic-treated individuals (7, 31). Live L. crispatus biotherapeutics used in conjunction with metronidazole can reduce the recurrence of BV and CST IV microbiota (7), although how L. crispatus probiotics might influence HIV susceptibility is unknown.
NHPs have been an invaluable animal model for infectious disease research and in understanding host-pathogen-microbiome interactions. Our data indicate that the vaginal microbiome of NHPs is naturally deficient in Lactobacillus spp. and is resistant to long-term colonization by exogenously applied L. crispatus, highlighting the coevolution of the microbiome with the host. We saw that following metronidazole treatment, the relative abundance of Firmicutes, in particular Lactobacillus, increased significantly. However, endogenous and exogenously applied Lactobacillus bacteria were unable to repopulate that space at a higher abundance than before treatment.
Vaginal pH is considered an important indicator of vaginal health. Women with Lactobacillus-deficient BV-associated CSTs tend to have higher vaginal pH values than women with Lactobacillus-dominated CSTs, and having a vaginal pH of >4.5 is one of the Amsel criteria used clinically to diagnose BV (32). In humans, the production of lactic acid by lactobacilli lowers pH, inhibiting the outgrowth of pathogenic bacteria and possibly inhibiting viral infection (1, 33). Unlike in humans, in our attempts at colonization of NHPs, we did not see a significant reduction in pH after administering L. crispatus and the level of L. crispatus did not correlate with vaginal pH, even if only rhesus macaques were included in the analysis.
Lactobacillus species, including L. crispatus, utilize glycogen as a food source, and the scarcity of Lactobacillus in the FGT of NHPs correlates with low levels of glycogen in the vagina (34, 35). Glycogen in the vagina is broken down by α-amylase of host or microbial origin and is metabolized by lactic acid-producing microbes like Lactobacillus. It is possible that supplementing vaginal glycogen during and after L. crispatus administration might increase the degree to which the FGT is colonized by L. crispatus in both humans and NHPs.
Lactobacillus dominance has so far only been observed in humans. Furthermore, Lactobacillus-dominant CSTs I, II, III, and V occur more frequently in women of European or North American or Asian descent than in women of African or Hispanic descent, including those living in North America (1, 4). Moreover, the vaginal microbiome of an individual is dynamic and fluctuates with age, often changing during puberty or menopause (36). Though cultural norms and sanitation, such as use of feminine hygiene products, may play a role in determining the composition of the vaginal microbiome, more studies are needed to understand behavioral, environmental, or genetic factors that may influence the vaginal microbiome and reproductive health (36). While NHP models are useful for studies of human reproductive systems, further work is needed to understand why the vaginal microbiome compartment in NHPs does not maintain the high levels of Lactobacillus found in humans. The data we present here provide a framework for future studies.
MATERIALS AND METHODS
Animals.
Thirteen female rhesus macaques (Macaca mulatta) and four female African green monkeys (Chlorocebus pygerythrus and Chlorocebus sabaeus) were assigned to one of four treatment groups (Table 1). Group A received a 5-day course of metronidazole followed by 5 days (doses) of L. crispatus treatment (4 RM and 1 AGM). Group B received metronidazole with 1 dose of L. crispatus (4 RM and 1 AGM). Group C received 5 daily doses of L. crispatus treatment without metronidazole pretreatment (4 RM and 1 AGM). Group D received 1 dose of L. crispatus treatment without metronidazole pretreatment (4 RM and 1 AGM). Animals were monitored for menstruation, and treatments were timed such that Lactobacillus treatment was initiated 13 days after onset of menses.
The National Institute of Allergy and Infectious Diseases (NIAID) animal care and use committee, as part of the National Institutes of Health (NIH) intramural research program, approved all experimental procedures pertaining to the animals (protocol LVD 26E). The animals in this study were housed and cared for at the NIH animal center, under the supervision of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited division of veterinary resources and as recommended by the office of animal care and use nonhuman primate management plan. Care at this facility met the standards set forth by the Animal Welfare Act and animal welfare regulations (37), U.S. Fish and Wildlife Service regulations (38), and the Guide for the Care and Use of Laboratory Animals (39). The physical conditions of the animals were monitored daily. Animals in this study were exempt from contact social housing due to scientific justification according to the NIAID/NIH institutional animal care and use committee (IACUC) protocol and were housed in noncontact, social housing where the primary enclosures consisted of stainless-steel primate caging. The animals were provided continuous access to water and offered commercial monkey biscuits twice daily, as well as fresh produce, eggs, and bread products and a foraging mixture consisting of raisins, nuts, and rice. Enrichment to stimulate foraging and play activity was provided in the form of food puzzles, toys, cage furniture, and mirrors.
Lactobacillus crispatus.
Lactobacillus crispatus strain MV-3A-US was obtained from BEI resources as a glycerol stock, and cultures were maintained in Mann Rogosa Sharpe (MRS) medium (Teknova) by passaging every 3 to 4 days and incubating at 37°C without agitation. For vaginal administration, 200 ml MRS was inoculated with 1 ml of 24-h-old L. crispatus culture in an Erlenmeyer flask and incubated for 24 h at 37°C without shaking to approximately 1 × 109 CFU/ml at the end of the log phase of growth, according to predetermined bacterial growth curves. Bacterial concentrations were verified by plating 50 μl of 10-fold serial dilutions of culture on MRS agar plates using a spot plate method and counting CFU after 48 h of incubation at 37°C. The culture was mixed and then centrifuged at 1,000 × g for 10 min. Bacterial pellets containing 1 × 1011 CFU were resuspended in 1 ml 3% (wt/vol) hydroxyethyl cellulose (HEC; Aldrich) in PBS and 1 ml fresh MRS broth. HEC has previously been used as a placebo in the assessment of vaginal microbicide gels (40).
Bacterial suspensions were administered intravaginally using a 3-ml syringe. L. crispatus administration occurred once daily for 5 days or 1 day according to the treatment groupings. For animals in metronidazole-treated groups, metronidazole vaginal gel, 0.75% (Upsher-Smith) was administered using the provided applicator once daily for 5 days immediately prior to L. crispatus administration. The hind quarters were elevated for 15 min after administration of metronidazole or L. crispatus. The vaginal inflammatory milieu was sampled weekly (Fig. 1) by inserting a polyester-tipped swab (Fisher, Waltham, MA) into the vagina and swirling. The swab tip was cut from the stem into a 1.5-ml tube containing 300 μl sterile saline (McKesson, Irving, TX) and frozen at −80°C. For microbiome sampling, two additional vaginal swab samples were collected using the same procedure and the swabs placed into 1.5-ml Precellys tubes containing homogenization beads (Bertin Corp., Rockville, MD) and frozen at −80°C. Vaginal pH was measured by inserting colorimetric pH test strips (MicroEssential Laboratory, Brooklyn, NY) into the vagina and then comparing to the color chart.
Microbiome analysis.
Total DNA was extracted from vaginal swabs using a modified PowerFecal kit (Qiagen). Seven hundred fifty microliters of PowerBead solution and 60 μl of C1 solution were added to Precellys tubes containing vaginal swabs, and tubes were incubated for 10 min at 65°C. Mechanical homogenization was carried out using a Precellys 24 homogenizer (Bertin Corp., Rockville, MD), with 6 cycles of 30 s at 5,000 × g and 30 s of rest between cycles. The PowerFecal kit protocol was then followed. Extracted DNA was quantified by spectrophotometer (DeNovix, Wilmington, DE) and stored at −80°C.
The proportion of L. crispatus in the vaginal microbiome was determined through quantitative PCR (qPCR) using primers specifically targeting L. crispatus and universal 16S primers. Each reaction mixture contained SYBR green master mix (Applied Biosystems) at a final concentration of 1× and forward and reverse primers targeting either L. crispatus (forward, 5′-AGCGAGCGGAACTAACAGATTTAC-3′, and reverse, 5′-AGCTGATCATGCGATCTGCTT-3′) (41) or the universal 16S sequence (785F 5′-GGACTACGGATTAGATACCCTGGTAGTCC-3′, 919R 5′-CTTGTGCGGGTCCCCGTCAAT-3′) (42) at a final concentration of 500 nM per reaction mixture volume. Two microliters of DNA template was used per 20-μl reaction mixture volume. Measurement was assessed in triplicate on the Applied Biosystems StepOnePlus real-time PCR system with StepOne software (Thermo Fisher Scientific), using the following cycling parameters: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 60 s, and 72°C for 20 s.
Dual-index amplification and sequencing was performed on extracted DNA using the Illumina MiSeq platform with universal primers spanning the V4 region (515F to 806R) of the bacterial 16S rRNA as previously described (43). Illumina FASTQ files were demultiplexed with a custom R script. Paired-end FASTQ reads were filtered and processed using the dada2 package (version 1.18.0) in R (version 4.0.3). Before filtering, 14.2 million reads were included in 156 samples with an average of 91,000 reads per sample. After filtering and quality trimming, 9.2 million reads were included across all samples with an average of 59,000 reads per sample. Reads were binned into amplicon sequence variants (ASVs), and taxonomies were annotated with the SILVA taxonomic framework (release 132) and analyzed using the PhyloSeq (version 1.34.0) and DESeq packages (version 1.30.1) in R. FASTQ files and metadata are available in the NCBI Sequence Read Archive (accession number PRJNA734441).
Cytokine bead array.
Vaginal swabs frozen in saline were thawed and vortexed for 2 min and then centrifuged with the polyester tip up for 1 min at 1,000 × g to extract all fluids from the polyester-tipped swabs. The dry swab tips were discarded, and the fluids were centrifuged again for 1 min at 1,000 × g to pellet debris. Supernatant was transferred to a new tube and stored at −20°C. Undiluted extracts were analyzed using the nonhuman primate cytokine magnetic bead panel (Luminex; EMD Millipore, Billerica, MA) and the BioPlex 200 system (Bio-Rad, Hercules, CA) in technical duplicates. Only analytes that were in range for >70% of samples were included in downstream analyses.
Statistical analysis.
Paired Wilcox rank sum tests were carried out using the Benjamini-Hochberg correction for multiple comparisons where applicable (R, version 4.0.3). Spearman’s correlation coefficient was used to assess linear relationships between variables. Weighted UniFrac and alpha diversity analyses were conducted using the PhyloSeq package (version 1.34.0) in R. Differentially abundant taxa were assessed using the DESeq package (version 1.30.1) in R, with significance defined as an adjusted P value of <0.05 with Benjamini-Hochberg correction for multiple comparisons.
Data availability.
The study generated amplicon sequence data that have been deposited to SRA under PRJNA734441.
ACKNOWLEDGMENTS
We thank the NIAID Microbiome Program for technical and analytical assistance.
Funding for this study was provided in part by the Division of Intramural Research/NIAID/NIH. The content of this publication does not necessarily reflect the views or policies of DHHS, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Contributor Information
Jason M. Brenchley, Email: jbrenchl@mail.nih.gov.
Jan Claesen, Lerner Research Institute.
REFERENCES
- 1.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.France MT, Ma B, Gajer P, Brown S, Humphrys MS, Holm JB, Waetjen LE, Brotman RM, Ravel J. 2020. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. doi: 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donders GG, Bosmans E, Dekeersmaecker A, Vereecken A, Van Bulck B, Spitz B. 2000. Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol 182:872–878. doi: 10.1016/S0002-9378(00)70338-3. [DOI] [PubMed] [Google Scholar]
- 4.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, Padavattan N, Desai C, Droit L, Moodley A, Dong M, Chen Y, Ismail N, Ndung’u T, Ghebremichael MS, Wesemann DR, Mitchell C, Dong KL, Huttenhower C, Walker BD, Virgin HW, Kwon DS. 2017. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46:29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee WJY, Chew SY, Than LTL. 2020. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact 19:203. doi: 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, Grobler A, Westmacott G, Xie IY, Butler J, Mansoor L, McKinnon LR, Passmore JS, Abdool Karim Q, Abdool Karim SS, Burgener AD. 2017. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 356:938–945. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- 7.Cohen CR, Parks T, Hemmerling A. 2020. Randomized trial of Lactin-V to prevent recurrence of bacterial vaginosis. Reply. N Engl J Med 383:791–792. doi: 10.1056/NEJMc2021832. [DOI] [PubMed] [Google Scholar]
- 8.Berard AR, Miller C, Arainga M, Broedlow CA, Noel-Romas L, Schifanella L, Hensley-McBain T, Roederer A, Driscoll C, Coronado E, Manuzak J, McKinnon LR, Villinger FJ, Hope TJ, Burgener AD, Klatt NR. 2021. SIV susceptibility, immunology and microbiome in the female genital tract of adolescent versus adult pigtail macaques. AIDS Res Hum Retroviruses. doi: 10.1089/AID.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhoades NS, Hendrickson SM, Gerken DR, Martinez K, Slayden OD, Slifka MK, Messaoudi I. 2021. Longitudinal profiling of the macaque vaginal microbiome reveals similarities to diverse human vaginal communities. mSystems 6:e01322-20. doi: 10.1128/mSystems.01322-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Yeoh YK, Hui M, Wong PY, Chan MCW, Ip M, Yu J, Burk RD, Chan FKL, Chan PKS. 2018. Diversity of macaque microbiota compared to the human counterparts. Sci Rep 8:15573. doi: 10.1038/s41598-018-33950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spear GT, Kersh E, Guenthner P, Vishwanathan SA, Gilbert D, Zariffard MR, Mirmonsef P, Landay A, Zheng L, Gillevet P. 2012. Longitudinal assessment of pigtailed macaque lower genital tract microbiota by pyrosequencing reveals dissimilarity to the genital microbiota of healthy humans. AIDS Res Hum Retroviruses 28:1244–1249. doi: 10.1089/AID.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y, Tsai D, Rao SS, Hamer DH, Parks TP, Lee PP, Xu Q. 2006. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother 50:3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagenaur LA, Swedek I, Lee PP, Parks TP. 2015. Robust vaginal colonization of macaques with a novel vaginally disintegrating tablet containing a live biotherapeutic product to prevent HIV infection in women. PLoS One 10:e0122730. doi: 10.1371/journal.pone.0122730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brichacek B, Lagenaur LA, Lee PP, Venzon D, Hamer DH. 2013. In vivo evaluation of safety and toxicity of a Lactobacillus jensenii producing modified cyanovirin-N in a rhesus macaque vaginal challenge model. PLoS One 8:e78817. doi: 10.1371/journal.pone.0078817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu RR, Cheng AT, Lagenaur LA, Huang W, Weiss DE, Treece J, Sanders-Beer BE, Hamer DH, Lee PP, Xu Q, Liu Y. 2009. A Chinese rhesus macaque (Macaca mulatta) model for vaginal Lactobacillus colonization and live microbicide development. J Med Primatol 38:125–136. doi: 10.1111/j.1600-0684.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y, Yu R, Venzon D, Lee PP, Hamer DH. 2011. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol 4:648–657. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, Fan TY, Tan Y, Xiong Z, Wang Z. 2010. Seasonal changes in the reproductive physiology of female rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 49:289–293. [PMC free article] [PubMed] [Google Scholar]
- 18.Spear G, Rothaeulser K, Fritts L, Gillevet PM, Miller CJ. 2012. In captive rhesus macaques, cervicovaginal inflammation is common but not associated with the stable polymicrobial microbiome. PLoS One 7:e52992. doi: 10.1371/journal.pone.0052992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparman ML, Ramsey CM, Thomas CM, Mitalipov SM, Fanton JW, Maginnis GM, Stouffer RL, Wolf DP. 2007. Evaluation of the vervet (Clorocebus aethiops) as a model for the assisted reproductive technologies. Am J Primatol 69:917–929. doi: 10.1002/ajp.20413. [DOI] [PubMed] [Google Scholar]
- 20.Hadzic SV, Wang X, Dufour J, Doyle L, Marx PA, Lackner AA, Paulsen DB, Veazey RS. 2014. Comparison of the vaginal environment of Macaca mulatta and Macaca nemestrina throughout the menstrual cycle. Am J Reprod Immunol 71:322–329. doi: 10.1111/aji.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blakley GB, Beamer TW, Dukelow WR. 1981. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab Anim 15:351–353. doi: 10.1258/002367781780953059. [DOI] [PubMed] [Google Scholar]
- 22.Beaumier CM, Harris LD, Goldstein S, Klatt NR, Whitted S, McGinty J, Apetrei C, Pandrea I, Hirsch VM, Brenchley JM. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med 15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 24.Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, Rothwangl KB, Veazey RS, Hope TJ. 2014. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog 10:e1004440. doi: 10.1371/journal.ppat.1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nugeyre MT, Tchitchek N, Adapen C, Cannou C, Contreras V, Benjelloun F, Ravel J, Le Grand R, Marlin R, Menu E. 2019. Dynamics of vaginal and rectal microbiota over several menstrual cycles in female cynomolgus macaques. Front Cell Infect Microbiol 9:188. doi: 10.3389/fcimb.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, Mack M, Magaret A, Patton D, Agnew K, McElrath MJ, Hladik F, Eschenbach D. 2014. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis 210:651–655. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlow SD, Ephross SA. 1995. Epidemiology of menstruation and its relevance to women's health. Epidemiol Rev 17:265–286. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- 28.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung’u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. 2015. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagenaur LA, Hemmerling A, Chiu C, Miller S, Lee PP, Cohen CR, Parks TP. 2021. Connecting the dots: translating the vaginal microbiome into a drug. J Infect Dis 223:S296–S306. doi: 10.1093/infdis/jiaa676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman CD, Klutman NE, Lamp KC. 1997. Metronidazole. A therapeutic review and update. Drugs 54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 31.Coudray MS, Madhivanan P. 2020. Bacterial vaginosis—a brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol 245:143–148. doi: 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 33.Ñahui Palomino RA, Zicari S, Vanpouille C, Vitali B, Margolis L. 2017. Vaginal Lactobacillus inhibits HIV-1 replication in human tissues ex vivo. Front Microbiol 8:906. doi: 10.3389/fmicb.2017.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. 2012. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS Res Hum Retroviruses 28:76–81. doi: 10.1089/aid.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Veer C, Hertzberger RY, Bruisten SM, Tytgat HLP, Swanenburg J, de Kat Angelino-Bart A, Schuren F, Molenaar D, Reid G, de Vries H, Kort R. 2019. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: implications for in vivo dominance of the vaginal microbiota. Microbiome 7:49. doi: 10.1186/s40168-019-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis FMT, Bernstein KT, Aral SO. 2017. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 129:643–654. doi: 10.1097/AOG.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Department of Agriculture. 2020. USDA animal care. Animal Welfare Act and animal welfare regulations. Animal and Plant Health Inspection Service, USDA, Washington, DC. [Google Scholar]
- 38.The Fish and Wildlife Act of 1956. 16 U.S.C. 742a ‐ 742j. 2020.
- 39.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 40.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses 21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 41.Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N. 2004. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol 42:3128–3136. doi: 10.1128/JCM.42.7.3128-3136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klase Z, Ortiz A, Deleage C, Mudd JC, Quinones M, Schwartzman E, Klatt NR, Canary L, Estes JD, Brenchley JM. 2015. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol 8:1009–1020. doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortiz AM, Flynn JK, DiNapoli SR, Sortino O, Vujkovic-Cvijin I, Belkaid Y, Sereti I, Brenchley JM. 2019. Antiretroviral therapy administration in healthy rhesus macaques is associated with transient shifts in intestinal bacterial diversity and modest immunological perturbations. J Virol 93:e00472-19. doi: 10.1128/JVI.00472-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study generated amplicon sequence data that have been deposited to SRA under PRJNA734441.