ABSTRACT
Targeted gene insertion or replacement is a promising genome-editing tool for molecular breeding and gene engineering. Although CRISPR/Cas9 works well for gene disruption and deletion in Ganoderma lucidum, targeted gene insertion and replacement remain a serious challenge due to the low efficiency of homologous recombination (HR) in this species. In this work, we demonstrate that the DNA double-strand breaks induced by Cas9 were mainly repaired via the nonhomologous end joining (NHEJ) pathway, at a frequency of 96.7%. To establish an efficient target gene insertion and replacement tool in Ganoderma, we first inactivated the NHEJ pathway via disruption of the Ku70 gene (ku70) using a dual single guide RNA (sgRNA)-directed gene deletion method. Disruption of the ku70 gene significantly decreased NHEJ activity in G. lucidum. Moreover, ku70 disruption strains exhibited 96.3% and 93.1% frequencies of targeted gene insertion and replacement, respectively, when target DNA with the orotidine 5′-monophosphate decarboxylase (ura3) gene and 1.5-kb homologous 5′- and 3′-flanking sequences was used as a donor template, compared to 3.3% and 0%, respectively, at these targeted sites for a control strain (Cas9 strain). Our results indicated that ku70 disruption strains were efficient recipients for targeted gene insertion and replacement. This tool will advance our understanding of functional genomics in G. lucidum.
IMPORTANCE Functional genomic studies in Ganoderma have been hindered by the absence of adequate genome-engineering tools. Although CRISPR/Cas9 works well for gene disruption and deletion in G. lucidum, targeted gene insertion and replacement have remained a serious challenge due to the low efficiency of HR in these species, although such precise genome modifications, including site mutations, site-specific integrations, and allele or promoter replacements, would be incredibly valuable. In this work, we inactivated the NHEJ repair mechanism in G. lucidum by disrupting the ku70 gene using the CRISPR/Cas9 system. Moreover, we established a target gene insertion and replacement method in ku70-disrupted G. lucidum that possessed high-efficiency gene targeting. This technology will advance our understanding of the functional genomics of G. lucidum.
KEYWORDS: Ganoderma, higher fungi, medicinal mushroom, targeted gene insertion, gene replacement, CRISPR/Cas9
INTRODUCTION
Ganoderma lucidum, a well-known medicinal mushroom, has been used to improve health and to treat numerous diseases for over one millennium (1). The annual market size of Ganoderma products in Asia is more than $2.5 billion (2, 3). Due to its important pharmacological and economic values, it has received worldwide attention in recent years. The complete genome sequence of G. lucidum has recently been published (4, 5), providing a foundation for the further study of Ganoderma biology and the biosynthesis of secondary metabolites in this organism. Nevertheless, functional genomic studies in Ganoderma have been hindered by the absence of adequate genome-engineering tools.
The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system is an efficient tool for genome editing in mammals, plant, and microbes (6–10). The feasibility of using CRISPR/Cas9 to generate mutants through short insertions or deletions at targeted DNA double-strand breaks (DSBs) has also been illustrated in mushrooms such as Coprinopsis cinerea, Schizophuyllum commune, and Cordyceps militaris (11–13). Recently, gene disruption and gene deletion in G. lucidum using the CRISPR/Cas9 system have been reported (14–16). To date, however, targeted gene insertion and replacement have remained a serious challenge in Ganoderma, although such precise genome modifications, including site mutations, site-specific integrations, and allele or promoter replacements, would be incredibly valuable.
Targeted gene insertion and replacement can be achieved by homologous recombination (HR) using a donor template possessing homology arms. HR-mediated precision repairs in filamentous fungi, however, often occur at a lower frequency than nonhomologous end joining (NHEJ)-directed repairs, which involve an error-prone repair process (17, 18). Our previous work showed that DNA DSBs directed by CRISRP/Cas9 were predominantly repaired by NHEJ in G. lucidum (14). There is competition between NHEJ and HR when DNA DSBs are repaired in eukaryotes such as fungi (19). Therefore, inhibition of NHEJ would be expected to favorably increase the frequency of HR. The HR frequency was shown to be significantly improved by the inhibition of NHEJ in some fungal species such as S. commune, C. cinerea, Trichoderma reesei, and Pleurotus ostreatus (13, 20–23). However, blocking NHEJ has not yet been demonstrated in Ganoderma spp.
In this study, we inactivated the NHEJ repair mechanism in G. lucidum by disrupting one of the key genes, ku70, using the CRISPR/Cas9 system. Moreover, we established a target gene insertion and replacement method in ku70-disrupted G. lucidum with high-efficiency gene targeting. This technology will advance our understanding of the functional genomics of G. lucidum.
RESULTS AND DISCUSSION
CRISPR/Cas9-induced DSB repair in G. lucidum.
We initially tried to insert the hygR cassette into the orotidine 5′-monophosphate decarboxylase gene (ura3) by HR in wild-type G. lucidum. However, no expected mutants were obtained (data not shown), possibly due to the low frequency of HR in a wild-type strain. To quantitatively investigate the outcome of CRISPR/Cas9-induced DSB repair, we selected the G. lucidum ura3 gene as a target and transformed the plasmid pU6-ura3-sgRNA1 and the donor pMD19T-ura3-hr-d1 into G. lucidum pJW-EXP-intron-opCas9 (Cas9) protoplasts (1 × 107 cells). The strategy for targeted gene (ura3) insertion in G. lucidum is shown in Fig. 1A. After selection on complete yeast medium (CYM) plates with 5-fluoroorotic acid (5-FOA), we obtained a total of 60 5-FOA-resistant transformants from six independent experiments. Those transformants were confirmed by genomic PCR with the primers ura3-hru-500-F and ura3-hrd-500-R and Sanger sequencing. The results of the PCR analysis of some transformants are shown in Fig. 1B. Only transformant 3 showed a clear 6.9-kb band, but other transformants exhibited different band sizes. Sequence analysis of these PCR products confirmed that CRISPR/Cas9-induced DSBs were repaired by HR in transformant 3 and by NHEJ in other transformants. Some sequence results are summarized in Data Set S3 in the supplemental material. Of the 60 transformants selected, 2 exhibited DSB repair by HR and 58 showed DSB repair by NHEJ. We observed 3.3% and 96.7% frequencies of HR and NHEJ repair, respectively, in this study with this strain (Table 1). These results showed that CRISPR/Cas9-induced DSBs increased the frequency of HR in G. lucidum, which was similar to reports in plants (24, 25), and NHEJ is the dominant repair mechanism for DSBs in G. lucidum.
FIG 1.
(A) Strategy for targeted gene (ura3) insertion in the G. lucidum Cas9 strain. (B) PCR screening of transformants that underwent HR, using the primers ura3-hru-500-F and ura3-hrd-500-R. The hygromycin resistance (hygR) cassette fused to 1.5 kb of 5′ and 3′ DNA flanking the ura3 gene in the donor plasmid pMD19T-uras-hr-d1 and pU6-ura3-sgRNA1 were used for transformation. The arrow indicates the expected 6.8-kb band in transformant 3. WT, wild-type strain; M, size markers; N, negative control; 1-8, transformants 1 to 8.
TABLE 1.
Efficiency of NHEJ and HR repair of CRISPR/Cas9-induced DSBs in G. lucidum
| DSB repair | Mutation of ura3 | No. of mutants | Efficiency (%) |
|---|---|---|---|
| NHEJ | Insertion or deletion | 58 | 96.7% |
| HR | Target insertion | 2 | 3.3% |
Inactivation of the NHEJ pathway in G. lucidum.
To inactivate the NHEJ pathway in G. lucidum, we disrupted the G. lucidum ku70 homolog. The G. lucidum 5.260125-1 genome reference (4) was analyzed to find a homolog of the Coprinopsis cinerea Ku70 protein using BLASTP. The gl24233 gene was identified as a candidate ku70 homolog in G. lucidum 5.260125-1. Then, we cloned this putative ku70 (see Data Set S1) from the G. lucidum Cas9 strain by PCR using the primers ku70-F-F and ku70-F-R (Table 2) based on the sequence of the gl24233 gene. The gene encodes a protein of 786 amino acids, which showed 60.38, 61.47, and 59.43% identity to the Ku70 proteins from Coprinopsis cinerea (GenBank accession number KAG2017394.1), Tricholoma matsutake 945 (GenBank accession number KAF8224427.1), and Armillaria solidipes (GenBank accession number PBK67889.1), respectively.
TABLE 2.
Oligonucleotides used in this study
| Target and primer | Sequence (5′ to 3′) |
|---|---|
| ku70 | |
| ku70-F-F | ATGGCACCCTATGATGACTGGA |
| ku70-F-R | TCAGGCGTGGTTGTCCAGC |
| ophph cassette | |
| gpd-F | TCCAAAGCCGCTCTCATGG |
| ter-R | TGCTCTATGTCTTGCCTTGTCTCG |
| 5′-flanking region of ura3 sgRNA1 site | |
| ura3-hru-1-F | CGAATGGCGGTTCTGGTCC |
| ura3-hru-1-R | CCAAGGGGCAGACCGACAG |
| 3′-flanking region of ura3 sgRNA1 site | |
| ura3-hrd-1-F | CAGGGGGCTTCTGCTCCTC |
| ura3-hrd-1-R | GCGGTGAAGGTATGTCCCTAGGA |
| 3′-flanking region of ura3 sgRNA2 site | |
| ura3-hrd-2-F | CATACACGGAAGAGGCCGTCC |
| ura3-hrd-2-R | GCGGTGAAGGTATGTCCCTAGGA |
| ku70 fragment outside ku70-sgRNA1 target | |
| ku70-1-F | AGAAAGCCACTTTTCGGCAGTGAG |
| ku70-1-R | TCCTCAGTCTCCGCCTCCCG |
| ku70 fragment outside ku70-sgRNA2 target | |
| ku70-2-F | GCATTCAGTTGACCGCATGGTACA |
| ku70-2-R | CGGAAAGGAACGACCACCTCG |
| ura3 | |
| ura3-F | ATGGTGGCCGTGGCCAA |
| ura3-R | CTAATCCGAGATCCCAACCCTTT |
| ura3 and flanking regions | |
| ura3-hru-500-F | AGGGGTGTCCTTGGAAGAAAC |
| ura3-hrd-500-R | GGCACTTATACAACCTCATTCAGAC |
| ura3 and two homologous arms | |
| ura3-HR-F | CGAATGGCGGTTCTGGTCC |
| ura3-HR-R | GCGGTGAAGGTATGTCCCTAGGA |
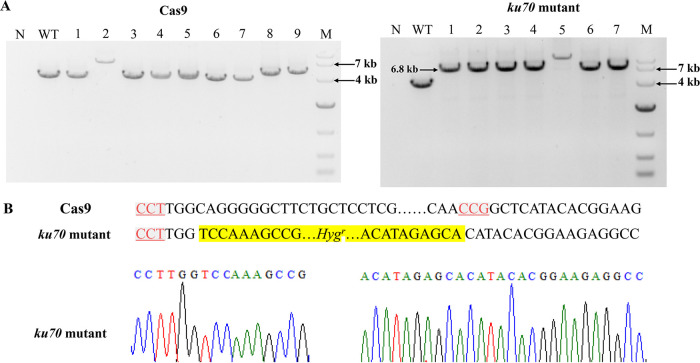
To disrupt the ku70 gene in G. lucidum Cas9, in vitro-transcribed ku70-sgRNA1, ku70-sgRNA2, and plasmid pMD19T-ophph were cotransformed into G. lucidum Cas9 strain protoplasts using the dual single guide RNA (sgRNA)-directed gene deletion method (14). Hygromycin-resistant colonies were isolated and analyzed by PCR using the primers ku70-F-F and ku70-1-R, ku70-2-F and ku70-F-R, and ku70-F-F and ku70-F-R (Table 2). Sequence analysis of the PCR products obtained using the primers ku70-F-F and ku70-F-R showed that the region (about 1.5 kb) between the ku70-sgRNA1 and ku70-sgRNA2 target sites was inverted in transformant 5 (Fig. 2). After five rounds of culturing transformant 5 in nonselective CYM medium, PCR amplification and sequence analysis were performed to confirm the stability of the ku70 disruption. The amplified sequences of ku70 in this G. lucidum ku70 mutant are shown in Data Set S3. No significant morphological difference between the G. lucidum Cas9 strain and the ku70 mutant was observed, although the growth rate of the ku70 mutant was slightly lower than that of the G. lucidum Cas9 strain (see Fig. S1 in the supplemental material). Similar results were also reported for S. commune, Penicillium chrysogenum, and Botrytis cinerea, in which the disruption of ku70 did not significantly affect mycelial growth or morphology (21, 26, 27). The ku70 mutant obtained was chosen for further analysis.
FIG 2.
(A) Strategy for ku70 disruption in the G. lucidum Cas9 strain. In vitro-transcribed sgRNA1 and sgRNA2 targeting ku70 and the plasmid pMD19T-ophph were delivered to G. lucidum Cas9 protoplasts. (B) Sequence analysis of the ku70 fragment in the ku70 mutant.
To evaluate the effect of the ku70 disruption on NHEJ in G. lucidum, the ura3 gene (see Data Set S1) was disrupted in the G. lucidum Cas9 strain and the ku70 mutant. The plasmid pU6-ura3-sgRNA1 in the absence of a repair template was introduced into protoplasts from the G. lucidum Cas9 strain and the ku70 mutant by the polyethylene glycol-mediated transformation (PMT) method mentioned previously (33). Transformation of G. lucidum Cas9 produced 13, 19, and 17 5-FOA-resistant colonies on a selective CYM plate in three independent experiments. Only one 5-FOA-resistant colony, however, was obtained when the plasmid pU6-ura3 sgRNA1 was transformed into the ku70 mutant under the same conditions (see Table S1). The ura3 gene was amplified from the genome of these transformants and sequenced to determine whether DSBs were repaired by NHEJ. Our results showed that the repair sites of the G. lucidum Cas9 transformants obtained were located 3 bp upstream of the protospacer-adjacent motif (PAM) sequence for the ura3 sgRNA1, suggesting that these DSBs were repaired by NHEJ. Nevertheless, a 1-bp insertion was found 222 bp downstream of the ura3 sgRNA1 target site in the ku70 mutant transformant, which might have been produced by spontaneous mutation. The low frequency of transformation in the Δku70 strain has also been observed in other fungi such as Aspergillus oryzae, S. commune, and Stagonospora nodorum (21, 28, 29). Our results illustrated that NHEJ repair was inactivated in G. lucidum by disruption of the Ku70 function, an essential component involved in NHEJ repair.
HR-mediated gene insertion in ku70-deficient G. lucidum.
To evaluate the effects of the inactivation of NHEJ on HR, we transformed the plasmid pU6-ura3-sgRNA1 and the repair template pMD19T-ura3-hr-d1 into the G. lucidum Cas9 strain and the ku70 mutant, respectively. The 5-FOA-resistant transformants obtained were examined by PCR with the primers ura3-hru-500-F and ura3-hrd-500-R (Table 2) and by sequence analysis. Figure 3 shows that all tested ku70 mutant transformants exhibited a 6.8-kb band, which indicated that this DSB had been repaired by HR-mediated gene insertion. Sequence analysis confirmed target gene insertion in these transformants (see Data Set S3). Table 3 shows that the efficiency of HR-mediated gene insertion was roughly 96.3% (26/27 transformants) in the ku70 mutant background, which was 29.2 time higher than that obtained (2/60 transformants) in the G. lucidum Cas9 background.
FIG 3.
PCR screening of transformants that underwent targeted gene (ura3) insertion in the ku70 mutant, using the primers ura3-hru-500-F and ura3-hrd-500-R. WT, wild-type strain; M, size markers; N, negative control; 1-10, transformants 1 to 10.
TABLE 3.
Efficiency of target gene insertion and replacement in the G. lucidum Cas9 strain and the ku70 mutanta
| Strain | Mutation of ura3 | Expt. 1 | Expt. 2 | Expt. 3 | Efficiency (%) |
|---|---|---|---|---|---|
| Cas9 | Insertion | 1/20 | 1/23 | 0/17 | 3.3 |
| ku70 mutant | Insertion | 10/10 | 8/8 | 8/9 | 96.3 |
| Cas9 | Replacement | 0/13 | 0/10 | 0/11 | 0 |
| ku70 mutant | Replacement | 7/8 | 9/9 | 11/12 | 93.1 |
Targeted gene insertions were screened for in 2 of 60 5-FOA-resistant G. lucidum Cas9 transformants produced and in 26 of 27 5-FOA-resistant ku70 mutant transformants produced, in three independent experiments. Targeted gene replacement was identified in 0 of 34 5-FOA-resistant G. lucidum Cas9 transformants produced and in 27 of 29 5-FOA-resistant ku70 mutant transformants produced, in three independent experiments.
Efficient gene replacement by HR in the ku70 mutant.
To investigate whether disruption of ku70 could lead to efficient gene replacement in G. lucidum, the plasmids pU6-ura3-sgRNA1 and pU6-ura3-sgRNA2, which targeted two different recognition sites of ura3, and the donor template pMD19T-ura3-hr-d2 were cotransformed into the G. lucidum Cas9 strain and the ku70 mutant. The strategy for targeted gene (ura3) replacement for G. lucidum is shown in Fig. 4. Following 5-FOA selection, the transformants obtained were subjected to PCR using the primers ura3-hru-500-F and ura3-hrd-500-R (Table 2), and the resulting PCR products were analyzed by Sanger sequencing. Some of the ku70 mutant transformants (Fig. 5A, lanes 1, 2, 3, 4, 6, and 7) showed 6.8-kb amplicons by PCR, which indicated that the region between the target sites of ura3-sgRNA1 and ura3-sgRNA2 was replaced with the hygR cassette (Fig. 5A). Sequencing results confirmed gene replacement by HR in these transformants (Fig. 5B; also see Data Set S3). However, no corresponding PCR products (6.8 kb) were found in Cas9 transformants (Fig. 5A). Genotyping of the transformants obtained by PCR and sequence analysis showed that 27 of 29 ku70 mutant transformants had the hygR cassette flanked by homologous regions at the expected locus in three independent experiments, while no corresponding transformants of the 34 Cas9 transformants analyzed were found (Table 3). Some sequence results are summarized in Data Set S3. These results showed that disruption of ku70 efficiently increased the efficiency of target gene replacement in G. lucidum. Previous reports showed that suppression or disruption of ku70 leads to increased HR efficiency in fungi (18, 22, 23). The disruption of ku70 in Ganoderma reduced the efficiency of NHEJ, leading to HR, and increased the efficiency of target gene insertion and replacement by HR (30, 31). Gene targeting is important for determining the functions of essential genes and for adding reporters and new markers in studies of gene expression. The ku70 mutant obtained greatly enhanced gene targeting by HR. Therefore, use of the ku70 mutant will greatly facilitate site-specific integration and site-specific substitution and gene replacement in G. lucidum. To eliminate the effect of the introduced plasmid pMD19T-ophph on gene functional analysis, we also constructed a ku70-deficient strain without the plasmid pMD19T-ophph in the genome. In vitro-transcribed ku70-sgRNA1, ku70-sgRNA2, and ura3-sgRNA1 were cotransformed into the G. lucidum Cas9 strain (Fig. 6). Then, 5-FOA-resistant transformants were isolated and analyzed by PCR using the primers ku70-1-F and ku70-2-R, ku70-1-F and ku70-1-R, and ku70-2-F and ku70-2-R (Fig. 7). Sequence analysis of ura3 and ku70 showed that the region between the ku70-sgRNA1 and ku70-sgRNA2 target sites was inverted in transformant 1 (Fig. 8). Moreover, an 11-bp deletion of ura3 was detected in transformant 1 (ura3-ku70 mutant). When the repair template pMD19T-ura3-HR (Fig. 9) was transformed into the ura3-ku70 mutant, we obtained the transformant ku70-mutant. The ku70-mutant did not grow on a minimal medium (MM) plate containing 400 mg/liter 5-FOA, whereas it did grow on an MM plate without uridine (Fig. 9). Sequence analysis confirmed that the ku70-mutant was complemented with ura3 (Fig. 10). Our work will contribute to the progress of the research on this medicinal mushroom.
FIG 4.
Strategy for targeted gene (ura3) replacement in G. lucidum. The hygR cassette fused to 1.5 kb of 5′ and 3′ DNA flanking ura3 (donor pMD19T-ura3-hr-d2), pU6-ura3-sgRNA1, and pU6-ura3-sgRNA2 were used for transformation.
FIG 5.
(A) PCR screening for transformants that underwent targeted gene (ura3) replacement in the G. lucidum Cas9 strain and the ku70 mutant. WT, wild-type strain; M, size markers; N, negative control; 1-9, transformants 1 to 9. (B) Sequence analysis of the targeted gene (ura3) replacement in the ku70 mutant. The primers ura3-hru-500-F and ura3-hrd-500-R were used for PCR amplification.
FIG 6.
Strategy for ku70 and ura3 disruption in the G. lucidum Cas9 strain.
FIG 7.
(A) Selection of 5-FOA-resistant mutants after ku70-sgRNA1, ku70-sgRNA2, and ura3-sgRNA1 were cotransformed into the G. lucidum Cas9 strain. (B to D) Determination of the ura3-ku70 mutant by PCR using primers ku70-1-F and ku70-2-R (B), ku70-1-F and ku70-1-R (C), and ku70-2-F and ku70-2-R (D). WT, wild-type strain; M, size markers; 1, 2, and 3, transformants 1, 2, and 3, respectively.
FIG 8.
Sequence analysis of the ura3 (A) and ku70 (B) genes in the wild-type (WT) strain and the ura3-ku70 mutant.
FIG 9.
(A) Donor plasmid pMD19T-ura3-HR used for transformation of the ura3-ku70 mutant. (B) Subculture of the ku70 mutant (1) obtained, the wild-type (WT) strain, and the ura3-deficient strain (ΔURA3) on an MM plate containing 400 mg/liter 5-FOA. (C) Subculture of the ku70 mutant obtained, the wild-type strain, and the ura3-deficient strain on an MM plate without uridine.
FIG 10.
Sequence analysis of the ura3 gene in the wild-type (WT) strain and the ku70 mutant obtained.
Conclusion.
In summary, we showed that the blocking of NHEJ was an efficient way to enhance the efficiency of HR repair of CRISPR/Cas9-induced DSBs in G. lucidum. In addition, the constructed ku70 mutant was suitable for target gene insertion and replacement in G. lucidum. This developed method should be helpful in advancing precise genome editing of Ganoderma for molecular breeding and biotechnological applications.
MATERIALS AND METHODS
Strains, growth conditions, and genomic DNA extraction.
The reference G. lucidum pJW-EXP-intron-opCas9 (Cas9) strain used in this study was grown and maintained on potato dextrose agar slants. In the G. lucidum Cas9 strain, Cas9 was efficiently expressed by codon optimization and addition of the gpd intron 1 upstream of the opCas9 gene (14). Escherichia coli DH5α (Promega, Madison, WI, USA) was used for cloning procedures according to the manufacturer’s instructions. G. lucidum transformants were then grown on CYM regeneration plates (0.6 M mannitol, 20 g/liter glucose, 10 g/liter maltose, 2 g/liter yeast extract, 2 g/liter tryptone, 4.6 g/liter KH2PO4, 0.5 g/liter MgSO4, and 10 g/liter agar). Genomic DNA was extracted from G. lucidum mycelia using the cetyltrimethylammonium bromide (CTAB) method described previously (32).
Cloning of G. lucidum ku70 and in vitro transcription of two ku70 sgRNA cassettes.
The ku70 gene of G. lucidum was first amplified by genomic PCR using the primers ku70-F-F and ku70-F-R (Table 2). The PCR products were cloned into the pMD19-T vector and confirmed by Sanger sequencing. sgRNAs against these target genes were designed using the web tool CRISPOR (http://crispor.tefor.net). The T7 promoter and two ku70 sgRNA cassettes, T7-ku70-sgRNA1 and T7-ku70-sgRNA2 (see Data Set S2 in the supplemental material), were synthesized by Sangon Biotech Corp. Ltd., (Shanghai, China). These ku70 sgRNAs were transcribed in vitro with a HiScribe T7 high-yield RNA synthesis kit (NEB, Beijing, China) and purified with an RNA Clean & Concentrator-25 kit (Zymo Research, Beijing, China) according to the manufacturer’s protocols.
Plasmid construction.
The sequence of an intron-codon-optimized hygromycin resistance gene (ophph) flanked by NheI and SmaI sites (see Data Set S2) was synthesized by Sangon Biotech Corp. This NheI-intron-ophph-SmaI fragment was ligated into the NheI and SmaI sites of the plasmid pJW-EXP (33, 34) to produce the pJW-EXP-ophph plasmid. The hygR cassette, including the glyceraldehyde-3-phosphate dehydrogenase (gpd) gene promoter of G. lucidum, a codon-optimized hph gene, and the iron-sulfur protein subunit of the succinate dehydrogenase (sdhB) gene terminator, was amplified from the pJW-EXP-ophph plasmid using the primers gpd-F and ter-R (Table 2). The pMD19T-ophph plasmid was made by ligating the hygR cassette into the pMD19-T plasmid (TaKaRa, Dalian, China). The sequences of pU6-ura3 sgRNA1 and pU6-ura3 sgRNA2 (see Data Set S2) were synthesized and ligated into the plasmid pUC57 (http://www.addgene.org/vector-database/4509) by Sangon Biotech Corp. to produce sgRNA expression plasmids pU6-ura3-sgRNA1 and pU6-ura3-sgRNA2, respectively.
The plasmids pMD19T-ura3-hr-d1 and pMD19T–ura3-hr-d2, which provide donor repair templates for DSBs, were constructed as follows. The 5′- and 3′-flanking sequences (1.5 kb) of the ura3 sgRNA1 target site were amplified from genomic DNA using the primers ura3-hru-1-F and ura3-hru-1-R and ura3-hrd-1-F and ura3-hrd-1-R, respectively (Table 2). The ophph cassette was amplified from pJW-EXP-ophph using the primers gpd-F and ter-R (Table 2). The resulting 5′-flanking sequence of the ura3 sgRNA1 target site, the ophph cassette, and the 3′-flanking sequence of the ura3 sgRNA1 target site were fused into the pMD19T plasmid using the ClonExpress MultiS one-step cloning kit (Vazyme, Nanjing, China) to produce the plasmid pMD19T-ura3-hr-d1. The plasmid pMD19T-ura3-hr-d2 was similar to pMD19T-ura3-hr-d1 except that the 3′-flanking sequence of the ura3 sgRNA1 target site was replaced with the 3′-flanking sequence of the ura3 sgRNA2 target site, which was amplified from genomic DNA using primers ura3-hrd-2-F and ura3-hrd-2-R (Table 2).
Genetic transformation and screening of transformants.
A plasmid or sgRNA was introduced into G. lucidum protoplasts (1 × 107) using the PMT method described previously (35, 36). For cotransformation experiments, 10 μg of plasmids and in vitro-transcribed sgRNA was used. ku70 mutants were selected on CYM plates containing 250 mg/liter hygromycin B. Transformants were selected for gene insertion or gene replacement on CYM plates containing 400 mg/liter 5-FOA and 100 mg/liter uridine, respectively (Sangon Corp.).
Identification of mutant strains.
For identification of the ku70 disruption transformants, PCR amplification from transformant genomic DNA was performed with the primers ku70-F-F and ku70-1-R (located outside the ku70-sgRNA1 target), ku70-2-F and ku70-F-R (located outside the ku70-sgRNA2 target site), and ku70-F-F and ku70-F-R (Table 2). The PCR products obtained by using primers ku70-F-F and ku70-F-R were sequenced directly by Sangon Corp.
To identify NHEJ or HR mutations, transformant genomic DNAs were extracted and amplified for ura3 using the primers ura3-F and ura3-R or ura3-hdu-500-F and ura3-hdd-500-R (Table 2), which were located about 500 bp outside the homology arms. The amplified products were sequenced by the Sanger method to confirm the mutations.
Data availability.
G. lucidum ku70 sequence data may be found in the supplemental material.
ACKNOWLEDGMENTS
The National Natural Science Foundation of China (grant 81860668) and the Yunnan Applied Basic Research Project (grant 2018FB065) supported this study. J.-W.X. also thanks the Yunnan Ten Thousand Talents Plan-Young and Elite Talents Project.
Footnotes
Supplemental material is available online only.
Contributor Information
Jun-Wei Xu, Email: xjuwei@163.com.
Haruyuki Atomi, Kyoto University.
REFERENCES
- 1.Hsu KD, Cheng KC. 2018. From nutraceutical to clinical trial: frontiers in Ganoderma development. Appl Microbiol Biotechnol 102:9037–9051. 10.1007/s00253-018-9326-5. [DOI] [PubMed] [Google Scholar]
- 2.Bishop KS, Kao CHJ, Xu Y, Glucina MP, Paterson RRM, Ferguson LR. 2015. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 114:56–65. 10.1016/j.phytochem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 3.de Mattos-Shipley KMJ, Ford KL, Alberti F, Banks AM, Bailey AM, Foster GD. 2016. The good, the bad and the tasty: the many roles of mushrooms. Stud Mycol 85:125–157. 10.1016/j.simyco.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, Li C, Wang L, Guo X, Sun YZ, Luo HM, Li Y, Song JY, Henrissat B, Levasseur A, Qian J, Li JQ, Luo X, Shi LC, He L, Xiang L, Xu XL, Niu YY, Li QS, Han MV, Yan HX, Zhang J, Chen HM, Lv AP, Wang Z, Liu MZ, Schwartz DC, Sun C. 2012. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat Commun 3:913. 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kües U, Nelson DR, Liu C, Yu G-J, Zhang J, Li J, Wang X-C, Sun H. 2015. Genome analysis of medicinal Ganoderma spp. with plant-pathogenic and saprotrophic life-styles. Phytochemistry 114:18–37. 10.1016/j.phytochem.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Arazoe T, Miyoshi K, Yamato T, Ogawa T, Ohsato S, Arie T, Kuwata S. 2015. Tailor-made CRISPR/Cas system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol Bioeng 112:2543–2549. 10.1002/bit.25662. [DOI] [PubMed] [Google Scholar]
- 7.Boontawon T, Nakazawa T, Inoue C, Osakabe K, Kawauchi M, Sakamoto M, Honda Y. 2021. Efficient genome editing with CRISPR/Cas9 in Pleurotus ostreatus. AMB Express 11:30. 10.1186/s13568-021-01193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortesi L, Zhu C, Zischewski J, Perez L, Bassie L, Nadi R, Forni G, Lade SB, Soto E, Jin X, Medina V, Villorbina G, Muñoz P, Farré G, Fischer R, Twyman RM, Capell T, Christou P, Schillberg S. 2016. Patterns of CRISPR/Cas9 activity in plants, animals and microbes. Plant Biotechnol J 14:2203–2216. 10.1111/pbi.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz JC, Cao M, Zhao H. 2019. Development of a CRISPR/Cas9 system for high efficiency multiplexed gene deletion in Rhodosporidium toruloides. Biotechnol Bioeng 116:2103–2109. 10.1002/bit.27001. [DOI] [PubMed] [Google Scholar]
- 10.Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kühn R. 2015. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol 33:543–548. 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- 11.Chen BX, Wei T, Ye ZW, Yun F, Kang LZ, Tang HB, Guo LQ, Lin JF. 2018. Efficient CRISPR-Cas9 gene disruption system in edible-medicinal mushroom Cordyceps militaris. Front Microbiol 9:1157. 10.3389/fmicb.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugano SS, Suzuki H, Shimokita E, Chiba H, Noji S, Osakabe Y, Osakabe K. 2017. Genome editing in the mushroom-forming basidiomycete Coprinopsis cinerea, optimized by a high-throughput transformation system. Sci Rep 7:1260. 10.1038/s41598-017-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonk PJ, Escobar N, Wosten HAB, Lugones LG, Ohm RA. 2019. High-throughput targeted gene deletion in the model mushroom Schizophyllum commune using pre-assembled Cas9 ribonucleoproteins. Sci Rep 9:7632. 10.1038/s41598-019-44133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Sun B, You H, Tu JL, Yu X, Zhao P, Xu JW. 2020. Dual sgRNA-directed gene deletion in basidiomycete Ganoderma lucidum using the CRISPR/Cas9 system. Microb Biotechnol 13:386–396. 10.1111/1751-7915.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin H, Xiao H, Zou G, Zhou Z, Zhong JJ. 2017. CRISPR-Cas9 assisted gene disruption in the higher fungus Ganoderma species. Process Biochem 56:57–61. 10.1016/j.procbio.2017.02.012. [DOI] [Google Scholar]
- 16.Wang PA, Xiao H, Zhong JJ. 2020. CRISPR-Cas9 assisted functional gene editing in the mushroom Ganoderma lucidum. Appl Microbiol Biotechnol 104:1661–1671. 10.1007/s00253-019-10298-z. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y, Wang KF, Wang WJ, Ma YR, Shi TQ, Huang H, Ji XJ. 2019. Increasing the homologous recombination efficiency of eukaryotic microorganisms for enhanced genome engineering. Appl Microbiol Biotechnol 103:4313–4324. 10.1007/s00253-019-09802-2. [DOI] [PubMed] [Google Scholar]
- 18.Kück U, Hoff B. 2010. New tools for the genetic manipulation of filamentous fungi. Appl Microbiol Biotechnol 86:51–62. 10.1007/s00253-009-2416-7. [DOI] [PubMed] [Google Scholar]
- 19.Morio F, Lombardi L, Butler G. 2020. The CRISPR toolbox in medical mycology: state of the art and perspectives. PLoS Pathog 16:e1008201. 10.1371/journal.ppat.1008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chum PY, Schmidt G, Saloheimo M, Landowski CP. 2017. Transient silencing of DNA repair genes improves targeted gene integration in the filamentous fungus Trichoderma reesei. Appl Environ Microbiol 83:e00535-17. 10.1128/AEM.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong JF, Ohm RA, de Bekker C, Wosten HAB, Lugones LG. 2010. Inactivation of ku80 in the mushroom-forming fungus Schizophyllum commune increases the relative incidence of homologous recombination. FEMS Microbiol Lett 310:91–95. 10.1111/j.1574-6968.2010.02052.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa T, Ando Y, Kitaaki K, Nakahori K, Kamada T. 2011. Efficient gene targeting in ΔCc.ku70 or ΔCc.lig4 mutants of the agaricomycete Coprinopsis cinerea. Fungal Genet Biol 48:939–946. 10.1016/j.fgb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Salame TM, Knop D, Tal D, Levinson D, Yarden O, Hadar Y. 2012. Predominance of a versatile-peroxidase-encoding gene, mnp4, as demonstrated by gene replacement via a gene targeting system for Pleurotus ostreatus. Appl Environ Microbiol 78:5341–5352. 10.1128/AEM.01234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang TK, Puchta H. 2019. CRISPR/Cas-mediated gene targeting in plants: finally a turn for the better for homologous recombination. Plant Cell Rep 38:443–453. 10.1007/s00299-019-02379-0. [DOI] [PubMed] [Google Scholar]
- 25.Svitashev S, Young JK, Schwartz C, Gao H, Falco SC, Cigan AM. 2015. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol 169:931–945. 10.1104/pp.15.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choquer M, Robin G, Le Pecheur P, Giraud C, Levis C, Viaud M. 2008. Ku70 or Ku80 deficiencies in the fungus Botrytis cinerea facilitate targeting of genes that are hard to knock out in a wild-type context. FEMS Microbiol Lett 289:225–232. 10.1111/j.1574-6968.2008.01388.x. [DOI] [PubMed] [Google Scholar]
- 27.de Boer P, Bastiaans J, Touw H, Kerkman R, Bronkhof J, van den Berg M, Offringa R. 2010. Highly efficient gene targeting in Penicillium chrysogenum using the bi-partite approach in Δlig4 or Δku70 mutants. Fungal Genet Biol 47:839–846. 10.1016/j.fgb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Feng J, Li W, Hwang SF, Gossen BD, Strelkov SE. 2012. Enhanced gene replacement frequency in KU70 disruption strain of Stagonospora nodorum. Microbiol Res 167:173–178. 10.1016/j.micres.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Masuda T, Koyama Y. 2006. Enhanced gene targeting frequency in ku70 and ku80 disruption mutants of Aspergillus sojae and Aspergillus oryzae. Mol Genet Genomics 275:460–470. 10.1007/s00438-006-0104-1. [DOI] [PubMed] [Google Scholar]
- 30.Bugeja HE, Boyce KJ, Weerasinghe H, Beard S, Jeziorowski A, Pasricha S, Payne M, Schreider L, Andrianopoulos A. 2012. Tools for high efficiency genetic manipulation of the human pathogen Penicillium marneffei. Fungal Genet Biol 49:772–778. 10.1016/j.fgb.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Rehman S, Tang X, Gu K, Fan Q, Chen D, Ma W. 2018. Methodologies for improving HDR efficiency. Front Genet 9:691. 10.3389/fgene.2018.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu JW, Xu N, Zhong JJ. 2012. Enhancement of ganoderic acid accumulation by overexpression of an N-terminally truncated 3-hydroxy-3-methylglutaryl coenzyme A reductase gene in the basidiomycete Ganoderma lucidum. Appl Environ Microbiol 78:7968–7976. 10.1128/AEM.01263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Ji SL, He YL, Ren MF, Xu JW. 2014. Development of an expression plasmid and its use in genetic manipulation of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher basidiomycetes). Int J Med Mushrooms 16:161–168. 10.1615/intjmedmushr.v16.i2.60. [DOI] [PubMed] [Google Scholar]
- 34.Zhang DH, Jiang LX, Li N, Yu X, Zhao P, Li T, Xu JW. 2017. Overexpression of the squalene epoxidase gene alone and in combination with the 3-hydroxy-3-methylglutaryl coenzyme A gene increases ganoderic acid production in Ganoderma lingzhi. J Agric Food Chem 65:4683–4690. 10.1021/acs.jafc.7b00629. [DOI] [PubMed] [Google Scholar]
- 35.Fei Y, Li N, Zhang DH, Xu JW. 2019. Increased production of ganoderic acids by overexpression of homologous farnesyl diphosphate synthase and kinetic modeling of ganoderic acid production in Ganoderma lucidum. Microb Cell Fact 18:115. 10.1186/s12934-019-1164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu JW, Zhong JJ. 2015. Genetic engineering of Ganoderma lucidum for the efficient production of ganoderic acids. Bioengineered 6:357–360. 10.1080/21655979.2015.1119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 to S3, Table S1, Fig. S1. Download aem.01510-21-s0001.pdf, PDF file, 0.2 MB (240KB, pdf)
Data Availability Statement
G. lucidum ku70 sequence data may be found in the supplemental material.