ABSTRACT
Composite microecological agents have received widespread attention due to their advantageous properties, including safety, multiple effects, and low cost. This study was conducted to evaluate the protective effects of selenium (Se) nanoparticle (SeNP)-enriched Lactococcus lactis NZ9000 (L. lactis NZ9000-SeNPs) against enterotoxigenic Escherichia coli (ETEC) K88-induced intestinal barrier damage in C57BL/6 mice. The oral administration of L. lactis NZ9000-SeNPs significantly increased the villus height and the number of goblet cells in the ileum; reduced the levels of serum and ileal interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ); and increased the activities of thioredoxin reductase (TrxR) and glutathione peroxidase (GSH-Px) compared with the ETEC K88-infected group not treated with L. lactis NZ9000-SeNPs. In addition, L. lactis NZ9000-SeNPs significantly attenuated the reduction of the expression levels of occludin and claudin-1, dysbiosis of the gut microbiome, and activation of the Toll-like receptor (TLR)/nuclear factor kappa B (NF-κB)-mediated signaling pathway induced by ETEC K88. These findings suggested that L. lactis NZ9000-SeNPs may be a promising and safe Se supplement for food or feed additives.
IMPORTANCE The beneficial effects of microecological agents have been widely proven. Se, which is a nutritionally essential trace element for humans and animals, is incorporated into selenoproteins that have a wide range of pleiotropic effects, ranging from antioxidant to anti-inflammatory effects. However, sodium selenite, a common addition form of Se in feed and food, has disadvantages such as strong toxicity and low bioavailability. We investigated the protective effects of L. lactis NZ9000-SeNPs against ETEC K88-induced intestinal barrier injury in C57BL/6 mice. Our results show that L. lactis NZ9000-SeNPs effectively alleviate ETEC K88-induced intestinal barrier dysfunction. This study highlights the importance of developing a promising and safe Se supplement for the substitution of sodium selenite applied in food, feed, and biomedicine.
KEYWORDS: probiotic, Lactococcus lactis NZ9000, intestinal barrier, selenium nanoparticles
INTRODUCTION
The intestine, as the largest immune and digestive organ, plays a crucial role in maintaining human and animal health (1). The intestinal barrier is the functional interface separating the luminal contents of the intestinal cavity and the intestinal mucosa harboring the intestine-associated immune system, which allows the absorption of nutrients and fluids but concurrently prevents harmful substances like toxins and bacteria from invading the organism and maintains intestinal homeostasis (2). Each part of the intestinal mucosal barrier has a corresponding structural basis (3). The integrity of the intestinal mucosal barrier is maintained by tight junction (TJ) proteins (4). TJ proteins are multiprotein complexes located around the top of the outer membrane of intestinal epithelial cells, composed mainly of occludin and claudin-1 (4, 5). Intestinal barrier dysfunction results in the production of diarrhea in weaned piglets and necrotizing enteritis in poultry and causes huge economic losses to the livestock and poultry industries. Therefore, it is urgently necessary to develop safe and efficient microecological agents to protect the integrity of the intestinal barrier.
Selenium (Se) is an essential trace element that is closely associated with multiple physiological functions, such as antioxidant activity, immunity enhancement, and the promotion of body metabolism (6). Se deficiency has become a problem in animal husbandry worldwide, which causes not only white muscle disease (WMD) but also abortion in ewes and a decline in fertility and pregnancy rates (7). The chemical species of Se mainly include inorganic Se (selenite, selenate, elemental Se, and nano-selenium) and organic selenium (selenocysteine [Sec], selenomethionine [SeMet], and selenoprotein, etc.). To date, studies have confirmed that the order of toxicity of Se species is as follows: selenite > selenate > SeMet > selenium nanoparticles (SeNPs) (8). Se is involved in the regulation of immune responses (9). Crude Ulva lactuca polysaccharide (ULP)-modified SeNPs inhibited macrophage activation by inhibiting the nuclear translocation of nuclear factor kappa B (NF-κB), thereby alleviating inflammation in a mouse model of colitis (10). In addition, it has also been shown that the regulatory effect of Se on Staphylococcus aureus-induced mastitis is mediated by inhibiting the NF-κB signaling pathway (11). Our previous studies showed that Lactococcus lactis NZ9000 has a high capacity to convert toxic sodium selenite into SeNPs with red color when cocultured with 0.6 mM Na2SeO3 for 48 h at 30°C (12). Additionally, L. lactis can induce mucosal immunity and has adjuvant properties (13). The oral administration of L. lactis NZ9000 effectively improved intestinal barrier function in rats (14). However, the protective effects of SeNP-enriched microorganisms on the function of the intestinal barrier remain to be elucidated.
In this study, we hypothesized that SeNP-enriched L. lactis NZ9000 (L. lactis NZ9000-SeNPs) could play a dual role as probiotics and nano-Se and produce a synergistic effect. This study aimed to evaluate the protective effects of SeNP-enriched L. lactis NZ9000 against intestinal barrier injury and its association with the Toll-like receptor (TLR)/NF-κB-mediated signaling pathway through the establishment of an enterotoxigenic Escherichia coli (ETEC) K88-induced intestinal barrier dysfunction model in C57BL/6 mice.
RESULTS
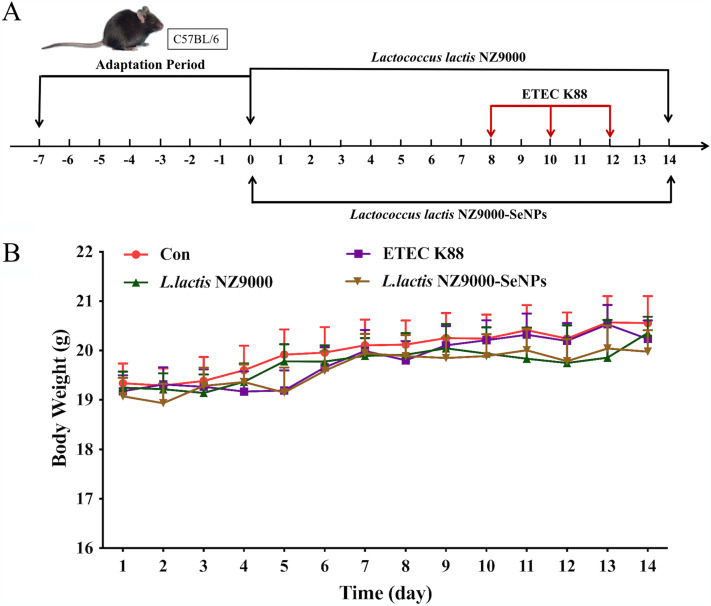
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the body weight of mice.
According to the overall trend observed in Fig. 1B, except for the normal control group, the body weight of mice in the other treatment groups decreased gradually at first and then increased slowly and finally did not change significantly. The average body weight of ETEC K88-infected mice was slightly lower than those of mice in the other treatment groups. Furthermore, the fecal material of mice from the ETEC K88-infected group was thin, soft, or irregularly shaped and showed a yellow-green color, which indicated symptoms of diarrhea, compared with the normal control group. However, the oral administration of L. lactis NZ9000 or L. lactis NZ9000-SeNPs effectively prevented the occurrence of diarrhea.
FIG 1.
Experimental scheme and change in body weight of mice during the experimental period. (A) Experimental scheme. (B) Change in body weight of mice. All data are presented as the means ± SEM (n = 10). The ETEC K88 group included mice that were orally administered ETEC K88 only. The L. lactis NZ9000+ETEC K88 group included mice that were pretreated with L. lactis NZ9000 before administration of ETEC K88. The L. lactis NZ9000-SeNP+ETEC K88 group includes mice that were pretreated with L. lactis NZ9000-SeNPs before administration of ETEC K88.
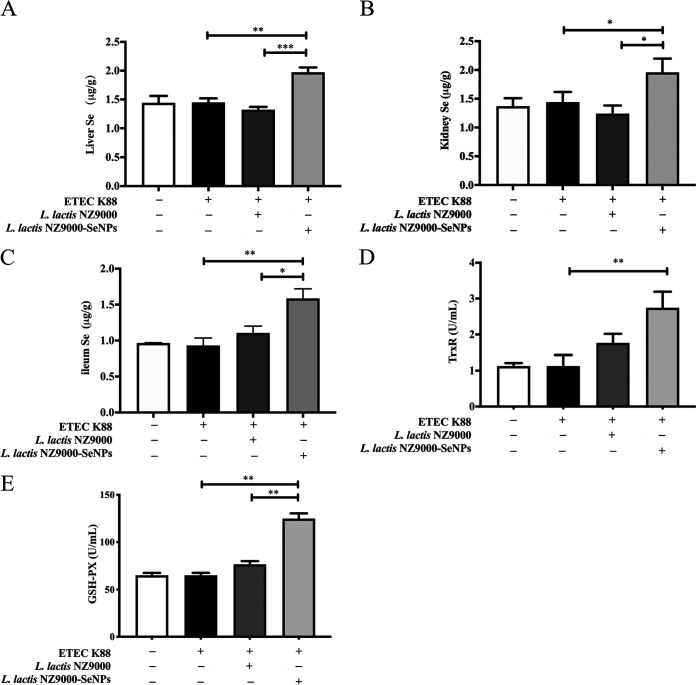
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on Se content in organs of mice.
Compared with other groups, the oral administration of L. lactis NZ9000-SeNPs significantly increased the Se levels in the liver, kidney, and ileum of mice (Fig. 2A to C). SeNPs may be absorbed by the small intestine tissue and participate in the metabolic network. Se is the active site of many selenoenzymes such as glutathione peroxidase (GSH-Px) and thioredoxin reductase (TrxR). Therefore, we detected the activities of GSH-Px and TrxR in the serum of mice. As shown in Fig. 2D and E, L. lactis NZ9000-SeNP supplementation significantly increased GSH-Px and TrxR activities in serum compared with the ETEC K88-infected group.
FIG 2.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNP supplementation on Se content. (A) Se content in liver. (B) Se content in kidney. (C) Se content in ileum. (D) Activity of TrxR in serum. (E) Activity of GSH-Px in serum. All data are presented as the means ± SEM (n = 6). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
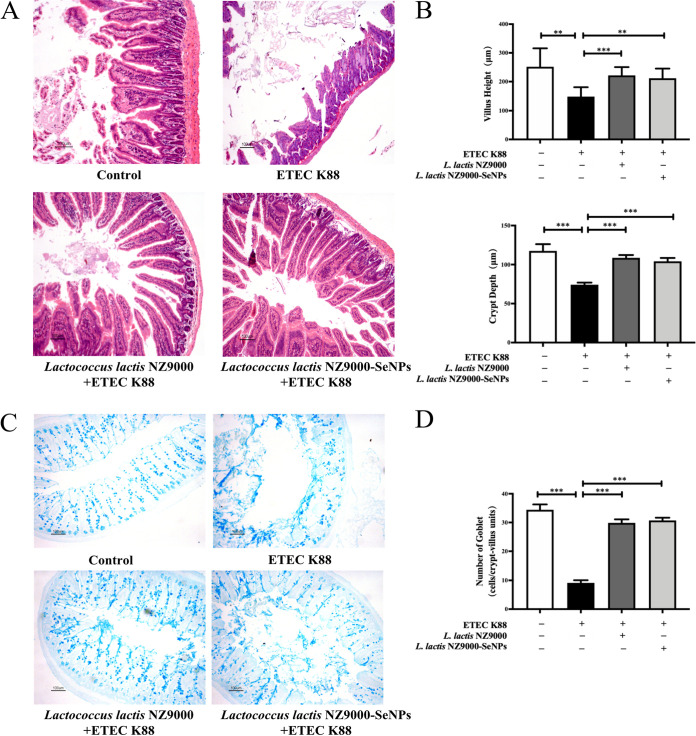
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the intestinal histology of mice.
Infection with ETEC K88 caused an increase in the crypt depth (CD) and a decrease in the villus height (VH) in the ileum as well as a decrease in the number of goblet cells in the ileum tissue compared with the normal control group. However, orally administered L. lactis NZ9000 or L. lactis NZ9000-SeNPs significantly alleviated the ETEC K88-induced increases in the VH and CD, and the intestinal villi were more regularly distributed (Fig. 3A and B). Moreover, the oral administration of L. lactis NZ9000 or L. lactis NZ9000-SeNPs significantly inhibited the ETEC K88 infection-induced decrease in the number of goblet cells in the ileum (Fig. 3C and D).
FIG 3.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on intestinal morphology in mice challenged with ETEC K88. (A) The histomorphology of the proximal ileum was observed by H&E staining. (B) Quantitative analysis of villus height and crypt depth. (C) Image of goblet cells in the ileum using alcian blue staining. (D) Quantitative analysis of the number of goblet cells in the ileum of each experimental group. All data are presented as the means ± SEM (n = 6). **, P < 0.01; ***, P < 0.001.
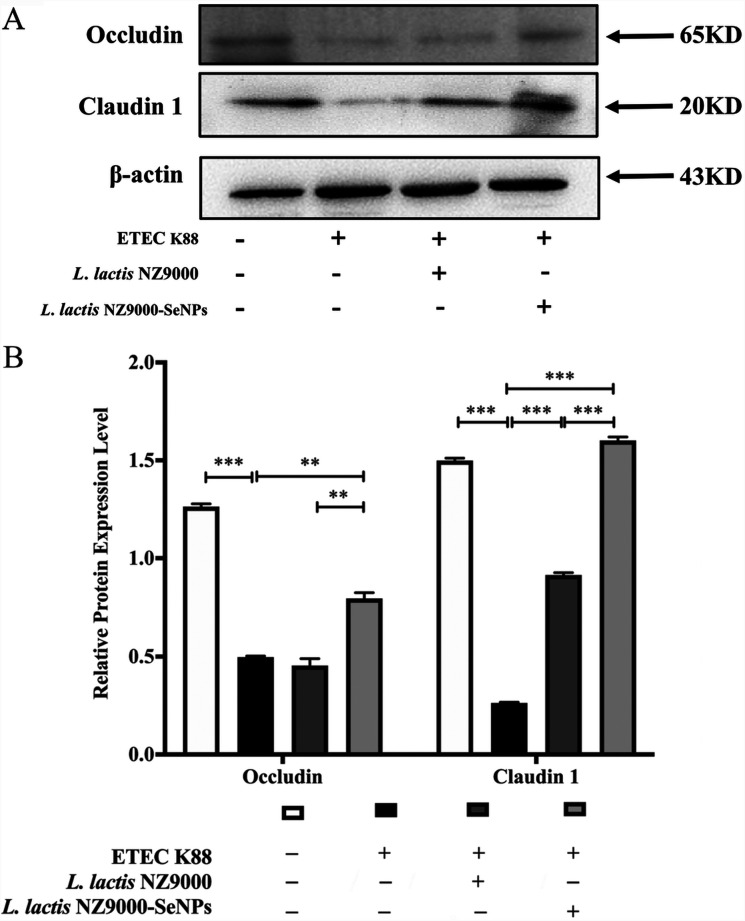
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the expression levels of TJ proteins in the ileum.
Occludin and claudin-1 are two key proteins that are involved in the maintenance of intestinal barrier function. As shown in Fig. 4, ETEC K88 infection resulted in significant decreases in the expression levels of occludin and claudin-1 in the ileum compared with the normal control group. The administration of L. lactis NZ9000 and L. lactis NZ9000-SeNPs significantly improved the expression levels of occludin and claudin-1 compared with the ETEC K88-infected group. Moreover, the upregulatory effect of L. lactis NZ9000-SeNPs on the expression levels of TJ proteins was more pronounced than that of L. lactis NZ9000. Furthermore, the improvement effect of L. lactis NZ9000-SeNPs is significantly better than that of L. lactis NZ9000.
FIG 4.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the expression of tight junction (TJ) proteins in the ileum of mice challenged with ETEC K88. (A) The expression levels of TJ proteins were determined by Western blotting. (B) Statistical results of gray value quantification. All data are presented as the means ± SEM (n = 3). **, P < 0.01; ***, P < 0.001.
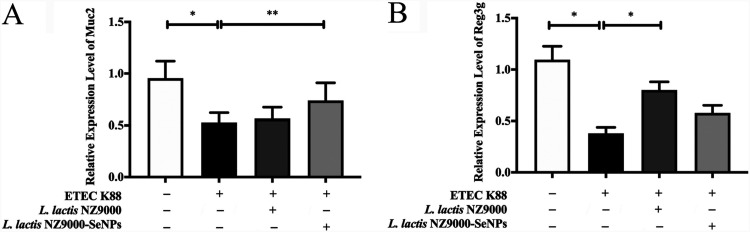
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the mRNA levels of MUC2 and Reg3g.
The mRNA expression levels of the mucin 2 (MUC2) and regenerating family member 3 gamma (Reg3g) genes in the ileum were determined by real-time quantitative PCR (qPCR). As shown in Fig. 5A and B, compared with the normal control group, ETEC K88-infected mice showed significantly reduced expression of MUC2 and Reg3g. However, compared with the group treated with ETEC K88 alone, pretreatment with L. lactis NZ9000 and L. lactis NZ9000-SeNPs significantly upregulated the mRNA levels of MUC2 and Reg3g in the ileum, respectively.
FIG 5.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the mRNA levels of MUC2 and Reg3g. (A) mRNA levels of MUC2 in the ileum analyzed by real-time qPCR. (B) mRNA levels of Reg3g in the ileum analyzed by real-time qPCR. All data are presented as the means ± SEM (n = 6). *, P < 0.05; **, P < 0.01.
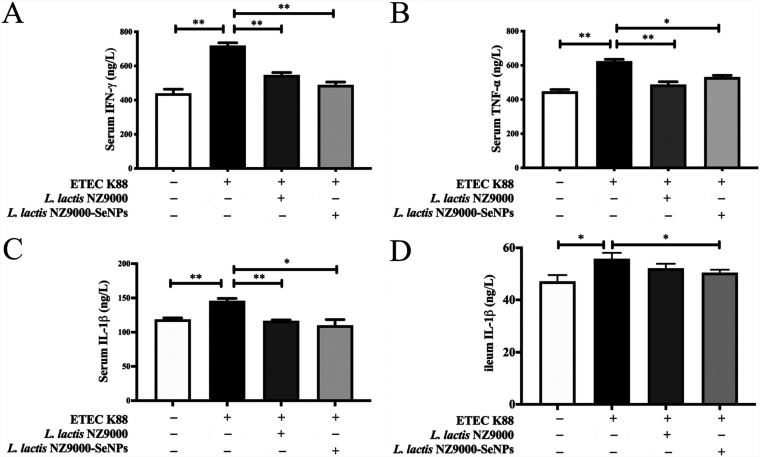
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the levels of serum and ileum cytokines.
As shown in Fig. 6A to D, compared with the normal control group, infection with ETEC K88 caused increases in the serum levels of interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-1β (IL-1β) and an increase in the ileal level of IL-1β. However, the oral administration of L. lactis NZ9000 or L. lactis NZ9000-SeNPs significantly inhibited the increases in IFN-γ, TNF-α, and IL-1β levels in the serum and ileum compared with those in the ETEC K88-infected group.
FIG 6.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on serum and ileum cytokine levels in mice challenged with ETEC K88. (A) Serum levels of IFN-γ. (B) Serum levels of TNF-α. (C) Serum levels of IL-1β. (D) Ileum levels of IL-1β. All data are presented as the means ± SEM (n = 6). *, P < 0.05; **, P < 0.01.
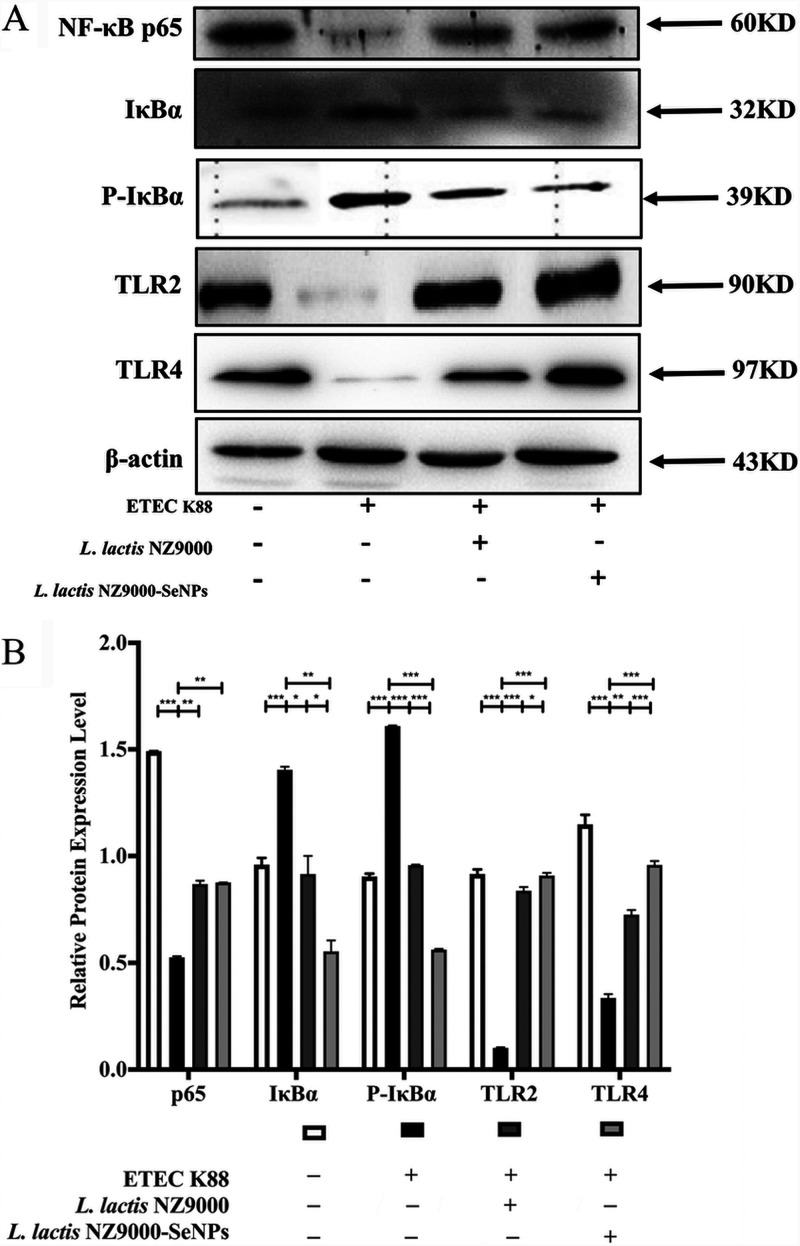
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the TLR/NF-κB signaling pathway.
As shown in Fig. 7, compared with the normal control group, ETEC K88 infection resulted in decreases in the expression levels of TLR2, TLR4, and NF-κB-p65 and increases in the expression levels of IκB and p-IκB in the ileum of mice. Moreover, compared with the ETEC K88-infected group, the administration of L. lactis NZ9000 or L. lactis NZ9000-SeNPs by gavage significantly improved the expression levels of TLR2 and TLR4 and decreased the expression levels of IκB and p-IκB. Furthermore, the improvement effect of L. lactis NZ9000-SeNPs is significantly better than that of L. lactis NZ9000.
FIG 7.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the TLR/NF-κB signaling pathway. (A) The expression levels of TLR/NF-κB-related proteins were determined by Western blot analysis. (B) Statistical results of gray value quantification. All data are presented as the means ± SEM (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
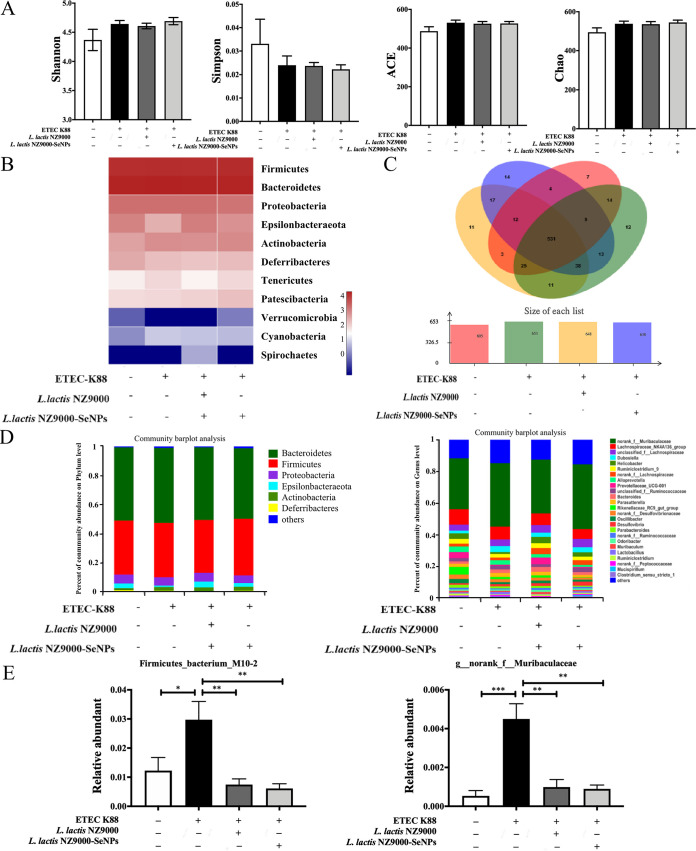
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the cecum microbiome.
Compared with the normal control group, orally administered ETEC K88 had no effect on α-diversity (Fig. 8A). Also, as shown in the community composition analysis heat map diagram (Fig. 8B), there were significant differences in intestinal flora structures among the experimental groups. A total of 531 operational taxonomic units (OTUs) were shared by each experimental group (Fig. 8C). The abundance analysis of the community composition at the level of phylum revealed that compared with the normal control group, ETEC K88 infection led to a marked decrease in the abundance of Epsilonbacteraeota in the ileum contents, while L. lactis NZ9000 and L. lactis NZ9000-SeNPs could reverse this trend. Meanwhile, L. lactis NZ9000 and L. lactis NZ9000-SeNPs could alleviate the increase in the abundance of Actinobacteria caused by ETEC K88 (Fig. 8D). The abundance analysis of the community composition at the level of genus revealed that compared with the normal control group, treatment with ETEC K88 significantly increased the abundances of g_norank_f_Muribaculaceae and Firmicutes_bacterium_M10-2. However, compared with the ETEC K88 group, supplementation with L. lactis NZ9000 or L. lactis NZ9000-SeNPs reduced the abundances of these two bacteria (Fig. 8E).
FIG 8.
Effects of L. lactis NZ9000 and L. lactis NZ9000-SeNPs on the microbial community in the cecum of mice challenged with ETEC K88. (A) α-Diversity analysis. (B) Heat map image at the phylum level in the cecum. (C) Shared operational taxonomic unit (OTU) analysis of the different libraries in the cecum. The Venn diagram shows the unique and shared OTUs (3% distance level) in the different libraries. (D) Relative abundances of intestinal microbiota at the phylum level and the genus level in the cecum. (E) Selected genera (g_norank_f_Muribaculaceae and Firmicutes_bacterium_M10-2) whose relative abundance values were significantly altered by L. lactis NZ9000-SeNP treatments. All data are presented as the means ± SEM (n = 6). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
The intestinal barrier is a complex multilayered structure that provides a physical and functional barrier to the transport of luminal contents to the systemic circulation (15). Therefore, maintaining the integrity of intestinal barrier function is critical for maintaining human and animal health. Alterations of the mucosal layer, as well as goblet cell pathology, have been associated with inflammatory bowel disease (IBD) (13). Numerous microbiotas inhabit the intestine or gastrointestinal tract, which are indispensable components of the intestinal barrier. Under normal physiological conditions, the relationship between the microorganism and the host is mutualistic and symbiotic. Gut microbiota can harvest energy, protect against pathogens, and regulate host immunity (14, 16–18). Since antibiotics are prohibited in animal feed, it is urgent to develop safe and efficient nutrition regulation strategies. Under pathological conditions, supplementation with exogenous probiotics is beneficial to regulate intestinal microecological dysbiosis. Se, as an essential micronutrient, is needed for the biosynthesis of selenoproteins, which contribute to antioxidant defense and immune function (19). The regulatory effects of dietary Se supplementation on intestinal barrier function and immune responses are associated with its regulation of the gut microbiota (20). Many studies have shown that patients with IBD have lower Se levels than healthy subjects (21, 22). An increase of dietary Se and vitamin E was shown to relieve the impact of heat stress on intestinal barrier integrity in pigs (23). MUC2 secreted by goblet cells can form a mucous layer on the surface of the intestinal epithelium, which is one of the main intestinal mucosal mechanical barriers (24). In addition, Reg3g is closely related to intestinal barrier function. A lack of Reg3g will increase the number of mucosa-associated bacteria, leading to intestinal barrier dysfunction (25). The oral administration of L. lactis NZ9000-SeNPs or L. lactis NZ9000 was found to increase the expression levels of TJ proteins and the mRNA levels of MUC2 and Reg3g in mice challenged with ETEC K88. The current results demonstrated that supplementation with exogenous L. lactis NZ9000-SeNPs or L. lactis NZ9000 effectively alleviates ETEC K88-induced intestinal barrier dysfunction.
Dietary supplementation with Se is essential for maintaining normal immune and antioxidant defense functions. Selenoproteins are involved in many physiological processes such as immune regulation and antioxidant defense. Se deficiency is strongly associated with increased incidences of placental retention and white muscle disease (WMD), lower fertility rates, and increased susceptibility to infections. The prevention of these disorders can be achieved by adequate supplementation of the diet with Se (26). In this study, we found that the oral administration of SeNP-enriched L. lactis NZ9000 attenuated the inflammatory response and enhanced the antioxidant capacity of C57BL/6 mice. Se plays a critical role in regulating immune function, especially nonspecific immune responses. A low level of Se was found to be closely associated with a weakening of the immune system (27–29). Decreased Se concentrations and impaired selenoprotein biosynthesis were observed in inflammatory diseases (30). Supplementation with Se alleviated the inflammatory response (29). Se deficiency was found to lead to decreased GSH-Px activity (31). To date, many genes that encode proteins associated with the antioxidant properties of Se have been identified (32). Also, Se supplementation was recently found to protect against oxidative stress in patients with Crohn’s disease (33).
Traditional Se supplements such as sodium selenite and selenomethionine (SeMet) generally have low bioavailability and high toxicity compared to other Se species such as organic Se and nano-Se (8). Therefore, it is urgent and significant to develop an innovative supplement of Se with high bioavailability and low toxicity. The biological activities and toxic effects of Se are strongly associated with its dosage and its chemical form (34). Recent research indicates that SeNPs have numerous advantages as a food or feed additive (35). For instance, SeNPs can be synthesized using a chemical, physical, or biological approach (so-called green synthesis) (36–39). Our previous studies demonstrated that Lactobacillus casei ATCC 393 and L. lactis NZ9000 effectively convert sodium selenite into SeNPs (12, 40). Biogenic SeNPs effectively relieve the intestinal barrier damage caused by oxidative stress and infection by pathogenic bacteria. Moreover, SeNPs exhibit low toxicity compared with sodium selenite and selenomethionine (40, 41). Also, SeNPs can theoretically pass through the intestinal epithelium in paracellular and transcellular ways (42). Our previous study indicated that SeNPs synthesized by Lactobacillus casei ATCC 393 could enter HepG2 cells by endocytosis (43).
SeNPs have higher antioxidant activity and lower toxicity than SeMet (44). Zhang et al. found that the activity of GSH-Px was higher in the livers of weanling pigs that were fed a diet with 0.5 or 1.0 mg Se/kg of body weight in the form of SeNPs than that in the form of Na2SeO3 (45). In this study, we found that the oral administration of L. lactis NZ9000-SeNPs increased the activities of GSH-Px and TrxR in the serum of ETEC K88-infected C57BL/6 mice. The biological activity of Se is exerted through the functions of selenoproteins, into which it is cotranslationally incorporated, as the amino acid residue Sec, during their synthesis. Se deficiency induces intestinal mucosal injury by affecting mucosal morphology, secretory IgA (S-IgA) secretion, and GSH-Px activity (46). SeNPs synthesized by Enterobacter cloacae Z0206 attenuated oxidative stress-induced intestinal barrier damage by activating the Nrf2 antioxidant signaling pathway (47). Se plays a crucial role in maintaining epithelial barrier integrity and protecting against inflammation and even against enteric infection (48). Supplementation with Se was found to restore the antioxidant capacity of the lungs and to further moderate the inflammatory response in respiratory distress syndrome patients (49).
In this study, we found that L. lactis NZ9000-SeNPs effectively relieve the increase in the serum levels of inflammatory cytokines in ETEC K88-infected mice. Moreover, the administration of L. lactis NZ9000-SeNPs attenuates both ETEC K88-induced intestinal microbiota dysbiosis and the activation of the TLR/NF-κB signaling pathway. Specific bacteria of the g_norank_f_Muribaculaceae and Firmicutes_bacterium_M10-2 families are correlated with intestinal barrier function and cytokine levels (50). The increases in these two bacteria will improve intestinal inflammatory cytokine levels and damage the intestinal mucus layer barrier function. Similar results were observed in our study in which Muribaculaceae and Firmicutes were more prevalent in mice treated with ETEC K88. Although the relationship between Se supplementation and increased immunity against pathogens has not been fully elucidated, it is established that Se deficiency leads to immune suppression and further increases the susceptibility to infections (51). Changes in the Se levels in the diet led to an alteration of the composition of the gut microbiota in mice (52). Sufficient or supranutritional Se supplementation can optimize the gut microbiota for protection against intestinal dysfunctions (20). Se-induced downregulation of the NF-κB pathways was associated with the eicosanoid class-switching phenomenon to differentially regulate pathways of inflammation and resolution in immune cells (53). The biological activity of Se, exerted through various selenoproteins, effectively resolves inflammation by driving the production of prostaglandin D2 (PGD2) and its cyclopentenone prostaglandin (CyPG) metabolites that potentially modulate the NF-κB- and peroxisome proliferator-activated receptor (PPAR)-dependent pathways (21). The protective effect of Se supplementation may be mediated through microbial metabolites that may not only impact Se species selection but also assist in attenuating inflammation or enhancing resolution through the regulation of the host immune response.
At present, selenium-enriched probiotics not only have a wide range of applications in the food industry but also have great potential in the biomedical field in the future (54). Simona et al. found that an L. casei strain is a potential SeNP-enriched probiotic and that the L. casei strain and L. casei strain SeNPs have the ability to annihilate the toxic effect of cadmium on the kidneys. L. casei strain SeNPs at 0.4 mg/kg significantly decreased the gene expression of kidney inflammatory markers (TNF-α, IL-6, and NF-κB) compared with an L. casei strain (55, 56). Yi et al. found that Bifidobacterium longum and selenium-B. longum have protective effects against alcohol- plus high-fat-diet (HFD)-induced hepatic injury in mice. Selenium can have a synergistic effect with B. longum, and the effect is better than that of the B. longum group (57). In this study, we found that L. lactis NZ9000-SeNPs effectively relieve the increased serum levels of inflammatory cytokines in ETEC K88-infected mice. Moreover, the administration of L. lactis NZ9000-SeNPs better attenuates both ETEC K88-induced intestinal microbiota dysbiosis and the activation of the TLR/NF-κB signaling pathway, and the effect has a significant advantage compared to L. lactis NZ9000.
Conclusion.
The oral administration of SeNP-enriched L. lactis NZ9000 and L. lactis NZ9000 was found to effectively alleviate ETEC K88-induced intestinal barrier dysfunction in C57BL/6 mice. Additionally, it was also found that the protective mechanism may be related to their antioxidant activities and their downregulatory effects on the TLR/NF-κB signaling pathway. Moreover, L. lactis NZ9000-SeNPs are more effective than L. lactis NZ9000 in regulating the expression of TJ proteins and the TLR/NF-κB signaling pathway. These findings suggested that SeNP-enriched microorganisms may be a promising and safe Se supplement for use as a food or feed additive. However, it is still necessary to conduct further studies on the effects of the application of SeNP-enriched L. lactis NZ9000.
MATERIALS AND METHODS
Bacterial strains and experimental animals.
The L. lactis NZ9000 strain was purchased from MoBiTec Company (Göttingen, Germany). The ETEC K88 strain was kept in our laboratory. Forty healthy 4-week-old male C57BL/6 mice weighing 20 ± 2 g used in this study were purchased from Keao Biotechnology Co. Ltd. (Xi’an, China).
Reagents.
M17 broth and Luria-Bertani (LB) medium were purchased from Oxoid Ltd. (Basingstoke, UK). Enzyme-linked immunosorbent assay (ELISA) kits for mouse tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and interleukin-1β (IL-1β) were purchased from Jianglaibio Co. Ltd. (Shanghai, China). The radioimmunoprecipitation assay (RIPA) lysis buffer and bicinchoninic acid (BCA) protein assay kit were purchased from Solarbio Life Sciences Co. Ltd. (Beijing, China). Thioredoxin reductase (TrxR) and glutathione peroxidase (GSH-Px) assay kits were purchased from the Nanjing Institute of Bioengineering (Jiangsu, China). Primary antibodies against occludin, claudin-1, β-actin, nuclear factor kappa B (NF-κB)-p65, IκBα, and p-IκBα and horseradish peroxidase (HRP)-labeled secondary antibody were purchased from Abclonal Biotechnology (Wuhan, China). Primary antibodies against Toll-like receptor 2 (TLR2) and TLR4 were purchased from Boster Biological Technology (Wuhan, China).
Bacterial culture conditions.
L. lactis NZ9000 was incubated in M17 broth at 30°C for 24 h without shaking. The synthesis process for SeNP-enriched L. lactis NZ9000 mainly included the following steps. First, monoclonal L. lactis NZ9000 was inoculated into M17 broth and cultured until an optical density at 600 nm (OD600) of 0.5 was reached, after which the bacteria were inoculated into a new flask with fresh M17 broth and cultured at 30°C for 24 h. Subsequently, a solution of sodium selenite (0.6 mM) was added to the L. lactis NZ9000 culture broth to stimulate the biosynthesis of the SeNPs. Next, SeNP-enriched L. lactis NZ9000 bacteria were obtained after coculturing for 24 h at 30°C. According to methods used previously in our laboratory, ETEC K88 was cultured in LB medium with shaking at a speed of 120 rpm at 37°C overnight. Bacteria were collected by centrifugation at 5,000 × g at 4°C for 10 min, and the pellets were then washed with phosphate-buffered saline (PBS) (pH 7.4) (58). The obtained bacteria were suspended in M17 broth medium. The concentrations of the bacterial resuspension solutions of L. lactis NZ9000, L. lactis NZ9000-SeNPs, and ETEC K88 were adjusted to 1.0 × 109 CFU/ml, 1.0 × 109 CFU/ml, and 1.0 × 108 CFU/ml, respectively.
Protective effects of SeNP-enriched L. lactis NZ9000 against ETEC K88-induced intestinal barrier dysfunction in C57BL/6 mice.
The animal experimental protocol used in this study was approved by the Laboratory Animal Welfare and Ethics Committee of Northwestern Polytechnical University, and the study was conducted strictly in accordance with International Laboratory Animal Assessment and Accreditation Committee guidelines for the care and use of laboratory animals. During the entire experimental period, the 40 healthy male C57BL/6 mice were maintained at the Experimental Animal Center of Northwestern Polytechnical University. The living conditions were as follows: ambient temperature of 25°C, relative humidity of 50%, and a 12-h light/dark cycle. The experimental scheme is depicted in Fig. 1A. After an adaptive period of 7 days, the mice were randomly assigned to four groups, with 10 mice per group, as follows: normal control group (M17 broth and LB medium), ETEC K88-infected model group (ETEC K88 resuspension solution), L. lactis NZ9000 treatment group (resuspension solution of L. lactis NZ9000 and ETEC K88), and SeNP-enriched L. lactis NZ9000 treatment group (resuspension solution of L. lactis NZ9000-SeNPs and ETEC K88). The experimental duration was 14 days. The mice in the L. lactis NZ9000 treatment group were administered 100 μl of 1.0 × 109 CFU/ml of an L. lactis NZ9000 resuspension solution per day by gavage. The mice in the L. lactis NZ9000-SeNP treatment group were orally administered 100 μl of 1.0 × 109 CFU/ml of an L. lactis NZ9000-SeNP resuspension solution containing 0.5 mg/kg SeNPs per day. The mice in the other groups were given the same volume of M17 broth per day by gavage. Mice in the ETEC K88-infected groups were orally administered 100 μl of 1.0 × 108 CFU/ml of an ETEC K88 resuspension solution on days 8, 10, and 12, and the other groups were orally administered the same volume of LB broth. Body weight, diarrhea, behavior, and mood were monitored and recorded daily. After the above-described treatments, all experimental mice were anesthetized with ether, and aliquots of their blood were rapidly drawn from their eyes for the isolation of serum. The mice were then sacrificed, and organs in their abdominal cavities were dissected. Portions of the ileum (0.5-cm sections) were immediately excised and stored in 4% paraformaldehyde for subsequent histological analysis. The appropriate amounts of cecal content samples were aseptically collected into a 1.5-ml sterile centrifuge tube and quickly frozen in liquid nitrogen. Finally, the remaining ileum tissue samples, cecum contents, and serum samples were stored at −80°C for subsequent analysis.
Organ selenium content determination.
The selenium content in the organs was measured by inductively coupled plasma mass spectrometry (ICP-MS). With surgical scissors, about 200 mg of the liver, kidney, and ileum was clipped and then dissolved into a nitric acid solution that was colorless and transparent, with 5 ml. The solution was then heated in boiling water (100°C) for 2 h to ensure that the acid was completely volatilized. Next, the sample solution was transferred to a 10-ml volumetric flask to make the volume up to 10 ml. Before the test, standard solutions with Se concentrations of 0 μg/ml, 5 μg/ml, 10 μg/ml, 20 μg/ml, 50 μg/ml, 100 μg/ml, 200 μg/ml, and 500 μg/ml were prepared. A standard curve based on the content of selenium in the standard solution was drawn. Finally, the selenium contents in the liver, kidney, and ileum were determined.
Evaluation of selenoenzyme activities.
GSH-Px and TrxR are important antioxidant enzymes containing Se. The activity of GSH-Px and TrxR in serum was assayed by using the corresponding kits.
Evaluation of intestinal morphology and goblet cell numbers in ileum tissue.
Proximal ileum tissue samples fixed in 4% paraformaldehyde were dehydrated, paraffin embedded, and cut into slices. Next, the tissue sections were stained with hematoxylin and eosin (H&E) and examined under a phase-contrast microscope for histological and morphological characteristics of the ileum. The villus height and crypt depth in the ileum were determined using ImageJ analyzer software (National Institutes of Health [NIH], Bethesda, MD, USA). The number of goblet cells in the proximal ileum tissue was determined by alcian blue staining and counting with a Nikon 80i fluorescence microscope.
Evaluation of the expression levels of TJ proteins by Western blotting.
About 50 mg of ileum tissue was suspended in 500 μl of RIPA lysis buffer solution containing 1% phenylmethylsulfonyl fluoride (PMSF) and homogenized on ice. Next, the supernatants were collected after centrifugation at 13,000 × g for 15 min at 4°C. The total protein concentration in the supernatant was determined using a BCA kit, and the protein concentration in each group was adjusted to the same level with PBS. The expression levels of TJ proteins were evaluated by Western blotting as described in our previous study (24). Briefly, protein samples were first denatured at 95°C for 10 min using 5× loading buffer, and 30 μg of the denatured protein solution was loaded onto a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Afterwards, the protein solution was transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% skim milk blocking buffer for 2 h, the membrane was washed three times with Tris-buffered saline containing Tween 20 (TBST) for 5 min each time and then incubated with primary antibodies against occludin, claudin-1, and β-actin overnight at 4°C. Subsequently, the PVDF membrane was washed three times with TBST, followed by incubation with HRP-labeled secondary antibody at room temperature for 2 h. After washing three times with TBST, the expression levels of the proteins of interest were measured using the Clarity Western ECL substrate kit (Bio-Rad Laboratories, Hercules, CA, USA), and the images of the blots were acquired using the Odyssey imaging system (Li-Cor Biosciences, Lincoln, NE, USA). Densitometry of the images of the blots was performed using the ImageJ system (NIH) by normalization to β-actin.
Analysis of MUC2 and Reg3g mRNA expression levels in the ileum.
The ileum segments were isolated and immediately stored in liquid nitrogen. Total RNA was extracted from the ileum using TRIzol reagent (Invitrogen, USA). The quality and concentration of purified total RNA were determined with a nanophotometer (Implen, Germany). cDNA was synthesized using a PrimeScript II 1st-strand cDNA synthesis kit. Next, real-time PCR was performed on a CFX96 Touch real-time PCR detection system (Bio-Rad, USA) according to the instructions of the ChamQ SYBR qPCR master mix kit. The primers used in the study are listed in Table 1. β-Actin was chosen as an internal reference. Data analysis was performed using the 2−ΔΔCT method.
TABLE 1.
Sequences of primers used for qPCRa
| Gene | Sequence (5′–3′) |
|---|---|
| β-Actin | F, TCACCCACACTGTGCCCATCTACGA |
| R, GGATGCCACAGGATTCCATACCCA | |
| MUC2 | F, CTGACCAAGAGCGAACACAA |
| R, CATGACTGGAAGCAACTGGA | |
| Reg3g | F, TCAGGACATCTTGTGTCTGTGCTC |
| R, CATCCACCTCTGTTGGGTTCA | |
Abbreviations: MUC2, mucin 2; Reg3g, regenerating family member 3 gamma; F, forward; R, reverse.
Analysis of cytokine levels by ELISAs.
The levels of serum IL-1β, IFN-γ, and TNF-α and ileal IL-1β were determined using ELISA kits according to the manufacturer’s instructions.
Detection of TLR/NF-κB signaling pathway-related proteins by Western blotting.
The expression levels of NF-κB, p65, IκBα, p-IκBα, TLR2, and TLR4 in the ileum tissue samples were determined by Western blotting as described above.
Analysis of bacterial diversity and community composition in the cecum.
The bacterial diversity and community composition in cecal contents were analyzed by the 16S rRNA gene amplicon pyrosequencing technique using the Illumina MiSeq platform for sequencing by Shanghai Majorbio Co. Ltd. (Shanghai, China). Specific steps are as follows. The microbial community DNA was extracted using MagPure stool DNA KF kit B (Magen, China) according to the manufacturer’s instructions. V3-V4 variable regions of the bacterial 16S rRNA gene were amplified with degenerate PCR primers 341F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Libraries were qualified using the Agilent 2100 bioanalyzer. The validated libraries were used for sequencing on the Illumina MiSeq platform according to standard pipelines for Illumina, generating 2- by 300-bp paired-end reads.
Statistical analysis.
All data were expressed as the means ± standard errors of the means (SEM). Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA). Comparison of the mean values was performed using one-way analysis of variance (ANOVA). A P value of <0.05 was considered to be statistically significant.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 32072746), the Key Research and Development Program of Shaanxi Province (no. 2021NY-004), and the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (no. CX2021029).
Contributor Information
Chunlan Xu, Email: clxu@nwpu.edu.cn.
Danilo Ercolini, University of Naples Federico II.
REFERENCES
- 1.Takiishi T, Fenero C, Camara NOS. 2017. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers 5:e1373208. 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. 2012. The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol 46(Suppl):S12–S17. 10.1097/MCG.0b013e31826ae849. [DOI] [PubMed] [Google Scholar]
- 3.Romero ES, Cotoner CA, Camacho CP, Bedmar MC, Vicario M. 2015. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig 107:686–696. 10.17235/reed.2015.3846/2015. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. 1988. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol 106:1141–1149. 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. 1998. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141:1539–1550. 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremonini E, Zonaro E, Donini M, Lampis S, Boaretti M, Dusi S, Melotti P, Lleo MM, Vallini G. 2016. Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb Biotechnol 9:758–771. 10.1111/1751-7915.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humann-Ziehank E. 2016. Selenium, copper and iron in veterinary medicine—from clinical implications to scientific models. J Trace Elem Med Biol 37:96–103. 10.1016/j.jtemb.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Nagy G, Pinczes G, Pinter G, Pocsi I, Prokisch J, Banfalvi G. 2016. In situ electron microscopy of lactomicroselenium particles in probiotic bacteria. Int J Mol Sci 17:1047. 10.3390/ijms17071047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu N, Han F, Lin X, Tang C, Ye J, Cai X. 2016. The association between serum selenium levels with rheumatoid arthritis. Biol Trace Elem Res 172:46–52. 10.1007/s12011-015-0558-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C, Zhang S, Song C, Zhang Y, Ling Q, Hoffmann PR, Li J, Chen T, Zheng W, Huang Z. 2017. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J Nanobiotechnology 15:20. 10.1186/s12951-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, Zhang Z, Li Y, Hu X, Shen P, Fu Y, Cao Y, Zhang N. 2016. Selenium deficiency deteriorate the inflammation of S. aureus infection via regulating NF-κB and PPAR-γ in mammary gland of mice. Biol Trace Elem Res 172:140–147. 10.1007/s12011-015-0563-5. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Qiao L, Ma L, Yan S, Guo Y, Dou X, Zhang B, Roman A. 2019. Biosynthesis of polysaccharides-capped selenium nanoparticles using Lactococcus lactis NZ9000 and their antioxidant and anti-inflammatory activities. Front Microbiol 10:1632. 10.3389/fmicb.2019.01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YS, Ho SB. 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12:319–330. 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervantes-García D, Jiménez M, Rivas-Santiago CE, Gallegos-Alcalá P, Hernández-Mercado A, Santoyo-Payán LS, Loera-Arias MDJ, Saucedo-Cardenas O, Montes de Oca-Luna R, Salinas E. 2021. Lactococcus lactis N9000 prevents asthmatic airway inflammation and remodelling in rats through the improvement of intestinal barrier function and systemic TGF-β production. Int Arch Allergy Immunol 182:277–291. 10.1159/000511146. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh SS, Wang J, Yannie PJ, Ghosh S. 2020. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc 4:bvz039. 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker Barbara M. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bäumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535:85–93. 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352:539–544. 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ala M, Kheyri Z. 2021. The rationale for selenium supplementation in inflammatory bowel disease: a mechanism-based point of view. Nutrition 85:111153. 10.1016/j.nut.2021.111153. [DOI] [PubMed] [Google Scholar]
- 20.Zhai Q, Cen S, Li P, Tian F, Zhao J, Zhang H, Chen W. 2018. Effects of dietary selenium supplementation on intestinal barrier and immune responses are associated with its modulation of gut microbiota. Environ Sci Technol Lett 5:724–730. 10.1021/acs.estlett.8b00563. [DOI] [Google Scholar]
- 21.Kudva AK, Shay AE, Prabhu KS. 2015. Selenium and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 309:G71–G77. 10.1152/ajpgi.00379.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stochel-Gaudyn A, Fyderek K, Kościelniak P. 2019. Serum trace elements profile in the pediatric inflammatory bowel disease progress evaluation. J Trace Elem Med Biol 55:121–126. 10.1016/j.jtemb.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U, Pustovit RV, Fothergill LJ, Bravo DM, Celi P, Leury BJ, Gabler NK, Dunshea FR. 2016. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat stressed pigs. Exp Physiol 101:801–810. 10.1113/EP085746. [DOI] [PubMed] [Google Scholar]
- 24.Qiao L, Dou X, Yan S, Zhang B, Xu C. 2020. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct 11:3020–3031. 10.1039/d0fo00132e. [DOI] [PubMed] [Google Scholar]
- 25.Chopyk DM, Grakoui A. 2020. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology 159:849–863. 10.1053/j.gastro.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Malevu TD, Sochor J, Baron M, Melcova M, Zidkova J, Kizek R. 2017. A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int J Mol Sci 18:2209. 10.3390/ijms18102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dercksen DP, Counotte GH, Hazebroek MK, Arts W, van Rijn T. 2007. Selenium requirements of dairy goats. Tijdschr Diergeneeskd 132:468–471. (In Dutch.) https://pubmed.ncbi.nlm.nih.gov/17626576. [PubMed] [Google Scholar]
- 28.Effraimidis G, Wiersinga WM. 2014. Mechanisms in endocrinology. Autoimmune thyroid disease: old and new players. Eur J Endocrinol 170:R241–R252. 10.1530/EJE-14-0047. [DOI] [PubMed] [Google Scholar]
- 29.Köhrle J, Gärtner R. 2009. Selenium and thyroid. Best Pract Res Clin Endocrinol Metab 23:815–827. 10.1016/j.beem.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Schomburg L. 2011. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol 8:160–171. 10.1038/nrendo.2011.174. [DOI] [PubMed] [Google Scholar]
- 31.Shalini S, Bansal MP. 2007. Alterations in selenium status influences reproductive potential of male mice by modulation of transcription factor NF-κB. Biometals 20:49–59. 10.1007/s10534-006-9014-2. [DOI] [PubMed] [Google Scholar]
- 32.Chan JM, Darke AK, Penney KL, Tangen CM, Goodman PJ, Lee G-SM, Sun T, Peisch S, Tinianow AM, Rae JM, Klein EA, Thompson IM, Jr, Kantoff PW, Mucci LA. 2016. Selenium- or vitamin E-related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in SELECT. Cancer Epidemiol Biomarkers Prev 25:1050–1058. 10.1158/1055-9965.EPI-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barros SEDL, Dias TMDS, de Moura MSB, Soares NRM, Pierote NRA, de Araujo COD, Maia CSC, Henriques GS, Barros VC, Moita Neto JM, Parente JML, Marreiro DDN, Nogueira NDN. 2020. Relationship between selenium status and biomarkers of oxidative stress in Crohn’s disease. Nutrition 74:110762. 10.1016/j.nut.2020.110762. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Li X, Wong YS, Zheng W, Zhang Y, Cao W, Chen T. 2012. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano 6:6578–6591. 10.1021/nn202452c. [DOI] [PubMed] [Google Scholar]
- 35.Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjørklund G, Sochor J, Kizek R. 2018. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomedicine 13:2107–2128. 10.2147/IJN.S157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quintana M, Haro-Poniatowski E, Morales J, Batina N. 2002. Synthesis of selenium nanoparticles by pulsed laser ablation. Appl Surf Sci 195:175–186. 10.1016/S0169-4332(02)00549-4. [DOI] [Google Scholar]
- 37.Ramamurthy C, Sampath KS, Arunkumar P, Kumar MS, Sujatha V, Premkumar K, Thirunavukkarasu C. 2013. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng 36:1131–1139. 10.1007/s00449-012-0867-1. [DOI] [PubMed] [Google Scholar]
- 38.Shoeibi S, Mashreghi M. 2017. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J Trace Elem Med Biol 39:135–139. 10.1016/j.jtemb.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S-Y, Zhang J, Wang H-Y, Chen H-Y. 2004. Synthesis of selenium nanoparticles in the presence of polysaccharides. Mater Lett 58:2590–2594. 10.1016/j.matlet.2004.03.031. [DOI] [Google Scholar]
- 40.Xu C, Qiao L, Guo Y, Ma L, Cheng Y. 2018. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr Polym 195:576–585. 10.1016/j.carbpol.2018.04.110. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, Qiao L, Ma L, Guo Y, Dou X, Yan S, Zhang B, Roman A. 2019. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int J Nanomedicine 14:4491–4502. 10.2147/IJN.S199193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieux AD, Fievez V, Garinot M, Schneider Y-J, Préat V. 2006. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 116:1–27. 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Guo Y, Qiao L, Ma L, Cheng Y, Roman A. 2018. Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front Microbiol 9:1129. 10.3389/fmicb.2018.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Zhang J, Yu H. 2007. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42:1524–1533. 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Xia M, Hu C. 2007. Effect of nano-selenium on the activities of glutathione peroxidase and type-I deiodinase in the liver of weanling pigs. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 24:153–156. https://schlr.cnki.net/zn/Detail/index/SJPD_04/SJPD12102103182782. [PubMed] [Google Scholar]
- 46.He XJ, Lin YC, Lian S, Sun DB, Guo DH, Wang JF, Wu R. 2020. Selenium deficiency in chickens induces intestinal mucosal injury by affecting the mucosa morphology, SIgA secretion, and GSH-Px activity. Biol Trace Elem Res 197:660–666. 10.1007/s12011-019-02017-6. [DOI] [PubMed] [Google Scholar]
- 47.Song DG, Cheng YZ, Li XX, Wang FQ, Lu ZQ, Xiao X, Wang YZ. 2017. Biogenic nanoselenium particles effectively attenuate oxidative stress-induced intestinal epithelial barrier injury by activating the Nrf2 antioxidant pathway. ACS Appl Mater Interfaces 9:14724–14740. 10.1021/acsami.7b03377. [DOI] [PubMed] [Google Scholar]
- 48.Nettleford SK, Zhao L, Qian F, Herold M, Arner B, Desai D, Amin S, Xiong N, Singh V, Carlson BA, Prabhu KS. 2020. The essential role of selenoproteins in the resolution of Citrobacter rodentium-induced intestinal inflammation. Front Nutr 7:96. 10.3389/fnut.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahmoodpoor A, Hamishehkar H, Shadvar K, Ostadi Z, Sanaie S, Saghaleini SH, Nader ND. 2019. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol Invest 48:147–159. 10.1080/08820139.2018.1496098. [DOI] [PubMed] [Google Scholar]
- 50.Sheng K, Zhang G, Sun M, He S, Kong X, Wang J, Zhu F, Zha X, Wang Y. 2020. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct 11:7817–7829. 10.1039/d0fo01418d. [DOI] [PubMed] [Google Scholar]
- 51.Avery JC, Hoffmann PR. 2018. Selenium, selenoproteins, and immunity. Nutrients 10:1203. 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kasaikina MV, Kravtsova MA, Lee BC, Seravalli J, Peterson DA, Walter J, Legge R, Benson AK, Hatfield DL, Gladyshev VN. 2011. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J 25:2492–2499. 10.1096/fj.11-181990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi UH, Kaushal N, Ravindra KC, Hegde S, Nelson SM, Narayan V, Vunta H, Paulson RF, Prabhu KS. 2011. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) γ. J Biol Chem 286:27471–27482. 10.1074/jbc.M111.260547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J, Yang H. 19 July 2021. Recent development in Se-enriched yeast, lactic acid bacteria and bifidobacteria. Crit Rev Food Sci Nutr 10.1080/10408398.2021.1948818. [DOI] [PubMed]
- 55.Vicas SI, Laslo V, Timar AV, Balta C, Herman H, Ciceu A, Gharbia S, Rosu M, Mladin B, Fritea L, Cavalu S, Cotoraci C, Prokisch J, Puschita M, Pop C, Miutescu E, Hermenean A. 2021. Functional food product based on nanoselenium-enriched Lactobacillus casei against cadmium kidney toxicity. Appl Sci 11:4220. 10.3390/app11094220. [DOI] [Google Scholar]
- 56.Vicas SI, Laslo V, Timar AV, Balta C, Herman H, Ciceu A, Gharbia S, Rosu M, Mladin B, Chiana L, Prokisch J, Puschita M, Miutescu E, Cavalu S, Cotoraci C, Hermenean A. 2021. Nano selenium-enriched probiotics as functional food products against cadmium liver toxicity. Materials (Basel) 14:2257. 10.3390/ma14092257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi HW, Zhu XX, Huang XL, Lai YZ, Tang Y. 2020. Selenium-enriched Bifidobacterium longum protected alcohol and high fat diet induced hepatic injury in mice. Chin J Nat Med 18:169–177. 10.1016/S1875-5364(20)30018-2. [DOI] [PubMed] [Google Scholar]
- 58.Xu C, Yan S, Guo Y, Qiao L, Ma L, Dou X, Zhang B. 2020. Lactobacillus casei ATCC 393 alleviates enterotoxigenic Escherichia coli K88-induced intestinal barrier dysfunction via TLRs/mast cells pathway. Life Sci 244:117281. 10.1016/j.lfs.2020.117281. [DOI] [PubMed] [Google Scholar]