ABSTRACT
Soybean root rot caused by the oomycete Phytophthora sojae is a serious soilborne disease threatening soybean production in China. Bacillus velezensis FZB42 is a model strain for Gram-positive plant growth-promoting rhizobacteria and is able to produce multiple antibiotics. In this study, we demonstrated that B. velezensis FZB42 can efficiently antagonize P. sojae. The underlying mechanism for the inhibition was then investigated. The FZB42 mutants deficient in the synthesis of lipopeptides (bacillomycin D and fengycin), known to have antifungal activities, and polyketides (bacillaene, difficidin, and macrolactin), known to have antibacterial activities, were not impaired in their antagonism toward P. sojae; in contrast, mutants deficient in bacilysin biosynthesis completely lost their antagonistic activities toward P. sojae, indicating that bacilysin was responsible for the activity. Isolated pure bacilysin confirmed this inference. Bacilysin was previously shown to be antagonistic mainly toward prokaryotic bacteria rather than eukaryotes. Here, we found that bacilysin could severely damage the hyphal structures of P. sojae and lead to the loss of its intracellular contents. A device was invented allowing interactions between P. sojae and B. velezensis FZB42 on nutrient agar. In this manner, the effect of FZB42 on P. sojae was studied by transcriptomics. FZB42 significantly inhibited the expression of P. sojae genes related to growth, macromolecule biosynthesis, pathogenicity, and ribosomes. Among them, the genes for pectate lyase were the most significantly downregulated. Additionally, we showed that bacilysin effectively prevents soybean sprouts from being infected by P. sojae and could antagonize diverse Phytophthora species, such as Phytophthora palmivora, P. melonis, P. capsici, P. litchi, and, most importantly, P. infestans.
IMPORTANCE Phytophthora spp. are widespread eukaryotic phytopathogens and often extremely harmful. Phytophthora can infect many types of plants important to agriculture and forestry and thus cause large economic losses. Perhaps due to inappropriate recognition of Phytophthora as a common pathogen in history, research on the biological control of Phytophthora is limited. This study shows that B. velezensis FZB42 can antagonize various Phytophthora species and prevent the infection of soybean seedlings by P. sojae. The antibiotic produced by FZB42, bacilysin, which was already known to have antibacterial effectiveness, is responsible for the inhibitory action against Phytophthora. We further showed that some Phytophthora genes and pathways may be targeted in future biocontrol studies. Therefore, our data provide a basis for the development of new tools for the prevention and control of root and stem rot in soybean and other plant diseases caused by Phytophthora.
KEYWORDS: Phytophthora sojae, Bacillus velezensis, FZB42, biocontrol, bacilysin, transcriptome, Phytophthora infestans
INTRODUCTION
Phytophthora is a eukaryotic pathogen harmful to a wide range of plants, many of which are important in agriculture and forestry (1). The damage caused by Phytophthora is often devastating, leading to severe declines in plant yields and even harvest failure. The Great Famine, famous in the history of Ireland, was caused by a dramatic yield reduction in potato due to Phytophthora infestans infection. Globally, soilborne potato disease has led to an economic loss of up to $6.7 billion (2). In China, root and stem rot of soybean owing to Phytophthora sojae seriously threatens Chinese soybean production. P. sojae causes damping off in soybean seedlings and root rot in older plants. It is able to persist in the soil for a long time and is extremely difficult to cure (3, 4). Owing to its economic importance, P. sojae, along with P. infestans, has been developed as a model species for the study of oomycete plant pathogens (3). Other notorious Phytophthora species include Phytophthora melonis and Phytophthora capsici, phytopathogens that endanger the production of common fruits and vegetables globally (5, 6). In forestry, there are also many hazardous Phytophthora diseases. For instance, Phytophthora cinnamomi can cause root rot in more than 3,000 species of plants, including eucalyptus and avocados (7, 8); Phytophthora palmivora can cause papaya disease and cassava root rot (9); and Phytophthora litchi can infect litchi, resulting in a severe decline in the quality and yield of litchi fruit (10).
Phytophthora forms mycelia morphologically similar to most fungi. From a taxonomic point of view, however, Phytophthora belongs to the phylum Oomycota (=Pseudofungi) in the kingdom Chromista (11). Therefore, Phytophthora is not a fungus; in fact, it is more phylogenetically related to diatoms (12). At present, the control of Phytophthora mainly depends on chemical pesticides, whose efficacy is not sufficient in many cases (13). Another main countermeasure against P. sojae disease is to breed resistant soybean varieties (14–16). However, a breeding cycle is usually time-consuming and expensive. As far as biological control is concerned, until now, only very limited reports have been focused on Phytophthora (17–21), whereas the rich knowledge derived from numerous studies on fungi cannot be applied to Phytophthora because it is phylogenetically distant from fungi.
Bacillus velezensis FZB42 is a prototype Gram-positive plant growth-promoting rhizobacterium (PGPR) and an excellent biocontrol strain (22–25). Currently, it has been found to produce at least 13 antimicrobial compounds, including three polyketides (bacillaene, difficidin, and macrolactin) synthesized by polyketide synthase (PKS) and some lipopeptides synthesized by nonribosomal peptide synthetases (NRPS) (26, 27). In addition, FZB42 can synthesize a dipeptide antibiotic, bacilysin, also through a nonribosomal pathway. Bacilysin has been reported to efficiently inhibit the growth of a variety of pathogenic bacteria, such as Erwinia amylovora, Xanthomonas oryzae pv. oryzae, X. oryzae pv. oryzicola, and Microcystis aeruginosa (27–30). Although there have been many reports on the antagonism of B. velezensis (22, 23, 31), only a few studies have examined its effects on Phytophthora spp (32). In this study, B. velezensis FZB42 was examined for its biocontrol potential against Phytophthora and the underlying mechanism.
RESULTS
B. velezensis FZB42 can efficiently inhibit the growth of P. sojae and other Phytophthora species.
The effect of B. velezensis FZB42 on P. sojae growth was tested on agar plates. While P. sojae was grown on V8 medium agar, FZB42 was grown in Landy medium agar confined in Oxford cups whose bottoms merged into V8 agar (Fig. 1a). In this way, the two microorganisms were supported by different media. Moreover, FZB42 growth was controlled by the cups so that its colony shape and size would not affect the measurement of the inhibition zone for quantitative comparisons. The results showed that P. sojae hyphae reached the edge of plates (∼90 mm) within 7 days in the controls, whereas its growth rate decreased after 2 to 3 days with FZB42 inoculation. In the next few days, the P. sojae colony size did not change, remaining at ∼34 mm in diameter on average. This indicated that FZB42 could produce diffusible metabolites that inhibited the growth of P. sojae. Statistical analysis revealed that the average colony size of P. sojae in the plates could be reduced by 66.8% by the presence of FZB42 (Fig. 1b).
FIG 1.
Inhibition of the growth of different Phytophthora species by B. velezensis FZB42 7 days after inoculation. (a) Growth of Phytophthora started from the center of the plates, while growth of B. velezensis FZB42 was restricted in Oxford cups, whose bottoms were merged in solid medium for Phytophthora, allowing contact of the two media. Sterile medium was added to the Oxford cups serving as the control (+Medium), while FZB42 was inoculated in the Oxford cups as the treatment. (b) Inhibition of different Phytophthora species by B. velezensis FZB42 (n = 3). ***, P < 0.001.
To determine whether the inhibitory effect of B. velezensis FZB42 can be generalized, we tested other Phytophthora species, including P. palmivora, P. melonis, P. capsici, P. litchi, and P. infestans, in the manner described above. All the pathogenic strains spread over the entire surface of the agar dish in the control groups, while their colonies were significantly smaller in the presence of FZB42 (Fig. 1a). The growth speed of the Phytophthora strains was obviously decreased by FZB42. The growth of P. palmivora and P. infestans almost ceased 2 days after inoculation. P. infestans was the species most affected by FZB42, with an average colony size of 11.30 mm after 15 days, corresponding to an inhibition rate of 93.7%, followed by P. palmivora, with an inhibition rate of 77.3% (Fig. 1b). P. melonis, P. capsici, and P. litchi were less sensitive to FZB42 and formed larger colonies, although they were also significantly suppressed with an inhibition rate of 50 to 60% (Fig. 1b). From the results, we concluded that B. velezensis FZB42 can inhibit the growth of not only P. sojae but also other Phytophthora species.
Inhibitory effect of FZB42 on P. sojae depends on bacilysin.
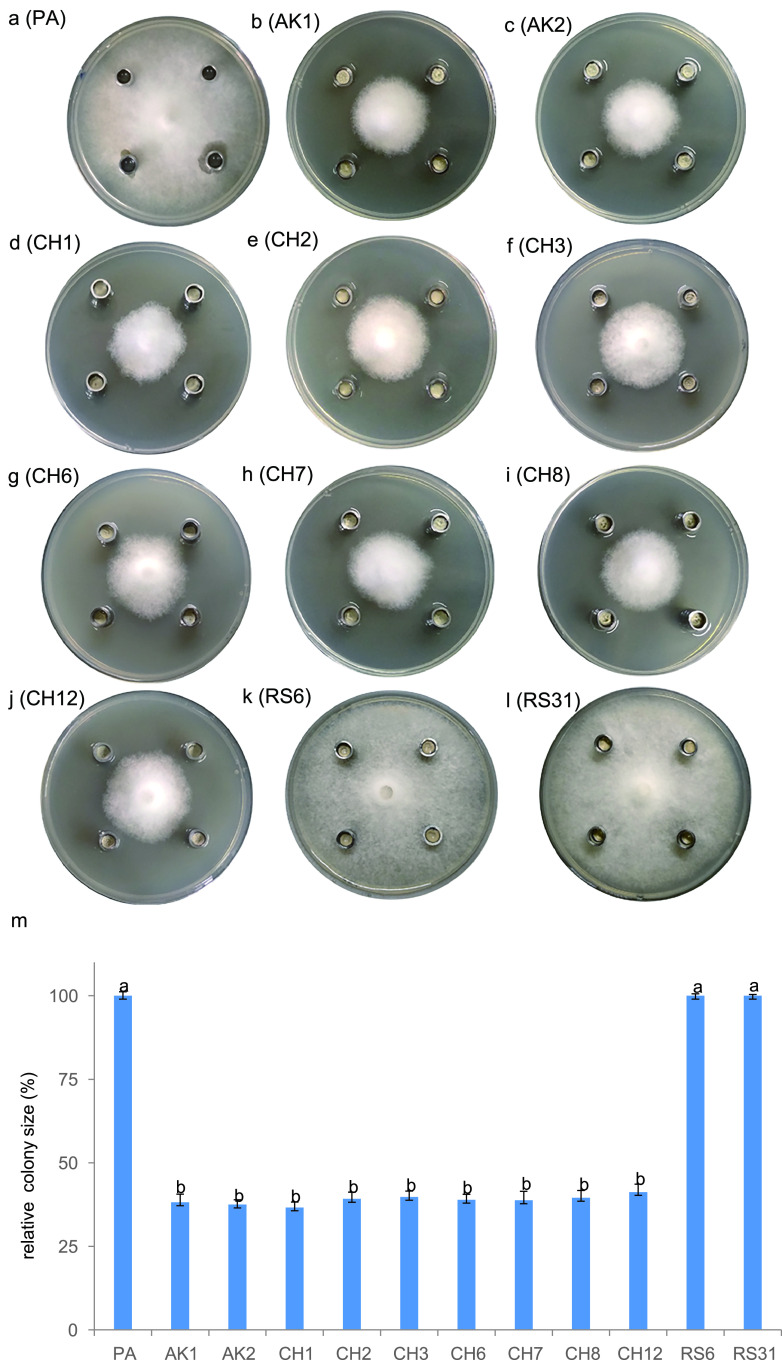
B. velezensis FZB42 can produce more than 10 antimicrobial compounds (22, 31), which may be responsible for its inhibitory effect on P. sojae. To pinpoint the relevant compound, we tested the inhibitory effects of 10 mutants defective in the synthesis of these compounds (Table 1) using the method described above. The results revealed that eight mutants (AK1, AK2, CH1, CH3, CH6, CH7, CH8, and CH12) exhibited inhibitory effects comparable to those of the wild type (Fig. 2). Remarkably, the sfp mutant strain CH3, which is unable to synthesize any polyketide and lipopeptide antibiotics, was still able to suppress the growth of P. sojae. In contrast, RS6 and RS31, both of which are incapable of synthesizing lipopeptides, polyketides, and bacilysin, did not show any inhibitory effects. Strain RS6 was constructed by knocking out bacA (encoding bacilysin biosynthesis protein BacA) of CH3, while RS31 was obtained by knocking out pznC (required for plantazolicin synthesis) of RS6. Since CH3 inhibited the growth of P. sojae but RS6 and RS31 did not, we conclude that the antibiotic bacilysin, which is absent in both RS6 and RS31, is the active compound suppressing the growth of P. sojae. To confirm this, we prepared HPLC (high-performance liquid chromatography)-purified bacilysin from CH3 and showed that bacilysin (200 μg/ml) could indeed inhibit P. sojae (Fig. 3).
TABLE 1.
Bacterial and oomycete strains used
| Strain | Species or genotype | Reference(s) |
|---|---|---|
| Bacteria | ||
| FZB42 | B. velezensis FZB42, wild type | 26 |
| ATCC 9144 | Staphylococcus aureus subsp. aureus | 62 |
| AK1 | FZB42 bmyA::Emr; deficient in bacillomycin D synthesis | 26 |
| AK2 | FZB42 fenA::Cmr; deficient in fengycin synthesis | 26 |
| CH1 | FZB42 srfAA::Emr; deficient in surfactin synthesis | 26 |
| CH3 | FZB42 sfp::Emr; deficient in all lipopeptides and polyketides synthesis | 26, 27 |
| CH6 | FZB42 bae::Cmr; deficient in bacillaene synthesis | 26 |
| CH7 | FZB42 mln::Cmr; deficient in macrolactin synthesis | 26 |
| CH8 | FZB42 dfn::Emr; deficient in difficidin synthesis | 26, 27 |
| CH12 | FZB42 mln::Cmr dfn::Emr, deficient in macrolactin and difficidin synthesis | 26 |
| RS6 | FZB42 sfp::Emr bacA::Cmr; deficient in all lipopeptides, polyketides, and bacilysin synthesis | 27 |
| RS31 | RS6 pznC::spc; deficient in all lipopeptides, polyketides, bacilysin and plantazolicin synthesis | 67 |
| Oomycetes | ||
| P6497 | P. sojae | 68 |
| 88069 | P. infestans | 69 |
| P01 | P. litchi | Laboratory stock |
| P32 | P. melonis | Laboratory stock |
| P60 | P. palmivora | Laboratory stock |
| P72 | P. capsici | Laboratory stock |
FIG 2.
Inhibition of P. sojae P6497 by different mutants of B. velezensis FZB42. Representatives (a to l) and the statistics (m) of colony size of P. sojae when challenged by (a) PA medium (CK), (b) AK1 (ΔbmyA), (c) AK2 (ΔfenA), (d) CH1 (ΔsrfAA), (e) CH2 (ΔfenA and ΔsrfAA), (f) CH3 (Δsfp), (g) CH6 (Δbae), (h) CH7 (Δmln), (i) CH8 (Δdfn), (j) CH12 (Δmln and Δdfn), (k) RS6 (Δsfp and Δbac), and (l) RS31 (ΔRBAM_007460). In panel m, averages with different letters are significantly different (P < 0.001).
FIG 3.

HPLC-MS analysis of prepared bacilysin and its antagonistic activity against P. sojae. (a) HPLC analysis of bacilysin prepared from CH3. The retention time of bacilysin is 4.75 min. Structure of bacilysin is showed on the left of the bacilysin peak. (b) Mass spectrum analysis of bacilysin and its antagonism toward P. sojae. On the plate, 30 μl bacilysin solution (200 μg/ml) was used to fill the holes on the left and right sides of the P. sojae colony, while sterile pure water was used to fill the holes above and below the P. sojae colony.
Microscopic observation revealed that bacilysin can disrupt P. sojae hyphae.
To understand the mechanism underlying the inhibition of P. sojae growth by bacilysin, we first compared the morphology of P. sojae hyphae treated with the supernatants of the two mutants, CH3 (Δsfp) and RS6 (Δsfp and Δbac). While hyphae soaked for 2 h in the RS6 supernatant appeared colorless, transparent, and smooth, similar to those treated with Perry and Abraham (PA) medium (Fig. 4a and b), the hyphae treated with CH3 supernatant for 2 h were unevenly dyed blue by Evans blue staining (Fig. 4c). Evans blue is able to penetrate ruptured or destabilized membranes. This result indicates that bacilysin can lead to the death of P. sojae hyphae and thus allow Evans blue to enter its cells through damaged membranes.
FIG 4.
Effect of bacilysin-containing supernatants on microscopic morphology of P. sojae visualized by light microscope (a to c), emission electronic microscope (d to i), and scanning electronic microscope (j to o). Mycelia of P. sojae were treated for 2 h with PA medium (CK) (a, d, g, and j), RS6 supernatants (b, e, h, and k) and CH3 supernatants (c, f, i, and l to o).
Scanning electron microscopy (SEM) showed that the P. sojae hyphae treated with PA medium (Fig. 4d and g) and the supernatant from bacilysin-deficient RS6 (Fig. 4e and h) remained smooth and healthy. However, after treatment with the CH3 supernatant containing bacilysin, nearly no intact hyphae could be observed. Instead, most hyphae displayed a collapsed, depressed, or distorted surface (Fig. 4f and i). At a magnification of ×2,400, broken hyphae losing cellular contents were clearly observed (Fig. 4f and i).
Transmission electron microscopy (TEM) revealed that the cell wall, as well as the organelles, of P. sojae looked intact after treatment with PA medium (Fig. 4j) and the RS6 supernatant (Fig. 4k). However, after treatment with the CH3 supernatant, broken cell walls were observed (Fig. 4m and n). Extravasation of cellular content due to broken cells should be responsible for the collapsed structure shown by SEM. Remarkably, inside some unbroken cells, large vacuoles with homogeneous contents occurred, but organelles almost disappeared (Fig. 4l and o), possibly due to degradation of the cellular content.
Both SEM and TEM results showed a severely damaged cell structure of P. sojae hyphae, which could account for their dead status, as revealed by light microscopy. Based on these microscopic observations, we propose that bacilysin is capable of impairing P. sojae hyphae, which can lead to their death.
Bacilysin protects soybean sprouts from infection by P. sojae.
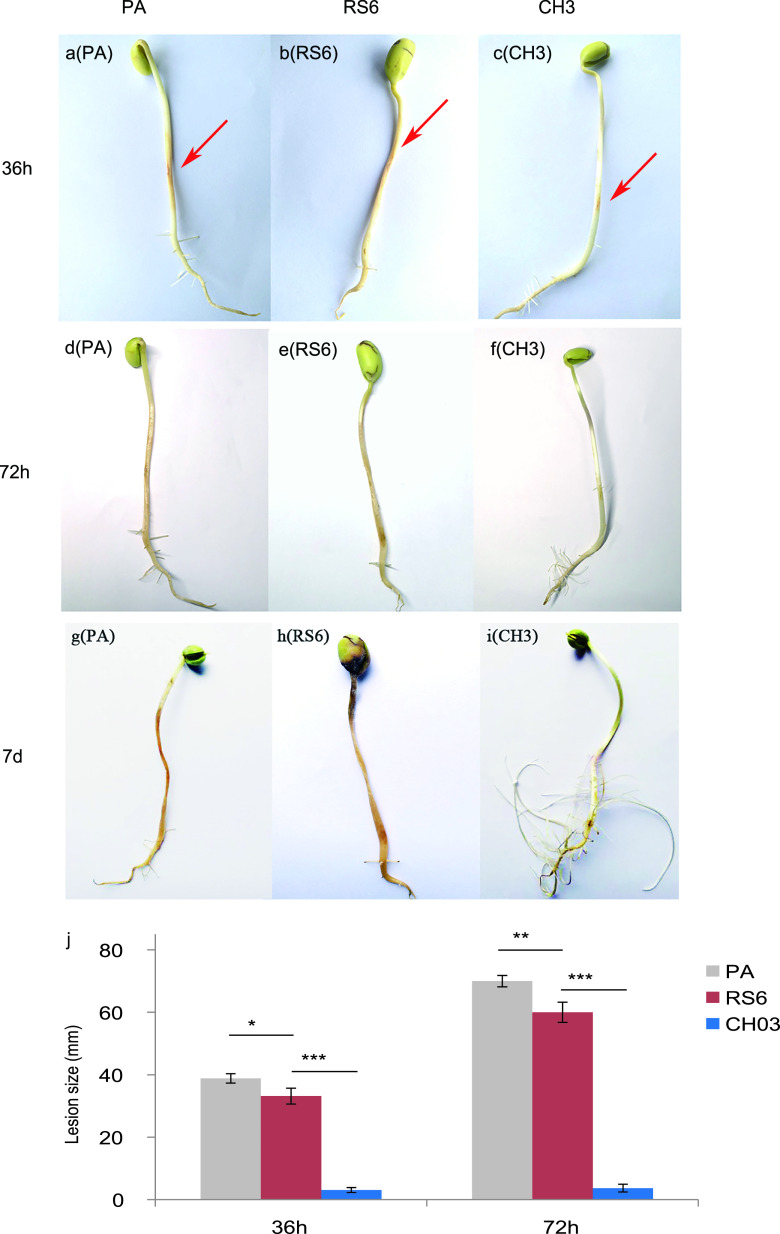
Zoospores of P. sojae P6497 were inoculated onto surfaces of soybean sprouts (soybean seedling hypocotyls) and observed for lesion formation over a 36-h period. The sprouts in the control group pretreated with sterile PA medium or with filtered RS6 supernatant showed obvious brown lesions 36 h after inoculation (Fig. 5a and b). By comparison, the sprouts pretreated with filtered CH3 supernatant showed only a slight yellow color within a much smaller zone (Fig. 5c). Statistical calculations revealed that the size of the lesions in the control groups (PA and RS6) was significantly larger (P < 0.01) than that in the group treated with bacilysin-containing CH3 supernatant (Fig. 5j).
FIG 5.
Effect of the bacilysin-containing supernatant on lesion formation on soybean sprouts infected by P. sojae. The sprouts were treated with PA medium (a, d, and g), RS6 supernatants (b, e, and h), and CH3 supernatants (c, f, and i) before they were infected with P. sojae. Representative images were taken 36 h (a to c), 72 h (d to f), and 7 days (g to i) after the infection. The arrows indicate the locations of P. sojae zoospore inoculation. (j) Statistics on the inhibition effect of bacilysin-containing supernatants on lesion formation on soybean sprouts infected by P. sojae (n = 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
After 72 h, the lesions in the P. sojae-infected bean sprouts pretreated with PA or RS6 increased and became more discolored (Fig. 5d and e). In contrast, the lesions on the sprouts pretreated with CH3 had no significant change and were still restricted to the original region. The sprouts treated with CH3 looked healthy and white and continued growing with cotyledons slightly stretched with dozens of newly formed fibrous roots (Fig. 5f). The quantitative analysis showed that the lesion size of the sprouts treated with CH3 was significantly smaller (P < 0.01) than that of sprouts treated with PA or RS6 (Fig. 5j).
By 7 days, sprout roots pretreated with PA or RS6 turned dark brown or completely black, and their cotyledons were partially infected (Fig. 5g and h). In contrast, the sprouts in the CH3 group were healthy with plenty of new lateral roots. The original lesion region began to fade, suggesting that the infection was cured (Fig. 5i). Combining all results, we concluded that the FZB42 culture containing bacilysin can effectively protect soybean sprouts from infection by P. sojae.
The transcriptome of P. sojae was affected when challenged with FZB42.
Since interactions between P. sojae and FZB42 in soil mostly occur on a solid surface, we designed a sieve-based device (see Fig. S1a and b in the supplemental material) to simulate this situation. In this device, P. sojae was precultured for 5 days on the side with V8 medium agar, reaching a diameter of 60 to 70 mm, before FZB42 was spread on the other side, which had Landy medium agar. The two sides were separated by a 500-mesh sieve, allowing an exchange of metabolites. This device allowed us to investigate the interplay of two microorganisms in more real and homogeneous conditions. After FZB42 inoculation, the growth of P. sojae almost ceased within 48 h (Fig. S1d and f), while P. sojae hyphae in the controls without FZB42 continued to grow to the edge of the boxes (Fig. S1c and e). There was no noticeable difference in the appearance of P. sojae between the treatments and controls, suggesting that FZB42 inhibited the growth of P. sojae rather than altering developmental responses, most likely by producing metabolites that could diffuse across the agar layers and the sieve.
Total RNAs of P. sojae from three treatments (PS42_1, PS42_2, and PS42_3) and three controls (PSCK_1, PSCK_2, and PSCK_3) grown on the device described above were isolated for Illumina HiSeq sequencing. The analyses showed that all sequencing data were of good quality (Q20 ≥ 93%) (Table S1 and Fig. S2). The number of clean reads (passing-filter reads) in each group varied from 5,072,091,304 to 8,893,846,008 with GC content between 32.9 and 49.2% (Table S1). The reads were mapped to the genome of P. sojae P6497 in the JGI database (12). Bacterial genes were detected in the PSCK_1 sample, indicating possible bacterial contamination, and therefore excluded from the analysis (Fig. S2).
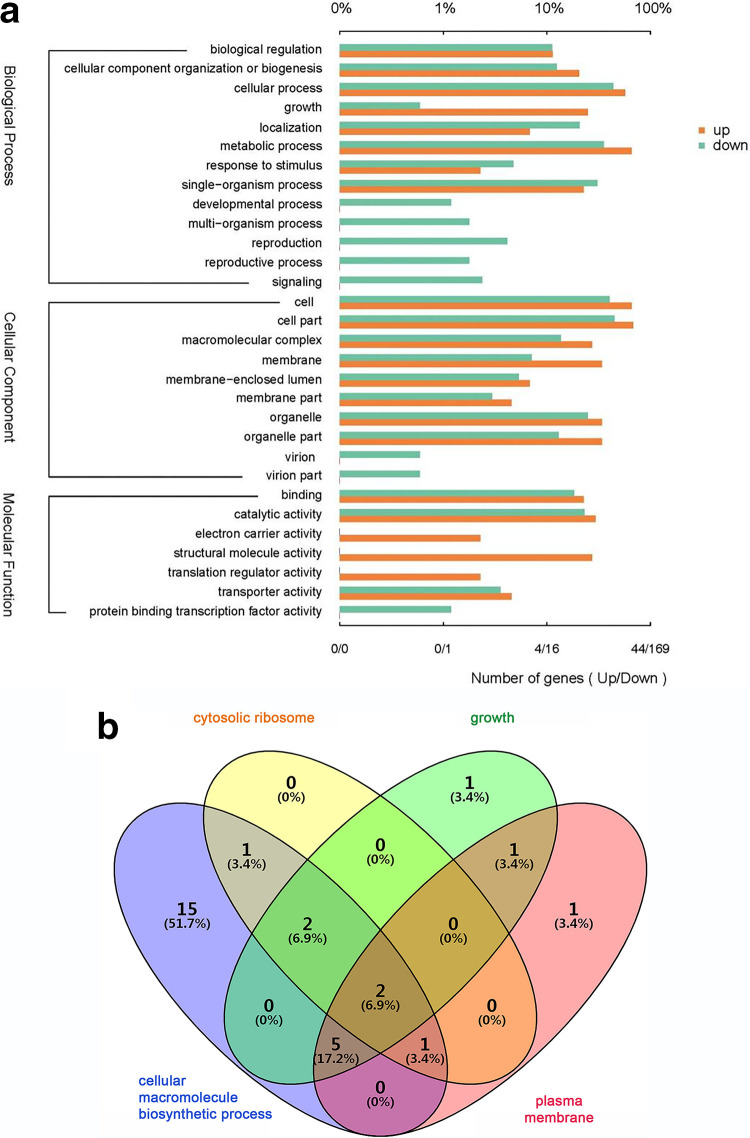
The number of downregulated genes (2,691) was more than three times higher than that of upregulated genes (642), indicating that FZB42 essentially inhibited the expression of P. sojae genes (Table S2 and Fig. S3). Gene ontology (GO) functional annotation of the differentially expressed genes (DEGs) showed that 1,840 genes, accounting for 55.19% of the total, are involved in biological processes (BPs); 1,257 (37.7%) DEGs are involved in cellular components (CCs); and only 237 (7.11%) genes are involved in molecular functions (MFs) (Fig. 6a). It is interesting that there were only downregulated genes but no upregulated genes present in the subgroups for developmental processes, multiorganism processes, reproduction, and signaling within the BP group (Fig. 6a), suggesting a repression of these processes upon confrontation with FZB42.
FIG 6.
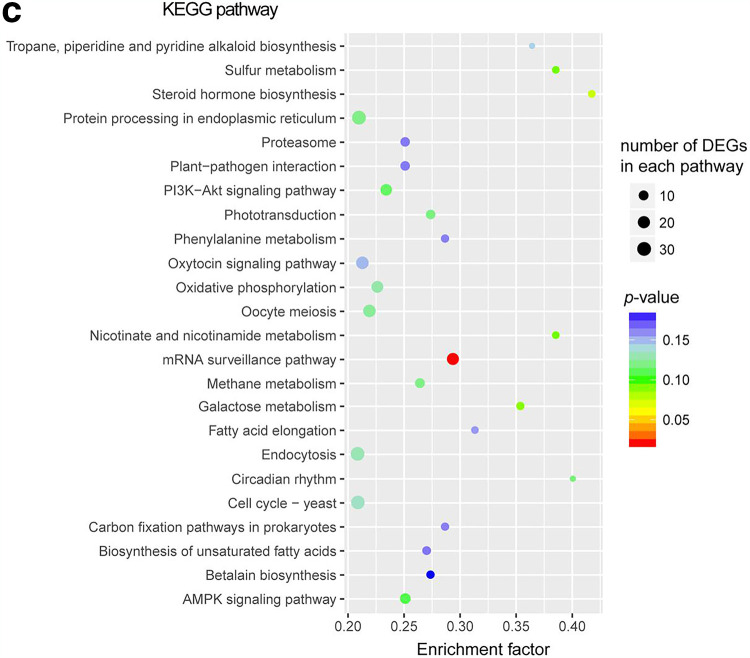
GO annotation and enrichment analysis for the DEGs of P. sojae when challenged with B. velezensis FZB42. (a) GO annotation analysis for the DEGs of P. sojae when challenged with B. velezensis FZB42. The top x axis represents the percentage of genes annotated to a GO term relative to the total number of GO-annotated genes, while the bottom x axis indicates the number of genes annotated to a GO term. (b) Venn diagram showing the relation between the numbers of DEGs in the four most enriched GO terms (growth, cellular macromolecule biosynthetic process, plasma membrane and cytosolic ribosome). (c) Enriched KEGG pathways for the DEGs. The size of the dots is proportional to the number of DEGs. The color spectrum from blue to red represents different P values.
GO enrichment analysis showed that 162 and 109 DEGs were significantly enriched in the BP and CC groups, respectively (Table S3). Growth (GO:0040007) and cellular macromolecule biosynthetic process (GO:0034645) were the two most enriched subgroups in BP, while plasma membrane (GO:0005886) and cytosolic ribosome (GO:0022626) were the two most enriched subgroups in CC (Fig. S4). The results imply that FZB42 can significantly affect the physiological functions and metabolism of P. sojae. A large portion of the genes in the four subgroups overlapped, most of which were ribosomal proteins or mitochondrial ribosomal proteins (Fig. 6b and Tables S2 and S3), such as small subunit ribosomal proteins S4, S12, and S13 and large subunit ribosomal proteins L3, L5, and L6. This result implies that ribosomal proteins, and thus translational processes, are important targets inhibited by FZB42.
Furthermore, we performed KEGG pathway enrichment analysis for the DEGs (Fig. 6c). The genes were mapped to 267 KEGG pathways but significantly enriched only in the mRNA surveillance pathway when taking a P value of <0.05 as the threshold (Fig. 6c; Fig. S5). The mRNA surveillance pathway is a quality control mechanism that detects and degrades abnormal mRNAs to ensure the fidelity and quality of mRNA molecules. More specifically, the genes for cleavage and polyadenylation specificity factors (CPSF), involved in the 3′ end processing machinery of a newly synthesized pre-mRNA, were significantly altered in expression (Fig. S5). Downregulation of many genes in the mRNA surveillance pathway implies a potential dysfunction of the quality control machinery of P. sojae mRNA synthesis.
qPCR verified the downregulation of pathogenicity-related genes of P. sojae.
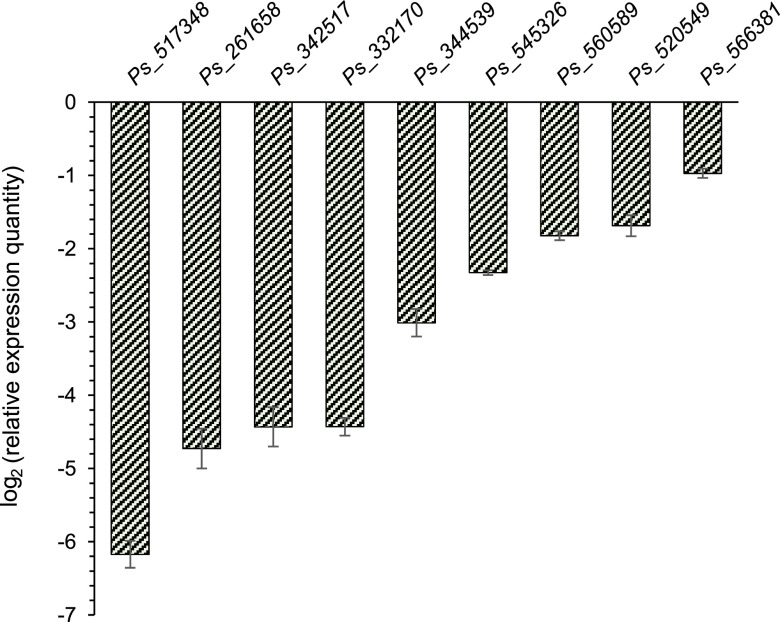
To examine the reliability of the transcriptome results, we selected nine genes of interest for validation by quantitative PCR (qPCR) (Table 2). Expression or upregulation of these genes has been connected with the pathogenic process caused by P. sojae or molds in previous reports (33–42). B. velezensis FZB42 lowered the expression of these genes (Fig. 7), suggesting that FZB42 can antagonize the pathogenic process of P. sojae. Pectate lyase (PL) plays an important role in the invasion of plants by pathogenic fungi (33). Remarkably, repression of the gene for PL (ps_517348) was highest (fold change, >60) among all differentially expressed genes. In addition, there were another four PL genes and four putative PL genes that were downregulated (Table S2). The strong downregulation of so many PL or putative PL genes suggests that FZB42 is perhaps able to directly hinder the invasion by P. sojae of host plants, while all pathogenicity-related genes were confirmed, indicating a reduced level of pathogenicity of P. sojae after FZB42 treatment.
TABLE 2.
Differentially expressed genes of interest selected from the transcriptome data for qPCR verification
| Gene | Gene product | P value | Fold change |
|---|---|---|---|
| ps_517348 | Pectate lyase | 0.0104 | −36.8 |
| ps_261658 | Vesicular GABA transporter | 0.00145 | −19.7 |
| ps_342517 | Glucan 1,3-beta-glucosidase | 5.00E−05 | −17.1 |
| ps_332170 | Glycoside hydrolase family 5 protein | 5.00E−05 | −16.0 |
| ps_560589 | CMGC/MAPK protein kinase | 5.00E−05 | −2.6 |
| ps_566381 | Heat shock transcription factor | 0.00015 | −2.1 |
| ps_ 520549 | Calmodulin | 5.00E−05 | −2.8 |
| ps_ 545326 | Vacuolar calcium ion transporter | 5.00E−05 | −3.5 |
| ps_ 344539 | MFS transporter | 5.00E−05 | −3.0 |
FIG 7.
qPCR verification of the DEGs of P. sojae detected by the transcriptome sequencing. The genes are ps_517348 for pectate lyase, ps_261658 for vesicular GABA transporter, ps_342517 for glucan 1,3-beta-glucosidase, ps_332170 for glycoside hydrolase family 5 protein (GHF5), ps_560589 for CMGC/MAPK protein kinase, ps_566381 for heat shock transcription factor, ps_520549 calmodulin, ps_545326 for vacuolar calcium ion transporter, and ps_344539 for MFS transporter. The actin gene was used as an internal control.
DISCUSSION
Due to the ability to produce heat-resistant endospores and a broad spectrum of antimicrobial secondary metabolites, Bacillus velezensis is the bacterial species that has become the most successfully commercialized biocontrol agent. However, its action on Phytophthora remains largely unknown. In this study, we used a representative strain of B. velezensis, FZB42, to study its relationship with Phytophthora. Our results demonstrate that FZB42 has antagonistic activity against P. sojae and that the activity is mainly mediated by bacilysin, a dipeptide antibiotic. Bacilysin damages P. sojae mycelium, affecting its cell integrity and leading to hyphal death. FZB42 inhibits the expression of a large number of Phytophthora genes, many of which are involved in plant infection. The application of FZB42 could protect soybean sprouts from Phytophthora infection. We also show that FZB42 can efficiently inhibit the growth of different species of Phytophthora in the laboratory, suggesting the promising biocontrol potential of B. velezensis strains against Phytophthora disease.
To date, FZB42 is known to be capable of producing 13 antimicrobial compounds, including three polyketides (bacillaene, difficidin, and macrolactin), several lipopeptides (surfactin, bacillomycin D, and fengycin) and bacteriocins (amylocyclicin and plantazolicin) (22, 25). These compounds can antagonize the growth of a wide range of phytopathogens, including fungi and bacteria (22, 27, 28, 43–45). Bacilysin, another antibiotic produced by FZB42, is a rather simple dipeptide composed of only one l-alanine and one unusual amino acid called anticapsin (30, 46). In previous studies, bacilysin was found to suppress various bacteria because it inhibits glucosamine synthetase, thus interfering with bacterial peptidoglycan biosynthesis (47). In contrast to true fungi, which have chitin as a major cell wall component, the cell walls of oomycetes consist primarily of glucans, such as β-(1→3)-d-glucans, β-(1→6)-d-glucans, and cellulose; only a small amount of chitin is present in the cell walls of oomycetes (48–50). The reason why bacilysin inhibits Phytophthora growth is perhaps because it interferes with the biosynthesis of glucans using a mechanism similar to the one it uses to interfere with bacterial peptidoglycan biosynthesis. It was shown recently that among 22 Bacillus species strains positive for bacilysin-related genes detected by PCR, 14 were very effective against P. infestans; that is, the genes for bacilysin biosynthesis were correlated with the strong antagonism of the Bacillus strains toward P. infestans (32). This is obviously consistent with the results here. The greenhouse assays and field trials performed in that report showed that the Bacillus strains significantly decreased late blight severity, exemplifying the promising use of bacilysin-producing bacteria in protecting plants from oomycete pathogens.
Although we showed inhibition of P. sojae by bacilysin, we were not able to exclude additional mechanisms for suppressing Phytophthora infection. For example, the P. sojae transcriptome data showed that more than 3,000 genes were altered in transcription by FZB42, which may not all be related to bacilysin.
Among the regulated genes discovered in the transcriptome data, the most attractive ones are those for pectate lyase. Pectin is a fundamental plant cell wall structural polysaccharide. In the process of infecting plants, Phytophthora secretes pectin lyase to degrade plant cell walls to assist itself in entering plant cells (33). The downregulation of a total of eight genes for pectate lyase with a high fold change suggests an additional mechanism of FZB42’s protective effect; i.e., it may directly lower the pathogenicity of P. sojae to host plants. Similarly, five genes for glycoside hydrolase family 5 proteins (GHF5), important components of Phytophthora cell walls and involved in plant infection (50), were downregulated. It is unknown whether bacilysin is involved in this action.
Two other regulated genes, encoding mitogen-activated protein kinase (MAPK) and cyclic AMP (cAMP)-dependent protein kinase (PKA), are also noteworthy. Both enzymes play critical roles in the oocyte meiosis pathway (Fig. S4b) and in signal transduction. The downregulation of the two genes indicated a compromised status of P. sojae proliferation. MAPK is highly conserved in all eukaryotes. There are 14 MAPK genes in P. sojae, among which PsSAK1 is involved in the regulation of P. sojae cell integrity, zoospore formation, hyphal growth, and pathogenicity (51, 52). In general, the MAPKs of P. sojae play an important role in its growth and development and infection cycles (53). PKA is also a highly conserved regulator whose targets involve various enzymes/proteins or transcriptional regulators in response to various stresses (54–56). Loss of a subunit of PKA can lead to a decrease in spore survival and hyphal extension of Aspergillus fumigatus (57).
Other regulated genes related to P. sojae pathogenicity include the ones for heat shock factor (HSF), cell wall protein GP42, GABA (γ-aminobutyric acid) transporters and MFS (major facilitator superfamily) transporters. Ubiquitous in animals, plants, fungi, and other organisms, HSFs are end components of signaling pathways responding to diverse stresses, such as heat shock, oxidative stress, and chemical stimulation (37–39). HSF1 can regulate the expression of heat shock proteins and repair intracellular protein damage caused by external pressure (40). P. sojae PsHSF1 was reported to participate in the response to oxidative stress applied by reactive oxygen species in the plant defense process (41). The P. sojae cell wall protein GP42 is a Ca2+-dependent glutamine transaminase (TGase). The short peptide of its C terminus (EPE-13) is highly conserved and able to trigger pattern-triggered immunity (PTI) in plants, which has been shown to activate the defense response of parsley and potato (58, 59). Reiss et al. proposed using GP42 as a target to develop new agents that can inhibit the growth of oomycetes and reduce their pathogenicity (60). Here, the expression of four GP42 genes was downregulated 16-fold by FZB42, suggesting that one or more FZB42 metabolites can inhibit GP42 expression, which supports the idea that interfering with GP42 expression is a good option.
In summary, the reduced expression of the genes above clearly indicates reduced pathogenicity of P. sojae in the presence of B. velezensis FZB42. Together with the evidence that FZB42 can inhibit P. sojae growth due to bacilysin production, this study favors the use of B. velezensis FZB42 and other Bacillus strains able to produce bacilysin for biocontrol of Phytophthora-caused plant diseases of soybean and other plants. However, although the primary mechanisms of the inhibitory effect of B. velezensis on Phytophthora were elucidated in this study, many questions remain unanswered. For example, we suspect that bacilysin may not be the only factor that affects Phytophthora growth and pathogenicity according to the transcriptome data. In this respect, we are only beginning to understand their interaction and possible application.
MATERIALS AND METHODS
Strains and growth conditions.
All bacteria and oomycete strains used as well as their sources are listed in Table 1. Deletion mutants of FZB42 were generally constructed by insertion of an antibiotic cassette in place of the genes noted. As described previously (26), standard growth in Luria-Bertani (LB) broth at 37°C was used for FZB42 wild type and its derivative mutants except where otherwise indicated. Landy medium was used as described in a previous report (27). For the production of bacilysin, PA medium was used with shaking at 165 rpm and 29°C for 24 h (27). While P. infestans was cultured in rye grain agar (61), all other Phytophthora species were cultured in vegetable juice (V8) agar plates (10% [vol/vol] V8 juice, 0.1% CaCO3, 0.8% agar). The following antibiotics were supplemented where appropriate: erythromycin (5 μg/ml), chloramphenicol (5 μg/ml), and spectinomycin (100 μg/ml).
Preparation and assay of bacilysin.
The mutant CH3 was incubated in PA medium at 28°C and 175 rpm for 24 h before the culture was centrifuged at 6,000 rpm for 30 min to remove bacterial cells. Four volumes of absolute ethanol was added to the supernatant to precipitate impurities. Subsequently, the supernatant was concentrated 50-fold before being subjected to XAD1600N resin (Amberlite, Rohm & Haas, USA) separation. The eluate was passed through SP207 resin (Sepabeads; Mitsubishi Chemical Corporation, Japan) and washed with 3 bed volumes (BV) of water. Then, the resin column was eluted with 3 BV of 10% ethanol at a rate of 0.5 BV/h. The eluate was lyophilized to dryness for use. High-performance liquid chromatography (HPLC) analysis was performed with a Syncronis HILIC (150 by 4.6 mm) column using acetonitrile-isopropanol-water (70:5:25) as the mobile phase at a flow rate of 1 ml/min. By monitoring the absorbance values at 230 nm and 205 nm, the molecule in the peak at the retention time of 4.707 min was collected.
The collected bacilysin was further identified with HPLC-mass spectrometry (HPLC-MS). The sample was injected into Hypersil GOLD (100*2.1 mm) at a flow rate of 0.4 ml/min, using 5% acetonitrile as the mobile phase. Molecules with an m/z range from 100 to 400 Da were detected. The bacilysin sensitive Staphylococcus aureus subsp. aureus ATCC 9144 was used to verify bacilysin activity as previously described (29, 62).
Antagonism test.
Four Oxford cups were evenly placed, 30 mm from the middle point, on a plate prepared with 10 ml of V8 agar. Then, another 10 ml of V8 agar was poured onto the plate to fix the Oxford cups. An agar stub 6 mm in diameter with P. sojae mycelium was punched out and placed in the middle of the V8 plates with Oxford cups, which were subsequently filled with 0.25 ml of Landy medium agar. Three microliters of bacterial cultures (OD600 = 1) grown in Landy medium was inoculated into Oxford cups. Each treatment was set up in triplicate and incubated at 25°C in the dark. Sterile Landy medium was added to the cups as a control. When P. sojae colonies in the blank group grew to the edge of the plates, the diameter of the colonies was measured. Inhibition rate was calculated as (1 − colony diameter of P. sojae in the treatment/colony diameter of P. sojae in the control) × 100. Similar tests were performed with pure bacilysin. After P. sojae was grown for 4 days, 30 μl bacilysin solution at different concentrations was filled in the holes beside the P. sojae colony in the middle. Sterile Millipore pure water was used as the control. The results were photographed 2 days after the addition of the bacilysin solution.
Viability staining and light microscopy.
CH3 and RS6 culture filtrates were prepared as follows. A single colony of the mutants was transferred from an LB plate to 10 ml PA medium. After overnight incubation, 2 ml of the precultures was inoculated into 200 ml PA medium and shaken at 165 rpm and 29°C for 24 h. Then, the cultures were collected and centrifuged at 12,000 rpm for 10 min. The supernatants were filtered with 0.22-μm sterile films to prepare culture filtrates. P. sojae was cultured for 2 days in liquid V8 medium before the hyphae were collected and washed 2 or 3 times with sterile PBS buffer. The washed hyphae were soaked in 200 μl of CH3 and RS6 culture filtrates for 2 h. An equal volume of PA medium was used as the control to soak the hyphae. Three repetitions were done for each treatment. The treated hyphae were picked out and then stained with 0.05% Evans blue for another 2 h before being washed with sterile deionized water. A Zeiss microscope was used to visualize the stained hyphae. Three replicates were carried out for each sample. A total of 90 images were taken and analyzed.
TEM.
P. sojae hyphae were prepared and treated as described for light microscopy. The treated hyphae were fixed in a 2.5% glutaraldehyde solution at 4°C for 3 days and then rinsed with 100 mM phosphate buffer three times for 10 min each time. The rinsed hyphae were fixed in 1% osmic acid solution for 1 to 2 h and then rinsed with 100 mM phosphate buffer three times. Subsequently, the samples were dehydrated with ethanol solutions of different concentrations (50%, 70%, 80%, 90%, and 95%) for 15 min each and then treated with 100% ethanol twice, each for 20 min. Finally, the samples were embedded in a mixture of the embedding resin and acetone. The embedded sample was sliced with a Reichert ultramicrotome and observed with TEM (JEM-1230; JEOL, Japan). Three replicates were carried out for each sample. A total of 154 images were taken and analyzed.
SEM.
P. sojae was grown on V8 medium agar for 3 days before the hyphae in a region of 5 mm by 5 mm were taken to be soaked in the supernatants as described above. The treated hyphae were fixed in a 2.5% glutaraldehyde solution at 3°C for 3 days. The next fixation procedures were the same as for TEM. Finally, the hyphae were dehydrated as described above for TEM. After treatment with a mixture of isoamyl acetate-ethanol (1:1 [vol/vol]) for 30 min and then with pure isoamyl acetate for 2 h, the samples were sprayed with gold on the surface before being subjected to scanning electron microscopy (Quanta 2000; FEI, USA). Three replicates were carried out for each sample. A total of 30 images was taken and analyzed.
P. sojae and B. velezensis FZB42 interaction.
A round box-like device was manufactured for interaction between phytopathogens and biocontrol bacteria (Fig. S1). The box was made of stainless steel, 95 mm in diameter and 73 mm in height. The box was open at both ends but separated in the middle into two compartments by a 500-mesh sieve, which allows microbial metabolites to easily pass through. After autoclaving, V8 agar was poured on one side of the sieve at a low temperature. After V8 agar solidified, Landy medium agar was poured on the other side. When the double-sided plate was prepared, glass paper (cellophane paper) was mounted on the V8 agar side, and then P. sojae was inoculated in the middle. After 5 days of incubation in the dark, 500 μl of B. velezensis culture, which was obtained by growing FZB42 in Landy medium until the OD600 reached 1.0 and then diluted 10-fold with fresh Landy medium, was spread on the other side. The same volume of diluted LB was spread as the control. Finally, all P. sojae mycelium on the glass paper was harvested for RNA isolation after 48 h.
Biocontrol test with soybean sprouts.
Seeds of the soybean variety “Hefeng 47,” which are sensitive to P. sojae, were disinfected (63) and then planted in pots containing sterile vermiculite. The pots were incubated at 25°C in the dark to cultivate bean sprouts (chlorosis seedlings), which were then used when they were approximately 6 cm in length. Roots of the sprouts were rinsed with sterile water to remove vermiculite adhering to their surface. After drying, the roots were soaked in filtered supernatants, as prepared above, of CH3 or RS6 for 10 min. PA medium was used as a control treatment. The soaked seedlings were taken out and air dried before they were inoculated with P. sojae zoospores at the hypocotyledonary axis (64). The inoculated seedlings were placed on autoclaved filter paper in petri dishes containing 1 ml sterile water to maintain humidity. The dishes were kept at 25°C, and the length of the lesions was measured with a ruler over time. Five seedlings were assayed for each treatment. The assays were performed twice to determine their reproducibility.
Transcriptome sequencing.
RNA extraction of P. sojae was performed using the TRIzol method (65). DNA residues were digested according to the instructions of the TaKaRa RR07A DNA digestion kit. After digestion, 25 to 50 μg RNA of each sample was sent to the transcriptome sequencing pipeline. cDNA was prepared according to the instructions of the TaKaRa PrimeScript RT master mix kit. RNA sequencing was performed at Shanghai Biozeron Technology Co., Ltd. In general, cDNA libraries were constructed using the Illumina TruSeq RNA sample prep kit. RNA sequencing was performed with the Illumina HiSeq sequencing platform. Sequencing saturation and coverage were analyzed with RSeQC software (http://code.google.com/p/rseqc/) for quality control. Expression levels of each transcript were calculated using the FPKM (fragments per kilobase of exon per million reads mapped) method with Cuffdiff software (http://cufflinks.cbcb.umd.edu/). Genes with a false discovery rate (FDR) of ≤0.05 and a fold change of ≥2 were regarded as differentially expressed genes (DEGs). Fisher’s exact test integrated in GOatools (https://github.com/tanghaibao/GOatools) was used for GO enrichment analysis. The enrichment factor (that is, the ratio of the number of DEGs enriched in a GO term to the number of all genes in this GO term), was calculated. Four multiple tests (Bonferroni, Holm, Šidák, and FDR) were used to correct the P value to control the false-positive rate. KOBAS (http://kobas.cbi.pku.edu.cn/home.do) was used for KEGG pathway enrichment analysis. The enrichment factor was calculated as the ratio of DEGs enriched in a KEGG pathway to the number of all genes in this KEGG pathway. The Benjamini-Hochberg procedure was used for the FDR test. When the adjusted P value was ≤0.05, the GO term or KEGG pathway was regarded as significantly enriched. The results of the GO enrichment analysis are shown by a directed acyclic graph (DAG) (Fig. S4).
qPCR.
Total RNAs were isolated from the samples prepared using the same method for the transcriptome experiment. The TaKaRa fluorescence quantification kit SYBR Premix Ex Taq was used to perform qPCR verification. All qPCR primers were designed using the online tool Primer 3 (https://bioinfo.ut.ee/primer3/) and are listed in Table 3 with their amplification efficiencies. The actin gene was used as an internal control. Reactions were carried out with a StepOnePlus system (ABI, USA). The 2−ΔΔCT method was used to analyze the PCR data (66).
TABLE 3.
Primers
| Gene | Sequences (forward, reverse; 5′ to 3′) | Amplification efficiency (%) |
|---|---|---|
| ps_517348 | CTCGTGACGGTCAACAAGAA, GTGATGGTGCTGGTGGAGTA | 98.7 |
| ps_261658 | AGCGCTCGTACATCCAGAGT, CCAGAACCACAATGATGCAG | 99.1 |
| ps_342517 | CGTCTCCGCGAATTACTTTT, CCAGCGTGTAGAAGTCGTGA | 98.3 |
| ps_332170 | GCTCATGACCAGCAACAAGA, ACCATAGCGGAGTTGGTGTC | 95.3 |
| ps_560589 | AGTACATCCACTCGGCCAAC, TCCTTCGGGGAGTACAAGTG | 97.2 |
| ps_566381 | GGTTGGCGGATTTCTTACAA, CTTCGTCCAAAAATGCCAGT | 99.6 |
| ps_ 520549 | GGGATGACACTGGACGAGTT, AGCTTCGTCTTCCACCAGAA | 97.3 |
| ps_ 545326 | TACCACATGCTCAAGGACCA, GAAGATGGCGACAATCACCT | 93.5 |
| ps_ 344539 | AATGGTTGGGTTCTGAGTGC, CGATTGATTCCGTGATGATG | 95.4 |
Statistics.
Student's t test was used to determine the significance of the difference between the means from two groups with small sample sizes. Analysis of variance (ANOVA) was used to determine the significance of the differences between the means of three or more groups.
Data availability.
Raw transcriptome data of the six samples were deposited in the SRA database (Sequence Read Archive, NCBI) with the accession numbers SRR14494445, SRR14494779, SRR14494778, SRR14513876, SRR14521120, and SRR14521338.
ACKNOWLEDGMENTS
We thank Dong Suomeng at Nanjing Agricultural University for providing important advice and reviewing the manuscript.
This work was supported by the National Natural Science Foundation of China (no. 31970097) and the Key Scientific Project for Jiangsu Provincial Universities (17KJA220001).
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Ben Fan, Email: fanben2000@gmail.com.
Gladys Alexandre, University of Tennessee at Knoxville.
REFERENCES
- 1.Kroon L, Brouwer H, de Cock A, Govers F. 2012. The genus Phytophthora Anno 2012. Phytopathology 102:348–364. 10.1094/PHYTO-01-11-0025. [DOI] [PubMed] [Google Scholar]
- 2.Haverkort A, Boonekamp P, Hutten R, Jacobsen E, Lotz L, Kessel G, Visser R, Van der Vossen E. 2008. Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Potato Res 51:47–57. 10.1007/s11540-008-9089-y. [DOI] [Google Scholar]
- 3.Tyler BM. 2007. Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8:1–8. 10.1111/j.1364-3703.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang YC, Zhang WL, Wang Y, Zheng XB. 2006. Rapid and sensitive detection of Phytophthora sojae in soil and infected soybeans by species-specific polymerase chain reaction assays. Phytopathology 96:1315–1321. 10.1094/PHYTO-96-1315. [DOI] [PubMed] [Google Scholar]
- 5.Mirabolfathy M, Cooke DEL, Duncan JM, Williams NA, Ershad D, Alizadeh A. 2001. Phytophthora pistaciae sp nov and P-melonis: the principal causes of pistachio gummosis in Iran. Mycological Res 105:1166–1175. 10.1016/S0953-7562(08)61987-5. [DOI] [Google Scholar]
- 6.Hausbeck MK, Lamour KH. 2004. Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Dis 88:1292–1303. 10.1094/PDIS.2004.88.12.1292. [DOI] [PubMed] [Google Scholar]
- 7.Anderson P, Brundrett M, Grierson P, Robinson R. 2010. Impact of severe forest dieback caused by Phytophthora cinnamomi on macrofungal diversity in the northern jarrah forest of Western Australia. Forest Ecol Manage 259:1033–1040. 10.1016/j.foreco.2009.12.015. [DOI] [Google Scholar]
- 8.CABI. 2019. Phytophthora cinnamomi (Phytophthora dieback). Invasive species compendium. https://www.cabi.org/isc/datasheet/40957.
- 9.Guo H, Li CP, Shi T, Fan CJ, Huang GX. 2012. First report of Phytophthora palmivora causing root rot of cassava in China. Plant Dis 96:1072–1072. 10.1094/PDIS-09-11-0780-PDN. [DOI] [PubMed] [Google Scholar]
- 10.Vien NV, Benyon FHL, Trung HM, Summerell BA, Van NK, Burgess LW. 2001. First record of Peronophythora litchii on litchi fruit in Vietnam. Austral Plant Pathol 30:287–288. 10.1071/AP01034. [DOI] [Google Scholar]
- 11.Ruggiero MA, Gordon DP, Orrell TM, Bailly N, Bourgoin T, Brusca RC, Cavalier-Smith T, Guiry MD, Kirk PM. 2015. A higher level classification of all living organisms. PLoS One 10:e0119248. 10.1371/journal.pone.0119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyler BM, Tripathy S, Zhang X, Dehal P, Jiang RHY, Aerts A, Arredondo FD, Baxter L, Bensasson D, Beynon JL, Chapman J, Damasceno CMB, Dorrance AE, Dou D, Dickerman AW, Dubchak IL, Garbelotto M, Gijzen M, Gordon SG, Govers F, Grunwald NJ, Huang W, Ivors KL, Jones RW, Kamoun S, Krampis K, Lamour KH, Lee M-K, McDonald WH, Medina M, Meijer HJG, Nordberg EK, Maclean DJ, Ospina-Giraldo MD, Morris PF, Phuntumart V, Putnam NH, Rash S, Rose JKC, Sakihama Y, Salamov AA, Savidor A, Scheuring CF, Smith BM, Sobral BWS, Terry A, Torto-Alalibo TA, Win J, Xu Z, Zhang H, et al. 2006. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313:1261–1266. 10.1126/science.1128796. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto T, Watanabe K, Yoshida S, Aino M, Furiki M, Shiono M, Matoh T, Biggs AR. 2010. Field application of calcium to reduce phytophthora stem rot of soybean, and calcium distribution in plants. Plant Dis 94:812–819. 10.1094/PDIS-94-7-0812. [DOI] [PubMed] [Google Scholar]
- 14.Demirbas A, Rector BG, Lohnes DG, Fioritto RJ, Graef GL, Cregan PB, Shoemaker RC, Specht JE. 2001. Simple sequence repeat markers linked to the soybean Rps genes for Phytophthora resistance. Crop Sci 41:1220–1227. 10.2135/cropsci2001.4141220x. [DOI] [Google Scholar]
- 15.Burnham KD, Dorrance AE, VanToai TT, Martin SKS. 2003. Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci 43:1610–1617. 10.2135/cropsci2003.1610. [DOI] [Google Scholar]
- 16.Lamour KH, Finley L, Hurtado-Gonzales O, Gobena D, Tierney M, Meijer HJG. 2006. Targeted gene mutation in Phytophthora spp. Mol Plant Microbe Interact 19:1359–1367. 10.1094/MPMI-19-1359. [DOI] [PubMed] [Google Scholar]
- 17.Xiao K, Kinkel LL, Samac DA. 2002. Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol Control 23:285–295. 10.1006/bcon.2001.1015. [DOI] [Google Scholar]
- 18.Ezziyyani M, Requena ME, Egea-Gilabert C, Candela ME. 2007. Biological control of Phytophthora root rot of pepper using Trichoderma harzianum and Streptomyces rochei in combination. J Phytopathol 155:342–349. 10.1111/j.1439-0434.2007.01237.x. [DOI] [Google Scholar]
- 19.Smith V, Wilcox W, Harman G. 1990. Potential for biological control of Phytophthora root and crown rots of apple by Trichoderma and Gliocladium spp. Phytopathology 80:880–885. 10.1094/Phyto-80-880. [DOI] [Google Scholar]
- 20.Lee KJ, Kamala-Kannan S, Sub HS, Seong CK, Lee GW. 2008. Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using. World J Microbiol Biotechnol 24:1139–1145. 10.1007/s11274-007-9585-2. [DOI] [Google Scholar]
- 21.Liu D, Li K, Hu J, Wang W, Liu X, Gao Z. 2019. Biocontrol and action mechanism of Bacillus amyloliquefaciens and Bacillus subtilis in soybean Phytophthora blight. Int J Mol Sci 20:2908. 10.3390/ijms20122908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury SP, Hartmann A, Gao XW, Borriss R. 2015. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42. Front Microbiol 6:780. 10.3389/fmicb.2015.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan B, Wang C, Song XF, Ding XL, Wu LM, Wu HJ, Gao XW, Borriss R. 2018. Bacillus velezensis FZB42 in 2018: the Gram-positive model strain for plant growth promotion and biocontrol. Front Microbiol 9:2491. 10.3389/fmicb.2018.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan B, Blom J, Klenk H-P, Borriss R. 2017. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front Microbiol 8:22. 10.3389/fmicb.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Sussmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R. 2007. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014. 10.1038/nbt1325. [DOI] [PubMed] [Google Scholar]
- 26.Koumoutsi A, Chen XH, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R. 2004. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol 186:1084–1096. 10.1128/JB.186.4.1084-1096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XH, Scholz R, Borriss M, Junge H, Mogel G, Kunz S, Borriss R. 2009. Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol 140:38–44. 10.1016/j.jbiotec.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Wu H, Chen L, Yu X, Borriss R, Gao X. 2015. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci Rep 5:12975. 10.1038/srep12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Wu H, Chen L, Xie S, Zang H, Borriss R, Gao X. 2014. Bacilysin from Bacillus amyloliquefaciens FZB42 has specific bactericidal activity against harmful algal bloom species. Appl Environ Microbiol 80:7512–7520. 10.1128/AEM.02605-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenig M, Abraham EP. 1976. Antimicrobial activities and antagonists of bacilysin and anticapsin. J Gen Microbiol 94:37–45. 10.1099/00221287-94-1-37. [DOI] [PubMed] [Google Scholar]
- 31.Chen XH, Koumoutsi A, Scholz R, Borriss R. 2009. More than anticipated—production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol 16:14–24. 10.1159/000142891. [DOI] [PubMed] [Google Scholar]
- 32.Caulier S, Gillis A, Colau G, Licciardi F, Liepin M, Desoignies N, Modrie P, Legreve A, Mahillon J, Bragard C. 2018. Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front Microbiol 9:143. 10.3389/fmicb.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reignault P, Valette-Collet O, Boccara M. 2007. The importance of fungal pectinolytic enzymes in plant invasion, host adaptability and symptom type. Eur J Plant Pathol 120:1–11. 10.1007/s10658-007-9184-y. [DOI] [Google Scholar]
- 34.Yang KY, Liu YD, Zhang SQ. 2001. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 98:741–746. 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshioka H, Asai S, Yoshioka M, Kobayashi M. 2009. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol Cells 28:321–329. 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- 36.Meijer HJG, van de Vondervoort PJI, Yin QY, de Koster CG, Klis FM, Govers F, de Groot PWJ. 2006. Identification of cell wall-associated proteins from Phytophthora ramorum. Mol Plant Microbe Interact 19:1348–1358. 10.1094/MPMI-19-1348. [DOI] [PubMed] [Google Scholar]
- 37.Jedlicka P, Mortin MA, Wu C. 1997. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J 16:2452–2462. 10.1093/emboj/16.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller G, Mittler R. 2006. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot 98:279–288. 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nover L, Bharti K, Doring P, Mishra SK, Ganguli A, Scharf KD. 2001. Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaper 6:177–189. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandes M, Xiao H, Lis JT. 1994. Fine structure analyses of the Drosophila and Saccharomyces heat shock factor–heat shock element interactions. Nucleic Acids Res 22:167–173. 10.1093/nar/22.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng YT, Wang YL, Meijer HJG, Yang XY, Hua CL, Ye WW, Tao K, Liu XY, Govers F, Wang YC. 2015. The heat shock transcription factor PsHSF1 of Phytophthora sojae is required for oxidative stress tolerance and detoxifying the plant oxidative burst. Environ Microbiol 17:1351–1364. 10.1111/1462-2920.12609. [DOI] [PubMed] [Google Scholar]
- 42.Wang QH, Chen DP, Wu MC, Zhu JD, Jiang C, Xu JR, Liu HQ. 2018. MFS transporters and GABA metabolism are involved in the self-defense against DON in Fusarium graminearum. Front Plant Sci 9:438. 10.3389/fpls.2018.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhury SP, Uhl J, Grosch R, Alqueres S, Pittroff S, Dietel K, Schmitt-Kopplin P, Borriss R, Hartmann A. 2015. Cyclic lipopeptides of Bacillus amyloliquefaciens subsp plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol Plant Microbe Interact 28:984–995. 10.1094/MPMI-03-15-0066-R. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, Shao J, Li B, Yan X, Shen Q, Zhang R. 2013. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl Environ Microbiol 79:808–815. 10.1128/AEM.02645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Zhang R, Wang D, Qiu M, Feng H, Zhang N, Shen Q. 2014. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of Its DegU phosphorylation. Appl Environ Microbiol 80:2941–2950. 10.1128/AEM.03943-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Özcengiz G, Öğülür İ. 2015. Biochemistry, genetics and regulation of bacilysin biosynthesis and its significance more than an antibiotic. N Biotechnol 32:612–619. 10.1016/j.nbt.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Chmara H. 1985. Inhibition of glucosamine synthase by bacilysin and anticapsin. J Gen Microbiol 131:265–271. 10.1099/00221287-131-2-265. [DOI] [PubMed] [Google Scholar]
- 48.Bartnicki-Garcia S. 1968. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol 22:87–108. 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- 49.Bartnicki-Garcia S. 1966. Chemistry of hyphal walls of Phytophthora. J Gen Microbiol 42:57–69. 10.1099/00221287-42-1-57. [DOI] [PubMed] [Google Scholar]
- 50.Grenville-Briggs LJ, Anderson VL, Fugelstad J, Avrova AO, Bouzenzana J, Williams A, Wawra S, Whisson SC, Birch PRJ, Bulone V, van West P. 2008. Cellulose synthesis in Phytophthora infestans is required for normal appressorium formation and successful infection of potato. Plant Cell 20:720–738. 10.1105/tpc.107.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li A, Wang Y, Tao K, Dong S, Huang Q, Dai T, Zheng X, Wang Y. 2010. PsSAK1, a stress-activated MAP kinase of Phytophthora sojae, is required for zoospore viability and infection of soybean. Mol Plant Microbe Interact 23:1022–1031. 10.1094/MPMI-23-8-1022. [DOI] [PubMed] [Google Scholar]
- 52.Zhang M, Lu J, Tao K, Ye W, Li A, Liu X, Kong L, Dong S, Zheng X, Wang Y. 2012. A Myb transcription factor of Phytophthora sojae, regulated by MAP kinase PsSAK1, is required for zoospore development. PLoS One 7:e40246. 10.1371/journal.pone.0040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J, Cao M, Ye W, Li H, Kong L, Zheng X, Wang Y. 2015. PsMPK7, a stress‐associated mitogen‐activated protein kinase (MAPK) in P hytophthora sojae, is required for stress tolerance, reactive oxygenated species detoxification, cyst germination, sexual reproduction and infection of soybean. Mol Plant Pathol 16:61–70. 10.1111/mpp.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinan-Lucarre B, Baiguerie A, Clave C. 2005. Accelerated cell death in Podospora autophagy mutants. Eukaryot Cell 4:1765–1774. 10.1128/EC.4.11.1765-1774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bursch W. 2001. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 8:569–581. 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 56.Veneault-Fourrey C, Barooah M, Egan M, Wakley G, Talbot NJ. 2006. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 312:580–583. 10.1126/science.1124550. [DOI] [PubMed] [Google Scholar]
- 57.Shwab EK, Juvvadi PR, Waitt G, Soderblom EJ, Moseley MA, Nicely NI, Asfaw YG, Steinbach WJ. 2017. A novel phosphoregulatory switch controls the activity and function of the major catalytic subunit of protein kinase A in Aspergillus fumigatus. mBio 8:e02319-16. 10.1128/mBio.02319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brunner F, Rosahl S, Lee J, Rudd JJ, Geiler C, Kauppinen S, Rasmussen G, Scheel D, Nurnberger T. 2002. Pep-13, a plant defense-inducing pathogen-associated pattern from Phytophthora transglutaminases. EMBO J 21:6681–6688. 10.1093/emboj/cdf667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sacks W, Nurnberger T, Hahlbrock K, Scheel D. 1995. Molecular characterization of nucleotide sequences encoding the extracellular glycoprotein elicitor from Phytophthora megasperma. Mol Gen Genet 246:45–55. 10.1007/BF00290132. [DOI] [PubMed] [Google Scholar]
- 60.Reiss K, Kirchner E, Gijzen M, Zocher G, Löffelhardt B, Nürnberger T, Stehle T, Brunner F. 2011. Structural and phylogenetic analyses of the GP42 transglutaminase from Phytophthora sojae reveal an evolutionary relationship between oomycetes and marine Vibrio bacteria. J Biol Chem 286:42585–42593. 10.1074/jbc.M111.290544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deahl K, Goth R, Young R, Sinden S, Gallegly M. 1991. Occurrence of the A 2 mating type of Phytophthora infestans in potato fields in the United States and Canada. Am Potato J 68:717–725. 10.1007/BF02853803. [DOI] [Google Scholar]
- 62.Hilton MD, Alaeddinoglu NG, Demain AL. 1988. Bacillus subtilis mutant deficient in the ability to produce the dipeptide antibiotic bacilysin: isolation and mapping of the mutation. J Bacteriol 170:1018–1020. 10.1128/jb.170.2.1018-1020.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li D, Zhao Z, Huang Y, Lu Z, Yao M, Hao Y, Zhai C, Wang Y. 2013. PsVPS1, a dynamin-related protein, is involved in cyst germination and soybean infection of Phytophthora sojae. PLoS One 8:e58623. 10.1371/journal.pone.0058623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide. APS Press, St. Paul, MN. [Google Scholar]
- 65.Rio DC, Ares M, Jr, Hannon GJ, Nilsen TW. 2010. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc 2010:pdb.prot5439. 10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 66.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholz R, Molohon KJ, Nachtigall J, Vater J, Markley AL, Sussmuth RD, Mitchell DA, Borriss R. 2011. Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J Bacteriol 193:215–224. 10.1128/JB.00784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Connolly MS, Williams N, Heckman CA, Morris PF. 1999. Soybean isoflavones trigger a calcium influx in Phytophthora sojae. Fungal Genet Biol 28:6–11. 10.1006/fgbi.1999.1148. [DOI] [PubMed] [Google Scholar]
- 69.Kamoun S, van West P, de Jong AJ, de Groot KE, Vleeshouwers VG, Govers F. 1997. A gene encoding a protein elicitor of Phytophthora infestans is down-regulated during infection of potato. Mol Plant Microbe Interact 10:13–20. 10.1094/MPMI.1997.10.1.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5, Table S1. Download aem.01601-21-s0001.pdf, PDF file, 0.8 MB (849.2KB, pdf)
Table S2. Download aem.01601-21-s0002.xlsx, XLSX file, 0.4 MB (407.4KB, xlsx)
Table S3. Download aem.01601-21-s0003.xlsx, XLSX file, 0.03 MB (32.9KB, xlsx)
Data Availability Statement
Raw transcriptome data of the six samples were deposited in the SRA database (Sequence Read Archive, NCBI) with the accession numbers SRR14494445, SRR14494779, SRR14494778, SRR14513876, SRR14521120, and SRR14521338.