Abstract
Background:
Posttraumatic stress disorder (PTSD) is a highly disabling condition associated with alterations in multiple neurobiological systems, including increases in inflammatory and sympathetic function, responsible for maintenance of symptoms. Treatment options including medications and psychotherapies have limitations. We previously showed that transcutaneous Vagus Nerve Stimulation (tcVNS) blocks inflammatory (interleukin (IL)-6) responses to stress in PTSD. The purpose of this study was to assess the effects of tcVNS on PTSD symptoms and inflammatory responses to stress.
Methods:
Twenty patients with PTSD were randomized to double blind active tcVNS (N=9) or sham (N=11) stimulation in conjunction with exposure to personalized traumatic scripts immediately followed by active or sham tcVNS and measurement of IL-6 and other biomarkers of inflammation. Patients then self administered active or sham tcVNS twice daily for three months. PTSD symptoms were measured with the PTSD Checklist (PCL) and the Clinician Administered PTSD Scale (CAPS), clinical improvement with the Clinical Global Index (CGI) and anxiety with the Hamilton Anxiety Scale (Ham-A) at baseline and one-month intervals followed by a repeat of measurement of biomarkers with traumatic scripts. After three months patients self treated with twice daily open label active tcVNS for another three months followed by assessment with the CGI.
Results:
Traumatic scripts increased IL-6 in PTSD patients, an effect that was blocked by tcVNS (p<.05). Active tcVNS treatment for three months resulted in a 31% greater reduction in PTSD symptoms compared to sham treatment as measured by the PCL (p=0.013) as well as hyperarousal symptoms and somatic anxiety measured with the Ham-A p<0.05). IL-6 increased from baseline in sham but not tcVNS. Open label tcVNS resulted in improvements measured with the CGI compared to the sham treatment period p<0.05).
Conclusions:
These preliminary results suggest that tcVNS reduces inflammatory responses to stress, which may in part underlie beneficial effects on PTSD symptoms.
1. Introduction
Posttraumatic Stress Disorder (PTSD) is a disabling disorder that affects the quality of life and productivity of millions of Americans (Bremner, 2016). The standard of care for PTSD includes psychotherapy and/or medication (Ballenger et al., 2000; Foa et al., 1999; Foa et al., 2007; Foa and Rothbaum, 1998; Hembree et al., 2003; Lancaster et al., 2016; Schnurr et al., 2007), however current treatments are characterized by high rates of non-completion and/or limitations in efficacy (Ballenger et al., 2004; Davis et al., 2016; Hembree et al., 2003; Schottenbauer et al., 2008). Some reports concluded that there is insufficient evidence to conclude that first line medication treatment with Selective Serotonin Reuptake Inhibitors (SSRIs) are effective for PTSD (Institute of Medicine of the National Academies, 2014). New approaches to treatment are needed, especially those that target the underlying psychobiology of PTSD, involving core changes in brain and autonomic nervous system (Reinertsen et al., 2017; Shah et al., 2013) and immune function (Neigh and Ali, 2016), that maintain symptoms of the disorder (Bremner, 2016; Shah et al., 2013).
Neuromodulation is a new approach that may be particularly useful in addressing the underlying psychobiology of stress-related psychiatric disorders (Adair et al., 2020; Bikson et al., 2016; Bikson et al., 2017b; Bremner et al., 2020b; Krames et al., 2018; Schachter and Saper, 1998; Tortella et al., 2015; Woods et al., 2016). Vagal Nerve Stimulation (VNS) is a form of neuromodulation that has been shown to be efficacious in the treatment of epilepsy (Ben-Menachem et al., 1999; Ben-Menachem et al., 1994; George et al., 1994; Handforth et al., 1998; Salinsky et al., 1999; The Vagus Nerve Stimulation Study Group, 1995) and treatment-refractory major depression (Aaronson et al., 2017; Berry et al., 2013; Dell-Osso et al., 2013; George et al., 2005; George et al., 2003; George et al., 2000; Marangell et al., 2002; Rush et al., 2000; Rush et al., 2005a; Rush et al., 2005b; Sackeim et al., 2007; Sackeim et al., 2001a; Sackeim et al., 2001b). Implantable VNS devices are currently approved by the Food and Drug Administration (FDA) for treatment resistant major depression (Aaronson et al., 2017; George et al., 2003; Terry, 2014). Beneficial effects of VNS that may be particularly useful for stress-related psychiatric disorders including blocking of sympathetic (Pena et al., 2014; Peña et al., 2013; Schomer et al., 2014) and immune function (Bansal et al., 2012; Borovikova et al., 2000), and enhancement of cognition (Clark et al., 1999; Jacobs et al., 2015; Sackeim et al., 2001a; Sjögren et al., 2002; Smith et al., 2005; Sun et al., 2017; Vonck et al., 2014). Implantable devices have not been widely implemented in psychiatry, however, in part due to lack of reimbursement by Medicare and private insurers (Feldman et al., 2013).
A new generation of non-implantable VNS devices has the potential to be more widely implemented in psychiatry due to lower cost and greater convenience (Bremner and Rapaport, 2017). VNS can be applied to branches of the vagus nerve in the ear (transcutaneous auricular VNS, or taVNS) or in the neck, where it travels through the carotid sheath (transcutaneous cervical VNS, or tcVNS) (Adair et al., 2020; Badran et al., 2019; Bremner et al., 2020b). PTSD is associated with an increase in the blood concentrations of the inflammatory marker interleukin (IL)-6 at baseline (Gill et al., 2010; Gill et al., 2008; (Guo et al., 2012); Li et al., 2014; Lindqvist et al., 2017; Miller et al., 2001; Passos et al., 2015; Sutherland et al., 2003; Tucker et al., 2010; Vidovic et al., 2011; von Kanel et al., 2010) and in response to mental stress such as public speaking (Lima et al., 2019) or exposure to personalized traumatic scripts (Bremner et al., 2020a) as well as in diurnal cerebrospinal fluid (CSF) (Baker et al., 2001). Studies have shown increased blood concentrations of interferonγ (IFNγ) at baseline ( Guo et al., 2012; Hoge et al., 2009; Lindqvist et al., 2014; Passos et al., 2015; Woods et al., 2005; Zhou et al., 2014) and in response to traumatic script stress in PTSD (Bremner et al., 2020a). Other studies showed increased baseline Tumor Necrosis Factor (TNF)-α blood concentrations in PTSD (Gill et al., 2010; Lindqvist et al., 2017; Lindqvist et al., 2014; Passos et al., 2015; Sutherland et al., 2003; Vidovic et al., 2011; von Känel et al., 2007). Animal studies show VNS decreases both IL-6 (Borovikova et al., 2000; Brock et al., 2017; Corsi-Zuelli et al., 2017; Das and Basu, 2008; Das, 2007, 2011; Jan et al., 2010; Li and Olshansky, 2011; Marsland et al., 2007) and TNF-α (Bansal et al., 2012; Jan et al., 2010; Marsland et al., 2007). Studies using both implantable devices (De Herdt et al., 2009) and tcVNS (Brock et al., 2017; Lerman et al., 2016) show VNS also decreases TNF-α in human subjects, while another study showed long term treatment with tcVNS lowered both TNF-α and IL-6 in patients with Sjögren’s Syndrome (Tarn et al., 2019). We showed that tcVNS blocks IL-6 and IFNγ response to traumatic script stress in PTSD (Bremner et al., 2020a) and blocks the rise in Pituitary Adenylate Cyclase Activating Peptide (PACAP) over three days of stressful tasks in traumatized subjects with and without PTSD (Gurel et al., 2020c). We also previously reported that tcVNS in traumatized healthy human subjects with and without PTSD blocked peripheral sympathetic and enhanced parasympathetic responses both at baseline and in response to both personalized traumatic scripts and mental stressors (Gazi et al., 2020; Gurel et al., 2020a; Gurel et al., 2020b; Gurel et al., 2018; Gurel et al., 2020d; Gurel et al., 2020e) and modulated brain response to traumatic scripts (Wittbrodt et al., 2020), and other studies reported that tcVNS blocked sympathetic function in healthy subjects (Brock et al., 2017; Lerman et al., 2019) while taVNS blocked sympathetic function in healthy human subjects (Badran et al., 2018b; Bretherton et al., 2019; Clancy et al., 2014) and patients with co-morbid mild Traumatic Brain Injury (mTBI) and PTSD (Lamb et al., 2017). This work replicated findings in healthy subjects using implanted VNS (Schomer et al., 2014). A nonrandomized study of taVNS in patients with depression showed efficacy at four weeks for symptoms of depression when compared to sham stimulation (Rong et al., 2016). Other studies in patients with depression showed taVNS resulted in changes in brain regions implicated in that disorder (Fang et al., 2017; Fang et al., 2016; Liu et al., 2016; Tu et al., 2018; Wang et al., 2018). The purpose of the current study was to assess the efficacy of tcVNS, delivered in a longitudinal study, compared to sham stimulation for the treatment of PTSD, and to assess the effects on stress-induced inflammation. We hypothesized that tcVNS would be associated with improvement in symptoms associated with PTSD, in particular those driven by increased sympathetic function including hyperarousal and somatic anxiety, and a reduction in stress-induced IL-6 activation.
2. Materials and Methods
2.1. Human Subjects
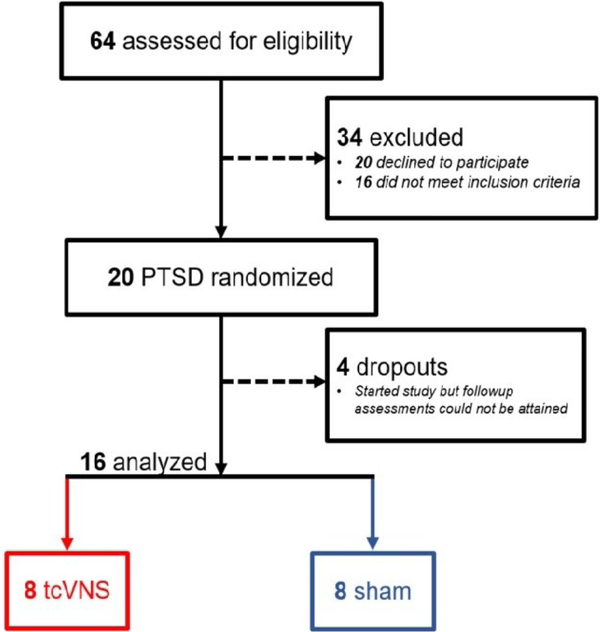
The research reported here was approved by the Institutional Review Boards of Emory University, Georgia Institute of Technology, and the Space and Naval Warfare Systems Command (SPAWAR) Systems Center of the Pacific and the Department of Navy Human Research Protection Program. Patients were studied between February 2019 and March 2020 at the Emory University School of Medicine. Subjects provided written, informed consent for participation. Subjects included physically healthy adults age 18–70 with a history of psychological trauma and the current diagnosis of posttraumatic stress disorder (PTSD) (Figure 1). Subjects were excluded with the diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, bulimia or anorexia, as defined by The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013) (American Psychiatric Association, 2013). Subjects were also excluded with current pregnancy, traumatic brain injury (TBI), meningitis, active implanted device, evidence or history of serious medical or neurological illness, such as cardiovascular, gastrointestinal, hepatic, renal, or other systemic illness; carotid atherosclerosis, cervical vagotomy or positive toxicology screen. Psychiatric diagnosis was evaluated with the Structured Clinical Interview for DSM (SCID) (First and Gibbon, 2004). The Clinician Administered PTSD Scale-5 (CAPS-5) was administered to evaluate for presence and severity of both current and lifetime PTSD (Blake et al., 1995; Weathers et al., 2018). The PTSD Checklist (PCL)-Civilian version was used to assess self-reported levels of PTSD symptoms (Ruggiero et al., 2003). Anxiety was measured with the Hamilton Anxiety Scale (Ham-A) (Hamilton, 1959) and depression with the Hamilton Depression Scale (Ham-D) (Hamilton, 1960). Somatic anxiety was measured by adding the Ham-A items for “gastrointestinal” (Item 11, nausea, heartburn, abdominal pain) and “autonomic” (item 13, flushing, rapid heart rate, faintness, sweaty skin, dry mouth) (Maier et al., 1988). Clinical improvement was assessed by study personnel using the Clinical Global Impressions (CGI) scale, a 7 point scale ranging from 1 for very much improved, 2 much improved, 3 minimally improved to 4 for no change and 7 very much worse (Busner and Targum, 2007; NIMH, 1970). Among 64 individuals who were screened for eligibility, 20 were enrolled and randomized to active (N=9) or sham (N=11) stimulation. Pre-treatment inflammatory biomarker data was previously reported in three patients randomized to sham {Bremner, 2020 #11163}. One in the tcVNS group and three in the sham group dropped out after starting the protocol and follow-up assessments were not attainable (Figure 1). In the active tcVNS group, three (33%) met criteria for current co-morbid major depression and six (66%) for a lifetime history of major depression, four (44%) for current generalized anxiety disorder, one (11%) for current panic disorder with agoraphobia, two (22%) for current panic disorder without agoraphobia, two (22%) for current social phobia, and one (11%) for current body dysmorphic disorder. In the sham stimulation group, four (36%) met criteria for lifetime major depression, one (9%) for current major depression, three (27%) for current generalized anxiety disorder, and one (9%) for current obsessive-compulsive disorder.
Figure 1.
CONSORT diagram showing flow of study participants screened, enrolled, and completing the protocol.
2.2. Study Design
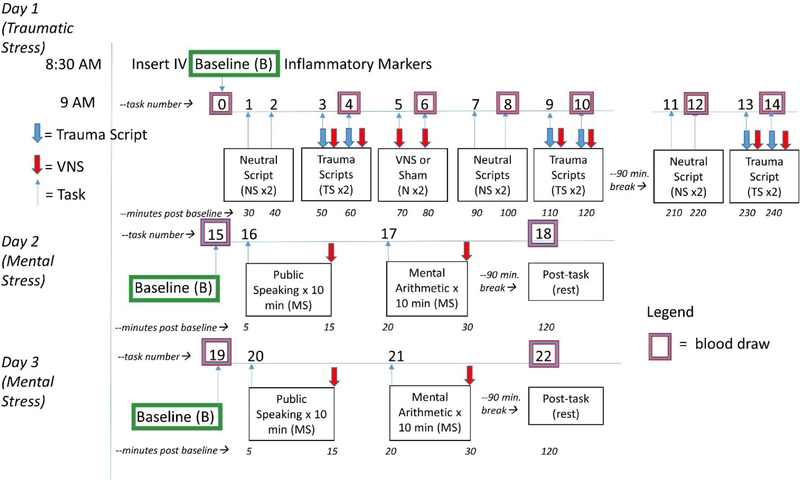
The participants provided their own traumatic experiences, and personalized voice recordings based on these experiences were presented as traumatic stress (Bremner et al., 1999; Orr et al., 1998). Subjects underwent exposure to personalized traumatic scripts in conjunction with tcVNS or sham on day 1, and “neutral” stressful tasks with tcVNS or sham on days 2 and 3 including public speech and mental arithmetic (Figure 2) (Bremner et al., 2009; Bremner et al., 2003; Burg and Soufer, 2014). We have described these paradigms in detail before and they have been shown to reliably produce behavioral and physiological responses consistent with a stress response (Bremner et al., 2009; Bremner et al., 2003; Hammadah et al., 2017b). The first day included six traumatic recall scripts (approximately one-minute each) and six neutral scripts presented audibly through headphones. The neutral scripts were designed to induce positive feelings to the subject, such as the description of pleasant scenery. Immediately after the traumatic stress recording ended, stimulation (active or sham) was applied by the researcher from the left side of the neck. Behavioral ratings after each task were performed using Visual Analogue Scales (VAS) rating subjective anger on a 0–100 scale with 100 being most extreme anger and 0 not at all (Southwick et al., 1993). On the same day two stimulation administrations (active or sham) were applied without any stressor. Blood draws were taken on the start of the day (baseline) and at multiple time points after. The second and third days were identical to each other. Baseline blood draws were taken both mornings. Afterwards, participants underwent a public speech task and mental arithmetic task, as previously described (Gurel et al., 2020b; Hammadah et al., 2017a). Participants were then instructed in use of the device for self-administration at home and received active or sham devices to take home. They were instructed to stimulate for two minutes on the left side, followed a one minute rest, and two minutes on the right side, and to do this once in the morning and once at night. They were further instructed to stimulate while listening to personalized traumatic scripts twice a week. Participants continued twice daily stimulation for three months and returned for behavioral assessments once a month. At the end of the three month period they were given an active device and instructed to continue twice daily stimulation treatments.
Figure 2.
Diagram of the baseline study protocol. PTSD patients underwent three days of stress, one day (Day 1) with neutral scripts (NS) and personalized traumatic scripts (TS), and two days (Days 2 and 3) with mental stress (MS) involving public speaking and mental arithmetic tasks. Participants underwent randomized, double-blind assignment to tcVNS or sham stimulation which was paired with stress tasks (or no task) on Days 1, 2 and 3. On Day 1 neutral and traumatic scripts lasted about one minute and occurred in pairs with 10 minutes in between. Stress tasks were paired with stimulation with tcVNS or sham which began immediately after termination of the task and continued for two minutes followed by a blood draw (purple/blue boxes signify pairing of task/stimulation/blood draw but blood draw actually occurred at the termination of stimulation). On Day 1 participants also underwent stimulation with tcVNS or sham for two minutes in the absence of a task (N) repeated twice with 10 minutes in between followed by a blood draw. Neutral and traumatic script pairs were repeated followed by a 60 minute rest and lunch break, with a repeat of neutral and traumatic script pairs in the afternoon each paired with blood draws. The neutral scripts tasks #11 and #12 were followed by a blood draw (which was about 110 minutes after the first trauma script pairs at tasks #3 and #4) and the trauma scripts tasks #13 and #14 paired with tcVNS or sham were followed by the final blood draw at 210 minutes into Day 1 (Traumatic Stress). On Day 2 after a baseline blood draw at rest (task #15) participants underwent mental stress (MS) involving five minutes of public speaking (task #16) with tcVNS or sham at the end, followed by an eight minute rest period, and another five minutes of mental arithmetic (task #17) followed by tcVNS or sham. After a 90 minute rest period participants underwent a blood draw at rest (task#18). This was repeated for Day 3 with baseline (task #19, public speaking (task #20), mental arithmetic (task #21) and a blood draw post-task at rest (task #22). The blood draws for all three days were timed to coincide with the roughly 90 minute time course of interleukin-6 (IL-6) response to stress based on prior studies. Patients then underwent three months of tcVNS/sham followed by a repeat of Day 1 only.
2.3. Blinding
The participants were randomized into active tcVNS or sham groups with pre-numbered devices by the manufacturer who were not involved in the research. Random allocation was carried out by personnel who did not take part in data collection or analyses. The participants and researchers were blinded to the stimulus type. Statistical analyses were carried out by a biostatistician who did not take part in data collection or processing. Stimulus groups was un-blinded for the interpretation of statistical analysis.
2.4. Transcutaneous Cervical Vagal Nerve Stimulation
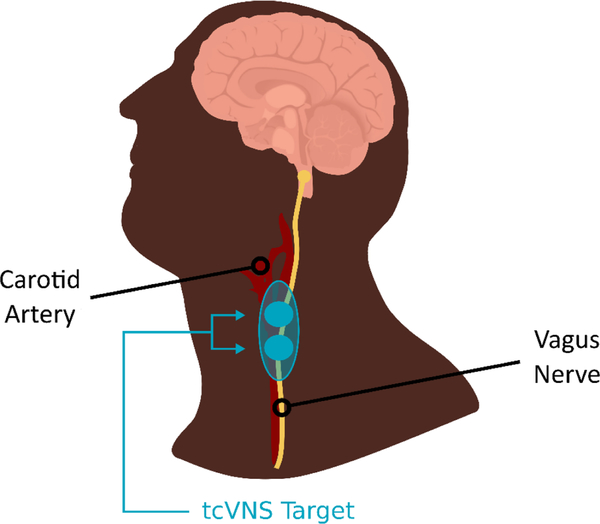
Both active tcVNS and sham stimuli were administered using hand-held devices that target the cervical portion of the vagus nerve from the skin (GammaCore, ElectroCore, Basking Ridge, New Jersey). Stimulation was applied using collar, stainless steel electrodes with a conductive electrode gel placed on the left side of the neck over the carotid sheath as determined by palpation of the carotid artery (Figure 3). Active tcVNS devices produced an alternating voltage signal consisting of five 5kHz sine bursts (1 ms of five sine waves with pulse width of 40 ms) repeating at a rate of 25 Hz envelopes. The frequency of 25 Hz was chosen based on prior studies showing optimization of effects on autonomic function and other measures at this frequency (Adair et al., 2020; Badran et al., 2019; Badran et al., 2018a; Badran et al., 2018b; Bikson et al., 2017a; Hays et al., 2014; Hays et al., 2013; Hulsey et al., 2017). The sham devices produce an alternating biphasic voltage signal consisting of 0.2 Hz square pulses (pulse width of 5 s) eliciting a mild sensation. The peak voltage amplitudes for active and sham device are 30V and 14V, respectively. An active stimulation amplitude higher than 15V using the studied device was previously reported to create vagal somatosensory evoked potentials associated with vagal afferent activation, that are also activated with VNS implants (Nonis et al., 2017). Both active and sham devices delivered two minutes of stimulation. The stimulation intensity (amplitude of the voltage wavefront) was adjustable using a roll switch that ranged from 0 to 5 a.u. (arbitrary units) with a corresponding peak output ranging from 0 to 30V for active tcVNS, and from 0 to 14 V for the sham device. During each application, the amplitude of the voltage waveform was increased to the maximum the subject could tolerate, without pain. The stimulation continued at the selected intensity.
Figure 3.
Diagram showing placement of tcVNS device on the neck to target the vagus nerve as it travels through the carotid sheath.
The rationale behind the frequency difference between active (5kHz) and sham (0.2Hz) device waveforms is based on the fact that high frequency voltage signals (such as the active stimulus, 5kHz) pass through the skin with minimal power dissipation due to the low skin-electrode impedance at kHz frequencies. In contrast, lower frequency signals (such as the sham stimulus, 0.2Hz) are mainly attenuated at the skin-electrode interface due to the high impedance (Rosell et al., 1988). Accordingly, the active device operating at higher frequencies can deliver substantial energy to the vagus nerve to facilitate stimulation, while the voltage levels appearing at the vagus would be expected to be orders of magnitude lower for the sham device and thus stimulation is unlikely. Nevertheless, since the sham device does deliver relatively high voltage levels directly to the skin, it activates skin nociceptors, causing a similar feeling to a pinch. This sensation is considered to be necessary for blinding of the participants, particularly longitudinal protocols such as in this manuscript.
2.5. Biomarker Assay
We employed the MesoScale system (MSD, Rockville, MD) multiplex assay to quantitate IL-6, IL-13, IL-22, IL-5, IL-12p70 and IFN-γ in EDTA plasma. The MesoScale system (https://www.mesoscale.com/) was performed according to the protocols supplied by the manufacturer, and uses electrochemiluminescence for high sensitivity and broad dynamic range. Intra-assay CVs were 5.5% for IL-6.
2.5.1. Statistical Analysis
Analysis of variance (ANOVA) and chi-square tests were used to compare the demographic and retention characteristics across the tcVNS treatment or sham stimulation group among patients with PTSD. Independent t-tests were used to compare the demographic characteristics across the tcVNS treatment or sham stimulation groups. Paired t-tests were used to compare behavioral responses before and after treatment with p<0.05 denoting statistical significance. CAll statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and MATLAB (R2017b, Natick, MA).
3. Results
Participant groups were similar in age, body mass index, race, sex, education level and marital status (Table 1). There were no statistically significant differences in baseline PTSD symptom levels as measured by the CAPS or PCL, depression as measured by the Ham-D or anxiety as measured by the Ham-A. Participants in the tcVNS and sham stimulation groups experienced similar primary traumas, including one in each group with combat trauma, three in each group with childhood sexual abuse, two in each group with rape in adulthood, one in tcVNS and two in sham with physical assault in adulthood, two in tcVNS and one in sham with injury or death of someone close to them, and one in sham with a traumatic failed suicide attempt (Table 2).
Table 1.
Baseline Demographic and Behavioral Variables in Active tcVNS and Sham Stimulation Groups
| tcVNS (n=9) | Sham (n=10) | |
|---|---|---|
| Age | ||
| Mean ± SD | 37 ± 13 | 40 ± 14 |
| Race | ||
| White | 5 (55%) | 5 (45%) |
| Black | 4 (45%) | 5 (45%) |
| Asian/Pacific Islander | 0 (0%) | 1 (9%) |
| Sex | ||
| Female | 6 (66%) | 7 (64%) |
| Male | 3 (33%) | 4 (36%) |
| BMI | ||
| Mean ± SD | 30 ± 7 | 30 ± 6 |
| Education Level | ||
| Some high school | 1 (11%) | 1 (9%) |
| High school graduate | 3 (33%) | 1 (9%) |
| Some college | 2 (22%) | 2 (18%) |
| College graduate | 3 (33%) | 7 (64%) |
| Marital Status | ||
| Never married | 3 (33%) | 5 (45%) |
| Married | 3 (33%) | 2 (18%) |
| Divorced / Separated | 2 (22%) | 3 (27%) |
| Widowed | 1 (11%) | 1 (9%) |
| PTSD Score (PCL) | ||
| Mean ± SD | 62 ± 14 | 61 ± 13 |
| CAPS Score | ||
| Intrusions-Mean ± SD | 11 ± 2 | 10 ± 3 |
| Avoidance-Mean ± SD | 5 ± 2 | 5 ± 1 |
| Negative Cognitions-Mean ± SD | 17 ± 4 | 14 ± 4 |
| Hyperarousal-Mean ± SD | 11 ± 3 | 10 ± 3 |
| Total-Mean ± SD | 44 ± 9 | 38 ± 9 |
| Ham-D Score | ||
| Mean ± SD | 19 ± 12 | 19 ± 5 |
| Ham-A Score | ||
| Mean ± SD | 23 ± 11 | 20 ± 6 |
tcVNS=transcutaneous Vagal Nerve Stimulation; sham=sham stimulation; BMI=body mass index; PCL=PTSD Checklist; CAPS=Clinician Administered PTSD Scale; Ham-D=Hamilton Depression Scale; Ham-A=Hamilton Anxiety Scale
Table 2.
Key Traumatic Events in Active tcVNS and Sham Stimulation Groups
| Subject/group/sex | Traumatic Event |
|---|---|
| 001 sham female | Husband left for hockey game, never returned |
| 002 active male | Iraq combat |
| 003 active female | Childhood sexual abuse from age 7 |
| 004 sham male | Shot, saw friend shot and killed |
| 005 active female | Sudden death of husband, received news while driving, almost crashed |
| 006 sham female | Sexual abuse by father from age 6 |
| 007 active female | Gang rape in adulthood |
| 008 sham female | Childhood sexual abuse |
| 009 active female | Raped in childhood, held at knifepoint. |
| 010 sham female | Raped at knifepoint at age 14. |
| 011 sham male | Failed suicide. |
| 012 active male | Childhood sexual abuse. Raised in religious cult. |
| 013 active female | Mother attempted suicide multiple times in childhood. |
| 014 sham female | Rape in adulthood. Physical and emotional abuse in childhood. |
| 015 sham male | Vietnam combat |
| 016 sham female | Child sexual abuse and assault, adult rape |
| 017 active female | Kidnapped and assaulted, abusive relationship |
| 018 active male | Stabbed twice in military, almost died |
| 019 sham male | Physical assault in military, stabbed in a fight |
| 020 sham female | Rape, parents in cult as child |
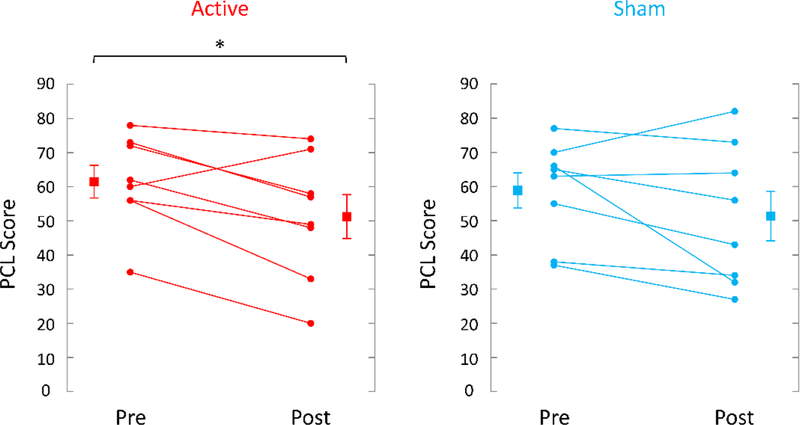
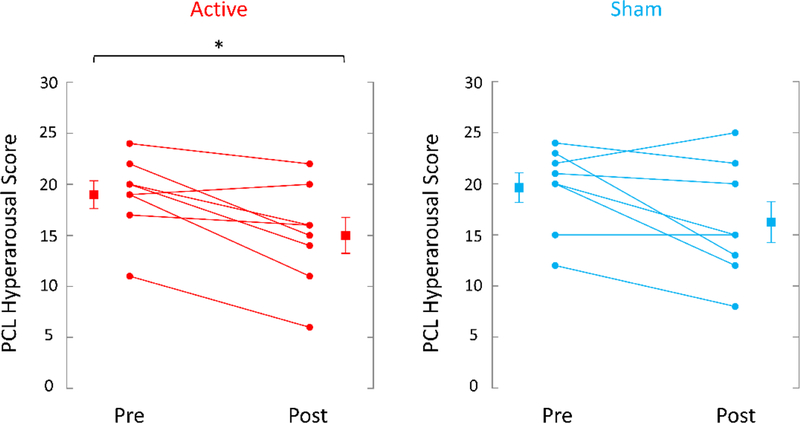
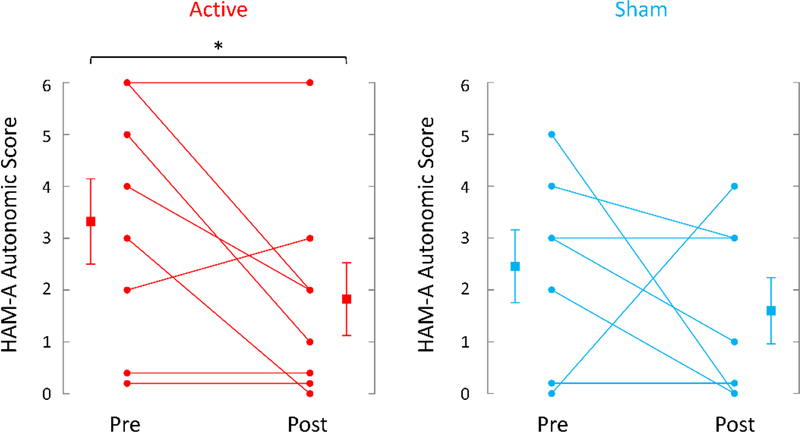
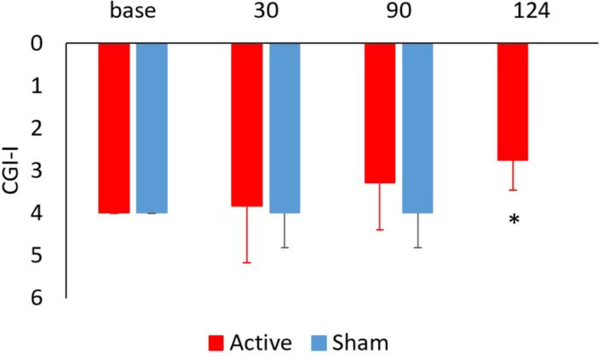
TcVNS was associated with greater retention, 8/9 (89%) completing three months of treatment versus 8/11 (73%) in the sham stimulation group. These patients dropped out of the protocol and further assessments were not attainable. Active tcVNS resulted in a 31% greater decrease in PTSD symptoms measured with the PCL (pretreatment: 62 (14 SD); posttreatment: 51 (18 SD), p=.013, effect size .79) compared to sham stimulation (pretreatment: 59 (15 SD); posttreatment: 51 (20 SD), p=.08) (Figure 4). There was a 31% decrease in PTSD symptoms on the CAPS in the tcVNS group (pretreatment: 46 (8 SD), posttreatment: 32 (17 SD)) versus a 23% decrease for sham (pretreatment: 38 (8 SD), posttreatment: 29 (11 SD), p<.05 for both groups). tcVNS resulted in a 21% decrease in hyperarousal symptoms measured with the PCL (pretreatment: 19 (4 SD), posttreatment: 15 (5 SD), p=.008, effect size 1.0) versus a 17% decrease with sham stimulation (pretreatment: 20 (4 SD), posttreatment: 16 (6 SD), p = .06) (Figure 5). tcVNS decreased overall anxiety as measured by the Ham-A in tcVNS (pretreatment: 23 (11 SD), posttreatment: 20 (9 SD), p=.10) and sham stimulation groups (pretreatment: 21 (6 SD), posttreatment: 16 (10 SD), p=.09). Active tcVNS resulted in a 46% decrease in somatic anxiety symptoms (autonomic/gastrointestinal) (pretreatment: 3.3 (2.4 SD), posttreatment: 1.8 (2.1 SD), p=.035, effect size .63) versus a 35% decrease with sham (pretreatment: 2.4 (1.9 SD), posttreatment: 1.6 (1.7 SD), p=.22) (Figure 6). Treatment did not result in significant changes in depression as measured by the Ham-D in either the tcVNS (pretreatment: 19 (12 SD), posttreatment: 17 (8 SD), p=.53) or sham stimulation groups (pretreatment: 18 (6 SD), posttreatment: 15 (8 SD), p=.23). The CGI showed a pattern of greater improvement in tcVNS versus sham at one month (3.83 (1.33 SD) versus 4.00 (0.82) and three months (3.29 (1.11 SD) versus 4.00 (0.82)) and significant improvement after three months of open label treatment following the double blind phase compared to three months of sham treatment (2.75 (0.71) versus 4.00 (0.82 SD) (p=0.003) (intermediate between 2 for much improved and 3 for minimally improved (Figure 7). Both active tcVNS and sham stimulation were well tolerated and there were no adverse effects.
Figure 4.
Effects of up to three months of twice daily transcutaneous cervical Vagal Nerve Stimulation (tcVNS) (on the left, red lines) or sham stimulation (on the right, blue lines) on symptoms of PTSD as measured with the PTSD Checklist (PCL). Individual participants are shown with lines separating pre- and post-treatment; lines with bars represent means and SD before and after treatment for both groups. Active tcVNS resulted in a 17% reduction in PTSD symptoms (p=.013) and sham stimulation a 13% reduction in PTSD symptoms after treatment (p=.15) (*p<.05 from pretreatment).
Figure 5.
Effects of up to three months of twice daily tcVNS (on the left, red lines) or sham stimulation (on the right, blue lines) on symptoms of PTSD as measured with the PTSD Checklist (PCL). Individual participants are shown with lines separating pre- and post-treatment; lines with bars represent means and SD before and after treatment for both groups. Active tcVNS resulted in a 21% reduction in hyperarousal symptoms (p=.008) while sham stimulation resulted in a 17% decrease (p=.06) (*p<.05 from pretreatment).
Figure 6.
Effects of tcVNS and sham on autonomic anxiety as measured with the Hamilton Anxiety Scale (Ham-A). Scores represent the sum of items for gastrointestinal and autonomic somatic anxiety (see text) at baseline and with three months of twice daily tcVNS (on the left, red lines) or sham stimulation (on the right, blue lines) on autonomic anxiety as measured with the Ham-A. Individual participants are shown with lines separating pre- and post-treatment; lines with bars represent means and SD before and after treatment for both groups. There was a −46% decrease in Ham-A somatic anxiety in the tcVNS group (p=.036) versus a −35% change in the sham stimulation group (p=.22) (*p<.05 from pretreatment).
Figure 7.
Effects of tcVNS and sham on clinical improvement as measured with the Clinical Global Impressions scale-improvements (CGI-I) in active tcVNS (red) and sham (blue) stimulation groups at baseline and 30 and 90 days after start of double-blind active (N=9) versus sham (N=11) treatment in patients with PTSD. The final measurement at 124 days (34 days after start of open label treatment) showed a significant improvement compared to after three months of sham stimulation (2.75 (0.71) versus 4.00 (0.82 SD) (*p=0.003). This score is intermediated between much improved (2) and minimally improved (3) compared to no improvement (4).
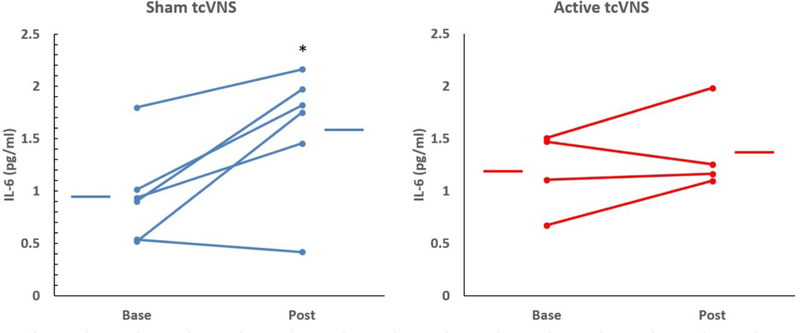
Exposure to personalized traumatic scripts in PTSD patients in conjunction with sham stimulation (but not tcVNS) resulted in a significant increase in IL-6 both pre-treatment (Figure 8) post-treatment (Figure 9). There were no statistically significant differences between tcVNS and sham stimulation groups in Interferon Gamma, IL-12, IL-13, IL22, or IL-5 (Supplementary Table).
Figure 8.
Effects of tcVNS (red lines, right side) or sham (blue lines, left side) on interleukin-6 (IL-6) at baseline (base) and following repeated exposure to traumatic script stress (post) in patients with PTSD. Lines connect pre and post stress in individual patients and bars represent the means for each group. There was a significant increase in IL-6 in the sham group (*p<0.05) not seen in the PTSD group.
Figure 9.
Effects of tcVNS (red lines, right side) or sham (blue lines, left side) on interleukin-6 (IL-6) at baseline (base) and following three months of double blind active tcVNS or sham treatment in patients with PTSD. Lines connect baseline to post-treatment and post traumatic script stress in 5/9 patients and bars represent the means for each group. There was a significant increase in IL-6 in the sham group (*p<0.05) not seen in the PTSD group.
4. Discussion
Non-invasive tcVNS in this study was associated with a decrease in PTSD symptoms with the greatest effects on hyperarousal and autonomic or somatic anxiety symptoms. tcVNS also blocked the interleukin (IL)-6 response to traumatic script stress in PTSD patients. tcVNS was well tolerated and there were no adverse effects of administration over three months. tcVNS may be useful for some patients with PTSD, including those who do not respond to medication treatments and patients with increased symptoms of hyperarousal and/or autonomic imbalance or patterns of elevated inflammatory biomarkers.
These findings suggest that tcVNS may target the underlying neurobiology of PTSD, in particular noradrenergic and peripheral sympathetic nervous system function. The findings are consistent with our prior studies showing a decrease in sympathetic nervous system activity when tcVNS is paired with traumatic reminders in traumatized individuals (Gazi et al., 2020; Gurel et al., 2020a; Gurel et al., 2020b; Gurel et al., 2020e), as well as numerous studies showing nVNS (taVNS) blocks peripheral sympathetic nervous system function and startle reflex (Bretherton et al., 2019; Clancy et al., 2014; Lamb et al., 2017). Symptoms of hyperarousal reduced by tcVNS in this study include increased attention and vigilance, being on guard, poor concentration and sleep. Autonomic symptoms captured by the Ham-A that were reduced by tcVNS include those in the somatic anxiety categories of “gastrointestinal” (nausea, heartburn, abdominal pain) and “autonomic” (flushing, rapid heart rate, faintness, sweaty skin, dry mouth). These symptoms are known to be associated with increased peripheral sympathetic nervous system function.
A key role of VNS is modulation of norepinephrine (NE) centrally in the brain and peripherally through the sympathetic nervous system (Follesa et al., 2007; Krahl et al., 1998; Manta et al., 2009a, 2009b; Manta et al., 2013). VNS modulates NE in the brain through effects on the locus coeruleus (LC), an area in the brainstem where the majority of NE neurons are located (Hulsey et al., 2017). The vagus nerve has efferent fibers that project to the periphery and modulate organ function and afferent fibers that relay through the Nucleus Tractus Solitarius (NTS) in the brainstem to affect central brain function (Roosevelt et al., 2006; Ura et al., 2013). The NTS has inputs to the LC and VNS acts through the LC to increase NE release in key brain areas implicated in stress, emotion, and PTSD, including the medial prefrontal cortex, amygdala and hippocampus (Hassert et al., 2004; Hulsey et al., 2017; Manta et al., 2009a; Roosevelt et al., 2006). Increased NE has a secondary effect on neurochemical systems that have been the target of medication treatments for PTSD, including the serotonin (5HT) system. NE acts through excitatory alpha-1 adrenoreceptors on serotonergic neurons to increase 5HT in the dorsal raphe, the major site of serotonin cell bodies in the brainstem, with secondary effects on the same target brain regions modulated by NE (Manta et al., 2009a, 2009b; Manta et al., 2013; McGaugh, 1985). Animal studies show that chronic VNS treatment increases firing rates of both NE neurons in the LC and 5HT neurons in the dorsal raphe (Dorr and Debonnel, 2006), resulting in increased extracellular NE in the hippocampus and prefrontal cortex, and 5HT in the dorsal raphe (Manta et al., 2009a; Manta et al., 2013; Nichols et al., 2011). Chronic VNS treatment increases metabolites of dopamine and 5HT in the cerebrospinal fluid (CSF) in patients with epilepsy (Hammond et al., 1992). VNS acts through these central brain areas in ways that are incompletely understood to decrease peripheral sympathetic function and enhance parasympathetic function (Brock et al., 2017; Clancy et al., 2014; Hammond et al., 1992; Pagani et al., 1986; Thayer and Lane, 2007; Weber et al., 2010). This fits with our findings that tcVNS blocks peripheral sympathetic activation and enhances parasympathetic tone (Gurel et al., 2020b).
Alterations in noradrenergic and peripheral sympathetic function play an important role in the maintenance of symptoms of PTSD (Bremner et al., 1996a, 2009b; Southwick et al., 1997). These systems represent key components of the stress response that ready the body to prepare to deal with potential threat (Bremner et al., 1996a, 1996b). NE cell bodies in the LC have axons that extend throughout the rest of the brain and are activated by stress, resulting in release of NE in the brain with associated increased attention, fear, and anxiety behaviors, as well as activation of the peripheral sympathetic system with increased heart rate, blood pressure, and respiration (Abercrombie and Jacobs, 1987a, 1987b; Aston-Jones et al., 1991; Foote et al., 1983; Jedema et al., 2001; Levine et al., 1990; Nisenbaum and Abercrombie, 1993; Redmond and Huang, 1979). Chronically stressed animals re-exposed to stress show a potentiated release of NE in brain areas involved in emotion and the stress response which is associated with anxiety like behaviors (Aston-Jones et al., 1991; Finlay et al., 1995; Miner et al., 2006; Nisenbaum et al., 1991; Petty et al., 1993; Tanaka et al., 2000; Torda et al., 1984; Weiss et al., 1981). Multiple lines of evidence support increased noradrenergic function in PTSD, including the fact that drugs of abuse and medications that inhibit LC firing reduce symptoms of hyperarousal, including opioids (Bremner et al., 1996c), and agonists of the α2 NE inhibitory autoreceptor on the LC, such as clonidine (Kinzie and Leung, 1989), while α2 NE antagonists, like yohimbine, have the opposite effect (Southwick et al., 1997; Southwick et al., 1993). Other studies in PTSD found increased peripheral concentrations of NE and its metabolites in urine and plasma (De Bellis et al., 1999; De Bellis et al., 1994; Lemieux and Coe, 1995; Mason et al., 1988; Yehuda et al., 1998) as well as cerebrospinal fluid at baseline (Geracioti et al., 2001). In PTSD patients, exposure to traumatic reminders increased symptoms of PTSD and increased NE and its metabolites, in addition to increasing heart rate, blood pressure, and skin conductance (Blanchard et al., 1986; Blanchard et al., 1982; Blanchard et al., 1991; Malloy et al., 1983; McFall et al., 1990; McFall et al., 1992; Orr et al., 1998; Orr et al., 1995; Orr et al., 1993; Orr and Roth, 2000; Shalev et al., 1998). Challenge to the NE system of patients with PTSD with the alpha2 adrenergic receptor antagonist, yohimbine, had similar effects (Bremner et al., 1997; Southwick et al., 1997; Southwick et al., 1993). These findings show that altered NE and sympathetic system function play an important role in PTSD symptoms, especially in the hyperarousal category, highlighting the potential utility of interventions such as tcVNS that modulate NE and block peripheral sympathetic function.
The current study is a partial replication of our prior report on its effects on IL-6 response to traumatic script stress in PTSD (Bremner et al., 2020a). IL-6 is linked to autonomic function, so it makes sense that VNS has effects on peripheral inflammation (Jan et al., 2010; Marsland et al., 2007). Our findgs add to the growing literature that VNS blocks inflammatory and autonomic responses in human (Frangos et al., 2015; Frangos and Komisaruk, 2017; Gurel et al., 2020b; Gurel et al., 2020e;; Lerman et al., 2019; Lerman et al., 2016; Yakunina et al., 2017) and animal studies (Brock et al., 2017; Chen et al., 2016; Oshinsky et al., 2014). Increased inflammatory function is increasing seen as being a response to stress and playing a role in stress-related psychiatric disorders like PTSD and depression (Miller et al., 2009; Miller and Raison, 2016; Raison and Miller, 2013). Studies in both animals and humans showed that catecholamines released during stress act through the adrenergic receptor to activate the transcription factor, nuclear factor-κB (NF-κB), which leads to increases in cytokines, including IL-6 (Bierhaus et al., 2003). Intervention at the level of IL-6 with VNS may be a useful intervention that reduces symptoms by targeting the underlying neurobiology of PTSD(Bremner et al., 2020b; Noble et al., 2019; Souza et al., 2019).
This is a pilot study with a small sample size that needs to be replicated with larger numbers of patients. The small sample size and multiple outcome measures could lead to spurious results. We also had imperfect follow-up, which is consistent with the highly symptomatic nature of the PTSD patients in this study. Future studies need to be performed with larger numbers of patients to replicate and extend these results.
Supplementary Material
Funding
This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Targeted Neuroplasticity Training (TNT) program through the Naval Information Warfare Center (NIWC) Cooperative Agreement No. N66001-16-4054 and with an Investigator Initiated Grant and device support from ElectroCore LLC, Basking Ridge, N.J., U.S.A. NIH sources of support R01 HL109413 R01 MH120262 UG3 DA048502.
Statement of interest
J.D.B has research funding support from ElectroCore LLC. Both active and sham stimulation devices used in this study were provided by ElectroCore free of charge.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jadr.2021.100190.
References
- Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, Reimherr FW, Schwartz TL, Zajecka JM, 2017. A five-year observational study of patients with treatment-resistant depression treated with VNS therapy or treatment-as-usual: comparison of response, remission, and suicidality. Am. J. Psychiatry 174, 640–648. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Jacobs BL, 1987a. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and non-stressful stimuli. J. Neurosci. 7, 2837–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie ED, Jacobs BL, 1987b. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J. Neurosci. 7, 2844–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, Ho L, Bremner JD, Badran BW, Napadow V, Clark VP, Bikson M, 2020. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul 13, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), 5 ed. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, VanBockstaele EJ, Pieribone V, Shiekhattar R, 1991. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog. Brain Res. 88, 47–75. [DOI] [PubMed] [Google Scholar]
- Badran BW, Alfred BY, Adair DN, Mappin G, DeVries WH, Jenkins DD, George MS, Bikson M, 2019. Laboratory administration of transcutaneous auricular Vagus Nerve Stimulation (taVNS): technique, targeting, and considerations. JoVE (Journal of Visualized Experiments) e58984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Jenkins DD, DeVries WH, Dancy M, Summers PM, Mappin GM, Bernstein H, Bikson M, Coker-Bolt P, George MS, 2018a. Transcutaneous auricular vagus nerve stimulation (taVNS) for improving oromotor function in newborns. Brain Stimul 11, 1198–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran BW, Mithoefer OJ, Summer CE, LaBate NT, Glusman CE, Badran AW, DeVries WH, Summers PM, Austelle CW, McTeague LM, Borckardt JJ, George MS, 2018b. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul 11, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD Jr., 2001. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 9, 209–217. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Foa EB, Kessler RC, McFarlane AC, Shalev AY, 2000. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatr. 61, 60–66. [PubMed] [Google Scholar]
- Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Marshall RD, Nemeroff CB, Shalev AY, Yehuda R, 2004. Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatry 65 (Suppl 1), 55–62. [PubMed] [Google Scholar]
- Bansal V, Ryu SY, Lopez N, Allexan S, Krzyzaniak M, Eliceiri B, Baird A, Coimbra R, 2012. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation 35, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem E, Hellström K, Waldton C, Augustinsson LE, 1999. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology 52, 1265–1267. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Mañon-Espaillat R, R R, Wilder BJ, Stefan H, Mirza W, Tarver WB, Wernicke JF, 1994. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 35, 616–626. [DOI] [PubMed] [Google Scholar]
- Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ, 2013. A patient‑level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med. Devices (Auckl) 6, 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP, 2003. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. U. S. A. 100, 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, Knotkova H, Liebetanz D, Liu A, Loo C, Nitsche MA, Reis J, Richardson JD, Rotenberg A, Turkeltaub PE, Woods AJ, 2016. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 9, 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Paneri B, Mourdoukoutas A, Esmaeilpour Z, Badran BW, Azzam R, Adair D, Datta A, Fang XH, Wingeier B, Chao D, Alonso-Alonso M, Lee K, Knotkova H, Woods AJ, Hagedorn D, Jeffery D, Giordano J, Tyler WJ, 2017a. Limited output transcranial electrical stimulation (LOTES-2017): engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimul 11, 134–157. [DOI] [PubMed] [Google Scholar]
- Bikson M, Unal G, Brunoni A, Loo C, 2017b. What psychiatrists need to know about transcranial direct current stimulation. Psychiatric Times 1–3. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, 1995. The development of a clinician-administered PTSD scale. J. Trauma. Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Gerardi RJ, Ryan P, Pallmeyer TP, 1986. Cardiac response to relevant stimuli as an adjunctive tool for diagnosing post-traumatic stress disorder in Vietnam veterans. Behav. Ther. 17, 592–606. [Google Scholar]
- Blanchard EB, Kolb LC, Pallmeyer TP, Gerardi RJ, 1982. A psychophysiological study of post-traumatic stress disorder in Vietnam veterans. Psychiatr. Q. 54, 220–229. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC, 1991. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J. Nerv. Ment. Dis. 179, 371–373. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Bremner JD, 2016. Posttraumatic Stress Disorder: From Neurobiology to Treatment, 1 ed. Wiley, Hoboken, New Jersey. [Google Scholar]
- Bremner JD, Cheema FA, Ashraf A, Afzal N, Fani N, Reed J, Musselman DL, Ritchie JC, Faber T, Votaw JR, Nemeroff CB, Vaccarino V, 2009. Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress Health 25, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, Huang M, Jung H, Shandhi MH, Beckwith J, Herring I, Rapaport MH, Murrah N, Driggers E, Ko Y-A, Alkhalaf ML, Soudan M, Song J, Ku BS, Shallenberger L, Hankus AN, Nye JA, Park J, Vaccarino V, Shah AJ, Inan OT, Pearce BD, 2020a. Transcutaneous vagal nerve stimulation blocks stress-induced activation of interleukin-6 and interferon-γ in posttraumatic stress disorder: a double-blind, randomized, sham-controlled trial. Brain Behav. Immun. Health 9, 100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Park J, Bikson M, Inan OT, 2020b. Application of non-invasive vagal nerve stimulation to stress-related psychiatric disorders. J. Pers. Med. 10, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, Bronen R, Zubal G, Rich D, Krystal JH, Dey H, Soufer R, Charney DS, 1997. PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Arch. Gen. Psychiatry 54, 246–256. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS, 1996a. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23, 28–38. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS, 1996b. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 23, 39–51. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS, 1999. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry 156, 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Rapaport MH, 2017. Vagus Nerve Stimulation: back to the future. Am. J. Psychiatry 174, 609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS, 1996c. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am. J. Psychiatry 153, 369–375. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Anderson G, Heninger GR, Southwick SM, Charney DS, 2003. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 28, 733–750. [DOI] [PubMed] [Google Scholar]
- Bretherton B, Atkinson L, Murray A, Clancy J, Deuchars S, Deuchars J, 2019. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging (Milano) 11, 4836–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Brock B, Aziz Q, Møller HJ, Pfeiffer Jensen M, Drewes AM, Farmer AD, 2017. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol. Motil. 29, 1–4. [DOI] [PubMed] [Google Scholar]
- Burg MM, Soufer R, 2014. Psychological stress and induced ischemic syndromes. Curr. Cardiovasc. Risk Rep. 8, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busner J, Targum SD, 2007. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 4, 28–37. [PMC free article] [PubMed] [Google Scholar]
- Chen SP, Ay I, de Morais AL, Qin T, Zheng Y, Sadeghian H, Oka F, Simon B, Eikermann-Haerter K, Ayata C, 2016. Vagus nerve stimulation inhibits cortical spreading depression. Pain 157, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J, 2014. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 7, 871–877. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA, 1999. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 2, 94–98. [DOI] [PubMed] [Google Scholar]
- Corsi-Zuelli FMG, Brognara F, Quirino GFS, Hiroki CH, Sobrano Fais R, Del-Ben CM, Ulloa L, Salgado HC, Kanashiro A, Loureiro CM, 2017. Neuroimmune interactions in schizophrenia: focus on vagus nerve stimulation and activation of the alpha-7 nicotinic acetylcholine receptor. Front. Immunol. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Basu A, 2008. Inflammation: a new candidate in modulating adult neurogenesis. J. Neurosci. Res. 86, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Das UN, 2007. Vagus nerve stimulation, depression, and inflammation. Neuropsychopharmacology 32, 2053–2054. [DOI] [PubMed] [Google Scholar]
- Das UN, 2011. Can vagus nerve stimulation halt or ameliorate rheumatoid arthritis and lupus? Lipids Health Dis 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Hamner M, Bremner JD, 2016. Pharmacotherapy for PTSD: effects on PTSD symptoms and the brain. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment. Wiley Blackwell, Hoboken, N,J,, pp. 389–412. [Google Scholar]
- De Bellis MD, Baum AS, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND, 1999. A.E. Bennett Research Award: developmental traumatology: Part I: biological stress systems. Biol. Psychiatry 45, 1259–1270. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Lefter L, Trickett PK, Putnam FW, 1994. Urinary catecholamine excretion in sexually abused girls. J. Am. Acad. Child Adolesc. Psychiatry 33, 320–327. [DOI] [PubMed] [Google Scholar]
- De Herdt V, Bogaert S, Bracke KR, Raedt R, De Vos M, Vonck K, Boon P, 2009. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J. Neuroimmunol. 214, 104–108. [DOI] [PubMed] [Google Scholar]
- Dell-Osso B, Oldani L, Palazzo MC, Balossi I, Ciabatti M, Altamura AC, 2013. Vagus nerve stimulation in treatment-resistant depression: acute and follow-up results of an Italian case series. J. ECT 29, 41–44. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G, 2006. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Therapeutics 318, 89–898. [DOI] [PubMed] [Google Scholar]
- Fang J, Egorova N, Rong P, Liu J, Hong Y, Fan Y, Wang X, Wang H, Yu Y, Ma Y, Xu C, Li S, Zhao J, Luo M, Zhu B, Kong J, 2017. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. Neuroimage Clin 14, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, Zhang G, Chen X, Shi S, Wang L, Liu R, Hwang J, Li Z, Tao J, Wang Y, Zhu B, Kong J, 2016. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol. Psychiatry 15, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RL, Dunner DL, Muller JS, Stone DA, 2013. Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. J. Med. Econ. 16, 63–74. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED, 1995. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 64, 619–628. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, 2004. The structured clinical interview for DSM-IV Axis I Disorders (SCID-I) and the structured clinical interview for DSM-IV Axis II Disorders (SCID-II). In: Segal MJHDL (Ed.), Comprehensive handbook of psychological assessment. John Wiley & Sons Inc., Hoboken, NJ, US, pp. 134–143. [Google Scholar]
- Foa EB, Davidson JRT, Frances A, Culpepper L, Ross R, Ross D, 1999. The expert consensus guideline series: treatment of posttraumatic stress disorder. J. Clin. Psychiatr. 60, 4–76. [PubMed] [Google Scholar]
- Foa EB, Hembree E, Rothbaum BO, 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. Oxford University Press, New York, NY. [Google Scholar]
- Foa EB, Rothbaum BO, 1998. Treating the Trauma of Rape: Cognitive-Behavioral Therapy for PTSD. The Guilford Press, New York. [Google Scholar]
- Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, Puligheddu M, Marrosu F, Biggio G, 2007. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res 1179, 28–34. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G, 1983. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol. Behav. 63, 844–914. [DOI] [PubMed] [Google Scholar]
- Frangos E, Ellrich E, Komisaruk BR, 2015. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 8, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos E, Komisaruk BR, 2017. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul 10, 19–27. [DOI] [PubMed] [Google Scholar]
- Gazi AH, Gurel NZ, Richardson KLS, Wittbrodt MT, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020. Digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: state-space modeling, prediction, and simulation. JMIR mHealth uHealth 8, e20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, Howland R, Kling MA, Moreno F, Rittberg B, Dunner D, Schwartz T, Carpenter L, Burke M, Ninan P, Goodnick P, 2005. A one-year comparison of Vagus Nerve Stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatry 58, 364–373. [DOI] [PubMed] [Google Scholar]
- George MS, Rush AJ, Sackeim HA, Marangell L, 2003. Vagus Nerve Stimulation (VNS): utility in neuropsychiatric disorders. Int. J. Neuropsychopharmacol. 6, 73–83. [DOI] [PubMed] [Google Scholar]
- George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lissanby SH, Burt T, Goldman J, Ballenger JC, 2000. Vagus nerve stimulation: a new tool for brain research and therapy. Biol. Psychiatry 47, 287–295. [DOI] [PubMed] [Google Scholar]
- George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver WB, Wernicke JF, 1994. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on the first 67 patients exiting a controlled study. Epilepsia 35, 637–643. [DOI] [PubMed] [Google Scholar]
- Geracioti TDJ, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PEJ, Kasckow JW, 2001. CSF norepinephrine concentrations in posttraumatic stress disorder. Am. J. Psychiatry 158, 1227–1230. [DOI] [PubMed] [Google Scholar]
- Gill J, Luckenbaugh D, Charney D, Vythilingam M, 2010. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol. Psychiatry 68, 999–1006. [DOI] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, Page GG, 2008. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J. Trauma. Stress 21, 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu L, Guo JC, Jiang XL, Chen F, Gao YS, 2012. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac. J. Trop. Med. 5, 323–325. [DOI] [PubMed] [Google Scholar]
- Gurel NZ, Gazi AH, Scott KL, Wittbrodt MT, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020a. Timing considerations for noninvasive Vagal Nerve Stimulation in clinical studies. AMIA Annual Sympos. Proc. 2019, 1061–1070. [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MH, Ko Y-A, Shallenberger L, Nye JA, Pearce B, Vaccarino V, Shah AJ, Bremner JD, Inan OT, 2020b. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul 13, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Jiao Y, Wittbrodt MT, Hankus A, Driggers EG, Shallenberger L, Murrah N, Huang M, Haffar A, Alkhalaf ML, Levantsevych OM, Nye JA, Vaccarino V, Shah AJ, Bremner JD, Inan OT, Pearce BD, 2020c. Effect of transcutaneous vagus nerve stimulation on the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) response to stress: a randomized, sham controlled, double blind pilot study. Comprehens. Psychoneuroendocrinol. 4, 100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Mobashir HS, Bremner JD, Vaccarino V, Ladd SL, Shah A, Inan OT, 2018. Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: a proof-of-concept study. IEEE: Body Sensor Networks (BSN) 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Wittbrodt MT, Jung H, Shandhi MH, Driggers EG, Ladd SL, Huang M, Ko Y-A, Shallenberger L, Becwith J, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Inan OT, Bremner JD, 2020d. Transcutaneous vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: a double-blind, randomized, sham controlled trial. Neurobiol. Stress 13, e100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Wittbrodt WT, Jung H, Ladd SL, Shah AJ, Vaccarino V, Bremner JD, Inan OT, 2020e. Automatic detection of target engagement in transcutaneous cervical Vagal Nerve Stimulation for traumatic stress triggers. IEEE J. Biomed. Health Inform. 24, 1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 12, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA, 2017a. The Mental Stress Ischemia Prognosis Study (MIPS): objectives, study design, and prevalence of inducible ischemia. Psychosom. Med. 79, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Samman Tahhan A, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko Y-A, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA, 2017b. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int. J. Cardiol. 243, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EJ, Uthman BM, Wilder BJ, Ben-Menachem E, Hamberger A, Hedner T, Ekman R, 1992. Neurochemical effects of vagus nerve stimulation in humans. Brain Res 583, 300–303. [DOI] [PubMed] [Google Scholar]
- Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris G.L.r., Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW, 1998. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 51, 48–55. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL, 2004. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav. Neurosci. 118, 79–88. [DOI] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, Kilgard MP, 2014. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. Neuroreport 25, 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Rennaker RL, Kilgard MP, 2013. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 207, 275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X, 2003. Do patients drop out prematurely from exposure therapy for PTSD? J. Trauma. Stress 16, 555–562. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM, 2009. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress. Anxiety 26, 447–455. [DOI] [PubMed] [Google Scholar]
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA, 2017. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Neurology 289, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies, 2014. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. National Academies of Science, Engineering and Medicine: Health and Medicine Division, Washington, D.C. [PubMed] [Google Scholar]
- Jacobs HIL, Riphagen JM, Razat CM, Wiese S, Sack AT, 2015. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol. Aging 36, 1860–1867. [DOI] [PubMed] [Google Scholar]
- Jan BU, Coyle SM, Macor MA, Reddell M, Calvano SE, Lowry SF, 2010. Relationship of basal heart rate variability to in vivo cytokine responses following endotoxin. Shock 33, 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Finlay JM, Sved AF, Grace AA, 2001. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol. Psychiatry 49, 351–359. [DOI] [PubMed] [Google Scholar]
- Kinzie JD, Leung P, 1989. Clonidine in Cambodian patients with posttraumatic stress disorder. J. Nerv. Ment. Dis. 177, 546–550. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA, 1998. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39, 709–714. [DOI] [PubMed] [Google Scholar]
- Krames E, Peckham PH, Rezai AR, 2018. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies, 2 ed. Academic Press (an imprint of Elsevier), London, United Kingdom. [Google Scholar]
- Lamb DG, Porges EC, Lewis GF, Williamson JB, 2017. Non-invasive Vagal Nerve Stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: preliminary evidence. Front. Med. 4, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster CL, Teeters JB, Gros DF, Back SE, 2016. Posttraumatic Stress Disorder: overview of evidence-based assessment and treatment. J. Clin. Med. 5, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL, 1995. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom. Med. 57, 105–115. [DOI] [PubMed] [Google Scholar]
- Lerman I, Davis B, Huang M, Huang C, Sorkin L, Proudfoot J, Zhong E, Kimball D, Rao R, Simon B, Spadoni A, Strigo I, Baker DG, Simmons AN, 2019. Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PLoS One 14, e0201212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, Huang A, Lam K, Simon B, Baker DG, 2016. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation 19, 283–290. [DOI] [PubMed] [Google Scholar]
- Levine ES, Litto WJ, Jacobs BL, 1990. Activity of cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res 531, 189–195. [DOI] [PubMed] [Google Scholar]
- Li W, Olshansky B, 2011. Inflammatory cytokines and nitric oxide in heart failure and potential modulation by vagus nerve stimulation. Heart Fail. Rev. 16, 137–145. [DOI] [PubMed] [Google Scholar]
- Li X, Wilder-Smith CH, Kan ME, Lu J, Cao Y, Wong RK, 2014. Combat-training stress in soldiers increases S100B, a marker of increased blood-brain-barrier permeability, and induces immune activation. Neuro Endocrinol. Lett. 35. [PubMed] [Google Scholar]
- Lima BB, Hammadah M, Wilmot K, Pearce BD, Shah A, Levantsevych O, Kaseer B, Obideen M, Gafeer MM, Kim JH, Sullivan S, Lewis TT, Weng L, Elon L, Li L, Bremner JD, Raggi P, Quyyumi A, Vaccarino V, 2019. Posttraumatic Stress Disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain. Behav. Immun. 75, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Dhabhar FS, Mellon SH, Yehuda R, Grenon M, Flory JD, Bierer LM, Abu-Amara D, Coy M, Makotkine I, Reus VI, Bersani FS, Marmar CR, Wolkowitz OM, 2017. Increased pro-inflammatory milieu in combat related PTSD – A new cohort replication study. Brain. Behav. Immun. 59, 260–264. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, Makotkine I, Reus VI, Yan X, Taylor NM, Marmar CR, Dhabhar FS, 2014. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain. Behav. Immun. 42, 81–88. [DOI] [PubMed] [Google Scholar]
- Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y, Wang X, Park J, Jin Y, Liu C, Zhu B, Kong J, 2016. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J. Affect. Disord. 205, 319–326. [DOI] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I, 1988. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 14, 61–68. [DOI] [PubMed] [Google Scholar]
- Malloy PF, Fairbank JA, Keane TM, 1983. Validation of a multimethod assessment of posttraumatic stress disorders in Vietnam veterans. J. Consult. Clin. Psychol. 51, 488–494. [DOI] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P, 2009a. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatr. Neurosci. 34, 272–280. [PMC free article] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P, 2009b. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur. Neuropsychopharmacol. 19, 250–255. [DOI] [PubMed] [Google Scholar]
- Manta S, El Mansari M, Debonnel G, Blier P, 2013. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int. J. Neuropsychopharmacol. 16, 459–470. [DOI] [PubMed] [Google Scholar]
- Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, Nahas Z, Lisanby SH, 2002. Vagus Nerve Stimulation (VNS) for major depressive episodes: Longer-term outcome. Biol. Psychiatry 51, 280–287. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB, 2007. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom. Med. 69, 709–716. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, 1988. Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. J. Nerv. Ment. Dis. 176, 498–502. [DOI] [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, Veith RC, 1990. Autonomic responses to stress in Vietnam combat veterans with posttraumatic stress disorder. Biol. Psychiatry 27, 1165–1175. [DOI] [PubMed] [Google Scholar]
- McFall ME, Veith RC, Murburg MM, 1992. Basal sympathoadrenal function in posttraumatic stress disorder. Biol. Psychiatry 31, 1050–1056. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, 1985. Peripheral and central adrenergic influences on brain systems involved in the modulation of memory storage. Ann. N. Y. Acad. Sci. 444, 150–161. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathphysiology of depression. Biol. Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Sutherland AG, Hutchison JD, Alexander DA, 2001. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine 13, 253–255. [DOI] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR, 2006. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J. Neurosci. 26, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GH, Ali FF, 2016. Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr. Opin. Pharmacol. 29, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzoni M, 2011. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 189, 207–214. [DOI] [PubMed] [Google Scholar]
- NIMH, 1970. CGI: clinical Global Impressions, In: Guy W, Bonato RR (Eds.), Manual for the ECDEU Assessment Battery. National Institute of Mental Health, Chevy Chase MD, pp. 1–6. [Google Scholar]
- Nisenbaum LK, Abercrombie ED, 1993. Presynaptic alterations associated with enhancement of evoked release and synthesis of NE in hippocampus of chemically cold stressed rats. Brain Res 608, 280–287. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED, 1991. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J. Neurosci. 11, 1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Souza RR, McIntyre CK, 2019. Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology (Berl.) 236, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonis R, D’Ostilio K, Schoenen J, Magis D, 2017. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: an electrophysiological study in healthy volunteers. Cephalagia 37, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Ahern CE, Berry NJ, Pitman RK, 1998. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. J. Consult. Clin. Psychol. 66, 906–913. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK, 1995. Physiological responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J. Abnorm. Psychol. 104, 75–82. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR, 1993. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J. Abnorm. Psychol. 102, 152–159. [DOI] [PubMed] [Google Scholar]
- Orr SP, Roth WT, 2000. Psychophysiological assessment: clinical applications for PTSD. J. Affect. Disord. 61, 225–240. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Murphy AL, Hekierski H, Cooper M, Simon BJ, 2014. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 155, 2037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Piccalluga E, Turiel M, Basellli G, Malliani A, 1986. Power spectral analysis of heart rate and arterial pressure variability as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59, 178–193. [DOI] [PubMed] [Google Scholar]
- Passos CI, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhães PV, Kapczinski F, Kauer-Sant’Anna M, 2015. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Pena DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S, 2014. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña DF, Engineer ND, McIntyre CK, 2013. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatry 73, 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F, Kramer G, Wilson L, Chae YL, 1993. Learned helplessness and in vivo hippocampal norepinephrine release. Pharmacol. Biochem. Behav. 46, 231–235. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH, 2013. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 18, 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond D, Huang Y, 1979. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci 25, 2149–2162. [DOI] [PubMed] [Google Scholar]
- Reinertsen E, Nemati S, Vest AN, Vaccarino V, Lampert R, Shah AJ, Clifford GD, 2017. Heart rate-based window segmentation improves accuracy of classifying posttraumatic stress disorder using heart rate variability measures. Physiol. Meas. 38, 1061–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, Zhao Y, Wang H, Vangel M, Sun S, Ben H, Park J, Li S, Meng H, Zhu B, Kong J, 2016. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J. Affect. Disord. 195, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA, 2006. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain 1119, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell J, Colominas J, Riu P, Pallas-Areny R, Webster JG, 1988. Skin impedance from 1 Hz to 1 MHz. IEEE Trans. Biomed. Eng. 35, 649–651. [DOI] [PubMed] [Google Scholar]
- Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE, 2003. Psychometric properties of the PTSD Checklist—Civilian version. J. Trauma. Stress 16, 495–502. [DOI] [PubMed] [Google Scholar]
- Rush AJ, George MS, Sackeim HA, Marangell LB, Husain M, Giller C, Nahas Z, Haines S, Simson RK, Goodman R, Burt T, 2000. Vagus Nerve Stimulation (VNS) for treatment-resistant depression: a multicenter study. Biol. Psychiatry 47, 276–286. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis S, Howland M, Kling R, Rittberg MA, Burke BR, Rapaport WJ, Zajecka MH, Nierenberg J, Husain AA, Ginsberg MM, Cooke DRG, 2005a. Vagus Nerve Stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol. Psychiatry 58, 347–354. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, Lavori P, Howland R, Kling MA, Rittberg B, Carpenter L, Ninan P, Moreno F, Schwartz T, Conway C, Burke M, Barry JJ, 2005b. Effects of 12 Months of Vagus Nerve Stimulation in treatment-resistant depression: a naturalistic study. Biol. Psychiatry 58, 355–363. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Brannan SK, Rush AJ, George MS, Marangell LB, Allen J, 2007. Durability of antidepressant response to vagus nerve stimulation (VNS). Int. J. Neuropsychopharmacol. 10, 817–826. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, Dormer JS, Burt T, Lisanby SH, Husain M, Collum M, Oliver NC, Zboyan HA, 2001a. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsych. Neuropsychol. Behav. Neurol. 14, 53–62. [PubMed] [Google Scholar]
- Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, Simpson RK, Goodman RR, 2001b. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25, 713–728. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB, 1999. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. The vagus nerve stimulation study group. Arch. Neurol. 53, 1176–1180. [DOI] [PubMed] [Google Scholar]
- Schachter SC, Saper CB, 1998. Vagus nerve stimulation. Epilepsia 39, 677–686. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea T, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N, 2007. Cognitive behavioral therapy for posttraumatic stress disorder in women. J. Am. Med. Assoc. 297, 820–830. [DOI] [PubMed] [Google Scholar]
- Schomer AC, Nearing BD, Schachter SC, Verrier RL, 2014. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug-resistant focal epilepsy. Epilepsia 55, 1996–2002. [DOI] [PubMed] [Google Scholar]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH, 2008. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry 71, 134–168. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V, 2013. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol. Psychiatry 73, 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, et al. , 1998. A prospective study of heart rate responses following trauma and the subsequent development of posttraumatic stress disorder. Arch. Gen. Psychiatry 55, 553–559. [DOI] [PubMed] [Google Scholar]
- Sjögren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E, 2002. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J. Clin. Psychiatr. 63, 972–980. [DOI] [PubMed] [Google Scholar]
- Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW, 2005. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J. Neurotrauma 22, 1485–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, Nicolaou A, Nagy LM, Johnson DR, Heninger GR, Charney DS, 1997. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry 54, 749–758. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS, 1993. Abnormal noradrenergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry 50, 266–274. [DOI] [PubMed] [Google Scholar]
- Souza RR, Robertson NM, Pruitt DT, Gonzales PA, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK, 2019. Vagus nerve stimulation reverses the extinction impairments in a model of PTSD with prolonged and repeated trauma. Stress 22, 509–520. [DOI] [PubMed] [Google Scholar]
- Sun L, Perakyla J, Holm K, Haapasalo J, Lehtimaki K, Ogawa KH, Peltola J, Hartikainen KM, 2017. Vagus nerve stimulation improves working memory performance. J. Clin. Exp. Neuropsychol. 954–964. Vagus nerve stimulation improves working memory performance.
- Sutherland AG, Alexander DA, Hutchison JD, 2003. Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine 24, 219–225. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H, 2000. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur. J. Pharmacol. 405, 397–406. [DOI] [PubMed] [Google Scholar]
- Tarn J, Legg S, Mitchell S, Simon B, Ng W-F, 2019. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary Sjögren’s Syndrome. Neuromodulation 22, 580–585. [DOI] [PubMed] [Google Scholar]
- Terry RS, 2014. Vagus Nerve Stimulation for Epilepsy. [Google Scholar]
- Thayer JF, Lane RD, 2007. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychiatry 74, 224–242. [DOI] [PubMed] [Google Scholar]
- The Vagus Nerve Stimulation Study Group, 1995. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 45, 224–230. [DOI] [PubMed] [Google Scholar]
- Torda T, Kvetnansky R, Petrikova M, 1984. Effect of repeated immobilization stress on rat central and peripheral adrenoceptors. In: Usdin E, Kvetnansky R, Axelrod J (Eds.), Stress: The Role of Catecholamines and Other Neurotransmitters. Gordon & Breach, New York, pp. 691–701. [Google Scholar]
- Tortella G, Casati R, Aparicio LVM, Mantovani A, Senço N, D’Urso G, Brunelin J, Guarienti F, Lorencini Selingardi PM, Muszkat D, de Sampaio Pereira B, Valiengo L, Moffa AH, Simis M, Borrione L, Brunoni AR, 2015. Transcranial direct current stimulation in psychiatric disorders. World J. Psychiatr. 5, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Fang J, Cao J, Wang Z, Park J, Jorgenson K, Lang C, Liu J, Zhang G, Zhao Y, Zhu B, Rong P, Kong J, 2018. A distinct biomarker of continuous transcutaneous vagus nerve stimulation treatment in major depressive disorder. Brain Stimul 11, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Jeon-Slaughter H, Pfefferbaum B, Khan Q, Davis NJ, 2010. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. Am. J. Disaster Med. 5, 113–125. [DOI] [PubMed] [Google Scholar]
- Ura H, Sugaya Y, Ohata H, Takumi I, Sadamoto K, Shibasaki T, Maru E, 2013. Vagus nerve stimulation induced long-lasting enhancement of synaptic transmission and decreased granule cell discharge in the hippocampal dentate gyrus of urethane-anesthetized rats. Brain Res 1492, 63–71. [DOI] [PubMed] [Google Scholar]
- Vidovic A, Gotovac K, Vilibic M, Sabioncello A, Jovanovic T, Rabatic S, Folnegovic-Smalc V, Dekaris D, 2011. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 18, 199–211. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Begre S, Abbas CC, Saner H, Gander ML, Schmid JP, 2010. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation 17, 39–46. [DOI] [PubMed] [Google Scholar]
- von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U, 2007. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 41, 744–752. [DOI] [PubMed] [Google Scholar]
- Vonck K, Raedt R, Naulaerts J, De Vogelaere F, Thiery E, Van Roost D, Aldenkamp B, Miatton M, Boon P, 2014. Vagus nerve stimulation. 25 years later! What do we know about the effects on cognition? Neurosci. Biobehav. Rev. 45, 63–71. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fang J, Liu J, Rong P, Jorgenson K, Park J, Lang C, Hong Y, Zhu B, J., K., 2018. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J. Psychiatr. Res. 102, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, Marx BP, 2018. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 30, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, Arck PC, Deter HC, 2010. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur. J. Appl. Physiol. 109, 201–211. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, 1981. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res. Rev. 3, 167–2015. [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, Huang M, Shah AJ, Pearce BD, Alam ZS, Rapaport MH, Murrah N, Ko Y-A, Haffer AA, Shallenberger LH, Vaccarino V, Inan OT, Bremner JD, 2020. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul 13, 1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AB, Page GG, O’Campo P, Pugh LC, Ford D, Campbell JC, 2005. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am. J. Community Psychol. 36, 159–175. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Mirandam PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA, 2016. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 127, 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N, Kim SS, Nam E-C, 2017. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 20, 290–300. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Siever LJ, Teicher MH, 1998. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry 44, 56–63. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M, 2014. Dysregulation in microRNA expression Is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.