Abstract
Glutamatergic synapses are key cellular sites where cocaine experience creates memory traces that subsequently promote cocaine craving and seeking. In addition to making across-the-board synaptic adaptations, cocaine experience also generates a discrete population of new synapses that selectively encode cocaine memories. These new synapses are glutamatergic synapses that lack functionally stable AMPARs, often referred to as AMPAR-silent synapses or, simply, silent synapses. They are generated de novo in the NAc by cocaine experience. After drug withdrawal, some of these synapses mature by recruiting AMPARs, contributing to the consolidation of cocaine-associated memory. After cue-induced retrieval of cocaine memories, matured silent synapses alternate between two dynamic states (AMPAR-absent vs AMPAR-containing) that correspond with the behavioral manifestations of destabilization and reconsolidation of these memories. Here, we review the molecular mechanisms underlying silent synapse dynamics during behavior, discuss their contributions to circuit remodeling, and analyze their role in cocaine-memory-driven behaviors. We also propose several mechanisms through which silent synapses can form neuronal ensembles as well as cross-region circuit engrams for cocaine-specific behaviors. These perspectives lead to our hypothesis that cocaine-generated silent synapses stand as a distinct set of synaptic substrates encoding key aspects of cocaine memory that drive cocaine relapse.
Introduction
Substance use disorder (SUD) is an acquired behavioral state that develops through repeated substance experience. While having substantial pathologic features, the development and maintenance of SUD share many key plasticity mechanisms and exhibit memory features that are commonly observed in learning and memory processes, including memory acquisition, consolidation, retrieval-induced destabilization, and reconsolidation (Torregrossa and Taylor, 2013; Everitt and Robbins, 2016). Indeed, SUD has been conceptualized as an extreme form of memory such that the underlying plasticity substrates can be targeted to decrease drug seeking and taking (Hyman et al., 2006).
In an attempt to identify neural underpinnings of SUD, intensive prior studies have identified several critical forms of drug-induced neural adaptations that promote the development of drug-associated behaviors (Wolf, 2016; Wright and Dong, 2020). Despite much exciting progress, our understanding has been partially limited to changes occurring in neuronal and synaptic populations that are broadly implicated in reward response, but not necessarily unique to SUD. For example, it has been shown that, following cocaine self-administration, synaptic potentiation is detected within the majority of hippocampal projections to medium spiny neurons (MSNs) that express D1 dopamine receptors (D1-MSNs) in the NAc, and this potentiation promotes cocaine seeking after cocaine withdrawal (Pascoli et al., 2014; Zhou et al., 2019). However, potentiating the majority of hippocampal inputs to NAc D1-MSNs also promotes seeking of nondrug rewards (LeGates et al., 2018). Are there any neuronal substrates or synaptic alterations that are unique to drug experience?
Recent studies have identified a population of immature glutamatergic synapses, namely, AMPAR-silent synapses, which are generated de novo within the NAc after cocaine administration (Y. H. Huang et al., 2009; J. Wang et al., 2021a) (Fig. 1). After drug withdrawal, these synapses exhibit three major dynamic changes corresponding to the consolidation, retrieval-induced destabilization, and reconsolidation of cocaine memories (Lee et al., 2013; Ma et al., 2014; Wright et al., 2020). Although new synapses are constitutively generated through metabolic turnover or by other experiences, here we discuss the possibility that cocaine-generated synapses represent a discrete population of neuronal substrates that encode cocaine-associated memories. We will make a conceptual generalization by exploring how a unique set of nascent synapses is generated by a particular experience to contribute to the formation of neuronal engrams and unique circuit activity patterns corresponding to this particular experience.
Figure 1.
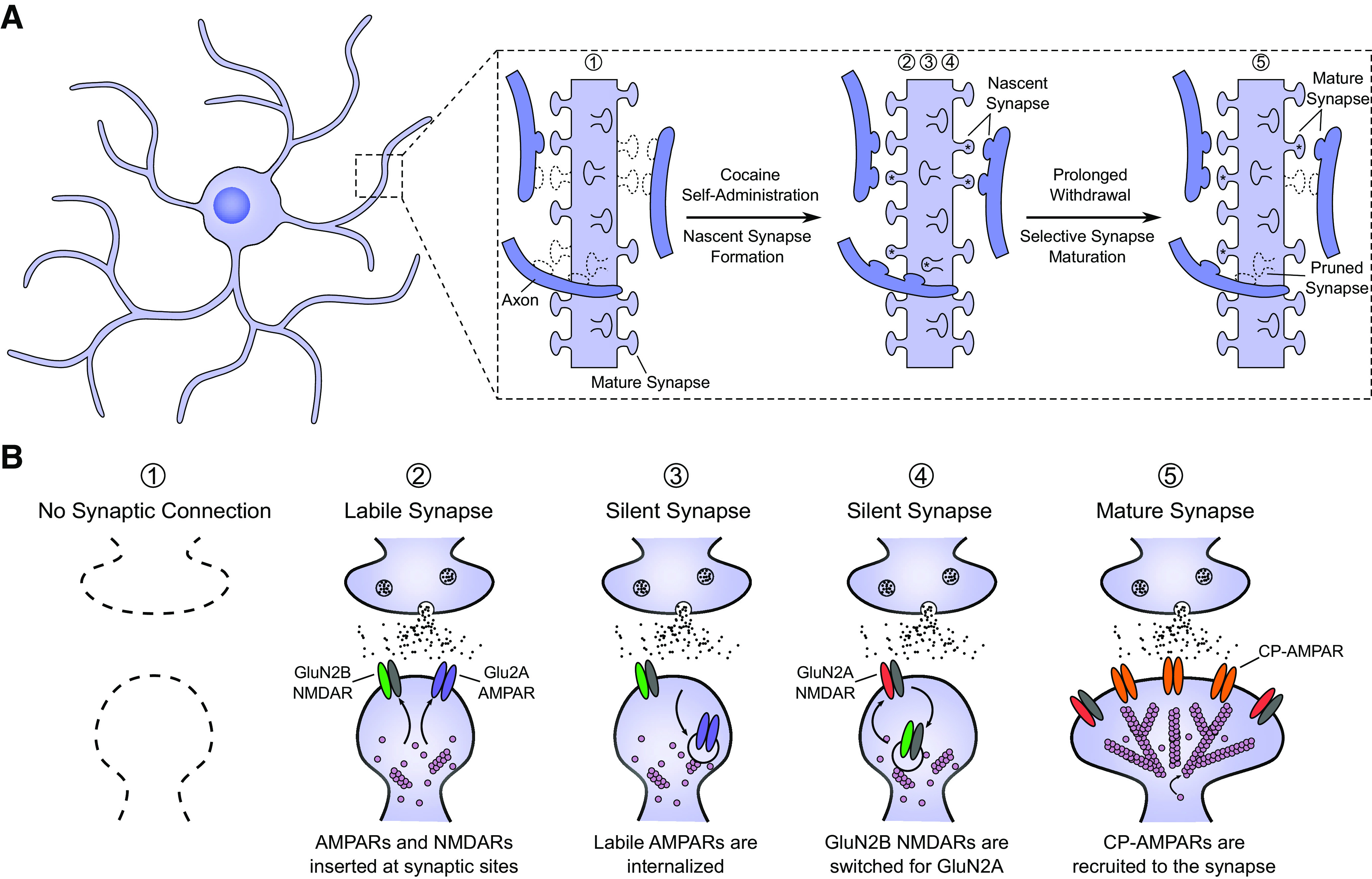
Cocaine-induced generation of silent synapses in the NAc. A, Schematic depiction of synaptogenesis in an MSN within the NAc during and following cocaine self-administration. During self-administration training, nascent, immature synapses are generated along the dendritic arbor, forming new connections. During withdrawal, some of these synapses mature into functional synapses, whereas others are presumably pruned away. Numbers correspond to the stages depicted in B. B, Illustration of the stages of cocaine-induced synapse generation and maturation. During cocaine self-administration, synaptogenic processes are triggered. This leads to the generation of new synapses, which contain both GluN2B-containing NMDARs and GluA2-containing AMPARs. These AMPARs, however, are highly labile and are soon internalized, resulting in silent synapses containing only NMDARs. During early withdrawal, GluN2B-containing NMDARs are replaced with GluN2A-containing NMDARs. This is critical for the subsequent functional maturation of the synapse mediated by the recruitment of CP-AMPARs to the synapse.
Memory encoding: role of synapses and synaptogenesis
A prominent goal of SUD neuroscientists is to determine the highly specific neuronal substrates that underlie drug-associated memories. Hope arises from the Engram Hypothesis, which proposes that a given memory is encoded by a select set of persistent structural and functional changes in the brain, termed the engram (Semon, 1921; Josselyn and Tonegawa, 2020). Synaptic connections between neurons have long been hypothesized to encode such memory traces through experience-induced plasticity (Ramon y Cajal, 1894; Hebb, 1949). Experience-induced synaptic plasticity can be expressed as changes in the weight of preexisting synapses without changing circuit connectivity or, alternatively, addition/elimination of synapses that results in circuit rewiring (Chklovskii et al., 2004). In either case, if unique to an experience, these synapses may bear a “synaptic engram” (Josselyn et al., 2015). On the other hand, sparsely distributed neurons that are selectively activated during formation or retrieval of a memory can bear a “neuronal engram” (Josselyn et al., 2015; Tonegawa et al., 2015). To capture engram neurons and synapses experimentally, many laboratories take advantage of activity-dependent biomarkers, with the assumption that the engram substrates would exhibit increased activities during memory formation and retrieval. Consequently, a relatively small number of engram neurons and synapses are tagged and defined among their numerous peers in response to a specific experience (Josselyn et al., 2015; Tonegawa et al., 2015). These synaptic and neuronal engrams are not mutually exclusive but are, at least in part, linked mechanistically. For example, some neurons can be “tuned” by remodeled synaptic inputs to selectively respond to a specific experience, thus becoming engram neurons. In this case, synaptic engrams may serve as the subcellular basis for neuronal engrams.
It has been demonstrated convincingly that selective inhibition of neurons in a neuronal engram (i.e., engram neurons) for a memory disrupts the behavioral expression of this memory (Tonegawa et al., 2015; Josselyn and Tonegawa, 2020). However, some neurons in one neuronal ensemble may also participate in another neuronal ensemble that encodes a different memory (Cai et al., 2016; Rashid et al., 2016; Yokose et al., 2017; Abdou et al., 2018). Such an overlap provides a mechanism for memory linkage or generalization (Cai et al., 2016; Yokose et al., 2017), but it also indicates the likelihood that targeting individual engram neurons may simultaneously interfere with nontargeted memories. Indeed, it is the temporal activities, rather than their physical existence, of engram neurons that express the memory for a neuronal engram. Specifically, neurons can participate in multiple engrams, but at a given time (e.g., during memory recall), activity of neurons within a pretuned neuronal engram supports a unique network activity pattern. The uniquely patterned temporal circuit dynamics are thought to be a circuit representation for a specific memory (Sussillo, 2014; Yuste, 2015; Kyriazi et al., 2018; Ruff et al., 2018; Gonzalez et al., 2019; Ju and Bassett, 2020). As such, the synaptic engram, neuronal engram, and circuit dynamics can be regarded as neural manifestations of the same memory perceived from three different anatomic angles, with synapses as the key subcellular basis defining the circuit connectivity that orchestrates the temporal dynamics of ensemble neurons and circuit activities.
In theory, generation of new memory-encoding circuit-activity patterns can be achieved by modification of the weight of preexisting synapses without changing the synaptic connectivity of the circuit. For example, experience-induced LTP and LTD may preferentially occur at different synapse populations to redefine the circuit activity dynamics. However, the fixed synaptic connectivity also entails a “range constraint” on a circuit and limits the encoding capacity (Sadtler et al., 2014; Oby et al., 2019). This constraint can be overcome at least in part by experience-dependent modification of the number of synapses. Indeed, experience-dependent synapse formation and elimination have been associated with the emergence of new circuit activity patterns (Peters et al., 2014) and formation of new memories (Yang et al., 2009; Fu et al., 2012; Lai et al., 2012; Parkhurst et al., 2013; Chen et al., 2015), including memories associated with SUD (Russo et al., 2010; Muñoz-Cuevas et al., 2013). Formation of neuronal engrams is also associated with synaptogenesis (Ryan et al., 2015; Choi et al., 2018), and reactivation of engram neurons during memory retrieval is dependent on these new synaptic connections (Ryan et al., 2015). Furthermore, synapses generated by different experiences may coexist on the same neurons but encode distinct memories, such that disrupting the function of one synapse population impairs only its associated memory (Yang et al., 2014; Cichon and Gan, 2015; Hayashi-Takagi et al., 2015). Such a compartmentalized arrangement allows different synaptic populations to embed different memory traces in the same neurons (Losonczy et al., 2008; Cichon and Gan, 2015), such that the same neurons may contribute to multiple memories (Abdou et al., 2018).
The above literature analysis supports the hypothesis that a memory can be selectively encoded in a specific synapse population, or a synaptic engram, that is generated de novo during learning. This hypothesis is similar to the previously proposed synaptic tagging hypothesis (Chklovskii et al., 2004; Rogerson et al., 2014; Holtmaat and Caroni, 2016) but conceptualizes new synapses generated by an experience as an independent synaptic engram that encodes nonoverlapping memory traces. Such synapses should have several key features: (1) they are generated by a learning experience, (2) they contribute to subsequent memory recall, and (3) changes in their functional state underlie the dynamics of a memory. Below, we review the features and mechanisms of cocaine-generated silent synapses, followed by our perspective of how these synapses may represent a synaptic engram encoding specific aspects of cocaine memories.
Mechanisms and functions of cocaine-generated silent synapses
Cocaine-induced generation of silent synapses
During brain development, immature glutamatergic synapses often contain only NMDARs without functionally stable AMPARs, and are thus called AMPAR-silent synapses (Kerchner and Nicoll, 2008). After development, synaptogenesis and silent synapses decline to low levels (Durand et al., 1996; Petralia et al., 1999), but the basic synaptogenic capacity remains throughout adulthood to contribute to experience-induced synaptic remodeling (Holtmaat and Svoboda, 2009). Under certain learning conditions, new glutamatergic synapses are generated in the adult brain, and these, like nascent synapses during development, often have thin or filopodia-like postsynaptic structures initially (Holtmaat et al., 2006; Knott et al., 2006), and then undergo gradual enlargement, a process corresponding to functional maturation via incorporation of AMPARs (Matsuzaki et al., 2004). Similar generation of silent synapses is observed during fear conditioning and likely other learning processes (Suvrathan et al., 2013; W. Ito et al., 2015; Y. Wang et al., 2018; W. Ito and Morozov, 2019). Thus, silent synapse-based synaptogenesis may serve as a general, basic strategy for certain learning processes to remodel related neural circuits during memory encoding.
Exposure to cocaine and other psychostimulants increases the density of dendritic spines in NAc MSNs (Robinson and Kolb, 1997, 1999; Robinson et al., 2001), suggesting that synaptogenesis occurs. Echoing these findings, cocaine experience induces generation of silent synapses in NAc MSNs (Y. H. Huang et al., 2009; Koya et al., 2012; Lee et al., 2013; Whitaker et al., 2016) (Fig. 1). Several features of these cocaine-generated silent synapses are consistent with their being nascent, immature synapses: (1) they are enriched in GluN2B-containing NMDARs (Y. H. Huang et al., 2009; Brown et al., 2011; Wright et al., 2020), a hallmark of immature glutamatergic synapses during development (Monyer et al., 1994; Kirson and Yaari, 1996; Tovar and Westbrook, 1999); (2) the majority of cocaine-generated silent synapses are not formed by AMPAR internalization at preexisting synapses (Graziane et al., 2016; Y. Q. Wang et al., 2021); (3) cocaine-induced generation of silent synapses is tightly correlated with a selective increase in thin and filopodia-like spines, which may represent immature glutamatergic synapses (Graziane et al., 2016; Wright et al., 2020); and (4) disrupting astrocytic thrombospondin-α2δ−1 signaling, a signaling pathway that promotes synaptogenesis during brain development, prevents both the generation of silent synapses and the increase in spine density after cocaine experience (J. Wang et al., 2021a). These results argue that cocaine-generated silent synapses are a discrete set of new synapses related to cocaine experience.
The mechanisms governing experience-induced synaptogenesis in the adult brain are complex and incompletely understood (Waites et al., 2005; Südhof, 2018). To date, several mechanistic processes that have been characterized for generating silent synapse in the adult brain are similar to those underlying synaptogenesis during development (Dong and Nestler, 2014). A prominent molecular mechanism is upregulation of the activity of CREB and other transcriptional signaling pathways, which promotes transcription of synaptogenic proteins (McClung and Nestler, 2003) and drives generation of silent synapses (Marie et al., 2005; Brown et al., 2011; Grueter et al., 2013; for thorough reviews, see Dong and Nestler, 2014; Nestler and Lüscher, 2019). Another cellular step is synaptic insertion of GluN2B-containing NMDARs (Y. H. Huang et al., 2009; Y. Q. Wang et al., 2021), an NMDAR subtype critically involved in synaptogenesis and silent synapse generation during both development and adulthood (Tovar and Westbrook, 1999; Nakayama et al., 2005; Gambrill and Barria, 2011; Chung et al., 2017). Recent results suggest that GluA2-containing AMPARs are inserted simultaneously with GluN2B-NMDARs during initial synapse generation, but the AMPARs are quickly internalized, resulting in an AMPAR-silent state shortly after synaptogenesis (Y. Q. Wang et al., 2021). Such a two-step process is again reminiscent of synaptogenesis during development (Xiao et al., 2004; Groc et al., 2006). In addition to neuronal mechanisms, cocaine-induced generation of silent synapses requires an astrocyte-mediated synaptogenic signaling pathway. Specifically, cocaine experience induces astrocytic release of thrombospondin-2, which, in turn, activates its neuronal receptor α2δ−1, promoting silent synapse generation in the NAc (J. Wang et al., 2021a). This and other astrocytic signaling pathways have been critically implicated in synapse generation, maintenance, and elimination during both development and adulthood (Eroglu and Barres, 2010; Allen and Eroglu, 2017; J. Wang et al., 2021b). Through localized activities, astrocyte processes can initiate and regulate synaptogenesis at specific dendritic segments (Eroglu and Barres, 2010; Martín et al., 2015; Allen and Eroglu, 2017; Martín-Fernández et al., 2017), and may even define the location within a circuit where new synapses are formed. These findings highlight that cocaine-induced generation of new synapses involves synaptogenic mechanisms normally used by the developing brain.
While the majority of silent synapses generated after cocaine experience are new, immature synapses, a portion of them originate from preexisting synapses after AMPAR internalization (Y.D., unpublished data), and this synaptic weakening process may eventually lead to synapse elimination. As another effective means to restructure the circuit connectivity, it is not surprising that experience-induced synapse elimination is also important for memory formation (Yang et al., 2009; Chen et al., 2015; Li et al., 2017). Related to this, morphine experience induces synapse elimination in NAc D2-MSNs, whereas disrupting this elimination compromises the retention of morphine-conditioned place preference, a reward conditioned spatial memory (Graziane et al., 2016).
Maturation or elimination: “to be or not to be” of cocaine-generated synapses
After cocaine experience, silent synapses do not persist in the AMPAR-silent state indefinitely but “disappear” after several days of withdrawal (Y. H. Huang et al., 2009; Lee et al., 2013). In general, nascent synapses have two possible fates: (1) to mature and, thus, be unsilenced and incorporated into the circuit; or (2) to be eliminated (Yang et al., 2009; Dong and Nestler, 2014). The first scenario is supported by observations that the increase in spine density following cocaine experience persists through withdrawal periods; and importantly, it is accompanied by a conversion of thin/filopodia-like spines to mushroom-like spines (Graziane et al., 2016; Wright et al., 2020), suggesting synaptic maturation. Furthermore, the disappearance of silent synapses corresponds with an upregulation of AMPARs, specifically atypical, Ca2+-permeable AMPARs (CP-AMPARs). Blocking CP-AMPARs restores high levels of silent synapses after drug withdrawal (Conrad et al., 2008; Lee et al., 2013; Wright et al., 2020).
Recent results have begun to reveal key mechanistic steps during maturation of cocaine-generated silent synapses. First, it appears that GluN2B-containing NMDARs regulate the pace of maturation. During development, GluN2B-NMDARs at nascent glutamatergic synapses inhibit AMPAR insertion, maintaining the synapses in the AMPAR-silent state (Adesnik et al., 2008; Gray et al., 2011). Subsequent synaptic maturation through AMPAR stabilization coincides with a switch of NMDARs from GluN2B- to GluN2A-containing subtypes (Monyer et al., 1994). Mirroring this developmental process, GluN2B NMDARs at cocaine-generated synapses are replaced with non-GluN2B, presumably GluN2A, NMDARs during early withdrawal from cocaine (Y. H. Huang et al., 2009; Wright et al., 2020). This replacement is required for the maturation of cocaine-generated synapses, such that disrupting this replacement keeps cocaine-generated synapses in the immature, silent state (Y. Q. Wang et al., 2021). Second, recruitment and stabilization of AMPARs at newly matured silent synapses during cocaine withdrawal may require coordinative interactions between postsynaptic density (PSD) scaffold proteins. Scaffold proteins, particularly PSD-95, stabilize synaptic AMPARs within the PSD, whereas genetic deletion of PSD-95 results in high basal levels of silent synapses in the adult brain (El-Husseini et al., 2000; Béïque et al., 2006; Ehrlich et al., 2007; Cane et al., 2014; Meyer et al., 2014; X. Huang et al., 2015; Favaro et al., 2018). Upon PSD-95 knockout or knockdown, cocaine experience still generates silent synapses in NAc MSNs, suggesting intact generation processes. However, these synapses do not mature after prolonged drug withdrawal, indicating failed insertion and/or stabilization of AMPARs (Shukla et al., 2017).
While a large portion of cocaine-generated silent synapses mature after drug withdrawal, it is speculated that some of them do not (Lee et al., 2013). This is not surprising because a similar phenomenon has been documented in the cortex, in which a portion of experience-generated synapses do not mature but are eliminated eventually (Yang et al., 2009; Li et al., 2017). Thus, selection mechanisms must exist for preservation versus elimination of these synapses. One potential mechanism involves activity-dependent synaptic maturation. It is believed that new synapse generation tends to be excessive, and only those that are frequently used eventually mature and are integrated into the circuit (Katz and Shatz, 1996; Cohen-Cory, 2002; Waites et al., 2005; Stephan et al., 2012; Holtmaat and Caroni, 2016). In this scenario, synapse generation has a degree of randomness, allowing sufficient connection opportunities, while maturation refines and specifies the circuit connectivity that supports consolidated/persistent memories.
However, memories are typically consolidated in the absence of direct exposure to related experience (Dudai et al., 2015; Klinzing et al., 2019). What activities drive synaptic maturation during such periods of quiescence? One possibility is the replay of patterned circuit activity that represents an experience. In the hippocampus, neuronal ensembles that are activated during learning are often reactivated to replay their temporal dynamics at later times in the absence of new learning (Ólafsdóttir et al., 2018), and this replay contributes to memory consolidation (Girardeau et al., 2009; Jadhav et al., 2012). Such replay may provide essential activity signals for selecting relevant synapses for maturation. In line with this viewpoint, it has been shown in the cortex that reactivation of a dendrite promotes the stabilization of nascent synapses on that dendrite (Cichon and Gan, 2015), and reactivation of a neuronal ensemble strengthens the functional connectivity between ensemble neurons to improve the circuit representation of the memory (Sugden et al., 2020). In the NAc, the neuronal ensembles related to rewarding experiences display reactivation dynamics during periods of quiescence (Pennartz et al., 2004; Lansink et al., 2008), suggesting the existence of similar replay mechanisms, which may contribute to the maturation of silent synapses and consolidation of drug-associated memories during withdrawal periods after cocaine self-administration training.
In addition to experience-dependent activity, homeostatic plasticity may contribute to synaptic maturation and circuit remodeling (Turrigiano and Nelson, 2004). By mobilizing a complex set of mechanisms, homeostatic plasticity regulates synaptic strength and intrinsic membrane excitability of neurons to maintain an output near a set point (Turrigiano, 2008; Davis, 2013). A form of homeostatic plasticity, termed synapse-membrane homeostatic crosstalk, has been characterized in NAc MSNs, through which the excitatory synaptic input and membrane excitability of MSNs are homeostatically adjusted to functionally compensate for changes in one or the other (Ishikawa et al., 2009). After short-term withdrawal from cocaine, upregulation of GluN2B NMDARs at cocaine-generated silent synapses produces a false signal of increased synaptic input that induces synapse-to-membrane homeostatic plasticity, resulting in decreased membrane excitability of NAc MSNs (J. Wang et al., 2018). This membrane change subsequently induces another round of membrane-to-synapse homeostatic plasticity, resulting in strengthening of excitatory synapses through insertion of CP-AMPARs (J. Wang et al., 2018). Such a large-scale upregulation of CP-AMPARs suggests a rather nonselective maturation of cocaine-generated synapses. This potential feature may contribute to the substantial and persistent increase in spine density after drug exposure, which is not typical for other memories but potentially critical for the robustness of cocaine-associated memories.
Dynamics of cocaine-generated synapses in cocaine memories
Cocaine-associated memories are multifaceted, containing information related to unconditioned responses, contextual and discrete cues, value, and actions. Cocaine-generated silent synapses and the resulting circuit remodeling contribute to several, but not all, aspects of cocaine-associated memories. Preventing cocaine-induced generation of silent synapses in the NAc does not prevent the acquisition of cocaine self-administration, suggesting that NAc silent synapses are not involved in unconditioned stimulus-driven instrumental learning (J. Wang et al., 2021a). This is not surprising since NAc lesions do not prevent the acquisition of cocaine self-administration (R. Ito et al., 2004), nor instrumental learning during other goal-directed behaviors (Corbit et al., 2001; Jonkman and Everitt, 2011). Instead, cocaine-generated NAc synapses may contribute to conditioned associations, particularly associations between conditioned stimuli and behavioral outcomes (Goldstein et al., 2012; West and Carelli, 2016; Gmaz et al., 2018). Supporting this viewpoint, animals trained in cue-conditioned self-administration exhibit robust cue-induced cocaine seeking after prolonged drug withdrawal, and preventing NAc silent synapse generation during self-administration or weakening these synapses after their maturation during withdrawal impairs cue-induced cocaine seeking (Lee et al., 2013; Ma et al., 2014; Wright et al., 2020; J. Wang et al., 2021a). Furthermore, these manipulations do not affect the learning that extinguishes cocaine seeking in the absence of conditioned cues, nor the general motivation to obtain cocaine (J. Wang et al., 2021a), further narrowing in on cue-conditioned associations as the key aspect of cocaine memories that cocaine-generated NAc synapses may encode.
NAc MSNs receive convergent glutamatergic projections from many limbic and paralimbic areas (Sesack and Grace, 2010; Xia et al., 2020). Cocaine-generated silent synapses have been detected in projections from all the brain regions examined thus far, including the BLA, prelimbic and infralimbic PFC, and paraventricular nucleus of the thalamus (Lee et al., 2013; Ma et al., 2014; Neumann et al., 2016), and may be present in projections from other brain regions, such as the hippocampus, which have not yet been investigated. Accumulating evidence suggests that cocaine-generated synapses in projections from different brain regions contribute differentially to cocaine-related behaviors. For example, BLA and prelimbic silent synapses promote cue-induced cocaine seeking, whereas infralimbic silent synapses suppress this behavior (Lee et al., 2013; Ma et al., 2014). These results raise the possibility that cocaine-generated synapses within NAc afferents from different brain regions transmit different information, such as discrete or spatial cues, to the same NAc MSNs to differentially regulate behavior. Thus, cocaine-generated synapses dispersed in different afferents may collectively form a synaptic engram that encodes a broader, multifaceted representation of the cocaine experience.
Memories are not static, but highly dynamic. For example, consolidated memories become destabilized and susceptible to modification on reactivation, and then restabilize through a reconsolidation process (Tronson and Taylor, 2007; Torregrossa and Taylor, 2013). If memories are indeed encoded in synaptic and neuronal engrams as theorized, the engram neurons and synapses would be expected to exhibit dynamic changes corresponding to these memory dynamics. Such functional dynamics of cocaine-generated NAc synapses are observed. Specifically, after formation and consolidation of cue-associated cocaine memories following withdrawal from cocaine self-administration, the already mature synapses are transiently resilenced on reactivation of cocaine memories, via internalization of CP-AMPARs; the synapses then mature again during the period of memory reconsolidation (Wright et al., 2020). This rematuration is essential for the reconsolidation of cue-associated cocaine memories, as preventing rematuration during the destabilization period impairs subsequent cue-induced cocaine seeking (Wright et al., 2020). Thus, the functional states of cocaine-generated synapses contribute to the dynamics of cocaine memories. Similar dynamics of AMPAR trafficking are observed during destabilization and reconsolidation of other types of memories (Rao-Ruiz et al., 2011), suggesting that the synaptic dynamics are a common mechanism underlying memory dynamics. Theoretically, resilencing of memory-encoding synapses provides an opportunity for certain synapses to be selected for rematuration and others to be pruned away, with the redefined circuit connectivity updating the memory content.
Another important phenomenon revealed by this line of studies is the dissociation between the functional state of experience-generated synapses and the behavioral expression of memory. During the 1 h testing of cue-induced cocaine seeking, which measures the behavioral expression of cue-associated cocaine memories, cue reexposure resilences cocaine-generated NAc synapses instantly (<10 min), but high levels of cocaine seeking persist beyond this initial 10 min and last throughout the testing (Wright et al., 2020). A similar dissociation is observed in the behavioral expression of fear memories, in which cue-conditioned freezing persists throughout the memory destabilization window, despite the AMPAR internalization-mediated synaptic weakening (Monfils et al., 2009; Rao-Ruiz et al., 2011). However, if cocaine-generated synapses are resilenced before memory reactivation, subsequent cue-induced cocaine seeking is impaired (Wright et al., 2020). These results suggest that cocaine-generated synapses are key substrates for the storage and/or reactivation of cocaine memories, but once the memories are reactivated, behavioral expression is maintained by an independent set of mechanisms. It remains unknown what these mechanisms are, but one possibility is that, once the memory-encoding neural substrates are activated, the resulting circuit dynamics are self-sustaining, supporting the ongoing behavior. Alternatively, other transient adaptations may compensate for the weakening of encoding neural substrates to support the ongoing behavior. Such “compensatory” adaptations are detected in the NAc as transient potentiation of potentially non–cocaine-generated glutamatergic synapses during cue-induced cocaine seeking (Gipson et al., 2013), as well as in the hippocampus as a transient increase in the membrane excitability of engram neurons following memory retrieval (Pignatelli et al., 2018). Regardless of the mechanisms, this division of labor allows memory encoding substrates to be modified without sacrificing the ongoing behavior, and thus has adaptive advantages.
A synaptic substrate for cocaine memories
The concept that memories are encoded by a select population of synapses, or a synaptic engram, has been elaborated in various forms (Chklovskii et al., 2004; Rogerson et al., 2014; Holtmaat and Caroni, 2016). However, it is technically challenging to identify and manipulate such a select set of synapses among large synapse populations. By taking advantage of their unique cellular features, targetable generation mechanisms, and robust behavioral correlates, investigating cocaine-generated synapses provides a compelling case that cocaine-generated synapses form a synaptic engram to encode aspects of cocaine memories. However, there still remains much to consider regarding how such synaptic engrams contribute to the circuit dynamics that support long-lasting memories.
Theoretical considerations of synaptic engrams
A key form of experience-dependent synaptic plasticity is achieved by modification of the weight at preexisting synapses. Through LTP, LTD, or other forms of weight changes at synapses, select routes of informational flow within the preexisting circuit can be strengthened or weakened, redefining the circuit activity dynamics. However, studies of artificial neural networks predict that such weight changes alone do not provide sufficient capacity to encode multiple memories over extended periods of time (French, 1999; Fusi and Abbott, 2007). This is partially because of spontaneous fluctuation in synaptic strength driven by spurious neural activity within the circuit. While the potential transition of synapses from a relatively plastic state to a rigid state promotes memory stability (Fusi et al., 2005; Benna and Fusi, 2016; Kirkpatrick et al., 2017; Masse et al., 2018), such spontaneous fluctuations, which are commonly detected in animal experiments, can still lead to degradation of precise synaptic weights that encode a given memory, resulting in catastrophic forgetting (Fusi and Abbott, 2007; Ziv and Brenner, 2018). These limitations restrain the capacity of preexisting synapses for forming new, functionally stable synaptic engrams.
On the other hand, experience-induced synapse formation or elimination may substantially rewire the circuit connectivity and provide improved capacity for memory encoding. Counterintuitively, the actual rate at which dendrites make synapses with bypassing axons is extremely low, such that neurons are connected in a spare wiring framework within a circuit (Markram et al., 1997; Holmgren et al., 2003). This is exemplified in individual NAc MSNs, in which the bypassing axons from the PFC, amygdala, or thalamus exhibit low rates (i.e., 1%-2%) in forming synapse-like connections on a given dendritic segment (Xia et al., 2020). Within such a sparse wiring setup, even moderate levels of synapse formation or elimination can effectively redefine the circuit matrix for new, distinct connectivity patterns with a much higher predicted capacity for memory encoding than the modification of synaptic weight alone (Chklovskii et al., 2004; Knoblauch et al., 2014; Knoblauch and Sommer, 2016). In animal experiments, nascent and immature synapses are shown to be generated by new experience (Arendt et al., 2013; Suvrathan et al., 2013; Chung et al., 2017), and high levels of synaptogenesis are detected during periods of novel learning (Holtmaat and Svoboda, 2009; Peters et al., 2014; Chen et al., 2015; Holtmaat and Caroni, 2016). We argue that these experience-generated synapses are key substrates in creating new connectivity patterns underlying unique circuit activity dynamics; they stand as a distinct synaptic population, a synaptic engram, that supports memory encoding (Fig. 2).
Figure 2.
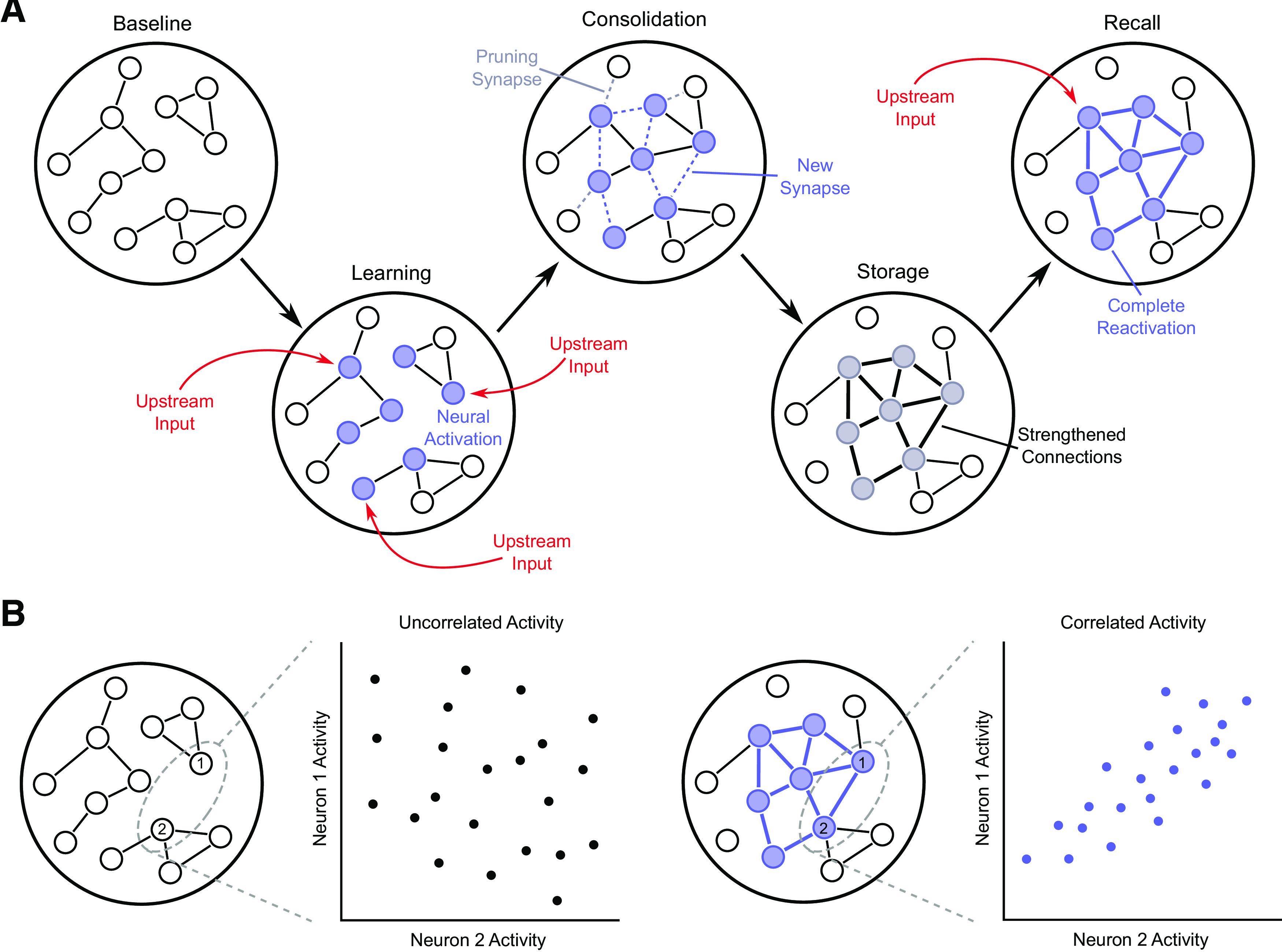
Potential role of synaptogenesis in circuit formation and dynamics. A, Schematic depiction of how synaptic remodeling may lead to the formation of a neuronal engram. During learning, strong inputs to the circuit from various upstream areas leads to the activation of select neurons. Activity triggers synaptic remodeling, with formation of new connections between neurons activated together while also potentially pruning away connections between neurons not activated together. This may connect neurons that previously did not interact with one another. Consolidation then proceeds to strengthen the new and preexisting connections between engram neurons while eliminating some connections with other neurons. The strong interconnectivity between engram neurons subsequently allows weaker and incomplete inputs to trigger complete reactivation of the neural engram and allow for memory recall. B, Example illustrating one way that synaptogenesis may reshape coactivity dynamics between neurons within a circuit. Before learning, neurons within a circuit may not be connected or share the same inputs. This results in their activity being largely independent of each other and uncorrelated. Following learning-induced formation of new synaptic connections between them, the activity of one of the neurons becomes dependent on the other, resulting in correlated dynamics. Such changes throughout an entire circuit may lead to dramatic changes in the neuronal coactivity patterns.
While generation and elimination of synapses are likely more metabolically costly than modifying synaptic weight, this cost is predicted by modeling work to favor circuit modularity, which can facilitate sparse encoding to reduce interference between memories and ultimately increase memory capacity (Ellefsen et al., 2015). Such connectivity-oriented models also emphasize the contribution of the modification of preexisting synapses. For example, formation of new connections may encode core and, particularly, novel information to form the skeleton of a memory, while modification of preexisting synapses may adjust and rearrange the already encoded information to flesh out the skeleton with details and richness. This is particularly important when considering that few experiences during adulthood are completely novel and new memories often incorporate previously learned information.
An unsolved issue for the connectivity model of memory is spontaneous synaptic turnover (Yang et al., 2009; Attardo et al., 2015). While some synapses persist for the lifetime of a memory, many others undergo continuous turnover (Xu et al., 2009; Yang et al., 2009; Attardo et al., 2015). If every individual synapse is essential for a complete memory, synaptic turnover would lead to progressive memory fragmentation. In the mammalian brain, two neurons are often connected through a compound connection comprising multiple synapses (Stepanyants et al., 2002). For NAc MSNs, compound connections can be achieved by short terminal branches (also called terminal tufts) from a single axonal projection, which forms multiple synapses onto the same dendritic segment (Tripathi et al., 2010; Aransay et al., 2015). Within a relatively homogeneous microenvironment, synapses in a compound connection may share similar spatial and temporal activation profiles. In this case, although activation of individual synapses is stochastic, the integrated activity of these synapses, referred to as the collective dynamics, is predicted to be strikingly stable, resilient to the probabilistic failure or reasonably paced synaptic turnover (Fauth et al., 2015). As a theoretical extrapolation, compound connections may serve as the basic functional modules for a synaptic engram to support stable circuit dynamics for a memory. Apparently, the compound connection is not the only anatomic organization for synapses. Mechanisms that determine how the activity of individual synapses is integrated to generate stable circuit dynamics remain a hot topic for current computational and experimental neuroscience.
The role of engram synapses in neuronal engram and circuit dynamics
Neuronal engrams are formed during learning when individual neurons are recruited into a functionally correlated population to encode a memory (Josselyn and Tonegawa, 2020). Several studies detect increased numbers and/or efficacy of synapses in engram neurons (Ryan et al., 2015; Kitamura et al., 2017; Roy et al., 2017; Choi et al., 2018; Zhou et al., 2019). Furthermore, experience-dependent synaptogenesis or synaptic strengthening appears to occur preferentially between engram neurons across different brain regions in support of the same memory (Kitamura et al., 2017; Choi et al., 2018; Zhou et al., 2019). On the other hand, disrupting experience-dependent synaptogenesis or synaptic potentiation prevents the effective reactivation of neuronal engrams by natural recall cues, resulting in memory failure (Ryan et al., 2015; Kitamura et al., 2017; Roy et al., 2017). While this memory failure can be rescued by strong optogenetic stimulation of engram neurons (Ryan et al., 2015; Roy et al., 2017), the rescue requires the preserved synaptic connections between engram neurons (Roy et al., 2017). These findings suggest that formation and potentiation of synaptic connections are key mechanistic steps in forming and maintaining neuronal engrams and related circuits for memory encoding and recall.
In the context of SUD-related memories, it is tempting to hypothesize that cocaine-generated synapses contribute to the formation of neuronal engrams that encode specific aspects of cocaine memory. As alluded to above, cocaine-generated silent synapses detected in randomly sampled NAc MSNs mature after withdrawal from cocaine self-administration through two cellular steps, switching GluN2B NMDARs to nonGluN2B NMDARs and recruiting AMPARs (Y. Q. Wang et al., 2021). After maturation, these synapses become resilenced on cue reexposure-induced retrieval of cocaine memories (Wright et al., 2020) (Fig. 1). However, another population of silent synapses is detected in a potential cocaine engram/ensemble in the NAc. After withdrawal from repeated intraperitoneal injections of cocaine, silent synapses are preferentially detected in NAc MSNs that express high levels of cFos within 2 h after a locomotor sensitization test, in which animals are reexposed to cocaine injection and/or cocaine-associated cues (Koya et al., 2012; Whitaker et al., 2016). These synapses do not express high levels of GluN2B NMDARs, but they do contribute to the decreased AMPAR-mediated responses and decreased frequencies of AMPAR-mediated EPSCs observed in cFos-positive neurons (Koya et al., 2012; Whitaker et al., 2016). Thus, rather than being formed by synaptogenesis, these synapses are generated through internalization of AMPARs from synapses that have already existed before the final sensitization test. In theory, these silent synapses in cFos-enriched neurons may not belong to the same population of synapses that are generated by the initial cocaine experience, but instead represent a new synapse population specific for the cFos-defined neuronal engram/ensemble. Alternatively, they may be part of the silent synapse population generated by the initial cocaine experience, mature after drug withdrawal (Fig. 1), but exhibit substantially robust resilencing dynamics within the cFos-defined engram neurons on reexposure to cocaine or cocaine-associated cues after drug withdrawal. In either case, silent synapse-mediated circuit remodeling is correlated with cocaine-induced formation of neuronal engram/ensembles in the NAc.
As discussed above, temporal circuit dynamics are possibly the circuit-level functional manifestation of synaptic and neuronal engrams. Recent results suggest that a unique set of temporal circuit dynamics is often formed gradually through learning processes, relying on synapse-mediated remodeling of circuit connectivity. For example, movement-related circuit activities in the motor cortex become progressively correlated, exhibiting consistent spatiotemporal sequences over repeated motor learning (Komiyama et al., 2010; Peters et al., 2014; Adler et al., 2019). In conditioned fear learning, coactivity patterns of the amygdala neuronal population that encode conditioned stimuli become progressively similar to those that encode unconditioned stimuli (Grewe et al., 2017). The range of coactivity patterns of a neural circuit is constrained by the synaptic connectivity, which can, theoretically, be expanded by experience-generated synapses. Consistent with this, disrupting synapse formation or elimination during motor learning prevents the emergence of stereotypical and spatiotemporal sequences in the motor cortex and impairs behavioral performance (Chen et al., 2015; Adler et al., 2019). These findings suggest a compelling possibility that experience-induced synapse formation and elimination provide key mechanisms for generation of new patterns of circuit activity dynamics that encode new memories.
In associative learning, the emergence of new circuit dynamics is, in part, driven by neurons acquiring stimulus- or behavior-specific responses over time (Vega-Villar et al., 2019; Ahmed et al., 2020). For cue-associated drug memories, following cocaine self-administration training and withdrawal, some prior cue-insensitive NAc neurons become cue-responsive, with the relative number of new responsive neurons predicting the magnitude of cue-induced cocaine seeking (Hollander and Carelli, 2005, 2007; Guillem et al., 2014). These newly recruited neurons may be particularly important in conditioning cues with cocaine to form a cue-cocaine association. To form such an association, neural substrates encoding cues versus cocaine must interact to establish a link between neurons of different engrams. Interestingly, the emergence of new, cue-responsive NAc neurons after cocaine experience follows a similar time course to the generation and maturation of silent synapses (Hollander and Carelli, 2007; Lee et al., 2013; Guillem et al., 2014), leading us to speculate that such an associative link is mediated by new synaptic connections.
Conclusions and future directions
In the context of a circuit- and engram-based understanding of memory, we have attempted to provide a conceptual perspective that silent synapses generated by cocaine experience stand as a distinct set of synapses, or a synaptic engram, that encodes key aspects of cocaine memories. Either by remodeling an established circuit or expanding the circuit through recruiting additional neurons, these synapses may create new connectivity patterns and, thus, new circuit activity dynamics that represent newly acquired cocaine-associated memories.
Our literature analyses reveal some key questions for future studies. A clearly important one is how synaptic remodeling creates new, memory-encoding circuit activity dynamics. Based on limited evidence, we speculate that new synaptic connections contribute to the formation of both neuronal engrams and new circuit activity patterns, yet direct evidence linking the synaptic modification to circuit dynamics remains lacking. This missing link is particularly prominent in the context of SUD-associated memories, in which the circuit activity patterns that are unique to drug experience have not been identified. Another question regards the relative contributions of experience-generated synapses versus experience-modified synapses to memory encoding. We speculate that modifications of preexisting synapses preferentially retune the established circuit connectivity for incorporating previously learned information while synaptogenesis preferentially creates new circuit connectivity patterns for novel information/experiences. This speculation needs to be tested and refined with empirical data. While exceedingly challenging, addressing these and other related questions will open the possibility to precisely target and manipulate memory traces uniquely associated with drug memories and treat addictive disorder.
Footnotes
This work was supported in part by National Institutes of Health Grants DA043940 to W.J.W., and Grants DA023206, DA040620, DA047861, and DA051010 to Y.D.
The authors declare no competing financial interests.
References
- Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu SI, Inokuchi K (2018) Synapse-specific representation of the identity of overlapping memory engrams. Science 360:1227–1231. 10.1126/science.aat3810 [DOI] [PubMed] [Google Scholar]
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA (2008) NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci USA 105:5597–5602. 10.1073/pnas.0800946105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A, Zhao R, Shin ME, Yasuda R, Gan WB (2019) Somatostatin-expressing interneurons enable and maintain learning-dependent sequential activation of pyramidal neurons. Neuron 102:202–223. 10.1016/j.neuron.2019.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MS, Priestley JB, Castro A, Stefanini F, Canales AS, Balough EM, Lavoie E, Mazzucato L, Fusi S, Losonczy A (2020) Hippocampal network reorganization underlies the formation of a temporal association memory. Neuron 107:283–291.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Eroglu C (2017) Cell biology of astrocyte-synapse interactions. Neuron 96:697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aransay A, Rodríguez-López C, García-Amado M, Clascá F, Prensa L (2015) Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front Neuroanat 9:59. 10.3389/fnana.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Sarti F, Chen L (2013) Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J Neurosci 33:2087–2096. 10.1523/JNEUROSCI.3880-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo A, Fitzgerald JE, Schnitzer MJ (2015) Impermanence of dendritic spines in live adult CA1 hippocampus. Nature 523:592–596. 10.1038/nature14467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL (2006) Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA 103:19535–19540. 10.1073/pnas.0608492103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benna MK, Fusi S (2016) Computational principles of synaptic memory consolidation. Nat Neurosci 19:1697–1706. 10.1038/nn.4401 [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas P, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schlüter OM (2011) A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci 31:8163–8174. 10.1523/JNEUROSCI.0016-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, Flores SE, Kim I, Sano Y, Zhou M, Baumgaertel K, Lavi A, Kamata M, Tuszynski M, Mayford M, Golshani P, et al. (2016) A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534:115–118. 10.1038/nature17955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane M, Maco B, Knott G, Holtmaat A (2014) The relationship between PSD-95 clustering and spine stability in vivo. J Neurosci 34:2075–2086. 10.1523/JNEUROSCI.3353-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Kim AN, Peters AJ, Komiyama T (2015) Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci 18:1109–1115. 10.1038/nn.4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K (2004) Cortical rewiring and information storage. Nature 431:782–788. 10.1038/nature03012 [DOI] [PubMed] [Google Scholar]
- Choi JH, Sim SE, Kim JI, Choi DI, Oh J, Ye S, Lee J, Kim T, Ko HG, Lim CS, Kaang BK (2018) Interregional synaptic maps among engram cells underlie memory formation. Science 360:430–435. 10.1126/science.aas9204 [DOI] [PubMed] [Google Scholar]
- Chung S, Jeong JH, Ko S, Yu X, Kim YH, Isaac JT, Koretsky AP (2017) Peripheral sensory deprivation restores critical-period-like plasticity to adult somatosensory thalamocortical inputs. Cell Rep 19:2707–2717. 10.1016/j.celrep.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon J, Gan WB (2015) Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520:180–185. 10.1038/nature14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S (2002) The developing synapse: construction and modulation of synaptic structures and circuits. Science 298:770–776. 10.1126/science.1075510 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121. 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW (2001) The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci 21:3251–3260. 10.1523/JNEUROSCI.21-09-03251.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW (2013) Homeostatic signaling and the stabilization of neural function. Neuron 80:718–728. 10.1016/j.neuron.2013.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ (2014) The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 35:374–383. 10.1016/j.tips.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Karni A, Born J (2015) The consolidation and transformation of memory. Neuron 88:20–32. 10.1016/j.neuron.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A (1996) Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381:71–75. 10.1038/381071a0 [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R (2007) PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA 104:4176–4181. 10.1073/pnas.0609307104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS (2000) PSD-95 involvement in maturation of excitatory synapses. Science 290:1364–1368. [PubMed] [Google Scholar]
- Ellefsen KO, Mouret JB, Clune J (2015) Neural modularity helps organisms evolve to learn new skills without forgetting old skills. PLoS Comput Biol 11:e1004128. 10.1371/journal.pcbi.1004128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468:223–231. 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2016) Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol 67:23–50. 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Fauth M, Wörgötter F, Tetzlaff C (2015) Formation and maintenance of robust long-term information storage in the presence of synaptic turnover. PLoS Comput Biol 11:e1004684. 10.1371/journal.pcbi.1004684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro PD, Huang X, Hosang L, Stodieck S, Cui L, Liu Y, Engelhardt KA, Schmitz F, Dong Y, Löwel S, Schlüter OM (2018) An opposing function of paralogs in balancing developmental synapse maturation. PLoS Biol 16:e2006838. 10.1371/journal.pbio.2006838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R (1999) Catastrophic forgetting in connectionist networks. Trends Cogn Sci 3:128–135. 10.1016/s1364-6613(99)01294-2 [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y (2012) Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483:92–95. 10.1038/nature10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusi S, Abbott LF (2007) Limits on the memory storage capacity of bounded synapses. Nat Neurosci 10:485–493. 10.1038/nn1859 [DOI] [PubMed] [Google Scholar]
- Fusi S, Drew PJ, Abbott LF (2005) Cascade models of synaptically stored memories. Neuron 45:599–611. 10.1016/j.neuron.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Gambrill AC, Barria A (2011) NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci USA 108:5855–5860. 10.1073/pnas.1012676108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW (2013) Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron 77:867–872. 10.1016/j.neuron.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12:1222–1223. 10.1038/nn.2384 [DOI] [PubMed] [Google Scholar]
- Gmaz JM, Carmichael JE, van der Meer MA (2018) Persistent coding of outcome-predictive cue features in the rat nucleus accumbens. eLife 7:1096. 10.7554/eLife.37275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Barnett BR, Vasquez G, Tobia SC, Kashtelyan V, Burton AC, Bryden DW, Roesch MR (2012) Ventral striatum encodes past and predicted value independent of motor contingencies. J Neurosci 32:2027–2036. 10.1523/JNEUROSCI.5349-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez WG, Zhang H, Harutyunyan A, Lois C (2019) Persistence of neuronal representations through time and damage in the hippocampus. Science 365:821–825. 10.1126/science.aav9199 [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA (2011) Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron 71:1085–1101. 10.1016/j.neuron.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, Nestler EJ, Wang YT, Schlüter OM, Dong Y (2016) Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci 19:915–925. 10.1038/nn.4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, Lüthi A, Schnitzer MJ (2017) Neural ensemble dynamics underlying a long-term associative memory. Nature: 543:670–675. 10.1038/nature21682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E (2006) AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci 29:132–139. 10.1016/j.tins.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC (2013) ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA 110:1923–1928. 10.1073/pnas.1221742110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH, Peoples LL (2014) Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol Psychiatry 76:31–39. 10.1016/j.biopsych.2013.08.032 [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333–338. 10.1038/nature15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949) The organization of behavior: a neuropsychological theory. New York: Wiley. [Google Scholar]
- Hollander JA, Carelli RM (2005) Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology 30:1464–1474. 10.1038/sj.npp.1300748 [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM (2007) Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci 27:3535–3539. 10.1523/JNEUROSCI.3667-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y (2003) Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol 551:139–153. 10.1113/jphysiol.2003.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Caroni P (2016) Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 19:1553–1562. 10.1038/nn.4418 [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10:647–658. 10.1038/nrn2699 [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K (2006) Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441:979–983. 10.1038/nature04783 [DOI] [PubMed] [Google Scholar]
- Huang X, Stodieck SK, Goetze B, Cui L, Wong MH, Wenzel C, Hosang L, Dong Y, Löwel S, Schlüter OM (2015) Progressive maturation of silent synapses governs the duration of a critical period. Proc Natl Acad Sci USA 112:E3131–E3140. 10.1073/pnas.1506488112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schlüter OM, Zukin RS, Dong Y (2009) In vivo cocaine experience generates silent synapses. Neuron 63:40–47. 10.1016/j.neuron.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. 10.1146/annurev.neuro.29.051605.113009 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y (2009) Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci 29:5820–5831. 10.1523/JNEUROSCI.5703-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ (2004) Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7:389–397. 10.1038/nn1217 [DOI] [PubMed] [Google Scholar]
- Ito W, Morozov A (2019) Prefrontal-amygdala plasticity enabled by observational fear. Neuropsychopharmacology 44:1778–1787. 10.1038/s41386-019-0342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W, Erisir A, Morozov A (2015) Observation of distressed conspecific as a model of emotional trauma generates silent synapses in the prefrontal-amygdala pathway and enhances fear learning, but ketamine abolishes those effects. Neuropsychopharmacology 40:2536–2545. 10.1038/npp.2015.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM (2012) Awake hippocampal sharp-wave ripples support spatial memory. Science 336:1454–1458. 10.1126/science.1217230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Everitt BJ (2011) Dorsal and ventral striatal protein synthesis inhibition affect reinforcer valuation but not the consolidation of instrumental learning. Learn Mem 18:617–624. 10.1101/lm.2269911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Tonegawa S (2020) Memory engrams: recalling the past and imagining the future. Science 367:eaaw4325. 10.1126/science.aaw4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW (2015) Finding the engram. Nat Rev Neurosci 16:521–534. 10.1038/nrn4000 [DOI] [PubMed] [Google Scholar]
- Ju H, Bassett DS (2020) Dynamic representations in networked neural systems. Nat Neurosci 23:908–910. 10.1038/s41593-020-0653-3 [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274:1133–1138. 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9:813–825. 10.1038/nrn2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick J, Pascanu R, Rabinowitz N, Veness J, Desjardins G, Rusu AA, Milan K, Quan J, Ramalho T, Grabska-Barwinska A, Hassabis D, Clopath C, Kumaran D, Hadsell R (2017) Overcoming catastrophic forgetting in neural networks. Proc Natl Acad Sci USA 114:3521–3526. 10.1073/pnas.1611835114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson ED, Yaari Y (1996) Synaptic NMDA receptors in developing mouse hippocampal neurones: functional properties and sensitivity to ifenprodil. J Physiol 497:437–455. 10.1113/jphysiol.1996.sp021779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, Tonegawa S (2017) Engrams and circuits crucial for systems consolidation of a memory. Science 356:73–78. 10.1126/science.aam6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing JG, Niethard N, Born J (2019) Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22:1598–1610. 10.1038/s41593-019-0467-3 [DOI] [PubMed] [Google Scholar]
- Knoblauch A, Sommer FT (2016) Structural plasticity, effectual connectivity, and memory in cortex. Front Neuroanat 10:63. 10.3389/fnana.2016.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch A, Körner E, Körner U, Sommer FT (2014) Structural synaptic plasticity has high memory capacity and can explain graded amnesia, catastrophic forgetting, and the spacing effect. PLoS One 9:e96485. 10.1371/journal.pone.0096485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K (2006) Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci 9:1117–1124. 10.1038/nn1747 [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K (2010) Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464:1182–1186. 10.1038/nature08897 [DOI] [PubMed] [Google Scholar]
- Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, Hope BT (2012) Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci 15:1556–1562. 10.1038/nn.3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazi P, Headley DB, Paré D (2018) Multi-dimensional coding by basolateral amygdala neurons. Neuron 99:1315–1328.e1315. 10.1016/j.neuron.2018.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB (2012) Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature 483:87–91. 10.1038/nature10792 [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Joosten R, McNaughton BL, Pennartz CMA (2008) Preferential reactivation of motivationally relevant information in the ventral striatum. J Neurosci 28:6372–6382. 10.1523/JNEUROSCI.1054-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schlüter OM, Dong Y (2013) Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 16:1644–1651. 10.1038/nn.3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, Thompson SM (2018) Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature: 564:258–262. 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma L, Yang G, Gan WB (2017) REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci 20:427–437. 10.1038/nn.4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC (2008) Compartmentalized dendritic plasticity and input feature storage in neurons. Nature 452:436–441. 10.1038/nature06725 [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467. 10.1016/j.neuron.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie H, Morishita W, Yu X, Calakos N, Malenka RC (2005) Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron 45:741–752. 10.1016/j.neuron.2005.01.039 [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B (1997) Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol 500:409–440. 10.1113/jphysiol.1997.sp022031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–734. 10.1126/science.aaa7945 [DOI] [PubMed] [Google Scholar]
- Martín-Fernández M, Jamison S, Robin LM, Zhao Z, Martín ED, Aguilar J, Benneyworth MA, Marsicano G, Araque A (2017) Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20:1540–1548. 10.1038/nn.4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse NY, Grant GD, Freedman DJ (2018) Alleviating catastrophic forgetting using context-dependent gating and synaptic stabilization. Proc Natl Acad Sci USA 115:E10467–E10475. 10.1073/pnas.1803839115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ (2003) Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat Neurosci 6:1208–1215. 10.1038/nn1143 [DOI] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V (2014) Balance and stability of synaptic structures during synaptic plasticity. Neuron 82:430–443. 10.1016/j.neuron.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324:951–955. 10.1126/science.1167975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12:529–540. 10.1016/0896-6273(94)90210-0 [DOI] [PubMed] [Google Scholar]
- Muñoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L (2013) Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci 16:1367–1369. 10.1038/nn.3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Kiyosue K, Taguchi T (2005) Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci 25:4040–4051. 10.1523/JNEUROSCI.4115-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Lüscher C (2019) The molecular basis of drug addiction: linking epigenetic to synaptic and circuit mechanisms. Neuron 102:48–59. 10.1016/j.neuron.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, Huang YH, Sesack SR, Schlüter OM, Dong Y (2016) Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology 41:2399–2410. 10.1038/npp.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oby ER, Golub MD, Hennig JA, Degenhart AD, Tyler-Kabara EC, Yu BM, Chase SM, Batista AP (2019) New neural activity patterns emerge with long-term learning. Proc Natl Acad Sci USA 116:15210–15215. 10.1073/pnas.1820296116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ólafsdóttir HF, Bush D, Barry C (2018) The role of hippocampal replay in memory and planning. Curr Biol 28:R37–R50. 10.1016/j.cub.2017.10.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR III, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Lüscher C (2014) Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509:459–464. 10.1038/nature13257 [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL (2004) The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci 24:6446–6456. 10.1523/JNEUROSCI.0575-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AJ, Chen SX, Komiyama T (2014) Emergence of reproducible spatiotemporal activity during motor learning. Nature 510:263–267. 10.1038/nature13235 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R (1999) Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci 2:31–36. 10.1038/4532 [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Ryan TJ, Roy DS, Lovett C, Smith LM, Muralidhar S, Tonegawa S (2018) Engram cell excitability state determines the efficacy of memory retrieval. Neuron 101:274–284.e5. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S (1894) The Croonian Lecture: La fine Structures des Centres Nerveux. Proc R Soc Lond 55:444–468. [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S (2011) Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci 14:1302–1308. 10.1038/nn.2907 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, Deisseroth K, Frankland PW, Josselyn SA (2016) Competition between engrams influences fear memory formation and recall. Science 353:383–387. 10.1126/science.aaf0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B (1997) Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 17:8491–8497. 10.1523/JNEUROSCI.17-21-08491.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11:1598–1604. 10.1046/j.1460-9568.1999.00576.x [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B (2001) Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39:257–266. [DOI] [PubMed] [Google Scholar]
- Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, Silva AJ (2014) Synaptic tagging during memory allocation. Nat Rev Neurosci 15:157–169. 10.1038/nrn3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Muralidhar S, Smith LM, Tonegawa S (2017) Silent memory engrams as the basis for retrograde amnesia. Proc Natl Acad Sci USA 114:E9972–E9979. 10.1073/pnas.1714248114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff DA, Ni AM, Cohen MR (2018) Cognition as a window into neuronal population space. Annu Rev Neurosci 41:77–97. 10.1146/annurev-neuro-080317-061936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ (2010) The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33:267–276. 10.1016/j.tins.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S (2015) Memory: engram cells retain memory under retrograde amnesia. Science 348:1007–1013. 10.1126/science.aaa5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, Yu BM, Batista AP (2014) Neural constraints on learning. Nature 512:423–426. 10.1038/nature13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon R (1921) The mneme: George Allen & Unwin, London. [Google Scholar]
- Sesack SR, Grace AA (2010) Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35:27–47. 10.1038/npp.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Beroun A, Panopoulou M, Neumann PA, Grant SG, Olive MF, Dong Y, Schlüter OM (2017) Calcium-permeable AMPA receptors and silent synapses in cocaine-conditioned place preference. EMBO J 36:458–474. 10.15252/embj.201695465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyants A, Hof PR, Chklovskii DB (2002) Geometry and structural plasticity of synaptic connectivity. Neuron 34:275–288. 10.1016/s0896-6273(02)00652-9 [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389. 10.1146/annurev-neuro-061010-113810 [DOI] [PubMed] [Google Scholar]
- Südhof TC (2018) Towards an understanding of synapse formation. Neuron 100:276–293. 10.1016/j.neuron.2018.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden AU, Zaremba JD, Sugden LA, McGuire KL, Lutas A, Ramesh RN, Alturkistani O, Lensjø KK, Burgess CR, Andermann ML (2020) Cortical reactivations of recent sensory experiences predict bidirectional network changes during learning. Nat Neurosci 23:981–991. 10.1038/s41593-020-0651-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussillo D (2014) Neural circuits as computational dynamical systems. Curr Opin Neurobiol 25:156–163. 10.1016/j.conb.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S (2013) Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci 369:20130151. 10.1098/rstb.2013.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R (2015) Memory engram cells have come of age. Neuron 87:918–931. 10.1016/j.neuron.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR (2013) Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 226:659–672. 10.1007/s00213-012-2750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL (1999) The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci 19:4180–4188. 10.1523/JNEUROSCI.19-10-04180.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Cebrian C, Mengual E (2010) Axonal branching patterns of nucleus accumbens neurons in the rat. J Comp Neurol 518:4649–4673. 10.1002/cne.22484 [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8:262–275. 10.1038/nrn2090 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135:422–435. 10.1016/j.cell.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB (2004) Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5:97–107. 10.1038/nrn1327 [DOI] [PubMed] [Google Scholar]
- Vega-Villar M, Horvitz JC, Nicola SM (2019) NMDA receptor-dependent plasticity in the nucleus accumbens connects reward-predictive cues to approach responses. Nat Commun 10:4429. 10.1038/s41467-019-12387-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC (2005) Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci 28:251–274. 10.1146/annurev.neuro.27.070203.144336 [DOI] [PubMed] [Google Scholar]
- Wang J, Ishikawa M, Yang Y, Otaka M, Kim JY, Gardner GR, Stefanik MT, Milovanovic M, Huang YH, Hell JW, Wolf ME, Schlüter OM, Dong Y (2018) Cascades of homeostatic dysregulation promote incubation of cocaine craving. J Neurosci 38:4316–4328. 10.1523/JNEUROSCI.3291-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li KL, Shukla A, Beroun A, Ishikawa M, Huang X, Wang Y, Wang YQ, Yue Y, Bastola ND, Huang HH, Kramer LE, Chao T, Huang YH, Sesack SR, Nestler EJ, Schlüter OM, Dong Y (2021a) Cocaine triggers astrocyte-mediated synaptogenesis. Biol Psychiatry 89:386–331. 10.1016/j.biopsych.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Holt LM, Huang HH, Sesack SR, Nestler EJ, Dong Y (2021b) Astrocytes in cocaine addiction and beyond. Mol Psychiatry Advance online publication. Retrieved Apr 9, 2021. doi: 10.1038/s41380-021-01080-7. 10.1038/s41380-021-01080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Wang L, Tang W, Wang Z (2018) Silent synapse unsilencing in hippocampal CA1 neurons for associative fear memory storage. Cereb Cortex 29:4067–4076. 10.1093/cercor/bhy288 [DOI] [PubMed] [Google Scholar]
- Wang YQ, Huang YH, Balakrishnan S, Liu L, Wang YT, Nestler EJ, Schluter OM, Dong Y (2021) AMPA and NMDA receptor trafficking at cocaine-generated synapses. J Neurosci 41:1996–2011. 10.1523/JNEUROSCI.1918-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Carelli RM (2016) Nucleus accumbens core and shell differentially encode reward-associated cues after reinforcer devaluation. J Neurosci 36:1128–1139. 10.1523/JNEUROSCI.2976-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Carneiro de Oliveira PE, McPherson KB, Fallon RV, Planeta CS, Bonci A, Hope BT (2016) Associative learning drives the formation of silent synapses in neuronal ensembles of the nucleus accumbens. Biol Psychiatry 80:246–256. 10.1016/j.biopsych.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365. 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WJ, Dong Y (2020) Psychostimulant-induced adaptations in nucleus accumbens glutamatergic transmission. Cold Spring Harb Perspect Med 10:a039255. 10.1101/cshperspect.a039255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WJ, Graziane NM, Neumann PA, Hamilton PJ, Cates HM, Fuerst L, Spenceley A, MacKinnon-Booth N, Iyer K, Huang YH, Shaham Y, Schlüter OM, Nestler EJ, Dong Y (2020) Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat Neurosci 23:32–46. 10.1038/s41593-019-0537-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia SH, Yu J, Huang X, Sesack SR, Huang YH, Schluter OM, Cao JL, Dong Y (2020) Cortical and thalamic interaction with amygdala-to-accumbens synapses. J Neurosci 40:7119–7132. 10.1523/JNEUROSCI.1121-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao MY, Wasling P, Hanse E, Gustafsson B (2004) Creation of AMPA-silent synapses in the neonatal hippocampus. Nat Neurosci 7:236–243. 10.1038/nn1196 [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y (2009) Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462:915–919. 10.1038/nature08389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924. 10.1038/nature08577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB (2014) Sleep promotes branch-specific formation of dendritic spines after learning. Science 344:1173–1178. 10.1126/science.1249098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokose J, Okubo-Suzuki R, Nomoto M, Ohkawa N, Nishizono H, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, Sasahara M, Kato F, Inokuchi K (2017) Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science 355:398–403. 10.1126/science.aal2690 [DOI] [PubMed] [Google Scholar]
- Yuste R (2015) From the neuron doctrine to neural networks. Nat Rev Neurosci 16:487–497. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, Tian Z, Huang B, Yan E, Liu X, Ma L (2019) A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci 22:1986–1999. 10.1038/s41593-019-0524-y [DOI] [PubMed] [Google Scholar]
- Ziv NE, Brenner N (2018) Synaptic tenacity or lack thereof: spontaneous remodeling of synapses. Trends Neurosci 41:89–11. 10.1016/j.tins.2017.12.003 [DOI] [PubMed] [Google Scholar]


