Abstract
Neuronal underpinning of learning cause-and-effect associations in the adolescent brain remains poorly understood. Two fundamental forms of associative learning are Pavlovian (classical) conditioning, where a stimulus is followed by an outcome, and operant (instrumental) conditioning, where outcome is contingent on action execution. Both forms of learning, when associated with a rewarding outcome, rely on midbrain dopamine neurons in the ventral tegmental area (VTA) and substantia nigra (SN). We find that, in adolescent male rats, reward-guided associative learning is encoded differently by midbrain dopamine neurons in each conditioning paradigm. Whereas simultaneously recorded VTA and SN adult neurons have a similar phasic response to reward delivery during both forms of conditioning, adolescent neurons display a muted reward response during operant but a profoundly larger reward response during Pavlovian conditioning. These results suggest that adolescent neurons assign a different value to reward when it is not gated by action. The learning rate of adolescents and adults during both forms of conditioning was similar, supporting the notion that differences in reward response in each paradigm may be because of differences in motivation and independent of state versus action value learning. Static characteristics of dopamine neurons, such as dopamine cell number and size, were similar in the VTA and SN of both ages, but there were age-related differences in stimulated dopamine release and correlated spike activity, suggesting that differences in reward responsiveness by adolescent dopamine neurons are not because of differences in intrinsic properties of these neurons but engagement of different dopaminergic networks.
SIGNIFICANCE STATEMENT Reckless behavior and impulsive decision-making by adolescents suggest that motivated behavioral states are encoded differently by the adolescent brain. Motivated behavior, which is dependent on the function of the dopamine system, follows learning of cause-and-effect associations in the environment. We find that dopamine neurons in adolescents encode reward differently depending on the cause-and-effect relationship of the means to receive that reward. Compared with adults, reward contingent on action led to a muted response, whereas reward that followed a cue but was not gated by action produced an augmented phasic response. These data demonstrate an age-related difference in dopamine neuron response to reward that is not uniform and is guided by processes that differentiate between state and action values.
Keywords: motivation, reinforcement learning, Pavlovian conditioning, addiction, schizophrenia, impulsivity
Introduction
Understanding how motivated behavioral states are encoded by the adolescent brain is critical for detection and prevention of brain disorders and reckless behaviors that emerge in this developmental stage. These include, but are not limited to, suicide attempts, addiction, mood disorders, and schizophrenia. What about the adolescent neural processing of motivated behavior predisposes them to these conditions? This is a question we are poorly equipped to answer because much of the data on neuronal representation of mental processes related to the operation of the motivational systems were generated using adult animal models (Robbins and Everitt, 1996; Dayan and Balleine, 2002; Berridge, 2004; Schultz, 2010; Flagel et al., 2011). These include data related to incentive motivation, reinforcement learning, and decision-making that factor prominently in potential models and influential theories that attempt to explain adolescent vulnerabilities and reckless behaviors (Ernst et al., 2011; Luciana and Collins, 2012; Naneix et al., 2012; Casey, 2015; Larsen and Luna, 2018; Hauser et al., 2019).
Motivated behavior, rudimentarily defined as an action taken toward an expected outcome, is constrained by learning. The organism can only be motivated about an outcome if it has learned that the outcome may be a consequence of an action or a context. Thus, the neuronal basis of adolescent-motivated behavior is guided by the previously learned cause-and-effect associations. Two fundamental and complementary forms of associative learning are Pavlovian (classical) and operant (instrumental) conditioning (Dickinson, 1981; Fanselow and Wassum, 2015; Corbit and Balleine, 2016). Pavlovian conditioning involves learning that a particular stimulus (conditioned stimulus [CS]) in the environment predicts the occurrence of an outcome (unconditioned stimulus [US]), independent of any action taken. Operant conditioning involves learning that a particular action by the organism leads to the occurrence of an outcome. Each learning process can be described by temporal difference learning algorithms that differentiate between state and action values (Averbeck and Costa, 2017). State values are defined by the information that predicts an upcoming outcome, whereas actions can take on different values depending on the state in which they are enacted.
Neuronal networks and circuits that contribute to these forms of learning are multidimensional, and involve multiple and distinct brain regions (Maren, 2001; Fanselow and Wassum, 2015; Corbit and Balleine, 2016; O'Doherty, 2016; Bouton et al., 2021). Both forms of conditioning, however, involve midbrain dopamine neurons. In the adult brain, dopamine neurons in the VTA, which project primarily to ventral (limbic) striatal regions and represent state and action values as well as reward prediction errors, are important for reward signaling during both types of conditioning (Schultz, 1998; Pessiglione et al., 2006; Keiflin et al., 2019). Emerging data suggest that adult dopamine neurons in another midbrain region, the substantia nigra (SN), are also involved in processing reward-related learning (Coddington and Dudman, 2018; Keiflin et al., 2019; van Zessen et al., 2021). Dopamine neurons in SN primarily project to the dorsal striatum (DS). Notably, adolescent rodents exhibit larger reward-related firing in the DS compared with adults (Sturman and Moghaddam, 2012), suggesting a nigrostriatal bias in encoding reinforcement learning.
To better understand if and how adolescent VTA and SN neurons encode cause-and-effect relationships differently than adults, we recorded from these regions simultaneously during Pavlovian and operant conditioning in both age groups. The US in the Pavlovian task and the action-led outcome in operant task involved the delivery of an identical food reward, allowing for comparison of the operational aspects of these learning paradigms independent of the expected outcome. The data across these two learning tasks suggest that, despite exhibiting the same learning rate as adults, adolescents use different patterns of dopamine neuron activation to reach the same reward endpoint.
Materials and Methods
Subjects
Experiments were started at the University of Pittsburgh and completed at Oregon Health and Science University. Subjects for all experiments were male Sprague Dawley rats (Harlan; Charles River Laboratories) housed in a humidity- and temperature-controlled conditions using a 12 h reverse light/dark cycle with lights off at 8:00 or 9:00 A.M. All procedures were approved by either the University of Pittsburgh Institutional Animal Care and Use Committee or the Oregon Health and Science University Institute Animal Care and Use Committee and were in accordance with the National Institutes of Health's Guide for the care and use of laboratory animals. For all experiments, adolescent animals were postnatal day (PND) 35-38 and adults were PND >65.
Conditioning behavior
Operant chambers (Coulbourn Instruments) equipped with a food trough and reward magazine opposite a nose-poke port with a cue light and infrared photo-detector unit, and a tone-generating speaker were used. One day before the start of habituation, animals were food restricted to 85% of their weight. After 2-3 d of habituation, wherein animals learned to retrieve reward from the food magazine, they completed either Pavlovian or operant conditioning. In the Pavlovian task, a light cue (CS) was presented for 10 s on the wall opposite the food trough; 500 ms after the termination of the CS, a sugar pellet reward (45 mg, Bio-Serv) was delivered. Reward retrieval was followed by a variable (9-12 s) intertrial interval. Each conditioning session consisted of 100 trials. In the operant task, rats were trained to execute an action (nose-poke into the lit port) to earn a single sugar pellet reward on a fixed ratio 1 schedule as described previously (Sturman and Moghaddam, 2012). Immediately after the action execution, the cue light was extinguished and the reward was delivered after a 1 s delay. Reward collection was followed by 10 s intertrial interval and initiation of the next trial. For each trial, the cue light remained illuminated until the rat responded. Each session lasted 45 min or 100 trials.
Learning rate
We modeled trial-by-trial changes in retrieval latencies concatenated across all of the sessions collected per animal. Animal learning behavior was simulated using Rescorla-Wagner model (Danks, 2003) as follows:
| (1) |
where V is the associative value of cue to reward, α represents learning rate, and λ is the maximum reward available. Learning rate is bounded by 0 and 1, where 0 indicates no learning and 1 indicates instant learning, and λ is 0 or 1 based on animal behavior in trial (explained below). Associative value V is set to 0 at the beginning of Session 1; and as learning takes place, this value updates using Equation 1.
Latency of animals to retrieve the reward after delivery (in the Pavlovian task) or to poke after cue (in the operant task) was used as an indicator of reinforcement learning stage. A latency threshold of 5 s was established to distinguish between random and aimed retrievals/pokes, so that the value of a retrieval/poke is 1 (λ = 1) if it happens within 5 s from delivery/cue, and zero (λ = 0) if after 5 s.
Given that trial-by-trial retrieval latencies may be a noisy measure of the rats' learning in each task, task sessions were stitched together to allow a better fit for the gradual decrease in latency, and a 10 trial moving average window was applied to smooth the latency data. A mapping function was used to make associative value V and latency data L comparable as follows:
| (2) |
Where maximum latency is set to 5 and minimum latency is computed for each individual and is the minimum of a 100 trial moving average window. Therefore, the estimated latency is equal to 5 when associative value is 0 in the beginning and is equal to individual's minimum latency when associative learning is 1, meaning the rat has reached its best performance and learning is complete.
Finally, the learning rate α is estimated for each animal by minimizing the sum of squared errors between latency data and the estimated latency.
Electrophysiology
Recording procedures
Electrophysiology recordings of single-unit spiking activity were conducted during both conditioning paradigms. Laboratory-made 8-channel electrode arrays (50-µm-diameter tungsten wire insulated with polyimide, California Fine Wire) were implanted in the VTA (AP −5.3, ML 0.8, DV −7.7) and SN (AP −5.2, ML 2.2, DV −7.4) under isoflurane anesthesia. All animals had 1 week to recover from surgery before the start of habituation. Recording began on the first day of conditioning training. During recordings, animals were connected via a field-effect transistor head-stage (Omnetics Connector) to a lightweight cable and a rotating motorized commutator to allow unrestricted movement during recording. Spikes were amplified at 1000× gain, digitized at 40 kHz, and single-unit data were bandpass filtered at 300 Hz. Single units were isolated in Kilosort (Allen et al., 2018) or Offline Sorter (Plexon) using a combination of manual and semiautomatic sorting techniques until each unit is well isolated in state space (minimum acceptable signal-to-noise ratio ∼2.5:1). Neurons were not screened for specific physiological characteristics or response properties before recording.
Dopamine classification
Neurons were classified as putative dopamine based on waveform width >1.2 ms and mean baseline firing rate slower than 12 Hz (Grace and Bunney, 1984; Schultz and Romo, 1987; Kim et al., 2016), consistent with the profile of optogenetically tagged dopamine neurons in our laboratory (Lohani et al., 2019). Additionally, a dopamine agonist drug study was conducted on a subset of subjects following the final recording session. After a 30 min baseline recording, animals were injected with 0.75 mg/kg apomorphine intraperitoneally and recorded for an additional 30 min (Grace and Bunney, 1984). Responsive units were defined through comparison of interspike interval distributions in a nonparametric Kolmogorov–Smirnov test (p < 0.05). The direction of modulation after apomorphine (inhibited or excited) was determined by whether the pre- or post-injection distribution had a larger cumulative distribution function. Only neurons characterized as putative dopamine are used for firing rate analyses.
Spike correlations
Spike correlations were computed by calculating the trial-by-trial correlation in spike counts between each pair of simultaneously recorded neurons as described previously (Kim et al., 2012). A Pearson's correlation of spike counts was calculated in the 500 ms window following behavioral epochs of interest, consistent with firing rate analyses. Spike count correlations are sensitive to outliers, so we excluded any trial in which either unit firing rate was >3 SDs away from its mean baseline firing rate (Kohn and Smith, 2005; Ruff and Cohen, 2014). In order to preserve sufficient sample sizes, unit pairs were not grouped based on neurotransmitter content.
Immunohistochemistry
Tissue preparation
All rats were anesthetized with chloral hydrate and perfused transcardially with PBS (pH 7.4, Sigma-Aldrich) followed by 4% PFA (Sigma-Aldrich). Brains were extracted and postfixed in 4% PFA overnight before being transferred and stored in a 20% sucrose solution at 4°C for cryoprotection; 40 μm serial coronal sections were sliced using a cryostat and stored as free-floating sections in PBS + 0.05% sodium azide (NaN3).
Immunohistochemistry
Free-floating brain sections were blocked and permeabilized in a solution of PBS with 0.05% NaN3, 3% BSA, 0.5% Triton-X, and 0.05% Tween-80 for 2 h at room temperature. Sections were then incubated with a chicken polyclonal primary antibody against TH (dilution 1:1000, Abcam, ab76442) for 48 h at 4°C. Sections were washed in a solution of PBS + 0.05% NaN3, 3% BSA, 0.1% Triton-X, and 0.01% Tween 80 before being incubated with an AlexaFluor-conjugated goat anti-chicken secondary antibody (dilution 1:1000, AlexaFluor-594, Abcam, ab150176) for 48 h at 4°C. Sections were again washed before being mounted and coverslipped with Vectashield hard-set mounting medium for fluorescence with DAPI (Vector Laboratories).
Microscopy
A Zeiss Axiovert 200 microscope (Carl Zeiss) with an Axiocam camera and Apotome II instrument with grid-based optical sectioning was used to visualize dopamine cells (TH-labeled cells) on the red channel. Each image was acquired with the 20× objective, and the Zen 2 software (Carl Zeiss) generated a Z-stack scan series, consisting of 25 1-μm scans, resulting in three-dimensional images with a total volume of 120 × 120 × 25 μm3 per image stack. In all cases, four images from each brain were analyzed and averaged for each rat.
Microdialysis
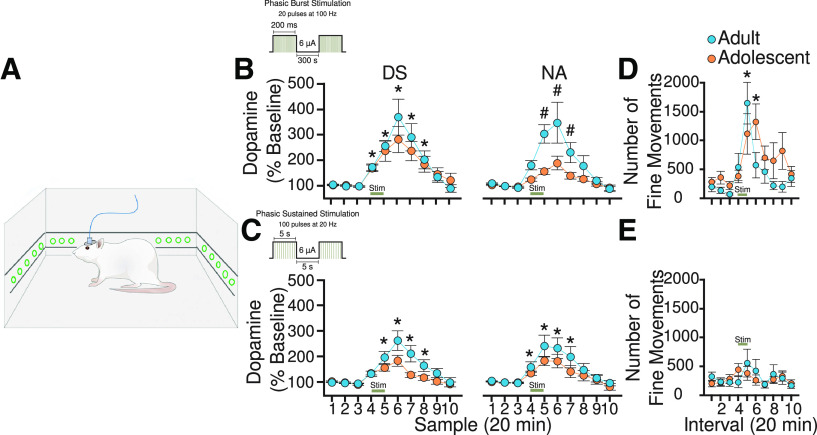
For microdialysis experiments, adolescent (PND 35-38) and adult (PND 65-70) rats were implanted with guide cannulas in both the medial area of the DS (AP = 1.6 mm, ML= 2.2 mm from bregma and DV = −4.0 mm from skull for adults; and AP = 0.7 mm, ML= 2.0 mm from bregma and DV = −3.0 mm from skull for adolescents) and the NAc (AP = 1.2 mm, ML = 1.1 mm from bregma and DV = −6.0 mm from skull for adults; and AP = 1.0 mm, ML = 0.9 mm from bregma and DV = −5.0 mm from skull for adolescents), plus a bipolar stimulating electrode in the VTA (AP = −5.3 mm, ML = 0.9 mm from bregma and DV = −8.3 mm from skull for adults; and AP = −4.2, ML = −0.6 from bregma and DV = −7.4 from skull for adolescents). One week after the surgery, microdialysis experiments on freely moving animals were conducted. Dialysis probes (CMA Microdialysis) with an active membrane length of 2 mm were inserted into the guide cannula and Ringer's solution (in mm as follows: 37.0 NaCl, 0.7 KCl, 0.25 MgCl2, and 3.0 CaCl2) was perfused at flow rate of 2.0 μl/min. After 60 min of stabilization, dialysis samples (20 min each) were collected and immediately injected into an HPLC system with electrochemical detection of dopamine as described previously (Adams and Moghaddam, 1998; Pehrson and Moghaddam, 2010). Once three consecutive stable baseline samples were observed, the electrical stimulation was delivered. The VTA was electrically stimulated for 20 min using one of two burst protocols: (1) the phasic burst stimulation (20 pulses at 100 Hz; pulse width = 1 ms, burst width = 200 ms, interburst interval = 500 ms) or (2) the phasic sustained stimulation (100 pulses at 20 Hz; pulse width = 5 ms, burst width = 5 s, interburst interval = 10 s) (Lohani et al., 2019). Microdialysis data were expressed as the percentage of baseline dopamine release, where baseline is defined as the mean of three consecutive samples obtained before the electrical stimulation. Motor behavior was measured during the microdialysis experiments by placing a stainless-steel frame with an array of infrared beams (Hamilton-Kinder) outside the rats' home-cage environment. Beam breaks were monitored over the entire course of the experiment using the Kinder Scientific Motor Monitor program. Locomotor activity data are expressed in terms of basic movements (total X/Y breaks) and values were pooled into 20 min bins corresponding to the collection of dialysis samples.
Quantification and statistical analyses
Behavior and electrophysiology
All analyses were performed in MATLAB (The MathWorks) and R (https://www.r-project.org/) NeuroExplorer (NEX Technologies) was used for preliminary analysis, such as perievent rasters. For electrophysiological recordings, we conservatively classified neurons recorded in consecutive recording sessions as different units, despite any indications that the same unit were recorded serially. Unit firing rates for both behaviors were analyzed in 25 ms bins, and aligned with behavioral events. Baseline rate for individual units was determined using the average firing rate during the middle 3 s of the intertrial interval. Isolated single-unit data were analyzed with custom-written MATLAB functions. Behavioral events of interest included stimulus onset, nose-poke response, and reward delivery. Statistical tests were performed using activity in 500 ms epoch windows, before, during and after the event of interest. To account for within subject variability, we computed the within-subject average baseline firing rate for each rat and analyzed age differences using Welch two-sample t test. To assess firing rate data collapsed across session, data were Z-score normalized, relative to baseline. Area under the curve was computed to assess group differences in firing rate. Group differences in physiology and behavior were assessed using mixed effect ANOVA models. Behavioral task and age were treated as between-subjects factors and brain region; session and epoch were treated as within-subjects factors. Factors were treated as fixed effects. Dunnett's post hoc and Bonferroni-corrected comparisons were used when appropriate.
Immunohistochemistry
IMARIS software (version 9.2.0; Bitplane) was used for image processing and quantification of the parameters of interest. Dopamine cells were visualized by TH staining visible on the red channel, and IMARIS analysis modules were used for automated quantification of volume and number of dopamine cells within the VTA and SN. Statistical analyses were conducted in R (https://www.r-project.org/). Two-way ANOVAs were used to compare dopamine cell size and number across adult and adolescent rats in the VTA and SN.
Microdialysis
Microdialysis data were expressed as a percentage of baseline dopamine release, with baseline defined as the mean of three consecutive samples obtained before the electrical stimulation. For locomotor activity, data are expressed as the number of infrared beam breaks within a 20 min bin (which correspond with the 20 min microdialysis sample collection). The statistical analysis of these dependent measures was conducted using two-factor repeated-measures ANOVA with age as the between-subjects factor and time as the within-subjects factor.
Histology
After experiments were complete, rats were anesthetized and transcardially perfused with 0.9% saline, followed by 10% buffered formalin. Brains were stored in this formalin and transferred to 30% sucrose for at least 24 h before brains were coronally sliced. Electrode and probe placement in the VTA, SN, DS, and NAc was confirmed for all animals who provided electrophysiological and microdialysis data.
Results
Learning rates and behavioral performance are similar in both adults and adolescents
Adult and adolescent rats were trained in Pavlovian or operant conditioning as we recorded from VTA and SN neurons. The learning paradigms were designed so that, while operationally distinct, they used the same behavioral apparatus and resulted in the delivery of the same reward (a sugar pellet) in each trial (Fig. 1A). We assessed learning during consecutive operant (n = 16 adults, n = 6 adolescents) and Pavlovian (n = 11 adults, n = 4 adolescents) conditioning sessions by measuring the latency to retrieve the reward after CS termination in Pavlovian sessions and the latency to nose-poke into the lit port after cue onset in operant sessions (Fig. 1B), and the total number of trials completed in either paradigm (Fig. 1C). Age did not influence latency to retrieve in Pavlovian conditioned animals (two-way repeated-measures ANOVA, main effect of age, F(1,15) = 0.14, p = 0.72). Both age groups decreased their latencies over sessions (two-way repeated-measures ANOVA, main effect of session: F(5,62) = 6.92, p < 0.0001). In operant conditioning, both adolescents and adults show a decrease in their latency to poke over sessions (two-way repeated-measures ANOVA, main effect of session: F(5,98) = 14.80, p < 0.0001), but adolescents showed longer latencies to make a response (two-way repeated-measures ANOVA, main effect of age: F(1,22) = 6.91, p = 0.02). Both age groups completed a comparable number of trials during Pavlovian (main effect of age: F(1,34) = 0.39, p = 0.54) and operant conditioning (main effect of age: F(1,61) = 0.02, p = 0.88). We then modeled learning based on performance latencies using the Rescorla-Wagner algorithm (Danks, 2003) to estimate individual learning rates across behavioral sessions in both tasks (Fig. 1D). Specifically, we estimated the rate at which adolescent and adult rats learned the Pavlovian and operant associations with the predictive cue or action. Adolescent and adult rats exhibited similar learning rates in the Pavlovian and operant tasks (age, F(1,15) = 0.003, p = 0.96; task: F(1,17) = 1.29, p = 0.27; age × task, F(1,7) = 1.40, p = 0.25). Repeating these analyses using latency to retrieve reward during both conditioning paradigms also did not support significance of age (p > 0.05).
Figure 1.
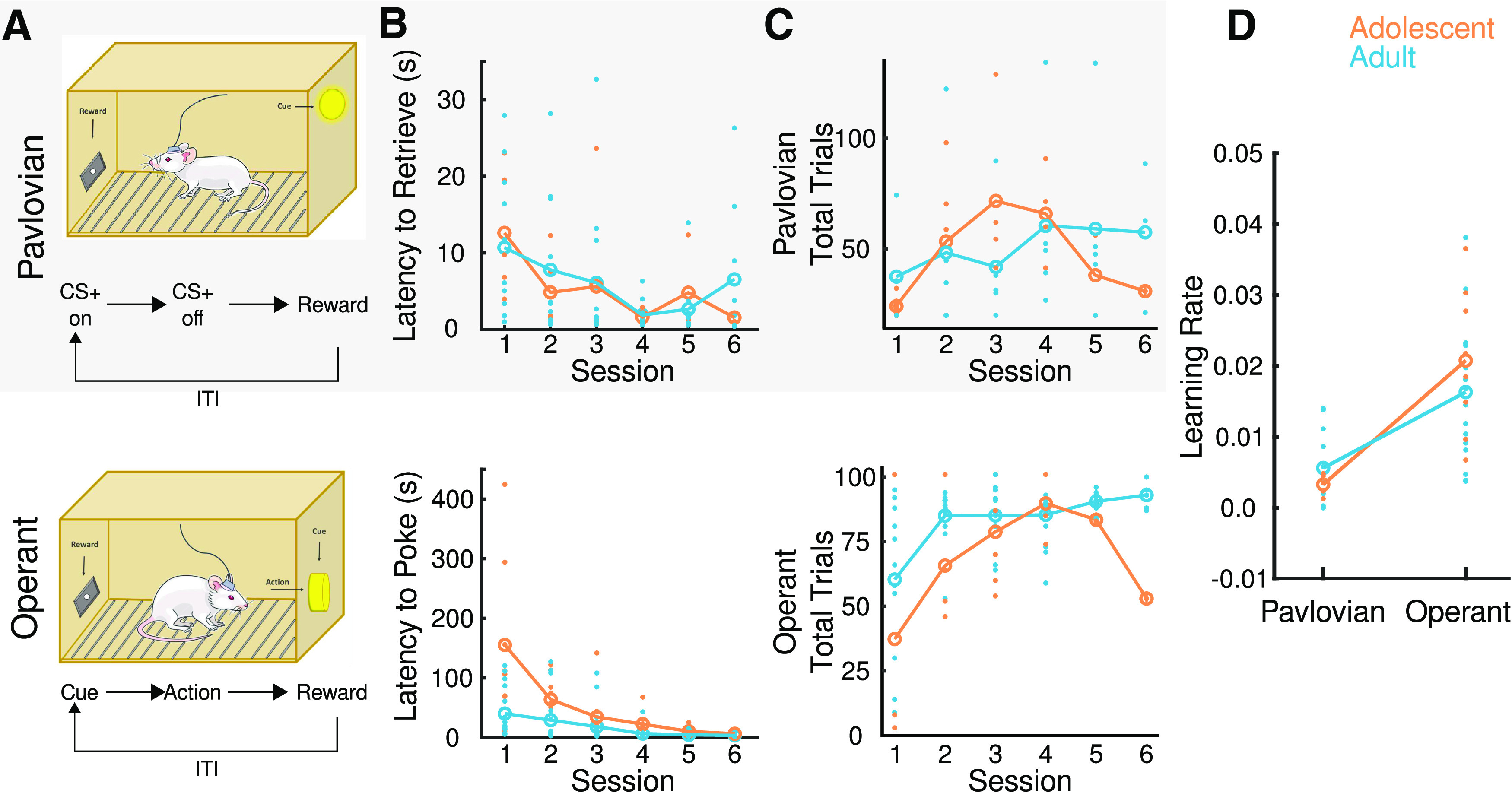
Experimental methods and behavior. A, Schematic of operant box and behavioral paradigm for Pavlovian (gray) and operant (white) conditioned adolescent (orange) and adult (blue) animals. During Pavlovian conditioning, a CS (a light on the opposite side of the food magazine) was presented for 10 s after which the US (sugar pellet) was delivered. During operant conditioning, a cue (also a light) was presented in a hole located in the opposite side of the food magazine. Executing an action (nose-poke) led to delivery of reward. In both paradigms, once the reward was retrieved, another trial was started after an intertrial interval. B, Pavlovian learning was assessed by measuring latency to retrieve a reward, and operant learning was assessed by measuring latency to poke across sessions. C, Total number of trials completed in both tasks was also assessed. D, Individual learning rate was estimated using the Rescorla-Wagner model (Eq. 1). Learning rate is scaled from 1 to 0, where 1 represents instant learning and 0 corresponds with no learning. There was no influence of age on learning rate of either task. Similarly, both adolescents and adults complete similar numbers of trials and decrease latency to perform either an action or conditioned approach, collectively demonstrating that both adolescents and adults learn each respective task. Individual points represent data from 1 animal. Values are means.
Static and dynamic characteristics of VTA and SN dopamine neurons are similar between adolescents and adults
Recordings were conducted in all the rats included in the behavioral analysis shown in Figure 1. Units were classified as putative dopamine neurons based on waveform width >1.2 ms and mean baseline firing rate slower than 12 Hz, as reported previously (Grace and Bunney, 1984; Schultz and Romo, 1987; Kim et al., 2016). This classical approach has been substituted in some recent papers by optogenetic classification of dopamine neurons (Coddington and Dudman, 2018; Mohebi et al., 2019). We and others have observed that the waveform and firing rate of optogenetically identified dopamine neurons is consistent with classically defined criteria (Stauffer et al., 2016; Hughes et al., 2020). Here, we were not able to repeat the opto-tagging characterization because the short time frame of the adolescent experiments (<3 weeks between weaning and the start of recording) does not allow for sufficient viral expression required for optogenetic tagging of dopamine neurons. Instead, we supplemented our waveform and firing rate characterization approach with examining the effect of the dopamine agonist apomorphine on the interspike interval of VTA and SN neurons in a separate group of rats (Fig. 2A,B). Consistent with previous reports (Guyenet and Aghajanian, 1978; Schultz and Romo, 1987), this treatment increased the interspike interval of neurons with dopamine-like waveforms without affecting neurons classified as fast spiking putative nondopamine neurons (Fig. 2A,B). Only units characterized as putative dopamine neurons (VTA: n = 272 adults, n = 241 adolescents; SN: n = 226 adults, n = 241 adolescents) were used for further analysis. These neurons displayed canonical phasic response to reward during both Pavlovian (Fig. 2C) and operant conditioning (Fig. 2D). For each session and animal, baseline firing rates of all units were averaged and a Welch two-sample t test was computed. Baseline firing rate of putative dopamine neurons was comparable between adults and adolescents in both the SN (t(72.63) = −0.21, p = 0.83) and VTA (t(91.64) = −0.06, p = 0.95; Fig. 2E). There was no effect of age (n = 11 adults, n = 14 adolescents) on number of dopamine cells in the SN (t(9) = −0.41, p = 0.69) or VTA (t(6) = 0.96, p = 0.37; Fig. 2F). Also, there was no effect of age on dopamine cell size in the SN (t(10) = 0.68, p = 0.51) or VTA (t(10) = 0.14, p = 0.89; Fig. 2F).
Figure 2.

Dopamine neuron classification and characterization. We classified neurons as putative dopamine or nondopamine based on waveform width and mean baseline firing rate. As a secondary classification metric, we measured the impact of the dopamine agonist apomorphine (0.75 mg/kg) on the interspike interval. Arrows indicate drug administration time point (A,B). Apomorphine increased the interspike interval in putative dopamine neurons but has no effect on nondopamine neurons. Raster plots of individual neuron firing in response to reward (C,D). As expected, neurons classified as dopamine exhibited a phasic response to reward during both Pavlovian (C) and operant (D) conditioning. E, Baseline firing rate in both the SN and VTA was similar in adults and adolescents. F, Dopamine cell volume and numbers were similar in the VTA and SN in adolescent (PND 35) and adult rats (PND 75). *p < 0.05 (t test). Data are mean ± SEM.
Session-by-session response of dopamine neurons revealed age- and task-specific differences during learning
Figure 3 shows the phasic response to presentation of the CS and US during Pavlovian conditioning. Phasic response to reward in the SN was larger in adolescents compared with adults (main effect of age: F(1,158) = 9.39, p = 0.003; Fig. 3A). A nonsignificant trend in peak firing rate changes across session was also observed (main effect of session: F(5,158) = 2.23, p = 0.05). In the VTA, peak firing rate was influenced by both age and session (F(5,227) = 2.37, p = 0.04; Fig. 3B). Age differences in phasic response to reward were most pronounced on Session 3 and later. The larger dopamine response in adolescents during and after Session 3 was specific to reward and did not generalize to phasic response to the other events, including CS initiation. Could differential valuation of the reward per se evoke a larger response by dopamine neurons? Session-by-session analysis of the response of dopamine during operant conditioning suggested that this is not case. As this form of conditioning progressed, dopamine neurons' response to reward was smaller in adolescents compared with adults in both absolute and normalized levels in SN and VTA (Fig. 4). In contrast to the Pavlovian conditioning, during operant conditioning, adults exhibited a larger phasic SN response to reward than adolescents (main effect of age: F(1,249) = 6.34, p = 0.01; Fig. 4A) and VTA (main effect of age: F(1,321) = 8.32, p = 0.004; Fig. 4B). There was no effect of session in either brain region (p values >0.05).
Figure 3.
Pavlovian firing rates across session. Session-by-session firing rates are in adolescent (orange) and adult (blue) rats in the SN (A) and VTA (B). Bar graphs represent mean peak phasic response 500 ms after reward. Dopamine neurons recorded in adolescents exhibited a larger response to reward receipt than dopamine neuron responses recorded in adults. This effect was more pronounced among dopamine neurons recorded in the SN versus the VTA. Data are mean ± SEM.
Figure 4.
Operant firing rates across session. Session-by-session firing rates are in adolescent (orange) and adult (blue) rats in the SN (A) and VTA (B). Bar graphs represent mean peak phasic response 500 ms after reward. Dopamine neurons recorded in adults exhibited a larger response to reward receipt than dopamine neuron responses recorded in adolescents. Data are mean ± SEM.
Comparison of adult and adolescent phasic response to reward and other key events during Pavlovian and operant conditioning was made by considering the normalized response across Sessions 3-6 in which performance in both tasks was stable. We first determined whether significant events, such as CS or reward presentation, evoked a significant change in firing rate. Firing rate was significantly altered by CS presentation in all animals (main effect of epoch: adolescent SN: F(2,82) = 7.62, p = 0.0009; adolescent VTA: F(2,68) = 7.93, p = 0.0008; adult SN: F(2,168) = 65.19, p < 0.01; adult VTA: F(2,194) = 68.86, p < 0.001) where a robust phasic response to presentation of the CS was observed in both the VTA and SN of both age groups (Fig. 5A). During cue presentation in operant conditioning, a main effect of epoch was observed in the SN of adults (F(2,186) = 15.15, p < 0.001) but not adolescents (F(2,114) = 2.076, p = 0.13) and the VTA of both age groups (adult: F(2,68) = 11.95, p < 0.001; adolescents: F(2,166) = 4.84, p = 0.009; Fig. 5B). A phasic response to Pavlovian reward delivery was observed in both adolescents and adults in both the SN (adolescents: F(2,82) = 21.56, p < 0.001; adults: F(2,168) = 14.29, p < 0.001) and VTA (adolescents: F(2,68) = 9.64, p = 0.0002; adults: F(2,194) = 39.78, p < 0.001; Fig. 5C). In the operant group, a main effect of epoch was observed in both brain regions in adults (SN: F(2,186) = 19.26, p < 0.001; VTA: F(2,126) = 12.63, p < 0.001) and the SN of adolescents (F(2,114) = 5.98, p = 0.003; Fig. 5D).
Figure 5.
Averaged firing rates around salient events. Adolescent (orange) and adult (blue) data were combined across Sessions 3-6, after both tasks were learned. To determine whether the phasic response following behavioral events of interest was significantly different from basal activity, data were divided into 500 ms epochs before, during, and after each event, and repeated-measures ANOVAs were performed in data stratified by brain region and age. Phasic responses to CS presentation in Pavlovian conditioning (A,E) and operant conditioning (B,F) were comparable in both ages. Compared with adults, adolescents exhibited a larger phasic response to reward during Pavlovian conditioning in both the SN and VTA (C,G). In contrast, adults exhibited a pronounced phasic response to reward during operant conditioning in both the SN and VTA, whereas the phasic response in adolescents was attenuated, relative to adults and only observed in the SN (D,H). #p < 0.05, main effect of age. Data are mean ± SEM.
We next compared firing rate between behavioral groups, brain regions, and age groups. Area under the curve (AUC) was computed for the 500 ms epoch following each event. Group differences were then assessed by ANOVA with Bonferroni-corrected post hoc tests performed as necessary. During Pavlovian CS presentation, there was no effect of age (main effect of age: F(1,710) = 1.16, p = 0.28) or brain on AUC firing rate (main effect of brain: F(1,710) = 0.30, p = 0.59; Fig. 5E). During cue presentation in operant conditioned rats, there was no effect of age (main effect of age: F(1,491) = 2.93, p = 0.09) or brain region on firing rate AUC (main effect of brain: F(1,491) = 0.71, p = 0.40; Fig. 5F). During reward delivery, overall firing rate was greater in the Pavlovian group, compared with the operant group (main effect of task: F(1,1146) = 66.42, p = 1.03 × 10−15). Data were therefore next stratified by behavioral task. In the Pavlovian group adolescents exhibited greater firing rate during reward (main effect of age: F(1,660) = 10.76, p = 0.001; Fig. 5G). There was no difference between brain regions in the Pavlovian animals (main effect of brain: F(1,660) = 0.0, p = 0.98). In contrast, adults in the operant group exhibited greater firing rate during reward delivery (main effect of age: F(1,486) = 18.95, p = 1.64 × 10−5), with a nonsignificant trend between brain region differences observed (main effect of brain: F(1,486) = 3.09, p = 0.07; Fig. 5H). In summary, there was no effect of age on CS presentation in either paradigm, but adolescents exhibited a larger phasic response to reward during Pavlovian conditioning in both the SN and VTA compared with adults, whereas adults exhibited a more pronounced phasic response to reward during operant conditioning, compared with adolescents. This analysis further established that the same reward achieved as a US, as opposed to that obtained after an action, selectively produces a larger response in adolescents.
Adolescent VTA and SN neurons exhibit different correlated activity to reward in different conditioning paradigms
Neuronal representation of behavioral events can be distributed across populations of neurons (Cohen et al., 2012). To assess population dynamics in response to reward during learning in SN and VTA of adults and adolescents, we computed spike correlation in simultaneously recorded neurons (Cohen and Kohn, 2011; Kim et al., 2012). Data were stratified by brain region and behavioral task, and two-way repeated measures ANOVAs were performed to determine whether spike correlation ratios after stimulus (cue or CS) presentation changed across session and whether this effect was influenced by age group. In all groups, the main effect of sessions was not significant (p values > 0.05). Adolescents in the Pavlovian group exhibited more correlated activity than adults in the SN (main effect of age F(1,22) = 42.34, p = 1.52 × 10−6) and the VTA (main effect of age (F(1,23) = 23.39, p = 7.01 × 10−5; Fig. 6A). Similarly, in the operant group, a main effect of age was observed in the SN (F(1,46) = 20.96, p = 3.56 × 10−5) and VTA (F(1,68) = 56.08, p = 1.86 × 10−10; Fig. 6B). Differences in population response to conditioned stimuli may reflect differences in encoding efficiency or the amount of information encoded by that population and received by downstream networks.
Figure 6.
Spike correlation ratios (correlated/noncorrelated neuron pairs) during Pavlovian and operant conditioning. The higher the ratio, the greater the number of correlated pairs, or the smaller the variance in population dynamics. Conversely, a lower ratio indicates fewer correlated pairs and a more heterogeneous population dynamic. In comparing coordinated activity in adults and adolescents during stimulus presentation, we observed an age-mediated different pattern of correlated activity in both Pavlovian (A) and operant (B) conditioning. *p < 0.05, main effect of age. Data are mean ± SEM.
Terminal dopamine release in response of activation of dopamine neurons is muted in adolescents
Motivated actions are mediated by dopamine release from the terminals. Mechanisms that govern dopamine release and volume transmission may be different in adults and adolescents (Robinson et al., 2011; Pitts et al., 2020). Our observation of increased dopamine neuron phasic response to reward in Pavlovian conditioning, but decreased phasic response in operant conditioning could be functionally amplified or muted if dopamine release is different in response to similar phasic activation of these neurons. We therefore determined whether the same pattern of activation of dopamine neurons in adults (N = 34) and adolescents (N = 31) produces similar increase in terminal release. Dopamine efflux was measured in NAc and DS in response to different patterns of stimulation which mimicked different patterns of dopamine neuron activation (Lohani et al., 2019). These two regions have been implicated in both forms of conditioning (O'Doherty et al., 2004; Day and Carelli, 2007; Corbit and Janak, 2010). Phasic burst stimulation (20 pulses at 100 Hz) altered dopamine release in the DS (main effect of time: F(9,135) = 21.14, p < 2 × 10−16) but was not influenced by age group (main effect of age: F(1,15) = 0.26, p = 0.62; Fig. 7B). Phasic burst stimulation also elicited an increase of dopamine in the NAc, which was influenced by rodent age (age × time interaction: F(9,135) = 2.81, p = 0.005). Specifically, both adults and adolescents exhibited an increase in dopamine at samples 5, 6, and 7 (Dunnett's post hoc, p values < 0.05). However, this increase was greater in adults (main effect of age: F(1,15) = 4.78, p = 0.04). Phasic sustained (100 pulses at 20 Hz) stimulation of the VTA produced a mild increase in dopamine levels in the DS (two-way repeated-measures ANOVA, main effect of time: F(9,108) = 18.16, p < 2 × 10−16) and the NAc (F(9,135) = 17.77, p < 2 × 10−16; Fig. 7C), which was similar between adolescents and adults (main effect of age, p values >0.05). With regard to locomotor activity, phasic burst stimulation increased the number of fine movements (main effect of time: F(10,160) = 6.34, p = 3.59 × 10−10) but was not influenced by age group (main effect of age: F(1,16) = 3.03, p = 0.10; Fig. 7D). Sustained stimulation did not increase locomotion in any of the groups (two-way repeated-measures ANOVA, main effect of time: F(10,132) = 1.17, p = 0.31; Fig. 7E). In summary, the general trend we observed was reduced dopamine release in adolescents with the most robust effect observed in NAc after the burst activation of dopamine neuron.
Figure 7.
Microdialysis measurement of evoked dopamine release. A, Schematic illustration of VTA electrical stimulation and simultaneous measurement of dopamine dialysates in the DS or NAc (NA) and locomotor activity in freely moving adolescent (orange) or adult (blue) rats. B, Phasic burst stimulation was associated with increased dopamine release in the DS and NAc in both adults and adolescents, but in the NAc the effect of stimulation was more pronounced in adults. C, Phasic sustained stimulation resulted in an increase in dopamine release in both the DS and NAc, comparable in both age groups. D, Phasic burst stimulation increased the number of fine motor movements in both age groups. E, Sustained stimulation had no effect on locomotor activity. Data are mean ± SEM. *p < 0.05 different from baseline (Dunnett's post hoc). #p < 0.05, main effect of age.
Discussion
Reckless behavior and impulsive decision-making by adolescents suggest that motivated behavioral states are encoded differently by the adolescent brain (Simon and Moghaddam, 2015). Motivated behavior follows learning of cause-and-effect associations in the environment. Here we sought to understand whether the learning of these associations, and response of dopamine neurons in VTA and SN during learning, differs in adolescents compared with adults. We focused this work on comparing two elementary forms of associative learning: Pavlovian and operant conditioning. In both conditioning paradigms, we used the same reward as the outcome. This ensured that the rewarding value of the outcome was identical; and therefore, behavioral or neural differences in the two paradigms were because of operational differences in the means to reach the outcome. We find that, while learning rate is similar in both ages, adolescent dopamine neurons encode reward differently depending on the cause-and-effect relationship of the means to receive that reward. Compared with adults, reward contingent on action led to a muted response, whereas reward that was not gated by action produced an augmented response, suggesting that adolescent dopamine neurons assign a higher value to rewards that are made available independent of actions.
Learning rate and response rate in Pavlovian and operant conditioning in adolescents and adults
Pavlovian conditioning involves learning that occurrence of a stimulus in the environment predicts the occurrence of an outcome, independent of taking any particular action. In the parlance of reinforcement learning, Pavlovian learning amounts to learning the value of state that occurs when the conditioned stimulus is presented. We found that the learning rate of adolescents is not different from adults during Pavlovian conditioning, suggesting that state value representations are similar in adults and adolescents.
During operant conditioning, the rats can control how and when a reinforcing event occurs by deciding to execute an action in a particular state. The action value of a nose-poke comes to define the ultimate value of the state in which that action is executed. We found that while adolescents exhibited longer latencies to execute an action, they displayed similar learning rate, suggesting that they can learn contextualized action values similarly to adults. The longer latencies to make an operant response, despite having learned the action-reward association, suggest lower motivation and slower capacity to update action values after action execution.
Adolescent VTA and SN neurons are engaged differently to reach the same behavioral endpoints as adults
Dopamine neurons in the VTA and SN have been implicated in operant and Pavlovian conditioning (Schultz, 1998; Dalley et al., 2002; Parkinson et al., 2002; Haruno and Kawato, 2006; Lex and Hauber, 2010; Coddington and Dudman, 2018; Keiflin et al., 2019; van Zessen et al., 2021). While much of the learning literature has focused on dopamine neurons in the VTA, multiple studies indicated that SN neurons also generate reward-related signals (Coddington and Dudman, 2018; Saunders et al., 2018). In adult rats, we find that neurons in VTA and SN have a near identical magnitude of response to reward during either conditioning paradigm. Both neuron groups displayed similar phasic responses to operant cue and Pavlovian CS. Moreover, phasic response of adult VTA and SN neurons to reward delivery was similar regardless of whether it was delivered as US or in response to action execution.
Adolescent neurons, however, had a different response to reward depending on the conditioning paradigm and contingencies that led to reward delivery. In operant conditioning, both VTA and SN cells displayed a smaller phasic response to reward compared with adults. The phasic response of adolescents to the operant cue was equally muted consistent with previous findings (Kim et al., 2016). The lower phasic dopamine activation in adolescents may provide a mechanism for our observation that the latency of action to reward retrieval was longer in adolescents during operant conditioning and is consistent with dopamine's role in motivation for effort-based behavior (Salamone et al., 2018). In contrast to the muted dopamine reward response in operant conditioning, adolescents had a robust reward response during Pavlovian conditioning in both regions, with the SN response being slightly larger than that observed in the VTA. Thus, adolescent dopamine neurons may assign higher value to a given reward when it is obtained independent of action.
What could be the potential mechanism for the difference in contingency-dependent signaling of dopamine neurons in response to the same reward? Importantly, there was no age difference in dopamine basal firing rate, cell number, or size. Thus, age differences in reward-evoked activity cannot be explained by static dopamine neuron characteristics and are likely because of different networks driving dopamine neurons during reward. Consistent with this notion, we observed age-specific changes in spike correlation. This measure reflects the strength of information provided by a population of neurons to their target regions (Cohen and Kohn, 2011) and thus may be an index of functional connectivity among networks of neurons. While the difference we observe was not task-specific, it suggests that activation of distinct networks contributes to the phasic activation of VTA and SN neurons in adults and adolescents. A relatively large literature has, indeed, implicated distinct striatal and cortical circuity in operant and Pavlovian conditioning (Cardinal et al., 2002; Shiflett and Balleine, 2011; Peak et al., 2019). In particular, operant conditioning relies on participation of prefrontal cortical regions, including the orbitofrontal cortex (OFC) and dorsal striatal regions (McDannald et al., 2005; Yin et al., 2005). These cortical and striatal regions, which have reciprocal connections with midbrain dopamine cells, are undergoing maturation during adolescence (Huttenlocher, 1979; Lebel et al., 2008). Notably, OFC and dorsal striatal neurons of adolescents display a large excitatory phasic response to reward during operant conditioning (Sturman and Moghaddam, 2011, 2012) compared with adults. Thus, it is tempting to speculate that a weaker dopamine reward response during operant conditioning reduces the postsynaptic dopamine-mediated inhibition on target regions, causing an exaggerated excitatory response in DS and OFC. A muted dopamine neuron response to reward during operant conditioning in adolescents may therefore lead to increased engagement of DS and OFC, two regions that have been strongly implicated in habit learning (Gremel and Costa, 2013; Barker et al., 2015).
Adolescents may exhibit nigrostriatal bias
Phasic response to Pavlovian conditioning in the SN was larger compared with the VTA response. Moreover, muted dopamine release in response to investigator-administered stimulation was only observed in ventral and not DS. Dopamine neurons in the SN preferentially innervate the DS, whereas VTA DA neurons project to the ventral striatum (Haber, 2016). Age-mediated differences in SN and VTA activity together with differences in dorsal and ventral striatal release suggest that adolescents may more prominently engage the SN-dorsal striatal as opposed to the VTA-accumbal pathway. Interestingly, the increase in reward-related firing in the DS of adolescents is not observed in ventral striatum (Sturman and Moghaddam, 2012), further suggesting a bias toward use of nigrostriatal systems in reward processing in adolescents. Future work delineating the roles of nigrostriatal and mesolimbic pathways in adolescents and adults motivated behaviors is warranted.
Caveats
Several limitations are associated with the current work. First, we do not have direct behavioral evidence that changes in responding for reward are because of learning about cues (or CS) per se and not related to learning about timing or other factors that could influence the behavior. Second, because of the technical challenges associated with adolescent recordings, the group sizes were higher for adults than adolescents, which may potentially influence the outcome of our analyses. Third, the rate of reward consumption was different between tasks because of operational differences between operant and Pavlovian conditioning. This difference may influence motivational properties of the reward. Finally, we did not include female subjects. While our previous electrophysiology work (Rivera-Garcia et al., 2020) had shown no sex differences in reward responsiveness of dopamine neurons in adult rats, future work should include female adolescent subjects.
Conclusions and potential interpretations
Adolescent learning rate was similar to adults during Pavlovian and operant conditioning paradigms, indicating that their capacity to learn state value representations and contextualized action values is similar to adults. During learning, however, adolescent VTA and SN dopamine neurons exhibited paradigm-specific phasic responses to reward. Whereas adult neurons responded similarly to reward in both paradigms, adolescent neurons had a larger response to reward delivered as a Pavlovian unconditioned stimulus, and a muted response when the same reward was delivered after an action. This observation has two implications. First, it invites the field to rethink influential theories that propose blanket dopamine hyper- or hypo-responsiveness to reward to explain adolescent behavior (Spear, 2000; McCutcheon et al., 2012; Ernst and Luciana, 2015; Luna et al., 2015). Our findings clearly demonstrate that, while there is an age-related difference in dopamine neuron response to reward, this difference is not uniform and is guided by network processes that differentiate between state and action values. Second, our findings may have evolutionary significance. Pavlovian associations allow organisms to make predictions about the occurrence of critical events, such as reward availability. Compared with operant conditioning, which may model aspects of foraging behavior, assignment of higher motivational value to unconditioned rewards during Pavlovian conditioning can be advantageous because it does not require an action (or foraging) and thus may help conserve energy. On the other hand, the lower response of dopamine neurons to reward delivery during operant conditioning may be consistent with adolescents being resistant to reward devaluation in instrumental responding (Serlin and Torregrossa, 2015; Marshall et al., 2020; Towner et al., 2020) because it suggests that adolescents assign lower value to the reward, as opposed to cue-action component of this form of conditioning. This may also be evolutionarily advantageous because it allows adolescents to explore (Gopnik et al., 2017) and persist in goal-directed actions and exploratory behavior in the absence of reward availability.
Footnotes
This work was supported by National Institute of Mental Health Grants R01MH048404 and R01MH115027 to B.M., and NIAAA F32AA027935 to A.M.M. We thank Alina Bogachuk for technical assistance.
The authors declare no competing financial interests.
References
- Adams B, Moghaddam B (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18:5545–5554. 10.1523/JNEUROSCI.18-14-05545.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M, Chowdhury T, Wegener M, Moghaddam BJ (2018) Efficient sorting of single-unit activity from midbrain cells using KiloSort is as accurate as manual sorting. BioRxiv 303479. [Google Scholar]
- Averbeck BB, Costa VD (2017) Motivational neural circuits underlying reinforcement learning. Nat Neurosci 20:505–512. 10.1038/nn.4506 [DOI] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ (2015) Corticostriatal circuitry and habitual ethanol seeking. Alcohol 49:817–824. 10.1016/j.alcohol.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2004) Motivation concepts in behavioral neuroscience. Physiol Behav 81:179–209. 10.1016/j.physbeh.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Maren S, McNally GP (2021) Behavioral and neurobiological mechanisms of Pavlovian and instrumental extinction learning. Physiol Rev 101:611–681. 10.1152/physrev.00016.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352. 10.1016/s0149-7634(02)00007-6 [DOI] [PubMed] [Google Scholar]
- Casey B (2015) Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol 66:295–319. 10.1146/annurev-psych-010814-015156 [DOI] [PubMed] [Google Scholar]
- Coddington LT, Dudman J (2018) The timing of action determines reward prediction signals in identified midbrain dopamine neurons. Nat Neurosci 21:1563–1573. 10.1038/s41593-018-0245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482:85–88. 10.1038/nature10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A (2011) Measuring and interpreting neuronal correlations. Nat Neurosci 14:811–819. 10.1038/nn.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW (2016) Learning and motivational processes contributing to Pavlovian–instrumental transfer and their neural bases: dopamine and beyond. In: Behavioral neuroscience of motivation (Simpson EH, Balsam PD, eds), pp 259–289. Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH (2010) Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur J Neurosci 31:1312–1321. 10.1111/j.1460-9568.2010.07153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Chudasama Y, Theobald DE, Pettifer CL, Fletcher CM, Robbins TW (2002) Nucleus accumbens dopamine and discriminated approach learning: interactive effects of 6-hydroxydopamine lesions and systemic apomorphine administration. Psychopharmacology (Berl) 161:425–433. 10.1007/s00213-002-1078-2 [DOI] [PubMed] [Google Scholar]
- Danks D (2003) Equilibria of the Rescorla–Wagner model. J Math Psychol 47:109–121. 10.1016/S0022-2496(02)00016-0 [DOI] [Google Scholar]
- Day JJ, Carelli RM (2007) The nucleus accumbens and Pavlovian reward learning. Neuroscientist 13:148–159. 10.1177/1073858406295854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Balleine BW (2002) Reward, motivation, and reinforcement learning. Neuron 36:285–298. 10.1016/S0896-6273(02)00963-7 [DOI] [PubMed] [Google Scholar]
- Dickinson A (1981) Conditioning and associative learning. Br Med Bull 37:165–168. 10.1093/oxfordjournals.bmb.a071695 [DOI] [PubMed] [Google Scholar]
- Ernst M, Daniele T, Frantz K (2011) New perspectives on adolescent motivated behavior: attention and conditioning. Dev Cogn Neurosci 1:377–389. 10.1016/j.dcn.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Luciana M (2015) Neuroimaging of the dopamine/reward system in adolescent drug use. CNS Spectr 20:427–441. 10.1017/S1092852915000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Wassum KM (2015) The origins and organization of vertebrate pavlovian conditioning. Cold Spring Harb Perspect Biol 8:a021717. 10.1101/cshperspect.a021717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H (2011) A selective role for dopamine in stimulus-reward learning. Nature 469:53–57. 10.1038/nature09588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopnik A, O'Grady S, Lucas C, Griffiths T, Wente A, Bridgers S, Aboody R, Fung H, Dahl R (2017) Changes in cognitive flexibility and hypothesis search across human life history from childhood to adolescence to adulthood. Proceedings of the National Academy of Sciences, Vol 114, pp 7892–7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4:2866–2876. 10.1523/JNEUROSCI.04-11-02866.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM (2013) Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4:2264. 10.1038/ncomms3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P, Aghajanian G (1978) Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res 150:69–84. 10.1016/0006-8993(78)90654-6 [DOI] [PubMed] [Google Scholar]
- Haber SN (2016) Corticostriatal circuitry. Dialogues Clin Neurosci 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kawato M (2006) Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol 95:948–959. 10.1152/jn.00382.2005 [DOI] [PubMed] [Google Scholar]
- Hauser TU, Will GJ, Dubois M, Dolan RJ (2019) Annual research review: developmental computational psychiatry. J Child Psychol Psychiatry 60:412–426. 10.1111/jcpp.12964 [DOI] [PubMed] [Google Scholar]
- Hughes RN, Bakhurin KI, Petter EA, Watson GD, Kim N, Friedman AD, Yin HH (2020) Ventral tegmental dopamine neurons control the impulse vector during motivated behavior. Curr Biol 30:2681–2694.e5. 10.1016/j.cub.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1979) Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Res 163:195–205. 10.1016/0006-8993(79)90349-4 [DOI] [PubMed] [Google Scholar]
- Keiflin R, Pribut HJ, Shah NB, Janak PH (2019) Ventral tegmental dopamine neurons participate in reward identity predictions. Curr Biol 29:93–103.e103. 10.1016/j.cub.2018.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wood J, Moghaddam B (2012) Coordinated activity of ventral tegmental neurons adapts to appetitive and aversive learning. PLoS One 7:e29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Simon NW, Wood J, Moghaddam B (2016) Reward anticipation is encoded differently by adolescent ventral tegmental area neurons. Biol Psychiatry 79:878–886. 10.1016/j.biopsych.2015.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Smith MA (2005) Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25:3661–3673. 10.1523/JNEUROSCI.5106-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Luna B (2018) Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94:179–195. 10.1016/j.neubiorev.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055. 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Lex B, Hauber W (2010) The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb Cortex 20:873–883. 10.1093/cercor/bhp151 [DOI] [PubMed] [Google Scholar]
- Lohani S, Martig AK, Deisseroth K, Witten IB, Moghaddam B (2019) Dopamine modulation of prefrontal cortex activity is manifold and operates at multiple temporal and spatial scales. Cell Rep 27:99–114.e116. 10.1016/j.celrep.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Collins PF (2012) Incentive motivation, cognitive control, and the adolescent brain: is it time for a paradigm shift? Child Dev Perspect 6:392–399. 10.1111/j.1750-8606.2012.00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R (2015) An integrative model of the maturation of cognitive control. Annu Rev Neurosci 38:151–170. 10.1146/annurev-neuro-071714-034054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- Marshall AT, Munson CN, Maidment NT, Ostlund SB (2020) Reward-predictive cues elicit excessive reward seeking in adolescent rats. Dev Cogn Neurosci 45:100838. 10.1016/j.dcn.2020.100838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M (2012) Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol 108:1620–1630. 10.1152/jn.00077.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Saddoris MP, Gallagher M, Holland PC (2005) Lesions of orbitofrontal cortex impair rats' differential outcome expectancy learning but not conditioned stimulus-potentiated feeding. J Neurosci 25:4626–4632. 10.1523/JNEUROSCI.5301-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebi A, Pettibone JR, Hamid AA, Wong JM, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD (2019) Dissociable dopamine dynamics for learning and motivation. Nature 570:65–70. 10.1038/s41586-019-1235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E (2012) Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci 32:16223–16232. 10.1523/JNEUROSCI.3080-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP (2016) Multiple systems for the motivational control of behavior and associated neural substrates in humans. Curr Top Behav Neurosci 27:291–312. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ (2004) Dissociable roles of ventral and dorsal striatum in instrumental conditioning. science 304:452–454. 10.1126/science.1094285 [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ (2002) Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res 137:149–163. 10.1016/s0166-4328(02)00291-7 [DOI] [PubMed] [Google Scholar]
- Peak J, Hart G, Balleine BW (2019) From learning to action: the integration of dorsal striatal input and output pathways in instrumental conditioning. Eur J Neurosci 49:658–671. 10.1111/ejn.13964 [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Moghaddam B (2010) Impact of metabotropic glutamate 2/3 receptor stimulation on activated dopamine release and locomotion. Psychopharmacology (Berl) 211:443–455. 10.1007/s00213-010-1914-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006) Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442:1042–1045. 10.1038/nature05051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts EG, Stowe TA, Christensen BA, Ferris MJ (2020) Comparing dopamine release, uptake, and D2 autoreceptor function across the ventromedial to dorsolateral striatum in adolescent and adult male and female rats. Neuropharmacology 175:108163. 10.1016/j.neuropharm.2020.108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Garcia MT, McCane AM, Chowdhury TG, Wallin-Miller KG, Moghaddam B (2020) Sex and strain differences in dynamic and static properties of the mesolimbic dopamine system. Neuropsychopharmacology 45:2079–2086. 10.1038/s41386-020-0765-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6:228–236. 10.1016/s0959-4388(96)80077-8 [DOI] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Smith KJ, Spear LP (2011) Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience 176:296–307. 10.1016/j.neuroscience.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff DA, Cohen MR (2014) Attention can either increase or decrease spike count correlations in visual cortex. Nat Neurosci 17:1591–1597. 10.1038/nn.3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yang JH, Rotolo R, Presby R (2018) Dopamine, effort-based choice, and behavioral economics: basic and translational research. Front Behav Neurosci 12:52. 10.3389/fnbeh.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Margolis EB, Janak PH (2018) Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci 21:1072–1083. 10.1038/s41593-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27. 10.1152/jn.1998.80.1.1 [DOI] [PubMed] [Google Scholar]
- Schultz W (2010) Multiple functions of dopamine neurons. F1000 Biol Rep 2:2. 10.3410/B2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R (1987) Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol 57:201–217. 10.1152/jn.1987.57.1.201 [DOI] [PubMed] [Google Scholar]
- Serlin H, Torregrossa MM (2015) Adolescent rats are resistant to forming ethanol seeking habits. Dev Cogn Neurosci 16:183–190. 10.1016/j.dcn.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW (2011) Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol 95:1–13. 10.1016/j.pneurobio.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Moghaddam B (2015) Neural processing of reward in adolescent rodents. Dev Cogn Neurosci 11:145–154. 10.1016/j.dcn.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. 10.1016/s0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, Schultz W (2016) Dopamine neuron-specific optogenetic stimulation in rhesus macaques. Cell 166:1564–1571.e1566. 10.1016/j.cell.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B (2011) Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. J Neurosci 31:1471–1478. 10.1523/JNEUROSCI.4210-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B (2012) Striatum processes reward differently in adolescents versus adults. Proc Natl Acad Sci USA 109:1719–1724. 10.1073/pnas.1114137109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner TT, Fager M, Spear LP (2020) Adolescent but not adult Sprague-Dawley rats display goal-directed responding after reward devaluation. Dev Psychobiol 62:368–379. 10.1002/dev.21912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Flores-Dourojeanni JP, Eekel T, van den Reijen S, Lodder B, Omrani A, Smidt MP, Ramakers GM, van der Plasse G, Stuber GD, Adan RA (2021) Cue and reward evoked dopamine activity is necessary for maintaining learned Pavlovian associations. J Neurosci 41:5004–5014. 10.1523/JNEUROSCI.2744-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW (2005) The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22:513–523. 10.1111/j.1460-9568.2005.04218.x [DOI] [PubMed] [Google Scholar]