Abstract
Objective:
The tolerability and efficacy of targeted therapy in older adults with cancer has not been adequately studied. Neratinib is a novel HER1, HER2, HER4 tyrosine kinase inhibitor that has recently been granted FDA approval for treatment of breast cancer. The major toxicity of neratinib is diarrhea, which affects up to 90% of patients. This phase II trial evaluates the safety and tolerability of neratinib in adults ≥ 60.
Methods:
Patients aged 60 or older with histologically proven metastatic breast cancer and HER2 amplification (defined by ASCO/CAP guideline) or HER2/HER3 activating mutation were enrolled to receive neratinib at 240 mg daily in 28-day cycles. The association between tolerability, defined as dose reduction and number of completed courses, and log2 Cancer and Aging Research Group (CARG) toxicity risk score was assessed using a Student’s t-test and linear regression, respectively. Response rate, progression free survival, and overall survival were also evaluated.
Results:
25 patients were enrolled with median age of 66 (range 60–79). Seventy-six percent of patients were white, 16% Asian, and 8% African-American. Seventy-six percent were patients with hormone receptor (HR) positive metastatic breast cancer (MBC) and 24% were patients with HR negative MBC. Median number of prior lines of metastatic therapy were 3 (range 0–11). 20/25 (80%) had worst grade toxicities ≥ 2. A total of 9/25 (36%) had grade 3 toxicities including 5/20 (20%) diarrhea, 2/20 (8%) vomiting, and 2/20 (8%) abdominal pain. There were no grade 4 or 5 toxicities. A total of 9/25 (36%) had dose reduction, and 2/25 (8%) discontinued therapy due to toxicity. The association between dose reductions and CARG toxicity score reached borderline statistical significance suggesting a trend with participants with higher CARG toxicity risk scores being more likely to require a dose modification (p=0.054). 1/25 (4%) had a partial response, 11/25 (44%) had stable disease, 12/25 (48%) had progression of disease, and 1/25 (4%) was not assessed. Median progression free survival (PFS) was 2.6 months (95% CI [2.56–5.26]), and median overall survival (OS) was 17.4 months (95% CI [10.3, NA]).
Conclusions:
Neratinib was safe in this population of older adults with HER2 amplified or HER2/3 mutated metastatic breast cancer (BC). Higher CARG toxicity risk score may be associated with greater need for dose adjustments. Future studies are needed to confirm this finding.
Keywords: Phase II trial, neratinib, metastatic breast cancer, CARG toxicity risk score
INTRODUCTION
Human epidermal growth factor receptor 2 positive (HER2+) breast cancer (BC) accounts for 15–20% of total breast cancers. The introduction of HER2-targeted therapies for BC patients with HER2 amplification and overexpression has led to significant improvements in oncologic outcomes1–3. Second generation HER2-targeted tyrosine kinase inhibitors (TKI) including neratinib and tucatinib have shown promising efficacy in recent clinical trials4–7. Neratinib is a potent oral small molecule TKI that targets HER1, HER2, and HER4 receptors at the intracellular tyrosine kinase domain through irreversible binding at a targeted cysteine residue on the receptor8,9. Somatic HER2 activating mutations, identified in 2% of BCs, could lead to constitutive HER2 activation in the absence of gene amplification10,11. A phase II trial of neratinib for HER2 mutated, non-amplified metastatic BC showed a clinical benefit rate (CBR) of 36% in a heavily pretreated population12. In the recent multicenter plasmaMATCH study, neratinib had a response rate (RR) of 25%, with 20% grade ≥3 diarrhea in patients with HER2 mutated BC using ctDNA testing13.
Older adults with BC are underrepresented in clinical trials14,15. There is significant interest in studying neratinib among older adults, as it is a targeted therapy which has become the standard of care for HER2+ BC6,16. However, there is a significant amount of gastrointestinal (GI) toxicity, including grades 1–4 diarrhea in 92% of patients. Grades 3–4 diarrhea occurred in over 30% of patients, and dose reductions secondary to diarrhea ranged from 20–53% across nine trials of neratinib alone or in combination with other therapies17–25. This side effect is likely to be significant in older adults who are particularly vulnerable to diarrhea. Therefore, there is a significant gap of knowledge regarding the toxicity profile of neratinib in older adults, as well as optimal supportive care medications to minimize side effects.
Consensus statements now recommend including a geriatric assessment (GA) as part of the evaluation of an older patient with cancer26–29. GA tools can be used to assess an older adult’s risk of significant toxicity resulting from chemotherapy, and to assist with the discussion of treatment options between the oncologist and the patient. The Cancer and Aging Research Group (CARG) developed and validated a chemotherapy toxicity score for older adults with cancer30. However, the CARG toxicity risk score has not been validated in older patients receiving most targeted therapies. Our group has recently presented a phase II trial evaluating tolerability of lapatinib and trastuzumab in older patients (≥ 60) with HER2+ metastatic BC31. The current phase II trial was designed to evaluate the tolerability of neratinib in patients ≥ 60 years of age with HER2 amplified and HER2/3 mutated metastatic breast cancer. In addition, the association of neratinib tolerability and CARG toxicity risk score was studied.
MATERIALS AND METHODS
Patients:
Patients with histologically proven metastatic breast cancer; HER2 amplification (defined by American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline) or HER2/HER3 activating mutation; 60 and older; Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2; life expectancy of > 12 weeks; RECIST 1.1 measurable or non-measurable disease; no limitation on the number of prior HER2-targeted therapy; left ventricular ejection fraction ≥ 50%; QTc interval ≤ 450 msec (men) or ≤ 470 msec (women); ≥ 3 week wash-out from radiotherapy or systemic therapy; and adequate organ function were eligible for enrollment. Patients with treated and stable brain metastasis were also eligible. Somatic HER2 or HER3 mutations were identified by Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories. HER2 mutations included but were not limited to the following: missense substitutions (G309A, G309E, S310F, S310Y, S653C, V659E, R678Q, V697L, T733I, L755S, L755P, D769H, D769Y, D769N, G776V, G776C, V777L, L841V, V842I, R849W, L869R); insertions/deletions (A775_G776insYVMA aka Y772_A755dup, G776VinsC, G776AinsVGC, G778_S779insCPG, P780_781insGSP aka G778_P780dup, L755_T759del); and/or HER3 activating mutations.
This study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice and was approved by City of Hope’s regulatory and ethics committees. All participants provided written informed consent.
Study design and treatment:
The primary objective was to estimate the rate of grade ≥ 2 toxicities attributed to neratinib. Secondary objectives were to estimate the rate of dose reduction and hospitalizations; pharmacokinetic parameters; overall response rate (ORR) defined by RECIST 1.1; progression free survival (PFS); and overall survival (OS). In addition, the role of a cancer-specific GA tool in predicting tolerability, defined as dose modifications and completed courses, was evaluated. Geriatric assessment (GA) was performed to determine CARG toxicity risk scores including measures of function, comorbidity, cognition, nutrition, and psychosocial status at baseline, cycle 4, and end of study. The activity based on response rate (RR), progression free survival (PFS), and overall survival (OS) was determined.
Patients were started with a neratinib dose of 240 mg oral daily in a 28-day cycle. Diarrhea prophylaxis with loperamide was mandatory during the first cycle of treatment. Loperamide was administered at an initial dose of 4 mg every eight hours for the first fourteen days, followed by 4 mg twice a day through the first cycle of therapy (day 28) from start of neratinib dosing. Thereafter, loperamide was administered as needed, with the goal of 1–2 bowel movements a day. A total of three dosing levels of neratinib (240 mg, 160 mg, and 120 mg) were tested. Adverse events (AEs) were assessed by NCI Common Terminology Criteria for Adverse Events (CTCAE) 4.0 weekly during the first cycle, and day 1 of subsequent cycles. Patients underwent tumor evaluation by RECIST 1.1 every twelve weeks, and echocardiograms every three months. Study treatment was continued until disease progression or unacceptable toxicity. Patients who permanently discontinued treatment entered a long-term follow-up phase until death or withdrawal of consent.
Geriatric Assessment:
Geriatric assessment surveys were administered at baseline, cycle 4 day 1 and at the end of the study. The measures in the geriatric assessment are outlined in prior publications and listed in Supplemental Table 132,33 and the variables used to generate the CARG toxicity risk score were listed previously30. These include: tumor type and stage, pretreatment laboratory data (WBC count, hemoglobin, blood urea nitrogen, creatinine, albumin, and liver function tests), and treatment characteristics (chemotherapy regimen, dosing, line of therapy, the use of WBC or RBC growth factors, and the timing of initiation of WBC growth factors). The CARG toxicity risk score calculator was accessed at the CARG website: http://www.mycarg.org/Chemo_Toxicity_Calculator.
Pharmacokinetics:
Pharmacokinetics (PK) samples were collected at the following time points: Cycle 1 Day 15 pre-dose, 6 hours post-dose, Day 1 Cycle 3 and Day 1 Cycle 4 pre-dose. Plasma neratinib concentrations were measured using a validated liquid chromatography/tandem mass spectrometry method34.
Statistical methods:
The original sample size of 40 subjects was chosen so the maximum half-width of the 95% confidence limits for the rate of grade 2 or higher toxicities was 0.16. With a sample size of 40, we would expect that toxicities with a true rate of occurrence of 0.05 in 87 of 100 trials. Rates and associated 95% exact Clopper and Pearson binomial confidence limits were estimated for 1) grade 2 or higher toxicities attributed to neratinib, 2) dose reductions and hospitalizations, 3) the objective response (CR+PR), and 4) PFS and OS estimated using the product limit method of Kaplan and Meier. The association between tolerability (dose reductions and number of completed courses) and log2 chemotherapy toxicity risk score was assessed using t-test and linear regression respectively. The study was stopped early (N = 25) due to sponsor’s decision to halt further accrual due to lack of funding.
RESULTS
Patients:
A total of 25 patients were accrued between December 2016 and March 2019 (Table 1). The median age was 66 (60–79), including 16/25 (64%) aged 60–69, 5/25 (20%) aged 70–74, and 4/25 (16%) aged ≥75. A total of 76% of patients were Caucasian, 16% Asian, and 8% African-American. Seventy-six percent were patients with hormone receptor positive metastatic breast cancer. The median lines of therapy for metastatic disease received prior to study drug was 3 (range 0–11). Thirty-six percent of patients had lung metastases, 36% had liver metastases, and 12% had brain metastases. Seventy-six percent received trastuzumab, pertuzumab containing regimen, and/or trastuzumab-emtansine (TDM-1) therapy prior to this trial.
Table 1:
Demographic and baseline clinical characteristics of patients
| Characteristic | Patients (N = 25) |
|---|---|
|
| |
| Age (years) | |
| Median (range) | 66 (60–79) |
| 60–69 | 16 (64%) |
| 70–74 | 5 (20%) |
| ≥75 | 4 (16%) |
|
| |
| Gender | |
| Female | 24 (96%) |
| Male | 1 (4%) |
|
| |
| Race | |
| Caucasian | 19 (76%) |
| Asian | 4 (16%) |
| African-American | 2 (8%) |
|
| |
| ECOG performance status | |
| 0 | 8 (32%) |
| 1 | 17 (68%) |
|
| |
| Hormone receptor status | |
| Positive | 19 (76%) |
| Negative | 6 (24%) |
|
| |
| Sites of metastasis1 | |
| Bone | 16 (64%) |
| Lung | 9 (36%) |
| Local | 9 (36%) |
| Liver | 9 (36%) |
| Distant | 5 (20%) |
| Chest wall/sternum | 4 (16%) |
| Skin | 3 (12%) |
| Brain | 3 (12%) |
| Peritoneum | 2 (8%) |
| Breast | 2 (8%) |
| Other2 | 5 (20%) |
|
| |
| Median number of prior lines of metastatic therapy (range) | 3 (0–11) |
|
| |
| Prior systemic therapy for metastatic disease3 | |
| Trastuzumab | 17 (68%) |
| Trastuzumab emtansine (TDM-1) | 8 (32%) |
| Pertuzumab | 13 (52%) |
| Hormone therapy | 7 (28%) |
| Other systemic therapy | 11 (44%) |
18/25 (72%) had multiple metastases
Other metastases include: left carotid artery, spleen, CNS, pleura, and clavicle
All 25 patients had multiple therapies
Adverse Events:
All 25 patients were evaluable for adverse events (AEs). Worst grade per adverse event type per subject were summarized. 20/25 patients (80%, 95% CI [59%, 93%]) experience a worst grade toxicities ≥ grade 2 attributed to neratinib. They are listed in Table 2. 9/25 patients (36%, 95% CI [18%, 57%]) had grade 3 toxicities attributed to neratinib (Figure 1 and Table 2). Grade 3 GI toxicities were: diarrhea (N =5, 20%), vomiting (N = 2, 8%), abdominal pain (N = 2, 8%), and nausea (N = 1, 4%). Grade 3 hematological toxicities were: anemia, leukopenia, neutropenia, and lymphopenia (N = 1, 4% each). Other grade 3 toxicities were fatigue, anorexia, dehydration, weight loss, acute kidney injury, and hypertension (N = 1, 4% each). There were no grade 4 or 5 toxicities. Four patients were hospitalized including two for neratinib-induced gastrointestinal toxicities, one for spinal cord compression, and one for appendicitis.
Table 2:
Grade 2 and 3 adverse events by CTCAE 4.0
| Adverse Events (CTCAE 4.0) | Grade 2, N (%) | Grade 3, N (%) |
|---|---|---|
|
| ||
| Blood and lymphatic system disorders: | ||
| Anemia | 3 (12%) | 1 (4%) |
| Lymphocyte count decreased | 5 (20%) | 1 (4%) |
| Neutrophil count decreased | 0 (0%) | 1 (4%) |
| Platelet count decreased | 1 (4%) | 0 (4%) |
| White blood cell count decreased | 3 (12%) | 1 (4%) |
|
| ||
| Gastrointestinal disorders: | ||
| Abdominal pain | 2 (8%) | 2 (8%) |
| Diarrhea | 11 (44%) | 5 (20%) |
| Nausea | 3 (12%) | 1 (4%) |
| Vomiting | 0 (0%) | 2 (8%) |
|
| ||
| General: | ||
| Anorexia | 2 (8%) | 1 (4%) |
| Dehydration | 2 (8%) | 1 (4%) |
| Fatigue | 2 (8%) | 1 (4%) |
| Generalized muscle weakness | 1 (4%) | 0 (0%) |
| Weight loss | 1 (4%) | 1 (4%) |
|
| ||
| Laboratory abnormalities: | ||
| Acute kidney injury | 0 (0%) | 1 (4%) |
| Aspartate aminotransferase increased | 1 (4%) | 0 (0%) |
| Creatinine increased | 1 (4%) | 0 (0%) |
| Hypoalbuminemia | 1 (4%) | 0 (0%) |
|
| ||
| Skin: | ||
| Rash maculo-papular | 1 (4%) | 0 (0%) |
| Skin laceration | 1 (4%) | 0 (0%) |
|
| ||
| Cardiovascular disorders: | ||
| Ejection fraction decreased | 1 (4%) | 0 (0%) |
| Hypertension | 0 (0%) | 1 (4%) |
| Syncope | 0 (0%) | 1 (4%) |
Figure 1.
Worst grade toxicities attributed to treatment at grade 2 and above.
Dose hold and dose reduction:
Of 25 patients, the median number of cycles completed was 3 (0–12). A total of 9/25 patients (36%, 95% CI [18%, 57%]) had dose modifications in at least 1 cycle. The dose reductions were due to diarrhea (N = 6), rash (N = 1), vomiting (N = 1), and trouble breathing (N = 1).
Response and survival:
24/25 patients had restaging imaging for RECIST 1.1 measure. 1/25 (4%, 95% CI [0%, 20%]) had a partial response, 11/25 (44%, 95% CI [24%, 65%]) achieved stable disease, 12/25 (48%, 95% CI [28%, 69%]) had disease progression, and 1/25 (4%, 95% CI [0%, 20%]) was not assessed. 22/25 (88%) went off treatment due to progression. 1/25 (4%) went off treatment for toxicity but progressed before being given further treatment; therefore, there was a total of 23 events in the PFS analysis. Median PFS was 2.6 months (95% CI [2.56–5.26]). At the time of this analysis, 16/25 were reported to have died (64%, 95% CI [43%, 82%]), including two that were within 30 days of treatment. The median overall survival was 17.4 months (95% CI [10.3, NA]). At time of data cut-off, one patient was on treatment and had been receiving treatment for ten months. 24 patients discontinued therapy including 4/24 (17%) for clinical progression, 18/24 (75%) for progression by RECIST, and 2/24 (8%) for neratinib GI toxicity (grade 3 diarrhea and nausea).
CARG toxicity risk scores (N = 22):
The average CARG toxicity risk score was 0.34 (min=0.19, max=0.59). There was a trend in the difference in CARG toxicity risk scores by whether a patient had a dose reduction (mean difference in log2 risk = no dose modification - dose modification = −0.41, 95% CI [−0.82, 0.008], p = 0.054). Patients with higher risk scores were more likely to require a dose reduction (Figure 2). The lack of significance is most likely due to the small sample size. Using linear regression, we did not find log2 CARG toxicity risk score to be a significant predictor of courses completed (slope = −1.29, se =1.44, p = 0.39).
Figure 2.
The association of CARG toxicity risk score and neratinib dose. Boxplots showing the difference in log2 risk score between patients with no dose reduction vs. patients with reduction.
Pharmacokinetics:
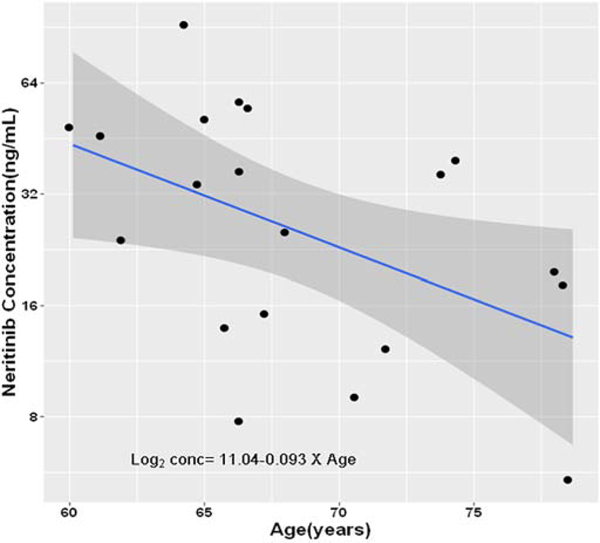
Neratinib concentrations at hour 0 measure at cycle 1 day 15 were used to define the concentration at steady state based on a 240 mg daily dose. Twenty-one participants had a concentration value at this time point; one participant’s dose had been modified so was removed. Neratinib concentration was skewed right, so we transformed it using a log base 2 transformation. Least squares regression was used to assess the relationship between steady state neratinib concentration and age (Figure 3). There was a linear relationship with a regression coefficient of −0.093 (se = 0.0398, p = 0.03). The antilog steady state values for participants aged 65 and 75 were 31.9 ng/mL and 16.7 ng/mL, respectively. The least squares regression was used to determine if CARG toxicity risk score at baseline was predictive of steady state neratinib concentration. The results showed CARG toxicity risk score was not predictive (1.40, se = 2.39, p = 0.57).
Figure 3.
The association between neratinib steady state concentrations and age. Graph presenting the least squares regression line, 95% confidence interval and data.
Mutation Analysis:
5/25 (20%) had activating HER2 or HER3 mutations: patient 1 (ERBB2 V777L and ERBB2 amplification); patient 2 (ERBB2 L755S and ERBB3 E928G); patient 3 (ERBB2 R784C); patient 4 (ERBB3 S846I); and patient 5 (ERBB2 V777L) (Supplemental Table 2). Responses in the HER2/3mut tumors were: 1 PR (20%), 3 SD (60%), and 1 PD (20%). CBR was 80% and 4/5 (80%, 95% CI [28%, 99%]) were alive at six months. Responses in the HER2amp group were: 8 SD (40%), 11 PD (55%), and 1 not assessed (5%). 17/20 (85%, 95% CI [62%, 97%]) were alive at six months.
DISCUSSION
With promising results from multiple clinical trials and recent FDA approval, neratinib has emerged as a standard of care therapy for patients with HER2+ breast cancer. The current study of single agent neratinib in older adults with breast cancer demonstrates the feasibility of neratinib therapy in patients ≥ 60. Grade ≥ 3 diarrhea was observed in 20% of patients with prophylactic use of Imodium, in comparison with 30.7% observed in the CONTROL trial. Only 2/25 (8%) discontinued therapy due to neratinib-induced GI toxicity. The efficacy of single agent neratinib in this heavily pretreated cohort of patients (median 3 lines of therapy; range 0–11) with mixed HER2 amplified or HER 2/3 mutated breast cancer appears to be modest.
Neratinib was initially FDA approved for extended adjuvant therapy in the phase III ExteNET trial which assessed the efficacy of neratinib as extended adjuvant therapy in patients with early-stage HER2+ breast cancer who had completed adjuvant therapy with trastuzumab. The neratinib treated patients had significantly higher rates of invasive DFS than placebo, corresponding to a relative reduction in the risk of invasive disease recurrence of 34% and 27% at 2 and 5 years, respectively. HER2-targeted therapies for metastatic BC continue to evolve over time4. The current standard of care treatment for patients with HER2+ metastatic breast cancer is first-line trastuzumab plus pertuzumab and a taxane, followed by second-line trastuzumab-emtansine35. After disease progression during treatment with the second-line metastatic therapy trastuzumab-emtansine, there are multiple treatment options available. These include third-line therapy drug-antibody conjugate fam-trastuzumab deruxtecan36 and the combination of neratinib plus capecitabine. In addition, the novel HER2-targeted TKI tucatinib has shown great efficacy in the phase III HER2CLIMB trial7. Burstein et al. reported neratinib as monotherapy in HER2-positive MBC with overall response rate of 24% in trastuzumab-refractory patients and 56% in trastuzumab-naive patients20. The modest response observed in our trial is comparable. In the Burstein trial, grades 3 to 4 diarrhea occurred in 30% of patients with prior trastuzumab treatment. Although older adults with mean age of 50 (range 31 – 83) were included, there is no specific toxicity data reported for the older adults. HER2-targeted therapy has been studied in older adults with breast cancer. O’Connor et al. reported the safety and toxicity of first-generation TKI lapatinib with trastuzumab in older adults with breast cancer31. To our knowledge, the current trial is the only study focusing on the safety and tolerability of neratinib in older adults.
The PK of TKIs in older adults with cancer has not been well studied. Lapatinib is a HER2 and EGFR TKI with variable bioavailability due to its low solubility and first-pass metabolism by CYP3A4/537. There are no dosing recommendations provided for older adults with breast cancer because no data is available on the age-related PK of lapatinib37. Available data indicate that advanced age has no relevant influence on the PK of TKIs such as lapatinib, imatinib, dasatinib, nilotinib, pazopanib and sunitinib38. In this study, the steady state neratinib concentrations for a patient aged 65 was twice that of a patient aged 75 based on least squares regression. The etiology is unclear and poor adsorption in older adults could contribute to the difference.
The epidermal growth factor receptor (EGFR) family of transmembrane receptor tyrosine kinases activates signaling pathways regulating cellular proliferation and survival39. The HER2 receptor is a non-ligand binding member of the EGFR family, and exerts its activity through heterodimerization with other EGFR family members. HER2 functional activation promotes oncogenesis, leading to the investigation of HER2-directed agents in cancers with HER2 alterations10. Somatic HER2 and HER3 mutations can lead to constitutive HER2 activation in the absence of gene amplification. Activating HER2 mutations occur in the HER2 receptor extracellular and transmembrane domains, and at multiple hotspots in the kinase domain with no single mutation predominating10. The rate of HER2/HER3 mutations in a variety of solid tumors has an incidence rate not exceeding 5–10% in any tumor type. HER2-activating mutations in primary breast cancer is approximately 2–3%, which translates to 4,000 to 6,000 patients with HER2-mutated MBC40–43. Neratinib is active in HER2 mutated, non-amplified MBC. In a phase II trial by Ma et al., neratinib demonstrated a CBR of 31% (90% CI [13%–55%]) in a heavily pretreated patient population with HER2 mutated non-amplified MBC12. The phase II basket SUMMIT trial is evaluating the efficacy of neratinib in HER2 mutated solid tumor regardless of tumor types (NCT01953926). Although neratinib can inhibit growth of HER2 and HER3 mutant tumors in preclinical models, the clinical activity was only observed in HER2 mutated, not HER3 mutated tumors. Clinical responses were observed in tumors harboring HER2 S310, L755, V777, P780_Y781insGSP, and A775_G776insYVMA mutations across different tumor types including breast, cervical, biliary, salivary, and non-small-cell lung cancers. More patients had stable disease than objective response. No objective responses were observed in bladder or colorectal cancers, or in the HER3 mutant. In the subgroup analysis of eleven patients with ER+ HER2 mutant MBC treated with neratinib plus fulvestrant, an overall RR of 18.2% (2/11, 95% CI [2.3 to 51.8]) and a CBR 54.5% (6/11, 95% CI [23.4 to 83.3]) was observed. SUMMIT may provide the largest body of clinical data to date on the use of a pan-HER inhibitor in solid tumors with somatic HER2/HER3 mutations, although variable responses in HER2 mutants varied by type of tumor and the specific HER2 mutation were observed. Importantly, not all HER2 mutations generate the same level of HER2 hyperactivity or oncogenic dependence44.
In the current study, the following diarrhea prophylaxis was used: Loperamide 4 mg three times a day for the first 14 days followed by 4 mg twice a day for days 14–28 on cycle 1. Beyond cycle 1, loperamide was used as needed. In the ExteNET trial, where antidiarrheal prophylaxis was not protocol-mandated, grade 3–4 diarrhea rate was 39.9%, median duration of grade ≥ 3 diarrhea was 5 days; and neratinib dose reductions and dose holds due to diarrhea occurred in 26.4% and 33.9% of patients, respectively. In this study, we observed 20% grade ≥ 3 diarrhea. In addition to Imodium, effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer was evaluated through the CONTROL trial45. Incidence of grade ≥ 3 diarrhea was 30.7% (95% CI [23.1–39.1]) with loperamide prophylaxis (loperamide cohort), 23.4% (95% CI [13.8–35.7]) with loperamide prophylaxis plus budesonide (budesonide cohort), and 11.5% (95% CI [2.4–30.2]) with loperamide prophylaxis plus colestipol (colestipol cohort) vs. 39.9% without protocol-mandated loperamide prophylaxis in the ExteNET trial. Based on the CONTROL trial result, it is reasonable to recommend addition of colestipol to loperamide as prophylaxis of diarrhea for older adults receiving neratinib.
The American Society of Clinical Oncology (ASCO) and the Institute of Medicine have identified therapeutic phase II trials as a key research priority to increase the evidence base for older adults with cancer14,46. While targeted therapies may represent a less toxic option for older patients, few trials have studied their tolerability and efficacy in older adults14,47. Older patients with breast cancer receiving adjuvant chemotherapy are at increased risk of chemotherapy toxicities, and currently there are limited BC-specific tools33,48,49,50. The Cancer and Aging Research Group (CARG) developed and validated a chemotherapy toxicity score for older adults with cancer30. The CARG toxicity risk score was associated with dose delay/reduction, chemotherapy discontinuation, hospitalization, and RDI < 85% (p<0.001) in patients with breast cancer51. Extending the validation of the CARG toxicity risk score to targeted therapies and immunotherapies would be a meaningful development in the era of precision medicine with broader application of targeted therapies. In a recent study using the CARG BC score in older adults with HER2 amplified breast cancer receiving the combination of lapatinib and trastuzumab, CARG-Basa was not associated with the toxicity profiles52. Our current study also showed a trend of increased CARG toxicity risk score with poor tolerance of neratinib, which calls for specific toxicity-predicting tools in the era of targeted therapy, including TKIs, immunotherapy, and CART therapy50,53.
The current study was limited by its small sample size and limited number of patients 70 and older. While on this trial, mandated management guideline using loperamide were used; however, other agents such budesonide and colestipol were not systemically addressed since the evidence of these supportive agents were not available at time of initial trial design in 2015. A shift in management guideline (dose titrating up from 160 mg to 240 mg if tolerated)54 was not used. In addition, FDA approved the combination of neratinib plus capecitabine based on the efficacy data observed from the NALA study55. Our single agent neratinib study may not be directly translated to combination use in older adults. A future study specifically assessing the combination of capecitabine and neratinib in older adults with cancer is needed. In addition, the oral bioavailability of HER-directed TKI, specifically lapatinib and erlotinib, is reduced in the presence of gastric acid-reducing agents. It is possible that neratinib absorption may also be decreased by reduced gastric acid secretion56,57. Similarly, several studies suggest age-related decreases in gastric acid secretion and reduced oral chemotherapy absorption consequently58–60. Lastly, the current study did not include quality of life assessment (QOL), which is a critical endpoint in older adults with advanced stage cancer.
CONCLUSION
Neratinib was safe in this population of older adults with HER2 amplified or HER2/3 mutated metastatic BC. Higher CARG toxicity risk score showed a trend towards a need for dose modification. Future studies with larger sample sizes are needed to confirm this finding.
Supplementary Material
Acknowledgements:
The authors gratefully acknowledge Puma for sponsoring the study and providing study drug, and Covance for analysis of correlatives. The authors also thank the STOP Cancer Foundation (PI Yuan Yuan), NCI K-12 Career Development Award (K12CA001727, PI Joanne Mortimer), and NIH 1R01CA206911–01A1 (PI Emily Wang). Research reported in this publication includes work performed at the Pathology and Biostatistics Core of City of Hope National Cancer Center, supported by the National Cancer Institute (NCI) under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
Funding: Puma sponsored the trial and provided neratinib. This study was supported by COH Pathology Research Services Core and Biostatistics and Mathematical Modeling Core (National Cancer Institute of the National Institutes of Health under award number P30CA033572). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of abbreviations
- AE
Adverse event
- ANC
Absolute neutrophil count
- ALT
Alanine transferase
- AST
Aspartate transferase
- BC
Breast cancer
- CLIA
Clinical Laboratory Improvement Amendments
- CT
computed tomography
- DLTs
Dose-limiting toxicities
- ECHO
echocardiogram
- ECOG
Eastern Oncology Group
- FISH
Fluorescence In-situ Hybridization
- HER2
Human epidermal growth factor receptor amplification
- IHC
Immunohistochemistry
- IRB
Institutional review board
- MUGA
multiple-gated acquisition scan
- MTD
Maximum tolerated dose
- MBC
Metastatic BC
- ORR
Overall response rate
- OS
Overall survival
- PFS
progression free survival
- PHI
Protected health information
- PK
Pharmacokinetics
- RR
response rate
- TEQR
Toxicity equivalence range
- UNL
Upper limit of normal
Footnotes
Ethics approval and consent to participate: The protocol and amendments were approved by City of Hope’s IRB-approved protocol 15342. All procedures were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. Privacy rights were observed and written informed consent was obtained from all participants of this study under IRB15342 and ClinicalTrials.gov NCT02673398.
Trial registration: ClinicalTrials.gov, NCT02673398. Registered 3 February 2016 https://clinicaltrials.gov/ct2/show/NCT02673398?term=neratinib&cond=metastatic+breast+cancer&rank=4
Conflict of Interest: Dr. Yuan has contracted research sponsored by Merck, Eisai, Novartis, Puma, Genentech, and Pfizer; is a consultant for Puma, and is on the Speakers Bureau for Eisai. The other authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meric-Bernstam F, Johnson AM, Dumbrava EEI, et al. : Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin Cancer Res 25:2033–2041, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brain E, Caillet P, de Glas N, et al. : HER2-targeted treatment for older patients with breast cancer: An expert position paper from the International Society of Geriatric Oncology. J Geriatr Oncol 10:1003–1013, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Pondé N, Wildiers H, Awada A, et al. : Targeted therapy for breast cancer in older patients. J Geriatr Oncol 11:380–388, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Chan A, Delaloge S, Holmes FA, et al. : Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology 17:367–377, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Freedman RA, Gelman RS, Anders CK, et al. : TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol 37:1081–1089, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saura C, Oliveira M, Feng YH, et al. : Neratinib plus capecitabine versus lapatinib plus capecitabine in patients with HER2+metastatic breast cancer previously treated with >= 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. Journal of Clinical Oncology 37:2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy RK, Loi S, Okines A, et al. : Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 382:597–609, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Rabindran SK, Discafani CM, Rosfjord EC, et al. : Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 64:3958–65, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Tiwari SR, Mishra P, Abraham J: Neratinib, A Novel HER2-Targeted Tyrosine Kinase Inhibitor. Clin Breast Cancer 16:344–348, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Connell CM, Doherty GJ: Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2:e000279, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Baruch NE, Bose R, Kavuri SM, et al. : HER2-Mutated Breast Cancer Responds to Treatment With Single-Agent Neratinib, a Second-Generation HER2/EGFR Tyrosine Kinase Inhibitor. Journal of the National Comprehensive Cancer Network 13:1061–1064, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma CX, Bose R, Gao F, et al. : Neratinib Efficacy and Circulating Tumor DNA Detection of HER2 Mutations in HER2 Nonamplified Metastatic Breast Cancer. Clin Cancer Res 23:5687–5695, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner NC, Kingston B, Kilburn LS, et al. : Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol 21:1296–1308, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurria A, Dale W, Mooney M, et al. : Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32:2587–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman RA, Foster JC, Seisler DK, et al. : Accrual of Older Patients With Breast Cancer to Alliance Systemic Therapy Trials Over Time: Protocol A151527. J Clin Oncol 35:421–431, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh H, Walker AJ, Amiri-Kordestani L, et al. : US Food and Drug Administration Approval: Neratinib for the Extended Adjuvant Treatment of Early-Stage HER2-Positive Breast Cancer. Clinical Cancer Research 24:3486–3491, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Awada A, Dirix L, Manso Sanchez L, et al. : Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol 24:109–16, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Jankowitz RC, Abraham J, Tan AR, et al. : Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol 72:1205–12, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Chow LW, Xu B, Gupta S, et al. : Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer 108:1985–93, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burstein HJ, Sun Y, Dirix LY, et al. : Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28:1301–7, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Wong KK, Fracasso PM, Bukowski RM, et al. : A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 15:2552–8, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Martin M, Bonneterre J, Geyer CE Jr., et al. : A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer 49:3763–72, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Blackwell KL, Zaman K, Qin S, et al. : Neratinib in Combination With Trastuzumab for the Treatment of Patients With Advanced HER2-positive Breast Cancer: A Phase I/II Study. Clin Breast Cancer 19:97–104.e4, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Saura C, Garcia-Saenz JA, Xu B, et al. : Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 32:3626–33, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Gandhi L, Bahleda R, Tolaney SM, et al. : Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol 32:68–75, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Extermann M, Aapro M, Bernabei R, et al. : Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55:241–52, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Decoster L, Van Puyvelde K, Mohile S, et al. : Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol 26:288–300, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology Summary. J Oncol Pract 14:442–446, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWalde N, Jagsi R, Dotan E, et al. : NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. J Natl Compr Canc Netw 14:1357–1370, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Hurria A, Mohile S, Gajra A, et al. : Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 34:2366–71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor T, Soto-Perez-de-Celis E, Blanchard S, et al. : Abstract P5–21-08: Tolerability of the combination of lapatinib and trastuzumab in older patients with HER2 positive metastatic breast cancer. Cancer Research 78:P5–21-08-P5–21-08, 2018 [Google Scholar]
- 32.Hurria A, Gupta S, Zauderer M, et al. : Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 104:1998–2005, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Hurria A, Cirrincione CT, Muss HB, et al. : Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. 29:1290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyvanjah K, DiPrimeo D, Li A, et al. : Pharmacokinetics of neratinib during coadministration with lansoprazole in healthy subjects. Br J Clin Pharmacol 83:554–561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giordano SH, Temin S, Davidson NE: Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract 14:501–504, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Fatah TM, Arora A, Moseley PM, et al. : DNA repair prognostic index modelling reveals an essential role for base excision repair in influencing clinical outcomes in ER negative and triple negative breast cancers. Oncotarget 6:21964–78, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crombag MR, Joerger M, Thurlimann B, et al. : Pharmacokinetics of Selected Anticancer Drugs in Elderly Cancer Patients: Focus on Breast Cancer. Cancers (Basel) 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Gion P, Kanefendt F, Lindauer A, et al. : Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet 50:551–603, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Gureasko J, Shen K, et al. : An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125:1137–49, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Desmedt C, Zoppoli G, Gundem G, et al. : Genomic Characterization of Primary Invasive Lobular Breast Cancer. J Clin Oncol 34:1872–81, 2016 [DOI] [PubMed] [Google Scholar]
- 41.The Cancer Genome Atlas N, Koboldt DC, Fulton RS, et al. : Comprehensive molecular portraits of human breast tumours. Nature 490:61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bose R, Kavuri SM, Searleman AC, et al. : Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 3:224–37, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross JS, Wang K, Sheehan CE, et al. : Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin Cancer Res 19:2668–76, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Hyman DM, Piha-Paul SA, Rodon J, et al. : Neratinib in HER2 or HER3 mutant solid tumors: SUMMIT, a global, multi-histology, open-label, phase 2 “basket” study. Cancer Research 77:2, 2017 [Google Scholar]
- 45.Hurvitz S, Chan A, Iannotti N, et al. : Abstract P3–14-01: Effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer: The CONTROL trial. Cancer Research 78:P3–14, 2018 [Google Scholar]
- 46.Hurria A, Levit LA, Dale W, et al. : Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. Journal of Clinical Oncology 33:3826-+, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Mohile S, Dale W, Hurria A: Geriatric oncology research to improve clinical care. Nature Reviews Clinical Oncology 9:571–578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan Y, Vora N, Sun CL, et al. : Association of pre-chemotherapy peripheral blood pro-inflammatory and coagulation factors with reduced relative dose intensity in women with breast cancer. Breast Cancer Res 19:101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soto-Perez-de-Celis E, Li D, Yuan Y, et al. : Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 19:e305–e316, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Kim KH, Extermann M: Predictive Tools for Older Cancer Patient Management, 2020, pp 463–476 [Google Scholar]
- 51.Hurria A, Magnuson A, Gross C, et al. : Abstract GS6–04: Development and validation of a chemotherapy toxicity (Chemo Tox) risk score for older patients (Pts) with breast cancer (BC) receiving adjuvant/neoadjuvant treatment (Adjuvant Tx): A R01 and BCRF funded prospective multicenter study. Cancer Research 79:GS6–04-GS6–04, 2019 [Google Scholar]
- 52.O’Connor T, Soto-Perez-De-Celis E, Blanchard S, et al. : Tolerability of the combination of lapatinib and trastuzumab in older patients with HER2 positive metastatic breast cancer. Cancer Research 78:1, 2018 [Google Scholar]
- 53.Soto-Perez-De-Celis E, Loh KP, Baldini C, et al. : Targeted agents for HER2-positive breast cancer in older adults: current and future perspectives. Expert Opin Investig Drugs 27:787–801, 2018 [DOI] [PubMed] [Google Scholar]
- 54.Barcenas CH, Hurvitz SA, Di Palma JA, et al. : Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann Oncol 31:1223–1230, 2020 [DOI] [PubMed] [Google Scholar]
- 55.Saura C, Oliveira M, Feng YH, et al. : Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 38:3138–3149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kletzl H, Giraudon M, Ducray PS, et al. : Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs 26:565–72, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Koch KM, Im YH, Kim SB, et al. : Effects of Esomeprazole on the Pharmacokinetics of Lapatinib in Breast Cancer Patients. Clin Pharmacol Drug Dev 2:336–41, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Skirvin JA, Lichtman SM: Pharmacokinetic considerations of oral chemotherapy in elderly patients with cancer. Drugs Aging 19:25–42, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Aymanns C, Keller F, Maus S, et al. : Review on pharmacokinetics and pharmacodynamics and the aging kidney. Clin J Am Soc Nephrol 5:314–27, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Kimmick GG, Fleming R, Muss HB, et al. : Cancer chemotherapy in older adults. A tolerability perspective. Drugs Aging 10:34–49, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.