Abstract
Background and Objectives:
The primary aim of this study is to assess the necessity of fundoplication for reflux in patients undergoing Heller myotomy for achalasia. The secondary aim is to assess the safety of the robotic approach to Heller myotomy.
Methods:
This is a single institution, retrospective analysis of 61 patients who underwent robotic Heller myotomy with or without fundoplication over a 4-year period (January 1, 2015 – December 31, 2019). Symptoms were evaluated using pre-operative and postoperative Eckardt scores at < 2 weeks (short-term) and 4 – 55 months (long-term) postoperatively. Incidence of gastroesophageal reflux and use of antacids postoperatively were assessed. Long-term patient satisfaction and quality of life (QOL) were assessed with a phone survey. Finally, the perioperative safety profile of robotic Heller myotomy was evaluated.
Results:
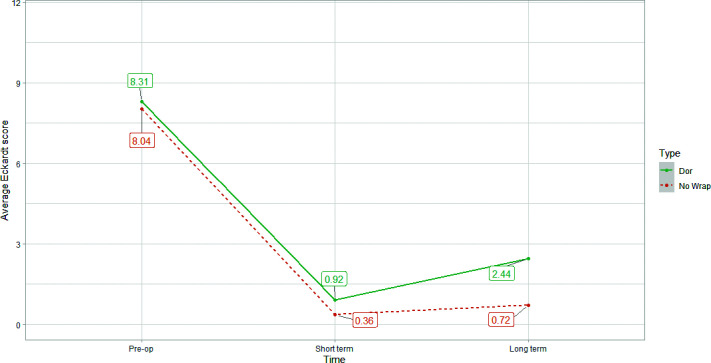
The long-term average Eckardt score in patients undergoing Heller myotomy without fundoplication was notably lower than in patients with a fundoplication (0.72 vs 2.44). Gastroesophageal reflux rates were lower in patient without a fundoplication (16.0% vs 33.3%). Additionally, dysphagia rates were lower in patients without a fundoplication (32.0% vs 44.4%). Only 34.8% (8/25) of patients without fundoplication continued use of antacids in the long-term. There were no mortalities and a 4.2% complication rate with two delayed leaks.
Conclusion:
Robotic Heller myotomy without fundoplication is safe and effective for achalasia. The rate of reflux symptoms and overall Eckardt scores were low postoperatively. Great patient satisfaction and QOL were observed in the long term. Our results suggest that fundoplication is unnecessary when performing Heller myotomy.
Keywords: Achalasia, Myotomy, Heller myotomy, Robotic Heller myotomy, Fundoplication
INTRODUCTION
Esophageal achalasia is a motility disorder characterized by absence or abnormal esophageal peristalsis and failure of the lower esophageal sphincter (LES) to relax.1 It occurs as result of a loss in inhibitory neurons in the esophageal myenteric plexus.1 Patients typically present with symptoms of dysphagia, gastroesophageal reflux (GER), chest pain, and weight loss.1 There is no cure to achalasia and it is palliatively managed surgically by a Heller myotomy, as first performed by Dr. Ernest Heller in 1913. Advances in minimally invasive surgery have allowed this procedure to be performed laparoscopically and robotically which significantly reduces risk and improves recovery times.
A wide range of reported postoperative reflux rates led to the adoption of performing a partial wrap with the procedure. Several studies suggest that a wrap may not be necessary and may in fact increase the risk for dysphagia.2–6 On the other hand, a recent meta-analysis by Campos et al. reviewed 39 observational studies with 3,086 patients that underwent laparoscopic myotomy and reported that postoperative rates of GER were 8.8% in the group that received a fundoplication and 31.5% in the group that did not.7 The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) strongly recommends the addition of fundoplication, favoring a partial over total fundoplication, to prevent postoperative reflux and minimize treatment failures.8
The introduction of Per-Oral Endoscopic Myotomy (POEM), which precludes the creation of a fundoplication, raised the question of whether a wrap is required.9,10 The aim of this study is to assess the necessity of a wrap when performing Heller myotomy and investigate the efficacy of a robotic approach to the procedure.
METHODS
With institutional review board approval, retrospective chart review and a long-term follow up questionnaire was undertaken. This is a single institution, retrospective analysis of 61 patients that underwent robotic Heller myotomy for achalasia over a 4-year period and follow up questionnaire (January 1, 2015 – December 31, 2019). Patients were grouped into those that underwent the addition of a Dor fundoplication and those that did not. The patients included in this study had their procedures performed by 3 surgeons, most being performed by the senior author (DRJ) who has performed roughly 200 robotic Heller myotomies at the time of this review. The decision to perform a fundoplication was made by surgeon preference at the time of surgery. Standard pre-operative workup for patients included esophagogastroduodenoscopy (EGD), upper gastrointestinal series (UGI), computed tomography of the chest, abdomen, and pelvis (CT CAP), esophageal manometry, and preoperative Eckardt score. The severity of the pre-operative symptoms and their improvement postoperatively was evaluated by comparing pre-operative Eckardt scores to short-term and long-term postoperative Eckardt scores. Short term follow-up was conducted with a patient survey within two weeks after surgery and included components of the Eckart score. A survey was then created and conducted over the phone with patients at a long-term postoperative point. The incidence of GER symptoms, their severity, and the frequency of antacid use postoperatively was assessed along with satisfaction and reported quality of life (QOL) [Figures 1a and 1b]. Perioperative complication rates, operative time, and estimated blood loss (EBL) were also compared between the two groups.
Figure 1.


Long-term Quality Of Life Phone Survey Questionnaire.
The operation was undertaken on the DaVinci (da Vinci™ Surgical Intuitive, Inc., Mountain View, CA) robotic platform in steep reverse Trendelenburg using a 5-port approach with the liver retractor placed via the AirSeal (AirSeal® Surgi-Quest, Inc., Milford, CT) port in the right upper quadrant. Standard approach at our institution begins via entry into the lesser sac and take-down of the short gastric vessels with intent to reach the left crus. Our energy instrument of choice for this procedure is the Harmonic Scalpel (Harmonic Scalpel™ Ethicon SAS, Issy-tes-Moutineaux, France), as it allows for fine dissection and excellent hemostasis. Trans-hiatal dissection is performed posteriorly to anteriorly to mobilize the esophagus intraperitoneally. The pars flaccida is then entered and the right crus is identified with the same maneuvers performed to fully mobilize the esophagus to the level of the mid-chest. Beginning at the level of the gastroesophageal junction with the nonactive blade of the Harmonic down, the myotomy is begun until the inner circular muscles are split and the mucosa is bulging. The myotomy is carried out for at least 8 cm into the esophagus and 4 cm onto the stomach. Intraoperative upper endoscopy is then performed to confirm mucosal integrity using the underwater leak test. In patients who underwent Dor fundoplication, this was done using 2-0 Ethibond to cover the myotomy. Postoperative care included a repeat UGI on day 1 followed by a liquid diet for 2 full weeks until seen in the office. All patients were discharged on antacid medication.
RESULTS
Sixty-one patients underwent robotic Heller myotomy for achalasia. Forty-eight (78.7%) patients underwent robotic Heller myotomy without fundoplication and 13 patients (21.3%) underwent surgery with Dor fundoplication.
In the without fundoplication group, there were 25 males (52.1%) and 23 females (47.9%) with a mean age of 51.4 years and a median American Society of Anesthesiologists (ASA) II classification [Table 1]. Pre-operatively, all patients reported dysphagia, 46 (95.8%) reported regurgitation, 33 (75%) reported gastrointestinal reflux disease (GERD), 28 (59.6%) reported weight loss, and 17 (36.2%) reported retrosternal pain. The average Eckardt score preoperatively was 8.04. The average operative time, EBL, and length of stay (LOS) were 97.0 minutes, 37.2 mL, and 3.3 days (median = 2.0), respectively [Table 2]. There were no 30-day mortalities or immediate postoperative leaks. Two patients (4.2%) developed delayed leaks identified between postoperative days 7 and 14. These leaks were delayed after normal immediate postoperative upper gastrointestinal studies. The first patient had free intraperitoneal air and left pleural effusion requiring an exploratory laparotomy, chest tube placement, and surgical drain placement. The second patient developed sudden onset abdominal pain followed by extravasation of contrast on an UGI requiring an exploratory laparotomy where a perforation was identified and sutured. Both did well after surgery.
Table 1.
Patient Demographics, Rate of Response to the Survey, and Average Time to Response to Survey
| No Wrap (n = 48) | Dor Fundoplication (n = 13) | ||
|---|---|---|---|
| Age in years (average) | 51.4 | 44.6 | p = 0.208 |
| Sex Male Female |
25 (52.1%) 23 (47.9%) |
7 (53.8%) 6 (46.2%) |
p = 0.910 |
| Body Mass Index (average) | 27.7 | 25.7 | p = 0.187 |
| ASA classification (median) | 2 | 2 | p = 0.744 |
| Responded to survey | 25 (52.1%) | 9 (69.2%) | |
| Average time from surgery to survey (months) | 35.3 (range: 4 – 55) | 15.2 (range: 3 – 26) |
ASA, American Society of Anesthesiologists.
Table 2.
Surgery Profiles
| No Wrap | Dor Fundoplication | Independent Samples T-Test | |
|---|---|---|---|
| Operative time (mins) | 97.0 (58.0 – 177.0) | 104.0 (71.0 – 160.0) | p = 0.402 |
| Average EBL (mL) | 37.2 (5.0 – 100.0) | 37.7 (10.0 – 50.0) | p = 0.542 |
| Median LOS (days) | 2.0 (1.0 – 26.0; M = 3.3) | 2.0 (2.0 – 6.0; M = 2.8) | p = 0.617 |
EBL, Estimated blood loss; LOS, length of stay.
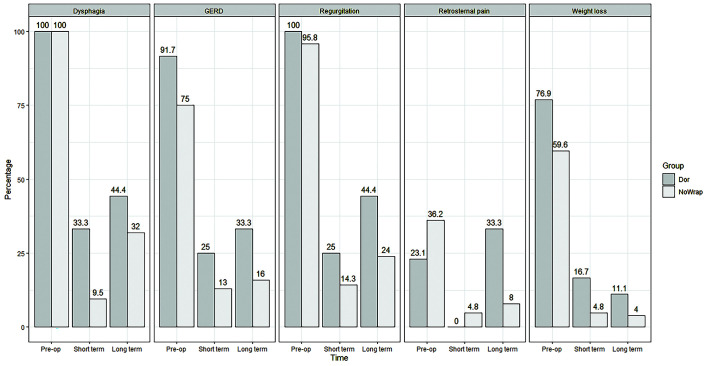
Forty-two (87.5%) patients within the no fundoplication group responded to the short-term (< 2 weeks) survey. Four (9.5%) reported dysphagia, 6 (14.3%) reported regurgitation, 13 (31.0%) reported GERD, 2 (4.8%) reported weight loss, and 2 (3.8%) reported retrosternal pain. The average Eckardt score at this time was 0.36. Twenty-five within the no fundoplication group (52.1%) patients responded to the long-term survey at an average of 35.3 (range: 4 – 55) months postoperatively. Eight (32.0%) patients reported dysphagia, 6 (24%) reported regurgitation, 4 (16%) reported GERD, 1 (4.0%) reported weight loss, and 2 (8.0%) reported retrosternal pain. The average Eckardt score at this time was 0.72 [Table 3]. Among the 8 patients who reported dysphagia in the long-term, 7 (87.5%) reported their symptoms as “occasional” and one (12.5%) reported their symptoms as “requires liquids to clear”. Six out of 25 (24%) patients in the no fundoplication group reported symptoms of regurgitation in the long-term survey. All 6 (24%) reported their symptoms as “occasional” and 8 out of the 25 patients (32%) reported using antacids for symptom control.
Table 3.
Summary of Symptoms Reported by Patients Pre-operatively, Short-Term Postoperatively, and Long-Term Postoperatively
| No Wrap (n = 48) |
Dor Fundoplication (n = 13) |
|||||
|---|---|---|---|---|---|---|
| Preop (n = 48) | ST Postop (n = 42) | LT Postop (n = 25) | Preop (n = 13) | ST Postop (n = 12) | LT Postop (n = 9) | |
| Dysphagia | 48 (100%) | 4 (9.5%) | 8 (32.0%) | 13 (100%) | 4 (33.3%) | 4 (44.4%) |
| Regurgitation | 46 (95.8%) | 6 (14.3%) | 6 (24%) | 13 (100%) | 3 (25%) | 4 (44.4%) |
| GERD | 33 (75%) | 13 (31.0%) | 4 (16%) | 11 (91.7%) | 3 (25%) | 3 (33.3%) |
| Weight loss | 28 (59.6%) | 2 (4.8%) | 1 (4.0%) | 10 (76.9%) | 2 (16.7%) | 1 (11.1%) |
| Retrosternal pain | 17 (36.2%) | 2 (4.8%) | 2 (8.0%) | 3 (23.1%) | 0 (0.0%) | 3 (33.3%) |
| Eckardt score | 8.04 | 0.36 | 0.72 | 8.31 | 0.92 | 2.44 |
ST, short-term; LT, long-term; GERD, gastroesophageal reflux disease.
In terms of QOL amongst long term survey respondents, all patients in the no fundoplication group reported improvement in their symptoms. Only 2 (8.0%) patients had to undergo another surgical procedure for similar symptoms. Most patients (76%) said that they would “probably” or “definitely” choose to do the surgery if they were in the same situation again. Almost all patients (92%) ranked their current QOL as “Good”, “Very good”, or “Excellent”. All 25 patients reported a “probable” or “definite” improvement in QOL directly attributable to surgery.
Thirteen patients underwent Heller myotomy with Dor fundoplication. Seven were males (53.8%) and 6 were (46.2%) females with an average age of 44.6 years, an average body mass index of 25.7, and a median ASA II classification [Table 1]. All patients initially presented with dysphagia and regurgitation, 11 (91.7%) reported GERD, 10 (7.9%) reported weight loss, and 3 (23.1%) reported retrosternal chest pain. The average Eckardt score preoperatively was 8.31. The average operative time, EBL, and LOS were 104.0 minutes, 37.7 mL, and 2.0 days, respectively [Table 2]. No complications or 30-day mortalities occurred in this group. The demographic and surgical profiles of patients who underwent Heller myotomy with fundoplication versus those who underwent the procedure without fundoplication did not differ significantly in any regard [Table 1 and Table 2].
Twelve out of the 13 patients in the fundoplication group (92.3%) responded to the short-term survey. Four (33.3%) reported dysphagia, 3 (25%) reported regurgitation, 3 (25%) reported GERD, and 2 (16.7%) reported weight loss. No patients complained of retrosternal pain at this time. The average Eckardt score at this time was 0.92. Nine out of the 13 patients (69.2%) responded to the long-term survey at an average of 15.2 months postoperatively. Four (44.4%) patients reported dysphagia, 4 (44.4%) reported regurgitation, 3 (33.3%) reported GERD, 1 (11.1%) reported weight loss, and 3 (33.3%) reported retrosternal pain. The average Eckardt score at this time was 2.44 [Table 3]. Of 4 total patients who reported dysphagia in the long-term survey, 1 patient (25.0%) reported their symptoms as “occasional”, 2 (50%) reported they “required liquids to clear”, and 1 patient (25%) reported “impaction requiring medical attention”. Of 4 total patients that reported regurgitation in the long-term survey, 2 reported their symptoms as “occasional” (50%), one reported their symptoms as “predictable by posture” (25.0%), and 1 reported that their symptoms “interferes with daily activities” (25.0%). Of 3 total patients who reported chest pain (33.3%), two described their symptoms as “occasional” (66.7%) and one described that their symptoms as “interferes with daily activities” (33.3%). Most patients (87.5%) were no longer using antacids in the long-term.
Most patients (77.8%) reported improvement in symptoms after the surgery but only 4 (44.4%) stated that they would undergo the same surgery again if they were in a similar situation. One (11.1%) patient reported undergoing a surgical procedure for recurrence of symptoms. Overall, 7 (77.8%) patients ranked their current QOL as “good”, “very good”, or “excellent”. Seven (77.8%) patients stated that their QOL was currently negatively affected by abdominal symptoms. Finally, 7 (77.8%) patients stated that their QOL was positively affected by surgery.
Figure 2 summarizes the improvement in symptoms over time in both groups. Figure 3 shows the drop in Eckardt score over the short and long-term in both groups.
Figure 2.
Rates of symptoms pre-operatively, short-term postoperatively, and long-term postoperatively in patients who underwent a dor fundoplication compared to patients who did not receive a fundoplication.
Figure 3.
Average Eckardt scores pre-operatively, short-term postoperatively, and long-term postoperatively.
DISCUSSION
This study found robotic Heller myotomy without fundoplication to have improved dysphagia and reflux rates over the long-term while maintaining great patient satisfaction and perioperative safety compared to Dor fundoplication. The drop in the Eckardt score in patients that underwent Heller myotomy without fundoplication from 8.04 pre-operatively to 0.72 in the long-term postoperative period demonstrates a clear remission of symptoms of achalasia as defined by an Eckardt score of less than 3.11, 12 It is also notably lower than the average Eckardt score seen in patients that underwent a Heller myotomy with Dor fundoplication (0.72 vs 2.44). Clinically significant dysphagia (33.3% vs 40.0%) and GERD (11.1% vs 00.0%) were also higher in the Dor fundoplication group in the long-term. It is important to note that most (88.9%) patients reporting GERD described their symptoms as “infrequent” and “occasional” with 70.9% not using any antacids for symptom relief. The fact that most patients complaining of reflux symptoms did not seek medical attention suggests the nonclinically significant nature of these symptoms.
The Eckardt score is a clinical scoring system developed by Eckardt et al. in 1992 as a predictive measure of treatment success in patients undergoing pneumatic dilation for achalasia.11 The score consists of 4 items: weight loss, dysphagia, retrosternal pain, and regurgitation.11 Each of these items can be score from 0 to 3 based on their severity for a maximum total score of 12.11 Typically, a score of 3 or less indicates successful treatment of achalasia while a score of 9 points or more indicates failure of treatment.13 Both groups in this study achieved an Eckardt score of < 3, but the group without a fundoplication had a more significant drop in Eckardt score to 0.72 compared to 2.44 in the fundoplication group.
Heller myotomy with fundoplication is suggested as the standard surgical treatment for achalasia. However, there are numerous studies suggesting that Heller myotomy without fundoplication is effective in the treatment of achalasia without resulting in significant heartburn.2,14 In a study conducted by Richards et al., only 13% of patient developed GER symptoms after Heller myotomy without an antireflux procedure and were treated medically with excellent results.15 Richards et al.’s argument against the addition of a fundoplication is the fact that it increases the resistance across the LES resulting in decreased symptom relief.15Also, adding a fundoplication to the remaining 87% of patients with no objective measure of reflux would be inappropriate.15 Treating such patients medically would be sufficient and spares them the risk of dysphagia down the line requiring more drastic management such as pneumatic dilatation or re-operation.15 In our study, patients that underwent a Heller myotomy without fundoplication had a higher rate of antacid use compared to the Dor fundoplication group over the long-term (34.8% vs 12.5%). Despite the higher proton pump inhibitor (PPI) use rate in the no fundoplication group, these patients had lower rates of GERD (16.0% vs 33.3%), regurgitation (24.0% vs 44.4%), dysphagia (32.0% vs 44.4%), and retrosternal chest pain (8.0% vs 33.3%) compared to the fundoplication group. This reinforces the notion that adding a fundoplication, or performing any intervention, in patients that can otherwise be managed medically may unnecessarily increase the risk of more significant symptoms such as dysphagia and expose them to the potential risk for an operative procedure.
A meta-analysis conducted by Lyass et al. in 2003 reviewed the 24-hour pH studies of 489 patients who underwent Heller myotomy with and without partial fundoplication performed between 1991 and 2001.16 Results revealed a GER rate of 7.9% (18/228) in the group that received partial fundoplication and 10% (4/40) in the group did not receive a fundoplication.16 This minimal increase was not statistically significant (P = .75).16 In addition, there was no significant difference in the rate of reported GER symptoms between the two groups.16
On the other hand, some authors argue that it is beneficial as it decreases the incidence of postoperative GER.17–20 A prospective randomized double-blind clinical trial conducted by Richards et al. in 2004 showed that Heller myotomy with a Dor fundoplication to be significantly superior to Heller myotomy alone in regard to the incidence of postoperative GER.21 These results are backed by objective measures of GER including an esophageal pH study and manometry.21 The incidence of GER symptoms was remarkably lower in the Heller myotomy plus Dor group (9.1% vs 47.6%) with lower DeMeester scores (6.5 vs 31.5).21 However, dysphagia symptoms were not significantly different between the two groups.21 Our results show a higher incidence of clinically significant dysphagia in the Dor fundoplication group (33%) compared to the no fundoplication group (4.0%) in the long-term.
The endoscopic alternative to laparoscopic fundoplication is the relatively new transoral incisional fundoplication (TIF) that was introduced in 2008.22 TIF has been shown to significantly decrease the rate of symptoms and PPI use in patients complaining of GER.23, 24 Although there have been no studies showing this technique used after a Heller myotomy, it has been performed successfully after peroral endoscopic myotomy procedures25. Some studies suggest that TIF is equivalent to a Dor fundoplication but remains inferior to a Nissen fundoplication.26 Despite good immediate results, there is some evidence to suggest that TIF’s efficacy fades over time and patients resort to PPI use eventually.27 Our results suggest that a fundoplication may not be necessary at all in most patients undergoing a Heller myotomy.
When it comes to QOL, a similar pattern of improvement was noted as seen above. In patient who underwent Heller myotomy without fundoplication, 84% reported their current QOL as “Very good” or “Excellent” compared to only 66.6% in patient that received a Dor fundoplication. Robert et al. reported good functional results per clinical examination in 91.4% of patients at one year and 78.6% at five years postoperatively.28 Ellis et al. reported partial or complete symptoms resolution in 92% of patients at a mean of 42 months with good or excellent results achieved in 84% of patients.29 Raiss et al. reported good to excellent results in 92% of patients undergoing Heller myotomy without fundoplication for reflux.30
Finally, robotic Heller myotomy maintained a safe perioperative profile without compromising efficacy. Mean operating room times, EBL, and other safety parameters were comparable to the current reported data in the literature which demonstrates the safety of a robotic approach.28, 31
There are a few limitations to this study. First, this is a single institution retrospective study looking at the practice of only three surgeons. All patients underwent surgery using the DaVinci platform which limits the ability to generalize the results of this study to other robotic platforms. The sample size is limited to 61 patients with complete surgical profiles but a long-term response rate of 55.7% (34/61) which makes it difficult to make a generalized statement. The two groups are disproportionate in size and only include one type of fundoplication. The long-term follow up phone survey assessed subjective GER symptoms reported by patients without any objective measures of reflux. Finally, our definition of “long-term” at 4 – 55 months with a mean of 35.3 months in the Heller myotomy without fundoplication group and 15.2 months in the fundoplication group may be considered relatively short.
Richards et al. presented an objective answer to the question of adding a fundoplication to Heller myotomy.21 Despite these results and as the author notes, there appears to be poor correlation between objective pathologic reflux measures and subjective symptoms reported by patients. Given this apparent disconnect and our finding of mild GER symptoms and low Eckardt scores in the long-term, it appears that Heller myotomy without fundoplication is sufficient in decreasing symptoms while sparing patients the potential complications of pneumatic dilatation or reoperation for the treatment of dysphagia down the line.
CONCLUSION
Robotic Heller myotomy alone without partial fundoplication appears to be safe and effective in the management of achalasia. The rate of reflux symptoms and overall Eckardt scores were low postoperatively. Great patient satisfaction and QOL were observed in the long-term. Randomized control trials comparing patients undergoing robotic Heller myotomy with and without fundoplication are needed to achieve more generalizable results.
Footnotes
*Contributed equally to this work.
Disclosure: none.
Funding sources: none.
Conflict of interests: none.
Informed consent: Dr. D. Rohan Jeyarajah declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Muhammad B. Darwish, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
Shankar I. Logarajah, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
Kei Nagatomo, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
Terence Jackson, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
Annie Laurie Benzie, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
Patrick James McLaren, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
Edward Cho, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX.; Department of Surgery, TCU/UNTHSC School of Medicine, Fort Worth, Texas, USA.
Houssam Osman, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX..
D. Rohan Jeyarajah, Department of Surgery, Methodist Richardson Medical Center, Richardson, TX.; Department of Surgery, TCU/UNTHSC School of Medicine, Fort Worth, Texas, USA.
References:
- 1.Park W, Vaezi MF. Etiology and pathogenesis of achalasia: The current understanding. Am J Gastroenterol. 2005;100(6):1404–1414. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, Sharp K, Holzman M, Clements R. The outcome of laparoscopic Heller myotomy without antireflux procedure in patients with achalasia/Discussion. The American Surgeon. 1998;64(6):515. [PubMed] [Google Scholar]
- 3.Dempsey DT, Delano M, Bradley K, et al. Laparoscopic esophagomyotomy for achalasia: does anterior hemifundoplication affect clinical outcome? Ann Surg. 2004;239(6):779-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp KW, Khaitan L, Scholz S, Holzman MD, Richards WO. 100 consecutive minimally invasive heller myotomies: Lessons learned. Ann Surg. 2002;235(5):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomston M, Rosemurgy A. Selective application of fundoplication during laparoscopic Heller myotomy ensures favorable outcomes. Surg Laparosc Endosc Percutan Tech. 2002;12(5):309-315. [DOI] [PubMed]
- 6.Diamantis T, Pikoulis E, Felekouras E, et al. Laparoscopic esophagomyotomy for achalasia without a complementary antireflux procedure. J Laparoendosc Adv Surg Tech A. 2006;16(4):345–349. [DOI] [PubMed] [Google Scholar]
- 7.Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and Surgical Treatments for Achalasia. Ann Surg. 2009;249(1):45–57. [DOI] [PubMed] [Google Scholar]
- 8.Stefanidis D, Richardson W, Farrell TM, et al. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc. 2012;26:296–311. [DOI] [PubMed] [Google Scholar]
- 9.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42(4):265–271. [DOI] [PubMed] [Google Scholar]
- 10.Minami H, Isomoto H, Yamaguchi N, et al. Peroral endoscopic myotomy for esophageal achalasia: clinical impact of 28 cases. Dig Endosc. 2014;26(1):43–51. [DOI] [PubMed] [Google Scholar]
- 11.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103(6):1732–1738. [DOI] [PubMed] [Google Scholar]
- 12.Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil. 2018;30(6):e13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren Y, Tang X, Chen Y, et al. Pre-treatment Eckardt score is a simple factor for predicting one-year peroral endoscopic myotomy failure in patients with achalasia. Surg Endosc. 2017;31(8):3234–3241. [DOI] [PubMed] [Google Scholar]
- 14.Brown J, Egger M, Kehdy FJ. Heller myotomy: to wrap or not to wrap? Am Surg. 2018;84(6):1022–1026. [PubMed] [Google Scholar]
- 15.Richards WO, Sharp KW, Holzman MD. An antireflux procedure should not routinely be added to a heller myotomy. J Gastrointest Surg. 2001;5(1):13–16. [DOI] [PubMed] [Google Scholar]
- 16.Lyass S, Thoman D, Steiner JP, Phillips E. Current status of an antireflux procedure in laparoscopic Heller myotomy. Surg Endosc. 2003;17(4):554–558. [DOI] [PubMed] [Google Scholar]
- 17.Kjellin AP, Granqvist S, Ramel S, Thor KB. Laparoscopic myotomy without fundoplication in patients with achalasia. Eur J Surg. 1999;165(12):1162–1166. [DOI] [PubMed] [Google Scholar]
- 18.Burpee SE, Mamazza J, Schlachta CM, et al. Objective analysis of gastroesophageal reflux after laparoscopic Heller myotomy: an anti-reflux procedure is required. Surg Endosc. 2005;19(1):9–14. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti G, Brusciano L, Amato G, et al. A total fundoplication is not an obstacle to esophageal emptying after heller myotomy for achalasia: results of a long-term follow up. Ann Surg. 2005;241(4):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V, Shimi SM, Cuschieri A. Does laparoscopic cardiomyotomy require an antireflux procedure? Endoscopy. 1998;30(1):8–11. [DOI] [PubMed] [Google Scholar]
- 21.Richards WO, Torquati A, Holzman MD, et al. Heller myotomy versus heller myotomy with dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240(3):405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue H, Minami H, Satodate H, Kudo S-E. First clinical experience of submucosal endoscopic esophageal myotomy for esophageal achalasia with no skin incision. Gastrointestinal Endoscopy. 2009;69(5):AB122. [Google Scholar]
- 23.Cadière GB, Buset M, Muls V, et al. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg. 2008;32(8):1676–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miutescu BP, Tantau MV, Khashab MA. Advances in POEM for achalasia: optimal technique, post-POEM GERD. Curr Treat Options Gastro. 2020;18(2):328–336. [Google Scholar]
- 25.Tyberg A, Choi A, Gaidhane M, Kahaleh M. Transoral incisional fundoplication for reflux after peroral endoscopic myotomy: a crucial addition to our arsenal. Endosc Int Open. 2018;6(5):E549–E552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter JE, Kumar A, Lipka S, Miladinovic B, Velanovich V. Efficacy of laparoscopic Nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology. 2018;154(5):1298–1308.e7. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Chen S, Zhao H, et al. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: a systematic review with meta-analysis. Surg Endosc. 2017;31(3):1032–1044. [DOI] [PubMed] [Google Scholar]
- 28.Robert M, Poncet G, Mion F, Boulez J. Results of laparoscopic Heller myotomy without anti-reflux procedure in achalasia. Monocentric prospective study of 106 cases. Surg Endosc. 2008;22(4):866–874. [DOI] [PubMed] [Google Scholar]
- 29.Ellis FH, Gibb SP, Crozier RE. Esophagomyotomy for achalasia of the esophagus. Ann Surg. 1980;192(2):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raiss M, Hrora A, Menfaa M, et al. L’opération de Heller sans système anti-reflux. À propos de 123 cas. Annales de Chirurgie. 2002;127(10):771–775. [DOI] [PubMed] [Google Scholar]
- 31.Horgan S, Galvani C, Gorodner MV, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: multicenter study. Journal of Gastrointestinal Surgery. 2005;9(8):1020–1030. [DOI] [PubMed] [Google Scholar]