Abstract
Background and Objectives:
The aim of this study was to demonstrate a detailed and elaborative step-wise laparoscopic surgical management technique of vault endometriosis.
Methods:
A total of 5 patients were operated on for laparoscopic management of vault endometriosis performed at our center between January 1 2015 and December 31, 2019.
Results:
There were no short or long term complications related to laparoscopic management of vault endometriosis with a satisfactory prognosis.
Conclusion:
This analysis explains the descriptive methodology of assessment of patients and operative technique for vault endometriosis.
Keywords: Vault endometriosis, Laparoscopic surgeries, Assessment of patients, Surgical technique
INTRODUCTION
Endometriosis is a chronic inflammatory gynecological condition common in women of reproductive age. It can manifest in the form of ovarian endometrioma, peritoneal implants, and deep endometriosis. Deep endometriosis is defined as a disease infiltrating 5 mm of retroperitoneum or pelvic viscera.1 It can occur at the rectum, bladder, ureter, and vault. The exact etiology for deep endometriosis at the vault is unknown, and multifactorial causes have been implicated. According to a few cases reports in the literature, it was hypothesized that it might occur in cases of hysterectomy for adenomyotic uterus where transplantation of endometrial cells can occur over the vault during colpotomy or vaginal morcellation of bulky specimen.2 There might also be a possibility of incomplete excision of the endometriotic tissue at the utero-sacral ligament or vagina during the hysterectomy procedure, which may lead to remnant endometriotic tissue at the vault. These remnant endometriotic tissue may regrow at the vault leading to symptomatology.3 It may also be referred to as a form of iatrogenic endometriosis,3 which is the presence of endometriotic tissue following specific gynecologic surgical interventions in case of benign uterine disease. Vault endometriosis can spread locally over time, leading to severe symptoms as chronic pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and can involve adjacent structures gradually leading to grave complications as the involvement of bladder leads to hematuria, involvement of bowel leads to hematochezia and ureter involvement which leads to dreadful consequences like hydroureter, hydronephrosis, or even kidney failure,4,5 and peritonitis.6 Owing to its infiltrating pathology and high recurrence rate, the absolute curative treatment of vault endometriosis requires its complete excision with disease free margin. To achieve this surgical outcome, detailed anatomical knowledge and experienced surgical expertise is required, especially in a post hysterectomized patient. Considering it, we have described a step-by-step surgical management technique for the excision of vault endometriosis.
MATERIALS, METHODS, AND SURGICAL TECHNIQUE
This is a retrospective analysis. Video records of all laparoscopic management of vault endometriosis performed at our center between January 1, 2015 and December 31, 2019 were analyzed, for a total of 5 patients, all of whom were operated on by the same surgeon. The laparoscopic vault endometriosis excision was done according to the technique described below with the principle of complete excision with a 1 – 2 cm disease free margin.
Assessment of Patient
Vault endometriosis usually manifests with symptoms suggestive of chronic pelvic pain. It does not have any specific symptoms, signs, or any particular findings on imaging study for its confirmatory diagnosis. It is a diagnosis of exclusion. A high index of suspicion and detailed assessment of the patient is required. The diagnostic perspective for vault endometriosis commences from evaluation of medical history and detailed symptomatology followed by elaborative clinical examination7 and appropriate imaging study. Common symptoms in vault endometriosis are chronic pelvic pain, dyspareunia, dysuria, and dyschezia.7,8 Symptomatology depends on the involvement of surrounding structures by endometriosis at uterosacral ligament, bladder, and bowel. Detailed past medical history for any medical management of deep endometriosis should be noted. On clinical examination, speculum examination may reveal grossly normal or bluish to brownish spots over the dorsal fornix of the vault. Vaginal examination may reveal irregular hypertrophied or nodular vault associated with tenderness. Rectal examination may reveal hard nodule, tender on palpation, usually felt along the anterior rectal wall, especially in cases accompanied with recto-vaginal nodule.9,10
Imaging of the abdomen-pelvis region (magnetic resonance imaging [MRI], abdominal ultrasound, transvaginal ultrasound) may be tried for supportive evidence, but usually, there are no specific findings related to vault endometriosis.11,12 However, few findings on imaging may be still suggestive of vault endometriosis or associated other endometriotic lesions. Abdominal and transvaginal ultrasound is the first imaging of choice in a suspected case of vault endometriosis as it may help in the assessment of site, size, approximate depth of nodule if present, and findings of ovarian endometrioma. Abdominal ultrasound should be done as baseline investigation and to diagnose if there is any silent hydronephrosis. Pelvic MRI should be used as second-line imaging, and may show irregular and hypointense soft tissue nodular thickening on T2- weighted sequences, as it occurs when the disease involves uterosacral ligament, vaginal, or rectal wall.1 Cystoscopy or colonoscopy may be performed pre-operatively if the patient has symptoms of bladder or bowel endometriosis. Laparoscopy remains the method of choice for diagnosis and treatment in deep endometriosis,13 and it is confirmed by histology.
SURGICAL TECHNIQUE
Under general anesthesia, the patient is placed in a modified Lloyd Davis position at a 30 degree angle with the horizontal. The basic angle is a 30 degree Trendelenburg with the hips flexed at 15 degrees; the legs abducted to allow access to the perineum. It is a common position for surgical procedures involving the pelvis and lower abdomen, as it gives a good exposure and may minimize pressure area damage in longer surgeries. Foley’s catheter is inserted into the urinary bladder. The operating surgeon stood on the left side of the patient. The first assistant held the camera and was positioned toward the head of the table, and the second assistant on the right side helped with manipulation, grasping, and retracting tissues. The third assistant aided the surgeon in vaginal manipulation.
The port positions were as follows: the primary trocar (10 mm) for the camera was inserted at 1 cm above the umbilicus. The 3 accessory trocars (5 mm) were inserted under direct vision, two on the left and one on the right side. The abdomen was insufflated with CO2 gas and maintained at a pressure of 12 mm Hg during the surgery. All cases were operated in a 3-dimensional laparoscopy set-up. The Mangeshkar vaginal cup was used for vaginal manipulation.
STEPS
1) Adhesiolysis with exploration and assessment of the extent of endometriosis.
With the operating surgeon on the left side, adhesiolysis begins by grasping the epiploic appendage of the large bowel and putting it under traction while simultaneously cutting the adhesions between the antimesenteric border of the large bowel and vault and pelvic sidewall. On completion of adhesiolysis, the large bowel is then mobilized out of the pelvis and placed into the abdomen, thereby facilitating the inspection of the vault. Detailed exploration and assessment were done for any signs/features suggestive of endometriosis, especially over pelvic peritoneum, sigmoid colon, rectum, uterosacral ligament, bilateral ovaries, and vault [Figure 1].
Figure 1.

Vault endometriosis with peritoneal implants.
2) Dissection of Para-vesical and lateral Para-rectal spaces [Figure 2].
Figure 2.
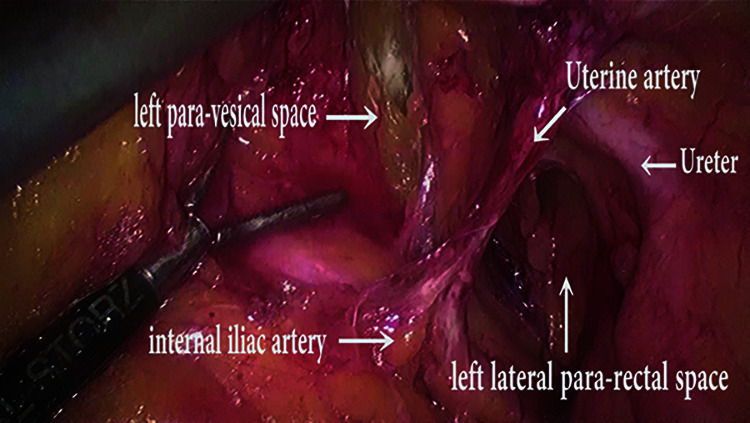
Dissection Of lateral para-rectal and para-vesical space.
The development of avascular spaces in the pelvis is preliminary for any difficult pelvic dissection. Retro-peritoneal space dissection begins at sacral promontory, at the entry level of the ureter into the pelvis and extending the incision caudally almost up to respective side uterosacral ligament. Keeping the ureter under the vision and retracted laterally, the loose areolar tissue within this region is cut to expose the lateral pararectal space (Latzko space), medial to internal iliac artery. The uterine artery originates from the internal iliac artery, which forms the common boundary between pararectal space and para-vesical space. Inferomedial to the terminal part of the internal iliac artery (also known as an obliterated umbilical artery), the operator grasps the fibro-fatty tissue with atraumatic forceps and dissect it to expose the para-vesical space, until the base of paravesical space (formed by the levator ani muscle) is visible.
3) Ureterolysis
The ureter is lateralized from its medial peritoneal attachments preserving its vascular adventitia intact. Meso-ureter is held under gentle traction by the surgeon using an atraumatic grasper, and the ureter is separated and lateralized. The dissection is proceeded in a craniocaudal direction, and the ureter is traced from its medial surface up to the uterosacral ligament. This step is repeated on the opposite side.
4) Nerve dissection [Figure 3]
Figure 3.
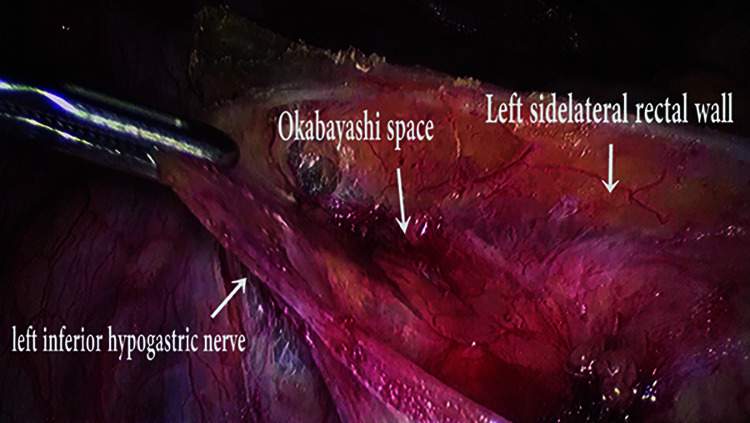
Left inferior hypogastric nerve dissection.
Bilateral Inferior Hypogastric Nerve (IHN) is identified in medial para-rectal space (Okabayashi space) as a white shiny cord like taut structures. Further bilateral IHNs are lateralized from their medial peritoneal attachment and safeguarded till hypogastric plexus at uterosacral ligament. If any entrapment/encasement of nerves is identified, adhesiolysis and release are performed for a better postoperative recovery.14
5) Rectovaginal space dissection [Figure 4]
Figure 4.

Rectovaginal space dissection.
Keeping the rectum under traction using bowel grasper, the peritoneum in the pouch of Douglas is incised. The areolar tissue within this space is cut and to expose the posterior vaginal wall. This step is greatly enhanced by the help of a vaginal assistant who keeps one small, folded gauze on a sponge holder in each posterior fornix and rectum, with traction being applied at both places in the opposite direction to delineate a surgical plane between posterior fornix and prerectal vaginal space.
6) Vescico-vaginal space dissection [Figure 5]
Figure 5.
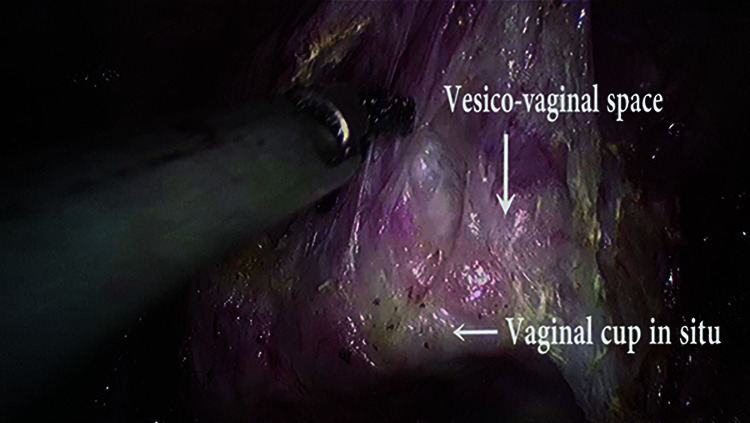
Vesico-vaginal space dissection.
The vaginal cup may be used at this step to enhance the surgical plane between the vagina and bladder for a safe and secure dissection. The anterior edge of the vaginal vault is grasped using atraumatic forceps and is pulled inferiorly while the assistant lifts the peritoneum over the bladder. This step facilitates the identification of the plane between the bladder and vagina. Using sharp dissection, the plane is developed, and the bladder is separated from the anterior vaginal wall to provide an adequate length of vaginal margins for excision. The plane is then extended laterally, taking care not to injure the ureters. The lateral extension of the plane is limited by the ureteric tunnel.
7) Ureteric tunnel dissection [Figure 6]
Figure 6.
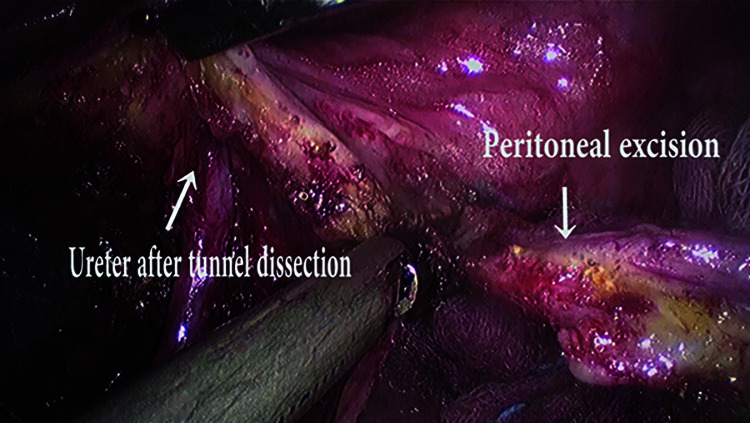
Left ureteric tunnel dissection with diseased peritoneal excision.
In order to achieve a successful circumferential vault excision with a disease-free margin, it is mandatory to secure the safety of the ureter near the angle of the vaginal vault. The roof of the ureteric tunnel comprising of the dense fibers of the vesico-vaginal ligament is identified by applying upward and lateral traction over the bladder peritoneum. Using cold scissors/harmonic scalpel, the left ureteric tunnel is deroofed, and the ureter lateralized to expose the lateral margin of the left angle of the vaginal vault. Care should be taken not to injudiciously use the delivery of thermal or ultrasonic energy in this region as it can compromise the vascularity of the ureter and lead to uretero- vaginal fistula. Similar steps are repeated on the opposite side.
8) Diseased posterior peritoneal dissection
Associated diseased posterior pelvic peritoneum is excised, lying adjacent to the ureter and uterosacral ligament. Excision depends on the extent of the involvement, approximately posterior peritoneum from sacral promontory till utero-sacral ligament, adjacent to the ureter, is excised along with uterosacral nodule if present. Traction is applied with atraumatic forceps over the diseased peritoneum to be excised in the opposite direction, and dissection is completed.
9) Vault excision [Figure 7]
Figure 7.
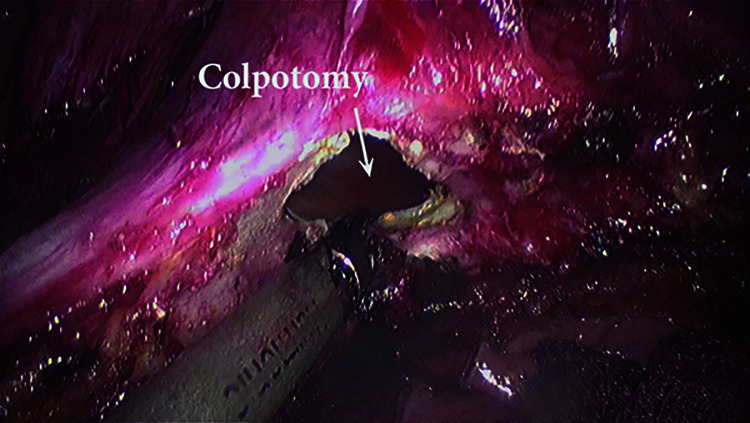
Circumferential diseased vault excision.
Beginning from the left angle of the vaginal vault, nearly 1 – 2 cm below the laparoscopically visible disease margin, vault excision is commenced. The cut is extended anteriorly, keeping the bladder retracted superiorly. The cut is then extended in a clockwise manner to achieve a circumference of disease-free margin of the vaginal vault, ensuring that the surrounding vital structures bladder, rectum, and bilateral ureter are away from the excision site. The excised vault is then examined for its adequacy and delivered out vaginally [Figure 8].
Figure 8.

Final specimen of diseased vault with peritoneum along with circumferential normal looking vagina.
10) Vault closure
Beginning from either end of the newly created vault, the needle is passed through the angle of the vault and the vault is closed with 1-0 vicryl suture in a continuous non locking manner. The vault is then examined and hemostasis is confirmed.
RESULTS
A total of 5 patients were operated on for vault endometriosis in the last 5 years at our center. The median age of the patients was 43 years (range, 38 – 46 years), and the median body mass index was 20.4 kg/m2 (range, 17 – 31.6 kg/m2). [Table 1]
Table 1.
Patients’ Past Medical and Surgical History
| Case | Age | Primary Surgery | Symptoms | Medical Management | Imaging Study: Noninvasive, Invasive | Surgery for vaginal vault excision |
|---|---|---|---|---|---|---|
| 1 | 43 | Total Abdominal Hysterectomy with Left Salpingo-oophorectomy in 2014 |
-Dyspareunia since 8 months -Irregular bleeding per vaginum for past 3 months |
Symptomatic treatment for and dyspareunia and bleeding per vaginum | Transvaginal ultrasound, MRI pelvis: no significant finding | was performed in 2015 - Ovary was preserved and only surface fulguration was done. |
| 2 | 46 | Vaginal Hysterectomy with Bilateral Salpingo-oophorectomy done in 2015 | - Chronic pelvic pain and dyspareunia for past 6 months - Irregular spotting and bleeding per vaginum for past 2 months |
Symptomatic treatment for and dyspareunia and bleeding per vaginum | MRI Pelvis: T2 image showed -Soft tissue nodular lesion on right side of vaginal vault |
was performed in 2016 |
| 3 | 43 | Total Abdominal Hysterectomy in 2013 | - Chronic pelvic pain, dyspareunia, severe dyschezia for past 36 months - Irregular spotting per vaginum for past 6 months |
-Injection GnRH analog 11.25 mg 2 doses,-Oral Dienogest for 6 months-OCPs for 1 year-Symptomatic treatment for bleeding per vaginum | - Transvaginal ultrasound Bilateral ovarian endometrioma, Recto-vaginal nodule -Colonoscopy:no significant findings |
was performed in 2017 |
| 4 | 45 | Total Laparoscopic Hysterectomy in 2016 | - Chronic pelvic pain, dyspareunia, severe dyschaezia for past 24 months -Bleeding per vaginum and spotting for past 3 months |
-Oral Dienogest for 6 months-OCPs for 1 year-Symptomatic treatment for bleeding per vaginum | - Transvaginal ultrasound: Bilateral ovarian endometrioma, Recto-vaginal nodule -Colonoscopy: no significant findings |
was performed in 2019 |
| 5 | 38 | Total Abdominal Hysterectomy in 2017 | -Chronic pelvic pain, dyspareunia, severe dyschezia for past 18 months -Bleeding per vaginum and irregular spotting for past 4 months |
-Injection GnRH analog 11.25 mg 2 doses, -Oral Dienogest for 6 months -OCPs for 6 months-Symptomatic treatment for bleeding per vaginum |
- Transvaginal ultrasound: Bilateral ovarian endometrioma, Recto-vaginal nodule -Colonoscopy: no significant findings |
was performed in 2019 |
OCP, oral contraceptive pills; MRI, magnetic resonance imaging; GnRH, gonadotrophin releasing hormone; IHN, inferior hypogastric nerve; MIS, minimal invasive surgery.
Common symptoms found in our study were chronic pelvic pain, dyspareunia, dyschezia, irregular spotting, and vaginal bleeding. There was a history of prior medical management in the form of hormonal and symptomatic treatment in our all patients. But none of the patients had symptomatic relief. Even some of the cases complained of worsening of the symptoms in spite of continuing medical management. The average duration of symptomatology in our study is from 3.6 months to 17 months.
Physical examination revealed some common findings in almost all of the patients. Speculum examination showed a bluish-brownish spot at dorsal fornix in 3 cases. Vaginal examination revealed vault thickening in all cases. Nodularity was felt in 3 patients per rectal examination. Transvaginal ultrasound was done as a primary imaging study in all cases. Three cases had ovarian endometriosis with a recto-vaginal nodule. MRI of the pelvis revealed soft tissue like nodular lesion in the right side of the vaginal vault in 1 case. Three cases had a history of colonoscopy in view of severe dyschezia. Abdominal ultrasound was done as a pre-operative baseline investigation in all cases, but no significant findings related to vault endometriosis were found. Peroperatively, all patients had bluish to blackish, flat to a nodular lesion at the vault. All the patients also had discolored, puckered, nonelastic peritoneal implant on the posterior broad ligament peritoneum, which were excised with negative margins. Two patients had left IHN entrapped with an endometriotic nodule at the level of the respective uterosacral ligament. Adhesiolysis and nodule excision with the release of involved nerves was performed. Three patients had a gross recto-vaginal nodule, approximately 3 × 2 cm in size, hard in consistency, densely adhered to adjacent structures, complete excision of the nodules was executed along with the vault excision. Bilateral salpingo-oophorectomy was done for ovarian endometrioma in 3 patients. Ovarian surface fulguration was performed for endometriotic surface implants in 1 patient. The respective ovary was preserved after explaining the risk of recurrence and taking proper consent for the same.
The average operative duration was 70 mins (range 60 – 90 mins). None of the cases was converted to laparotomy. The average blood loss during surgery was approximately 30 ml (20 –40 ml). Blood transfusion was not required in any of the patients.
The final histopathological report confirmed endometriosis in the specimen of vault, peritoneum, recto-vaginal nodule. The patients’ median hospital stay was two days (range 1– 4 days). There was no short term or long term complication related to the surgery. All patients were given injection gonadotropin hormone releasing hormone analogs, 11.25 mg, as a single dose 1 day following surgery. All patients are on regular follow-up without symptoms related to endometriosis until now.
DISCUSSION
Vault endometriosis has been documented in the literature since 1954, but a proper management protocol is not described in any study.2,3,15–18 Ideally, all cases of hysterectomy with peroperative findings suggestive of endometriosis should undergo complete excision of disease at the of primary surgery to reduce chances of recurrence. Also, any surgical intervention in a post-hysterectomized patient with vault endometriosis is itself a challenging procedure owing to distorted anatomy and the likelihood of dense adhesion peroperatively. Laparoscopy is considered a gold standard technique for diagnosis, to assess the severity and as a concluding surgical management tool.
All patients in our study had symptoms as irregular vaginal spotting, chronic pelvic pain, dyspareunia, dyschezia, as also found in other study by Riazi et al. in 2015.7 Medical management was not efficacious in our patients as elicited by the clinical history, which corresponded with the findings of other studies in the literature.19 Moreover, prolonged and unguided medical management may lead to tissue fibrosis which further makes the surgery technically demanding. Some of the review articles have documented the role of imaging in the pre-operative diagnosis, but no definite features suggestive of vault endometriosis has been specified.11,12
The average duration between primary surgery and the need for repeat surgery for vault endometriosis in our cases was almost comparable with the duration for the same mentioned in other studies.16 There were neither significant intra-operative nor postoperative complications related to the procedure. None of the patient required blood transfusion intra- or postoperatively. All cases in our study had histopathologically proven endometriotic components in the excised specimen (vault and pelvic peritoneum). Long term follow-up (minimum - 2 years to maximum - 5 years) of the patients was also uneventful.
Patients revealed complete resolution of their symptoms as chronic pelvic pain, dyspareunia, and dyschezia after definitive surgical management. These conclusions are consonant with other’s research works.16,20,21
This is a type of introductory study which exclusively enumerates explicit surgical steps for meticulous management of perplexing cases of vault endometriosis. A proper diagnosis and evaluation have also been elaborated, which can help the clinician suspect the case of vault endometriosis so that apt and timely management can be designed. Some noteworthy attributes of our study were the proposition for a detailed surgical approach with anatomical landmarks for the excision of a recto-vaginal nodule and IHN nerve entrapment release. This step is especially relevant in cases of fertility preserving surgeries for endometriosis with chief complaints of infertility, chronic pelvic pain, or dyspareunia. Proper anatomical knowledge of the pelvis regarding pelvic avascular spaces, IHN nerve, and ureter course in the pelvis are a prerequisite for this arduous, skillful surgery. The same output can be achieved with open laparotomy, but laparoscopy has the benefits of minimally invasive surgery. A few recent studies have tried to provide an anatomical description, but their replicability is practically challenging.22 We have tried to overcome these flaws in our study with detailed anatomical descriptions and explanatory surgical steps. In our study, we aimed to describe 10 surgical maneuvers to tackle these perplexing surgeries, safeguarding all surrounding vital structures like bladder, rectum, bilateral ureter, and IHN. Further studies with a large sample size will add detailed knowledge about this surgical technique.
CONCLUSION
Vault endometriosis is extremely rare owing to a lack of sufficient knowledge and paucity of adequate data. Detailed pre-operative assessment and a high degree of suspicion are required for diagnosis. Owing to its infiltrating pathological behavior and high recurrence rate, complete surgical excision with disease-free margins remains the treatment of choice. Our descriptive step wise surgical technique may be useful to achieve this outcome.
Footnotes
Disclosure: none.
Funding sources: none.
Conflict of interests: none.
Informed consent: Dr. Dipak Limbachiya declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
IRB/Ethics Committee approved this study.
Contributor Information
Dipak Limbachiya, Department of Gynecological Endoscopy, Eva Women's Hospital, Shahibaug, India..
Rajnish Tiwari, Department of Gynecological Endoscopy, Eva Women's Hospital, Shahibaug, India..
Rashmi Kumari, Department of Gynecological Endoscopy, Eva Women's Hospital, Shahibaug, India..
References:
- 1.Foti PV, Farina R, Palmucci S, et al. Endometriosis: clinical features, MR imaging findings and pathologic correlation. Insights Imaging. 2018;9(2):149–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Zhu J. Vaginal cuff endometriosis after laparoscopic-assisted vaginal hysterectomy: a case report and literature review. Int J Clin Exp Med. 2018;11(6):6336–6339. [Google Scholar]
- 3.Choi CH, Kim JJ, Kim WY, Min KW, Kim DH. A rare case of post-hysterectomy vault site iatrogenic endometriosis. Obstet Gynecol Sci. 2015;58(4):319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saavalainen L, Heikinheimo O, Tiitinen A, Härkki P. Deep infiltrating endometriosis affecting the urinary tract—surgical treatment and fertility outcomes in 2004–2013. Gynecol Surg. 2016;13(4):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ota K, Sato K, Tanaka M. Ureteral stenosis due to DIE (deep infiltrating endometriosis) with difficulty in treatment: case report and brief literature review. Gynecol Minim Invasive Ther. 2017;6(4):214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong KM, Chuang J, Tsai YL, Hwang JL, Chu CC. Vaginal cuff endometriosis resulting in a fistula between the vagina and abdominal cavity and presenting as peritonitis: a case report. J Reprod Med. 2007;52:439–440. [PubMed] [Google Scholar]
- 7.Riazi H, Tehranian N, Ziaei S, Mohammadi E, Hajizadeh E, Montazeri A. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Womens Health. 2015;15(39):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nnoaham KE, Hummelshoj L, Kennedy SH, Jenkinson C, Zondervan KT. Developing symptom-based predictive models of endometriosis as a clinical screening tool: results from a multicenter study. Fertil Steril. 2012;98(3):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92(6):1825–1833. [DOI] [PubMed] [Google Scholar]
- 10.Hudelist G, Ballard K, English J, et al. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2011;37(4):480–487. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, He T, Shen W. Comparison of physical examination, ultrasound techniques and magnetic resonance imaging for the diagnosis of deep infiltrating endometriosis: a systematic review and meta-analysis of diagnostic accuracy studies. Exp Ther Med. 2020;20(4):3208–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger J, Henneman O, Rhemrev J, Smeets M, Jansen FW. MRI-ultrasound fusion imaging for diagnosis of deep infiltrating endometriosis – a critical appraisal. Ultrasound Int Open. 2018;4(3):E85–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy JMN, Arambage K, Correa FJS. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;3(4):CD011031. [DOI] [PubMed] [Google Scholar]
- 14.Possover M, Andersson K, Forman A. Neuropelveology: an emerging discipline for the management of chronic pelvic pain. Int Neurourol J. 2017;21(4):243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver R, Coker A, Khoo D. Laparoscopic approach to an endometriotic vault fistula causing posthysterectomy “menstruation”. J Minim Invasive Gynecol. 2006;13(2):164–165. [DOI] [PubMed] [Google Scholar]
- 16.Trehan A, Sanaullah F. Laparoscopic posthysterectomy vaginal vault excision for chronic pelvic pain and deep dyspareunia. J Minim Invasive Gynecol. 2009;16(3):326–332. [DOI] [PubMed] [Google Scholar]
- 17.Aydin Y, Atis A, Ercan E, Donmez M. An endometriotic vault fistula presenting with monthly bleeding after hysterectomy. Arch Gynecol Obstet. 2009;280(6):1011–1014. [DOI] [PubMed] [Google Scholar]
- 18.Sidiropoulou Z, Setubal A, Acosta C, Roberto E. Post-hysterectomy vaginal haemorrhage: a case report. Cases J. 2009;2:7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wild M, Miskry T, Rose G, Crofton M, Al-Kufaishi A. Medical management of deeply infiltrating endometriosis – 7years experience in a tertiary endometriosis center in London. Gynecol Surg. 2019;16(1):12. [Google Scholar]
- 20.Lamvu G, Robinson B, Zolnoun D, Steege JF. Vaginal apex resection: a treatment option for vaginal apex pain. Obstet Gynecol. 2004;104(6):1340–1346. [DOI] [PubMed] [Google Scholar]
- 21.Sharp HT, Dodson MK, Langer KM, Doucette RC, Norton PA. The role of vaginal apex excision in the management of persistent posthysterectomy dyspareunia. Am J Obstet Gynecol. 2000;183(6):1385–1388. [DOI] [PubMed] [Google Scholar]
- 22.Keckstein J, Becker CM, Canis M, et al. Recommendations for the surgical treatment of endometriosis. Part 2: deep endometriosis. Hum Reprod Open. 2020(1):hoaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]


