Abstract
Objective.
The 2 pneumoproteins, KL-6 and CCL-18, are promising biomarkers in systemic sclerosis (SSc)-related interstitial lung disease (ILD). Our goal was to determine their predictive significance for forced vital capacity % (FVC%) decline within the first year of followup in patients with early SSc-ILD.
Methods.
Early SSc patients with imaging-verified ILD enrolled in the Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohort were included. Annualized rate of change in FVC% based on the baseline and followup measurement within 12–18 months was used as the surrogate outcomes for ILD progression.
Results.
Eighty-two early SSc-ILD patients with mean disease duration of 2.3 years were investigated. FVC% change ranged from −23% to 38%. Baseline KL-6 levels were higher in patients than healthy controls (p < 0.0001). Higher KL-6 levels were predictive of faster FVC% decline at the 1-year followup (r = −0.23, p = 0.037). Upon categorizing KL-6 using a previously published cutoff of 1273 U/ml, its predictive significance remained in the univariable model (b = −0.07, p = 0.01), indicating that patients with positive KL-6 had on average 7% more decline in annualized percent change of FVC%. Moreover, KL-6 remained an independent predictor after adjustment for sex, disease type, anti-Scl-70, and immunosuppressive treatment status in multivariable models. Although CCL-18 was higher in patients than controls (p < 0.001), its levels did not predict FVC decline rate (p = 0.458).
Conclusion.
KL-6 but not CCL-18 is predictive of early SSc-ILD progression. KL-6 is a promising pneumoprotein that can contribute to SSc-ILD clinical trial enrichment.
Keywords: INTERSTITIAL LUNG DISEASE, BIOMARKERS, SYSTEMIC SCLEROSIS
Interstitial lung disease (ILD) is a common organ manifestation of systemic sclerosis (SSc) and is the leading cause of disease-related mortality1. However, not all patients with SSc-ILD will experience progressive disease or die from this complication, and the treatment has side effects. Hence, biomarker enrichment and better understanding of the risk factors for disease progression in SSc-ILD are necessary to guide clinical management and trials.
KL-6 and CCL-18 are pneumoproteins linked to lung parenchymal injury and have been studied in various types of ILD, including SSc, as lung-specific biomarkers.
KL-6 is a glycoprotein secreted by type II pneumocytes (TIIP), which proliferate following lung injury. Therefore, it has been extensively studied as a biomarker of lung injury. A study following 50 patients with SSc and ILD over time observed that having a high KL-6 level was predictive of endstage lung disease (ESLD), defined as % forced vital capacity (FVC%) < 50%, requiring oxygen, or death as a result of ILD. They also found that a cutoff of 1273 U/ml had the ability to predict which patients would progress to ESLD2.
CCL-18, also known as pulmonary activation-regulated chemokine (PARC), is produced by alternatively activated alveolar macrophages (M2 type) and has been linked for this reason to lung injury. It has been studied in SSc-ILD by multiple groups and found to be predictive of worse prognosis. Further, lung microarray data showed that CCL-18 transcript levels are increased in SSc compared to controls3. Another study by Hoffmann-Vold, et al observed higher rate of FVC% decline and lower survival in patients with high CCL-18 levels4.
Although both of these cytokines have been observed to predict SSc-ILD progression, their predictive significance for short-term lung volume changes in early disease have not been studied. The objective of our study was to determine the predictive significance of KL-6 and CCL-18 for progression of early SSc-ILD to inform individualized care in routine clinical practice and to aid enrichment strategies in clinical trials.
MATERIALS AND METHODS
Study population.
All patients enrolled in the Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohort5 who had the following characteristics were included: (1) ILD verified by imaging; and (2) pulmonary function tests (PFT) at enrollment and a second set at 12–18 months. All patients in this prospective, multicenter observational cohort fulfilled the American College of Rheumatology/European League Against Rheumatism classification criteria for SSc6 and had disease duration ≤ 5 years (from the first non-Raynaud phenomenon symptom) at enrollment. Immunosuppressive therapy was defined as treatment with any immunosuppressive agents at the baseline visit, except for hydroxychloroquine or prednisone ≤ 5 mg daily. Forty age-, sex-, and ethnicity-matched healthy controls with no personal or family history of autoimmune disorders were also investigated. Approval by the Committee for the Protection of Human Subjects at the McGovern Medical School–University of Texas Medical School at Houston was obtained prior to initiation of the study (approval number HSC-MS-02-161).
KL-6 and CCL-18 measurement.
Plasma was obtained from patients and healthy controls upon study enrollment. CCL-18 was measured by a commercially available, validated ELISA kit (MIP-4/CCL-18 kit, Cell Sciences). KL-6 was measured by detection of agglutinates with mouse KL-6 monoclonal antibody–coated latex using an automated analyzer (Nanopia KL-6, Eidia)7. All measurements were performed in duplicate and coefficient of variation values were < 0.2.
Clinical manifestations.
Baseline demographic and clinical variables were recorded upon enrollment using a standard abstract form5. All PFT data were reviewed by a pulmonologist (REYM) and data that did not fulfill the American Thoracic Society/European Respiratory Society criteria were excluded. Predicted FVC% values were calculated according to the patient’s age, height, weight, sex, and ethnicity using consistent reference values8.
For this particular study, patients were included only if they had imaging changes consistent with SSc-ILD including honeycombing, increased interstitial markings, or ground glass opacity on high-resolution chest computed tomography (HRCT) or typical increased bibasilar markings on chest radiograph. Twenty-one patients fulfilled criteria based on chest radiograph abnormalities alone; 71 patients had an abnormal HRCT.
Analysis.
FVC expressed as percentage of predicted value was used as a surrogate of ILD progression. Additionally, DLCO% corrected for the current hemoglobin was investigated. Annualized percent change in FVC% and DLCO% at followup were calculated using the following formula: [(PFT1–PFT0)/PFT0)/(timePFT1–timePFT0)]. The followup FVC% and DLCO% at 1 year were used because this is the typical study duration of SSc-ILD trials. The annualized FVC% and DLCO% changes were considered the outcome, or dependent variables and the log-transformed or dichotomized levels of the 2 investigated pneumoproteins were incorporated into univariate and multivariable (after adjustment for baseline demographic and clinical variables) linear models as independent variables. All variables listed in Table 1 were examined for their univariable association with the annualized percent change in FVC%. Only variables that achieved p < 0.2 in the univariate analyses were included in the multivariable analysis. Analysis was carried out using the SAS 9.4 (SAS Institute Inc.) statistical package.
Table 1.
Baseline patient characteristics. Values are mean ± SD or n (%) unless otherwise specified.
| Characteristics | All, n = 82 | KL-6-positive, n = 29 | KL-6-negative, n = 53 | p |
|---|---|---|---|---|
|
| ||||
| Age at disease onset, yrs | 53.6 ± 11.0 | 53.6 ± 10.1 | 53.5 ± 11.5 | 0.88 |
| Disease duration, yrs | 2.4 ± 1.5 | 2.3 ± 1.4 | 2.4 ± 1.5 | 0.93 |
| Male sex | 18 (22) | 8 (28) | 10 (19) | 0.36 |
| African American | 18 (22) | 9 (31) | 9 (17) | 0.14 |
| Disease type, diffuse | 45 (55) | 17 (59) | 28 (53) | 0.61 |
| Immunosuppression | 37 (45) | 17 (59) | 20 (38) | 0.07 |
| FVC% at baseline | 75.2 ± 17.4 | 70.2 ± 14.4 | 77.9 ± 18.3 | 0.04 |
| mRSS | 16.7 ± 11.4 | 17 ± 10.5 | 16.5 ± 11.9 | 0.76 |
| Anti-Scl 70 | 17 (21) | 10 (35) | 7 (14) | 0.03 |
| Anti-RNA polymerase III | 20 (24.7) | 5 (17) | 15 (29) | 0.29 |
| Anticentromere | 5 (6.2) | 0 (0) | 5 (10) | 0.15 |
KL-6 positivity determined based on a level of 1273 U/ml. FVC%: % forced vital capacity; mRSS: Modified Rodnan skin score.
RESULTS
Eighty-two patients with SSc-ILD were included, 18 of whom were male, 18 African American, and 45 had diffuse cutaneous involvement. Mean disease duration was 2.4 years (Table 1). Table 2 shows baseline immunosuppressive regimen in the investigated patients. Rate of FVC%-predicted change over time ranged from −23% to 38%, indicating a highly variable course.
Table 2.
Immunosuppressive agents at the baseline visit. Values are n (%).
| Medication | n = 82 |
|---|---|
|
| |
| No immunosuppression | 45 (54.9) |
| Prednisone > 5 mg | 20 (24.4) |
| Methotrexate | 10 (12.2) |
| Mycophenolate mofetil | 9 (11) |
| Cyclophosphamide | 3 (3.7) |
| Azathioprine | 2 (2.4) |
| Other | 4 (4.9) |
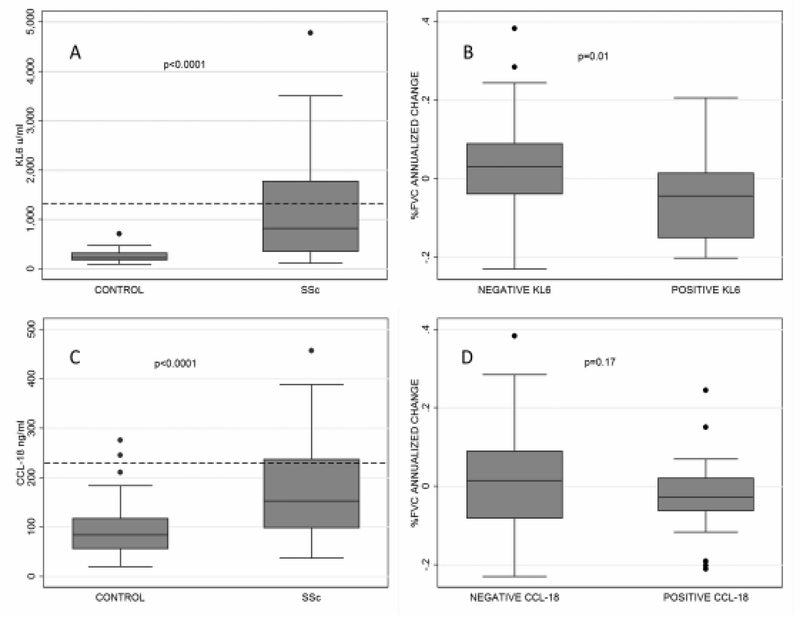
Baseline KL-6 levels were higher in patients than in healthy controls (p < 0.0001; Figure 1A), ranging from 116 to 4777 U/ml with a median of 772.9 in patients with SSc-ILD and from 97.9 to 712 U/ml with a median of 226.5 in controls. Similarly, CCL-18 was higher in patients than controls (p < 0.001, Figure 1C), ranging from 37.2 to 457.4 ng/ml with median of 152.6 in patients with SSc-ILD and from 20.2 to 275.8 ng/ml with median of 84.6 in controls. Neither baseline KL-6 nor baseline CCL-18 levels were associated with ethnicity (p = 0.446 and p = 0.457, respectively).
Figure 1.
A. KL-6 levels (U/ml) in patients with SSc versus controls (dashed line = 1273 cutoff). B. FVC% annualized percent change in KL-6-positive versus -negative patients based on a cutoff of 1273 U/ml. C. CCL-18 levels (ng/ml) in patients with SSc versus controls. D. FVC% annualized percent change in CCL-18-positive versus -negative patients based on a cutoff of 140 ng/ml. Lower values in FVC% percent change correspond to a faster FVC decline. SSc: systemic sclerosis; FVC%: % forced vital capacity.
Higher baseline KL-6 levels (log-transformed, as a continuous variable) were predictive of a faster rate of FVC% decline at the 1-year followup in the univariable model (b = −0.03, p = 0.037); specifically, the Pearson correlation coefficient with annualized percent change in FVC% was −0.23. Upon categorizing KL-6 using a cutoff of 1273 U/ml based on the optimal cutoff predicting risk of SSc-ILD progression as determined in a previous Japanese study2, the positive KL-6 remained significant in the univariable model (b = −0.07, 95% CI = −0.13 to −0.02, p = 0.01; Figure 1B). In other words, patients with positive KL-6 experienced 7% more decline in their annualized percent change in FVC%. Next, the multivariable model was extended by inclusion of disease type, Scl-70 positivity, and sex; these variables were included based on p < 0.2 in their univariable associations with annualized percent change in FVC%. As shown in Table 3, the predictive significance of positive KL-6 remained in this multivariable model. Moreover, positive KL-6 remained significantly associated with annualized percent change in FVC% when immunosuppressive treatment at the baseline visit was added to the multivariable model (b = −0.07, 95% CI −0.12 to −0.01, p = 0.022). Although KL-6 levels were predictive of FVC% decline, KL-6 levels as continuous or dichotomized variable did not predict DLCO% changes (p = 0.7 and p = 0.862, respectively). Twenty-nine (34.5%) patients had KL-6 levels ≥ 1273 U/ml. None of the controls had a level > 1273 U/ml.
Table 3.
Predictive significance of positive KL-6 for annualized percent change in FVC%.
| Variables | Estimate | 95% CI | p |
|---|---|---|---|
|
| |||
| Positive KL-6* | −0.07 | −0.12 to −0.01 | 0.016 |
| Sex, male | 0.06 | −0.01 to 0.12 | 0.053 |
| Disease type, limited | −0.03 | −0.08 to 0.02 | 0.261 |
| Scl-70 | −0.04 | −0.1 to 0.03 | 0.278 |
KL-6 was dichotomized based on the previously published value of 1273 U/ml. FVC%: % forced vital capacity.
Although CCL-18 was higher in patients than controls (Figure 1C), CCL-18 levels (log-transformed, as continuous variable) did not correlate with annualized percent change in FVC% (b = −0.41, r = −0.08, p = 0.458). Similarly, CCL-18 did not correlate with the annualized percent change in DLCO% (b = 0.12, r = 0.04, p = 0.756). In a followup analysis, the CCL-18 levels were dichotomized based on the value of 228 ng/ml, which corresponded to 95% value in unaffected controls. Twenty-four patients (29.3%) had positive CCL-18 levels. Table 4 shows the baseline demographic and clinical differences based on the CCL-18 status. Positive CCL-18 levels were not associated with annualized percent change in FVC% (b = −0.04, 95% CI −0.1 to 0.02, p = 0.17; Figure 1D).
Table 4.
Demographic and clinical characteristics of patients dichotomized based on CCL-18 status. Values are mean ± SD or n (%) unless otherwise specified.
| Characteristics | CCL-18-positive, n = 24 | CCL-18-negative, n = 58 | p |
|---|---|---|---|
|
| |||
| Age at disease onset, yrs | 55.5 ± 10.3 | 52.8 ± 11.3 | 0.25 |
| Disease duration, yrs | 2.3 ± 1.7 | 2.4 ± 1.4 | 0.55 |
| Male sex | 6 (25) | 12 (21) | 0.67 |
| African American | 6 (25) | 12 (21) | 0.67 |
| Disease type, diffuse | 13 (54) | 32 (55) | 0.93 |
| Immunosuppression | 7 (29) | 30 (52) | 0.06 |
| FVC% at baseline | 73.4 ± 15.2 | 76 ± 18.3 | 0.56 |
| mRSS | 17.7 ± 12.2 | 16.3 ± 11.1 | 0.67 |
| Anti-Scl 70 | 7 (30) | 10 (17) | 0.19 |
| Anti-RNA polymerase III | 4 (17) | 16 (28) | 0.40 |
| Anticentromere | 1 (4) | 4 (7) | 1.00 |
FVC%: % forced vital capacity; mRSS: modified Rodnan skin score.
DISCUSSION
In the well-characterized, prospective GENISOS cohort, plasma KL-6 levels but not CCL-18 were predictive of early SSc-ILD progression. We were also able to validate the proposed cutoff of 1273 U/ml for KL-6 as predictive for SSc-ILD progression.
KL6 and CCL-18 are pneumoproteins that have been previously studied in pulmonary disorders because they are primarily produced by TIIP and alternatively activated alveolar macrophages, respectively.
KL-6 is a glycoprotein secreted by TIIP that proliferates following lung injury. It is chemotactic and antiapoptotic for fibroblasts, potentially contributing to the fibrotic process in ILD9. KL-6 was capable of discriminating between ILD and other common lung diseases. A cutoff of 500 U/ml was established to distinguish patients with ILD from healthy controls and those with other lung disorders10. Using this cutoff, 70–100% of patients with ILD had abnormal KL-6 levels, as opposed to only 10% of patients with pneumonia, asthma, or chronic obstructive pulmonary disorder and 28% of patients with active pulmonary tuberculosis9. Further, KL-6 levels in patients with connective tissue disorders (CTD) without ILD were not statistically different from controls11. KL-6 levels > 1000 were associated with increased mortality in patients with idiopathic pulmonary fibrosis (IPF)12,13. Higher serum KL-6 but not CCL-18 levels were also significantly associated with higher risk of developing acute exacerbation in a prospective study of IPF patients. Specifically, a cutoff value of 1300 for KL-6 had a sensitivity and specificity of 92% and 61% for identifying those with acute exacerbation, respectively14. Moreover, among patients with IPF experiencing an acute exacerbation, high KL-6 levels predicted 3-month mortality, and positive KL-6 was included in a composite staging system combining clinical variables and HRCT findings that can be used for identifying patients at mortality risk15. Serial measurements of KL-6 have been also studied in IPF; patients with both high baseline KL-6 as well as serial increases in its serum levels experienced the steepest FVC decline16. In SSc, few studies favor the utility of KL-6 as a biomarker for SSc-ILD (Table 5)17,18,19. In the Scleroderma Lung Study I (SLSI) cohort, KL-6 levels were higher in patients with evidence of alveolitis (defined by the following SLS criteria: BAL analysis with > 3 neutrophils, > 2 eosinophils or both, or by ground glass opacities per thoracic HRCT) than SSc-ILD patients without alveolitis18. In a Japanese cohort, the frequency of ILD in SSc patients with high KL-6 was higher than those with normal levels (78% vs 14%, p < 0.001)17.
Table 5.
Studies published to date on KL-6 and CCL-18 in systemic sclerosis-related interstitial lung disease.
| Author | Population | Outcomes Studied | Results |
|---|---|---|---|
|
| |||
| KL-6 | |||
| Sato, et al17 | 45 Japanese SSc, 15 HC | Cross-sectional correlation with fibrosis scores and PFT | Inverse correlation between KL-6 and DLCO% and VC |
| Hant, et al18 | 66 SSc,10 HC | Concomitant alveolitis based on BAL or HRCT findings | KL-6 was associated with concomitant alveolitis |
| Hesselstrand, et al19 | 15 early SSc,12 HC | BAL measures, HRCT and PFT findings | KL-6 correlated with BAL eosinophil concentration. |
| Kuwana, et al2 | 50 Japanese early (< 3 yrs) SSc with ILD | ESLD (FVC < 50%, oxygen requirement | KL-6 predictive of ESLD development or death) |
| CCL-18 | |||
| Kodera, et al24 | 123 SSc (86 with ILD), 37 HC | SSc to HC comparison, fibrosis score per imaging (HRCT), DLCO, VC | CCL-18 associated with decreased DLCO, VC, and higher fibrosis scores |
| Prasse, et al22 | 12 SSc-ILD, 43 IIP, 23 HC | CCL-18 levels in IIP, SSc and HC, correlation with PFT | Higher in SSc serum and BAL and inversely correlated with concomitant TLC |
| Tiev, et al20 | 83 SSc (46 with ILD) | 10% decrease of baseline TLC and/or FVC% or death | CCL-18 was predictive of ILD event |
| Elhaj, et al21 | 266 early SSc (with or without ILD) | Decline in FVC% | CCL18 predicted short-term but not longterm decline in FVC |
| Schupp, et al23 | 96 SSc | > 10% FVC drop or death | Higher risk of death or lung function worsening within 2 yrs |
| Hoffmann-Vold, et al4 | 298 SSc (with or without ILD) | Rates of FVC decline and cumulative survival | Higher rate of decline in FVC and lower overall survival in patients with high CCL-18 |
SSc: systemic sclerosis; HC: healthy controls; ILD: interstitial lung disease; BAL: bronchoalveolar lavage; HRCT: high-resolution computed tomography; PFT: pulmonary function tests; VC: vital capacity; ESLD: endstage lung disease; IIP: idiopathic interstitial pneumonia; TLC: total lung capacity; FVC%:% forced vital capacity.
In a previously published Japanese study, 50 patients with early SSc-ILD not treated with immunosuppressive agents were examined2. The primary outcome was ESLD defined as SSc-ILD resulting in at least 1 of the following: an FVC < 50%, continuous oxygen supplementation, or death as a result of ILD. In this observational cohort, 32% (16 patients) developed ESLD and higher KL-6 levels were associated with ESLD. Receiver-operating characteristic (ROC) curve revealed that a threshold of 1273 U/ml for KL-6 had sensitivity of 87.5% and specificity of 100% for identifying patients who developed ESLD during followup. An annualized rate of decline in FVC%, which is frequently the primary outcome in clinical trials, was not investigated.
In our present study, we did not calculate ROC for KL-6 because a widely accepted cutoff for dichotomizing the outcome variable (i.e., FVC% annualized rate of change) does not exist in SSc-ILD. However, our present study validates the proposed KL-6 cutoff of 1273 U/ml in the Japanese study for identifying SSc-ILD patients with progressive disease. Moreover, the predictive significance of KL-6 in our present study was independent of other potential risk factors such as disease type and Scl-70 positivity or factors that could blunt ILD progression such as immunosuppression, which could not be investigated in the Japanese study2. While SSc-ILD has generally a more indolent course than IPF and it is not typically associated with acute exacerbations similar to those observed in IPF, our findings in conjunction with the study by Kuwana, et al2 support the notion that high baseline KL-6 levels predict more active disease that is associated with subsequent deterioration of lung function and development of respiratory failure.
CCL-18 (or PARC) is produced by alternatively activated alveolar macrophages (M2 type) and promotes production of collagen by lung fibroblasts17. Six previous studies have explored CCL-18 as a biomarker for SSc-ILD (Table 5)4,20,21,22,23,24. All observed significance of CCL-18 for different measures of SSc-ILD.
A study performed in 83 patients with SSc, 46 of whom had ILD, observed that CCL-18 was predictive of an “ILD event” (defined as 10% decrease of baseline total lung capacity and/or FVC% or death)20. Another study with 298 SSc patients with and without ILD (mean baseline HRCT fibrosis score was 6.6%) observed higher rate of decline in FVC% and lower overall survival in patients with high CCL-184. Our group previously performed a study with a similar number of patients with SSc (n = 266) that included patients with or without ILD, and CCL-18 was observed to predict short-term but not longterm decline in FVC%21.
Although CCL-18 levels were higher in patients than unaffected controls in our present study, we did not observe a relationship between CCL-18 levels and rate of decline in FVC%. The observed difference between our negative finding and the previously published studies (including the study performed within our own cohort) likely stems from the type of investigated patients. The previous studies included SSc patients with and without ILD while our present study exclusively investigated patients with imaging-confirmed ILD. This indicates that CCL-18 is better at discriminating SSc patients with and without ILD than predicting the rate of decline in FVC% within the SSc-ILD group.
Our present study has several strengths. It was conducted in a well-characterized cohort of early SSc patients with confirmed ILD. Further, GENISOS is a multiethnic cohort with important implications for the generalizability of study results. However, our study also has some weaknesses. The GENISOS study sites are tertiary referral centers, which create a bias toward more severe cases. Further, as is common in observational studies, the treatment regimens were not uniform in our present study, but the ability of KL-6 to discriminate patients with progressive ILD was independent of immunosuppression.
KL-6 but not CCL-18 has predictive value for disease progression in early SSc-ILD. This study also validates the cutoff of > 1273 U/ml proposed by a Japanese cohort as an independent prognostic factor for SSc-ILD progression. KL-6 is a promising pneumoprotein that can not only help detect presence or absence of ILD in patients with SSc14,15, but also has predictive significance for short-term decline in lung volume in SSc-ILD. Measuring KL-6 might inform individualized clinical care and contribute to enrichment strategies in clinical trials of SSc-ILD.
Acknowledgments
Supported by grants from the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) K23 AR061436 (Assassi), NIH/NIAMS P50-AR05414, and the Department of Defense Congressionally Directed Medical Research Programs (W81XWH-07-01-0111-Mayes and W81XWH-16-1-0296-Assassi).
Contributor Information
Gloria A. Salazar, The University of Texas Health Science Center at Houston, McGovern Medical School
Masataka Kuwana, Department of Allergy and Rheumatology, Nippon Medical School.
Minghua Wu, The University of Texas Health Science Center at Houston, McGovern Medical School.
Rosa M. Estrada-Y-Martin, The University of Texas Health Science Center at Houston, McGovern Medical School
Jun Ying, The University of Texas Health Science Center at Houston, McGovern Medical School.
Julio Charles, The University of Texas Health Science Center at Houston, McGovern Medical School.
Maureen D. Mayes, The University of Texas Health Science Center at Houston, McGovern Medical School
Shervin Assassi, The University of Texas Health Science Center at Houston, McGovern Medical School.
REFERENCES
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007;66:940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuwana M, Shirai Y, Takeuchi T. Elevated serum Krebs von den Lungen-6 in early disease predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. J Rheumatol 2016;43:1825–31. [DOI] [PubMed] [Google Scholar]
- 3.Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, et al. Association of interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol 2014;66:714–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann-Vold AM, Tennoe AH, Garen T, Midtvedt O, Abraityte A, Aalokken TM, et al. High level of chemokine CCL 18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest 2016; 150:299–306. [DOI] [PubMed] [Google Scholar]
- 5.Assassi S, Del Junco D, Sutter K, McNearney TA, Reveille JD, Karnavas A, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum 2009;61:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013; 72:1747–55. [DOI] [PubMed] [Google Scholar]
- 7.Sekisui Diagnostics. Nanopia KL-6 Reagent. [Internet. Accessed May 17, 2018.] Available from: www.sekisuidiagnostics.com/writable/product_documents/files/kl-6reagenten_02.pdf
- 8.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3–13. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi J, Itoh Y, Kitamura S, Kawai T. [Establishment of reference intervals and cut-off value by an enzyme immunoassay for KL-6 antigen, a new marker for interstitial pneumonia.] [Article in Japanese] Rinsho Byori 1996;44:653–8. [PubMed] [Google Scholar]
- 11.Oguz EO, Kucuksahin O, Turgay M, Yildizgoren MT, Ates A, Demir N, et al. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: a cross-sectional study. Clin Rheumatol 2016;35:663–6. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006;11:164–8. [DOI] [PubMed] [Google Scholar]
- 13.Satoh H, Kurishima K, Ishikawa H, Ohtsuka M. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med 2006;260:429–34. [DOI] [PubMed] [Google Scholar]
- 14.Ohshimo S, Ishikawa N, Horimasu Y, Hattori N, Hirohashi N, Tanigawa K, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 2014;108:1031–9. [DOI] [PubMed] [Google Scholar]
- 15.Kishaba T, Tamaki H, Shimaoka Y, Fukuyama H, Yamashiro S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014;192:141–9. [DOI] [PubMed] [Google Scholar]
- 16.Wakamatsu K, Nagata N, Kumazoe H, Oda K, Ishimoto H, Yoshimi M, et al. Prognostic value of serial serum KL-6 measurements in patients with idiopathic pulmonary fibrosis. Respir Investig 2017;55:16–23. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Nagaoka T, Hasegawa M, Nishijima C, Takehara K. Elevated serum KL-6 levels in patients with systemic sclerosis: association with the severity of pulmonary fibrosis. Dermatology 2000;200:196–201. [DOI] [PubMed] [Google Scholar]
- 18.Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, et al. ; Scleroderma Lung Study Research Group. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol 2009;36:773–80. [DOI] [PubMed] [Google Scholar]
- 19.Hesselstrand R, Wildt M, Bozovic G, Andersson-Sjoland A, Andreasson K, Scheja A, et al. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease relate to severity of lung fibrosis. Respir Med 2013;107:1079–86. [DOI] [PubMed] [Google Scholar]
- 20.Tiev KP, Hua-Huy T, Kettaneh A, Gain M, Duong-Quy S, Toledano C, et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J 2011;38:1355–60. [DOI] [PubMed] [Google Scholar]
- 21.Elhaj M, Charles J, Pedroza C, Liu X, Zhou X, Estrada YMR, et al. Can serum surfactant protein D or CC-chemokine ligand 18 predict outcome of interstitial lung disease in patients with early systemic sclerosis? J Rheumatol 2013;40:1114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasse A, Pechkovsky DV, Toews GB, Schafer M, Eggeling S, Ludwig C, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum 2007;56:1685–93. [DOI] [PubMed] [Google Scholar]
- 23.Schupp J, Becker M, Gunther J, Muller-Quernheim J, Riemekasten G, Prasse A. Serum CCL-18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur Respir J 2014;43:1530–2. [DOI] [PubMed] [Google Scholar]
- 24.Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum 2005;52:2889–96. [DOI] [PubMed] [Google Scholar]