Abstract
As governments consider policy action to reduce smoking, a key factor in creating political will is the level of public support, particularly among smokers who are most affected by the policies. The goal of this paper is to assess and compare the level of support in Canada, the United States, England, and Australia for five smoking control policies: 1) banning menthol in cigarettes, 2) banning cigarette additives, 3) reducing nicotine in cigarettes to make them less addictive, 4) raising the minimum age to purchase cigarettes to 21 years and older, and 5) requiring pictorial warning labels on cigarette packs (examined in the US only). Data for these analyses come from 8,165 daily cigarette smokers who responded to the 2016 International Tobacco Control Four Country Smoking and Vaping Survey. In all countries, the highest level of support was for raising the legal age for purchase to 21 years and older (62–70%) and reducing the nicotine content of cigarettes to make them less addictive (57–70%). Smokers who were less dependent on cigarettes and those expressing interest in quitting were more likely to support all policies. When asked how they would respond to a nicotine reduction policy, the most common response given was to try the non-nicotine cigarettes to see how they liked them (42–48%), with the next most common response being to quit smoking entirely (16–24%). The high level of support for these proposed policies among daily smokers provides important evidence for policymakers to counteract claims that such policies would be unpopular.
Introduction
Approximately one billion people will die from smoking in this century unless comprehensive actions are taken to curb the consumption of conventional cigarettes1,2. In 2003, the World Health Assembly adopted the World Health Organization Framework Convention on Tobacco Control (WHO FCTC), the first-ever WHO treaty that obligates 182 Parties (as of July 2020) to implement a comprehensive set of tobacco control policies3. An analysis of 126 countries found a strong association between the number of FCTC policies implemented, and the corresponding reduction in smoking prevalence in the first decade of the treaty (2005 to 2015)4. Canada, England, and Australia have all ratified the FCTC, while the US has not. However, in 2009 the US Congress gave the US FDA authority to regulate tobacco products as appropriate for the protection of public health5.
In some high-income countries that have already implemented most of the basic FCTC policies (smoke-free air laws, high taxes, pictorial warnings ), additional measures are now being considered to further reduce smoking prevalence and attributable burden of tobacco-related death and disease. First, Article 9 of the FCTC calls for the regulation of product constituents within tobacco, and tobacco control advocates have long argued that menthol specifically should be banned from cigarettes6. In 2011, the Tobacco Product Scientific Advisory Committee to the United States (US) Food and Drug Administration (FDA) argued that removing menthol cigarettes from the market would benefit public health7. So far, the FDA has not acted on the recommendation of its advisory committee to ban menthol in cigarettes, although some cities (e.g., San Francisco) and states (e.g., Massachusetts) within the US have enacted menthol bans. In 2017, Canada implemented a nationwide menthol ban, although the overall prevalence of menthol cigarette use in Canada is relatively low (approximately 5% compared to 30% in the US7–10). A few studies have found that menthol bans in Canada increased 11,12. The European Union (which included England at the time) implemented a menthol cigarette ban in May 202013. Menthol cigarettes constitute a relatively small share of the Australian market so have not been a major focus of regulatory action.
Second, rather than focusing on a single additive such as menthol, some advocates have recommended a broader ban of all cigarette additives (such as preservatives, moisturizers, and flavors)14,15. Like a menthol ban, an additive ban would fall under Article 9 of the FCTC. Additives used in cigarette manufacturing serve a variety of functions such as reducing the harshness of smoke, optimizing nicotine delivery, enhancing appeal, and modifying flavor, and thus would be expected to impact smoking through multiple mechanisms. Though Brazil was the first country to adopt a ban on additives, Canada was the first country to implement such regulation, with Brazil still working on implementation16. European Union legislation13 prohibited characterizing flavors other than menthol, and prohibited vitamins, colorings and some specified additives in tobacco products in May 2016.
Third, nicotine in cigarettes is the main reason smokers find it hard to stop smoking and persist in smoking to the point where they get sick and die prematurely17,18. In 2017, the US FDA announced that it was considering adopting a regulation that would reduce the nicotine content of cigarettes to very low levels to render them minimally- or non-addictive19,20. A nicotine ban may be considered in countries outside the US under Article 9 of the FCTC. Clinical trials suggest that the proposed nicotine standard to reduce the addictiveness of cigarettes would markedly decrease smoking prevalence21–25. Despite this evidence from clinical trials, as of 2021, no country has yet adopted or implemented this regulation, so we have no data on the impact of such a policy on population-level smoking behavior26.
Fourth, most adult smokers’ report initiating smoking prior to being 21 years old, and evidence shows that smoking earlier in life leads to a greater likelihood of becoming strongly addicted to cigarettes and developing a smoking-related disease later in life18. Given the well-established relationship between age and smoking, public health groups have advocated for raising the minimum age to purchase tobacco products to 21 years and older27,28. Age restrictions would fall under Article 16 of the FCTC (sales to and by minors). In 2019, the US Congress passed legislation increasing the minimum age to purchase tobacco products to 21 years and older. In Canada, the national minimum age remains 18, but some provinces have raised the minimum age to 19. Other jurisdictions have or are considering similar measures. The legal age required to purchase tobacco in England29 and Australia30 is 18. In Australia, the state of Tasmania is currently considering raising the minimum age to purchase tobacco to 2131,32.
Fifth, strong evidence suggests that pictorial health warnings promote cigarette cessation by encouraging smokers to quit33,34. Pictorial health warnings fall under Article 11 of the FCTC (packaging and labeling of tobacco products). Over 100 countries have implemented pictorial warnings on cigarette packs, including Canada, England, and Australia35. In June 2020, the US FDA announced a final rule for pictorial health warning content on cigarette packs, to go into effect in 2021 – this was their second proposed rule after their first attempt in 2011 was successfully challenged in court by cigarette companies. US cigarette companies have again challenged this revised final rule, which may delay or block them from appearing on packs sold in the US beyond 202136,37.
While these and other policies have received support from public health groups, a better understanding of the level of public support for these policies is needed, especially among smokers, the group most affected by these policies. Understanding the level of support is critical because public opinion on potential policies can influence policy change38–40. Further, lobbying efforts designed to block tobacco control policies often claim that such policies will be unpopular. Strong evidence to the contrary may effectively increase political will. Studies on public support for different smoking control measures not surprisingly show that smokers are generally less supportive compared to nonsmokers41. However, several studies have found that the majority of the public, including smokers, favor regulations that would increase the minimum age to purchase tobacco to 21 years of age and older42–46, require pictorial health warnings47, and reduce nicotine levels in cigarettes12,48–55. However, support for a menthol ban or an additive ban is lower, especially among smokers45,52,53,55. While there are many studies that queried the public about different smoking control measures, few assessed support for multiple policies at the same time, making comparisons of the support level across policies difficult. Further, we know relatively little about how levels of support for these policies differ across countries.
The goal of the present paper was to assess support for different smoking control policies among current daily cigarette smokers in four countries: Canada, the US, England, and Australia. We have previously reported the level of support for these policies in Canada45, and the level of support for a policy increasing the minimum age to purchase tobacco to 2146. Here, we report the level of support for a group of policies across countries and include comparisons across countries. Five policies are included in this study: 1) banning menthol in cigarettes, 2) banning cigarette additives, 3) establishing a minimum level of nicotine allowed in cigarettes to make them less addictive if other nicotine products were available, 4) raising the minimum age to purchase cigarettes to 21 years and older, and 5) requiring pictorial warning labels on cigarette packs (examined in the US only). After reporting the level of support for each policy, we examined correlates of support for key baseline demographic and tobacco use variables. For one policy, reducing the nicotine level in cigarettes, we also report on smokers’ behavioral intentions in response to the implementation of the policy.
Methods
Study participants
Data were collected as part of the 2016 (Wave 1) International Tobacco Control (ITC) Four Country Smoking and Vaping Survey (4CV1). The 4CV1 Survey is an ongoing panel-based survey including respondents 18 years or older and who currently smoke, recently quit smoking (quit in previous 2 years), and/or currently vape. Details of the design of the 4CV1 survey can be found elsewhere56. The survey protocols and all materials including the survey questionnaires, were cleared for ethics by Institutional Review Board, Medical University of South Carolina; Research Ethics Office, King’s College London, UK; Office of Research Ethics, University of Waterloo, Canada; and Human Research Ethics, Cancer council Victoria Australia. All participants provided consent to participate. For this paper we restricted our analyses to participants who reported smoking cigarettes daily (N=8165) and compared support for different policies between Canada (n=2220), the US (n=1850), England (n=2880), and Australia (n=1215). Data from smokers in Canada have been reported previously, and we include them here for comparisons across countries45.
Tobacco Control Policy Measures
This study examined support for four tobacco control policies asked of smokers in all four countries, and support for pictorial warnings in US respondents only. The four policies compared across respondents in the four countries include: 1) ban on menthol in cigarettes; 2) ban on all cigarette additives, 3) reducing nicotine in cigarettes if other nicotine products were available, and 4) raising the minimum legal age for purchase of cigarettes to age 21 years and older. Since the US was the only country without pictorial health warnings on cigarette packs out of the four countries surveyed, questions about health warnings on cigarette packs were restricted to US respondents only. Table 1 provides the wording for survey measures of policy support. Response options for all survey questions ranged from “strongly support” to “strongly oppose” with a “don’t know” option also provided. For the purposes of analyses, we collapsed responses to examine percentage of respondents who indicated any support for the policy.
Table 1.
Questions assessing support for tobacco control policies
| Policy | This next set of items is about possible laws that could be used to control tobacco products and tobacco companies. |
| Menthol Ban | Would you support or oppose a law that bans the use of menthol in cigarettes/ tobacco? |
| Additive Ban | Would you support or oppose a law that bans all additives, including flavorings, in cigarettes/ tobacco? |
| Legal Age 21+ | Would you support or oppose a law that raises the legal age of purchasing cigarettes/ tobacco to 21 years and older? |
| Nicotine Standard | If you could get nicotine in products other than tobacco, would you support or oppose a law that reduced the amount of nicotine in cigarettes and tobacco, to make them less addictive? |
| Pictorial Warning | Would you support or oppose a law that places pictorial health warnings on the front of all cigarette/ tobacco packs? |
We also examined how the following characteristics of participants influenced support for a given policy: country, gender, age, heaviness of smoking index (HSI), vaping status, and intention to quit smoking. HSI is calculated from the number of cigarettes smoked per day (1–10=0, 11–20=1, 31–30=2, 31+=3), and the time to first cigarettes after waking (<5 min=3, 6–30 min=2, 31–60 min=1, 61+=0). For the policies banning menthol and additives, we also looked at how support varied by the use of flavored cigarettes, including menthol. For the policy reducing nicotine content, we also looked at how support varied by the perception of nicotine as harmful.
Participants were also asked: “Which of the following describes what you would be likely to do if nicotine in cigarettes was reduced to non-addictive levels?: Quit smoking entirely / Try the non-nicotine cigarettes to see how I liked them / Switch to an electronic cigarette or vaping device that has nicotine in it / Switch to a nicotine medication like the gum or patch / Find a way to get cigarettes with nicotine / Don’t know.” Respondents could select only one response.
Data Analysis
For each policy, a dichotomous variable depicting support was created by grouping together the options “strongly support” and “support” as “support” and the options “strongly oppose”, “oppose”, and “don’t know” as “not support”. Data were weighted to be representative of smoker population in each country in terms of sex, age, education, and geography. Weighted generalized logistic regression for survey data was performed accounting for missing data being missing not at random. For all policies, support for each policy was the dependent variable; country was the independent variable. Countries were compared to each other by varying the referent group. For all policies, we adjusted for gender, age, HSI, vaping status, and intention to quit. For menthol ban and additive ban policies, we also adjusted for usual brand flavor. For a nicotine reduction policy, we also adjusted for perceptions about the harmfulness of nicotine. Vaping status was categorized as either former (someone who vaped at least weekly in the past, but not at all now), current (someone who vapes now daily, weekly, or occasionally), or never (someone who has never heard of e-cigarettes, someone who has never tried e-cigarettes, someone who has vaped only once, or someone who vaped more than once but never weekly and not at all now). Intention to quit was categorized as either within the next 6 months (combines “Within the next month” and “Between 1–6 months from now”) or > 6 months from now (combines “Sometime in the future, beyond 6 months” and “Not planning to quit”). Usual brand flavor was categorized as either just tobacco (“Just Tobacco”) or menthol or another flavor (combines “Tobacco and menthol” and “Tobacco and some other flavor”). Perceptions about the harmfulness of nicotine was assessed by asking “How harmful do you think nicotine is –or was, or would be—to your health?” Response options were categorized as not very harmful (combines “Not at all harmful,” “Slightly harmful,” and “Moderately harmful”) or more harmful (combines “Very harmful” and “Extremely harmful”).
Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were weighted (Table 3 and Supplementary Table 2). Adjusted prevalences for level of support were obtained from the same logistic regression models reported in Table 3 by least square means method (Figure 1). In supplementary material, we report unweighted frequencies for all response options along with weighted percentages. We also report a sensitivity analysis which compared Support vs. Oppose (excluding Don’t Know, reported in Supplementary Table 2). All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC), and additional information on how weights are calculated can be found in Thompson et al. (2019)56.
Table 3.
Adjusted Odds Ratios and Confidence Intervals for Correlates of Policy Support1
| Menthol Ban | Additive Ban | Nicotine Standard | Legal Age 21+ | Pictorial Warning | ||
|---|---|---|---|---|---|---|
| Variable | Comparison | aOR 95% CI | aOR 95% CI | aOR 95% CI | aOR 95% CI | aOR 95% CI |
| Country | US vs Canada | 0.83 (0.66, 1.04) | 1.00 (0.82, 1.22) | 0.57 (0.47, 0.69) | 0.72 (0.59, 0.87) | NA |
| England vs Canada | 0.49 (0.41, 0.60) | 0.69 (0.59, 0.81) | 0.70 (0.60, 0.83) | 0.82 (0.70, 0.96) | NA | |
| Australia vs. Canada | 0.45 (0.34, 0.60) | 0.76 (0.60, 0.95) | 0.62 (0.49, 0.78) | 0.80 (0.64, 1.00) | NA | |
| England vs. US | 0.60 (0.47, 0.76) | 0.69 (0.57, 0.84) | 1.24 (1.02, 1.49) | 1.14 (0.94, 1.38) | NA | |
| Australia vs. US | 0.54 (0.39, 0.74) | 0.76 (0.59, 0.98) | 1.09 (0.85, 1.40) | 1.12 (0.88, 1.43) | NA | |
| Australia vs. England | 0.91 (0.67, 1.22) | 1.10 (0.87, 1.38) | 0.88 (0.70, 1.11) | 0.98 (0.79, 1.23) | NA | |
| Gender | Female vs Male | 0.64 (0.54, 0.75) | 0.92 (0.80, 1.06) | 1.25 (1.10, 1.44) | 1.12 (0.98, 1.28) | 0.80 (0.64, 0.99) |
| Age | 25–39 vs 18–24 | 1.06 (0.81, 1.39) | 0.99 (0.76, 1.30) | 1.22 (0.94, 1.57) | 1.86 (1.46, 2.37) | 0.80 (0.57, 1.14) |
| 40–54 vs 18–24 | 0.87 (0.67, 1.13) | 1.13 (0.87, 1.45) | 1.24 (0.97, 1.59) | 2.06 (1.64, 2.59) | 0.70 (0.51, 0.97) | |
| 55+ vs 18–24 | 0.76 (0.58, 0.99) | 1.22 (0.95, 1.57) | 1.54 (1.22, 1.96) | 2.03 (1.62, 2.54) | 0.67 (0.49, 0.61) | |
| HSI | 3+ vs 0–2 | 0.70 (0.60, 0.83) | 0.79 (0.68, 0.90) | 0.79 (0.69, 0.91) | 0.93 (0.81, 1.07) | 0.62 (0.44, 0.86) |
| Vaping Status | Former vs Current | 0.71 (0.53, 0.95) | 0.86 (0.69, 1.09) | 1.03 (0.83, 1.29) | 1.03 (0.82, 1.29) | 0.92 (0.57, 1.47) |
| Never vs Current | 0.85 (0.71, 1.01) | 1.09 (0.93, 1.27) | 0.91 (0.78, 1.06) | 0.91 (0.78, 1.06) | 1.25 (0.86, 1.82) | |
| Intention to Quit | > 6 months vs. ≤ 6 months | 0.59 (0.50, 0.70) | 0.66 (0.57, 0.76) | 0.59 (0.51, 0.68) | 0.59 (0.51, 0.68) | 0.39 (0.28, 0.55) |
| Flavor | Tobacco only vs. Menthol or Another Flavor | 1.65 (1.30, 2.08) | 1.85 (1.53, 2.25) | |||
| Perceptions about Harmfulness of Nicotine | More Harmful vs Not Very Harmful | 1.83 (1.59, 2.09) |
Comparisons are for Support vs. Oppose/Don’t know (combined). Sensitivity Analyses compared Support vs. Oppose, and found a similar pattern (Supplementary Table 2).
NA: not applicable since questions about pictorial HWLs were only asked of US respondents
Figure 1.
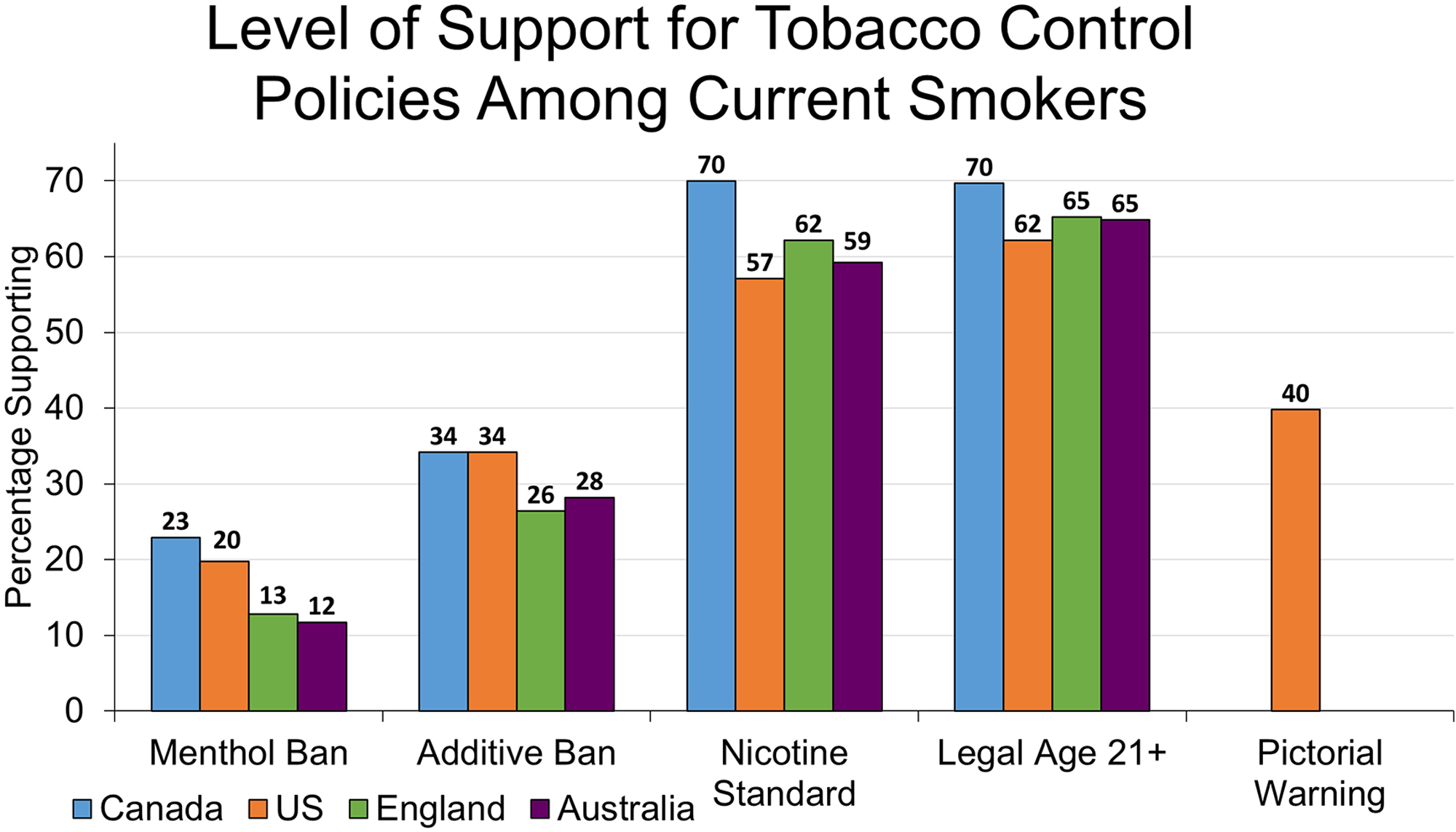
The adjusted prevalence of daily smokers in each country who “Support” or “Strongly Support” each policy. See Table 1 for questionnaire items, and Supplementary Table 1 for percentages endorsing each response option. Respondents who refused are excluded from graph and data analysis, but are included in Supplementary Table 1.
Results
Table 2 reports demographic and other smoking characteristics for this sample by country.
Table 2.
Demographics and Smoking Characteristics by Country
| Variable | Group | Canada (CA) n=2220 | United States (US) n=1850 | England (EN) n=2880 | Australia (AU) n=1215 | Total N=8165 |
|---|---|---|---|---|---|---|
| Gender | Female | 41.7% | 47.2% | 45.3% | 44.0% | 44.5% |
| Male | 58.3% | 52.8% | 54.7% | 56.0% | 55.5% | |
| Age | 18–24 | 9.9% | 9.4% | 14% | 13.1% | 11.6% |
| 25–39 | 25.4% | 26.4% | 30.6% | 33.9% | 28.7% | |
| 40–54 | 33.2% | 30.9% | 28.4% | 28.3% | 30.3% | |
| 55+ | 31.6% | 33.2% | 24.7% | 24.7% | 29.4% | |
| Race | White (CA, US, EN) or English only (AU) | 85.1% | 80.1% | 91.9% | 89.8% | 86.9% |
| Non-white (CA, US, EN) or non-English (AU) | 13.3% | 19.4% | 6.1% | 10.1% | 11.8% | |
| Refused/Don’t Know | 1.6% | 0.5% | 2.0% | 0.1% | 1.2% | |
| Income 1 | Low | 21.5% | 40.8% | 23.0% | 19.8% | 26.2% |
| Moderate | 30.8% | 31.2% | 31.0% | 27.5% | 30.4% | |
| High | 40.5% | 27.0% | 36.9% | 47.2% | 37.2% | |
| No Answer | 7.2% | 1.1% | 9.1% | 5.5% | 6.1% | |
| Education 2 | Low | 31.7% | 56.2% | 21.3% | 42.1% | 35.6% |
| Moderate | 44.9% | 32.3% | 63.0% | 36.6% | 46.8% | |
| High | 22.6% | 11.4% | 12.0%) | 20.5% | 16.1% | |
| No Answer | 0.8% | 0.0% | 3.7% | 0.8% | 1.6% | |
| HSI | 0–2 | 45.1% | 41.4% | 47.1% | 39.8% | 44.1% |
| 3+ | 50.6% | 51.5% | 47.7% | 55.1% | 50.6% | |
| Missing | 4.2% | 7.1% | 5.1% | 5.1% | 5.4% | |
| Vaping Status | Current | 41.0% | 27.2% | 40.8% | 18.3% | 34.2% |
| Former | 10.5% | 18.2% | 13.1% | 13.3% | 13.6% | |
| Never | 48.5% | 54.6% | 46.0% | 68.4% | 52.2% | |
| Intention To Quit | ≤ 6 months | 39.1% | 28.7% | 34.3% | 39.1% | 35.0% |
| > 6 months | 50.9% | 57.1% | 55.0% | 47.8% | 53.2% | |
| Refused/Don’t Know | 10.1% | 14.2% | 10.8%) | 13.2% | 11.7% | |
| Menthol Status | Tobacco + Flavor | 81.8% | 63.7% | 80.5% | 77.3% | 76.4% |
| Just Tobacco | 9.4% | 29.3% | 12.3% | 14.3% | 15.8% | |
| Missing | 8.8% | 7.0% | 7.2% | 8.4% | 7.8% | |
| Perceptions about Harmfulness of Nicotine | Not Very Harmful | 57.8% | 52.8% | 47.9% | 53.1% | 52.6% |
| More Harmful | 40.2% | 44.0% | 48.1% | 43.5% | 44.2% | |
| Missing | 2.1% | 3.2% | 4.0% | 3.4% | 3.2% |
Table Note: Percentages are weighted.
Canada: Low=<45,000 CAD, Moderate=45,000–75,000 CAD, High=75,000+ CAD; US: Low=<30,000 USD, Moderate=30,000–59,999 USD, High=60,000+ USD; England: Low=<30,000 GBP, Moderate=30,000–45,999 GBP, High=45,000+ GBP; Australia: Low=<45,000 AUD, Moderate=45,000–75,000 AUD, High=75,000+ AUD
Low=high school or less, moderate=technical degree or some university, high=completed university
Predictors of support for different policies
Table 3 shows aORs and 95% CIs for variables we tested as predictors of support for each policy. For a menthol ban, support was greater among respondents from the US and Canada (compared to Australia and England), males, smokers aged 55 and older (compared to those 18–24), those with lower HSI, those using tobacco only flavored cigarettes (compared to menthol and other flavors), those smokers who also currently vaped (compared to former smokers), and those planning to quit within the next six months.
For an additive ban, support was greater among respondents from the US and Canada (compared to Australia and England), those with lower HSI, those using tobacco flavored cigarettes (compared to menthol and other flavors), and those planning to quit within the next six months (gender, age, and vaping status not significant predictors).
For a nicotine reduction policy, support was greater among respondents in Canada (compared to the other three countries) and England (compared to the US), females, adults over the age of 55 (compared to 18–24), those with lower HSI, those who believe nicotine is more harmful, and those planning to quit within the next six months (vaping status not a significant predictor).
For an increase in the minimum purchase age to 21, support was greater in Canada (compared to England and the US), older adults, and those planning to quit within the next six months (gender, HSI, and vaping status not significant predictors).
Among US respondents, support for pictorial health warnings on cigarettes was greater among males, younger adults (18–24 vs. 40+), those with lower HSI, and those planning to quit within the next six months (vaping status not a significant predictor).
Support for different policies by country
Figure 1 shows the adjusted prevalence of participants in each country who support each of the five policies. The majority of smokers in all four countries supported a policy to raise the legal age of purchase to age 21 years and older (62–70%), as well as a policy that would reduce the level of nicotine in cigarettes to render them less addictive (57–70%). Level of support was less than 50% for banning menthol (12–23%), banning cigarette additives (26–34%), and (in the US) pictorial health warnings (40%). Supplementary Table 1 provides the distribution to the full set of response options by respondents in each country.
Behavioral intentions in response to a very low nicotine cigarette policy
Figure 2 reports what smokers say they would do if a reduced nicotine policy were implemented. In all four countries, the most common response given by daily smokers was that they would try the non-nicotine cigarettes to see how they liked them or not. The next most frequently mentioned response was to stop smoking, followed by finding a way to get cigarettes with nicotine, and switching to another source of nicotine. A sizeable portion of people (between 13–20% in each country) said that they did not know how they might react.
Figure 2.
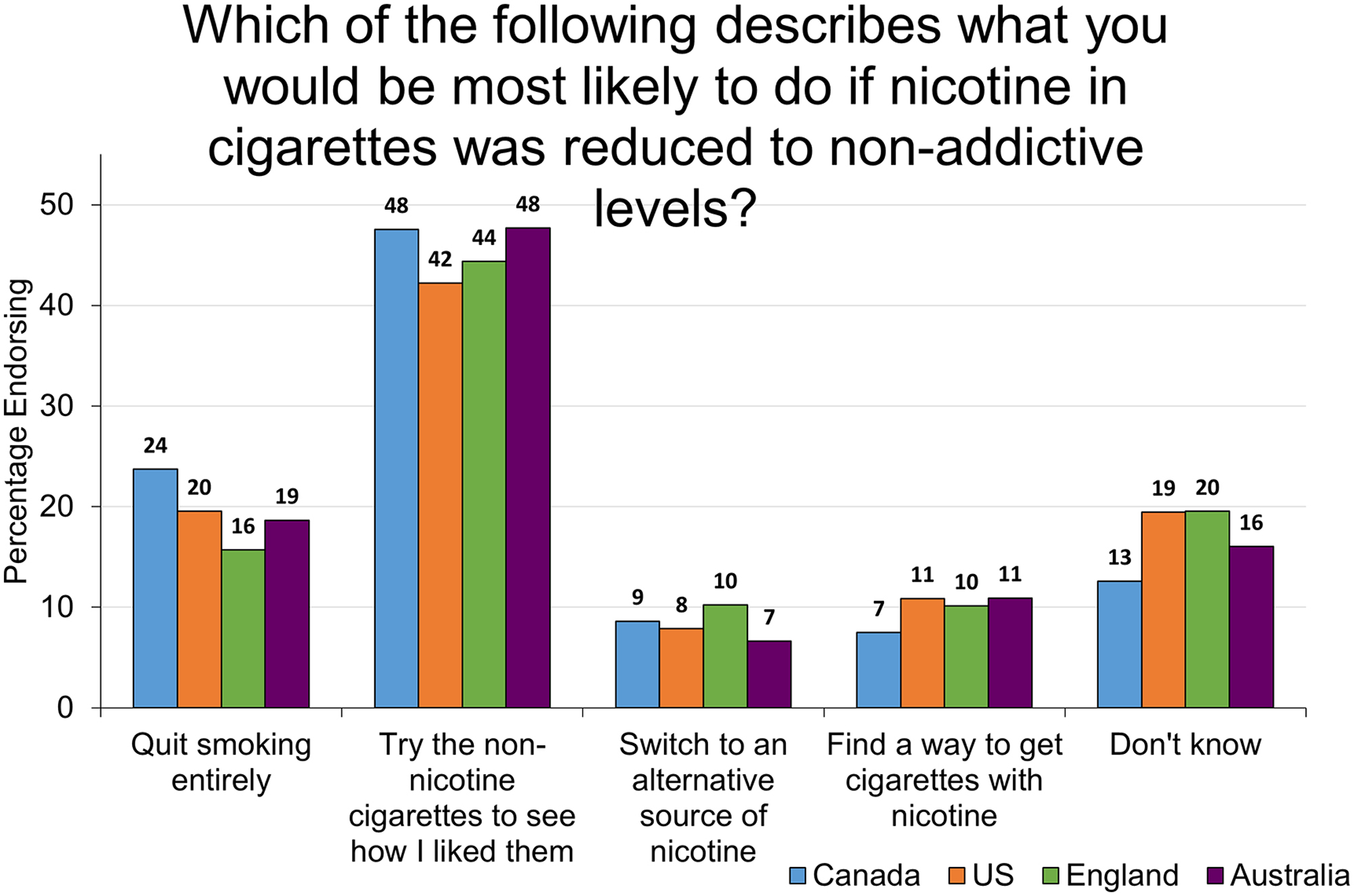
Weighted percentage of daily smokers who endorsed each response option. Two options: “Switch to an electronic cigarette or vaping device which has nicotine in it” and “Switch to a nicotine medication like the gym or patch” were combined to form the response option “Switch to an alternative source of nicotine.” Responses for each country do not always sum to 100 due to rounding.
Discussion
Most daily cigarette smokers in all four countries supported raising the minimum age of purchase for tobacco products to 21 and older (62–70%) and having government mandate a limit on the amount of nicotine allowed in cigarette tobacco to render them less addictive (57–70%). Our findings on the level of support for raising the legal age for purchasing tobacco to 21 years and older and reducing the amount of nicotine in cigarettes were consistent with results reported in previous studies42–44,48–55. In this study, smokers were less supportive of policies that would ban menthol or other additives in cigarettes; although support for banning all additives (26–34%) was stronger than for menthol banning alone (12–23%). This result might reflect publicity around government plans to ban menthol in cigarettes and could also reflect a general misunderstanding about the role of additives in addiction and harm57,58. This is also consistent with the finding that in some cases support is stronger for general policies than it is for specific sub-instances59. In the US, only 40% of smokers expressed support for pictorial warnings, which is lower than policy support reported previously (over 80%)47.
Correlates of support reported here are in line with correlates of support for tobacco control policies reported elsewhere42,49. Across the four policies that were assessed in the four countries, support was highest in Canada. Those who were less cigarette dependent (i.e., low HSI) and those interested in quitting within the next six months were more likely to support all policies. Smokers of flavored cigarettes including menthol were less likely to support a menthol and additive ban, which is not surprising as the policy would directly impact their choice of cigarette. Young adults (18–20 years) were significantly less likely to support a policy raising the minimum age to purchase cigarettes to 21 years of age and older, likely because the policy would directly impact their access to cigarettes. Those who perceived nicotine to be very harmful to their health were more likely to support a policy reducing nicotine in cigarettes, suggesting that if such a policy is implemented, it will be important to correct misperceptions about the role of nicotine in harm from tobacco so that reduced nicotine cigarettes will not be viewed as reduced harm products.
The present study has several limitations. First, some respondents might have not fully understood the implications of each policy, potentially limiting the validity of study measures. For example, a poor understanding of the role of additives and nicotine in cigarettes on health outcomes could contribute to whether someone supports a ban on additives or a reduction in nicotine content. Health education campaigns surrounding these policies might have also influenced the level of support, although our finding that support for a nicotine standard policy was associated with intention to quit suggests that at least smokers recognize their dependence on nicotine. Second, for this analysis we chose to focus on the level of support among smokers, and long-term quitters and nonsmokers would be expected to have higher levels of support for all policies. Third, some of these policies have been implemented since the survey was administered. In 2019, the US Congress passed legislation to increase the minimum age of purchase to 21 years and older60. In 2020, the US FDA recently announced a final ruling that will require cigarette packs to include pictorial warnings by the year 202137. In 2018, the FDA asked for comment on a proposed rule to reduce the level of nicotine in cigarettes19. Characterizing flavors (except menthol) were banned in England in May 2016 and nationwide bans on menthol cigarettes were implemented in Canada in 2017, and in England in 2020. Level of support for these policies may have changed following experience with the policy. Fourth, these findings may not generalize to low and middle income countries where progress in implementation of key tobacco control policies still lags behind high income countries.
The evidence base for most of these policies is strong and suggests that banning menthol, raising the minimum age for tobacco purchase, and using pictorial health warnings will reduce the prevalence of smoking and save lives7,14,15,27,28,33. Clinical trials assessing the impact of nicotine reduction although suggest that it will reduce the number of cigarettes smoked per day, increase quit attempts, and increase the ability to quit among smokers who are motivated to do so21,22,61–63. The high level of support for these proposed policies among daily smokers themselves provides important evidence for policymakers and advocates to counteract claims that such policies would be unpopular.
Supplementary Material
Highlights:
We assessed the level of support for tobacco policies among daily smokers in Canada, the US, England, and Australia.
Support was strongest for raising the minimum age to purchase tobacco to 21 and reducing nicotine in cigarettes.
Smokers in Canada were more likely than smokers in England and Australia to support most policies.
Smokers who were less dependent on cigarettes and those interested in quitting were more likely to support all policies.
The high level of support provides evidence for policymakers to counteract claims that such policies would be unpopular.
Funding:
The ITC Four Country Smoking and Vaping Survey was supported by grants from the US National Cancer Institute (P01 CA200512 and P30 CA138313), the Canadian Institutes of Health Research (FDN-148477), and the National Health and Medical Research Council of Australia (APP1106451). This research was partially supported by the National Cancer Institute of the National Institutes of Health and Food and Drug Administration Center for Tobacco Products (R01 CA239308). The funders had no role in the design, analysis, preparation, or decision to publish the manuscript.
Footnotes
Conflicts of Interest:
KMC has received payment as a consultant to Pfizer, Inc., for service on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. KMC also has served as a paid expert witness in litigation filed against cigarette manufacturers. GTF and JFT have served as expert witnesses on behalf of governments in litigation involving the tobacco industry. AM is a National Institute for Health Research (NIHR) senior investigator. The views expressed in this article are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care in England.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Department of Health and Human Services. The health consequences of smoking−-50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. [Google Scholar]
- 2.Work Health Organization. WHO report on the global tobacco epidemic 2017. Tobacco Free Initiative (TFI). 2017. [Google Scholar]
- 3.WHO Framework Convention on Tobacco Control. 2003.
- 4.Gravely S, Giovino GA, Craig L, et al. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. Lancet Public Health. 2017;2(4):e166–e174. [DOI] [PubMed] [Google Scholar]
- 5.US Congress. The Family Smoking Prevention and Tobacco Control Act. 2009.
- 6.Malone RE. It’s the 21st century: isn’t it past time to ban menthol cigarette sales? Tob Control. 2017;26(4):359–369. [DOI] [PubMed] [Google Scholar]
- 7.The Food and Drug Administration Tobacco Products Scientific Advisory Comitee. Menthol cigarettes and public health: Review of the scientific evidence and recommendations.. 2011.
- 8.Bird Y, May J, Nwankwo C, Mahmood R, Moraros J. Prevalence and characteristics of flavoured tobacco use among students in grades 10 through 12: a national cross-sectional study in Canada, 2012–2013. Tob Induc Dis. 2017;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nugent T, Tremblay G. Tobacco Sales in Canada 2014. In: Ottawa, Canada: Health Canada; 2016. [Google Scholar]
- 10.Minaker LM, Ahmed R, Hammond D, Manske S. Flavored tobacco use among Canadian students in grades 9 through 12: prevalence and patterns from the 2010–2011 youth smoking survey. Prev Chronic Dis. 2014;11:E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaiton MO, Nicolau I, Schwartz R, et al. Ban on menthol-flavoured tobacco products predicts cigarette cessation at 1 year: a population cohort study. Tob Control. 2020;29(3):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung-Hall J, Fong GT, Meng G, et al. Evaluating the impact of menthol cigarette bans on cessation and smoking behaviours in Canada: Findings from the 2016–18 ITC 4 Country Smoking and Vaping Surveys. Symposium presentation at the Society for Research on Nicotine and Tobacco Annual Meeting. 2020. [Google Scholar]
- 13.The European Parliament. Tobacco Products Directive. Official Journal of the European Union. 2014. [Google Scholar]
- 14.Work Health Organization (WHO) WHO. Fact sheet on ingredients in tobacco products. 2015.
- 15.Chapman S “Keep a low profile”: pesticide residue, additives, and freon use in Australian tobacco manufacturing. Tob Control. 2003;12 Suppl 3:iii45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Report of the Working Group on Tobacco Additives. 2014.
- 17.US Department of Health and Human Services, Surgeon General. General.The Health Consequences of Smoking: Nicotine Addiction. 1988.
- 18.The Surgeon General. The Health Consequences of Smoking−-50 Years of Progress: A Report of the Surgeon General. 2014.
- 19.Food and Drug Administration. (FDA) Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. A proposed rule by the Food and Drug Administration. [Google Scholar]
- 20.Gottlieb S, Zeller M. A Nicotine-Focused Framework for Public Health. N Engl J Med. 2017;377(12):1111–1114. [DOI] [PubMed] [Google Scholar]
- 21.Smith TT, Koopmeiners JS, Tessier KM, et al. Randomized Trial of Low-Nicotine Cigarettes and Transdermal Nicotine. Am J Prev Med. 2019;57(4):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TT, Hatsukami DK, Benowitz NL, et al. Whether to push or pull? Nicotine reduction and non-combusted alternatives - Two strategies for reducing smoking and improving public health. Prev Med. 2018;117:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith TT, Rupprecht LE, Denlinger-Apte RL, et al. Animal Research on Nicotine Reduction: Current Evidence and Research Gaps. Nicotine Tob Res. 2017;19(9):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatsukami DK, Luo X, Heskin AK, et al. Effects of immediate versus gradual nicotine reduction in cigarettes on biomarkers of biological effects. Addiction. 2019;114(10):1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong D U.S. Shelves Plan to Sharply Cut Nicotine in Cigarettes. Bloomberg. November 20, 2019, 2019. [Google Scholar]
- 27.Winickoff JP, Gottlieb M, Mello MM. Tobacco 21--an idea whose time has come. N Engl J Med. 2014;370(4):295–297. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds MJ, Crane R, Winickoff JP. The Emergence of the Tobacco 21 Movement From Needham, Massachusetts, to Throughout the United States (2003–2019). Am J Public Health. 2019;109(11):1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millett C, Lee JT, Gibbons DC, Glantz SA. Increasing the age for the legal purchase of tobacco in England: impacts on socioeconomic disparities in youth smoking. Thorax. 2011;66(10):862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Australia YL. Cigarettes.
- 31.Tobacco21.
- 32.Public Health Amendment (Prevention of Sale of Smoking Products to Underage Persons) Bill 2018.
- 33.Hammond D Health warning messages on tobacco products: a review. Tob Control. 2011;20(5):327–337. [DOI] [PubMed] [Google Scholar]
- 34.Yong HH, Borland R, Thrasher JF, et al. Mediational pathways of the impact of cigarette warning labels on quit attempts. Health Psychol. 2014;33(11):1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Canadian Cancer Society. Cigarette Package Health Warnings: International Status Report, Sixth Edition. September 2018. [Google Scholar]
- 36.R.J. Reynolds Tobacco Co. vs. Food and Drug Administration. In. Vol Case 6:20-cv-00176-JCB2020.
- 37.The US Food and Drug Administration (FDA) FDA requires new health warnings for cigarette packages and advertisements. FDA News; Release. 2020. [Google Scholar]
- 38.Petry F, Mendelsohn M. Public opinion and policy making in Canada 1994–2001. Can J Polit Sci. 2004;37:505–529. [Google Scholar]
- 39.Petry F The opinion-policy relationship in Canada. J Polit. 1999;61:540–550. [Google Scholar]
- 40.Burstein P The impact of public opinion on public policy: a review and an agenda. Polit Res Q. 2003;56:29–40. [Google Scholar]
- 41.Diepeveen S, Ling T, Suhrcke M, Roland M, Marteau TM. Public acceptability of government intervention to change health-related behaviours: a systematic review and narrative synthesis. BMC Public Health. 2013;13:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winickoff JP, McMillen R, Tanski S, Wilson K, Gottlieb M, Crane R. Public support for raising the age of sale for tobacco to 21 in the United States. Tob Control. 2016;25(3):284–288. [DOI] [PubMed] [Google Scholar]
- 43.Kuijpers TG, Willemsen MC, Kunst AE. Public support for tobacco control policies: The role of the protection of children against tobacco. Health Policy. 2018;122(8):929–935. [DOI] [PubMed] [Google Scholar]
- 44.Farley SM, Coady MH, Mandel-Ricci J, et al. Public opinions on tax and retail-based tobacco control strategies. Tob Control. 2015;24(e1):e10–13. [DOI] [PubMed] [Google Scholar]
- 45.Chung-Hall J, Fong GT, Driezen P, Craig L. Smokers’ support for tobacco endgame measures in Canada: findings from the 2016 International Tobacco Control Smoking and Vaping Survey. CMAJ Open. 2018;6(3):E412–E422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkins SS, Chung-Hall J, Craig L, et al. Support for minimum legal sales age laws set to age 21 across Australia, Canada, England and US: Findings from the 2018 ITC Four Country Smoking and Vaping Survey. Nicotine Tob Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall MG, Marteau TM, Sunstein CR, et al. Public support for pictorial warnings on cigarette packs: an experimental study of US smokers. J Behav Med. 2018;41(3):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fix BV, O’Connor RJ, Fong GT, Borland R, Cummings KM, Hyland A. Smokers’ reactions to FDA regulation of tobacco products: findings from the 2009 ITC United States survey. BMC Public Health. 2011;11:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denlinger-Apte RL, Tidey JW, Koopmeiners JS, et al. Correlates of support for a nicotine-reduction policy in smokers with 6-week exposure to very low nicotine cigarettes. Tob Control. 2019;28(3):352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connolly GN, Behm I, Healton CG, Alpert HR. Public attitudes regarding banning of cigarettes and regulation of nicotine. Am J Public Health. 2012;102(4):e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson JL, Abrams DB, Niaura RS, Richardson A, Vallone DM. Public support for mandated nicotine reduction in cigarettes. Am J Public Health. 2013;103(3):562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolcic-Jankovic D, Biener L. Public opinion about FDA regulation of menthol and nicotine. Tob Control. 2015;24(e4):e241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt AM, Kowitt SD, Myers AE, Goldstein AO. Attitudes towards Potential New Tobacco Control Regulations among U.S. Adults. Int J Environ Res Public Health. 2018;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fraser T, Kira A. Perspectives of key stakeholders and smokers on a very low nicotine content cigarette-only policy: qualitative study. N Z Med J. 2017;130(1456):36–45. [PubMed] [Google Scholar]
- 55.Pacek LR, Oliver JA, Sweitzer MM, McClernon FJ. Young adult dual combusted cigarette and e-cigarette users’ anticipated responses to a nicotine reduction policy and menthol ban in combusted cigarettes. Drug Alcohol Depend. 2019;194:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson ME, Fong GT, Boudreau C, et al. Methods of the ITC Four Country Smoking and Vaping Survey, wave 1 (2016). Addiction. 2019;114 Suppl 1:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byron MJ, Baig SA, Moracco KE, Brewer NT. Adolescents’ and adults’ perceptions of ‘natural’, ‘organic’ and ‘additive-free’ cigarettes, and the required disclaimers. Tob Control. 2016;25(5):517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDaniel PA, Malone RE. “I always thought they were all pure tobacco”: American smokers’ perceptions of “natural” cigarettes and tobacco industry advertising strategies. Tob Control. 2007;16(6):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore K, Borland R, Yong HH, et al. Support for tobacco control interventions: do country of origin and socioeconomic status make a difference? Int J Public Health. 2012;57(5):777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The US Food and Drug Administration. (FDA) Newly signed legislation raises federal minimum age of sale of tobacco products to 21. CTP Newsroom. 2019. [Google Scholar]
- 61.Hatsukami DK, Luo X, Jensen JA, et al. Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levy DT, Cummings KM, Heckman BW, et al. The Public Health Gains Had Cigarette Companies Chosen to Sell Very Low Nicotine Cigarettes. Nicotine Tob Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


