Abstract
Objective:
Race-related lifetime stress exposure including racial discrimination, trauma, and stressful life events have been shown to contribute to racial health disparities. However, little is known about associations between race-related stressors and premature biological aging that confer risk for adverse health outcomes. Even less is known about the mechanisms through which race-related stressors may be associated with accelerated aging. Early evidence suggests psychological processes such as anger, and particularly the internalization of anger, may play a role.
Methods:
In a community sample of predominantly low-income Black adults (n = 219; age 45.91 ± 12.33; 64% female), the present study examined the association of race-related lifetime stress exposure (LSE, as defined by exposure to racial discrimination, trauma, and stressful life events) and epigenetic age acceleration through anger expression.
Results:
Internalized and externalized anger expression were each significantly associated with LSE and age acceleration. Although LSE was not directly associated with age acceleration (ΔR2 = 0.001, p = 0.64), we found that greater LSE was indirectly associated with age acceleration through increases in internalized, but not externalized, anger (indirect effect: β = 0.03, SE = 0.02, 95% CI [0.003, 0.08]; total effect: β = 0.02, 95% CI [−0.25, 0.31]).
Conclusions:
These results suggest race-related LSE may elicit the internalization of anger, which, along with the externalization of anger, may initiate detrimental epigenetic alterations that confer risk for adverse health outcomes. These findings lay the groundwork for longitudinal studies of the association between race-related stress and racial health disparities.
Keywords: epigenetic age acceleration, anger, racial discrimination, stress, trauma, health disparities
INTRODUCTION
Within the United States, Black individuals disproportionately suffer from adverse health outcomes, develop chronic diseases at significantly earlier ages, and experience earlier mortality compared to White individuals (1,2). These health disparities were historically thought to be explained by disproportionate economic insecurity within the Black population, resulting in greater exposure to health adversities (e.g., reduced access to nutritional food, recreation activities, and quality healthcare) and health risk behaviors (e.g., substance use), and in turn earlier and higher rates of chronic disease (3). However, studies consistently demonstrate that Black individuals experience health disparities regardless of socioeconomic status (SES; 3–5), which suggests that additional race-related factors confer risk for adverse health outcomes. Given that genetic differences have demonstrated minimal bearing on the health disparities that exist between individuals of different racial and ethnic backgrounds (5-7), it is likely that experiences related to racial group membership confer risk for negative health outcomes. In the current study, we examine the possibility that lifetime cumulative stress exposure, including experiences of racial discrimination, contribute to premature biological aging processes among Black individuals.
Racial discrimination has been identified as a unique source of psychological stress that stems from chronic experiences of disrespect or inferior treatment due to one’s racial group membership (8,9). In addition to chronic experiences of racial discrimination, Black individuals are also disproportionately exposed to both traumatic and stressful life experiences (10,11). Compared to White individuals, Black individuals report higher rates of trauma exposure including assault, childhood maltreatment, and polytraumatization (12,13) that are often linked to living in neighborhoods of concentrated disadvantage. The lifetime trauma exposure rate for Black adults, particularly those in low-income urban settings, is close to 15% higher than that of White adults (14,15). Moreover, in addition to chronic stressors such as neighborhood disorder (16), Black individuals experience significantly higher rates of stressful life events such as witnessing violence, receiving bad news, and losing a loved one prematurely (17). Although these exposures can be partially attributed to socioeconomic disadvantages that disproportionately impact Black individuals (18,19), it is well established that these inequities stem from generations of racial discrimination and residential segregation (20). As such, it is difficult to separate the impact of trauma and life stress from the impacts of racism. To better understand how racism impacts the health of Black adults, a more comprehensive approach may be to view racial discrimination, trauma, and stressful life events as interrelated forms of a broader construct of race-related lifetime stress exposure (LSE).
Previous studies have established a link between race-related LSE and negative health outcomes (10,21,22). However, it remains unclear how experiences of race-related stress can exert negative influences on physical health. Existing evidence suggests that chronic experiences of racial discrimination, stress, and trauma may lead to overactivation of the physiological stress response (8,23) which, in turn, escalates the progression of aging and disease (24,25). In line with this evidence, the weathering hypothesis posits that Black individuals’ chronic exposure to racism may lead to a faster deterioration of physiological systems that are associated with age-related chronic diseases that disproportionately affect Black populations (26,27). This theory has been supported by several recent studies (28,29), including findings that, even after controlling for differences in SES, Black individuals exhibit greater physiological aging compared to White individuals (3) and that racial discrimination is significantly associated with physiological aging in Black young adults (30). In each of these studies, physiological aging was measured through a variety of stress-related biomarkers, including indicators of inflammation, blood pressure, and hypothalamic-pituitary-adrenal (HPA) axis activity (e.g., cortisol, norepinephrine), which were combined to reflect individuals’ allostatic load or accelerated aging.
Recent evidence suggests that epigenetic alterations may also play a role in the link between LSE and age-related disease. Epigenetic alterations include changes to DNA methylation (DNAm), which can regulate the expression of genes and, in turn, influence physiological functioning (31). Studies show that age-related changes to DNAm are reliable biomarkers of physiological aging (32) and that individuals with accelerated DNAm aging (i.e., epigenetic age acceleration, EAA) are at an increased risk for adverse health outcomes including cardiovascular disease and early mortality (33,34). Combined with evidence that chronic stress and other health risks are associated with EAA (35,36), researchers are increasingly turning to methylation-based measures of physiological aging to better understand the biological processes contributing to age-related diseases (37). To date, numerous measures of EAA have been developed through large-scale methylation studies (32,38,39). Of these measures, recent evidence suggests that AgeAccelGrim demonstrates the greatest utility for predicting physiological aging and risk for age-related disease (34). AgeAccelGrim is the age-adjusted version of the novel lifespan predictor DNAm GrimAge, which, compared to alternative measures of epigenetic age that are trained on chronological age, is trained on time-to-death and is thus more predictive of mortality and disease. However, given the novelty of this measure, few studies have examined how AgeAccelGrim relates to LSE or other psychosocial risk factors.
Importantly, although measures of EAA are valid across racial and ethnic groups (for instance, 40% of the individuals in the AgeAccelGrim validation study identified as African American (34)), the majority of studies investigating EAA have focused on predominantly White samples (40). In line with the weathering hypothesis, the few studies that have focused on Black samples found that youth exposed to greater trauma (41) and greater racial discrimination (42,43) demonstrate EAA, even after controlling for SES. Moreover, one study found that discrimination was indirectly associated with EAA through depressive symptoms, which suggests the emotional sequalae of race-related stress exposure may play a role in accelerated aging.
One emotional sequela that may be particularly relevant to understanding the mechanisms linking LSE and accelerated aging is anger, which has been identified as a normative emotional response to race-related stress (45) and a risk factor for age-related conditions that demonstrate racial disparities. For example, it is well established that individuals who experience more frequent and severe episodes of anger are at an elevated risk for cardiovascular disease (46-48), which disproportionately affects Black adults (1,2) and is linked to accelerated aging (34). In addition, decades of research suggest the expression of anger (i.e., internalized versus externalized) may differentially contribute to poor age-related health outcomes, although findings have been inconsistent (49). Researchers have theorized that the chronic internalization (i.e., suppression) of anger places cumulative stress on the body, which increases risk for negative health outcomes (50,51). In support of this theory, numerous studies have identified associations between internalized anger and poorer physiological health (e.g., 52,53). However, other studies have demonstrated stronger associations between externalized anger and adverse health outcomes (e.g., 54) and others have found that neither internalized nor externalized anger expression confers significant risk (e.g., 55). While numerous factors may contribute to these inconsistent findings, evidence suggests that racial factors may play an important role (50).
Anger is posited to have evolved as an adaptive emotional response to threat that, when externally expressed, can mobilize an individual to respond to a threatening situation (56). However, racially marginalized individuals often contend with barriers to directly addressing threats via outward expressions of anger. Studies indicate that stereotypes of Black individuals as more “angry” or “aggressive” than other demographic groups have led to a lower tolerance for Black expressions of anger (57,58). As a result of this societal intolerance, Black individuals are more likely to internalize – rather than externalize – anger expression to maintain their safety and livelihood (45,57,59,60). For example, greater levels of perceived racism have been associated with increases in anger inhibition (i.e., internalized anger expression) but not greater levels of expressed anger (i.e., externalized anger expression) in Black individuals (61). Considering evidence from multiple studies that, among Black Americans in particular, anger inhibition is associated with markers of physiological weathering and negative health outcomes (50,62,63), it is important to more thoroughly understand how differences in anger expression may contribute to racial health disparities.
Although associations between LSE, anger expression, and physiological health primarily stem from correlational studies, findings from a randomized experimental study suggest a causal role of internalized anger in the link between race-related stress exposure and negative health outcomes (64). Researchers compared cardiovascular recovery between Black and White men who were instructed to either internalize or externalize their anger in response to a racially-themed debate. Results indicated that delayed cardiovascular recovery (a predictor of poor cardiovascular health) was not only greater in Black men compared to White men, but was also greater in the internalized (versus externalized) anger group (64). These findings suggest that internalizing anger may play a unique role in the previously documented association between race-related stress exposure and physiological weathering (e.g., EAA). However, these relationships – in addition to those involving externalized anger expression – remain to be studied.
Despite evidence that race-related lifetime stressors can negatively impact both psychological and physiological functioning, there remains a dearth of studies examining these mechanisms in conjunction, particularly among Black individuals with significant exposure to trauma and stress. The present study aims to combine and extend evidence from the social and health sciences to examine the relationships between lifetime experiences of race-related stressors (i.e., racial discrimination, stressful life events, and trauma exposure) and physiological aging (i.e., EAA) as a first step to identifying how racism can impact health and disease. Moreover, given that Black individuals may be more likely to internalize anger, we will examine whether internalized anger expression may be particularly detrimental to epigenetic aging in the context of race-related LSE. We hypothesized that, within a sample of Black American individuals:
More frequent experiences of LSE will be associated with greater EAA, and
LSE will be indirectly associated with EAA through higher internalized, but not externalized, anger.
Method
Participants and Procedure
Individuals included in the current study were part of a larger cohort of the Grady Trauma Project, which investigates the influence of genetic and environmental factors on response to stressful life events in a predominantly Black, urban population of low socioeconomic status (65). Over the course of fifteen years (i.e., 2005-2020), interviews were conducted with participants (n = 10,331) in waiting rooms of primary care or obstetrical-gynecological clinics of a large, urban, public hospital in Atlanta, GA. Information was collected on participants’ demographics and life experiences (e.g., exposure to trauma and life stressors) among other clinical information relevant to the broader aims of the Grady Trauma Project (e.g., symptoms of PTSD, depression). From January 2006 to March 2010, a subset of these participants (n = 238) attended a lab visit, where they completed additional questionnaires (e.g., measures of racial discrimination and anger expression) and provided a blood sample for sequencing of DNA methylation and other biological markers. Non-Black participants (n = 19) were excluded from the present study, resulting in a final sample of 219 individuals (Table 1). All studies encompassed by the overarching research program have been approved by the Institutional Review Board of Emory University and all participants provided written informed consent.
Table 1.
Descriptive Statistics (n = 219)
| Demographic Characteristics | M ± SD | N (%) |
|---|---|---|
| Age | 45.91 ± 12.33 | |
| Sex | ||
| Male | 79 (36.1%) | |
| Female | 140 (63.9%) | |
| Monthly Income | ||
| $0 - $249 | 68 (31.1%) | |
| $250 - $499 | 22 (10.0%) | |
| $500 - $999 | 59 (26.9%) | |
| $1,000 - $1,999 | 50 (22.8%) | |
| $2,000 or more | 20 (9.1%) | |
| Predictors / Outcomes | M ± SD | |
| Lifetime Stress Exposure | 0.09 ± 0.77 | |
| Racial Discrimination | 6.43 ± 6.29 | |
| Life Stress | 10.86 ± 4.05 | |
| Trauma Exposure | 3.18 ± 2.27 | |
| Anger Expression | ||
| Anger Expression-In | 2.10 ± 0.64 | |
| Anger Expression-Out | 1.93 ± 0.56 | |
| Epigenetic Age Acceleration | 0.28 ± 4.97 |
Measures
Race-Related LSE
Racial Discrimination.
Personal experiences of racial discrimination were measured using the Experiences of Discrimination self-report instrument (EOD; 66), a 9-item questionnaire that asks participants to indicate whether they have (1 = Yes) or have not (0 = No) experienced discrimination in nine settings (e.g., at school, getting hired, from the police). It has demonstrated good internal consistency (Cronbach’s α > 0.70) and has been well validated in studies of working class and low-income Black adults (66). Consistent with epidemiological evidence that experiences of discrimination across a greater number of situations is associated with more adverse outcomes in Black samples (67), responses were summed to create a total Racial Discrimination score.
Stressful Life Events.
Lifetime experiences of objective life stressors were measured using the Stressful Events Questionnaire I (SEQ-I). The SEQ-I was developed for the Grady Trauma Project (68). Briefly, it is a 39-item self-report instrument that measures a wide range of stressors including personal life events (e.g., divorce, miscarriage), family life events (e.g., having a child or significant-other in prison), and network life events (e.g., knowing someone who was murdered). Participants report whether they have experienced each event at any time in their life. Life events were summed to create a total Stressful Life Events score that reflects the cumulative number of stressors experienced throughout the participants’ lifetime.
Trauma Exposure.
Lifetime history of traumatic experiences was measured using the Traumatic Events Inventory (TEI; 15), a 19-item screening instrument that assesses experiences of violence, physical and sexual abuse, life-threatening accidents or injuries, and other traumatic events. Participants were asked to indicate whether they had (Yes = 1) or had not (No = 0) experienced each trauma at any point in their lifetime. A total Trauma Exposure score was calculated by summing the scores from each question related to personal experiences of a traumatic event.
LSE Composite.
Due to the moderate-to-strong correlations between Racial Discrimination, Stressful Life Events, and Trauma Exposure (see Table 2), a composite LSE variable was created to reflect a single race-related lifetime stress factor. Scores for each variable were standardized and averaged to create a composite score in which higher scores indicated greater exposure to stressful experiences.
Table 2.
Bivariate Correlations (n = 219)
| Anger Expression- In |
Anger Expression- Out |
Racial Discrimination |
Life Stress | Trauma Exposure |
Lifetime Stress Exposure |
|
|---|---|---|---|---|---|---|
| Epigenetic Age Acceleration | 0.23** p = 0.001 |
0.13* p = 0.049 |
0.06 p = 0.39 |
−0.04 p = 0.55 |
0.06 p = 0.37 |
0.04 p = 0.59 |
| Anger Expression-In | 0.51*** p < 0.001 |
0.12* p = 0.068 |
0.16* p = 0.017 |
0.16* p = 0.022 |
0.19** p = 0.006 |
|
| Anger Expression-Out | 0.11 p = 0.098 |
0.11 p = 0.098 |
0.09 p = 0.17 |
0.13* p = 0.048 |
||
| Racial Discrimination | 0.30*** p < 0.001 |
0.34*** p < 0.001 |
0.70*** p < 0.001 |
|||
| Life Stress | 0.65*** p < 0.001 |
0.82*** p < 0.001 |
||||
| Trauma Exposure | 0.85*** p < 0.001 |
Anger Expression
Self-reported anger was measured using the State-Trait Anger Expression Inventory-2 (STAXI-2; 69), a 57-item questionnaire that assesses overall state and trait anger, in addition to anger expression and anger control. Item responses are scored along a 4-point Likert scale (1 = Almost never; 2 = Sometimes; 3 = Often; 4 = Almost always). Anger Expression-In was calculated as the sum of eight items including statements such as “I keep things in,” “I boil inside,” “I tend to harbor grudges,” and “I am irritated a great deal more than people are aware.” Anger Expression-Out was calculated as the sum of eight separate items including “I strike out at whatever infuriates me,” “I do things like slam doors,” and “I lose my temper.” The STAXI-2 has been well validated in non-clinical samples and has demonstrated good internal consistency (Cronbach’s α = 0.73 to 0.95) for each subscale (69).
Premature Biological Aging (i.e., Epigenetic Age Acceleration)
DNA was extracted from whole blood and sequenced according to manufacturer’s instructions using either the Illumina HumanMethylation450 or MethylationEPIC BeadChip, which capture >450,000 and >850,000 CpG sites across the genome, respectively. Out of 30,084 CpGs used to calculate GrimAge, the EPIC array captures 27,550 (92%) CpGs and the 450k array captures 28,484 (95%). To evaluate consistency across array types, samples from 121 subjects were sequenced using both 450k and EPIC arrays and compared. We observed that GrimAge values were highly correlated between arrays (r = 0.98). Raw methylation beta values were determined via GenomeStudio. Samples with probe detection call rates <90% and those with an average intensity value of either <50% of the experiment-wide sample mean or <2,000 arbitrary units (AU) were removed using R package CpGassoc (70). Probes with detection p-values >0.01 were set to missing. CpG sites that cross hybridize between autosomes and sex chromosomes were removed (71).
EAA was measured using AgeAccelGrim, the age-adjusted version of GrimAge. GrimAge is a lifespan predictor trained on methylation-based surrogates of tobacco exposure – given robust findings that tobacco smoking contributes to premature biological aging (34,73) – and 12 mortality-related plasma proteins. The surrogates are regressed on time-to-death such that GrimAge values represent an age estimate, with higher values representing a higher physiological age (34). AgeAccelGrim further adjusts for an individual’s chronological age, so higher values represent premature (i.e., accelerated) physiological aging.
To calculate GrimAge and AgeAccelGrim estimates, non-normalized methylation beta values were submitted to the new DNA methylation age calculator developed by Horvath (https://dnamage.genetics.ucla.edu/new). The calculator conducts a normalization step, applies the methylation data to the trained model (32,34), and returns estimates of GrimAge, AgeAccelGrim, and the proportions of CD8T, CD4T, natural killer (NK), B cells, monocytes (mono), and granulocytes using the methodology described by Houseman et al. (72). Estimated proportions of cell types, along with sex and array type, were included as covariates in all analyses with EAA. Given that AgeAccelGrim inherently accounts for chronological age, participant age was not included as a covariate for hypothesis testing. Similarly, given that AgeAccelGrim is trained on a methylation-based estimator of tobacco exposure, self-reported tobacco use was not included as a covariate for hypothesis testing.
Data Analysis
Analyses were completed in SPSS Statistics (Version 25). In line with prior evidence that SES may confound tests of race-related stress and EAA (3,34), all analyses adjusted for income. Hypothesis 1 was tested using hierarchical linear regressions, with covariates entered in the first step and the primary independent variable entered in the second step. Tests of significance were based on a two-sided p-value, with an alpha of .05. Testing for Hypothesis 2 was noncontingent on support for Hypothesis 1. This approach was consistent with evidence that a significant mediation can exist even in the absence of a significant direct association (74). Hypothesis 2 was tested using Model 4 within the SPSS PROCESS macro (Version 3.3; 75), which includes bootstrapping with 5,000 samples to ensure that results are not driven by outliers. Significance of direct and indirect associations was determined through 95% bootstrap confidence intervals, such that interval estimates that were entirely above or below (i.e., non-overlapping with) zero were deemed statistically significant (75).
Results
Preliminary Analyses
All primary variables satisfied the assumptions of normality as examined by visual inspection (i.e., histograms) and descriptive statistics (i.e., skewness and kurtosis). Zero-order correlations were then used to examine initial relationships between the primary variables of interest (Table 2).
Associations Between LSE, Anger Expression, and EAA
LSE was not significantly associated with EAA, neither before (Table 2) nor after (Table 3) accounting for SES and other relevant covariates. For completeness, we also examined how Anger Expression-In and Anger Expression-Out related to LSE and EAA after accounting for SES. LSE remained significantly associated with Anger Expression-In and Anger Expression-Out (Table 4), with small-to-medium effects for internalized anger (ΔR2 = 0.04, p < 0.001) and small effects for externalized anger (ΔR2 = 0.01, p = 0.017). In addition, after accounting for SES, Anger Expression-In and Anger Expression-Out were both significantly associated with EAA (Table 5), with higher levels of anger expression associated with greater EAA. Both Anger Expression-In (ΔR2 = 0.03, p = 0.001) and Anger-Expression-Out (ΔR2 = 0.02, p = 0.003) demonstrated small effects.
Table 3.
Association between Lifetime Stress Exposure and Epigenetic Age Acceleration: Multiple regression analyses (n = 219)
| Variable | ΔF | ΔR2 | t | p-value |
|---|---|---|---|---|
| Block 1 (Blood Sample Covariates) | 22.1 | 0.13 | <0.001*** | |
| CD4+ T cells | 0.28 | 0.78 | ||
| CD8+ T cells | 1.16 | 0.25 | ||
| Natural Killer Cells | −1.88 | 0.060 | ||
| B Cells | 0.28 | 0.78 | ||
| Monocytes | 2.42 | 0.016* | ||
| Granulocytes | 3.97 | <0.001*** | ||
| Sex | −1.86 | 0.063 | ||
| Block 2 (Income) | 5.09 | 0.004 | −2.26 | 0.024* |
| Block 3 (Lifetime Stress Exposure) | 0.23 | 0.001 | 0.48 | 0.64 |
Table 4.
Association between Lifetime Stress Exposure and Anger Expression: Multiple regression analyses (n = 219)
| Variable | ΔF | ΔR2 | t | p-value |
|---|---|---|---|---|
| Anger Expression-In | ||||
| Block 1 (Income) | 7.43 | 0.013 | −2.73 | 0.007** |
| Block 2 (Lifetime Stress Exposure) | 14.90 | 0.041 | 3.86 | <0.001*** |
| Anger Expression-Out | ||||
| Block 1 (Income) | 7.43 | 0.013 | −2.73 | 0.007** |
| Block 2 (Lifetime Stress Exposure) | 5.76 | 0.013 | 2.40 | 0.017* |
Table 5.
Association between Anger Expression and Epigenetic Age Acceleration: Multiple regression analyses (n = 219)
| Variable | ΔF | ΔR2 | t | p-value |
|---|---|---|---|---|
| Anger Expression-In | ||||
| Block 1 (Blood Sample Covariates) a | 22.1 | 0.13 | <0.001*** | |
| Block 2 (Income) | 5.09 | 0.004 | −2.26 | 0.024* |
| Block 3 (Anger Expression-In) | 12.00 | 0.03 | 3.46 | 0.001** |
| Anger Expression-Out | ||||
| Block 1 (Blood Sample Covariates) a | 22.1 | 0.13 | <0.001*** | |
| Block 2 (Income) | 5.09 | 0.004 | −2.26 | 0.024* |
| Block 3 (Anger Expression-Out) | 8.67 | 0.02 | 2.94 | 0.003** |
Note.
Table 3 displays all covariates included in Block 1.
Anger Expression as a Mediator Between LSE and EAA
Although LSE was not directly associated with EAA, we conducted mediation analyses to assess whether LSE and EAA were indirectly associated via anger expression. Results indicated that LSE was indirectly associated with EAA through Anger Expression-In (Figure 1a, path ab; β = 0.03, SE = 0.02, 95% CI [0.003, 0.08]). This was not the case for Anger Expression-Out (Figure 1b, path ab; β = 0.02, SE = 0.02, 95% CI [0.000, 0.06]). To probe potential directionality of the indirect association via internalized anger – in other words, whether individuals who internalize anger demonstrate accelerated aging because they are more likely to report experiences of lifetime stress exposure – we tested whether LSE mediated the relationship between Anger Expression-In and EAA. This mediation was nonsignificant (Figure 2, path ab; β = 0.003, SE = 0.01, 95% CI [−0.03, 0.02]).
Figure 1.
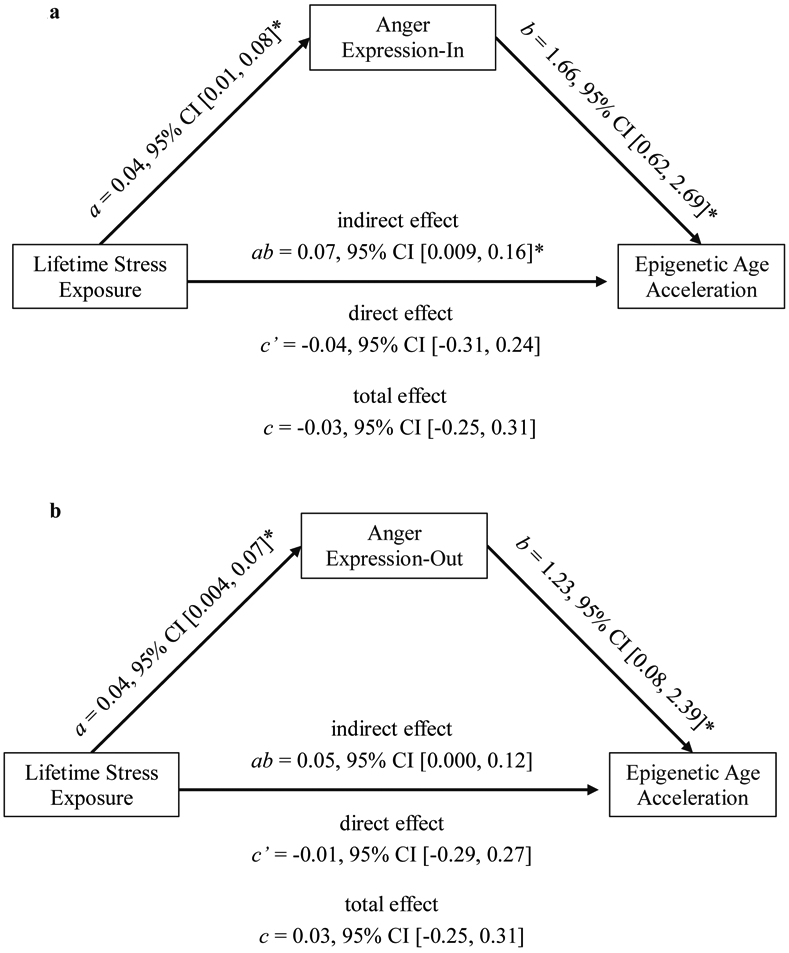
Lifetime Stress Exposure was indirectly associated with Epigenetic Age Acceleration through elevated internalized, but not externalized, anger expression. Asterisks (*) signify statistical significance according to 95% bootstrap confidence intervals (n = 219).
Figure 2.

To assess whether individuals who internalize anger demonstrate accelerated epigenetic aging because they are more likely to report experiences of lifetime stress exposure, we tested whether Lifetime Stress Exposure mediated the relationship between Anger Expression-In and Epigenetic Age Acceleration. Results indicated that this was not the case. Asterisks (*) signify statistical significance according to 95% bootstrap confidence intervals (n = 219).
Discussion
Race-related stressors such as racial discrimination, trauma exposure, and stressful life events are associated with deleterious mental and physical health outcomes, yet, little is known about the processes through which race-related stress exposure may be associated with these adverse outcomes. The current paper examined psychological processes (i.e., anger expression) through which LSE may be associated with an important biological outcome, epigenetic age acceleration (EAA), in a sample of Black adults who have experienced significant life stress and trauma. By examining these processes among this marginalized population, the present study contributes to a more representative and generalizable body of epigenetic research, which has typically relied on White, mid-to-high SES samples (40). Furthermore, given the typically strong effects of SES on health disparities and the fact that SES is often confounded with race (76), the present study clarifies the role of race-related LSE independent of SES. We expected to find a positive association between LSE and EAA (Hypothesis 1) that was mediated by internalized anger (Hypothesis 2). Although our results did not provide evidence supporting our first hypothesis, our second hypothesis was partially supported by our findings that: 1) LSE was significantly associated with both internalized and externalized anger, and 2) LSE was indirectly associated with EAA via internalized anger. These findings offer novel insights on the potential consequences of race-related stress exposure.
Based on increasing evidence in support of the weathering hypothesis (77), which posits that race-related LSE may confer risk for poorer health outcomes by triggering stress-induced physiological deterioration (e.g., allostatic load; 78), we expected LSE to be associated with EAA. Our results, however, did not support this hypothesis. One possible explanation for the nonsignificant association could be that our assessment of LSE relied on event-based reports of stressors that do not capture subjective stress associated with those experiences (79). It is possible that such individual differences influence the degree to which experiences of race-related stress are associated with aging. This interpretation is consistent with meta-analytic evidence (k = 9, N = 2,186) that childhood trauma – assessed using relatively subjective assessments (e.g., degree of agreement with statements about abuse) – is associated with EAA, whereas lifetime trauma – assessed using event-based measures (e.g., event happened or did not happen) – is not (40). Alternatively, and consistent with previous literature documenting indirect associations with accelerated aging via psychopathology (42), it is possible that the pernicious effects of LSE on accelerated aging occurs indirectly through stress exposure’s impact on key psychological variables.
In line with research highlighting the relevance of psychological mechanisms, our results indicate that experiencing greater LSE is associated with higher internalized and externalized anger, which, in turn, are each associated with greater EAA. These findings align with previous evidence that anger, regardless of expression, is characterized by arousal of physiological stress systems, including overactivation of the HPA-axis (80-82) and inflammatory response (83-85), which in turn contribute to physiological weathering and premature biological aging (34,40,83,86,87). In support of our second hypothesis, although LSE was directly associated with both internalized and externalized anger, the indirect effect was only found for internalized anger. This effect is consistent with evidence that higher internalization of strong negative emotions is associated with greater EAA (88,89) and that, among Black adults, inhibition - but not outward expression - of anger is associated with higher ambulatory blood pressure (61) and allostatic load (90). Our findings pertaining to the externalization of anger were more nuanced. We found that greater externalized anger was associated with greater EAA, which aligns with previous evidence that higher levels of outward anger expression are linked to adverse health outcomes in Black individuals (91). A recent study, however, found that externalized anger may actually be protective of cardiovascular health among Black individuals in the context of racial discrimination (92). Notably, neither of the former studies assessed the role of internalized anger. However, collectively these findings suggest that externalized anger may function differently in the face of race-related stress. Indeed, externalized anger did not mediate the association between race-related LSE and EAA in the current study, which is also consistent with previous evidence that Black individuals are more likely to internalize anger in the context of race-related stressors (61).
Together, our results indicate that elevated anger, expressed both internally and externally, may contribute to premature biological aging, yet the contingencies of these processes may differ. Anger expression may function differently according to racial and ethnic factors, for instance, given the societal barriers to expressing anger (50) which may be tied to experiences of stigmatization that Black individuals face regarding the outward expression of anger (59). In line with the weathering hypothesis, it is possible that the added pressure of suppressing such emotional reactions may erode resources in ways that accelerate epigenetic aging. Additional research is needed to further clarify these processes and identify potentially exacerbating or ameliorating contextual factors that may contribute to these contingencies.
Implications
The results of the current study have several implications. First, our results highlight how different types of dysregulation related to anger expression may have unique sequalae ultimately leading to EAA. Our operationalization of internalized and externalized anger both represent different elements of unsuccessful emotional processing and thus their direct associations with EAA is not surprising (93). Identifying strategies to promote successful anger processing may help minimize the adverse health outcomes associated with anger dysregulation in either type of expression. Moreover, the indirect effect of race-related LSE via internalized anger highlights the importance of assessing and intervening on dysregulated anger expression for individuals with disproportionate exposure to highly stressful experiences. Numerous psychological interventions are available to promote successful processing and expression of difficult emotions including anger (e.g., Dialectical Behavior Therapy, Emotion-Focused Therapy; 94,95). At the same time, it is crucial that clinicians consider the broader societal context when considering the utilization of such strategies when working with Black clients. Although encouragement of effective anger expression may in general be justified as a therapeutic intervention target, it also has the potential for harm within the context of a society that is still contending with racism. For example, there is evidence that in Black compared to White faces, anger is detected more quickly (96), is more likely to be misperceived (58), and is more likely to be attributed to dispositional (rather than situational) factors (97). As a result of this bias, individuals may then act aggressively toward Black individuals (98) and use (a biased perception of) perceived threat to discredit Black individuals (99) and justify anti-Black aggression and violence (100).
Although the onus of eliminating racialized anger bias and stereotypes is on the individuals who perpetrate it, Black individuals may nonetheless bear the burden of its consequences. While encouragement of anger inhibition to avoid bias is ethically questionable and, per our findings, may have detrimental health consequences, encouragement of anger expression without consideration of the broader societal context can also cause undue harm, over and above the health consequences associated with high levels of externalized anger. Taken together and in line with culturally sensitive care, clinicians should take such contextual considerations in their interventions and identify strategies that can maximize benefits and minimize harm.
Limitations and Future Directions
Our findings must be considered in light of several limitations. Most importantly, our analyses relied on cross-sectional data, limiting our ability to draw conclusions about the temporal progression of accelerated aging, or to draw causal conclusions about the associations between anger expression and EAA. Our findings lay a foundation for future longitudinal studies to examine how EAA may shift over time in relation to changes in anger expression. Experimental studies, in particular, could inform clinical interventions aimed at reducing the potential impact of race-related stress on premature aging. For example, research may examine whether interventions designed to reduce internalized anger in the context of racial discrimination can reduce EAA. In addition, many of our measures were crude assessments of complex constructs (e.g., using income as a proxy for socioeconomic status) and life events (e.g., summing racial discrimination types rather than examining frequency and perceived stress). Although our composite score approach was justified both quantitatively and theoretically (i.e., based on consistent evidence that racial discrimination, trauma exposure, and stressful life events are closely intertwined with the effects of systemic racism), it nevertheless may obfuscate unique associations for trauma, racial discrimination, and life stressors. Further research using comprehensive, nuanced measures is necessary to develop a clearer understanding of how different types of stress may be differentially or similarly associated with outcomes. Finally, additional research is needed to better understand the physiological mechanisms that link internalized and externalized anger to alterations in DNAm. Our utilization of transdiagnostic endophenotypes (e.g., anger, EAA; 101) – as opposed to symptomatically-heterogenous clinical diagnoses (e.g., depression, PTSD; 102) and broad health outcomes (e.g., cardiovascular disease) – puts us one step closer to understanding the complex processes underlying racial health disparities. However, understanding the mechanisms that confer risk for racial health disparities requires continued investigation.
Conclusion
Racial discrimination and other lifetime stressors affect Black individuals in complex ways that have consequences on biological and psychological levels. Better understanding the mechanisms through which these complex processes confer risk for various outcomes has the potential to deepen our understanding of health and wellness among Black populations and develop empirically-based intervention and prevention efforts.
Conflicts of Interest and Source of Funding:
The authors have no financial interests or potential conflicts of interest to disclose. This work was supported by the National Institute of Mental Health (MH071537; MH100122; MH102890; MH101380, MH115174), the National Institute of Child Health and Human Development (HD071982), the National Institute of Aging (AG062334) and the National Center for Complementary & Integrative Health (K23AT009713). Author BGM was supported by the NSF Graduate Research Fellowship Program (DGE-1444932) during the completion of this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the entire Grady Trauma Project team for their assistance in data collection and management of this project and our participants for their willingness to be a part of our study.
Abbreviations:
- LSE
lifetime stress exposure
- EAA
epigenetic age acceleration
- PTSD
posttraumatic stress disorder
References
- 1.Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. Am Sociol Rev 2000;65(6):910–30. [Google Scholar]
- 2.Williams DR. Miles to Go before We Sleep: Racial Inequities in Health. J Health Soc Behav 2012. September;53(3):279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine ME, Crimmins EM. Evidence of accelerated aging among african americans and its implications for mortality. Soc Sci Med 2014;118(C):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Affairs 2005;24(2):343–52. [DOI] [PubMed] [Google Scholar]
- 5.Batai K, Hooker S, Kittles RA. Leveraging genetic ancestry to study health disparities. Am J Phys Anthropol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper RS. Race and genomics. The New England Journal of Medicine 2003;348(12):1166. [DOI] [PubMed] [Google Scholar]
- 7.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Human Genomics 2015;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mays VM, Cochran SD, Barnes NW. Race, Race-Based Discrimination, and Health Outcomes Among African Americans. Annu Rev Psychol 2007;58(1):201–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pager D, Shepherd H. The sociology of discrimination: Racial discrimination in employment, housing, credit, and consumer markets. Annu. Rev. Sociol 2008;34:181–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389:1453–63. [DOI] [PubMed] [Google Scholar]
- 11.Bryant-Davis T, Ocampo C. The trauma of racism: Implications for counseling, research, and education. The Counseling Psychologist 2005;33(4):574–8. [Google Scholar]
- 12.López CM, Andrews Iii AR, Chisolm AM, De Arellano MA, Saunders B, Kilpatrick DG. Racial/Ethnic Differences in Trauma Exposure and Mental Health Disorders in Adolescents HHS Public Access. Cult Divers Ethn Minor Psychol 2017;23(3):382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychological medicine 2011;41(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breslau N The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, Violence, Abus 2009;10(3):198–210. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic Stress Disorder Among African Americans in an Inner City Mental Health Clinic. Psychiatr Serv 2005;56(2):212–5. [DOI] [PubMed] [Google Scholar]
- 16.Gapen M, Cross D, Ortigo K, Graham A, Johnson E, Evces M, Ressler KJ, Bradley B. Perceived neighborhood disorder, community cohesion, and PTSD symptoms among low-income African Americans in an urban health setting. American Journal of Orthopsychiatry 2011;81(1):31. [DOI] [PubMed] [Google Scholar]
- 17.Turner RJ, Avison WR. Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. J Health Soc Behav 2003;44(4):488–505. [PubMed] [Google Scholar]
- 18.Alim TN, Graves E, Mellman TA, Aigbogun N, Gray E, Lawson W, Charney DS. Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. Journal of the National Medical Association 2006;98(10):1630. [PMC free article] [PubMed] [Google Scholar]
- 19.Williams DR. Race, socioeconomic status, and health the added effects of racism and discrimination. 1999. [DOI] [PubMed] [Google Scholar]
- 20.Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing “Neighborhood Effects”: Social Processes and New Directions in Research. Annu Rev Sociol 2002. August 28;28(1):443–78. [Google Scholar]
- 21.Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, Gupta A, Kelaher M, Gee G. Racism as a determinant of health: a systematic review and meta-analysis. PloS one 2015;10(9):e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones SCT, Anderson RE, Gaskin-Wasson AL, Sawyer BA, Applewhite K, Metzger IW. From “crib to coffin”: Navigating coping from racism-related stress throughout the lifespan of Black Americans. Am J Orthopsychiatry 2020;90(2):267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun 2010. March 1;24(3):438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J Gerontol A Biol Sci Med Sci 2014;69(S1). [DOI] [PubMed] [Google Scholar]
- 25.Tomiyama AJ, O'Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Dhabhar FS, Wolkowitz O, Kirschbaum C, Blackburn E, Epel E. Does cellular aging relate to patterns of allostasis?: An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & behavior 2012;106(1):40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 2006;96(5):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons RL, Lei MK, Klopack E, Zhang Y, Gibbons FX, Beach SRH. Racial Discrimination, Inflammation, and Chronic Illness Among African American Women at Midlife: Support for the Weathering Perspective. J Racial Ethn Heal Disparities. 2020; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons RL, Lei MK, Beach SR, Philibert RA, Cutrona CE, Gibbons FX, Barr A. Economic hardship and biological weathering: the epigenetics of aging in a US sample of black women. Social Science & Medicine 2016;150:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpe VV., Lee DB, Hoggard LS, Rahal D. Racial Discrimination and Acute Physiological Responses Among Black Young Adults: The Role of Racial Identity. J Adolesc Heal 2019;64(2):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SRH. Perceived Discrimination Among African American Adolescents and Allostatic Load: A Longitudinal Analysis With Buffering Effects. Child Dev 2014;85(3):989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics 2007;253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horvath S DNA methylation age of human tissues and cell types. Genome Biol 2013;14(10):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clinical epigenetics 2016;8(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019;11(2):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biology 2015;16(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katrinli S, Stevens J, Wani AH, Lori A, Kilaru V, van Rooij SJ, Hinrichs R, Powers A, Gillespie CF, Michopoulos V, Gautam A. Evaluating the impact of trauma and PTSD on epigenetic prediction of lifespan and neural integrity. Neuropsychopharmacology 2020;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gassen NC, Chrousos GP, Binder EB, Zannas AS. Life stress, glucocorticoid signaling, and the aging epigenome: Implications for aging-related diseases. Vol. 74, Neuroscience and Biobehavioral Reviews. Elsevier Ltd; 2017. p. 356–65. [DOI] [PubMed] [Google Scholar]
- 38.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol Cell. 2013. January 24;49(2):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Q, Weidner CI, Costa IG, Marioni RE, Ferreira MR, Deary IJ, Wagner W. DNA methylation levels at individual age-associated CpG sites can be indicative for life expectancy. Aging 2016;8(2):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, Ashley-Koch AE, Garrett M, Kimbrel NA, Lori A, Workgroup VM. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology 2018;92:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, Klengel T, Smith AK. Exposure to violence accelerates epigenetic aging in children. Scientific Reports 2017;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter SE, Ong ML, Simons RL, Gibbons FX, Lei MK, Beach SRH. The effect of early discrimination on accelerated aging among African Americans. Health Psychol 2019;38(11):1010–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brody GH, Miller GE, Yu T, Beach SRH, Chen E. Supportive Family Environments Ameliorate the Link Between Racial Discrimination and Epigenetic Aging: A Replication Across Two Longitudinal Cohorts. Psychol Sci 2016. April 25;27(4):530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas SA, Gonzalez-Prendes AA. Powerlessness, anger, and stress in African American women: Implications for physical and emotional health. Health Care Women Int 2009;30(1–2):93–113. [DOI] [PubMed] [Google Scholar]
- 46.Kawachi I, Sparrow D, Spiro III A, Vokonas P, Weiss ST. A prospective study of anger and coronary heart disease: the Normative Aging Study. Circulation 1996;94(9):2090–5. [DOI] [PubMed] [Google Scholar]
- 47.Williams JE, Paton CC, Siegler IC, Eigenbrodt ML, Nieto FJ, Tyroler HA. Anger proneness predicts coronary heart disease risk: prospective analysis from the atherosclerosis risk in communities (ARIC) study. Circulation 2000;101(17):2034–9. [DOI] [PubMed] [Google Scholar]
- 48.Consedine NS, Moskowitz JT. The role of discrete emotions in health outcomes: A critical review. Applied and Preventive Psychology 2007;12(2):59–75. [Google Scholar]
- 49.al’Absi M, Bongard S. Neuroendocrine and behavioral mechanisms mediating the relationship between anger expression and cardiovascular risk: Assessment considerations and improvements. Journal of Behavioral Medicine 2006;29(6):573–91. [DOI] [PubMed] [Google Scholar]
- 50.Magai C, Kerns MD, Gillespie M, Huang B. Anger experience and anger inhibition in sub-populations of African American and European American older adults and relation to circulatory disease. J Health Psychol 2003;8(4):413–32. [DOI] [PubMed] [Google Scholar]
- 51.Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Annals of Behavioral Medicine 1998;20(4):326–32. [DOI] [PubMed] [Google Scholar]
- 52.Hosseini SH, Mokhberi V, Mohammadpour RA, Mehrabianfard M, Lashak NB. Anger expression and suppression among patients with essential hypertension. International journal of psychiatry in clinical practice 2011;15(3):214–8. [DOI] [PubMed] [Google Scholar]
- 53.Harburg E, Julius M, Kaciroti N, Gleiberman L, Schork AM. Expressive/suppressive anger-coping responses, gender, and types of mortality: a 17-year follow-up (Tecumseh, Michigan, 1971-1988). Psychosomatic medicine 2003;65(4):588–97. [DOI] [PubMed] [Google Scholar]
- 54.Keith F, Krantz DS, Chen R, Harris KM, Ware CM, Lee AK, Bellini PG, Gottlieb SS. Anger, hostility, and hospitalizations in patients with heart failure. Health Psychology 2017;36(9):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haukkala A, Konttinen H, Laatikainen T, Kawachi I, Uutela A. Hostility, anger control, and anger expression as predictors of cardiovascular disease. Psychosomatic Medicine 2010;72(6):556–62. [DOI] [PubMed] [Google Scholar]
- 56.Nesse RM, Ellsworth PC. Evolution, emotions, and emotional disorders. American Psychologist 2009;64(2):129. [DOI] [PubMed] [Google Scholar]
- 57.Walley-Jean JC. Debunking the Myth of the “Angry Black Woman”: An Exploration of Anger in Young African American Women. Women, Gend + Fam 2009;3(2):68–86. [Google Scholar]
- 58.Halberstadt AG, Cooke AN, Garner PW, Hughes SA, Oertwig D, Neupert SD. Racialized emotion recognition accuracy and anger bias of children’s faces. Emotion 2020. [DOI] [PubMed] [Google Scholar]
- 59.Corbin NA, Smith WA, Garcia JR. Trapped between justified anger and being the strong Black woman: Black college women coping with racial battle fatigue at historically and predominantly White institutions. International Journal of Qualitative Studies in Education. 2018. August 9;31(7):626–43. [Google Scholar]
- 60.Wingfield AH. The modern mammy and the angry Black man: African American professionals' experiences with gendered racism in the workplace. Race, Gender & Class 2007;196–212. [Google Scholar]
- 61.Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosomatic medicine 2003;65(5):746–50. [DOI] [PubMed] [Google Scholar]
- 62.Johnson EH, Greene A. The Relationship between Suppressed Anger and Psychosocial Distress in African American Male Adolescents. J Black Psychol 1991;18(1):47–65. [Google Scholar]
- 63.Poole JC, Snieder H, Davis HC, Treiber FA. Anger suppression and adiposity modulate association between ADRB2 haplotype and cardiovascular stress reactivity. Psychosomatic Medicine 2006;68(2):207–12. [DOI] [PubMed] [Google Scholar]
- 64.Dorr N, Brosschot JF, Sollers III JJ, Thayer JF. Damned if you do, damned if you don't: The differential effect of expression and inhibition of anger on cardiovascular recovery in Black and White males. International Journal of Psychophysiology 2007;66(2):125–34. [DOI] [PubMed] [Google Scholar]
- 65.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry 2009;31(6):505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;1576–96. [DOI] [PubMed] [Google Scholar]
- 67.Ertel KA, James-Todd T, Kleinman K, Krieger N, Gillman M, Wright R, Rich-Edwards J. Racial discrimination, response to unfair treatment, and depressive symptoms among pregnant black and African American women in the United States. Annals of epidemiology 2012;22(12):840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2011;156(6):700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spielberger CD, Reheiser EC. Measuring anxiety, anger, depression, and curiosity as emotional states and personality traits with the STAI, STAXI, and STPI. Compr Handb Psychol Assess 2003;2:70–86. [Google Scholar]
- 70.Barfield RT, Kilaru V, Smith AK, Conneely KN, Barrett J. CpGassoc: an R function for analysis of DNA methylation microarray data. Bioinforma Appl 2012;28(9):1280–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCartney DL, Walker RM, Morris SW, McIntosh AM, Porteous DJ, Evans KL. Identification of polymorphic and off-target probe binding sites on the Illumina Infinium MethylationEPIC BeadChip. Genomics Data 2016;9:22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 2012;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei MK, Gibbons FX, Simons RL, Philibert RA, Beach SR. The Effect of Tobacco Smoking Differs across Indices of DNA Methylation-Based Aging in an African American Sample: DNA Methylation-Based Indices of Smoking Capture These Effects. Genes 2020;11(3):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu. Rev. Psychol 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications; 2017. [Google Scholar]
- 76.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences 2010;69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity & disease 1992; 207–21. [PubMed] [Google Scholar]
- 78.Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SRH. Perceived Discrimination Among African American Adolescents and Allostatic Load: A Longitudinal Analysis With Buffering Effects. Child Dev 2014;85(3):989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinberg M, Gil S. Trauma as an objective or subjective experience: The association between types of traumatic events, personality traits, subjective experience of the event, and posttraumatic symptoms. J Loss Trauma 2016;21(2):137–46. [Google Scholar]
- 80.Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, behavior, and immunity 2010;24(2):215–9. [DOI] [PubMed] [Google Scholar]
- 81.Lupis SB, Lerman M, Wolf JM. Anger responses to psychosocial stress predict heart rate and cortisol stress responses in men but not women. Psychoneuroendocrinology 2014;49(1):84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raspopow K, Abizaid A, Matheson K, Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: Influence of anger and shame. Horm Behav 2010;58(4):677–84. [DOI] [PubMed] [Google Scholar]
- 83.Boylan JM, Ryff CD. Varieties of anger and the inverse link between education and inflammation: toward an integrative framework. Psychosomatic Medicine 2013;75(6):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barlow MA, Wrosch C, Gouin JP, Kunzmann U. Is anger, but not sadness, associated with chronic inflammation and illness in older adulthood?. Psychology and aging 2019;34(3):330. [DOI] [PubMed] [Google Scholar]
- 85.Wolf EJ, Morrison FG. Traumatic stress and accelerated cellular aging: from epigenetics to cardiometabolic disease. Current psychiatry reports 2017;19(10):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang RC, Lillycrop KA, Beilin LJ, Godfrey KM, Anderson D, Mori TA, Rauschert S, Craig JM, Oddy WH, Ayonrinde OT, Pennell CE. Epigenetic age acceleration in adolescence associates with BMI, inflammation, and risk score for middle age cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism 2019;104(7):3012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das A How does race get “ under the skin” ?: Inflammation, weathering, and metabolic problems in late life. Soc Sci Med 2013;77(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller GE, Yu T, Chen E, Brody GH. Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proc Natl Acad Sci 2015;112(33):10325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dich N, Doan SN, Evans GW. In risky environments, emotional children have more behavioral problems but lower allostatic load. Heal Psychol 2017. May 1;36(5):468–76. [DOI] [PubMed] [Google Scholar]
- 90.Zilioli S, Imami L, Ong AD, Lumley MA, Gruenewald T. Discrimination and anger control as pathways linking socioeconomic disadvantage to allostatic load in midlife. J Psychosom Res 2017. December 1;103:83–90. [DOI] [PubMed] [Google Scholar]
- 91.Johnson EH, Broman CL. The relationship of anger expression to health problems among black Americans in a national survey. Journal of Behavioral Medicine 1987;10(2):103–16. [DOI] [PubMed] [Google Scholar]
- 92.Park J, Flores AJ, Aschbacher K, Mendes WB. When anger expression might be beneficial for African Americans: The moderating role of chronic discrimination. Cult Divers Ethn Minor Psychol 2018;24(3):303–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rachman S Emotional processing. Behav Res Ther 1980;18(1):51–60. [DOI] [PubMed] [Google Scholar]
- 94.Frazier SN, Vela J. Dialectical behavior therapy for the treatment of anger and aggressive behavior: A review. Aggression and Violent Behavior 2014;156–63. [Google Scholar]
- 95.Pascual-Leone A, Paivio SC. Emotion-focused therapy for anger in complex trauma. Treatments for anger in specific populations: Theory, application, and outcome 2013;33–51. [Google Scholar]
- 96.Kang SK, Chasteen AL. Beyond the double-jeopardy hypothesis: Assessing emotion on the faces of multiply-categorizable targets of prejudice. Journal of Experimental Social Psychology 2009;45(6):1281–5. [Google Scholar]
- 97.Motro D, Evans JB, Ellis AP, & Benson L III (2021). Race and reactions to women’s expressions of anger at work: Examining the effects of the “angry Black woman” stereotype. Journal of Applied Psychology. [DOI] [PubMed] [Google Scholar]
- 98.Halberstadt AG, Castro VL, Chu Q, Lozada FT, Sims CM. Preservice teachers’ racialized emotion recognition, anger bias, and hostility attributions. Contemp Educ Psychol 2018;54:125–38. [Google Scholar]
- 99.Salerno JM, Peter-Hagene LC, Jay AC V. Women and African Americans are less influential when they express anger during group decision making. Gr Process Intergr Relations 2019;22(1):57–79. [Google Scholar]
- 100.Mekawi Y, Bresin K, Hunter CD. Dehumanization of African-Americans Influences Racial Shooter Biases. Race Soc Probl 2019;11(4):299–307. [Google Scholar]
- 101.Waszczuk MA, Eaton NR, Krueger RF, Shackman AJ, Waldman ID, Zald DH, Lahey BB, Patrick CJ, Conway CC, Ormel J, Hyman SE. Redefining phenotypes to advance psychiatric genetics: Implications from hierarchical taxonomy of psychopathology. Journal of abnormal psychology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kendler KS. DSM disorders and their criteria: how should they interrelate? 2017;2054–2060. [DOI] [PubMed] [Google Scholar]


