Abstract
This study, a secondary analysis of the HPTN 068 randomized control trial, aimed to quantify the association of father and male presence with HIV incidence and first pregnancy among 2533 school-going adolescent girls and young women (AGYW) in rural South Africa participating in the trial between March 2011 and April 2017. Participants’ ages ranged from 13 to 20 years at study enrollment and 17 to 25 at the post-intervention visit. HIV and pregnancy incidence rates were calculated for each level of the exposure variables using Poisson regression, adjusted for age using restricted quadratic spline variables, and, in the case of pregnancy, also adjusted for whether the household received a social grant. Our study found that AGYW whose fathers were dead and adult males were absent from the household were most at risk for incidence of first pregnancy and HIV (pregnancy: aIRR=1.30, Wald 95% CI=1.05, 1.61, Wald chi-square p=0.016; HIV: aIRR=1.27, Wald 95% CI=0.84, 1.91, Wald chi-square p=0.263) as compared to AGYW whose biological fathers resided with them. For AGYW whose fathers were dead, having other adult males present as household members seemed to attenuate the incidence (pregnancy: aIRR=0.92, Wald 95% CI=0.74, 1.15, Wald chi-square p=0.462; HIV: aIRR=0.90, Wald 95% CI=0.58,1.39, Wald chi-square p=0.623) such that it was similar, and therefore not statistically significantly different, to AGYW whose fathers were present in the household.
Keywords: Father absence, HIV, pregnancy, South Africa, longitudinal
BACKGROUND
Of the estimated 37.9 million people living with HIV worldwide in 2018, 2.8 million were children and adolescents under 20 years of age and about 18.8 million (more than half) were female.(1) In 2017 alone, 510,000 young people between the ages of 10 to 24 were newly infected with HIV globally, of whom 190,000 were adolescents between the ages of 10 and 19.(2) Adolescent girls and young women (AGYW) between the ages of 15 to 24 in Sub-Saharan Africa have a high burden of HIV and during these years the risk of HIV acquisition increases rapidly, with a quarter or more infected by their early to mid-twenties.(3–5)
South Africa carries the highest burden of HIV in the world, with 7.7 million HIV-positive individuals as of 2018.(6) Mpumalanga province, where this study is located, has one of the highest HIV prevalence levels (35.6% overall) among all provinces in South Africa.(7) Recent research supports that a woman’s risk of HIV infection increases with pregnancy and is even higher during the postpartum period.(8) A new maternal HIV infection during these periods has negative consequences for the mother and child, as it would put the child at risk for perinatal HIV transmission. Incidence of HIV infection during pregnancy has been shown to be around twice as high as incidence in nulliparous sexually active women.(9–12) In addition, it has been shown that young women who give birth in South Africa have twice the odds of school drop-out and five times the odds of failing to matriculate.(13) Further, young women who dropped out of school had a higher weighted hazard of acquiring HIV (HR 3.25, Wald 95% CI=1.67, 6.32).(14)
There is considerable literature that father absence during childhood might influence the timing of menarche (15–25), and be associated with sexual violence (26), earlier first sexual experience(21,24,27–29), earlier first pregnancy or birth (24,27,29–32), higher fertility (23), earlier marriage or partnership (30,31) and more sexual partners.(24,28,33) Paternal absence is wide-spread and persistent in post-apartheid South Africa due to rural-urban labor migration, undisclosed paternity, denied responsibility of fatherhood, denied access to the child, dissolution of households, and divorce.(26,34) Life-course adversity models suggest that father absence is a proxy for other associated stressors (e.g., divorce, poverty, conflictual family relationships, erosion of parental monitoring and control) that foster early sexual activity and pregnancy in daughters.(27,35–38)
Due to apartheid era policies, up to 42% of rural African male laborers migrate internally within South Africa for work, which moves them to urban industrial centers. In the study area, around 60% of men 30 to 49 years old migrate to work elsewhere for at least 6 months of every year.(39) Formal employment requiring migration in the areas surrounding Mpumalanga include the mining sector, construction, security firms in larger towns, and work on nearby agricultural and game farms.(40) Migrant fathers can be temporary or permanent migrants, some of whom send remittances to support the family, leaving mothers or other family members to serve as primary caregiver to their children. (14,41)
Understanding whether fathers’ physical absence from the household is associated with incidence of HIV or first pregnancy in AGYW can inform interventions and policies aimed at improving how fathers meet the needs of their children, even if not residing in the home. In South Africa, the dialogue of responsible fatherhood began before 2003, when The Fatherhood Project was established by Human Sciences Research Council.(42) The dialogue has continued since, including the high profile campaign, Brothers For Life, supported by South African National AIDS Council, Johns Hopkins Health and Education in South Africa, and Sonke Gender Justice.(42) These projects and campaigns not only aim to reach fathers with positive fathering messages, but to change social norms among men on parenting and prevention of violence against women and children. Additional understanding of the risks associated with father absence can help to tailor messages to biological fathers and other adult men that live in households with children and adolescents.
This study will quantify the association of a biological father’s physical presence or absence from a household, as well as the presence or absence of other adult men in father-absent households, with incidence of HIV or first pregnancy among a longitudinal cohort of rural South African AGYW.
METHODS
This study is a secondary data analysis of longitudinal data collected between March 5, 2011 and April 26, 2017 from the HPTN 068 phase III randomized controlled trial that was implemented to assess the impact of a conditional cash transfer intervention on HIV incidence among AGYW in rural South Africa. The trial took place in the Agincourt Health and Socio-Demographic Surveillance System (HDSS) site in the rural Bushbuckridge subdistrict in Mpumalanga province, South Africa. Methods of the study are described below. Additional information on study and recruitment procedures, and methods for HIV testing not covered in this paper can be found in the baseline and final papers.(41,43)
The hypothesis for this paper is:
AGYW whose fathers and adult males were physically absent from the home, would have a higher incidence rate for (1) HIV and (2) first pregnancy than AGYW whose father was a physically present household member.
Institutional Review Board approval for this secondary data analysis was obtained from the University of North Carolina at Chapel Hill; the parent study received approval from University of North Carolina at Chapel Hill as well as the University of the Witwatersrand in South Africa and the Mpumalanga Province’s Research and Ethics Committee.
Study Participants
Participants in the HPTN068 parent study included 2533 AGYW aged 13 to 20 years enrolled in grades 8 to 11 who were neither married nor pregnant at the time of baseline enrollment. Participant consent (≥18 years) and assent (<18 years) with parent or guardian consent were obtained prior to enrollment. Eligibility criteria also included that the AGYW be able to read (either Shangaan or English), have the documentation necessary to open a bank account (i.e. a national ID card or passport), have a parent or guardian with documentation necessary to open a bank account, and currently reside in the study area with the intention of remaining there for at least three years, until trial completion.
Data Collection
Study visits included a baseline visit at enrollment, three follow-up visits, a graduation visit (HIV test only), and a post-intervention visit. The cash transfer intervention occurred from March 2011 until March 2015. The post-intervention visits took place between April 2015 and April 2017 with the same cohort of AGYW and their households. All visits included biomarker data collection of the AGYW participant with pre-test HIV counseling conducted in group sessions and post-test counseling conducted individually with the participant. At each study visit, each participant received education on HIV prevention and treatment, HIV testing and counseling (if negative at the previous visit), as well as referrals for HIV treatment if they tested HIV-positive. (41,43) HIV screening was done at the study site by the HPTN068 study team with two HIV rapid tests completed in parallel. If one or both tests were reactive or positive, samples were collected and confirmatory HIV testing was done at the study site with the FDA-cleared GS HIV-1 western blot assay (Bio-Rad Laboratories Inc., Redmond Redmond, WA, USA). (41,43) Samples from all participants at all study visits were also tested at the HPTN Laboratory Center to confirm baseline HIV status and incident HIV infections.(41,43) With the exception of the graduation visit, each visit included a questionnaire administered to the AGYW using audio computer-assisted self-interviewing (ACASI) and a household questionnaire administered to the parent guardian. The household questionnaire included a household roster, to track migration into and out of the home. These visits occurred approximately annually until the young woman was scheduled to finish high school or until study completion in March 2015, whichever came first. All participants were invited to attend a post-intervention visit, which happened approximately 1 to 2 years after the main visit.
Variables
Main Exposures
Three exposure variables of father presence are examined: (1) father’s living status, (2) father and adult male presence in the household, and (3) father and adult male presence combined with father’s living status.
Father’s living status (exposure 1) is defined as a categorical variable with categories of: 1=father is present (resides) in the household; 2=father lives elsewhere; 3=father is deceased. This variable was determined using the following two questions from the AGYW Survey:
-
A
“Is your father alive?” (1=yes, 2=no, D=don’t know). For values of “don’t know”, 4.2% at baseline and less than 1.5% after baseline, the variable is retrospectively corrected if at a future visit the participant provides her age at which her father died. After this correction, values of “don’t know” were coded as father absent from the household.
-
B
If alive, “Where does your father live now?” (1 = your household, 2 = household elsewhere in Bushbuckridge, 3 = other urban area in South Africa, 4 = other rural area in South Africa, 5 = Mozambique, D = don’t know). Responses of household elsewhere in Bushbuckridge, other urban area in South Africa, other rural area in South Africa, Mozambique, and don’t know were coded as father lives elsewhere.
For the father living status exposure, a sensitivity analysis was carried out for each outcome assigning “don’t know” to “Father is deceased” no significant differences were found between the model outcomes (results not shown).
Father and adult male presence (exposure 2) is defined as a categorical variable with categories of: 1=Father present (resides) in the household; 2=Father absent from household, adult males present (reside) in household; 3=Father and adult males absent from household. Adult males are defined to be ≥18 years.
The first category of exposure 2 is created using the same two questions from the AGYW survey as outlined for exposure 1. The second and third categories for father and adult male presence are determined from four questions from the household survey roster:
-
C
“Is […]’s biological father listed on this roster?” (Response is the father’s roster line number in household roster of enumerated family members having ever lived in the household since the participants baseline visit)
-
D
Sex of household member
-
E
Age of household member
-
F
Compared to the roster completed 12 months ago at the last interview, is the household member 1=new to the household, 2=still a member of the household, 3=no longer a member of the household because member has left, 4=no longer a member of the household due to YW moving households, 5=has returned to household and was reported as left household at last visit, or 6=reported as died at last visit?
Based on these questions, a binary indicator is created for whether any adult male ≥18 years is a household member at time of each visit. Finally, this information is combined with the AGYW survey data to create the second and third categories of father and adult male presence.
Father and adult male presence combined with father’s living status (exposure 3), is a 5-category variable created by combining the two above measures, such that we are able to tell if the father is absent from the household due to living elsewhere or being deceased. Categories are 1=Father present in the household; 2=Father lives elsewhere, adult males absent from household; 3=Father lives elsewhere, adult males present in household; 4=Father dead, adult males absent from household, and 5=Father dead, adult males present in household.
Outcome Variables
HIV incidence:
The bivariate HIV status (0=negative, 1=positive), was determined at each study visit. For calculation of incidence, AGYW who were HIV positive at baseline were excluded from analysis. Once an AGYW tests positive, future study visits are censored.
Incidence of first pregnancy:
The bivariate pregnancy outcome, Ever Pregnant (0=no, 1=yes), was self-reported at each study visit and determined by the survey question: 1. “Have you ever been pregnant?” 2. “What was your age at first pregnancy?” (asked only at baseline) or 3. “What was your age at most recent pregnancy?” (asked only at follow-up). For calculation of incidence, AGYW who reported having been pregnant before or at baseline were excluded from analysis. Future study visits are censored after the AGYW reports her first pregnancy during the study period.
Covariates
Covariates that were considered in the directed acyclic graph included, age and grade level of the AGYW, and whether the AGYW had been sexually active (vaginal or anal), experienced an early vaginal sexual debut, had used a condom at last sex (among AGYW who have reported vaginal or anal sex), had ever participated in transactional sex (determined by the AGYW reporting that one of her most recent sex partners was a sex client, or that the work she did to earn money was sex work), had ever been forced to have sex (determined by a positive answer to the questions “Has anyone ever physically forced you to have sexual intercourse when you did not want to?” or “Have you ever had sexual intercourse that you did not want because you were afraid of what the other person might do?”), had a current boyfriend, or was depressed or not, as determined by the Child Depression Inventory (CDI) score.(44) The Children’s Depression Inventory is measured with a 10-item scale with each item assigned a numerical value from 0 to 2, with the total scale ranging from 0 to 20, with the higher value assigned to more clinically severe behavior.(44) Those with a score of 7 or above are categorized as depressed. Additional covariates included the number of lifetime partners she had and the age difference of her sex partners (age difference is reported as ≥5 years if at least one of the young women’s last three sexual partners was ≥5 years older than her). Household level covariates considered were household size and household expenditure. The characteristics of these covariates for the AGYW are all summarized in Table 1. Table 2 shows how some of the main characteristics vary over time.
Table 1.
Baseline characteristics of AGYW by father and adult male presence, who enrolled in the HPTN 068 conditional cash transfer trial in Bushbuckridge District, Mpumalanga, South Africa between March 2011 and December 2012
| Father present in household (n=989) | Father absent; adult males present in household (n=756) | Father and adult males absent from household (n=786) | All1 (n=2531) | |
|---|---|---|---|---|
|
|
||||
| Age at enrollment | ||||
| Mean (SD) | 15.5 (1.6) | 15.5 (1.6) | 15.6 (1.7) | 15.5 (1.7) |
| Median (IQR) | 15.0 (3.0) | 15.0 (3.0) | 15.0 (3.0) | 15.0 (3.0) |
| Grade at enrollment | ||||
| Grade 8 | 244 (24.7) | 183 (24.2) | 213 (27.1) | 640 (25.3) |
| Grade 9 | 268 (27.1) | 223 (29.5) | 191 (24.3) | 682 (26.9) |
| Grade 10 | 270 (27.3) | 218 (28.8) | 210 (26.7) | 698 (27.6) |
| Grade 11 | 207 (20.9) | 132 (17.6) | 172 (21.9) | 511 (20.2) |
| Ever repeated a grade | ||||
| Yes | 334 (33.8) | 271 (35.9) | 280 (35.6) | 885 (35.0) |
| No | 654 (66.2) | 485 (64.2) | 506 (64.4) | 1645 (65.0) |
| Missing | 1 | 0 | 0 | 1 |
| LN of total household expenditures | ||||
| Mean (SD) | 5.8 (0.86) | 5.7 (0.78) | 5.8 (0.79) | 5.8 (0.82) |
| Median (IQR) | 5.7 (1.00) | 5.7 (0.95) | 5.7 (0.93) | 5.68 (0.97) |
| Missing | 8 | 3 | 5 | 16 |
| Have current boyfriend | ||||
| Yes | 292 (29.6) | 258 (34.1) | 278 (35.4) | 828 (32.7) |
| No | 696 (70.5) | 498 (65.9) | 508 (64.6) | 1702 (67.3) |
| Missing | 1 | 0 | 0 | 1 |
| Ever had vaginal sex | ||||
| Yes | 256 (25.9) | 201 (26.7) | 242 (30.8) | 699 (27.7) |
| No | 731 (74.1) | 553 (73.3) | 542 (69.1) | 1826 (72.3) |
| Missing | 2 | 2 | 2 | 6 |
| Ever had anal sex | ||||
| Yes | 37 (3.7) | 35 (4.6) | 48 (6.1) | 120 (4.8) |
| No | 951 (96.3) | 721 (95.4) | 734 (93.9) | 2406 (95.3) |
| Missing | 1 | 0 | 4 | 5 |
| Early vaginal sexual debut (<15 years) | ||||
| Yes | 60(6.1) | 51 (6.8) | 55 (7.1) | 166 (6.6) |
| No | 923 (93.9) | 698 (93.2) | 724 (92.9) | 2345 (93.4) |
| Missing | 6 | 7 | 7 | 20 |
| Ever pregnant | ||||
| Yes | 112 (11.4) | 96 (12.9) | 103 (13.3) | 311 (12.4) |
| No | 873 (88.6) | 647 (87.1) | 671 (86.7) | 2191 (87.6) |
| Missing | 4 | 13 | 12 | 29 |
| HIV positive | ||||
| Yes | 22 (2.2) | 27 (3.6) | 32 (4.1) | 81 (3.2) |
| No | 966 (97.8) | 727 (96.4) | 753 (95.9) | 2448 (96.8) |
| Missing | 1 | 2 | 1 | 4 |
| Number of lifetime partners | ||||
| 0 | 729 (74.7) | 549 (73.9) | 535 (69.5) | 1813 (72.9) |
| 1 | 147 (15.1) | 106 (14.3) | 114 (14.8) | 367 (14.8) |
| 2+ | 100 (10.3) | 88 (11.8) | 120 (15.6) | 308 (12.4) |
| Missing | 13 | 13 | 17 | 43 |
| Used condom at last sex | ||||
| Yes | 144 (57.6) | 127 (64.1) | 136 (57.3) | 407 (59.3) |
| No | 106 (42.4) | 71 (35.9) | 102 (42.7) | 279 (40.6) |
| Missing | 8 | 8 | 10 | 26 |
| Transactional sex | ||||
| Yes | 49 (5.0) | 32 (4.2) | 23 (2.9) | 104 (4.1) |
| Not reported | 940 (95.1) | 724 (95.8) | 763 (97.1) | 2427 (95.9) |
| Missing | 0 | 0 | 0 | 0 |
| Ever forced sex | ||||
| Yes | 89 (9.1) | 73 (9.8) | 81 (10.6) | 243 (9.7) |
| No | 885 (90.9) | 674 (90.2) | 692 (89.4) | 2251 (90.3) |
| Missing | 15 | 9 | 13 | 37 |
| Age difference with partner | ||||
| ≥ 5 years older | 47 (19.0) | 42 (21.7) | 49 (20.8) | 138 (20.4) |
| <5 years older | 200 (81.0) | 152 (78.4) | 187 (79.2) | 539 (79.6) |
| Missing | 6 | 5 | 4 | 15 |
| Household size | ||||
| Mean (SD) | 6.2 (2.69) | 6.7 (2.75) | 5.1 (1.99) | 6.2 (2.61) |
| <=4 members | 194 (19.6) | 171 (22.6) | 324 (41.3) | 689 (27.3) |
| 5 – 7 members | 503 (50.9) | 335 (44.3) | 381 (48.5) | 1219 (48.2) |
| 8 – 10 members | 217 (21.9) | 182 (24.1) | 66 (8.4) | 465 (18.4) |
| 11+ members | 75 (7.6) | 68 (9.0) | 14 (1.8) | 157 (6.2) |
| Missing | 0 | 0 | 1 | 1 |
| Depression score (CDI) | ||||
| Mean (SD) | 4.5 (2.68) | 4.6 (2.87) | 4.8 (3.10) | 4.6 (2.87) |
| Median (IQR) | 4.0 (4.0) | 4.0 (4.3) | 4.0 (4.7) | 4.0 (4.0) |
| Depressed | 174 (17.6) | 157 (20.9) | 177 (22.6) | 508 (20.2) |
| Not Depressed | 813 (82.4) | 594 (79.1) | 606 (77.4) | 2013 (79.9) |
| Missina | 2 | 5 | 3 | 10 |
Note: Percentages are calculated based on non-missing responses.
Two AGYW had missing values for this exposure variable therefore the total column includes 2531 AGYW.
Table 2.
Characteristics by visit of AGYW who participated in the HPTN 068 conditional cash transfer trial and post-intervention visits in Agincourt, South Africa, which took place between March 2011 and April 2017
| Baseline (n=2533) | Follow-up visit 1 (n=2279) | Follow-up visit 2 (n=1870) | Follow-up visit 3 (n=899) | Post-intervention visit (n=1942) | |
|---|---|---|---|---|---|
|
|
|||||
| Household size | |||||
| Mean (SD) | 6.2 (2.61) | 6.3 (2.63) | 6.5 (2.68) | 6.5 (2.61) | 6.6 (2.70) |
| Min-Max | 2 – 20 | 2 – 23 | 1 – 22 | 2 – 21 | 1 – 23 |
| Missing | 1 | 0 | 8 | 9 | 0 |
| Age (years) | |||||
| Mean (SD) | 15.5 (1.66) | 16.6 (1.65) | 17.0 (1.49) | 17.5 (1.19) | 20.1 (1.42) |
| Min-Max | 13 – 20 | 13 – 22 | 14 – 22 | 16 – 23 | 17 – 26 |
| Father and adult male presence (exposure 1) | |||||
| Father present in household | 989 (39.1) | 873 (38.3) | 749 (40.1) | 384 (42.7) | 828 (42.6) |
| Father absent; adult males present in household | 756 (29.9) | 694 (30.5) | 580 (31.0) | 270 (30.0) | 620 (31.9) |
| Father and adult males absent from household | 786 (31.0) | 712 (31.2) | 541 (28.9) | 245 (27.3) | 493 (25.4) |
| Missing | 2 | 0 | 0 | 0 | 1 |
| Father’s living status (exposure 2) | |||||
| Father present in household | 989 (39.1) | 873 (38.3) | 749 (40.1) | 384 (42.7) | 828 (42.7) |
| Father lives elsewhere | 836 (33.0) | 736 (32.3) | 551 (29.5) | 228 (25.4) | 479 (24.7) |
| Father deceased | 706 (27.9) | 670 (29.4) | 570 (30.5) | 287 (31.9) | 634 (32.7) |
| Missing | 2 | 0 | 0 | 0 | 1 |
| Ever had vaginal sex | |||||
| Yes | 700 (27.7) | 949 (41.7) | 853 (45.7) | 478 (53.2) | 1508 (77.7) |
| No | 1827 (72.3) | 1327 (58.3) | 1015 (54.3) | 421 (46.8) | 431 (22.3) |
| Missing | 6 | 3 | 2 | 0 | 3 |
| Ever had anal sex | |||||
| Yes | 120 (4.8) | 115 (5.1) | 99 (5.3) | 39 (4.3) | 156 (8.1) |
| No | 2408 (95.3) | 2160 (95.0) | 1771 (94.7) | 859 (95.7) | 1783 (91.9) |
| Missing | 5 | 4 | 0 | 1 | 3 |
| Ever pregnant | |||||
| Yes | 311 (12.4) | 471 (20.7) | 437 (23.6) | 238 (26.6) | 895 (46.1) |
| No | 2193 (87.6) | 1803 (79.3) | 1412 (76.3) | 658 (73.4) | 1047 (53.9) |
| Missing | 29 | 5 | 21 | 3 | 0 |
Statistical Analysis
Frequency counts and summary statistics are used to characterize the study population. To quantify the association of the exposures with HIV and first pregnancy, incidence rates were calculated for each level of the exposure variables using Poisson regression with a log of person years as the offset term. Wald chi-square test p-values are presented along with Wald 95% confidence intervals (alpha=.05) for the adjusted incident rate ratios in each model. The exposure of father absence is time varying, therefore an AGYW can contribute to multiple risk sets over time. All models control for the age of the AGYW at each study visit. Rather than assuming the age distribution was a linear relationship, we utilized restricted quadratic splines with four knots (cut points) which allowed for non-linear relationships of age.(45) This allowed for a more parsimonious model, as an alternative to using indicator variables for each 1-year age group.
While AGYW report their age of first pregnancy, which could occur before baseline, we do not have household membership before baseline. For AGYW who acquired HIV before baseline, we do not know the age when she seroconverted. Therefore, to enable calculation of incidence since study start, AGYW who had experienced the outcomes at or before baseline were excluded from analysis. With both outcomes, once the AGYW experienced the event, her future study visits are not included in analysis. We also lagged the exposure one visit, to ensure temporality in the exposure-outcome association.
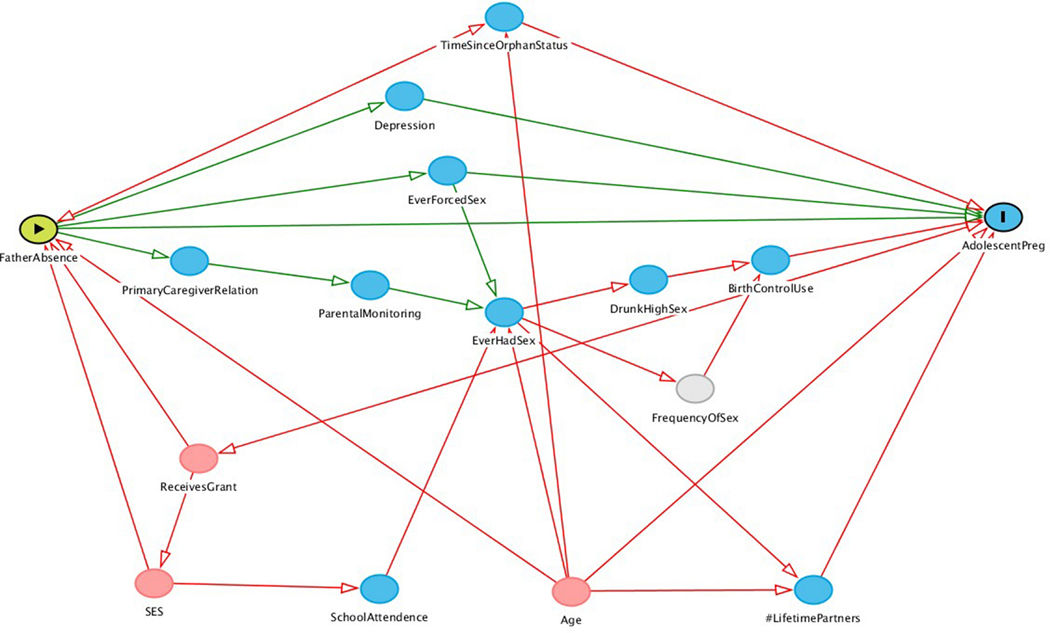
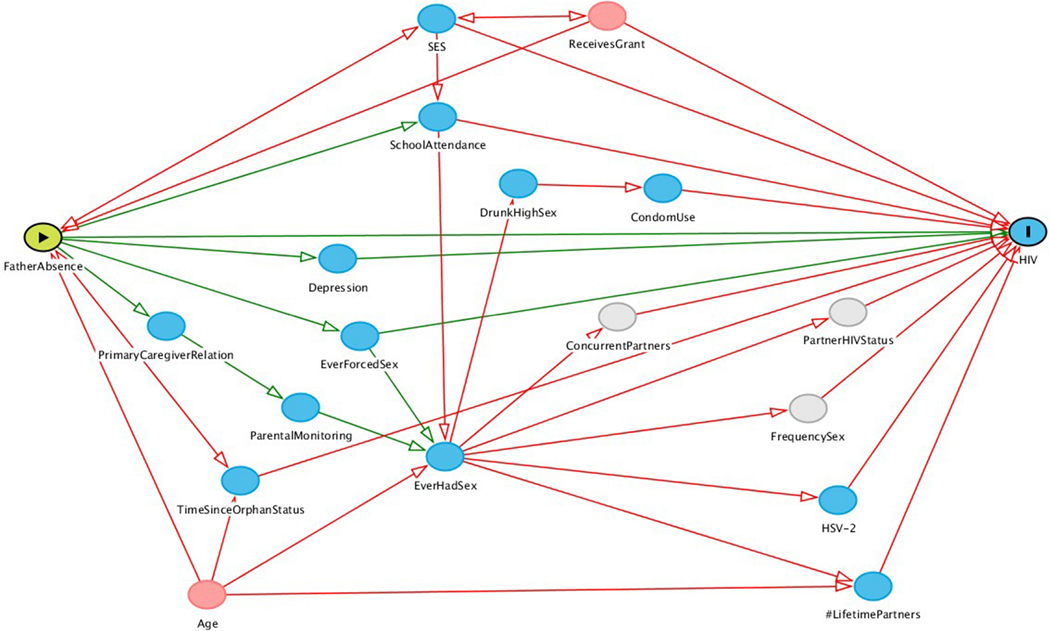
For both HIV and pregnancy models, directed acyclic graphs were produced with potential confounders and mediators informed by the literature (Appendix, Figures 3 and 4). The R package,“DAGgity“, was used to derive the minimally sufficient covariate adjustment sets; these included age, household expenditures, whether the AGYW’s household received at least one grant, and years since orphan status.(46,47) Then hierarchical backwards elimination was used to eliminate potential confounders that did not change the primary effect estimates by more than 10%.
The analysis for this paper was generated using SAS software version 9.4 and SAS/STAT software version 14.1, copyright © 2002–2012, SAS Institute Inc., Cary, NC, USA.
RESULTS
Study Participant Characteristics
Participant characteristics are provided in Table 1 by father and male presence categories and in Table 2 by study visit, to show differences in characteristics and sexual behaviors across the main exposure variable (at baseline) as well as over time.
At baseline (Table 1), when comparing AGYW with no males residing in the household to AGYW residing with their father, a higher proportion had a current boyfriend (35.4% vs. 29.6%), vaginal sex (30.8% vs. 25.9%), anal sex (6.1% vs. 3.7%), been pregnant (13% vs 11.4%), 2+ lifetime partners (15.6% vs. 10.3%), forced sex (10.6% vs. 9.1%), depression (22.6% with a CDI score >=7 vs. 17.6%), and were HIV positive (4% vs. 2.2%), while a lower proportion reported transactional sex (2.9% vs. 5.0%). A higher proportion of AGYW whose father was absent but other adult males were present in the household reported using a condom at last sex (64.1%) than AGYW whose father was present (57.6%) and AGYW with no adult male household members (57.3%). Significance testing was not done on the baseline attributes, as our main analysis, presented below, is a longitudinal multivariate analysis which considered confounders, of data collected between March 2011 and April 2017.
Table 2 summarizes the change in AGYW characteristics over the course of the study. At baseline, the mean age of the population was 15.5 years, while at the post-intervention visit the mean age was 20.1 years. Across all visits, the average household size was roughly 6.5 members. At the baseline visit, only 700 (27.7%) of AGYW reported having previously had vaginal sex, but this proportion grew to 77.7% by the post-intervention visit (n=1508).
HIV Analysis
Longitudinal analysis of HIV incidence included 2317 AGYW who were HIV-negative at baseline and had at least one follow-up visit with non-missing data on both HIV test results and father and male presence. From the full sample of 2533 AGYW, we excluded one participant who did not have corresponding household roster records, 4 participants who had unknown HIV status, and 81 who were HIV positive at baseline. Visits were excluded from the analysis for AGYW who lived in a household where the roster made it impossible to tell if an adult male also lived in the household, cases where household members were ≥ 18 years but sex was missing, or cases where age was missing and gender was male. This removed 1 AGYW, where this was the case at all of her study visits. It affected another 36 reducing their study visits to only one visit in the analysis dataset. A total of 129 AGYW were removed for having no follow up visits. After the above exclusions, from the baseline visit to the post-intervention visit, there were 197 incident HIV infections. Including the post-intervention visit, the crude HIV incidence rate for the 2317 AGYW included in the HIV analysis is 2.17 per 100 person years. Incident rate ratios were adjusted for potential confounders of the AGYW’s age, using restricted quadratic splines with 4 knots. Adjusted results are given in Table 3.
Table 3.
Incidence rate ratios of HIV based on father and adult male presence in the household among AGYW who participated in the HPTN 068 conditional cash transfer trial in Agincourt, South Africa, which took place between March 2011 and December 2015, including post-intervention visits conducted until April 2017
| Crude | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Cases | Person years | IR / 100PY1 | IRR | Wald 95% CI | aIRR2 | Wald 95% CI | Wald chi-square p-value | |
| All AGYW | 197 | 9093.6 | 2.17 | -- | -- | -- | -- | -- |
| Father’s living status (model 1) | ||||||||
| Father present in household (ref) | 76 | 3708.2 | 2.05 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father lives elsewhere | 62 | 2743.3 | 2.26 | 1.10 | (0.79, 1.54) | 1.16 | (0.83, 1.62) | 0.394 |
| Father deceased | 59 | 2642.1 | 2.23 | 1.09 | (0.78, 1.53) | 1.06 | (0.76, 1.50) | 0.719 |
| Father and adult male presence (model 2) | ||||||||
| Father present in household (ref) | 76 | 3708.2 | 2.05 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father absent; adult males present in household | 57 | 2812.7 | 2.03 | 0.99 | (0.70, 1.39) | 1.00 | (0.71, 1.41) | 0.995 |
| Father and adult males absent from household | 64 | 2572.7 | 2.49 | 1.21 | (0.87, 1.69) | 1.23 | (0.88, 1.71) | 0.224 |
| Father and adult male presence combined with father’s living status (model 3) | ||||||||
| Father present in household (ref) | 76 | 3708.2 | 2.05 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father lives elsewhere; adult males absent from household | 32 | 1366.9 | 2.34 | 1.14 | (0.76, 1.73) | 1.19 | (0.79, 1.80) | 0.400 |
| Father lives elsewhere, adult males present in household | 30 | 1376.3 | 2.18 | 1.06 | (0.70, 1.62) | 1.12 | (0.73, 1.71) | 0.600 |
| Father dead; adult males absent from household | 32 | 1205.8 | 2.65 | 1.29 | (0.86, 1.96) | 1.27 | (0.84, 1.91) | 0.263 |
| Father dead; adult males present in household | 27 | 1436.4 | 1.88 | 0.92 | (0.59, 1.42) | 0.90 | (0.58, 1.39) | 0.623 |
Father’s physical living status (exposure 1):
Adjusted results show that AGYW whose fathers lived elsewhere (aIRR=1.16, Wald 95% CI=0.83 – 1.62, Wald chi-square p=0.394) had the highest adjusted incidence rate ratio, and were 1.16 times as likely to become infected with HIV than AGYW whose fathers were present in the household.
Father and adult male presence (exposure 2):
Adjusted results show that AGYW whose fathers and other adult males were absent from the household were 1.23 times as likely to have experienced HIV infection (aIRR=1.23, Wald 95% CI=0.88 – 1.71, Wald chi-square p=0.224) than AGYW whose father was present in the household. AGYW whose fathers were absent but other adult males were present had the same adjusted rate ratio (aIRR=1.00, Wald 95% CI=0.71 – 1.41, Wald chi-square p=0.995) as AGYW whose fathers were present in the household.
Father and adult male presence combined with father’s living status (exposure 3):
Combining the main two exposure variables summarized above permits distinguishing between households where fathers were absent due to living elsewhere or death. AGYW whose fathers were dead and adult males were absent from the household had the highest adjusted incident rate ratio, and were 1.27 times as likely to become HIV infected (aIRR=1.27, Wald 95% CI=0.84 – 1.91, Wald chi-square p=0.263) than AGYW whose fathers were present in the household. The most protected group of AGYW were AGYW whose fathers were dead, but adult males were present in the household, they were 0.90 times as likely than AGYW whose fathers were present to experience HIV infection (aIRR=0.90, Wald 95% CI=0.58 – 1.39, Wald chi-square p=0.623). For AGYW whose fathers were alive but living elsewhere, those who had adult males present were 1.12 times as likely (aIRR=1.12, Wald 95% CI=0.73 – 1.71, Wald chi-square p=0.600) and those who had adult males absent from the household were 1.19 times (aIRR=1.19, Wald 95% CI=0.79 – 1.80, Wald chi-square p=0.400) as likely to experience HIV infection as compared to AGYW whose fathers were present. There was no difference when HIV incidence was stratified by intervention arm. Results of this stratification can be found in Table 6 in the Appendix.
Pregnancy Analysis
Of 2533 participants, 1086 AGYW reported having ever been pregnant, but only 740 eligible pregnancies were included in the final incidence analysis. Five participants who had missing pregnancy status, one who was missing roster data at all visits, and 313 who reported having been pregnant before baseline were excluded from the analysis dataset. Visits were excluded from the analysis for AGYW who lived in a household where partial roster data made it impossible to tell if an adult male also lived in the household. This removed an additional participant where this was the case at all of her study visits. It affected another 34 reducing their study visits to only one visit in the analysis dataset. Therefore, a total of 119 who did not have at least two study visits were also deleted from the analysis dataset. Therefore, 2094 AGYW with 740 first pregnancies were in the final analysis dataset. The crude pregnancy incidence rate for all AGYW is 10.55 per 100 person years. Incident rate ratios were adjusted for potential confounders of whether the household received at least one grant and the AGYW’s age, using restricted quadratic splines with 4 knots. Adjusted results are shown in Table 4.
Table 4.
Incidence rate ratios of pregnancy based on father and adult male presence in household among AGYW who participated in the HPTN 068 conditional cash transfer trial in Agincourt, South Africa, which took place between March 2011 and December 2015, including post-intervention visits conducted until April 2017
| Crude | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Cases | Person years | IR/100PY1 | IRR | Wald 95% CI | aIR R2 | Wald 95% CI | Wald chi-square p-value | |
| All AGYW | 740 | 7012.3 | 10.55 | -- | -- | -- | -- | -- |
| Father’s living status (model 1) | ||||||||
| Father present in household (ref) | 285 | 2867.2 | 9.94 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father lives elsewhere | 232 | 2124.5 | 10.92 | 1.10 | (0.92, 1.31) | 1.12 | (0.95, 1.34) | 0.286 |
| Father deceased | 223 | 2020.6 | 11.04 | 1.11 | (0.93, 1.32) | 1.09 | (0.92, 1.30) | 0.241 |
| Father and adult male presence (model 2) | ||||||||
| Father present in household (ref) | 285 | 2867.2 | 9.94 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father absent from household; adult males present in household | 212 | 2205.1 | 9.61 | 0.97 | (0.81, 1.16) | 0.96 | (0.81, 1.15) | 0.713 |
| Father and adult males absent from household | 243 | 1940.0 | 12.53 | 1.26 | (1.06, 1.50) | 1.27 | (1.07, 1.51) | <0.01 |
| Father and adult male presence combined with father’s living status (model 3) | ||||||||
| Father present in household (ref) | 285 | 2867.2 | 9.26 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father lives elsewhere; adult males absent from household | 125 | 1045.4 | 10.77 | 1.16 | (0.86, 1.58) | 1.23 | (0.99, 1.52) | 0.056 |
| Father lives elsewhere, adult males present in household | 107 | 1079.0 | 10.22 | 1.10 | (0.81, 1.50) | 1.02 | (0.82, 1.27) | 0.087 |
| Father dead; adult males absent from household | 118 | 894.5 | 12.45 | 1.34 | (0.99, 1.82) | 1.30 | (1.05, 1.61) | 0.016 |
| Father dead; adult males present in household | 105 | 1126.1 | 9.34 | 1.01 | (0.74, 1.38) | 0.92 | (0.74, 1.15) | 0.462 |
Father’s living status (exposure 1):
The crude incidence rate of first pregnancy was highest among AGYW whose fathers were deceased (11.04 per 100 person-years), and lowest for AGYW whose fathers were present in the household (9.94 per 100 person-years). AGYW whose fathers lived elsewhere were 1.12 times as likely to experience first pregnancy during the study period as compared to AGYW whose fathers were present in the household in adjusted models (aIRR=1.12, Wald 95% CI=0.95 – 1.34, Wald chi-square p=0.286). AGYW whose fathers were deceased had 1.09 times the incidence rate during the study period as compared to AGYW whose fathers were present in the household (aIRR=1.09, Wald 95% CI=0.92 – 1.30, Wald chi-square p=0.241).
Father and adult male presence (exposure 2):
The crude incidence was highest among AGYW whose fathers and other adult males were absent from the household (12.53 per 100 person-years) and lowest among AGYW whose fathers were absent but other adult males were present in the household (9.61 per 100 person-years). AGYW who lived in households where both fathers and other adult males were absent were 1.27 times as likely to experience first pregnancy (aIRR=1.27, Wald 95% CI=1.07 – 1.51, p<0.01) during the study period as compared to AGYW whose fathers were present in the household. This finding is statistically significant. AGYW whose fathers were absent, but other adult males were present in the household, had 0.96 times the incidence of first pregnancy during the study period as compared to AGYW whose fathers lived in the household (aIRR=0.96, Wald 95% CI=0.81 – 1.15, Wald chi-square p=0.713).
Father and adult male presence combined with father’s living status (exposure 3):
By combining the two main exposure variables to a 5-category variable, we are able to distinguish incidence rates of first pregnancy by whether the father was dead or alive and living elsewhere. AGYW whose fathers were dead and adult males were absent from the household had the highest incidence rate of first pregnancy (crude IR=12.45 per 100 person-years) and were 1.30 times as likely to experience adolescent pregnancy (aIRR=1.30, Wald 95% CI=1.05 – 1.61, Wald chi-square p=0.016) as AGYW whose father lived in the household (statistically significant), followed by AGYW whose fathers lived elsewhere and adult males were absent from the household (crude IR=10.77, aIRR=1.23, Wald 95% CI=0.99 – 1.52, Wald chi-square p=0.056). AGYW whose fathers were dead and adult males present in the household were the least likely to experience their first pregnancy during the study period (crude IR=9.34, aIRR=0.92, Wald 95% CI=0.74 – 1.15, Wald chi-square p=0.462) followed by AGYW whose fathers lived elsewhere and adult males were present in the household (crude IR=10.22, aIRR=1.02, Wald 95% CI=0.82 – 1.27, Wald chi-square p=0.087).
There was no difference when incidence of first pregnancy was stratified by intervention arm. Results can be found in Table 7 of the Appendix.
DISCUSSION
This study aimed to distinguish whether AGYW are at higher risk of sexually acquiring HIV or experiencing their first pregnancy depending on father and other adult male household membership. Our analysis allowed us to distinguish risk based on whether the AGYW resided in households with their biological fathers or not, distinguishing between fathers that were alive and dead, as well as whether other adult males lived in a father-absent household or not.
We hypothesized, based on our literature review, that AGYW whose fathers were absent would have a higher incidence of both HIV acquisition and first pregnancy. Even among a school going population that was likely less vulnerable for pregnancy risk than the population of AGYW at large, we found that AGYW who had no adult males residing in the household were most at risk for incidence of first pregnancy. The effects were similar for HIV incidence in these groups of AGYW, however not statistically significant. Additionally, the risk of HIV and pregnancy incidence for AGYW with adult males living in father absent households is similar to AGYW living with their fathers (aIRRs close to the null). Confidence intervals around the adjusted rate ratios for HIV incidence all included the null, indicating that the incidence rates for HIV could be interpreted to be similar across categories of father absence. With aIRRs close to the null, suggesting incidence is similar to that of the referent category, we would not expect the confidence intervals to exclude the null. Ideally studies aiming to quantify HIV incidence should have a larger sample and more follow-up time that covers the span of adolescence into young adulthood, however If there is no difference across the father and male absence categories then such a study may also not find statistical significance.
This was the first longitudinal study to our knowledge that examined the effect of father and adult male household membership on HIV acquisition. One study using longitudinal data in Cape Town found that for every increase in the number of times a father transitioned in or out of the household the odds of sexual initiation by age 17 increased by 23% (Wald chi-square p<0.05) for black African female adolescents; however, this study did not consider HIV or pregnancy outcomes.(48) Another study using the same urban Cape Town data with a cox proportional hazard model found that father absence did not significantly predict first pregnancy; black African young women who had never lived with their father had a relative hazard of 0.90 (Wald chi-square p=0.353), and those who lived with their father but not their entire life had a relative hazard of 1.02 (Wald chi-square p=0.935). (49) Our study is situated in a rural area where labor migration is very common, therefore we would expect some differences compared to an urban population.
In 2016 in South Africa, at least 53% of children under 18 years lived in a father absent home, yet the father was alive; and at least 10% lived in a father absent home due to the father being deceased.(50) In Mpumalanga, 60% of black African children between the ages 0 – 9 years do not live with their fathers.(51) Father absence has been found to increase risk of children being exposed to neglect and has been indirectly linked to sexual abuse due to reduced supervision and lack of boundary setting for children.(37,51,52) There is a long history in South Africa of children, especially those living in poverty, not being continuously parented by either one or both of their biological parents.(53,54)
Understanding the mechanism whereby father absence, and adult male absence in general, increases pregnancy risk may suggest how we can best intervene to delay pregnancies in AGYW, whether it be through changes in policy, funding, and/or programs. Parent-child communication about sexuality has been identified as a protective factor for a range of sexual behaviors, including a delayed sexual debut, particularly for females.(55) Many first pregnancies among AGYW in the study area are unintended, and AGYW say that a contributor to this is poor communication with their parents with discussions of menarche and contraceptives being avoided.(56)
Although we were unable to explore pathways in this study, potential mechanisms that may increase incidence of first pregnancy, and therefore should be explored in future analysis include: lack of paternal communication, lack of paternal monitoring and paternal care, decreased socioeconomic status due to lack of income from the father, and increased exposure to sexual violence for AGYW who do not have a father or another adult male living in the home. Adult males may act as guardians or a resource for AGYW who don’t know who else to turn to and could potentially provide more communication and monitoring than is available to AGYWs who lack any adult male figures in the household.
If future research confirms that lack of paternal communication and monitoring is a pathway through which father and adult male presence is associated with HIV and first pregnancy, then family based interventions could focus on helping remote fathers engage with their daughters using positive parenting techniques and approaches that educate daughters about the risks of early pregnancies and HIV.
Another possibility for increased pregnancy incidence among AGYW with no adult male presence in the home could be an increase in sexual violence. At baseline, among AGYW who had no adult male presence in the household, 10.6%, had ever experienced forced sex, about 1 to 2 percent higher than other AGYW. In a pooled analysis across 13 countries in Sub-Saharan Africa, Kidman and Palermo found that paternal absence, but not maternal absence, was significantly associated with greater sexual violence among adolescent girls 15 to 17 years old (OR 1.28; Wald chi-square p<=0.01), though rates varied greatly by country and year in non-pooled analysis.(26) Kidman and Palermo suggest that AGYW with an absent father may exhibit greater dependence on other males, leaving them more vulnerable to abuse, or they may seek out unhealthy relationships with older males.(26) If future research does find that paternal and other adult male presence in the household increases sexual violence, then the many active interventions in South Africa dealing with sexual violence could target homes with no father or adult male present.
STRENGTHS AND LIMITATIONS
Strengths of this study, which utilizes the HPTN 068 cohort, include the use of longitudinal data which provide the ability to determine incidence rates and the ability to disaggregate orphan status and household membership for the AGYW women over the course of the study. With the goal of reducing social desirability bias, survey data were collected using Audio Computer Assisted Self-Interview (ACASI) with the AGYW participants. Our study findings could be generalizable to other school going populations in poor rural African settings. As well, we used robust methods in our data analysis, including Poisson and Log-binomial methods and the models controlled for potential confounding.
There are several limitations to this study data and analysis. One important limitation is that this study required AGYW to be enrolled in school at study entry, therefore girls who dropped out of school before the study began are not included which may be associated with the exposure and outcome. Another limitation is that study participants were required to have documentation that enabled them to open a bank account, and so those without were not included in the study.
It is possible that some events may have been misclassified to the wrong father absence category, due to changes in household membership between study visits. However, a sensitivity analysis was done to determine the stability of an AGYWs father absence category over time, and due to relatively high stability in the households (around 85% from visit to visit remained in the same father absence category) we believe any misclassification would be very low (Appendix Table 5).
FUTURE RESEARCH
Further mixed methods analyses could explore potential factors and pathways that increase the risk of pregnancy among AGYW living in homes without adult men, including poor parental communication, low parental monitoring, increased sexual violence, or lower socio-economic status. As well, among those AGYW living in father-absent households with other adult males present, it would be helpful to understand what type of relationship these males have to the AGYW, and if the type of relationship has an effect on sexual and reproductive health outcomes. Such work could help identify which of the many current interventions could be tailored to reduce the risk of adolescent pregnancy in cases of father absence, especially if no other adult males co-reside in the household.
CONCLUSION
Regarding household membership of AGYW, in this study, having a father or male adult living in the home, appears to offer protection to AGYW against the risk of early pregnancy. It does not appear to be relevant why fathers are absent from the home—death or for another reason—what matters is that fathers not living in the home puts AGYW at higher risk for pregnancy.
Prevention programs among AGYW in high HIV prevalence areas often focus on targeting orphans as a key population for HIV and pregnancy prevention efforts. While this is only one study conducted among a school going population of AGYW in rural South Africa, it does raise important considerations for the field with regard to assumptions about orphaning and HIV/pregnancy risk. Our findings suggest that having other adult males residing in a father-absent household seems to provide some protection against pregnancy compared to AGYW living in homes with no father and no other adult males. AGYW who do not have any adult male residing in the home is a new key population of AGYW to consider for prevention programming.
Our hope is that our study informs next steps for future research, which may lead to better understanding of the mechanisms that drive HIV acquisition and pregnancy among AGYW. Future programs and research should consider the role of parents and caregivers in adolescent’s lives more broadly, in particular the role of key family members in raising young people, and how this larger context may influence HIV and pregnancy risk for AGYW.
ACKNOWELDGEMENTS
This research would not have been possible without the mentorship of the HPTN068 study team, particularly Amanda Selin who served as project manager for the HPTN068 study, as well as the contributions from the team at MRC/Wits-Agincourt Research Unit and Health and Socio-Demographic Surveillance System. Overall support for the HIV Prevention Trials Network (HPTN) was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health (NIMH) and the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) under Award Numbers UM1AI068619 (HPTN Leadership and Operations Center), UM1AI068617 (HPTN Statistical and Data Management Center), and UM1AI068613 (HPTN Laboratory Center). The study was also funded under Award Number 5R01MH087118-02 and R24 HD050924 to the Carolina Population Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The MRC/Wits-Agincourt Research Unit and Health and Socio-Demographic Surveillance System is supported by the School of Public Health, University of the Witwatersrand, and Medical Research Council, South Africa, and the UK Wellcome Trust (grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; and 085477/B/08/Z)
Funding
Appendix
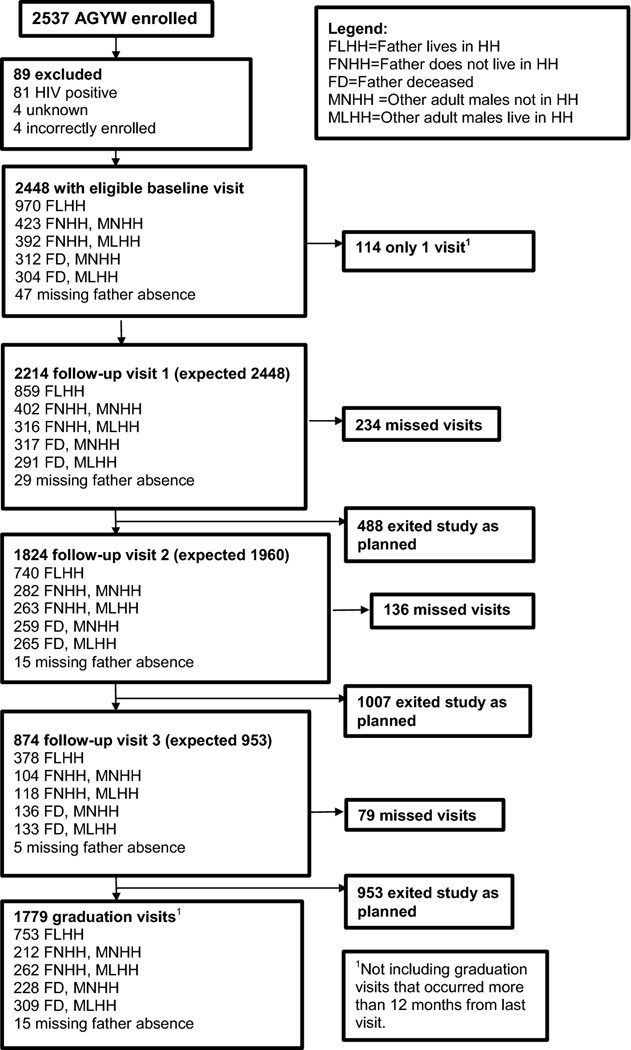
Figure 1.
Consort diagram for HIV outcome by 5-category father and adult male presence combined with father’s living status (exposure 3)
Figure 2.
Consort diagram for pregnancy outcome by the 5-category father and adult male presence combined with father’s living status (exposure 3)
Figure 3.
Directed acyclic graph depicting the hypothesized relationship between father presence and pregnancy
Figure 4.
Directed acyclic graph depicting the hypothesized relationship between father presence and HIV incidence
Table 5.
Stability of 5-category father presence (exposure 3) from visit to visit.
| Father Presence (5 category exposure) | 1st to 2nd visit | 2nd to 3rd visit | 3rd to 4th visit | 4th to 5th visit |
|---|---|---|---|---|
| Category remained stable | 82% | 83% | 85% | 84% |
| Category changed | 18% | 17% | 15% | 16% |
Note: We measured stability between non-missing study visits.
Table 6.
Stratified incidence rate ratios of HIV based on father and adult male presence among AGYW who participated in the HPTN 068 conditional cash transfer trial in Agincourt, South Africa, which took place between March 2011 and April 2017.
| Crude | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Cases | Person Years | IR / 100PY1 | IRR | Wald 95% CI | aIRR2 | Wald 95% CI | Wald chi-square p-value | |
| All AGYW | 197 | 9093.6 | 217 | -- | -- | -- | -- | -- |
| Father and adult male presence combined with father’s living status | ||||||||
| Control Arm | 94 | 4407.9 | 2.13 | -- | -- | -- | -- | -- |
| Father present in household (ref) | 31 | 1824.1 | 1.70 | (ref) | (ref) | (ref) | (ref) | |
| Father lives elsewhere; adult males absent from household | 20 | 658.5 | 3.04 | 1.79 | (1.02, 3.14) | 1.87 | (1.07, 3.28) | 0.043 |
| Father lives elsewhere, adult males present in household | 17 | 679.8 | 2.50 | 1.47 | (0.81, 2.66) | 1.55 | (0.86,2.81) | 0.201 |
| Father dead; adult males absent from household | 9 | 584.2 | 1.54 | 0.91 | (0.43, 1.90) | 0.88 | (0.42, 1.85) | 0.795 |
| Father dead; adult males present in household | 17 | 661.3 | 2.57 | 1.51 | (0.84, 2.73) | 1.48 | (0.82, 2.67) | 0.170 |
| Intervention Arm | 103 | 4685.7 | 2.20 | -- | -- | -- | -- | -- |
| Father resides in household | 45 | 1884.1 | 2.39 | 1.41 | (0.89, 2.22) | 1.43 | (0.90, 2.26) | 0.145 |
| Father lives elsewhere; adult males absent from household | 12 | 708.4 | 1.69 | 1.00 | (0.51, 1.94) | 1.06 | (0.54, 2.06) | 0.994 |
| Father lives elsewhere, adult males present in household | 13 | 696.5 | 1.87 | 1.10 | (0.57, 2.10) | 1.17 | (0.61, 2.24) | 0.777 |
| Father dead; adult males absent from household | 23 | 621.5 | 3.70 | 2.18 | (1.27, 3.73) | 2.17 | (1.26, 3.72) | 0.005 |
| Father dead; adult males present in household | 10 | 775.1 | 1.29 | 0.76 | (0.37, 1.55) | 0.75 | (0.37, 1.53) | 0.449 |
Table 7.
Stratified incidence rate ratios of pregnancy based on father and adult male presence among young women who participated in the HPTN 068 conditional cash transfer trial in Agincourt, South Africa, which took place between March 2011 and April 2017.
| Crude | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Cases | Person Years | IR / 100PY1 | IRR | Wald 95% CI | aIRR2 | Wald 95% CI | Wald chi-square p-value | |
| All AGYW | 740 | 7012.3 | 10.55 | -- | -- | -- | -- | -- |
| Father and adult male presence combined with father’s living status | ||||||||
| Control Arm | 372 | 3354.1 | 11.09 | -- | -- | -- | -- | -- |
| Father present in household (ref) | 147 | 1377.4 | 10.67 | (ref) | (ref) | (ref) | (ref) | (ref) |
| Father lives elsewhere; adult males absent from household | 66 | 497.5 | 13.27 | 1.24 | (0.93, 1.66) | 1.27 | (0.95, 1.70) | 0.102 |
| Father lives elsewhere, adult males present in household | 50 | 521.4 | 9.59 | 0.90 | (0.65, 1.24) | 0.93 | (0.67, 1.28) | 0.635 |
| Father dead; adult males absent from household | 59 | 420.7 | 14.02 | 1.31 | (0.97, 1.78) | 1.30 | (0.96, 1.76) | 0.085 |
| Father dead; adult males present in household | 50 | 537 | 9.31 | 0.87 | (0.63, 1.20) | 0.85 | (0.62, 1.17) | 0.324 |
| Intervention Arm | 368 | 3658.2 | 10.06 | -- | -- | -- | -- | -- |
| Father present in household (ref) | 138 | 1489.8 | 9.26 | 0.87 | (0.69, 1.09) | 0.88 | (0.7, 1.11) | 0.279 |
| Father lives elsewhere; adult males absent from household | 59 | 547.9 | 10.77 | 1.01 | (0.75, 1.36) | 1.04 | (0.77, 1.40) | 0.806 |
| Father lives elsewhere, adult males present in household | 57 | 557.6 | 10.22 | 0.96 | (0.71, 1.30) | 0.98 | (0.72, 1.34) | 0.914 |
| Father dead; adult males absent from household | 59 | 473.8 | 12.45 | 1.17 | (0.86, 1.58) | 1.15 | (0.85, 1.55) | 0.368 |
| Father dead; adult males present in household | 55 | 589 | 9.34 | 0.87 | (0.64, 1.19) | 0.87 | (0.64, 1.19) | 0.340 |
Footnotes
DECLARATION
Conflicts of Interest
The authors declare that they have no competing interests.
Ethics Approval
Institutional Review Board approval for this secondary data analysis was obtained from the University of North Carolina at Chapel Hill; the parent study received approval from University of North Carolina at Chapel Hill as well as the University of the Witwatersrand in South Africa and the Mpumalanga Province’s Research and Ethics Committee.
Informed Consent
Each young woman and her parent or guardian provided written informed consent for participation in the parent study at the home visit. Written assent was obtained for young women younger than 18 years. Consent and assent forms were available in English and Shangaan.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.UNICEF. Global and Regional Trends July 2019: The AIDS epidemic continues to take a staggering toll, but progress is possible. Vol. 2019. 2019. [Google Scholar]
- 2.Adolescent HIV Prevention July 2018: Turning the tide against AIDS will require more concentrated focus on adolescents and young people.
- 3.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS Lond Engl. 2005. September 23;19(14):1525–34. [DOI] [PubMed] [Google Scholar]
- 4.Karim QA, Kharsany AB, Frohlich JA, Werner L, Mashego M, Mlotshwa M, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2010;40(4):922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. The Gap Report [Internet]. 2014. Available from: https://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf
- 6.UNAIDS. UNAIDS data [Internet]. 2020. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica
- 7.Health SAND of. The 2012 National Antenatal Sentinel HIV and Herpes Simplex type-2 prevalence Survey. 2014; Available from: https://www.health-e.org.za/wp-content/uploads/2014/05/ASHIVHerp_Report2014_22May2014.pdf
- 8.Allergy NI of, Diseases (NIAID) I. Risk of Acquiring HIV Increases During and After Pregnancy, Research Suggests. 2018;
- 9.Rehle T, Shisana O, Pillay V, Zuma K, Puren A, Parker W. National HIV incidence measures-new insights into the South African epidemic. S Afr Med J. 2007;97(3):194–199. [PubMed] [Google Scholar]
- 10.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. The Lancet. 2005;366(9492):1182–8. [DOI] [PubMed] [Google Scholar]
- 11.Mmbaga EJ, Leyna GH, Mnyika KS, Klepp KI. Comparison of HIV-1 prevalence and risk factors between pregnant, non-pregnant, all women and the general population in Tanzania: implications for second-generation surveillance. Int J STD AIDS. 2009. July;20(7):483–8. [DOI] [PubMed] [Google Scholar]
- 12.Maman S, Moodley D, Groves AK. Defining male support during and after pregnancy from the perspective of HIV-positive and HIV-negative women in Durban, South Africa. 2011;56:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timaeus IM, Moultrie TA. Teenage Childbearing and Educational Attainment in South Africa. Stud Fam Plann. 2015. June;46(2):143–60. [DOI] [PubMed] [Google Scholar]
- 14.Stoner MC, Pettifor A, Edwards JK, Aiello AE, Halpern CT, Julien A, et al. The effect of school attendance and school dropout on incident HIV and HSV-2 among young women in rural South Africa enrolled in HPTN 068. Aids. 2017;31(15):2127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogaert AF. Age at puberty and father absence in a national probability sample. J Adolesc. 2005;28(4):541–6. [DOI] [PubMed] [Google Scholar]
- 16.Chisholm JS, Quinlivan JA, Petersen RW, Coall DA. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum Nat. 2005;16(3):233–65. [DOI] [PubMed] [Google Scholar]
- 17.Culpin I, Heron J, Araya R, Melotti R, Lewis G, Joinson C . Father absence and timing of menarche in adolescent girls from a UK cohort: The mediating role of maternal depression and major financial problems. J Adolesc. 2014;37(3):291–301. [DOI] [PubMed] [Google Scholar]
- 18.Doughty D, Rodgers JL. Behavior genetic modeling of menarche in US females. In Springer; 2000. p. 169–81. (Genetic influences on human fertility and sexuality). [Google Scholar]
- 19.Hoier S. Father absence and age at menarche. Hum Nat. 2003;14(3):209–33. [DOI] [PubMed] [Google Scholar]
- 20.Maestripieri D, Roney JR, DeBias N, Durante KM, Spaepen GM. Father absence, menarche and interest in infants among adolescent girls. Dev Sci. 2004;7(5):560–6. [DOI] [PubMed] [Google Scholar]
- 21.Matchock RL, Susman EJ. Family composition and menarcheal age: Anti-inbreeding strategies. Am J Hum Biol. 2006;18(4):481–91. [DOI] [PubMed] [Google Scholar]
- 22.Moffitt TE, Caspi A, Belsky J, Silva PA. Childhood experience and the onset of menarche: A test of a sociobiological model. Child Dev. 1992;63(1):47–58. [DOI] [PubMed] [Google Scholar]
- 23.Pesonen A-K, Räikkönen K, Heinonen K, Kajantie E, Forsén T, Eriksson JG. Reproductive traits following a parent–child separation trauma during childhood: a natural experiment during World War II. Am J Hum Biol. 2008;20(3):345–51. [DOI] [PubMed] [Google Scholar]
- 24.Quinlan RJ. Father absence, parental care, and female reproductive development. Evol Hum Behav. 2003;24(6):376–90. [Google Scholar]
- 25.Surbey MK . Family composition, stress, and the timing of human menarche. 1990;
- 26.Kidman R, Palermo T. The relationship between parental presence and child sexual violence: Evidence from thirteen countries in sub-Saharan Africa. Child Abuse Negl. 2016. January;51:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis BJ, Bates JE, Dodge KA, Fergusson DM, Horwood LJ, Pettit GS, et al. Does father absence place daughters at special risk for early sexual activity and teenage pregnancy? Child Dev. 2003;74(3):801–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvergne A, Faurie C, Raymond M. Developmental plasticity of human reproductive development: effects of early family environment in modern-day France. Physiol Behav. 2008;95(5):625–32. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard P, Snopkowski K, Sear R. Father absence and reproduction-related outcomes in Malaysia, a transitional fertility population. Hum Nat. 2014;25(2):213–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiernan KE, Hobcraft J. Parental divorce during childhood: age at first intercourse, partnership and parenthood. Popul Stud. 1997;51(1):41–55. [Google Scholar]
- 31.Noll JG, Shenk CE, Putnam KT. Childhood sexual abuse and adolescent pregnancy: a meta-analytic update. J Pediatr Psychol. 2009. May;34(4):366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waynforth D. Life-history theory, chronic childhood illness and the timing of first reproduction in a British birth cohort. ProceedingsBiological Sci. 2012. August 7;279(1740):2998–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehler N, Chisholm JS. Early psychosocial stress predicts extra-pair copulations. Evol Psychol. 2007;5(1):147470490700500100. [Google Scholar]
- 34.Padi T, Nduna M, Khunou G, Kholopane P. Defining absent, unknown and undisclosed fathers in South Africa. South Afr Rev Sociol. 2014;45(2):44–59. [Google Scholar]
- 35.Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Dev. 1991;62(4):647–70. [DOI] [PubMed] [Google Scholar]
- 36.Chisholm JS. Death, hope and sex: Steps to an evolutionary ecology of mind and morality. Cambridge University Press; 1999. [Google Scholar]
- 37.Draper P, Harpending H. Father absence and reproductive strategy: An evolutionary perspective. J Anthropol Res. 1982;255–73.
- 38.Draper P, Harpending H. A sociobiological perspective on the development of human reproductive strategies. In: Sociobiological perspectives on human development. Springer; 1988. p. 340–372. [Google Scholar]
- 39.Collinson MA, White MJ, Mcgarvey ST, Afolabi SA, Clark SJ, Kahn K, et al. SPECIAL ISSUE: EPIDEMIOLOGICAL TRANSITIONS BEYOND OMRAN’S THEORY Migration and the epidemiological transition: insights from the Agincourt sub-district of northeast South Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collinson MA. Striving against adversity: the dynamics of migration, health and poverty in rural South Africa. 2010;3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20531981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettifor A, MacPhail C, Selin A, Gomez-Olive FX, Rosenberg M, Wagner RG, et al. HPTN 068: A Randomized Control Trial of a Conditional Cash Transfer to Reduce HIV Infection in Young Women in South Africa-Study Design and Baseline Results. AIDS Behav. 2016. February 18; [DOI] [PMC free article] [PubMed]
- 42.Richter L, Chikovore J, Makusha T. The status of fatherhood and fathering in South Africa. Child Educ. 2010;86(6):360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettifor A, MacPhail C, Hughes JP, Selin A, Wang J, Gómez-Olivé FX, et al. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health. 2016;4(12):e978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cluver L, Gardner F, Operario D. Psychological distress amongst AIDS-orphaned children in urban South Africa. J Child Psychol Psychiatry. 2007. August;48(8):755–63. [DOI] [PubMed] [Google Scholar]
- 45.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, EJJ J. Splines for trend analysis and continuous confounder control. Epidemiol Camb Mass. 2011. November;22(6):874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiol Camb Mass. 2011. Sep;22(5):745. [DOI] [PubMed] [Google Scholar]
- 47.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol. 2016;45(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 48.Marteleto LJ, Cavanagh S, Prickett K, Clark S. Instability in Parent-Child Coresidence and Adolescent Development in Urban South Africa. Stud Fam Plann. 2016. March;47(1):19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson KG. Father Absence, Childhood Stress, and Reproductive Maturation in South Africa. Hum Nat Hawthorne N. 2015. December;26(4):401–25. [DOI] [PubMed] [Google Scholar]
- 50.ICF. The DHS Program STATcompiler. Funded by USAID. [Internet]. 2012. [cited 2020 Oct 3]. Available from: http://www.statcompiler.com
- 51.Makiwane M, Makoae M, Botsis H, Vawda M. A baseline study on families in Mpumalanga. Hum Sci Res Counc Pretoria Hum Soc Dev Popul Health Health Syst Innov CeSTii Viewed. 2015;2.
- 52.Artz L, Ward CL, Leoschut L, Kassanjee R, Burton P. The prevalence of child sexual abuse in South Africa: the Optimus Study South Africa. SAMJ South Afr Med J. 2018;108(10):791–792. [DOI] [PubMed] [Google Scholar]
- 53.Department of Social Development SA. Green Paper on Families: Promoting Family Life and Strengthening Families in South Africa. Gov Gaz. 2011;Gazette No. 34692 – Notice 756(10/23/2016). [Google Scholar]
- 54.Giese S, Meintjes H, Croke R, Chamberlain R. Health and social services to address the needs of orphans and other vulnerable children in the context of HIV/AIDS. Chapter. 2003;8:213. [Google Scholar]
- 55.Markham CM, Lormand D, Gloppen KM, Peskin MF, Flores B, Low B, et al. Connectedness as a predictor of sexual and reproductive health outcomes for youth. J Adolesc Health. 2010;46(3):S23–41. [DOI] [PubMed] [Google Scholar]
- 56.Margherio C. Centering female agency while investigating contraceptive use: a case study in Agincourt, South Africa. Int J Equity Health. 2019;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]