The original cohort
For more than three decades, extensive translational research has identified strong associations between fetal growth and development and fetal programming of health and diseases in later life [Developmental Origins of Health and Disease (DOHAD) hypothesis].1,2 More recently, the focus of DOHAD research has shifted to the periconceptional period, defined as the time window of 14 weeks before up to 10 weeks after conception, thereby covering the vulnerable processes of gametogenesis, embryogenesis and initiation of placentation. During this period, numerous molecular and biological processes are initiated, such as epigenetic modification (e.g. genome-wide methylation) and also unique transcriptional and translational activities. The periconceptional period as such represents a critical and vulnerable time window for a diversity of exposures with potentially large effects during the entire prenatal as well as postnatal life course. So far significant associations have been detected between maternal and paternal periconceptional exposures, pregnancy outcome and non-communicable diseases over the entire life course and in later life.3–6
The Rotterdam Periconceptional Cohort (Predict study) was initiated because most birth cohorts start enrolment and data collection during the second half of pregnancy or at birth, thereby ignoring the periconceptional window and the first half of pregnancy.7 Within this cohort we have designed serial patient consultations, including ultrasound measurements starting in the embryonic period. Morphological parameters as well as early placentation are being studied using innovative virtual reality imaging techniques. The Predict study is designed as a tertiary hospital-based, prospective open birth cohort study, with a focus on three research areas: (i) determinants of maternal and paternal periconceptional health; (ii) reproductive performance, pregnancy course and outcome; and (iii) underlying molecular biological mechanisms, such as 1-carbon metabolism and epigenetics and also cardiovascular and inflammatory mechanisms. The strength of this open and ongoing cohort lies in the fact that the various outcomes within these three research areas are of equal significance for the periconceptional period. Hypothesis-driven specific outcome parameters are defined before initiation of new studies within the cohort. Only after approval of a new study proposal can the particular study be initiated within the cohort. The achieved output very much depends on the topic of (inter)national scientific research calls, and thus funding.
The study started in November 2010 (after a pilot phase initiated in December 2009) and its ongoing cohort design serves as an infrastructure for embedding other subcohort studies, investigating research questions and measuring additive outcome parameters in more detail.7
All participants are recruited from the outpatient clinic of the Erasmus MC, University Medical Center, a Dutch tertiary referral centre for predominantly high risk pregnancies. Inclusion criteria are: (i) women (and their partner) should at least have reached the age of 18; (ii) proficiency in speaking and reading the Dutch language. Annually, about 2500 couples visit the Erasmus MC outpatient clinic for preconception or pregnancy consultation. The tertiary setting allows for a yearly capacity of first-trimester serial ultrasound measurements in at least 250 pregnancies.
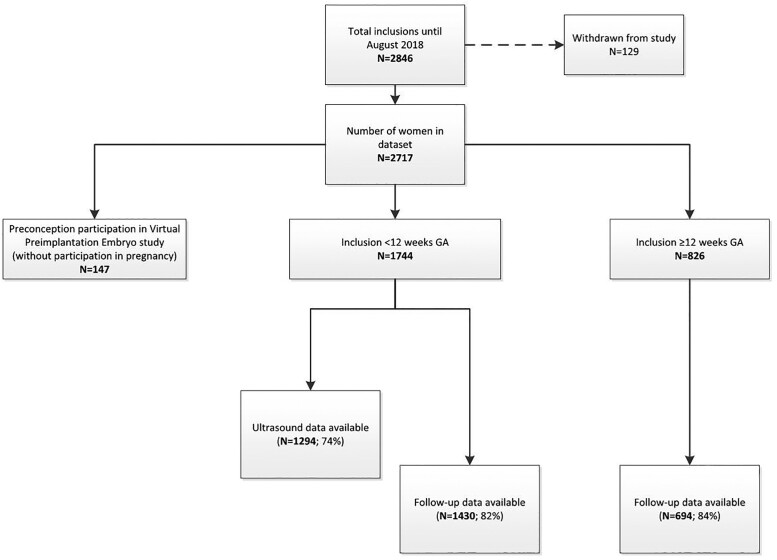
In total, 2950 couples have been included in the period between December 2009 and August 2018; 233 pregnancies from the preconceptional period up to 8 weeks of gestation were enrolled during the pilot phase (December 2009 and November 2010). A total of 2717 couples participated in this cohort between November 2010 and August 2018. Of these couples, 1891 women were included during the periconceptional period and 826 pregnancies ranging from 13 weeks of gestation until term. For the current profile update paper, it was decided to include all results until August 2018, since all participants had delivered by that time.
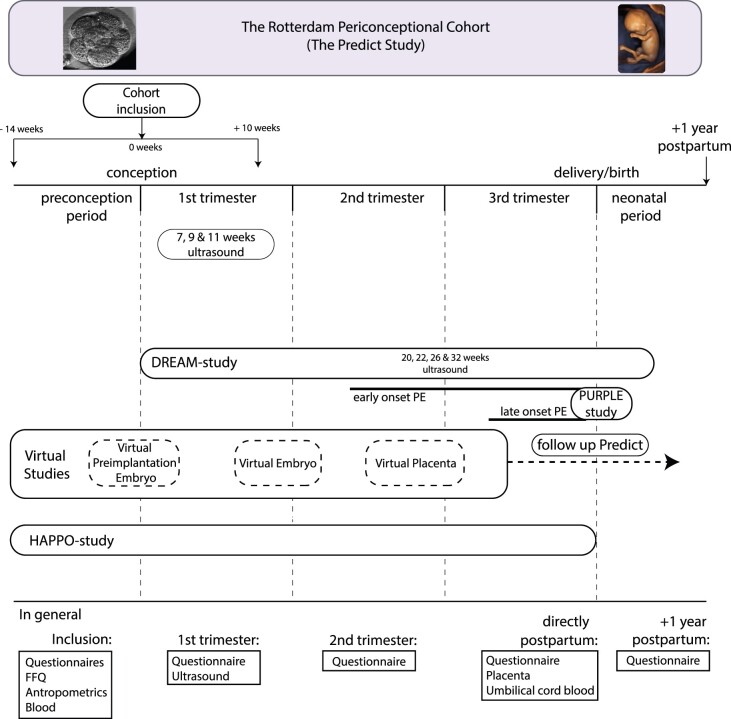
During the pilot phase, women underwent weekly 3 D transvaginal ultrasound examinations between 6 + 0 and 12 + 6 weeks of gestational age and inclusion was required <8 weeks of gestation (Figure 1). Serial investigation during the first trimester included two-dimensional (2 D) and 3 D transvaginal ultrasound examinations at 7, 9 and 11 weeks of gestational age. The subcohorts of the Dream and Virtual Placenta study included additional 2 D and 3 D transabdominal ultrasound examinations at 22, 26 and 32 weeks of gestation (Figure 2).
Figure 1.
Flowchart of included participants from November 2010 until August 2018 (without the pilot study participants). GA, gestational age
Figure 2.
Study design of Predict Cohort; including all subcohorts. General investigations of all subcohorts during the preconception period, 1st, 2nd and 3rd trimester, postpartum period, including follow-up at 1 year after delivery are shown. PE, preeclampsia; FFQ, Food Frequency Questionnaire
A more detailed description of the cohort is provided by the cohort profile article of Steegers-Theunissen et al.7 In short, women, and their partners, who are scheduled for a first prenatal visit at the outpatient clinic, are actively invited to participate (by means of an information brochure). Eligible couples undergo a standardized booking visit, in which height, weight, waist and hip circumference and systolic and diastolic blood pressure are determined. Before this booking visit, participants, including both women and their partners, are asked to fill out an extensive online questionnaire concerning both parental characteristics, medical (and obstetric) history, pre-pregnancy body mass index (BMI) and lifestyle behaviours. Moreover, a 196-item Food Frequency Questionnaire (FFQ) is completed for information about parental dietary patterns. Follow-up questionnaires are sent at 24 weeks of gestational age, around the expected delivery date and 1 year after actual delivery. Follow-up questionnaires collect information on health and environmental exposures and the course and outcome of pregnancy. One year after delivery, final information on general health of the offspring, including growth, congenital malformations and development is requested.
Preconceptionally and/or during the first trimester, both maternal and paternal venous blood samples are collected. At birth, umbilical cord blood is collected and, depending on the subcohort, the placenta is sent for pathological examination. Table 1 shows the numbers and percentages of available questionnaires and samples that have been collected up until August 2018 (excluding the pilot phase).
Table 1.
Data collection and general characteristics of the participants included between November 2010 and August 2018
| Data collected | Women (N = 1744) | Men (N = 1557) |
|---|---|---|
| First trimester | N (%) | N (%) |
|
General questionnaires |
1535 (88.0) | 1329 (85.4) |
| Food Frequency Questionnaires | 1421 (81.5) | 1186 (76.2) |
| Anthropometric measurements | 1630 (93.5) | 1331 (85.5) |
| Venous blood samples | 1405 (80.6) | 1272 (81.7) |
| (Serial) ultrasound scans | 1285 (73.7) | NA |
| 24 weeks of gestation | ||
| Questionnaires | 1149 (65.9) | NA |
| Structural ultrasound records | 1278 (73.3) | |
| 36 weeks of gestation to delivery | ||
| Questionnaire | 977 (56.0) | NA |
| Delivery reports | 1101 (63.1) | NA |
| Umbilical cord blood | 529 (30.3) | NA |
| Placenta | 147 (8.4) | NA |
| 1 year after delivery | ||
| Questionnaires | 756 (43.3) | NA |
| General characteristics | Women Mean (SD) or N (%) | Men Mean (SD) or N (%) |
|---|---|---|
| Age, years | 32.4 (4.5) | 35.3 (6.1) |
| Geographical origin | ||
| Dutch | 1201 (80.0) | 1091 (83.5) |
| Other Western | 68 (4.5) | 41 (3.1) |
| Non-Western | 233 (15.5) | 174 (13.3) |
| Education | ||
| Low | 126 (8.5) | 181 (14.0) |
| Middle | 529 (35.5) | 474 (36.8) |
| High | 835 (56.0) | 634 (49.2) |
| BMI, kg/m2 | 25.9 (5.2) | 26.4 (4.1) |
| Parity | NA | |
| Nulliparous | 678 (53.9) | |
| Multiparous | 580 (46.1) | |
| Mode of conception | NA | |
| Spontaneous | 1037 (67.6) | |
| IVF/ICSI | 496 (32.4) | |
| First trimester outcome | NA | |
| Miscarriage | 182 (10.4) | |
| Pregnancy outcome | NA | |
| Birthweight, g | 3270 (680) | |
| Gestational age at birth, days | 273 (61) | |
| PIH/preeclampsia | 117 (9.4) | |
| Preterm birth (<37weeks GA) | 118 (9.5) | |
| Small for gestational age (<p10) | 154 (12.3) | |
| Congenital anomaly | 54 (4.3) |
SD, standard deviation; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; BMI, body mass index; PIH, pregnancy-induced hypertension; GA, gestational age.
Update until August 2018, since all participants delivered and we could include the pregnancy outcome data in this manuscript.
As mentioned previously, the Predict study serves as an infrastructure for embedding new subcohorts. The first subcohort study, the Dream study, focused on detailed markers of embryonic and fetal brain development in the first, second and third trimester of pregnancy and continuing into the neonatal period (N = 227).8–12 The second subcohort study, the Purple study, aimed at the occurrence of early-onset and late-onset preeclampsia. Placenta and umbilical cord (postpartum) pathological examination, umbilical cord cell populations and the tissue-specific epigenome of the placenta and newborn were studied (N = 448).13–15 The third subcohort study, the Virtual Placenta study, concentrated on placental growth and development starting as early as the first-trimester (N = 241).16 Ongoing subcohorts are the Virtual Pre-implantation Embryo study, focusing on (pre-implantation) embryonic growth and development from the preconception period onwards (N = 360, number until 1 January 2019, still ongoing) and the HAPPO study, concentrating on maternal haemodynamic parameters before and during pregnancy (N = 25, number until October 2019, still ongoing).17
In addition, intervention studies designed as survey and multicentre randomized controlled trials are conducted using the mHealth coaching platform Smarter Pregnancy to empower couples to adapt a healthier lifestyle from the periconceptional period onwards.18,19 Also pregnant women with or without their male partner could participate in the study.20,21
What is the reason for the new focus?
Over the past years, data have been collected on 233 (pilot phase study) and 2717 (Predict study) couples and their pregnancies. Data have been used in multiple published articles in which several associations between exposures and both periconceptional and pregnancy outcomes have been studied (Supplementary File 1, available as Supplementary data at IJE online).
This cohort profile update paper not only represents an update of these results, but also provides description and discussion of our 3 D ultrasound and virtual reality measurement protocols. The results of the studies within the Predict cohort necessitated a re-evaluation and new strategies within the cohort. To optimize, further understand and unravel the potentials of maternal and paternal periconceptional health, an additional new study design of the original cohort is required. The new strategy and reason for the current update profile paper are aimed at studying the effects of an intervention on growth and development of the unborn child. Therefore, in 2021 the study will be extended with a nested randomized controlled trial, using a periconceptional blended lifestyle care approach in a specific high-risk population (BMI >25 kg/m²). Apart from the previous mentioned intervention, the design will be as the general observational cohort. The nested randomized controlled trial is considered the best design to show effectiveness, and has been developed to study the outcomes as defined for the cohort by integrating a blended lifestyle care approach into the current level of standardized health care.22 The study will be conducted during the periconceptional period in women at high risk of pregnancy complications and including their partners. The control group will receive standard care as also provided to the participants of the cohort according to local and national antenatal care protocols. The intervention group of high risk women will receive a combination of a personalized eHealth coaching programme for a period of 26 weeks [www.smarterpregnancy.co.uk] and a face-to-face counselling session with focus on lifestyle improvements. This approach allows for analyses of subgroups and associations between lifestyle changes and reproductive outcomes in the women, their male partners and their offspring.
To gain further insight into fetal and placental development, 3 D ultrasound examinations at 24 and 32 weeks of gestation will additionally be performed among all participants.
What will be the new areas of research?
Due to a 10-year anniversary evaluation, new areas in the dataset will be explored. In ongoing both uncomplicated and complicated pregnancies (i.e. miscarriage, intra-uterine fetal death, congenital anomalies, hypertensive disorders, large for gestational age, fetal growth restriction and preterm birth), serial parameters have been collected. Unique data are available for analysis since collection was already initiated during the periconceptional period. Until now, data have been published of subsets of these pregnancies focusing on the ongoing uncomplicated pregnancy group. In the existing dataset, as well as in new subcohorts, unique areas of research will be explored, by studying determinants of maternal and paternal periconceptional health in association with reproductive performance, pregnancy course and outcome, as well as underlying molecular biological mechanisms, including cardiovascular and metabolic pathways, inflammation and epigenetic profiles.
The Virtual Pre-implantation Embryo study investigates pre-implantation embryonic development using the EmbryoScope™, a time-lapse incubator providing a controlled culture environment and capturing comprehensive information on morphokinetics of the pre-implantation embryo. In animal models, the timing of embryonic developmental events has been shown to reflect embryonic metabolism and genetic integrity.23 The use of the EmbryoScope™ will enable us to further unravel the link between periconceptional parental exposures (such as obesity), in vitro environment, pre-implantation and post-implantation embryonic growth and development.
In collaboration with the Biomedical Imaging Group of the Erasmus MC, new innovative research will use deployment of artificial intelligence (AI) to focus on fully automated analyses of 3 D ultrasound data, with the goal to develop a spatiotemporal 3 D ultrasound embryonic and fetal brain atlas. The new 3 D atlas will enable fully automated pattern recognition within the developing embryonic and fetal brain. The use of AI will enable more reliable, less time-consuming and more independent measurements of brain structures and allow for putting the development of the entire prenatal brain into perspective regarding different parental exposures as well as maternal, fetal and neonatal outcomes.
The maternal environment and its cardiovascular system adaptation to pregnancy are crucial in the development of the embryo, the fetus and the placenta. The general aim of the Virtual Placenta study is to study periconceptional and (patho)physiological maternal cardiovascular and uterine vascular placental (adaptation) mechanisms that contribute to the origin of placenta-related pregnancy outcomes. Within this study, first-trimester reference values for longitudinal uterine vascular placental parameters will be determined using 3 D power Doppler ultrasound and virtual reality. To get more insight into vascular processes involved in in utero vascular placental development, placental parameters and embryonic growth trajectories, pregnancy outcome and periconceptional maternal vascular adaptation mechanisms will be studied. In addition to uterine vascular placental development studied using state-of-the-art 3 D ultrasound and virtual reality techniques, maternal haemodynamic parameters before and during pregnancy are studied in the HAPPO study.
The HAPPO study investigates the haemodynamic adaptation of women to pregnancy by examination of the maternal macro-circulatory and micro-circulatory system and uterine vascular placental development in the preconception period, during pregnancy and 3 months after delivery. The main outcome measures are differences in maternal haemodynamic adaptation to pregnancy between women with and without placenta-related pregnancy complications. A more detailed description of the HAPPO study protocol has been published separately.17
Finally, we want to address Smarter pregnancy. This is an example of a platform that has been first studied in a research setting and is now being used in regular clinical care at our outpatient clinic [www.smarterpregnancy.co.uk].18,19 The platform has been incorporated in the administrative database of the Erasmus MC since 2019; so every health care professional can see when a woman and her partner were included in this platform, together with the outcome of the visit and future goals. With permission of the woman and her partner, the information is also incorporated in national clinical and epidemiological databases.
What are the new measurements?
Two-dimensional and 3 D transvaginal and transabdominal ultrasound examinations are performed and subsequently analysed offline. Embryonic and fetal structures of interest include strictly defined and clinically important biometric measurements and also novel biometric and volumetric parameters. Examples of these novel parameters are first-trimester head volume and uterine vascular placental volume. Parameters still being developed are measurements of inner organ systems like the first-trimester kidney (length and volume) and lung (length and volume). Table 2 provides an overview of structures for which reliability analysis has been performed. Supplementary File 2, available as Supplementary data at IJE online, contains all measuring protocols of measurements as described in Table 2.
Table 2.
Reliability of different (newly) introduced measures performed using 3D ultrasound and virtual reality techniques
| Article | Year | Measures | Technique(s) used | Intraclass correlation coefficient (95% CI) | Mean difference | 95% CI for mean difference | Limits of agreement | Year of collection | Gestational weeks | Number of datasets |
|---|---|---|---|---|---|---|---|---|---|---|
| Verwoerd et al. | 2008 | Crown-rump length (mm) | 4D View, I-Spacea | 1,000 (0.982–0.997) | −0.07 | −0.32 to 0.18 | −1.37 to 1.22 | 2008 | 6 to 14 | 28 |
| Biparietal diameter (mm) | 4D View, I-Spacea | 0.987 (0.971–0.994) | −0.47 | −0.99 to 0.05 | −3.06 to 2.11 | 2008 | 6 to 14 | 26 | ||
| Head circumference (mm) | 4D View, I-Spacea | 0.994 (0.987–0.997) | −1.07 | −2.17 to 0.04 | −6.58 to 4.45 | 2008 | 6 to 14 | 26 | ||
| Abdominal circumference (mm) | 4D View, I-Spacea | 0.998 (0.997–0.999) | −0.49 | −0.95 to −0.04 | −2.76 to 1.77 | 2008 | 6 to 14 | 26 | ||
| Yolk sac diameter (mm) | 4D View, I-Spacea | 0.993 (0.982–0.997) | −0.04 | −0.09 to 0.02 | −0.29 to 0.22 | 2008 | 6 to 14 | 20 | ||
| Rousian et al. | 2009 | Yolk sac volume (cm3) | VOCAL, inversion mode, SonoAVC, I-Spacec | 0.992 (0.981–0.996) | −0.00 | −0.00 to 0.00 | −0.005 to 0.004 | 2008 | 6 to 11 | 24 |
| Balloons (cm3) | VOCAL, inversion mode, SonoAVC, I-Spacea | 0.997 (0.982–0.999) | −0.01 | −0.03 to 0.00 | NA | 2008 | NA | 7 | ||
| Rousian et al. | 2010 | Embryonic volume | I-Space | 0.999 (0.997–0.999) | NA | NA | NA | 2008 | 6 to 12 | 20 |
| Reus et al. | 2013 | Trophoblast volume (cm3) | VOCAL | 0.976 (0.945–0.989) | 1.57 | −0.81 to 3.94 | −9.69 to 12.82 | 2009 | 6 to 11 | 24 |
| Rousian et al. | 2013 | Total cerebellar diameter (mm) | 4D View, I-Spacea | 0.996 (0.986–0.999) | −0.12 | −0.18 to −0.06 | −0.45 to 0.22 | 2009 | 7 to 12 | 35 |
| Left hemispheric diameter (mm) | 4D View, I-Spacea | 0.992 (0.984–0.996) | −0.02 | −0.06 to 0.02 | −0.26 to 0.23 | 2009 | 7 to 12 | 35 | ||
| Left hemispheric thickness (mm) | 4D View, I-Spacea | 0.985 (0.968–0.992) | −0.04 | −0.09 to 0.00 | −0.31 to 0.22 | 2009 | 7 to 12 | 33 | ||
| Right hemispheric diameter (mm) | 4D View, I-Spacea | 0.985 (0.970–0.992) | −0.03 | −0.09 to 0.03 | −0.37 to 0.31 | 2009 | 7 to 12 | 35 | ||
| Right hemispheric thickness (mm) | 4D View, I-Spacea | 0.986 (0.971–0.993) | −0.05 | −0.09 to 0.00 | −0.29 to 0.20 | 2009 | 7 to 12 | 34 | ||
| Baken et al. | 2014 | Wrist width (mm) | I-Space | 0.984 (0.960–0.994) | −1.47 | −4.08 to 11.40 | −12.40 to 9.46 | 2009 | 9 to 12 | 20 |
| Hand width (mm) | I-Space | 0.992 (0.981–0.997) | −1.14 | −3.02 to 0.96 | −9.95 to 7.67 | 2009 | 9 to 12 | 20 | ||
| Hand length (mm) | I-Space | 0.997 (0.994–0.999) | −0.25 | −1.94 to 1.43 | −7.31 to 6.81 | 2009 | 9 to 12 | 20 | ||
| Hand index | I-Space | 0.984 (0.956–0.993) | −0.86 | −2.88 to 1.16 | −9.34 to 7.62 | 2009 | 9 to 12 | 20 | ||
| Gijtenbeek et al. | 2014 | Left thickness diencephalon (mm) | 4D View | 0.999 (0.998–0.999) | −0.01 | −0.03 to 0.01 | −0.12 to 0.11 | 2009 | 6 to 12 | 30 |
| Right thickness diencephalon (mm) | 4D View | 0.997 (0.994–0.999) | −0.03 | −0.06 to 0.00 | −0.19 to 0.14 | 2009 | 6 to 12 | 30 | ||
| Total diencephalon diameter (mm) | 4D View | 0.998 (0.996–0.999) | 0.04 | −0.00 to 0.08 | −0.19 to 0.27 | 2009 | 6 to 12 | 30 | ||
| Left thickness mesencephalon (mm) | 4D View | 0.992 (0.982–0.996) | −0.03 | −0.06 to 0.00 | −0.20 to 0.14 | 2009 | 6 to 12 | 30 | ||
| Right thickness mesencephalon (mm) | 4D View | 0.988 (0.974–0.994) | 0.00 | −0.04 to 0.04 | −0.21 to 0.21 | 2009 | 6 to 12 | 30 | ||
| Total mesencephalon diameter (mm) | 4D View | 0.994 (0.987–0.997) | 0.05 | −0.01 to 0.11 | −0.27 to 0.37 | 2009 | 6 to 12 | 30 | ||
| Left telencephalon thickness (mm) | 4D View | 0.980 (0.950–0.991) | −0.05 | −0.09 to -0.01 | −0.26 to 0.16 | 2009 | 6 to 12 | 30 | ||
| Right telencephalon thickness (mm) | 4D View | 0.982 (0.963–0.992) | 0.03 | −0.01 to 0.07 | −0.20 to 0.26 | 2009 | 6 to 12 | 30 | ||
| Reus et al. | 2014 | Trophoblast volume (cm3) | I-Space | 0.928 (0.829–0.971) | 3.88 | −1.41 to 9.17 | −18.74 to 26.49 | Nov 2009–Dec 2010 | 12 | 20 |
| Uteroplacental bed vascular volume (cm3) | I-Space | 0.994 (0.985–0.998) | −0.64 | −1.25 to -0.03 | −3.23 to 1.95 | Nov 2009–Dec 2010 | 12 | 20 | ||
| Fetal vascular volume (cm3) | I-Space | 0.964 (0.913–0.986) | 0.18 | −0.04 to 0.40 | −0.74 to 1.10 | Nov 2009–Dec 2010 | 12 | 20 | ||
| Baken et al. | 2015 | Crown-rump length (mm) | I-Space VS 3 D VR Desktop | 1.000 (0.999–1.000) | −0.34 | −0.77 to 0.08 | −2.58 to 1.89 | Jan 2009–Dec 2009 | 6 to 12 | 30 |
| Embryonic volume (cm3) | I-Space VS 3 D VR Desktop | 0.999 (0.998–1.000) | −0.92 | −2.07 to 0.23 | −6.97 to 5.13 | Jan 2009–Dec 2009 | 6 to 12 | 30 | ||
| Cohort profile update measurements | ||||||||||
| Koning et al. | 2016 | Head volume (cm3) | I-Space | 0.99 (NA) | 0.01 | −0.03 to 0.05 | −7.56 to 6.74 | 2009–10 | 9 to 12 | 34 |
| Koning et al. | 2017 | Sylvian fissure depth (mm) | 4D view | 0.846 (NA) | 0.51 | 0.13 to 0.89 | −2.18 to 3.19 | Nov 2013–March 2015 | 22 to 32 | 30 |
| Insula depth (mm) | 4D view | 0.879 (NA) | 0.31 | −0.12 to 0.74 | −2.73 to 3.35 | Nov 2013-March 2015 | 22 to 32 | 30 | ||
| Parieto-occipital fissure depth (mm) | 4D view | 0.841 (NA) | −0.48 | −1.05 to 0.09 | −4.44 to 3.91 | Nov 2013–March 2015 | 22 to 32 | 30 | ||
| Koning et al. | 2017 | Corpus callosum length (mm) | 4D view | 0.970 (NA) | −1.11 | −1.70 to -0.51 | −4.55 to 2.33 | Nov 2013–July 2015 | 22 to 42 | 30 |
| Corpus callosum fastigium length (mm) | 4D view | 0.970 (NA) | −0.13 | −0.74 to 0.49 | −3.59 to 3.34 | Nov 2013–July 2015 | 22 to 42 | 30 | ||
| Roelants et al. | 2017 | Fractional thigh volume (cm3) | 4D View | Excellent | NA | NA | NA | Nov 2013–July 2015 | 22 to 32 | 30 |
| Bogers et al. | 2018 | Genital tubercle angle | I-Space | 0.95 (0.90–0.98) | NA | NA | NA | 2009 | 9 to 12 | 30 |
| Qualitative sex predictionb | I-Space | NA | NA | NA | NA | 2009 | 9 to 12 | 30 | ||
| Bogers et al. | 2018 | Umbilical cord insertion width (mm) | I-Space | 0.917 (NA) | 0.01 | −0.08 to 0.10 | −0.48 to 0.49 | 2009 | 6 to 12 | 31 |
| Maximum diameter umbilical cord (mm) | I-Space | 0.962 (NA) | 0.04 | −0.04 to 0.12 | −0.40 to 0.48 | 2009 | 6 to 12 | 31 | ||
| Volume midgut herniation (cm3) | I-Space | 0.997 (NA) | 0.29 | −0.46 to 1.03 | −3.75 to 4.32 | 2009 | 6 to 12 | 31 | ||
| 2018 | Foot position—left frontal | I-Space | 0.935 (NA) | 0.14 | −10.56 to 10.85 | NA | 2009 | 8 to 13 | 30 | |
| Foot position—right frontal | I-Space | 0.941 (NA) | 0.33 | −9.28 to 9.95 | NA | 2009 | 8 to 13 | 30 | ||
| Foot position—left lateral | I-Space | 0.872 (NA) | −0.39 | −6.38 to 5.60 | NA | 2009 | 8 to 13 | 30 | ||
| Foot position—right lateral | I-Space | 0.909 (NA) | 0.57 | −6.17 to 7.32 | NA | 2009 | 8 to 13 | 30 | ||
| Reijnders et al. | 2018 | Uterine vascular volume (cm3) preconceptional | I-Space,a 3 D VR Desktop | 0.93 (NA) | −0.27 | −0.55 to 0.01 | NA | NA | 7 to 11 | 35 |
| Uteroplacental vascular volume (cm3) at 11 weeks GA | I-Space,a 3 D VR Desktop | 0.69 (NA) | 2.56 | 0.90 to 4.21 | NA | NA | 7 to 11 | 35 | ||
| Embryonic vascular volume (cm3) at 11 weeks GA | I-Space,a 3 D VR Desktop | 0.99 (NA) | 0.00 | −0.05 to 0.06 | NA | NA | 7 to 11 | 35 | ||
Intracorrelation coefficient, mean difference and limits of agreement for interobserver differences (between the measurements performed by two independent examiners).
GA, gestational age; NA, not available.
The results are shown for the I-Space measurements.
Cohen’s kappa = 024 (95% CI: 0.13–0.34).
I-Space measurements are compared with the golden standard, namely VOCAL.
Examinations are performed on a Voluson E8 or E10 (GE Healthcare, Austria) ultrasound machine. Measurements are carried out by ultrasound or on a personal computer using 4 D view software (GE Healthcare, Austria). This software program allows offline 3 D measurements after collection of the datasets and is only available for datasets generated on GE Healthcare ultrasound machines. The 3 D measurements are performed on a 2 D computer screen, not allowing real depth perception. In addition, complex and volumetric structures are measured using virtual reality techniques. The I-Space, one of the used systems, is a virtual reality room in which eight projectors project stereoscopic images on three walls and the floor of a small room, creating a so-called hologram of the embryo or fetus (Figure 3). V-Scope is the volume-rendering software package used for virtual reality measurements, allowing interaction with the dataset in an intuitive manner.24 Within the I-Space, different structures are measured using a wireless joystick. Length measurements can be performed using a tracing tool, and (semi-)automated volume measurements use an algorithm based on ultrasound-related grey scale differences.25–48 Finally, morphological examinations can be performed using all interaction modalities.49 As the I-Space is a physically static, large and expensive system, a 3 D virtual reality desktop system has been developed, using a 3 D monitor and a tracking system (Figure 4). The software program used on the I-Space and virtual reality desktop system are the same (V-Scope), which means that the measurements are performed in the same exact way. However, the virtual reality desktop system is smaller in size, which entails measuring taking place on a smaller screen.
Figure 3.

I-Space virtual reality system. A fetus of 12 weeks’ gestational age is displayed on the front wall. The researcher is wearing 3D glasses to observe real depth perception and interact with the dataset by using a joystick. Be aware that real depth perception cannot be shown on paper
Figure 4.

Three-dimensional virtual reality desktop system. An embryo of 9 weeks’ gestational age is displayed on the 3D monitor; the brain cavities are coloured in light blue (and the brain cavity volume is measured automatically)
Importantly, the software has not changed during the previous years; only new modalities have been added. Baken et al. validated the desktop system for crown-rump length (CRL) and embryonic volume (EV) measurements in 30 patients, showing excellent reliability: intraclass correlation coefficient (ICC) CRL 1.000 (95% confidence interval (CI): 0.999–1.000) and ICC EV 0.999 (95% CI : 0.998–1.000).50,51 Since the 3 D virtual reality desktop system uses exactly the same software program, we did not publish reliability data regarding other structures if these were measured on the desktop system instead of the I-Space. However, each researcher who is involved in the ultrasound measurements is obliged to pass the learning curve set in order to test their reproducibility of the measurements. Assessment of the intra-observer reliability involves measuring the structure of interest three times. Inter-observer reliability is assessed by comparing with multiple measurements previously performed in the I-Space or on the desktop system by other researchers. Only when an excellent reliability (intraclass correlation coefficient >0.90) is met, the researcher can start measuring the structure of interest in the research set.
Complex structures, like brain cavity volume, Sylvian fissure depth, genital tubercle angle and different dimensions of the cerebellum can also be measured with high ICCs.10,33,43,44Table 2 shows the mean difference (including 95% CI), limits of agreement between observers, gestational age of measurement performance and number of datasets used for various morphological, biometric and volumetric structures. For each study parameter, different pregnancies were used to perform the reliability analysis. Pregnancies were randomly chosen based on gestational age and only one dataset per pregnancy could be included in the measuring set, to exclude selection bias. During the 10-year period a considerable amount of experience was gained, and we have learned to sophisticate our ultrasound examinations and study protocols in such a way that, in case of specific, small structures, additional 3 D sweeps are necessary to measure the specific structure of interest. Therefore we included new pregnancy datasets when measuring these structures. After proving that these structures could be reliably measured, they are being measured in a larger subset.
What has been found? Key findings and publications
To date, numerous original papers have been published including reliability analyses of newly introduced measurements (Table 2).
Determinants of maternal and paternal periconceptional health
We studied associations between several exposures, such as maternal age, diet, smoking and BMI, and embryonic (CRL, EV, Carnegie stages), brain (head volume, cerebellum, cortical folding, corpus callosum, corpus callosum fastigium) and placental development (uterine vascular placental volume).52–64Supplementary File 1 shows the results of various studies with regard to these domains of intrauterine growth and development.
Koning et al. showed that congenital heart defects are associated with cortical folding (growth trajectories of left insula depth and right parieto-occipital fissure depths were lower).10 Fractional thigh volume, measured in the fetal period using 3 D ultrasound, showed to be a promising marker for prediction of neonatal adiposity.65 Finally, in a subset of women with preeclampsia, a nested case-control study was performed consisting of 15 patients with early-onset and 15 with late-onset preeclampsia, respectively.14,15 Placental and newborn vascular health was studied, showing associations between preeclampsia and (a smaller) umbilical vein area and wall thickness. These parameters serve as a proxy of disturbed cardiovascular development within the newborn.
Reproductive performance and pregnancy course and outcome
The link between parental periconceptional exposures and fertility parameters has been studied. A positive association between strong adherence to a healthy dietary pattern and semen parameters in men with poor semen quality has been shown.66 With regards to female fertility, strong periconceptional adherence to a healthy diet showed an inverse association with the hyperandrogenic polycystic ovarian syndrome (PCOS) phenotype. In women with PCOS, a strong periconceptional adherence to a healthy dietary pattern showed a 3-fold higher chance of an ongoing pregnancy.67 We also focused on endocrine and cardiometabolic cord blood characteristics of PCOS offspring, and compared these with characteristics of healthy controls.68 Androstenedione concentrations were increased in cord blood of both male and female PCOS offspring, supporting the hypothesis that maternal hyperandrogenism during pregnancy may predispose to fetal hyperandrogenism.
Underlying epigenetic profiles
Herzog et al. studied epigenetic programming of placental and fetal tissues in women with early-onset and late-onset preeclampsia, and identified loci known to be associated with cardiovascular system pathways.14,15 In these women, umbilical cord blood cell populations collected postpartum showed derangements of fetal haematopoiesis, in particular of neutrophil and nucleated red blood cell counts. The heterogeneity in umbilical cord blood cell populations should be considered a confounder in epigenetic association studies.13 A methylation study of umbilical cord blood leukocytes by van den Berg et al. in early-onset preeclampsia demonstrated differences in DNA methylation of circadian clock and clock-controlled genes.69,70
What are the main strengths and weaknesses?
The study design, being a unique periconceptional prospective cohort study, fully embedded in standard (tertiary) patient care, is the main strength of the study. Besides periconceptional questionnaires and biomarkers, study parameters include longitudinal 2 D and 3 D ultrasound examinations already from 6 + 0 weeks of gestation onwards. Measurements are performed using high-frequency ultrasound probes and virtual reality, allowing visualization and innovative measurement of structures for the first time. Besides virtual reality imaging techniques, biochemical, dietary, molecular biological and (epi)genetic data are available for integrated analysis, contributing to our further understanding of the epigenome as a predictor of reproductive and pregnancy outcomes.
Since we are interested in understanding the causation of complicated reproduction and pregnancy outcome, the cohort is embedded in tertiary care, which ensures a high internal validity but a limited external generalizability. All findings emphasize the importance of implementing periconceptional care in general medicine in order to prevent and ameliorate adverse pregnancy and future health outcomes in the offspring. Although the Predict study is a prospective cohort study, a validated intervention is needed to elucidate and enrich the findings.
Can I get hold of the data? Where can I find out more?
Colleagues interested in collaboration can contact us by sending a brief research proposal (Supplementary File 3, available as Supplementary data at IJE online). Approval depends on quality and feasibility, as assessed by the Principal Investigators of the Predict study. A formal contract is needed for collaboration, including mutual obligations. For more information contact the Principal Investigators at [predictstudie@erasmusmc.nl]; and for possibilities regarding harmonization of data go to [www.maelstrom-research.org].
Key Features
The Rotterdam Periconceptional Cohort study was set up in 2009 to investigate maternal and paternal periconceptional health and the impact on reproduction, pregnancy and neonatal outcome.
In the pilot phase 233 pregnancies were included (2009–10) and in the study phase 2717 pregnancies were included (2010–18), participants being aged between 19 and 48.
-
What is new in the cohort:
Novel three-dimensional (3D) ultrasound and virtual reality measurements and their protocols are introduced, such as the first-trimester head volume and uterine vascular placental volume. The measurements of the inner organ systems, e.g. the kidneys and lungs, are studies under development.
A new focus is the fully automated analyses of 3D ultrasound data, resulting in a 3D ultrasound embryonic and fetal brain atlas by using artificial intelligence.
Ongoing unique subcohorts comprise: (i) the Virtual Placenta study aiming to establish periconceptional and (patho)physiological maternal cardiovascular and uterine vascular placental mechanisms contributing to the origin of placenta-related pregnancy outcome; (ii) the Virtual Pre-implantation Embryo study focusing on (pre-implantation) embryonic growth and development; and (iii) the HAPPO study, focusing on maternal haemodynamic parameters during the preconception period and pregnancy.
The introduction of the mHealth online lifestyle coaching platform, Smarter Pregnancy, at the outpatient clinic and linkage to the administrative database of the Erasmus MC allow access of all health care personnel to personalized coaching advice.
To study the impact of above-mentioned associations, a nested randomized controlled trial will start in 2021. The trial is designed as a survey and will use a periconceptional blended lifestyle care approach.
The Predict study can be contacted for collaborations: [predictstudie@erasmusmc.nl].
Supplementary data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Professor J. W. Wladimiroff for his English grammar expertise. The authors also acknowledge all PhD students who contributed to the collection of all ultrasound data and performed/supervised all measurements (PhD students Amra Sabanovic, Averil Reus, Caroline van den Berg, Damiat Alouad Fares, Eline Oostingh, Emilie Herzog, Eveline van Uitert, Fatima Hammiche, Fieke Husen, Francesca Parisi, Hein Bogers, Igna Reijnders, Irene Koning, Jeffrey Hoek, John Twigt, Jorine Roelants, Katinka Snoek, Kim Wijnands, Leonie Baken, Linette van Duijn, Matthijs van Dijk, Melissa van der Windt, Nicole Huijgen, Wietske Bastiaanse, Rianne Bijl, Sharissa Smith) and supervisors Attie Go, Irene Groenenberg, Sten Willemsen, Niek Exalto.
Ethics approval
The study was approved by the Central Committee on Research in The Hague and the local Medical Ethics Committee of the Erasmus Medical Center (MC) in Rotterdam, The Netherlands (MEC-2004-227).
Funding
This work was supported by the Department of Obstetrics and Gynaecology of the Erasmus MC, University Medical Center in Rotterdam, The Netherlands. Additional support was provided by Sophia Foundation for Scientific Research (2010), Erasmus MC Medical Efficacy (2012), the Dutch Organisation for Health Care Research ad Care innovation (ZonMW) (2013, 2018), Foundation Born Healthy (2015), Erasmus MC Grants (2016, 2017, 2018), Horizon 2020 (2018) [project number 812660].
Conflict of interest
None declared.
References
- 1. Hales CN, Barker DJ.. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- 2. Gluckman PD, Hanson MA, Buklijas T.. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis 2010;1:6–18. [DOI] [PubMed] [Google Scholar]
- 3. Steegers-Theunissen RPM, Twigt J, Pestinger V, Sinclair KD.. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update 2013;19:640–55. [DOI] [PubMed] [Google Scholar]
- 4. Hart R, Norman RJ.. The longer-term health outcomes for children born as a result of IVF treatment. Hum Reprod Update 2013;19:232–43. [DOI] [PubMed] [Google Scholar]
- 5. Fleming TP, Watkins AJ, Sun C, Velazquez MA, Smyth NR, Eckert JJ.. Do little embryos make big decisions? How maternal dietary restriction can permanently change an embryo’s potential, affecting adult health. Reprod Fertil Dev 2015;27:684–92. [DOI] [PubMed] [Google Scholar]
- 6. Rousian M, Koster MPH, Mulders A, Koning AHJ, Steegers-Theunissen RPM, Steegers EAP.. Virtual reality imaging techniques in the study of embryonic and early placental health. Placenta 2018;64:S29–S35. [DOI] [PubMed] [Google Scholar]
- 7. Steegers-Theunissen RP, Verheijden-Paulissen JJ, van Uitert EM. et al. Cohort Profile: The Rotterdam Periconceptional Cohort (Predict Study). Int J Epidemiol 2016;45:374–81. [DOI] [PubMed] [Google Scholar]
- 8. Koning IV, Groenenberg IAL, Gotink AW. et al. Periconception maternal folate status and human embryonic cerebellum growth trajectories: The Rotterdam Predict Study. PLoS One 2015;10: e0141089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koning IV, Baken L, Groenenberg IAL. et al. Growth trajectories of the human embryonic head and periconceptional maternal conditions. Hum Reprod 2016;31:968–76. [DOI] [PubMed] [Google Scholar]
- 10. Koning IV, van Graafeiland AW, Groenenberg IAL. et al. Prenatal influence of congenital heart defects on trajectories of cortical folding of the fetal brain using three-dimensional ultrasound. Prenat Diagn 2017;37:1008–16. [DOI] [PubMed] [Google Scholar]
- 11. Koning IV, Roelants JA, Groenenberg IAL. et al. New ultrasound measurements to bridge the gap between prenatal and neonatal brain growth assessment. AJNR Am J Neuroradiol 2017;38:1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koning IV, Dudink J, Groenenberg IAL, Willemsen SP, Reiss IKM, Steegers-Theunissen RPM.. Prenatal cerebellar growth trajectories and the impact of periconceptional maternal and fetal factors. Hum Reprod 2017;32:1230–37. [DOI] [PubMed] [Google Scholar]
- 13. Herzog EM, Eggink AJ, van der Zee M. et al. The impact of early- and late-onset preeclampsia on umbilical cord blood cell populations. J Reprod Immunol 2016;116:81–85. [DOI] [PubMed] [Google Scholar]
- 14. Herzog EM, Eggink AJ, Willemsen SP. et al. Early- and late-onset preeclampsia and the tissue-specific epigenome of the placenta and newborn. Placenta 2017;58:122–32. [DOI] [PubMed] [Google Scholar]
- 15. Herzog EM, Eggink AJ, Reijnierse A. et al. Impact of early- and late-onset preeclampsia on features of placental and newborn vascular health. Placenta 2017;49:72–79. [DOI] [PubMed] [Google Scholar]
- 16. Reijnders IF, Mulder A, Koster MPH. et al. New imaging markers for preconceptional and first-trimester utero-placental vascularization. Placenta 2018;61:96–102. [DOI] [PubMed] [Google Scholar]
- 17. Bijl RC, Cornette JMJ, van den Bosch AE. et al. Study protocol for a prospective cohort study to investigate hemodynamic adaptation to pregnancy and placenta-related outcome: the HAPPO study. BMJ Open 2019;9: e033083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Dijk MR, Huijgen NA, Willemsen SP, Laven JS, Steegers EAP, Steegers-Theunissen RP.. Impact of an mHealth platform for pregnancy on nutrition and lifestyle of the reproductive population: a survey. JMIR Mhealth Uhealth 2016;4: e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Dijk MR, Oostingh EC, Koster MPH, Willemsen SP, Laven JS, Steegers-Theunissen RP.. The use of the mHealth program Smarter Pregnancy in preconception care: rationale, study design and data collection of a randomized controlled trial. BMC Pregnancy Childbirth 2017;17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Dijk MR, Borggreven NV, Willemsen SP, Koning AHJ, Steegers-Theunissen RPM, Koster MPH.. Maternal lifestyle impairs embryonic growth: the Rotterdam periconception cohort. Reprod Sci 2018;25:916–22. [DOI] [PubMed] [Google Scholar]
- 21. Gootjes DV, van Dijk MR, Koster MP, Willemsen SP, Steegers EA, Steegers-Theunissen RP.. Neighborhood deprivation and the effectiveness of mobile health coaching to improve periconceptional nutrition and lifestyle in women: survey in a large urban municipality in the Netherlands. JMIR Mhealth Uhealth 2019;7: e11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ioannidis JPA, Adami HO.. Nested randomized trials in large cohorts and biobanks: studying the health effects of lifestyle factors. Epidemiology 2008;19:75–82. [DOI] [PubMed] [Google Scholar]
- 23. D’Souza F, Pudakalakatti SM, Uppangala S. et al. Unraveling the association between genetic integrity and metabolic activity in pre-implantation stage embryos. Sci Rep 2016;6:37291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koning AHJ, Rousian M, Verwoerd-Dikkeboom CM, Goedknegt L, Steegers EAP, Van der Spek PJ.. V-scope: design and implementation of an immersive and desktop virtual reality volume visualization system. Stud Health Technol Inform 2009;142:136–38. [PubMed] [Google Scholar]
- 25. Baken L, Van Heesch PN, Wildschut HI. et al. First-trimester crown-rump length and embryonic volume of aneuploidy fetuses measured in virtual reality. Ultrasound Obstet Gynecol 2013;41:521–25. [DOI] [PubMed] [Google Scholar]
- 26. Baken L, Rousian M, Kompanje EJ. et al. Diagnostic techniques and criteria for first-trimester conjoined twin documentation: a review of the literature illustrated by three recent cases. Obstet Gynecol Surv 2013;68:743–52. [DOI] [PubMed] [Google Scholar]
- 27. Baken L, Benoit B, Koning AHJ. et al. First-trimester hand measurements in euploid and aneuploidy human fetuses using virtual reality. Prenat Diagn 2014;32:1–9. [DOI] [PubMed] [Google Scholar]
- 28. Baken L, Groenenberg IAL, Hoogeboom AJ, Koning AHJ, Exalto N.. First-trimester diagnosis of thrombocytopenia - absent radius syndrome using virtual reality. Clin Dysmorphol 2014;23:71–73. [DOI] [PubMed] [Google Scholar]
- 29. Baken L, Exalto N, Benoit B, Van der Spek PJ, Steegers EAP, Groenenberg IAL.. Differentiation of early first-trimester cranial neural tube defects. Ultrasound Obstet Gynecol 2014;43:711–12. [DOI] [PubMed] [Google Scholar]
- 30. Baken L, Benoit B, Koning AHJ, Van der Spek PJ, Steegers EAP, Exalto N.. First-trimester crown-rump length and embryonic volume of fetuses with structural congenital abnormalities measured in virtual reality: an observational study. Biomed Res Int 2017;2017:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bogers H, Baken L, Cohen-Overbeek TE. et al. Evaluation of first-trimester physiological midgut herniation using three-dimensional ultrasound. Fetal Diagn Ther 2018;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bogers H, Van Uitert EM, Van Ginkel S. et al. Human embryonic curvature studied with 3D ultrasound in ongoing pregnancies and miscarriages. Reprod Biomed Online 2018;36:576–83. [DOI] [PubMed] [Google Scholar]
- 33. Bogers H, Rifouna MS, Koning AHJ. et al. Accuracy of fetal sex determination in the first trimester of pregnancy using 3D virtual reality ultrasound. J Clin Ultrasound 2018;46:241–46. [DOI] [PubMed] [Google Scholar]
- 34. Bogers H, Rifouna MS, Cohen-Overbeek TE. et al. First trimester physiological development of the fetal foot position using three-dimensional ultrasound in virtual reality. J Obstet Gynaecol Res 2019;45:280–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reus AD, El-Harbachi H, Rousian M. et al. Early first-trimester trophoblast volume in pregnancies that result in live birth or miscarriage. Ultrasound Obstet Gynecol 2013;42:577–84. [DOI] [PubMed] [Google Scholar]
- 36. Reus AD, Klop-van der Aa J, Rifouna MS. et al. Early pregnancy placental bed and fetal vascular volume measurements using 3-D virtual reality. Ultrasound Med Biol 2014;40:1796–803. [DOI] [PubMed] [Google Scholar]
- 37. Rifouna MS, Reus AD, Koning AHJ. et al. First trimester trophoblast and placental bed vascular volume measurement in IVF of IVF/ICSI pregnancies. Hum Reprod 2014;29:2644–49. [DOI] [PubMed] [Google Scholar]
- 38. Rousian M, Verwoerd-Dikkeboom CM, Koning AHJ. et al. Early pregnancy volume measurements: validation of ultrasound techniques and new perspectives. BJOG 2009;116:278–85. [DOI] [PubMed] [Google Scholar]
- 39. Rousian M, Koning AHJ, Van Oppenraaij RH. et al. An innovative virtual reality technique for automated human embryonic volume measurements. Hum Reprod 2010;25:2210–16. [DOI] [PubMed] [Google Scholar]
- 40. Rousian M, Verwoerd-Dikkeboom CM, Koning AHJ. et al. First trimester umbilical and vitelline duct measurements using virtual reality. Early Hum Dev 2011;87:77–82. [DOI] [PubMed] [Google Scholar]
- 41. Rousian M, Koning AHJ, Hop WC, Van der Spek PJ, Exalto N, Steegers EAP.. Gestational sac fluid volume measurements in virtual reality. Ultrasound Obstet Gynecol 2011;38:524–29. [DOI] [PubMed] [Google Scholar]
- 42. Rousian M, Koning AHJ, Van der Spek PJ, Steegers EAP, Exalto N.. Virtual reality for embryonic measurements requiring depth perception. Fertil Steril 2011;95:773–74. [DOI] [PubMed] [Google Scholar]
- 43. Rousian M, Hop WC, Koning AHJ, Van der Spek PJ, Exalto N, Steegers EAP.. First trimester brain ventricle fluid and embryonic volumes measured by three-dimensional ultrasound with the use of I-Space virtual reality. Hum Reprod 2013;28:1181–89. [DOI] [PubMed] [Google Scholar]
- 44. Rousian M, Groenenberg IAL, Hop WC. et al. Human embryonic growth and development of the cerebellum using 3-dimensional ultrasound and virtual reality. Reprod Sci 2013;20:899–908. [DOI] [PubMed] [Google Scholar]
- 45. Verwoerd-Dikkeboom CM, Koning AHJ, Hop WC. et al. Reliability of three-dimensional sonographic measurements in early pregnancy using virtual reality. Ultrasound Obstet Gynecol 2008;32:910–16. [DOI] [PubMed] [Google Scholar]
- 46. Verwoerd-Dikkeboom CM, Van Heesch PN, Koning AHJ, Galjaard RJ, Exalto N, Steegers EAP.. Embryonic delay in growth and development related to confined placenta trisomy 16 mosaicism, diagnosed by I-Space virtual reality. Fertil Steril 2008;90:2017. [DOI] [PubMed] [Google Scholar]
- 47. Verwoerd-Dikkeboom CM, Koning AHJ, Groenenberg IAL. et al. Using virtual reality for evaluation of fetal ambiguous genitalia. Ultrasound Obstet Gynecol 2008;32:510–14. [DOI] [PubMed] [Google Scholar]
- 48. Verwoerd-Dikkeboom CM, Koning AHJ, Hop WC, Van der Spek PJ, Exalto N, Steegers EAP.. Innovative virtual reality measurements for embryonic growth and development. Hum Reprod 2010;25:1404–10. [DOI] [PubMed] [Google Scholar]
- 49. Verwoerd-Dikkeboom CM, Koning AHJ, Van der Spek PJ, Exalto N, Steegers EAP.. Embryonic staging using a 3D virtual reality system. Hum Reprod 2008;23:1479–84. [DOI] [PubMed] [Google Scholar]
- 50. Baken L, Rousian M, Koning AHJ. et al. First trimester detection of surface abnormalities: a comparison of 2- and 3-dimensional ultrasound and 3-dimensional virtual reality ultrasound. Reprod Sci 2014;21:993–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baken L, Van Gruting IM, Steegers EAP, Van der Spek PJ, Exalto N, Koning AHJ.. Design and validation of a 3D virtual reality desktop system for sonographic length and volume measurements in early pregnancy evaluation. J Clin Ultrasound 2015;43:164–70. [DOI] [PubMed] [Google Scholar]
- 52. Eindhoven SC, Van Uitert EM, Laven JS. et al. The influence of IVF/ICSI treatment on human embryonic growth trajectories. Hum Reprod 2014;29:2628–36. [DOI] [PubMed] [Google Scholar]
- 53. Gijtenbeek M, Bogers H, Groenenberg IAL. et al. First trimester size charts of embryonic brain structures. Hum Reprod 2014;29:201–07. [DOI] [PubMed] [Google Scholar]
- 54. Oostingh EC, De VI, Ham AC. et al. No independent associations between preconception paternal dietary patterns and embryonic growth; the Predict Study. Clin Nutr 2018;S0261. [DOI] [PubMed] [Google Scholar]
- 55. Parisi F, Rousian M, Koning AHJ, Willemsen SP, Cetin I, Steegers-Theunissen RPM.. Periconceptional maternal one-carbon biomarkers are associated with embryonic development according to the Carnegie stages. Hum Reprod 2017;32:523–30. [DOI] [PubMed] [Google Scholar]
- 56. Parisi F, Rousian M, Koning AHJ. et al. Periconceptional maternal biomarkers of one-carbon metabolism and embryonic growth trajectories: the Rotterdam Periconceptional Cohort (Predict Study). Fertil Steril 2017;107:691–98. [DOI] [PubMed] [Google Scholar]
- 57. Parisi F, Rousian M, Huijgen NA. et al. Periconceptional maternal ‘high fish and olive oil, low meat’ dietary pattern is associated with increased embryonic growth: The Rotterdam Periconceptional Cohort (Predit Study). Ultrasound Obstet Gynecol 2017;50:709–16. [DOI] [PubMed] [Google Scholar]
- 58. Parisi F, Rousian M, Steegers-Theunissen RPM. et al. Early first trimester maternal ‘high fish and olive oil and low meat’ dietary pattern is associated with accelerated human embryonic development. Eur J Clin Nutr 2018;72:1655–62. [DOI] [PubMed] [Google Scholar]
- 59. Parisi F, Rousian M, Koning IV. et al. Periconceptional maternal dairy-rich dietary pattern is associated with prenatal cerebellar growth. PLoS One 2018;13: e0197901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Uitert EM, Van der Elst-Otte N, Wilbers JJ. et al. Periconception maternal characteristics and embryonic growth trajectories: the Rotterdam Predict study. Hum Reprod 2013;28:3188–96. [DOI] [PubMed] [Google Scholar]
- 61. Van Uitert EM, Exalto N, Burton GJ. et al. Human embryonic growth trajectories and associations with fetal growth and birthweight. Hum Reprod 2013;28:1753–61. [DOI] [PubMed] [Google Scholar]
- 62. Van Uitert EM, Van Ginkel S, Willemsen SP. et al. An optimal periconception maternal folate status for embryonic size: The Rotterdam predict study. BJOG 2014;121:821–29. [DOI] [PubMed] [Google Scholar]
- 63. Wijnands KP, Van Uitert EM, Roeters van Lennep JE. et al. The periconception maternal cardiovascular risk profile influences human embryonic growth trajectories in IVF/ICSI pregnancies. Hum Reprod 2016;31:1173–81. [DOI] [PubMed] [Google Scholar]
- 64. Willemsen SP, Eilers PH, Steegers-Theunissen RPM, Lesaffre E.. A multivariate Bayesian model for embryonic growth. Stat Med 2015;34:1351–65. [DOI] [PubMed] [Google Scholar]
- 65. Roelants JA, Vermeulen MJ, Koning IV. et al. Foetal fractional thigh volume: an early 3D ultrasound marker of neonatal adiposity. Pediatr Obes 2017;12:65–71. [DOI] [PubMed] [Google Scholar]
- 66. Oostingh EC, Steegers-Theunissen RPM, De Vries JHM, Laven JS, Koster MPH.. Strong adherence to a healthy dietary pattern is associated with better semen quality, especially in men with poor semen quality. Fertil Steril 2017;107:916–23. [DOI] [PubMed] [Google Scholar]
- 67. Huijgen NA, Louwers YV, Willemsen SP, De Vries JHM, Steegers-Theunissen RPM, Laven JSE.. Dietary patterns and the phenotype of polycystic ovary syndrome: the chance of ongoing pregnancy. Reprod Biomed 2017;34:668–76. [DOI] [PubMed] [Google Scholar]
- 68. Daan NM, Koster MPH, Steegers-Theunissen RPM, Eijkemans MJ, Fauser BC.. Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil Steril 2017;107:261–68. [DOI] [PubMed] [Google Scholar]
- 69. Van den Berg CB, Chaves I, Herzog EM, Willemsen SP, Van der Horst GT, Steegers-Theunissen RPM.. Early- and late-onset preeclampsia and the DNA methylation of circadian clock and clock-controlled genes in placental and newborn tissues. Chronobiol Int 2017;14:1–12. [DOI] [PubMed] [Google Scholar]
- 70. Güzel C, den Berg CB, Duvekot JJ. et al. Quantification of calcyclin and heat shock protein 90 in sera from women with and without preeclampsia by mass spectrometry. Proteomics Clin Appl 2019;13: e1800181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.