Unique circulating regulatory T cell phenotypes distinguish hospitalized patients with SARS-CoV-2.
Abstract
Despite recent studies of immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), little is known about how the immune response against SARS-CoV-2 differs from other respiratory infections. We compare the immune signature from hospitalized SARS-CoV-2–infected patients to patients hospitalized prepandemic with influenza or respiratory syncytial virus (RSV). Our in-depth profiling indicates that the immune landscape in SARS-CoV-2 patients is largely similar to flu or RSV patients. Unique to patients infected with SARS-CoV-2 who had the most critical clinical disease were changes in the regulatory T cell (Treg) compartment. A Treg signature including increased frequency, activation status, and migration markers was correlated COVID-19 severity. These findings are relevant as Tregs are considered for therapy to combat the severe inflammation seen in COVID-19 patients. Likewise, having defined the overlapping immune landscapes in SARS-CoV-2, existing knowledge of flu and RSV infections could be leveraged to identify common treatment strategies.
INTRODUCTION
The current coronavirus (CoV) pandemic began in Wuhan, China in 2019 with an outbreak of what would later be designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1–3). To date, SARS-CoV-2 has led to devastating disease [called coronavirus disease 2019 (COVID-19)], death, and economic instability on a global scale (4). Despite the unprecedented rapid design and large-scale testing of SARS-CoV-2 vaccines, vaccine hesitancy, delays in global implementation, and emerging variants raise concerns that SARS-CoV-2, as well as the next pandemic respiratory infection, will continue to pose a threat to humans, underscoring the vital need for identification of additional therapeutics.
Many investigations to date have focused on characterizing the immune responses to natural SARS-CoV-2 infection in an effort to understand disease pathogenesis and reveal potential therapeutic targets. We reasoned that these extraordinary efforts to understand COVID-19 disease pathogenesis and improve treatment options could be leveraged for other respiratory infections if there is substantial congruence in the underlying immune response. In the vast majority of such SARS-CoV-2 studies, immune responses have been measured using human blood samples, comparing healthy controls to asymptomatic patients with SARS-CoV-2 or COVID-19 patients with varying degrees of disease severity (5). Both the innate and adaptive arms of the immune response to SARS-CoV-2 have been profiled to date. Initial studies focusing on innate immunity demonstrated that the type I and type III interferon (IFN) response is blunted in early stages of the response to SARS-CoV-2, although interleukin-6 (IL-6) and chemokines are elevated (6, 7). Notably, this varied from the response to other respiratory viral infections, including human parainfluenza virus 3 and respiratory syncytial virus (RSV), which induced potent type I and III IFN responses (6) and could suggest fundamental differences in the immune landscape of different respiratory infections. Further, data derived from comparing healthy controls and patients with severe COVID-19 identified an early reduction in type I IFNs in patients with the most severe or critical disease, as well as enhanced proinflammatory IL-6 and tumor necrosis factor (TNF) responses (8–11). In association with this depressed type I IFN response, patients with critical cases of COVID-19 have a corresponding decrease in frequency of professional type I IFN–producing cells, plasmacytoid dendritic cells (pDCs) (8). The frequency of natural killer (NK) cells was significantly diminished in SARS-CoV-2 patients with acute respiratory distress syndrome (ARDS) as compared to healthy donors, although patients with more severe disease had NK cells with increased expression of activation and cytotoxic molecules (12, 13). In addition, increased frequencies of neutrophils have been identified in patients with severe COVID-19 as compared to patients with milder disease or healthy donors (9, 10, 12, 14), congruent with a hyperinflammatory state.
Patients infected with SARS-CoV-2 also raise detectable adaptive immune responses in the form of both B and T cell responses specific to SARS-CoV-2 (15–23). In addition, circulating conventional T cell phenotypes have been extensively profiled in patients infected with SARS-CoV-2 with asymptomatic or mild to critical disease, and several differences in the dynamics of immune cells have been noted, including increased abundance of activated T cells in patients with the most severe COVID-19 disease as compared to healthy controls (9, 12, 22–24). Moreover, two groups have noted a significant decrease in abundance of circulating regulatory T cells (Tregs) (CD3+CD4+CD25+CD127lo) in patients with severe COVID-19 as compared to patients with non-severe disease or healthy donors (10, 25). Notably, one study identified a decrease in airway Tregs in patients with COVID-19 compared to healthy controls (26), raising the possibility that a Treg deficit at the lung site could be contributing to the disease.
Altogether, these data suggest that a dysregulated state of hyperinflammation is associated with severe COVID-19. However, it remains largely unclear whether this dysregulated hyperinflammatory state is unique to COVID-19 or is a feature of severe disease with respiratory viral infections more generally. A recent study comparing inflammatory profiles in patients infected with SARS-CoV-2 or influenza virus (flu) found several notable differences between such patients. These consisted of lower cytokine levels and reduced circulating monocyte counts in patients with SARS-CoV-2 as compared to flu, although circulating lymphocyte counts did not differ in patients with the two distinct infections (27). They concluded that SARS-CoV-2 patients have a less inflamed peripheral immunotype as compared to patients infected with flu, although other respiratory viruses were not examined. Thus, we designed a study wherein circulating immune signatures were compared among healthy human donors and hospitalized patients with SARS-CoV-2, flu, or RSV infection. Hospitalized patients infected with either of the three viruses were further classified as having moderate, severe, or critical disease on the basis of the type of provided oxygen supplementation, thereby allowing for comprehensive comparisons of immune cell abundance and phenotype across a range of disease severity. In general, our deep immune profiling revealed similar cellular and cytokine immune landscapes in hospitalized patients infected with SARS-CoV-2, flu, or RSV compared to healthy donors. However, unique to COVID-19 patients with the most critical disease was a significant increase in the frequency of Tregs in the circulation, as well as phenotypic changes indicating increased suppressive capacity and tissue-migratory patterns. Our previously uninvestigated findings have clinical implications, as treatments used for COVID-19 may be useful in mitigating severe flu or RSV, as well as future pandemic respiratory diseases. Furthermore, Tregs may provide a potential therapeutic target for COVID-19.
RESULTS
Deep immune profiling of a unique cohort of patients reveals shared circulating immune cell composition between respiratory infections
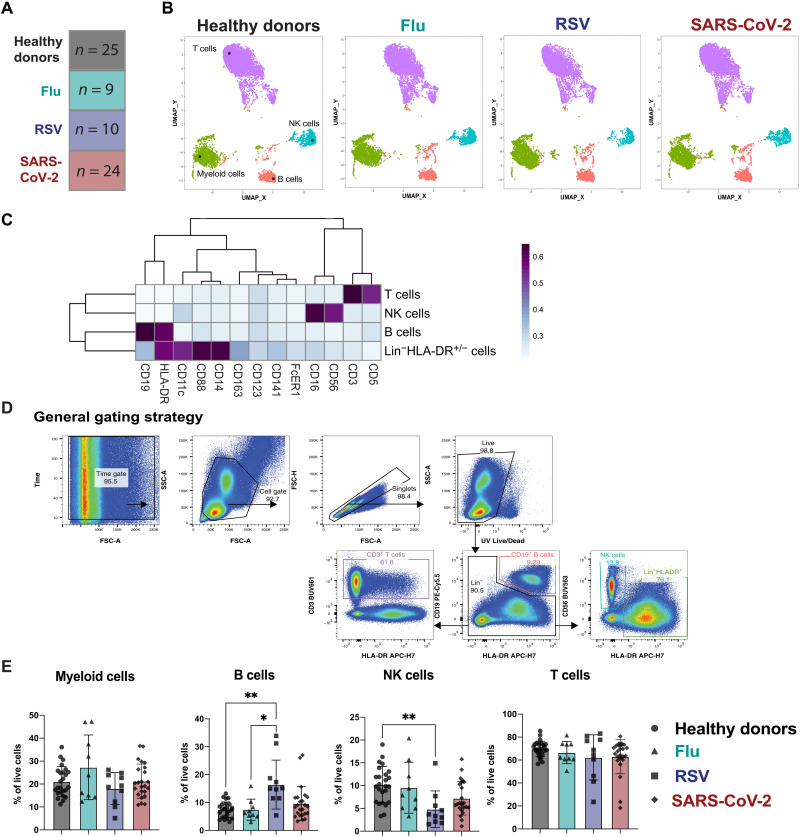
We analyzed peripheral blood mononuclear cells (PBMCs) from a unique cohort of age- and sex-matched patients hospitalized with respiratory infections including flu, RSV, or SARS-CoV-2 compared to PBMCs from healthy donors (Fig. 1A). The patients infected with SARS-CoV-2, flu, or RSV required varying degrees of oxygen supplementation, and patients experienced varying clinical outcomes, from moderate disease to death (Table 1). The PBMCs from hospitalized patients with RSV or flu were all collected before the SARS-CoV-2 pandemic. To extensively characterize the cellular immunotypes present in the peripheral blood of patients hospitalized with flu, RSV, or SARS-CoV-2 infection compared to healthy donors, we combined several high-parameter flow cytometry panels to profile myeloid cells [adapted from (28)], T cells, NK cells, and Tregs (table S1). For exploratory analysis of this high-dimensional dataset, we used clustering by FlowSOM (29, 30) and dimensionality reduction with uniform manifold approximation projection (UMAP) (31), which revealed similar distributions of cell populations between healthy donors and all three respiratory infections (Fig. 1B). A heatmap of markers to identify cell populations distinguished the main clusters as Lin−HLADR+ myeloid cells, B cells, T cells, and NK cells (Fig. 1C). A key feature of SARS-CoV-2 infection that has emerged through recent studies is lymphopenia (2, 7, 32, 33). Lymphopenia can also result from other respiratory infections such as flu and RSV, but this generally occurs early after onset of symptoms and is rapidly resolved (34). Thus, we wanted to compare alterations in immune subsets across respiratory infections to determine whether patients within our SARS-CoV-2 cohort were experiencing similar levels of immune alteration to patients with RSV or flu versus healthy donors. Manual gating of the flow cytometry data by a conventional gating strategy to determine the main immune populations confirmed the observations seen in the metaclustering data from FlowSOM (Fig. 1D). In the Lin−HLA-DR+ compartment, we did not see significant alterations in the overall frequency across groups. We observed a significant increase in the frequency of B cells in patients hospitalized with RSV compared to patients hospitalized with flu or to healthy donors. We saw no significant alterations in the frequency of total circulating T cells across all respiratory infections compared to healthy donors. Last, the frequency of NK cells was significantly reduced only in patients infected with RSV compared to healthy donors (Fig. 1E). While previous studies have indicated a reduction in NK cell populations after infection with SARS-CoV-2 as compared to healthy donors (12, 13), our data indicate that this phenomenon is likely not specific to SARS-CoV-2 infection but is seen across additional respiratory infections as well. While the frequencies of these cell populations may not directly correlate with the total number of cells found in the blood, we concluded that the overall immune populations remain similar between respiratory infections.
Fig. 1. Deep immunological profiling of a unique cohort of patients reveals circulating immune profiles between respiratory infections.
(A) Overview of the cohort. The number in each box indicates the number of donors per group. Criteria of inclusion are depicted in Table 1. Among the SARS-CoV-2 patients, four died from COVID-19. (B to D) Previously frozen PBMCs isolated from each group of the cohort were stained using the APC panel (see table S1). (B) FlowSOM was used to visualize the main immune cell populations found in the PBMCs from healthy donors or infected patients. (C) Heatmaps generated by FlowSOM and used to identify the main immune population. (D) Manual gating used to assess the frequencies of the main immune subsets; (E) bar graphs showing these frequencies for each group of our cohort. All data include at least nine patients per group (Table 1) and are represented as means ± SD. Statistical analyses were performed using Kruskal-Wallis test with Dunn’s multiple comparisons test. *P < 0.05; **P < 0.01.
Table 1. Demographic and clinical information for study patient cohorts.
n.a., not applicable for healthy donors; ACTT, Adaptive COVID-19 Treatment Trial.
| Healthy donors | Flu | RSV | SARS-CoV-2 | |
| Number of patients | 25 | 9 | 10 | 24 |
| Age, median (range) | 60 (33–79) | 59 (36–70) | 57.5 (38–71) | 62 (23–88) |
| Sex (female/male) | (12/13) | (4/5) | (4/6) | (11/13) |
| Race, n (%) | ||||
| White | 20 (80) | 4 (44.4) | 5 (50) | 11 (45.9) |
| Black/African American | 1 (4) | 2 (22.3) | 3 (30) | 3 (12.5) |
| Native Hawaiian/Pacific Islander | 1 (11) | 1 (4.1) | ||
| American Indian/Alaskan Native | 2 (20) | |||
| Hispanic | 1 (4) | 2 (22.3) | 6 (25) | |
| Asian | 3 (12) | 3 (12.5) | ||
| Days since symptom onset to sample collection, median (range) |
n.a. | 7+ (0–7+)* | 14 (2–21) | 14.5 (2–47) |
| Comorbidities, n | ||||
| None | 17 | 3 | 2 | 7 |
| Diabetes mellitus | 1 | 1 | 9 | |
| Hypertension | 3 | 12 | ||
| Chronic liver disease | 4 | 2 | ||
| Chronic kidney disease | 2 | 5 | ||
| Congestive heart failure | 1 | 2 | 6 | |
| Cardiovascular disease | 3 | 3 | 3 | |
| Asthma | 3 | 3 | 2 | 2 |
| COPD/emphysema | 1 | 1 | 3 | |
| Other chronic lung disease | 3 | |||
| Sleep apnea | 7 | |||
| Malignancy | 2 | 3 | ||
| ACTT clinical status categories, n | n.a. | |||
| Not requiring supplemental O2–ongoing medical care |
4 | 6 | 6 | |
| Required supplemental O2 | 3 | 0 | 8 | |
| Noninvasive ventilation or high flow O2 device |
1 | 1 | 4 | |
| Intubation | 1 | 3 | 3 | |
| ECMO | 0 | 0 | 1 | |
| Death | 0 | 0 | 4 | |
| Outcome, n (%) | n.a. | |||
| Discharged | 9 (100) | 10 (100) | 20 (83.3) | |
| Death | 0 (0) | 0 (0) | 4 (16.7) | |
| Treatment, n | n.a. | |||
| Tocilizumab | 0 | 0 | 4 | |
| Steroids | 1 | 4 | 4 | |
| Remdesivir | 0 | 0 | 8 | |
| Hydroxychloroquine | 0 | 0 | 10 | |
| Convalescent plasma | 0 | 0 | 1 | |
| Oseltamivir | 9 | 0 | 0 |
*Hospitalized flu patients were given the option “a week or more” for symptom duration.
A decreased frequency of dendritic cell subsets is common across hospitalized individuals with respiratory infections compared to healthy donors
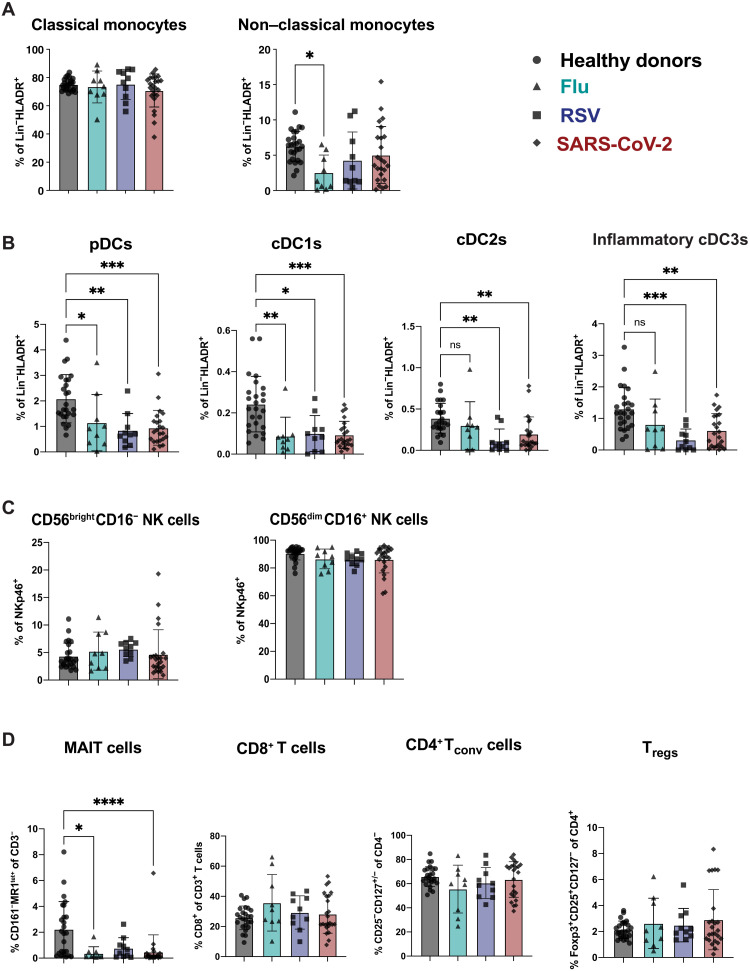
For an in-depth analysis of these immune cell populations, we leveraged four high-parameter flow cytometry panels focusing on antigen-presenting cells (APCs) (APC panel), NK cells (NK cell panel), T cells (T cell panel), and Tregs (Treg panel) (table S1). Our analysis of APC followed recently suggested phenotyping guidelines to separate classical and non-classical monocytes, as well as four distinct dendritic cell (DC) subsets: pDC, cDC1, cDC2, and the newly defined inflammatory cDC3 (fig. S1A) (35, 36). None of the infections led to a significant alteration in frequency of classical monocytes compared to healthy donors. While a decrease in non-classical monocyte frequency has been reported with SARS-CoV-2 infection (37), our data only showed a significant decrease of the non-classical monocytes for flu while a trend toward reduction was observed for RSV and SARS-CoV-2–infected patients (Fig. 2A). In a similar manner to Zhou et al. (38), we observed a reduced frequency across several DC subsets in SARS-CoV-2 compared to healthy donors. Of note, this decrease in the frequency of pDC, cDC1, cDC2, and inflammatory cDC3 was also observed in flu- and RSV-infected patients (Fig. 2B). When we assessed the frequencies of the CD56brightCD16− as well as the CD56dimCD16+ NK cell subsets (fig. S1B), we found no significant differences when compared across respiratory infections and healthy donors (Fig. 2C).
Fig. 2. Decreased frequency of DC subsets is common across respiratory infections compared to healthy donors.
Previously frozen PBMCs isolated from each group of the cohort were stained using four high-parameter flow cytometry panels. Manual gating was used to estimate the frequencies of (A) the monocyte family (fig. S1A), (B) the DC family (fig. S1A), (C) the NK cell family (fig. S1B), and (D) the T cell family (fig. S1C) for each group of the cohort. All data include at least nine patients per group (Table 1) and are represented as means ± SD. Depending on the distribution of our data, statistical analyses were performed using Kruskal-Wallis test with Dunn’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ns, not significant.
Last, we observed a reduction in peripheral mucosal-associated invariant T cells across all respiratory infections (Fig. 2D and fig. S1C), similar to what has been previously documented in patients with severe COVID-19 compared to healthy donors (39). We did not observe significant reductions compared to healthy donors in the T cell compartment of patients with any respiratory infection when we examined individual CD4 and CD8 T cell subsets (Fig. 2D). Furthermore, there was no statistically significant difference in the frequency of CD25+CD127−Foxp3+ Tregs in patients with any of the respiratory infections compared to healthy donors (Fig. 2D). These trends held true when examining frequency on the basis of the parent gate or the frequency of these populations as percentage of live cells (fig. S2). Thus, in terms of overall immune cell subset distribution, we found that the relative abundance of NK cell family and T cell family subsets remained unchanged between infected patients and healthy donors. We also found a congruent reduction in circulating DC subsets for flu, RSV, and SARS-CoV-2 patients as compared to healthy donors. Overall, our results indicate that these immune cell changes are a general feature of immune responses to respiratory virus infections rather than a unique signature of SARS-CoV-2 infection.
Immune cell phenotypic changes are similar between respiratory infections consistent with a respiratory virus signature
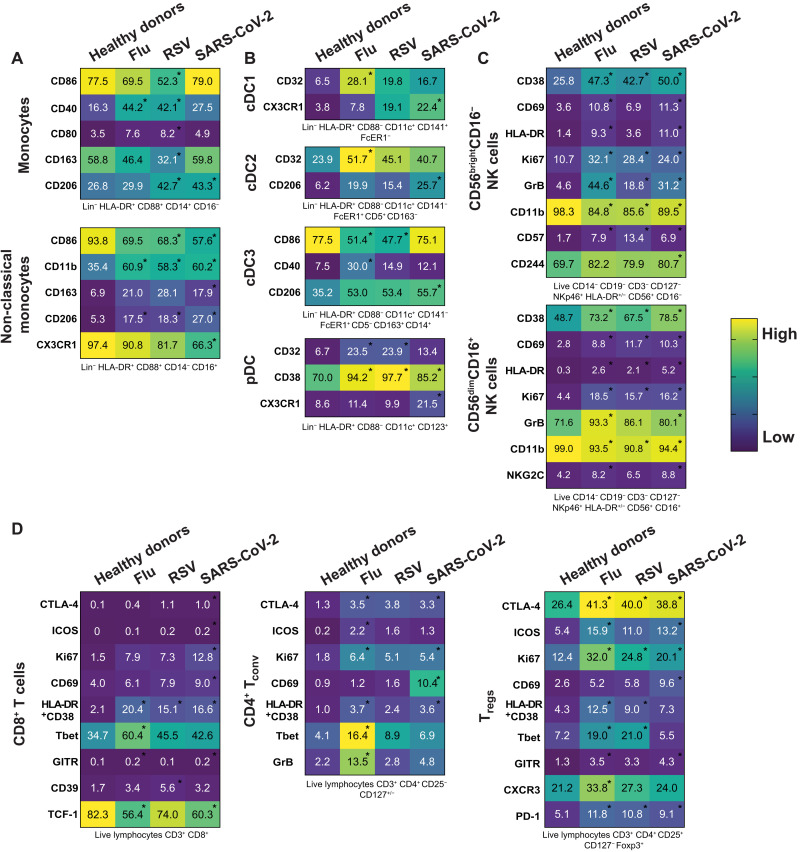
After observing minimal changes in the frequency of various immune cell subsets between respiratory viral infections, we next wanted to more comprehensively assess the expression of various markers of activation, maturation, and migration among monocytes, DC, NK cells, CD8+ T cells, CD4+ conventional T cells (Tconv), and CD4+ Tregs. Some markers did not show any change either across infection or compared to healthy donors and are displayed unabridged in figs. S3 to S5. In instances wherein we observed a significant difference for one of the infections compared to healthy donors, this difference was usually seen across multiple respiratory infections, consistent with a respiratory virus signature (Fig. 3, A to D). Specifically, we observed in the monocyte population an increase in CD40 and CD206 expression in multiple, but not all, respiratory infections. Non-classical monocytes had a significant increase in CD11b and CD206 across all respiratory infections compared to healthy donors (Fig. 3A). We observed few significant changes in the DC subsets, one being in the cDC3 population; the fraction of CD86-expressing cells was significantly lower in patients with flu and RSV, while CD206 was significantly higher in patients with SARS-CoV-2 (Fig. 3B). The frequency of pDCs expressing CD32 or CD38 was significantly increased in patients with any respiratory infection as compared to healthy donors (Fig. 3B). In the two NK cell subsets, we observed a strong NK cell activation signature in patients hospitalized with respiratory viral infections, characterized by an increased frequency of CD38, CD69, HLA-DR, Ki67, and granzyme B (Fig. 3C). While others have shown this activated phenotype in NK cells after SARS-CoV-2 infection (12, 13), we demonstrate that this phenotype is also a feature of NK cells in patients with other severe respiratory infections compared to healthy donors (Fig. 3C).
Fig. 3. Immune cell phenotypic changes are consistent with a respiratory virus signature.
(A to D) Heatmaps representing the expression pattern for all the indicated molecules within the main subsets of (A) the monocyte family, (B) the DC family, (C) the NK cell family, and (D) the T cell family for each group of the cohort. Gating strategy of the different subsets can be found under each heatmap (also see fig. S1), and numbers inside boxes represent the mean of frequency for each marker among the specific subsets. Depending on the distribution of our data, statistical analyses were performed using either one-way ANOVA or Kruskal-Wallis test with Tukey’s or Dunn’s multiple comparisons test, respectively. Asterisk inside the boxes is indicative of a significant difference compared to the healthy donors’ group and can include a P value from 0.05 to 0.0001.
Last, we found T cell subsets to have increased frequency of markers related to activation and effector function in individuals who were hospitalized for SARS-CoV-2 and other respiratory virus infections compared to healthy donors (Fig. 3D). In particular, CD8 and CD4 Tconv cells positive for HLA-DR and CD38 were increased in patients hospitalized with flu, RSV, and SARS-CoV-2 compared to healthy donors. There was also an increase in the fraction of CD4 Tconv cells expressing cytotoxic T-lymphocyte associated protein-4 (CTLA-4) or Ki67 across all infections compared to healthy donors (Fig. 3D). Last, we also observed an increased frequency of Tregs expressing activation and suppression markers CTLA-4, inducible T cell costimulator (ICOS), Ki67, HLA-DR/CD38, and programmed cell death protein 1 (PD-1) in patients with respiratory viruses compared to healthy donors (Fig. 3D). It has been demonstrated during SARS-CoV-2 infection that NK and T cell subsets have increased activation and function compared to healthy donors (12, 13, 40), and we hereby demonstrate that this phenomenon is not specific to SARS-CoV-2 infection but rather spans multiple respiratory viral infections. We highlighted phenotypic marker alterations consistent between flu, RSV, and SARS-CoV-2 infections compared to healthy donors, and we propose that these markers are consistent with a common circulating immune signature to respiratory virus infection.
Unsupervised complex phenotype discovery analysis reveals a SARS-CoV-2–specific signature including complex Treg phenotypes
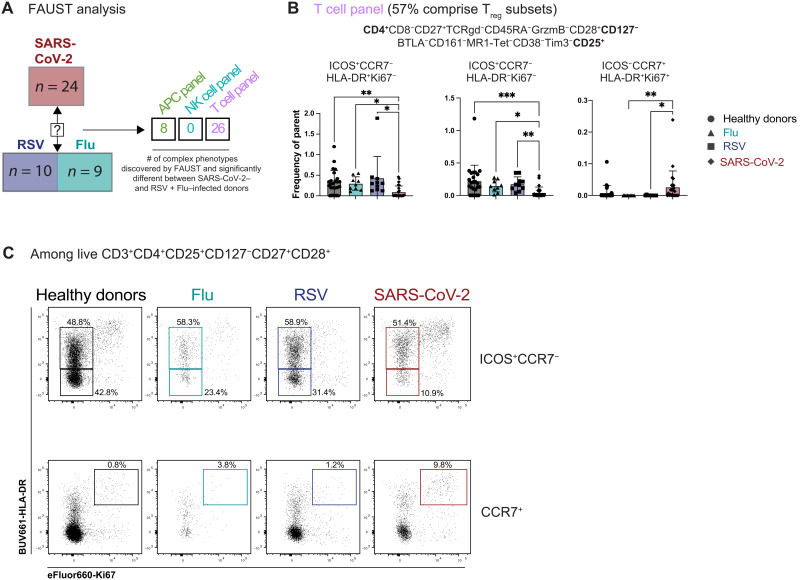
To specifically search for infection-specific changes in immune cell subsets in an unsupervised manner, we applied a recently developed nonparametric method for unbiased complex phenotype discovery called full annotation using shape-constrained trees (FAUST) (41). Briefly, FAUST performs data-driven phenotype discovery and annotation on a per-sample basis, enabling the identification of statistically different complex immune phenotypes between the different groups of our cohort (table S2). We tested for differences in immune phenotypes between SARS-CoV-2 cohorts relative to flu and RSV to determine whether there were any complex immune cell phenotypes unique to SARS-CoV-2 infection using our high-parameter flow panels (table S1). Data from the APC panel revealed eight distinct phenotypes with significant differences in cell frequency when comparing SARS-CoV-2 (all severity levels) to flu and RSV (Fig. 4A and table S2). There were no significant complex phenotypes discovered using the NK cell panel when patients with all SARS-CoV-2 severity levels were compared to patients with RSV or flu (Fig. 4A and table S2), in agreement with the NK cell analysis shown in Fig. 3, demonstrating that alterations in immune cell populations are largely consistent between respiratory infections. However, using the T cell panel in combination with FAUST analysis revealed 26 complex immune cell phenotypes that differed significantly in patients with SARS-CoV-2 infection with any level of disease severity compared to flu and RSV (Fig. 4A). Notably, most of these significantly different T cell phenotypes were CD4+CD25+CD127− and thus comprising a Treg population (Fig. 4B and table S2). Several subsets of CD4+CD25+CD127− Treg that were CD45RA−CCR7− (effector memory phenotype) were decreased in SARS-CoV-2 samples compared to the other respiratory infections and healthy donors, including a CD27+CD28+ICOS+HLA-DR+Ki67− and a CD27+CD28+ICOS+HLA-DR−Ki67− subset (Fig. 4B). In contrast, a subset of CD4+CD25+CD127− Treg that is CD45RA−CCR7+ (central memory phenotype) that coexpresses CD27, CD28, Ki67, and HLA-DR was significantly increased in the circulation of patients with SARS-CoV-2 compared to flu or RSV (Fig. 4B). We confirmed these populations by manual gating of our flow cytometry data (Fig. 4C). While the functional relevance of these cells is unclear, it is noteworthy that these complex Treg phenotypes distinguish SARS-CoV-2 infection compared to other respiratory infections, flu, and RSV. In summary, while much of the immune landscape is shared across these respiratory infections, our unsupervised analysis approach reveals unique complex phenotypes in the circulating Treg population that distinguishes patients infected with SARS-CoV-2 compared to flu or RSV.
Fig. 4. Unsupervised analysis reveals a SARS-CoV-2–specific signature including complex Treg phenotypes.
(A) FAUST analysis was used to discover complex phenotypes in the APC, NK, and T cell panels. The multiple comparisons were adjusted using the Bonferroni correction, and the numbers in the table are showing the number of identified phenotypes that are significantly different for SARS-CoV-2–infected patients compared to the flu- and RSV-infected patients for each panel, with Bonferroni-adjusted P values under 0.05 considered significant. (B) Example of three Treg phenotypes identified by FAUST within the T cell panel as shown in table S2. Bar graphs display the frequency of the phenotype for each group of the cohort among live CD3+ cells. Data are represented as means ± SD. Statistical analyses displayed were performed using Kruskal-Wallis test with Dunn’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001. (C) Representative flow plots showing the expression pattern of Ki67+HLA-DR+ cells among either CCR7−ICOS+ or CCR7+, live, CD3+CD4+CD25+CD127−CD27+CD28+ among the different groups of the cohort. Manual gating was performed using the T cell panel.
Proinflammatory cytokines and chemokines are increased during respiratory infections compared to healthy donors
We next sought to determine whether measuring cytokine and chemokine concentrations in the serum would provide additional insight to explain the overlap in immune phenotypes as well as the differences in Treg phenotypes. We tested serum samples from a subset of the cohort described in Table 1 to quantify 71 different cytokines and chemokines (table S3). This analysis revealed a significant increase in serum IL-6 levels compared to healthy controls in both SARS-CoV-2 and RSV patients (fig. S6A). Others have shown an increase in IL-6 to be consistent with SARS-CoV-2 infection (7, 42, 43), and here, we demonstrate that IL-6 is significantly elevated in the serum of RSV patients as well. Because IL-6 is an important proinflammatory cytokine during mucosal infections (44), we wanted to examine whether other proinflammatory cytokines were increased during respiratory viral infections compared to SARS-CoV-2. We observed a significant increase in IL-8, IL-15, and IL-10 during both SARS-CoV-2 infection and RSV infection (fig. S6A). We did not see a large increase in proinflammatory cytokine levels in patients infected with flu. However, this could be due to the reduced number of serum samples available in our cohort from flu patients (n = 3). Inflammatory mediators such as IFNs, IL-1α, and IL-1β have also been reported to be increased in patients with COVID-19 (2), although some reports have demonstrated very low levels of type I IFNs (IFNα and IFNβ) (6). In our cohort of patients, we observed increased levels of IL-1β and IL-1α in patients with RSV compared to healthy donors, but no difference between healthy donors and SARS-CoV-2 or flu patients. Furthermore, there was no difference in type I or type II IFNs in patients infected with SARS-CoV-2 compared to healthy donors, although serum IFNα was significantly increased during RSV infection compared to either healthy donors or SARS-CoV-2 patients (fig. S6A). We also observed a significant elevation of IL-1RA during both SARS-CoV-2 and RSV infection (fig. S6A). Inflammatory chemokines were significantly increased during respiratory infection compared to healthy donors, with serum levels of CXCL9 and CXCL10 elevated during SARS-CoV-2 infection, although CXCL9 was also significantly increased in the context of RSV infection compared to healthy donors (fig. S6B). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has previously been correlated with viral load during SARS-CoV-2 infection (9), but we found it to be significantly decreased in the serum of RSV and SARS-CoV-2 patients compared to healthy donors in our cohort (fig. S6B). In addition, CCL17 and CCL22, chemokines known to be involved in the mobilization of immune cells to the lungs (45, 46) and reported to be increased in SARS-CoV-2 infection (9, 47, 48), were decreased or unchanged in the serum compared to healthy donors in our cohort. These findings could be due to the timing of the sample collection from symptom onset; studies have shown that trafficking of immune cells by these chemokines are most elevated as early as 1 week after infection (47), and because all of our samples came from hospitalized patients, some were collected weeks after symptom onset (Table 1). To test whether cytokines from SARS-CoV-2 patients significantly different from healthy controls were due to the different times of sample collection after symptom onset, we graphed IL-6, IL-8, and IL-10 by days after symptom onset (fig. S6C). We saw no significant difference in cytokine expression between different days after symptom onset. Last, because of variability in the quantities of these cytokines and chemokines detected in serum of SARS-CoV-2 patients, we next assessed whether levels of these cytokines and chemokines differed by severity of COVID-19, as defined by oxygen supplementation requirements (COVID-19 moderate, severe, or critical). Serum levels of IL-6, IL-8, and IL-10 were all significantly increased in patients with critical COVID-19 as compared to healthy donors, thereby suggesting that these cytokines are a feature of critical disease (fig. S6D). In addition, the chemokines CXCL9 and CXCL10 were significantly elevated in patients with increased COVID-19 severity (fig. S6E). Overall, we demonstrated that several cytokines and chemokines previously associated with SARS-CoV-2 infection were also elevated in the serum of patients hospitalized with other respiratory infections and so may not be a unique feature of COVID-19.
Markers of cellular activation among NK and T cells are increased after COVID-19 to varying degrees
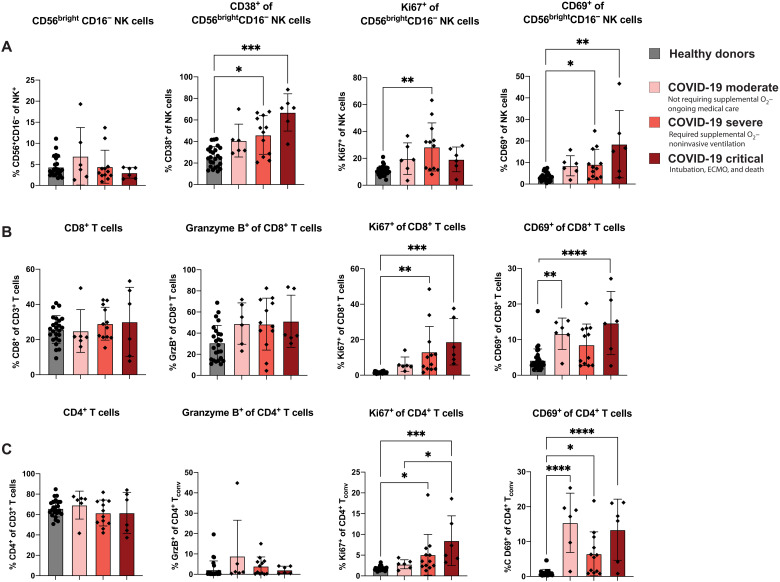
On the basis of the increase in proinflammatory cytokines and chemokines with increasing COVID-19 disease severity (fig. S6, D and E), we wanted to determine whether effector immune cell subsets were also altered with COVID-19 severity in our cohort. In the CD56brightCD16− population of NK cells, characterized as being cytokine producers with proliferative potential (49), we observed an increased expression of both CD38 and CD69 for patients with severe and critical COVID-19 compared to healthy donors (Fig. 5A). We also observed an increased expression of Ki67 among CD56brightCD16− NK cells of patients with severe COVID-19 compared to healthy donors.
Fig. 5. Markers of cellular activation among NK and T cells are increased after COVID-19 to varying degrees.
SARS-CoV-2–infected patients were grouped based on the severity of the disease, and markers of cellular activation were analyzed among (A) CD56brightCD16− NK cells, (B) CD8+ T cells, and (C) CD4+ T cells for healthy donors as well as moderate COVID-19 (n = 6), severe COVID-19 (n = 12), and critical COVID-19 (n = 6). All data are represented as means ± SD. Depending on the distribution of our data, statistical analyses were performed using either one-way ANOVA or Kruskal-Wallis test with Tukey’s or Dunn’s multiple comparisons test, respectively. *P< 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Upon examination of T cell activation status, we observed that the frequency of CD8+ T cells was not altered based on COVID-19 severity, nor was the frequency of CD8+ T cells expressing the cytotoxic molecule granzyme B (Fig. 5B). However, the frequency of CD8+ T cells expressing Ki67 was significantly elevated for both patients with severe and critical COVID-19 (Fig. 5B). We also found an increased CD69 expression among circulating CD8+ T cells in both patients with moderate and critical COVID-19 (Fig. 5B). A similar expression pattern of activation markers was observed in CD4+ Tconv cells; there was no change in the frequency of CD4+ T cells based on COVID-19 severity, and there was limited expression of granzyme B within the CD4+ T cell subset that did not vary by disease severity. However, the frequency of CD4+ T cells expressing either Ki67 or CD69 was increased in patients with COVID-19, with Ki67 increasing with disease severity (Fig. 5C). Again, consistent with a common respiratory virus infection signature, we found similar patterns of expression of these activating markers among NK cells, CD8+ T cells, and CD4+ T cells when comparing flu- and RSV- to SARS-CoV-2–infected samples based on severity (fig. S7, A to C). We next sought to confirm that any increase in markers of cellular activation was due to the severity of the disease and not related to days after symptom onset. When we examined these markers of cellular activation on NK cells and T cells by days after symptom onset to sample collection, we found no significant difference in cellular activation relating the days after symptom onset in our SARS-CoV-2 cohort (fig. S8). This suggests that our findings are associated with COVID-19 severity rather than timing of sample collection.
Tregs in patients with critical COVID-19 disease are increased in frequency and display a heightened activation signature
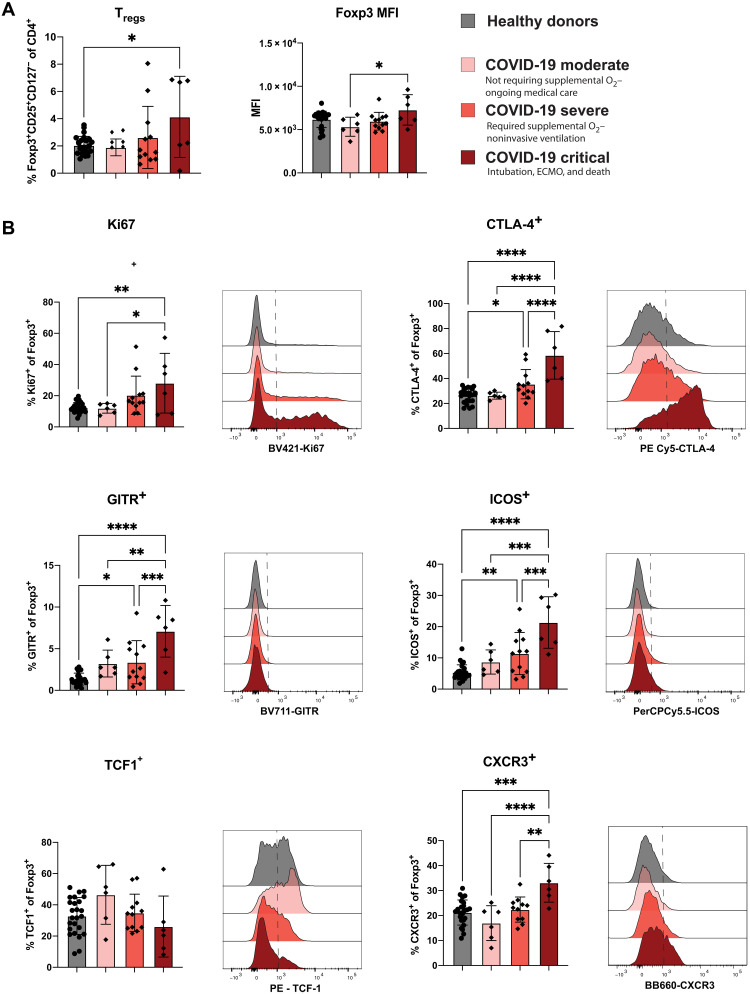
A hallmark of the immune response to SARS-CoV-2 infection in individuals with severe disease has been identified as a state of dysregulated and proinflammatory immunity (6–9, 12, 13, 24, 27, 32, 40, 50). We and others (51–56) have previously demonstrated that Tregs play a role in orchestrating the antiviral immune response by potentiating the antigen-specific T cell response. However, it is also evident that in the context of infections, including RSV and flu, Tregs can assist in restraint of immunity to reduce immunopathogenesis associated with a robust immune response (52, 56–62). Because we identified significant changes in the frequency of complex Treg phenotypes in patients with SARS-CoV-2 compared to flu and RSV infection (Fig. 4), we sought to further examine Treg phenotype based on COVID-19 severity. Because we also observed increased proliferation of CD8+ and CD4+ Tconv cells with increased COVID-19 severity, we hypothesized that Tregs could be involved in restraining this exuberant anti–SARS-CoV-2 immune response. A previous study found no significant difference in the frequency of Tregs in the circulation by COVID-19 severity (7), while two subsequent studies have identified a slight decrease in Treg frequency with increasing COVID-19 severity (10, 25). However, when we measured Treg frequency in healthy donors compared to patients with COVID-19 disease, we saw a significant increase in the frequency of CD25+CD127−Foxp3+ Tregs in COVID-19 critical patients only (Fig. 6A). This is consistent with a recent preprint report showing increased Treg frequency in COVID-19 patients with severe disease (63). We additionally measured the median fluorescent intensity of Foxp3 in the Tregs, as this has been shown to be an indicator of suppressive capabilities (64). We detected an increase in the level of Foxp3 expression by Tregs in the COVID-19 critical patients compared to patients with moderate disease (Fig. 6A). To test whether circulating Tregs from COVID-19 patients showed a more suppressive phenotype, we measured Ki67, CTLA-4, glucocorticoid-induced TNFR-related protein (GITR), and ICOS, all of which were significantly increased with COVID-19 disease severity (Fig. 6B). T cell factor 1 (TCF1), a transcription factor that has been shown to dampen Foxp3 activity (65, 66), appeared to be decreased with COVID-19 severity, albeit nonsignificantly, consistent with the notion of more functional Tregs in COVID-19 critical patients (Fig. 6B). Last, we wanted to determine whether Tregs were licensed to migrate to the lungs in COVID-19 patients by examining CXCR3 expression. We observed a significant increase in the frequency of CXCR3 expressing Tregs with increasing COVID-19 severity (Fig. 6B). Finally, we examined the Treg phenotype in samples from patients with flu and RSV to determine whether increasing disease severity results in an activation phenotype on Tregs across multiple respiratory infections (fig. S9). We found that Tregs were not increased with disease severity in flu/RSV infection (fig. S9A). While the frequency of Tregs expressing Ki67, CTLA-4, GITR, ICOS, and CXCR3 was increased in patients with flu/RSV compared to healthy donors, there were no statistically significant stepwise increase in moderate, severe, and critical flu/RSV disease outcomes (fig. S9B), and thus, the increase in activation phenotype on Tregs with increasing disease severity appears to be unique to COVID-19. Our data indicated that Tregs from patients infected with SARS-CoV-2 are highly activated in patients with critical COVID-19 and are potentially able to migrate toward a gradient of increasing CXCL9 and CXCL10 during SARS-CoV-2 infection.
Fig. 6. Tregs in patients with critical COVID-19 disease are increased in frequency and display a heightened activation signature.
(A) Bar graphs showing (left) the frequency of Treg and (right) the MFI of Foxp3 among parent for healthy donors and severity-based groups of COVID-19. (B) Representative histograms and quantification of the expression of activation and suppressive markers within Tregs for healthy donors as well as moderate COVID-19 (n = 6), severe COVID-19 (n = 12), and critical COVID-19 (n = 6). All data and are represented as means ± SD. Depending on the distribution of our data, statistical analyses were performed using either one-way ANOVA or Kruskal-Wallis test with Tukey’s or Dunn’s multiple comparisons test, respectively. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
DISCUSSION
More than a year into the COVID-19 pandemic, numerous studies of peripheral blood from individuals infected with SARS-CoV-2 have revealed that a hyperinflammatory and dysregulated immunotype is characteristic of COVID-19 patients compared to healthy donors. To identify unique aspects of anti–SARS-CoV-2 immunity that could underlie disease presentation and severity, a comparison with other common respiratory virus infections is required. However, there have only been a limited number of studies comparing immune phenotypes generated after SARS-CoV-2 infection to other respiratory viral infections (6, 27, 67). It was first demonstrated by Blanco-Melo et al. (6) that compared to other respiratory viral infections, including human parainfluenza virus 3 and RSV, SARS-CoV-2 elicits a blunted early type I and type III IFN response in vitro and in animal models. Through a single-cell RNA sequencing study of PBMCs from individuals with COVID-19 or severe flu, another group demonstrated that cells from COVID-19 patients had a predominantly IL-1β and TNF inflammatory signature, whereas flu patients had an increased IFN-stimulated gene response (67), thereby uncovering differential proinflammatory pathways elicited by distinct respiratory viral infections. Last, a recent study comparing immune responses in patients with severe flu or COVID-19 found that the latter exhibited similar lymphocyte counts but fewer monocytes and reduced HLA-DR expression on monocyte subsets as compared to flu patients (27). To link extensive immunophenotyping with inflammatory processes, we designed a study to comprehensively examine serum cytokines and chemokines as well as the immunotypes of myeloid cells, NK cells, T cells, and Tregs in the peripheral blood of patients hospitalized with flu or RSV compared to SARS-CoV-2 or healthy controls. We used high-parameter flow cytometry coupled with both unbiased computational analysis approaches as well as traditional manual gating to perform a comprehensive examination of many immune cell subsets as well as complex phenotypes. We reasoned that comparison of immune phenotypes between patients hospitalized with COVID-19 versus other severe respiratory virus infections could potentially reveal common immunotherapeutic strategies that can thus be leveraged in the battle against SARS-CoV-2 and future pandemic viruses. For example, knowledge of immune-targeting therapeutic strategies to treat SARS-CoV-2 could potentially be applied to the next pandemic respiratory virus, which may be flu or another CoV.
Analysis of our cohort demonstrates that the reduced frequencies of DC populations in PBMCs of infected patients compared to healthy donors is a common feature of all three different respiratory viral infections (Fig. 2). Specifically, the decreased numbers of pDCs and cDC1s is likely to contribute to impaired antiviral immune responses. pDCs are a critical early source of type 1 IFN, which induces several mechanisms aiming to limit viral replication (68). For cDC1s, a decrease in these cells can lead to impaired cross-presentation of viral antigens and, as a result, to suboptimal activation and priming of SARS-CoV-2–specific cytotoxic CD8 T cells. The role of inflammatory cDC3s during viral infection has not been systematically addressed. However, DC3s have been shown to specifically produce high amounts of IL-12 (35), which stimulate efficient T helper cell 1 responses, including the release of IFNg from activated CD4 helper T cells. In addition, we observed increased expression of the mannose receptor on DC3s (Fig. 3), and among other roles, CD206 has been suggested to inhibit effector T cell function (69). Thus, we hypothesize that the decreased numbers of pDCs, cDC1s, and DC3s together with their observed surface protein phenotype overall contribute to suboptimal induction of an antiviral immune response in SARS-CoV2 patients. Our results also revealed that most of the previously identified phenotypical alterations in peripheral immune populations during SARS-CoV-2 infection are not a distinguishing feature of the anti–SARS-CoV-2 immune response but rather indicate a more common immune landscape consistent with respiratory viruses in general. We have identified a respiratory virus–induced immune signature consistent across three different respiratory viral infections compared to healthy donors (Fig. 3, A to D), although future work with samples from additional patient cohorts is required to determine whether this is a more generalized signature of respiratory infection, including bacterial and fungal pathogens, or even a shared response to virus infection.
Despite this largely shared immune signature, the application of an unsupervised phenotype discovery (FAUST) tool revealed previously undescribed alterations of Tregs with various complex phenotypes in SARS-CoV-2 patients compared to those with flu or RSV (Fig. 4). More specifically, we detected a reduced frequency of effector memory phenotype (CD45RA−CCR7−) Tregs coexpressing various markers of activation, including ICOS, CD27, CD28, and HLA-DR present in the blood of patients with SARS-CoV-2 compared to flu or RSV (Fig. 4, B and C). This could reflect a reduction in activated Tregs able to migrate to the peripheral tissues including the lung, whereby they could participate in restraining immunopathology and limiting ARDS. In contrast, we identified a unique population of blood Tregs with a central memory phenotype (CD45RA−CCR7+) coexpressing CD27, CD28, Ki67, and HLA-DR that was present at a significantly increased frequency in SARS-CoV-2 patients compared to those with flu or RSV (Fig. 4, B and C). We speculate that these Tregs represent a circulating population of activated suppressive cells that may participate in restraining the inflammatory response in the context of COVID-19. However, whether this is of benefit to the host in the context of disease is an open question. Thus, additional studies are required to determine whether Treg-modulating therapies could be of benefit in limiting COVID-19 severity. We recently demonstrated that in a mouse model of SARS-CoV, an elevated steady-state, preinfection frequency of Tregs correlates with protection from high viral loads and disease upon infection (70), thereby contributing to the notion that Tregs could play a protective role in limiting disease. However, examination of prospectively collected pre–COVID-19 pandemic samples from humans that went on to become infected would be required to establish whether Treg abundance is predictive of viral loads or disease severity upon SARS-CoV-2 infection. Recent evidence suggests that the airways of patients with severe COVID-19 have reduced Treg frequencies compared to healthy airways (26), leading us to speculate that there may be a defective trafficking of Tregs from the circulation into the respiratory tract in the context of COVID-19, thus contributing to lung immunopathogenesis. We hypothesize that this may be due to the increased levels of CXCL10 and CXCL9 found in peripheral blood (fig. S6, B and E) that may retain CXCR3+ Treg in the periphery and prevent them from entering the airways via a chemokine gradient. In addition, the reduced frequency of effector memory phenotype Treg present in the blood of patients infected with SARS-CoV-2 compared to flu and RSV may indicate that Treg able to migrate to tissue sites may be diminished in the context of SARS-CoV-2. Thus, while additional studies of the mucosal immune response to SARS-CoV-2 are warranted, we speculate that immunotherapies designed to attract Treg out of the circulation and into the respiratory tract could be beneficial in limiting disease. Of note, there are several ongoing clinical trials that target various chemokine receptors, including CCR2 and CCR5, in an effort to minimize immune-mediated lung tissue damage (NCT04435522 and NCT04500418).
Our study has some limitations, the first of which is our exclusive focus on peripheral blood immune responses rather than tissue-specific responses. In addition, our cohort includes patient sample collection from variable times after symptom onset, from 0 to 47 days. This variability could clearly affect the types of immune phenotypes detected, as could variability in viral loads and durability of viral shedding, for which we are lacking data from most of the patients due to scarcity of testing in the early days of the pandemic. Last, while we were powered to uncover unique aspects of the circulating Treg phenotypes of patients with SARS-CoV-2 compared to flu or RSV, our relatively small N (Table 1) may have precluded identification of other distinguishing immune phenotypes. We also acknowledge that the different sampling time frames after infection or after symptom onset, as well as differing classifications of disease severity could account for differences in our results as compared to some previously published studies. Of note, a recent preprint corroborates our finding of increased Treg frequency in PBMCs from patients with severe COVID-19 (63).
In summary, our study based on high-dimensional flow cytometry data combined with several analysis methods reveals a largely similar immune landscape of patients hospitalized with respiratory virus infections, including SARS-CoV-2. This is further supported by our analysis of 71 soluble cytokines and chemokines in the blood of patients with SARS-CoV-2, flu, or RSV. The recent identification of novel SARS-CoV-2 variants that may increase transmission and alter vaccine efficacy (71) underscores the need for continued development of treatment strategies specifically for severe COVID-19 disease course. Thus, we speculate that the overlapping immune landscapes in SARS-CoV-2, flu, and RSV infections could be leveraged to identify and hasten common treatment strategies that could be leveraged for the response to the next pandemic respiratory virus. Unexpectedly, we identified that SARS-CoV-2 patients with the most critical disease presented with unique alterations in the Treg compartment, including an increase in a population of CD45RA−CCR7+Ki67+HLA-DR+ Tregs within the circulation compared to patients with flu or RSV, and an increase in CXCR3+ Tregs in the blood of patients with COVID-19, thus leading us to predict that Treg-targeting therapies could be useful in limiting disease associated with SARS-CoV-2. Additional studies of Tregs present in the respiratory tract of COVID-19 patients, as well as investigations into immunotherapeutic approaches to target multiple respiratory virus infections, will be useful in identifying additional therapeutic avenues that can curtail viral infection–mediated severe lung disease, including disease induced by future pandemic viruses.
MATERIALS AND METHODS
Design and sample collection
Study population
Study samples were collected as part of the prospective longitudinal cohort study HAARVI (Hospitalized or Ambulatory Adults with Respiratory Viral Infections) in Seattle, WA. Individuals 18 years or older were eligible for inclusion and were recruited from two groups: inpatients with laboratory confirmed respiratory viral infection and healthy controls. Inpatients were hospitalized at Harborview Medical Center, University of Washington Medical Center, or Northwest Hospital and identified through a laboratory alert system. A cohort of healthy individuals were enrolled in this study and were recruited through email and flyer advertising. They were considered eligible if they had no history of laboratory-confirmed SARS-CoV-2 infection and had not presented with flu-like illness in the past 30 days.
Participants or their legally authorized representatives completed informed consent. Sociodemographic and clinical data were collected from electronic chart review and from participants via a data collection questionnaire (Project REDCap) (72) at the time of enrollment. The questionnaire collected data on the nature and duration of symptoms, medical comorbidities, and care-seeking behavior. On the basis of these data, individuals were classified by disease severity using an eight-point ordinal clinical assessment scale (73). For our study, a clinical assessment score of 1 (death) or 2 [intubation and extracorporeal membrane oxygenation (ECMO)] was categorized as critical COVID-19. A score of 3 (noninvasive ventilation or high flow O2 device) or 4 (required supplemental O2) was categorized as severe COVID-19. Last, a score or 5 (not requiring supplemental O2) or 6 (no longer requires ongoing medical care) was categorized as moderate COVID-19. There were no participants with a clinical assessment score of 7 or 8 because our cohort solely consisted of hospitalized patients. All SARS-CoV-2 patient samples were collected after 1 March 2020. All flu and RSV patient samples were collected between 2017 and 2019 during flu season.
Ethics
The studies were approved by the University of Washington Human Subjects Institutional Review Board, IRB numbers STUDY00000959 and STUDY00002929.
Sample processing
Participant samples obtained before 1 March 2020 were collected in mononuclear cell processing (CPT, BD Biosciences) and serum tubes and immediately transferred to the University of Washington. Whole blood in serum tubes was allowed to clot by incubating for at least 1 h at room temperature, then centrifuged at 700g for 15 min, aliquoted, and stored at −20°C. CPT tubes were incubated for 2 h at room temperature before centrifuging at 2000g for 40 min. Purified PBMCs were transferred to a 15-ml conical tube, washed twice with phosphate-buffered saline (PBS), resuspended in recovery freezing medium (Thermo Fisher Scientific, Waltham, MA), and stored in liquid nitrogen until use. Participant samples obtained after 1 March 2020 were collected in acid citrate dextrose and serum-separating tubes (SSTs, BD Biosciences) and immediately transferred to the University of Washington. Whole blood in SSTs was allowed to clot by incubating for at least 1 h at room temperature, then centrifuged at 700g for 10 min, aliquoted, and stored at −20°C. PBMCs were isolated by density-gradient centrifugation using Histopaque (Sigma-Aldrich, St. Louis, MO). After washing, purified PBMCs were resuspended in 90% heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, MO) with 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) cryopreservation media and stored in liquid nitrogen until use. All samples were frozen within 6 h of collection time.
Flow cytometry
For flow cytometric analysis, good practices were followed as outlined in the guidelines for use of flow cytometry (74). Directly after thawing, cells were incubated with Fc-blocking reagent (BioLegend Trustain FcX, #422302) and fixable UV Blue LIVE/DEAD reagent (Thermo Fisher Scientific, #L34961) in PBS (Gibco, #14190250) for 15 min at room temperature. After this, cells were incubated for 20 min at room temperature with 50 μl total volume of antibody master mix freshly prepared in Brilliant staining buffer (BD Biosciences, #563794), followed by two washes. All antibodies were titrated and used at optimal dilution, and staining procedures were performed in 96-well round-bottom plates. A detailed list of the main panels used, including fluorochromes and final dilutions of all antibodies, is provided in table S1.
The stained cells were fixed with 4% paraformaldehyde (Cytofix/Cytoperm, BD Biosciences) for 20 min at room temperature, washed, resuspended in fluorescence-activated cell sorting (FACS) buffer, and stored at 4°C in the dark until acquisition. For panels with intranuclear staining, the cells were fixed with an intranuclear transcription factor staining kit (eBioscience Foxp3/Transcription Factor Staining Buffer Set, Thermo Fisher Scientific, #00-5532-00) following the manufacturer’s protocols.
Single-stained controls were prepared with every experiment using antibody capture beads diluted in FACS buffer [BD Biosciences, anti-mouse, #552843; anti-rat, #552844; and Miltenyi Biotec, anti-REA (recombinant engineered antibody), #130-1040693]. Beads (ArC Amine Reactive Compensation Bead Kit, Thermo Fisher Scientific, #A10346) or cells were used for Live/Dead single-stained control and treated exactly the same as the samples (including fixation procedures). All samples were acquired using FACSymphony A5 (BD Biosciences) equipped with 30 detectors and 355-nm (65 mW), 405-nm (200 mW), 488-nm (200 mW), 532-nm (200 mW), and 628-nm (200 mW) lasers and FACSDiva acquisition software (BD Biosciences). Detector voltages were optimized using a modified voltage titration approach (75) and standardized from day to day using median fluorescence intensity (MFI) target values and six-peak Ultra Rainbow Beads (SpheroTec, #URCP-38-2 K) (76). After acquisition, data were exported in FCS (flow cytometry standard) 3.1 format and analyzed using FlowJo (version 10.7.x, BD Biosciences). Doublets were excluded by forward scatter area (FSC-A) versus forward scatter height (FSC-H) gating.
As the samples were stained and acquired in two different batches, each experiment was conducted along with a technical control: a cryopreserved vial of PBMCs collected via leukapheresis from one single healthy donor. This method is valuable to ensure that the variability of expression of the different markers is neither due to variability on the instrument side nor staining-related and allows the comparison of data from biological samples stained on different days. For samples acquired on different experiment days, files were exported as compensated data and analyzed and combined together in a new workspace. Gates were kept the same across all samples except where changes in the density distribution clearly indicated the need for adjustment.
Cytokine and chemokine measurements
Patient serum aliquots were stored at −80°C. Serum samples were shipped to Eve Technologies (Calgary, Alberta, Canada) on dry ice, and levels of cytokines and chemokines were measured using the Human Cytokine Array/Chemokine Array 71-403 Plex Panel (HD71). All samples were measured upon the first thaw.
Statistical analysis
After testing the normal distribution of our data using the D’Agostino-Person test, statistical analyses were performed using either an ordinary one-way analysis of variance (ANOVA) (parametric test) or Kruskal-Wallis (nonparametric test) with Tukey’s or Dunn’s multiple comparisons test using the GraphPad Software. Data are expressed as means ± SD. Significant P values were annotated as follows: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
FAUST analysis
FAUST was used to discover and annotate phenotypes in the four tested panels. Manual gating was first used to define on which cell type FAUST should be run. FAUST was applied to live cells for the APC panel, live CD3+ cells for the T cell panel, live CD3+CD4+CD25+CD127− for the Treg panel, and live CD14−CD19−CD3−CD127− for the NK panel. After incorporating expert information about the panel design, FAUST selected markers to be used for annotation and discovery of phenotypes. The markers identified for the different panels were the following:
APC panel: CD1c, CD5, CD11b, CD11c, CD14, CD16, CD32, CD38, CD46. CD85k, CD86, CD88, CD123, CD141, CD163, CD301, CX3CR1, FcER1, PD-L1, and Sirpa
T cell panel: BTLA, CCR7, CD4, CD8, CD25, CD27, CD28, CD38, CD45RA, CD127, CD161, granzyme B, HLA-DR, ICOS, Ki67, MR1-tet, TCRgd, and Tim3
Treg cell panel: CCR5, CCR7, CD39, CD101, CTLA-4, CXCR3, GITR, Ki67, Tbet, and TCF-1
NK cell panel: CD2, CD16, CD38, CD56, CD57, CD69, granzyme B, NKG2A, NKG2C, and Ki67
Then, within a given panel, a binomial generalized linear mixed-effects model with a subject-level random effect was used to test for association between counts of the discovered phenotypes and COVID-19 patients relative to flu and RSV patients.
The severity of the COVID-19 disease was also tested in comparison to the other groups of the cohort (i.e., when the moderate COVID-19 patients were tested in comparison to flu and RSV patients, the severe and critical COVID-19 patients were removed from the analysis). The entire collection of tested hypotheses was then adjusted using the Bonferroni adjustment. Within a given panel, discovered FAUST phenotypes with Bonferroni-adjusted P values under 0.05 were selected.
FlowSOM, UMAP, and heatmap generation
Pipelines outlined in the Spectre R package were used to generate UMAP and FlowSOM clusters (29, 30, 77). In FlowJo from the APC panel, cells were gated by time (time and FSC-A), cell size (SSC-A and FSC-A), singlets (FSC-H and FSC-A), and live (SSC-A and dead) after compensation was applied. Each sample was then downsampled to 20,000 events if able and then exported as CSV files for the compensated channel values of all parameters. Channel values in R were transformed by ArcSinH using a cofactor of 1000. Batches were normalized based on a reference sample run on both days using CytoNorm (77). For clustering with FlowSOM and dimensionality reduction with UMAP, only lineage markers CD3, CD19, HLA-DR, CD56, CD14, CD88, CD16, CD11c, CD123, CD141, FcER1, CD5, and CD163 were used. FlowSOM was initiated on default parameters where the number of clusters was automatically generated and produced five. Clusters were then reannotated to four, labeling NK cells, T cells, B cells, and Lin−HLA-DR+/− cells. A UMAP was generated with default parameters on 40,000 events from downsampling 10,000 events per study group (healthy, RSV, flu, and SARS-CoV-2) so as to be equally represented. A heatmap was generated representing the MFI of the lineage markers.
Acknowledgments
We thank the members of the Lund and Prlic laboratories for helpful discussions, the HAARVI study team, and the patients and healthy donors for providing samples.
Funding: This work was supported by NIH grants R01 AI121129 and R01 AI141435.
Author contributions: S.C.V., M.F., and F.M. performed experiments. S.C.V., M.F., F.M., A.J.K., and E.G. performed formal data analysis. C.R.W., J.K.L., N.M.F., and J.B. collected clinical specimens and data and curated clinical data. R.G., J.T.S., H.Y.C., M.P., and J.M.L. conceptualized the study and supervised the study and study staff. S.C.V., M.F., and J.M.L. wrote the original draft of the manuscript, and all authors reviewed and edited the manuscript.
Competing interests: R.G. has received consulting income from Takeda and Merck and declares ownership in Ozette Technologies. E.G. declares ownership in Ozette Technologies. All other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Flow cytometry data are available on FLOWRepository (FR-FCM-Z47E, FR-FCM-Z4JV, FR-FCM-Z4JS, and FR-FCM-Z4KY).
Supplementary Materials
This PDF file includes:
Figs. S1 to S9
Legends for tables S1 to S3
Other Supplementary Material for this manuscript includes the following:
Tables S1 to S3
REFERENCES AND NOTES
- 1.Chan J. F., Yuan S., Kok K.-H., To K. K.-W., Chu H., Yang J., Xing F., Liu J., Yip C. C.-Y., Poon R. W.-S., Tsoi H.-W., Lo S. K. F., Chan K.-H., Poon V. K.-M., Chan W.-M., Ip J. D., Cai J.-P., Cheng V. C.-C., Chen H., Hui C. K.-M., Yuen K.-Y., A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 395, 514–523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., Tan W.; China Novel Coronavirus Investigating and Research Team , A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong E., Du H., Gardner L., An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 20, 533–534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vabret N., Britton G. J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M. D., Pia L., Risson E., Saffern M., Salomé B., Esai Selvan M., Spindler M. P., Tan J., van der Heide V., Gregory J. K., Alexandropoulos K., Bhardwaj N., Brown B. D., Greenbaum B., Gümüş Z. H., Homann D., Horowitz A., Kamphorst A. O., Curotto de Lafaille M. A., Mehandru S., Merad M., Samstein R. M., Agrawal M., Aleynick M., Belabed M., Brown M., Casanova-Acebes M., Catalan J., Centa M., Charap A., Chan A., Chen S. T., Chung J., Bozkus C. C., Cody E., Cossarini F., Dalla E., Fernandez N., Grout J., Ruan D. F., Hamon P., Humblin E., Jha D., Kodysh J., Leader A., Lin M., Lindblad K., Lozano-Ojalvo D., Lubitz G., Magen A., Mahmood Z., Martinez-Delgado G., Mateus-Tique J., Meritt E., Moon C., Noel J., O’Donnell T., Ota M., Plitt T., Pothula V., Redes J., Reyes Torres I., Roberto M., Sanchez-Paulete A. R., Shang J., Schanoski A. S., Suprun M., Tran M., Vaninov N., Wilk C. M., Aguirre-Ghiso J., Bogunovic D., Cho J., Faith J., Grasset E., Heeger P., Kenigsberg E., Krammer F., Laserson U., Immunology of COVID-19: Current state of the science. Immunity 52, 910–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco-Melo D., Nilsson-Payant B. E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T. X., Oishi K., Panis M., Sachs D., Wang T. T., Schwartz R. E., Lim J. K., Albrecht R. A., tenOever B. R., Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q., Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T. A., Merkling S. H., Treluyer J. M., Veyer D., Mouthon L., Blanc C., Tharaux P. L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas C., Wong P., Klein J., Castro T. B. R., Silva J., Sundaram M., Ellingson M. K., Mao T., Oh J. E., Israelow B., Takahashi T., Tokuyama M., Lu P., Venkataraman A., Park A., Mohanty S., Wang H., Wyllie A. L., Vogels C. B. F., Earnest R., Lapidus S., Ott I. M., Moore A. J., Muenker M. C., Fournier J. B., Campbell M., Odio C. D., Casanovas-Massana A.; Yale IMPACT Team, Obaid A., Lu-Culligan A., Nelson A., Brito A., Nunez A., Martin A., Watkins A., Geng B., Kalinich C., Harden C., Todeasa C., Jensen C., Kim D., McDonald D., Shepard D., Courchaine E., White E. B., Song E., Silva E., Kudo E., DeIuliis G., Rahming H., Park H. J., Matos I., Nouws J., Valdez J., Fauver J., Lim J., Rose K. A., Anastasio K., Brower K., Glick L., Sharma L., Sewanan L., Knaggs L., Minasyan M., Batsu M., Petrone M., Kuang M., Nakahata M., Campbell M., Linehan M., Askenase M. H., Simonov M., Smolgovsky M., Sonnert N., Naushad N., Vijayakumar P., Martinello R., Datta R., Handoko R., Bermejo S., Prophet S., Bickerton S., Velazquez S., Alpert T., Rice T., Khoury-Hanold W., Peng X., Yang Y., Cao Y., Strong Y., Herbst R., Shaw A. C., Medzhitov R., Schulz W. L., Grubaugh N. D., dela Cruz C., Farhadian S., Ko A. I., Omer S. B., Iwasaki A., Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D. S., Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 71, 762–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laing A. G., Lorenc A., del Molino del Barrio I., Das A., Fish M., Monin L., Muñoz-Ruiz M., McKenzie D. R., Hayday T. S., Francos-Quijorna I., Kamdar S., Joseph M., Davies D., Davis R., Jennings A., Zlatareva I., Vantourout P., Wu Y., Sofra V., Cano F., Greco M., Theodoridis E., Freedman J. D., Gee S., Chan J. N. E., Ryan S., Bugallo-Blanco E., Peterson P., Kisand K., Haljasmägi L., Chadli L., Moingeon P., Martinez L., Merrick B., Bisnauthsing K., Brooks K., Ibrahim M. A. A., Mason J., Lopez Gomez F., Babalola K., Abdul-Jawad S., Cason J., Mant C., Seow J., Graham C., Doores K. J., di Rosa F., Edgeworth J., Shankar-Hari M., Hayday A. C., A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 26, 1623–1635 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Wilk A. J., Rustagi A., Zhao N. Q., Roque J., Martínez-Colón G. J., McKechnie J. L., Ivison G. T., Ranganath T., Vergara R., Hollis T., Simpson L. J., Grant P., Subramanian A., Rogers A. J., Blish C. A., A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 26, 1070–1076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maucourant C., Filipovic I., Ponzetta A., Aleman S., Cornillet M., Hertwig L., Strunz B., Lentini A., Reinius B., Brownlie D., Gomez A. C., Ask E. H., Hull R. M., Haroun-Izquierdo A., Schaffer M., Klingström J., Folkesson E., Buggert M., Sandberg J. K., Eriksson L. I., Rooyackers O., Ljunggren H. G., Malmberg K. J., Michaëlsson J., Marquardt N., Hammer Q., Strålin K., Björkström N. K.; Karolinska COVID-19 Study Group , Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 5, eabd6832 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuri-Cervantes L., Pampena M. B., Meng W., Rosenfeld A. M., Ittner C. A. G., Weisman A. R., Agyekum R. S., Mathew D., Baxter A. E., Vella L. A., Kuthuru O., Apostolidis S. A., Bershaw L., Dougherty J., Greenplate A. R., Pattekar A., Kim J., Han N., Gouma S., Weirick M. E., Arevalo C. P., Bolton M. J., Goodwin E. C., Anderson E. M., Hensley S. E., Jones T. K., Mangalmurti N. S., Luning Prak E. T., Wherry E. J., Meyer N. J., Betts M. R., Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 5, eabd7114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sette A., Crotty S., Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184, 861–880 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens D. S., McElrath M. J., COVID-19 and the path to immunity. JAMA 324, 1279–1281 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Grifoni A., Weiskopf D., Ramirez S. I., Mateus J., Dan J. M., Moderbacher C. R., Rawlings S. A., Sutherland A., Premkumar L., Jadi R. S., Marrama D., de Silva A. M., Frazier A., Carlin A. F., Greenbaum J. A., Peters B., Krammer F., Smith D. M., Crotty S., Sette A., Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S. I., Dan J. M., Burger Z. C., Rawlings S. A., Smith D. M., Phillips E., Mallal S., Lammers M., Rubiro P., Quiambao L., Sutherland A., Yu E. D., da Silva Antunes R., Greenbaum J., Frazier A., Markmann A. J., Premkumar L., de Silva A., Peters B., Crotty S., Sette A., Weiskopf D., Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiskopf D., Schmitz K. S., Raadsen M. P., Grifoni A., Okba N. M. A., Endeman H., van den Akker J. P. C., Molenkamp R., Koopmans M. P. G., van Gorp E. C. M., Haagmans B. L., de Swart R. L., Sette A., de Vries R. D., Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 5, eabd2071 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Y., Mentzer A. J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., López-Camacho C., Slon-Campos J., Zhao Y., Stuart D. I., Paesen G. C., Grimes J. M., Antson A. A., Bayfield O. W., Hawkins D. E. D. P., Ker D. S., Wang B., Turtle L., Subramaniam K., Thomson P., Zhang P., Dold C., Ratcliff J., Simmonds P., de Silva T., Sopp P., Wellington D., Rajapaksa U., Chen Y. L., Salio M., Napolitani G., Paes W., Borrow P., Kessler B. M., Fry J. W., Schwabe N. F., Semple M. G., Baillie J. K., Moore S. C., Openshaw P. J. M., Ansari M. A., Dunachie S., Barnes E., Frater J., Kerr G., Goulder P., Lockett T., Levin R., Zhang Y., Jing R., Ho L. P.; Oxford Immunology Network Covid-19 Response T cell Consortium, Barnes E., Dong D., Dong T., Dunachie S., Frater J., Goulder P., Kerr G., Klenerman P., Liu G., McMichael A., Napolitani G., Ogg G., Peng Y., Salio M., Yao X., Yin Z.; ISARIC4C Investigators, Kenneth Baillie J., Klenerman P., Mentzer A. J., Moore S. C., Openshaw P. J. M., Semple M. G., Stuart D. I., Turtle L., Cornall R. J., Conlon C. P., Klenerman P., Screaton G. R., Mongkolsapaya J., McMichael A., Knight J. C., Ogg G., Dong T., Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 21, 1336–1345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rydyznski Moderbacher C., Ramirez S. I., Dan J. M., Grifoni A., Hastie K. M., Weiskopf D., Belanger S., Abbott R. K., Kim C., Choi J., Kato Y., Crotty E. G., Kim C., Rawlings S. A., Mateus J., Tse L. P. V., Frazier A., Baric R., Peters B., Greenbaum J., Saphire E. O., Smith D. M., Sette A., Crotty S., Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183, 996–1012.e19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., Wullimann D. J., Kammann T., Emgård J., Parrot T., Folkesson E.; Karolinska COVID- Study Group, Rooyackers O., Eriksson L. I., Henter J.-I., Sönnerborg A., Allander T., Albert J., Nielsen M., Klingström J., Gredmark-Russ S., Björkström N. K., Sandberg J. K., Price D. A., Ljunggren H.-G., Aleman S., Buggert M., Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183, 158–168.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodda L. B., Netland J., Shehata L., Pruner K. B., Morawski P. A., Thouvenel C. D., Takehara K. K., Eggenberger J., Hemann E. A., Waterman H. R., Fahning M. L., Chen Y., Hale M., Rathe J., Stokes C., Wrenn S., Fiala B., Carter L., Hamerman J. A., King N. P., Jr M. G., Campbell D. J., Rawlings D. J., Pepper M., Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 184, 169–183.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew D., Giles J. R., Baxter A. E., Oldridge D. A., Greenplate A. R., Wu J. E., Alanio C., Kuri-Cervantes L., Pampena M. B., D’Andrea K., Manne S., Chen Z., Huang Y. J., Reilly J. P., Weisman A. R., Ittner C. A. G., Kuthuru O., Dougherty J., Nzingha K., Han N., Kim J., Pattekar A., Goodwin E. C., Anderson E. M., Weirick M. E., Gouma S., Arevalo C. P., Bolton M. J., Chen F., Lacey S. F., Ramage H., Cherry S., Hensley S. E., Apostolidis S. A., Huang A. C., Vella L. A.; UPenn COVID Processing Unit, Betts M. R., Meyer N. J., Wherry E. J., Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369, eabc8511 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghi A., Tahmasebi S., Mahmood A., Kuznetsova M., Valizadeh H., Taghizadieh A., Nazemiyeh M., Aghebati-Maleki L., Jadidi-Niaragh F., Abbaspour-Aghdam S., Roshangar L., Mikaeili H., Ahmadi M., Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 236, 2829–2839 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Szabo P. A., Dogra P., Gray J. I., Wells S. B., Connors T. J., Weisberg S. P., Krupska I., Matsumoto R., Poon M. M. L., Idzikowski E., Morris S. E., Pasin C., Yates A. J., Ku A., Chait M., Davis-Porada J., Guo X. V., Zhou J., Steinle M., Mackay S., Saqi A., Baldwin M. R., Sims P. A., Farber D. L., Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 54, 797–814.e6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudd P. A., Crawford J. C., Turner J. S., Souquette A., Reynolds D., Bender D., Bosanquet J. P., Anand N. J., Striker D. A., Martin R. S., Boon A. C. M., House S. L., Remy K. E., Hotchkiss R. S., Presti R. M., O’Halloran J. A., Powderly W. G., Thomas P. G., Ellebedy A. H., Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci. Adv. 6, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mair F., Prlic M., OMIP-044: 28-color immunophenotyping of the human dendritic cell compartment. Cytometry A 93, 402–405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gassen S., Callebaut B., Van Helden M. J., Lambrecht B. N., Demeester P., Dhaene T., Saeys Y., FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 87, 636–645 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Ashhurst T. M., Marsh-Wakefield F., Putri G. H., Spiteri A. G., Shinko D., Read M. N., Smith A. L., King N. J. C., Integration, exploration, and analysis of high-dimensional single-cell cytometry data using Spectre. bioRxiv 2020.10.22.349563 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Becht E., Innes L. M., Healy J., Dutertre C.-A., Kwok I. W. H., Ng L. G., Ginhoux F., Newell E. W., Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Giamarellos-Bourboulis E. J., Netea M. G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A., Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y. Q., Wang Q., Miao H., Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 5, 33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClain M. T., Park L. P., Nicholson B., Veldman T., Zaas A. K., Turner R., Lambkin-Williams R., Gilbert A. S., Ginsburg G. S., Woods C. W., Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J. Clin. Virol. 58, 689–695 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Bourdely P., Anselmi G., Vaivode K., Ramos R. N., Missolo-Koussou Y., Hidalgo S., Tosselo J., Nuñez N., Richer W., Vincent-Salomon A., Saxena A., Wood K., Lladser A., Piaggio E., Helft J., Guermonprez P., Transcriptional and functional analysis of CD1c+ human dendritic cells identifies a CD163+ subset priming CD8+CD103+ T cells. Immunity 53, 335–352.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villani A. C., Satija R., Reynolds G., Sarkizova S., Shekhar K., Fletcher J., Griesbeck M., Butler A., Zheng S., Lazo S., Jardine L., Dixon D., Stephenson E., Nilsson E., Grundberg I., McDonald D., Filby A., Li W., de Jager P. L., Rozenblatt-Rosen O., Lane A. A., Haniffa M., Regev A., Hacohen N., Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Cerrillo I., Landete P., Aldave B., Sánchez-Alonso S., Sánchez-Azofra A., Marcos-Jiménez A., Ávalos E., Alcaraz-Serna A., de Los Santos I., Mateu-Albero T., Esparcia L., López-Sanz C., Martínez-Fleta P., Gabrie L., Del Campo Guerola L., de la Fuente H., Calzada M. J., González-Álvaro I., Alfranca A., Sánchez-Madrid F., Muñoz-Calleja C., Soriano J. B., Ancochea J., Martín-Gayo E.; REINMUN-COVID and EDEPIMIC groups , COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J. Clin. Invest. 130, 6290–6300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou R., To K. K.-W., Wong Y.-C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.-Y., Lau T. T.-K., Yeung P., Chan W.-M., Wu A. K.-L., Lung K.-C., Tsang O. T.-Y., Leung W.-S., Hung I. F.-N., Yuen K.-Y., Chen Z., Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity 53, 864–877.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flament H., Rouland M., Beaudoin L., Toubal A., Bertrand L., Lebourgeois S., Rousseau C., Soulard P., Gouda Z., Cagninacci L., Monteiro A. C., Hurtado-Nedelec M., Luce S., Bailly K., Andrieu M., Saintpierre B., Letourneur F., Jouan Y., Si-Tahar M., Baranek T., Paget C., Boitard C., Vallet-Pichard A., Gautier J. F., Ajzenberg N., Terrier B., Pène F., Ghosn J., Lescure X., Yazdanpanah Y., Visseaux B., Descamps D., Timsit J. F., Monteiro R. C., Lehuen A., Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol. 22, 322–335 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Wherry E. J., T cell responses in patients with COVID-19. Nat. Rev. Immunol. 20, 529–536 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene E., Finak G., D’Amico L. A., Bhardwaj N., Church C. D., Morishima C., Ramchurren N., Taube J. M., Nghiem P. T., Cheever M. A., Fling S. P., Gottardo R., New interpretable machine learning method for single-cell data reveals correlates of clinical response to cancer immunotherapy. bioRxiv , 702118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F., Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 71, 1937–1942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herold T., Jurinovic V., Arnreich C., Lipworth B. J., Hellmuth J. C., von Bergwelt-Baildon M., Klein M., Weinberger T., Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 146, 128–136.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose-John S., Winthrop K., Calabrese L., The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 13, 399–409 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Imai T., Baba M., Nishimura M., Kakizaki M., Takagi S., Yoshie O., The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 272, 15036–15042 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Imai T., Chantry D., Raport C. J., Wood C. L., Nishimura M., Godiska R., Yoshie O., Gray P. W., Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 273, 1764–1768 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Fahlberg M. D., Blair R. V., Doyle-Meyers L. A., Midkiff C. C., Zenere G., Russell-Lodrigue K. E., Monjure C. J., Haupt E. H., Penney T. P., Lehmicke G., Threeton B. M., Golden N., Datta P. K., Roy C. J., Bohm R. P., Maness N. J., Fischer T., Rappaport J., Vaccari M., Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nat. Commun. 11, 6078 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugiyama M., Kinoshita N., Ide S., Nomoto H., Nakamoto T., Saito S., Ishikane M., Kutsuna S., Hayakawa K., Hashimoto M., Suzuki M., Izumi S., Hojo M., Tsuchiya K., Gatanaga H., Takasaki J., Usami M., Kano T., Yanai H., Nishida N., Kanto T., Sugiyama H., Ohmagari N., Mizokami M., Serum CCL17 level becomes a predictive marker to distinguish between mild/moderate and severe/critical disease in patients with COVID-19. Gene 766, 145145 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper M. A., Fehniger T. A., Caligiuri M. A., The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Chen J., Zhang Z. Z., Chen Y. K., Long Q. X., Tian W. G., Deng H. J., Hu J. L., Zhang X. X., Pu-Liao, Xiang J. L., Wang D. X., Hu P., Zhou F. C., Li Z. J., Xu H. M., Cai X. F., Wang D. Q., Hu Y., Tang N., Liu B. Z., Wu G. C., Huang A. L., The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis. 7, 535–541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Da Costa A. S., Graham J. B., Swarts J. L., Lund J. M., Regulatory T cells limit unconventional memory to preserve the capacity to mount protective CD8 memory responses to pathogens. Proc. Natl. Acad. Sci. U.S.A. 116, 9969–9978 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham J. B., Da Costa A., Lund J. M., Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J. Immunol. 192, 683–690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lund J. M., Hsing L., Pham T. T., Rudensky A. Y., Coordination of early protective immunity to viral infection by regulatory T cells. Science 320, 1220–1224 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soerens A. G., Da Costa A., Lund J. M., Regulatory T cells are essential to promote proper CD4 T-cell priming upon mucosal infection. Mucosal Immunol. 9, 1395–1406 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruckwardt T. J., Bonaparte K. L., Nason M. C., Graham B. S., Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 83, 3019–3028 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fulton R. B., Meyerholz D. K., Varga S. M., Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185, 2382–2392 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belkaid Y., Tarbell K., Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 27, 551–589 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Richert-Spuhler L. E., Lund J. M., The immune fulcrum: Regulatory T cells tip the balance between Pro- and anti-inflammatory outcomes upon infection. Prog. Mol. Biol. Transl. Sci. 136, 217–243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smigiel K. S., Srivastava S., Stolley J. M., Campbell D. J., Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunol. Rev. 259, 40–59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee D. C., Harker J. A. E., Tregoning J. S., Atabani S. F., Johansson C., Schwarze J., Openshaw P. J. M., CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J. Virol. 84, 8790–8798 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loebbermann J., Thornton H., Durant L., Sparwasser T., Webster K. E., Sprent J., Culley F. J., Johansson C., Openshaw P. J., Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 5, 161–172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brincks E. L., Roberts A. D., Cookenham T., Sell S., Kohlmeier J. E., Blackman M. A., Woodland D. L., Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J. Immunol. 190, 3438–3446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galvan-Pena S., Leon J., Chowdhary K., Michelson D. A., Vijaykumar B., Yang L., Magnuson A., Manickas-Hill Z., Piechocka-Trocha A., Worrall D. P., Hall K. E., Ghebremichael M., Walker B. D., Li J. Z., Yu X. G., Mathis D., Benoist C., Profound Treg perturbations correlate with COVID-19 severity. bioRxiv , (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chauhan S. K., Saban D. R., Lee H. K., Dana R., Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 182, 148–153 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Loosdregt J., Fleskens V., Tiemessen M. M., Mokry M., van Boxtel R., Meerding J., Pals C. E. G. M., Kurek D., Baert M. R. M., Delemarre E. M., Gröne A., Koerkamp M. J. A. G., Sijts A. J. A. M., Nieuwenhuis E. E. S., Maurice M. M., van Es J. H., ten Berge D., Holstege F. C., Staal F. J. T., Zaiss D. M. W., Prakken B. J., Coffer P. J., Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 39, 298–310 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Yang B. H., Wang K., Wan S., Liang Y., Yuan X., Dong Y., Cho S., Xu W., Jepsen K., Feng G.-S., Lu L.-F., Xue H.-H., Fu W., TCF1 and LEF1 control Treg competitive survival and Tfr development to prevent autoimmune diseases. Cell Rep. 27, 3629–3645.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J. S., Park S., Jeong H. W., Ahn J. Y., Choi S. J., Lee H., Choi B., Nam S. K., Sa M., Kwon J. S., Jeong S. J., Lee H. K., Park S. H., Park S. H., Choi J. Y., Kim S. H., Jung I., Shin E. C., Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 5, eabd1554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colonna M., Trinchieri G., Liu Y. J., Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Schuette V., Embgenbroich M., Ulas T., Welz M., Schulte-Schrepping J., Draffehn A. M., Quast T., Koch K., Nehring M., König J., Zweynert A., Harms F. L., Steiner N., Limmer A., Förster I., Berberich-Siebelt F., Knolle P. A., Wohlleber D., Kolanus W., Beyer M., Schultze J. L., Burgdorf S., Mannose receptor induces T-cell tolerance via inhibition of CD45 and up-regulation of CTLA-4. Proc. Natl. Acad. Sci. U.S.A. 113, 10649–10654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham J. B., Swarts J. L., Leist S. R., Schäfer A., Menachery V. D., Gralinski L. E., Jeng S., Miller D. R., Mooney M. A., McWeeney S. K., Ferris M. T., Pardo-Manuel de Villena F., Heise M. T., Baric R. S., Lund J. M., Baseline T cell immune phenotypes predict virologic and disease control upon SARS-CoV infection in Collaborative Cross mice. PLOS Pathog. 17, e1009287 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fontanet A., Autran B., Lina B., Kieny M. P., Karim S. S. A., Sridhar D., SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 397, 952–954 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G., Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beigel J. H., Tomashek K. M., Dodd L. E., Mehta A. K., Zingman B. S., Kalil A. C., Hohmann E., Chu H. Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R. W., Dierberg K., Tapson V., Hsieh L., Patterson T. F., Paredes R., Sweeney D. A., Short W. R., Touloumi G., Lye D. C., Ohmagari N., Oh M. D., Ruiz-Palacios G. M., Benfield T., Fätkenheuer G., Kortepeter M. G., Atmar R. L., Creech C. B., Lundgren J., Babiker A. G., Pett S., Neaton J. D., Burgess T. H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H. C.; ACTT-1 Study Group Members , Remdesivir for the treatment of Covid-19—Final report. N. Engl. J. Med. 383, 1813–1826 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cossarizza A., Chang H. D., Radbruch A., Acs A., Adam D., Adam-Klages S., Agace W. W., Aghaeepour N., Akdis M., Allez M., Almeida L. N., Alvisi G., Anderson G., Andrä I., Annunziato F., Anselmo A., Bacher P., Baldari C. T., Bari S., Barnaba V., Barros-Martins J., Battistini L., Bauer W., Baumgart S., Baumgarth N., Baumjohann D., Baying B., Bebawy M., Becher B., Beisker W., Benes V., Beyaert R., Blanco A., Boardman D. A., Bogdan C., Borger J. G., Borsellino G., Boulais P. E., Bradford J. A., Brenner D., Brinkman R. R., Brooks A. E. S., Busch D. H., Büscher M., Bushnell T. P., Calzetti F., Cameron G., Cammarata I., Cao X., Cardell S. L., Casola S., Cassatella M. A., Cavani A., Celada A., Chatenoud L., Chattopadhyay P. K., Chow S., Christakou E., Čičin-Šain L., Clerici M., Colombo F. S., Cook L., Cooke A., Cooper A. M., Corbett A. J., Cosma A., Cosmi L., Coulie P. G., Cumano A., Cvetkovic L., Dang V. D., Dang-Heine C., Davey M. S., Davies D., de Biasi S., del Zotto G., dela Cruz G. V., Delacher M., Della Bella S., Dellabona P., Deniz G., Dessing M., di Santo J. P., Diefenbach A., Dieli F., Dolf A., Dörner T., Dress R. J., Dudziak D., Dustin M., Dutertre C. A., Ebner F., Eckle S. B. G., Edinger M., Eede P., Ehrhardt G. R. A., Eich M., Engel P., Engelhardt B., Erdei A., Esser C., Everts B., Evrard M., Falk C. S., Fehniger T. A., Felipo-Benavent M., Ferry H., Feuerer M., Filby A., Filkor K., Fillatreau S., Follo M., Förster I., Foster J., Foulds G. A., Frehse B., Frenette P. S., Frischbutter S., Fritzsche W., Galbraith D. W., Gangaev A., Garbi N., Gaudilliere B., Gazzinelli R. T., Geginat J., Gerner W., Gherardin N. A., Ghoreschi K., Gibellini L., Ginhoux F., Goda K., Godfrey D. I., Goettlinger C., González-Navajas J. M., Goodyear C. S., Gori A., Grogan J. L., Grummitt D., Grützkau A., Haftmann C., Hahn J., Hammad H., Hämmerling G., Hansmann L., Hansson G., Harpur C. M., Hartmann S., Hauser A., Hauser A. E., Haviland D. L., Hedley D., Hernández D. C., Herrera G., Herrmann M., Hess C., Höfer T., Hoffmann P., Hogquist K., Holland T., Höllt T., Holmdahl R., Hombrink P., Houston J. P., Hoyer B. F., Huang B., Huang F. P., Huber J. E., Huehn J., Hundemer M., Hunter C. A., Hwang W. Y. K., Iannone A., Ingelfinger F., Ivison S. M., Jäck H. M., Jani P. K., Jávega B., Jonjic S., Kaiser T., Kalina T., Kamradt T., Kaufmann S. H. E., Keller B., Ketelaars S. L. C., Khalilnezhad A., Khan S., Kisielow J., Klenerman P., Knopf J., Koay H. F., Kobow K., Kolls J. K., Kong W. T., Kopf M., Korn T., Kriegsmann K., Kristyanto H., Kroneis T., Krueger A., Kühne J., Kukat C., Kunkel D., Kunze-Schumacher H., Kurosaki T., Kurts C., Kvistborg P., Kwok I., Landry J., Lantz O., Lanuti P., LaRosa F., Lehuen A., LeibundGut-Landmann S., Leipold M. D., Leung L. Y. T., Levings M. K., Lino A. C., Liotta F., Litwin V., Liu Y., Ljunggren H. G., Lohoff M., Lombardi G., Lopez L., López-Botet M., Lovett-Racke A. E., Lubberts E., Luche H., Ludewig B., Lugli E., Lunemann S., Maecker H. T., Maggi L., Maguire O., Mair F., Mair K. H., Mantovani A., Manz R. A., Marshall A. J., Martínez-Romero A., Martrus G., Marventano I., Maslinski W., Matarese G., Mattioli A. V., Maueröder C., Mazzoni A., McCluskey J., McGrath M., McGuire H. M., McInnes I. B., Mei H. E., Melchers F., Melzer S., Mielenz D., Miller S. D., Mills K. H. G., Minderman H., Mjösberg J., Moore J., Moran B., Moretta L., Mosmann T. R., Müller S., Multhoff G., Muñoz L. E., Münz C., Nakayama T., Nasi M., Neumann K., Ng L. G., Niedobitek A., Nourshargh S., Núñez G., O’Connor J. E., Ochel A., Oja A., Ordonez D., Orfao A., Orlowski-Oliver E., Ouyang W., Oxenius A., Palankar R., Panse I., Pattanapanyasat K., Paulsen M., Pavlinic D., Penter L., Peterson P., Peth C., Petriz J., Piancone F., Pickl W. F., Piconese S., Pinti M., Pockley A. G., Podolska M. J., Poon Z., Pracht K., Prinz I., Pucillo C. E. M., Quataert S. A., Quatrini L., Quinn K. M., Radbruch H., Radstake T. R. D. J., Rahmig S., Rahn H. P., Rajwa B., Ravichandran G., Raz Y., Rebhahn J. A., Recktenwald D., Reimer D., Reis e Sousa C., Remmerswaal E. B. M., Richter L., Rico L. G., Riddell A., Rieger A. M., Robinson J. P., Romagnani C., Rubartelli A., Ruland J., Saalmüller A., Saeys Y., Saito T., Sakaguchi S., Sala-de-Oyanguren F., Samstag Y., Sanderson S., Sandrock I., Santoni A., Sanz R. B., Saresella M., Sautes-Fridman C., Sawitzki B., Schadt L., Scheffold A., Scherer H. U., Schiemann M., Schildberg F. A., Schimisky E., Schlitzer A., Schlosser J., Schmid S., Schmitt S., Schober K., Schraivogel D., Schuh W., Schüler T., Schulte R., Schulz A. R., Schulz S. R., Scottá C., Scott-Algara D., Sester D. P., Shankey T. V., Silva-Santos B., Simon A. K., Sitnik K. M., Sozzani S., Speiser D. E., Spidlen J., Stahlberg A., Stall A. M., Stanley N., Stark R., Stehle C., Steinmetz T., Stockinger H., Takahama Y., Takeda K., Tan L., Tárnok A., Tiegs G., Toldi G., Tornack J., Traggiai E., Trebak M., Tree T. I. M., Trotter J., Trowsdale J., Tsoumakidou M., Ulrich H., Urbanczyk S., Veen W., Broek M., Pol E., van Gassen S., van Isterdael G., Lier R. A. W., Veldhoen M., Vento-Asturias S., Vieira P., Voehringer D., Volk H. D., Borstel A., Volkmann K., Waisman A., Walker R. V., Wallace P. K., Wang S. A., Wang X. M., Ward M. D., Ward-Hartstonge K. A., Warnatz K., Warnes G., Warth S., Waskow C., Watson J. V., Watzl C., Wegener L., Weisenburger T., Wiedemann A., Wienands J., Wilharm A., Wilkinson R. J., Willimsky G., Wing J. B., Winkelmann R., Winkler T. H., Wirz O. F., Wong A., Wurst P., Yang J. H. M., Yang J., Yazdanbakhsh M., Yu L., Yue A., Zhang H., Zhao Y., Ziegler S. M., Zielinski C., Zimmermann J., Zychlinsky A., Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 49, 1457–1973 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perfetto S. P., Ambrozak D., Nguyen R., Chattopadhyay P. K., Roederer M., Quality assurance for polychromatic flow cytometry using a suite of calibration beads. Nat. Protoc. 7, 2067–2079 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Mair F., Tyznik A. J., High-Dimensional Immunophenotyping with fluorescence-based cytometry: A practical guidebook. Methods Mol. Biol. 2032, 1–29 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Van Gassen S., Gaudilliere B., Angst M. S., Saeys Y., Aghaeepour N., CytoNorm: A normalization algorithm for cytometry data. Cytometry A 97, 268–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S9
Legends for tables S1 to S3
Tables S1 to S3