Abstract
Introduction:
Teaching dermatology to medical students entails a series of lectures, pictures, and hands-on skin examinations to convey a sense of skin features and textures, often by use of simulated skin models. However, such methods can often lack accurate visual and tactile texture representation of skin lesions. To facilitate learning, we have developed a smartphone-based skin simulation model, which provides a configurable visual and tactile sense of a lesion by using the ubiquitous availability of smartphone-based mobile platforms.
Methods:
A polydimethylsiloxane (PDMS) overlay was used as a configurable translucent elastomer material to model the stiffness and texture of skin. A novel custom smartphone-based app was developed to capture images of various skin lesions, which were subsequently displayed on a tablet or second smartphone, over which the PDMS model skin elastomer was placed. Using the local Bluetooth connection between mobile devices, an iterative feedback algorithm corrected the visual distortion caused by the optical scattering of the translucent elastomer, enabling better virtual visualization of the lesion.
Results:
The developed smartphone-based app corrected the distortion of images projected through the simulated skin elastomer. Surface topography of the developed PDMS elastomer provided a more accurate representation of skin texture.
Conclusions:
In this investigation, we developed a smartphone-based skin lesion visualization app with a simulated skin elastomer for training/education in not only dermatology but also all general medical specialties that examine the skin. This technique has the potential to advance the educational experience by giving students the ability to see, touch, and feel pragmatic skin textures and lesions.
Keywords: Skin simulation, elastomer, smartphone app
Teaching dermatology to medical students or primary care physicians still necessitates presenting sets of photographs, conducting a series of lectures, and hands-on skin training models to comprehend the visual appearance and texture of skin lesions. Physicians in the clinic encounter a mixed population of patients having common and/or rare skin conditions and lesions. Educators in primary care and surgery have also found insufficient skin simulation material.1 Improvement in dermatology education is critical as most of the current teaching modes are lacking. Plausibly, this is one of the likely rationales for the misdiagnosis of skin pathologies by primary care physicians. Developing an appropriate evaluative technique before examining patients will reduce diagnostic errors and improve patient treatment outcomes and prognosis.2–5 An extensive amount of investigation has led to the development of skin simulation models. Of the many simulation models, 3D silicone models have led to a significant improvement in diagnostic outcomes and have enhanced knowledge among medical students, when compared with a 2D photographic method.6 Commercially available simulated tissue phantoms such as SurgiReal Products, Inc, Colorado, have simulated tissue with dermal lesions, having moles and skin tags to demonstrate surface and topographic features for dermatology training programs.7 However, simulated phantoms are opaque and do not always provide accurate color information, limiting their utility for accurately representing skin lesions, moles, or tags.
There is rising interest among medical educators to use a mobile-based platform for teaching. Virtual simulation methods are of significant interest among medical schools in the United States,8 and students find this to be beneficial for integrating clinical knowledge.9 A 3D virtual reality–based method was recently developed, wherein 2D still camera images of a lesion were used to generate a 3D surface image based on a model and passive stereo photogrammetry.10 This generated a 3D image that can be zoomed in and panned and can be viewed at different angles. Furthermore, several app-based learning methods for analyzing skin lesions have also been developed.11 These mobile applications can determine whether the mole is normal or abnormal based on fractal patterns of the skin or based on telemedicine where an image of a mole is sent to a dermatologist for evaluation. Unlike the previously mentioned image-based analysis, a haptic device model has been developed to convert a captured 2D image into a virtual 3D image. This could be felt using a haptic device. This enables the user to both visualize and feel the skin texture, such as roughness, simultaneously.12,13
For all of the previously mentioned approaches, the learning method either involves visualization of photographs or simulated tissue models, which either lack the feel of surface topography, or are opaque. In this regard, we investigated the development of a model with equitable surface topography but also with optical properties that enable visualization of skin lesion images. Polydimethylsiloxane (PDMS) was used to develop a skin simulation model because of its translucent nature and its moldable properties for mimicking surface texture and topography of skin and skin lesions. However, as a result of the surface texture, the underlying transmitted image is optically distorted. A custom iterative algorithm, written as an application for the Android operating system, enabled correction of some of these distortions, including color distortion, resulting in a training model having both visual representation and surface topography of skin and skin lesions. The resulting technology potentiates a new mobile and configurable dermatology teaching platform for medical students and physicians.
METHODS
Development of PDMS Elastomer-Based Skin Model
We developed a PDMS elastomer-based skin model to depict accurate features, topography, and textures of skin, including elevated skin tags, moles, and lesions. The PDMS elastomer was developed as a cast, using a commercially available simulated skin elastomer (SurgiReal Products, Inc, Colorado) to first create a mold, shown in Figure 1A. To accurately derive the features, a paraffin mold of the simulated elastomer was first generated (Fig. 1B). The elastomer and curing agent (Sylgard 184, in a ratio of 7:1) were thoroughly mixed for 5 minutes and placed inside a vacuum desiccator for 15 minutes to eliminate any air bubbles. This PDMS mixture was then poured into the paraffin-based mold and allowed to set at room temperature according to the manufacturer’s instructions. The cured PDMS elastomer cast [7 cm (x) × 6.5 cm (y) × 1 cm (z)] had a tactile representation of skin texture and topography of elevated moles, as shown in Fig. 1C. The PDMS elastomer was then used as a semitransparent skin model that was placed over the screen of a smartphone (Fig. 1D), which was used to display various images of acquired skin and skin lesions. Together, this combination platform was used to provide both a realistic tactile and visual sense of a skin lesion.
FIGURE 1.
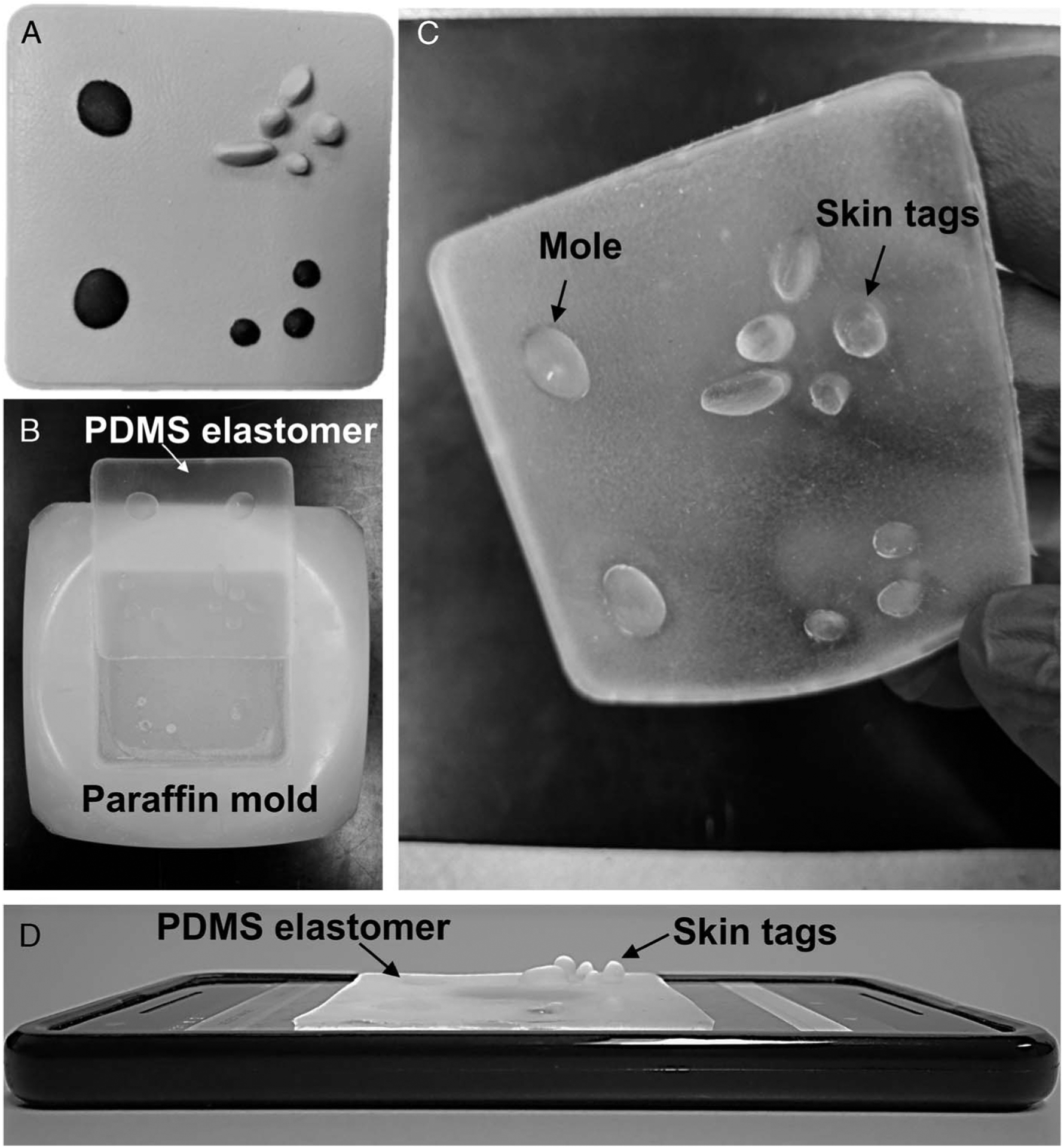
Development of a PDMS elastomer skin overlay. A, Photograph of a commercially available nontransparent silicone-based elastomer. B, Photograph of the simulated PDMS skin elastomer and paraffin mold. C, Photograph of the thin, translucent, stretchable PDMS skin elastomer showing representation of skin texture and elevated skin features such as moles and skin tags. D, Application of the simulated skin PDMS elastomer placed on a smartphone screen to simulate both the tactile nature and visual appearance of skin lesions.
Development of SkinSimulator App
The semitransparent simulated skin elastomer placed over the screen of a smartphone or tablet provided a textured and topographic surface to simulate the feel of skin and skin lesions. However, the image displayed on the smartphone (or tablet) and visualized through the elastomer skin model was distorted because of the optical scattering properties of the elastomer. To compensate for the feature and color distortion caused by the elastomer, we developed an Android-based app called “SkinSimulator,” which corrected the optical distortions by adjusting the image sharpness, contrast, and color using an iterative feedback loop. Figure 2 illustrates the sequence of using the smartphone-based skin simulation model. The Android program was written in the Java platform (Android SDK) and 2 smartphones (Google Pixel 2) were used for this study. One of the smartphones (Master) was used to display the color skin image, and the other smartphone (Capture) was used to capture the distorted image of the skin displayed on the Master phone with the elastomer overlay. The distortion was then iteratively corrected by the Bluetooth-linked Capture smartphone, which was positioned over the Master phone, while adjustments were made to the brightness and contrast of the image projected on the Master phone. This enabled better virtual visualization of the skin and lesion, while providing a textured, biomechanically appropriate tactile representation of the skin and lesion.
FIGURE 2.
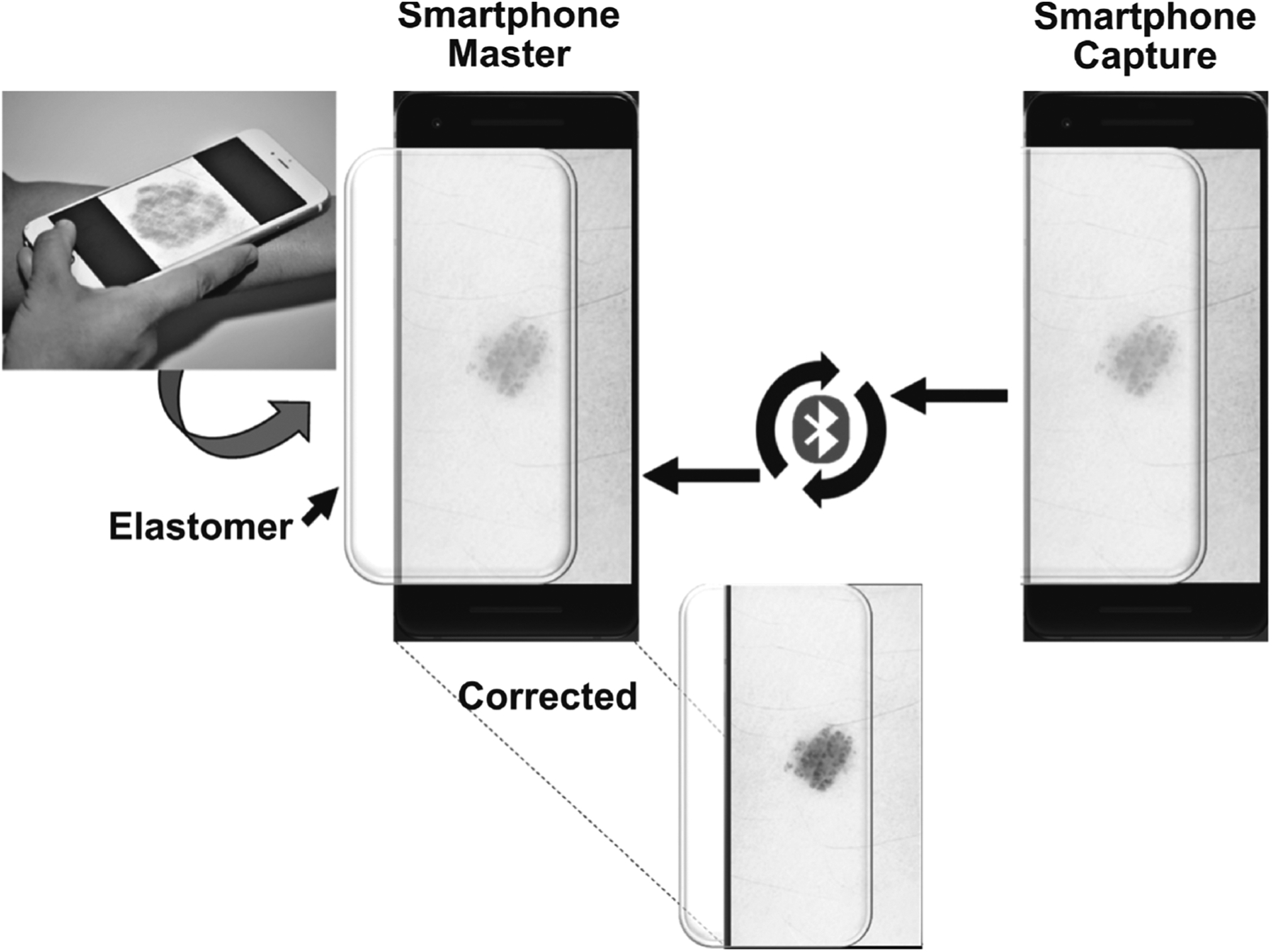
Illustration of the smartphone-based skin simulation model. An original digital image of a skin lesion visualized through the semitransparent elastomer will be distorted. The distortion is corrected through an iterative feedback algorithm, which simultaneously corrects the color, contrast, and intensity, until the corrected visualized image closely matches the image characteristics of the skin and lesion in the original digital image.
RESULTS
Characterization of PDMS Skin Elastomer
Optical and surface characterization of the PDMS elastomer was done using spectral-domain optical coherence tomography (OCT), an optical imaging technique that provides noninvasive images of surface topography and cross-sectional images of scattering tissues, such as skin,14 as well as scattering materials, such as the elastomer.15 Imaging was done using a custom-developed 1310-nm OCT system over a region of approximately 10 mm (x) × 10 mm (y) × 3 mm (z).16 The OCT system had an axial and lateral resolution of approximately 6 μm and approximately 16 μm, respectively. Figure 3 (first column) shows the digital image of the silicone and PDMS elastomers, whereas the second column shows cross-sectional OCT images of each. The silicone elastomer shows more scattering (brighter signal) in depth, whereas the PDMS elastomer shows largely only the surface interface, with low scattering interior due to the optical semitransparency of the PDMS material. The third and fourth columns show the maximum intensity projections of both elastomers and show similar surface topography between the silicone elastomer and the PDMS elastomer cast.
FIGURE 3.
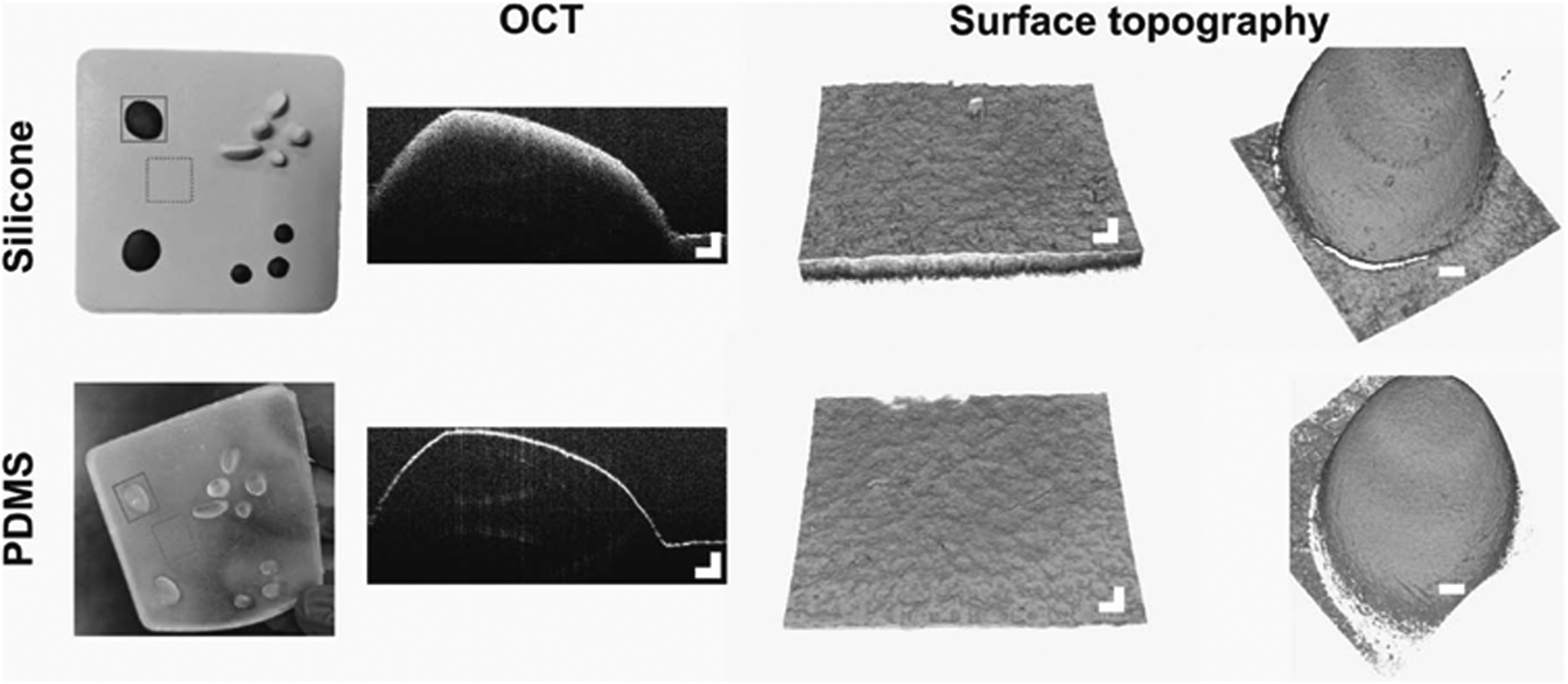
Characterization of a simulated PDMS skin elastomer. The top row shows cross-sectional and surface topography of the silicone elastomer skin model. The bottom row shows cross-sectional and surface topography of the fabricated semitransparent PDMS elastomer. The solid line boxes highlight the raised topography imaged with OCT and shown in the second and fourth columns, from the left. The dotted line boxes indicate the flat regions of the elastomers that were imaged with OCT and shown in the third column from the left. Scale bars represent 500 μm.
Assessment of Developed Skin Simulator Model
To demonstrate the practicality of the developed simulation methodology, skin images with different textural, topographic, and pathologic features such as pyogenic granuloma, superficial basal cell carcinoma, cutaneous horn with squamous cell carcinoma, and seborrheic keratosis were displayed on the Master smartphone. A PDMS elastomer was then placed over the Master smartphone, and the visualized images through the elastomer were expectedly distorted. By regulating the brightness, contrast, and color through iterative feedback, the developed algorithm was able to accurately correct the distortion. Figure 4 shows the results after the distorted images were corrected using the iterative feedback algorithm. Figures 4A, C, and D show the image correction with a uniform (flat surface) elastomer, and Figures 4B and E show the correction with a nonuniform (elevated topography) surface of the elastomer. A mean square error (MSE) metric was obtained from a region of interest (ROI, square box) to compare the image sharpness between the original image and the distorted/corrected one. The corrected image had a low MSE value, compared with the original image, indicating improvement after distortion correction. To further validate the improvement, a histogram plot was obtained from the uniform and nonuniform regions in Figure 4. Figures 5A and B correspond to the ROI shown in Figures 4B and E. The developed algorithm had corrected the distortion, which is evident from both the histogram profile and the visual appearance of the corrected image.
FIGURE 4.
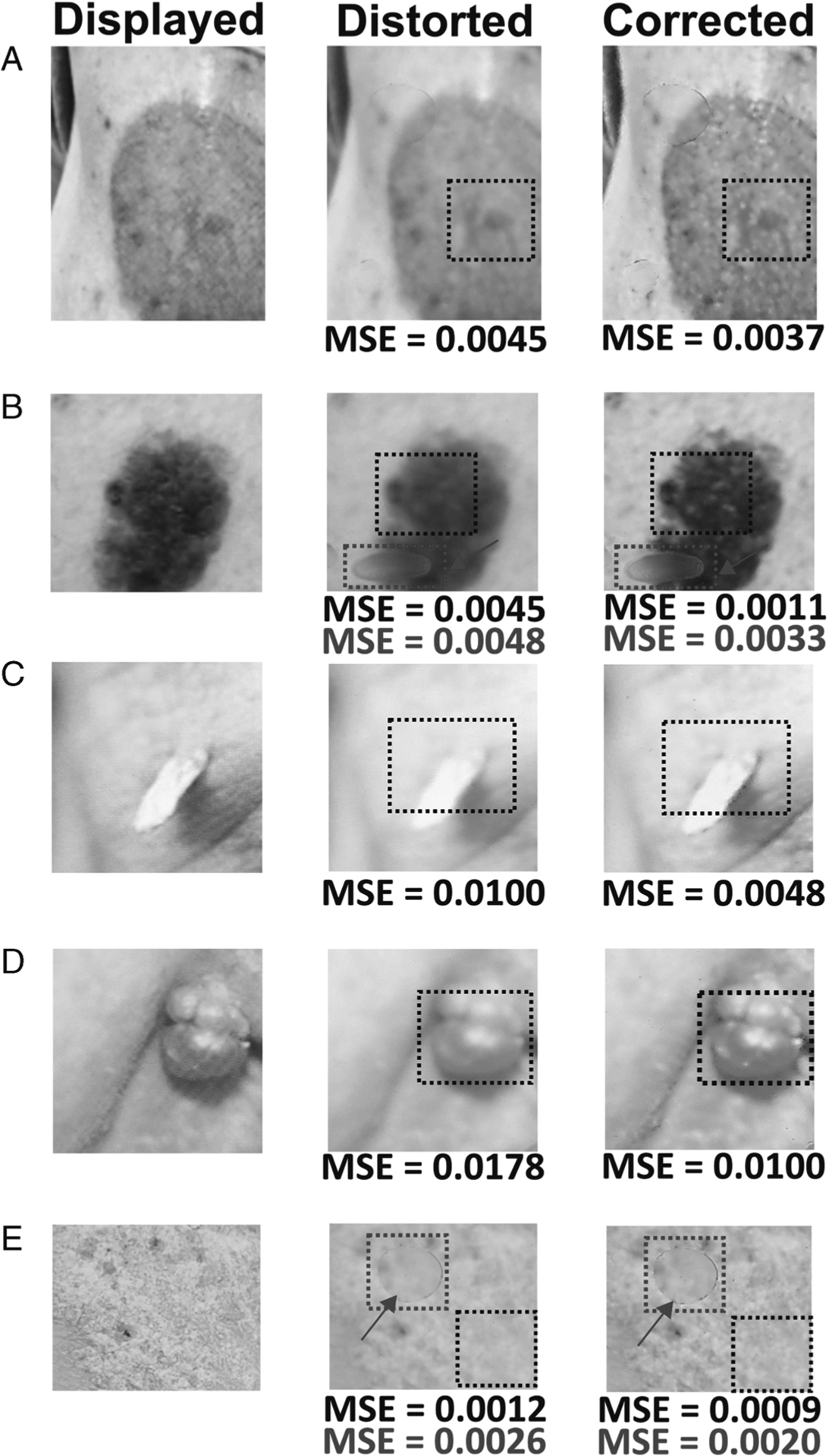
Representative images showing displayed, distorted, and corrected images of various skin conditions. A, Superficial basal cell carcinoma. B, Seborrheic keratosis. C, Cutaneous horn with squamous cell carcinoma. D, Pyogenic granuloma. E, Psoriasis. An MSE was calculated for the ROIs shown by the square dotted boxes.
FIGURE 5.
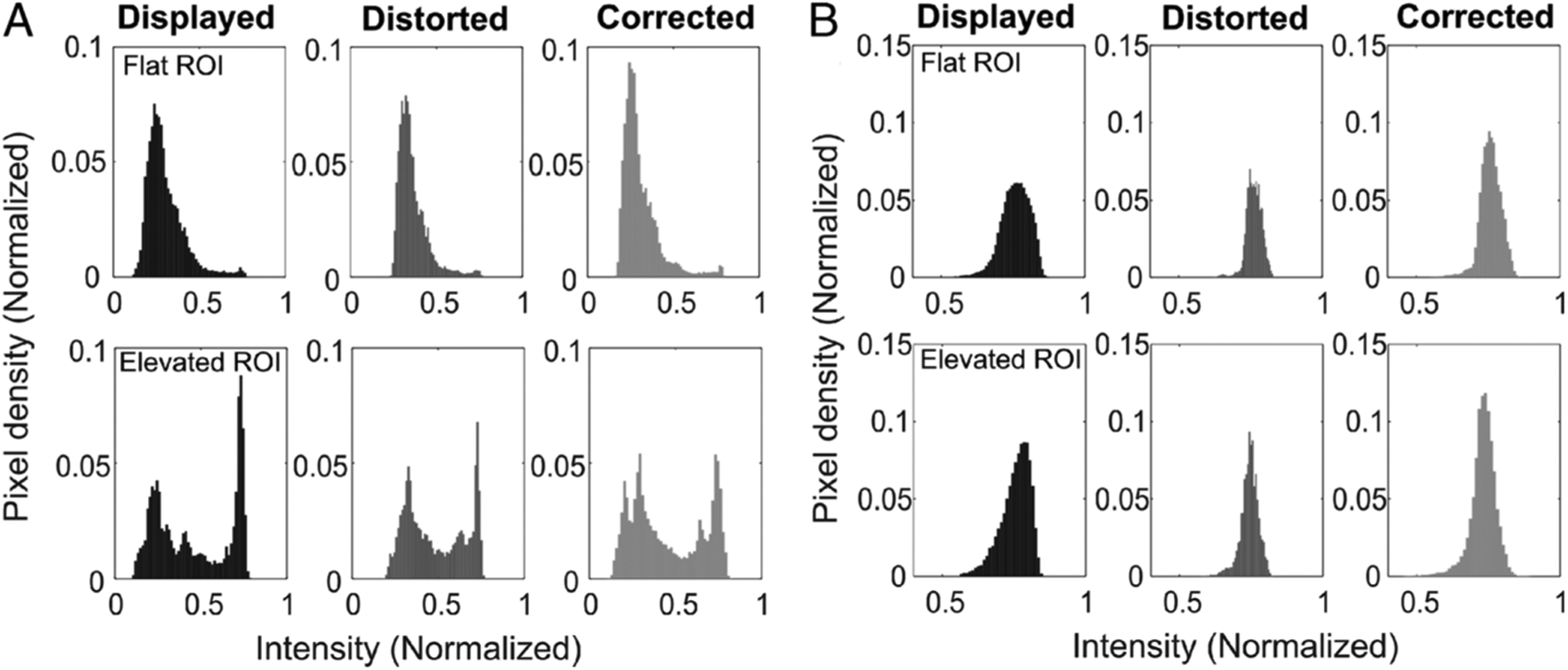
Histogram plots of the displayed, distorted, and corrected images from uniform and nonuniform surfaces. A, Corresponds to Figure 4B. B, Corresponds to Figure 4E. The plots were generated based on the pixel values within the ROI.
DISCUSSION
The development and practical use of a simulated skin model will play a key role in introducing dermatology to medical students as well as educating and training them with various examples of dermatopathology.17 Given the unmet need for the development of a realistic skin simulator model, our approach using a smartphone-based imaging algorithm coupled with a semitransparent or translucent PDMS elastomer overlay to represent skin texture and topography provides a novel simulator and a means to teach dermatology physical examination skills to medical students and healthcare providers. Importantly, this new technology and methodology will enable a more realistic feel of skin and clinical skin conditions, along with projected and corrected images that can be acquired immediately from patients, or downloaded from skin lesion databases with a smartphone or tablet. In the future, this smartphone or tablet-based skin simulator technique could provide new point-of-care training for students.
For decades, several materials and skin models have been used to train medical students. However, most have been developed after trial and error and are often research specific. The simulation-based surgical training methods can be of immense help in acquiring and maintaining surgical skills in safe environments without incurring any human health risk.18 However, they do not always provide accurate and true representation of skin biomechanics, texture/topography, and visual appearance for these needs.19
Interactive real-time skin simulation based on finite element solutions is also of interest, as these can provide accurate visualization of skin but fail to provide a real tactile perception of skin texture.20,21 In contrast, our approach offers the potential to provide both a visual and tactile representation of skin conditions. In this study, our developed algorithm corrected the distortion induced by the scattering properties of the semitransparent elastomer and closely displayed the features, sharpness, contrast, and color of the original image. Nevertheless, this technique also depends on how well the original image was captured, as our correction algorithm uses the original image as a reference target.
Spatial augmented reality (SAR)–based techniques provide a unique way of health care training as they project textures and augment stationary objects.22 A multitouch detection platform for interacting on a human head-shaped surface directly with the fingers provides hands-on touch-sensitive health care training.23 Similarly, a physical-virtual patient simulator has been developed, which combines the tangible object with a virtual patient and exhibits a wide range of multisensory cues.24 Haptic-based softness perception in SAR has also been developed, which visually manipulates the sense of softness by pushing a soft physical object.25 These technologies hold promise in the future and can also be potential methods to teach dermatology to students and practitioners. Although there has been a report on a mobile-based SAR system,26 most SAR systems are bulky and require complex components. On the other hand, our technology is simple and compact in nature and provides an alternative way of learning. Although our current elastomer is static and is limited in the types of lesions that it can represent, there is the potential for this static elastomer to be replaced by a programmable elastomer in the future.
Despite the noted advantages, the present study has some limitations. The topographical features of the developed PDMS elastomer are based on the molding of the skin phantom, and each elastomer overlay is designed specifically for only one type of skin color and skin condition. Although skin color can be adjusted by doping the elastomer material with different particles, this will also alter the transparency of the material, thereby affecting the color and presentation of the displayed imaging. It should also be possible to alter the skin color of the displayed image. Further studies are needed to optimize this trade-off between skin color representation and elastomer transparency. In the future, we plan to also explore lead zirconate titanate–based piezoelectric material–based actuated approaches,27,28 which will change the shape, texture, or topography of the elastomer based on applied voltages, and which could be co-registered with the displayed image pixels. To create an elevated lesion such as a mole, or any depth-based structure, it may be possible to incorporate OCT imaging or histology images of skin from our library and feed these metrics into the electroactive polymer to create a simulated elevated mole in the elastomer. Similarly, a soft, low-cost, pneumatic, or hydraulic-based elastomer approach could be an alternative for a reconfigurable, programmable elastomer.29,30 Other image correction and enhancement methods can also be explored, including deconvolution-based approaches to correct out-of-focus images displayed over the smartphone.31 In the future, we will incorporate this model in the IOS platform.
Acknowledgments
Supported by the Jump ARCHES endowment through the Health Care Engineering Systems Center, as well as by the National Institute for Biomedical Imaging and Bioengineering and the National Cancer Institute of the US National Institutes of Health under award numbers R01EB013723 and R01CA213149, respectively. Additional information can be found at http://biophotonics.illinois.edu.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Dąbrowska AK, Rotaru GM, Derler S, et al. Materials used to simulate physical properties of human skin. Skin Res Technol 2016;22:3–14. [DOI] [PubMed] [Google Scholar]
- 2.Garcia C, Poletti E. Surgical pearl: a model to practice the Mohs surgical technique. J Am Acad Dermatol 2006;55:313–314. [DOI] [PubMed] [Google Scholar]
- 3.Chen TM, Mellette JR. Surgical pearl: tomato—an alternative model for shave biopsy training. J Am Acad Dermatol 2006;54:517–518. [DOI] [PubMed] [Google Scholar]
- 4.Wanitphakdeedecha R, Nguyen TH, Chen TM. The banana: a surgery training model to refine blade control for Mohs layer removal and skin incisions. Dermatol Surg 2008;34:1088–1090. [DOI] [PubMed] [Google Scholar]
- 5.Bastos EM, Silva RDP. Proposal of a synthetic ethylene-vinyl acetate bench model for surgical foundations learning: suture training. Acta Cir Bras 2011;26:149–152. [DOI] [PubMed] [Google Scholar]
- 6.Garg A, Haley HL, Hatem D. Modern moulage: evaluating the use of 3-dimensional prosthetic mimics in a dermatology teaching program for second-year medical students. Arch Dermatol 2010;146:143–146. [DOI] [PubMed] [Google Scholar]
- 7.Dermal lesion simulated tissue pad. Available at: https://surgireal.com/products/dermal-lesion-simulated-tissue-pad. Accessed July 24, 2020.
- 8.Good ML. Patient simulation for training basic and advanced clinical skills. Med Educ 2003;37:14–21. [DOI] [PubMed] [Google Scholar]
- 9.Winston I, Szarek JL. Problem-based learning using a human patient simulator. Med Educ 2005;39:526–527. [DOI] [PubMed] [Google Scholar]
- 10.Aldridge RB, Li X, Ballerini L, et al. Teaching dermatology using 3-dimensional virtual reality. Arch Dermatol 2010;146:1184–1185. [DOI] [PubMed] [Google Scholar]
- 11.Reistad-Long S Diagnosing skin cancer via iPhone: the apps to know. The Atlantic. Available at: https://www.theatlantic.com/health/archive/2012/09/diagnosing-skin-cancer-via-iphone-the-apps-to-know/262325/2012. Accessed July 24, 2020. [Google Scholar]
- 12.Kim K Roughness based perceptual analysis towards digital skin imaging system with haptic feedback. Skin Res Technol 2016;22:334–340. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Lee S. Perception-based 3D tactile rendering from a single image for human skin examinations by dynamic touch. Skin Res Technol 2015;21:164–174. [DOI] [PubMed] [Google Scholar]
- 14.Alex A, Povazay B, Hofer B, et al. Multispectral in vivo three-dimensional optical coherence tomography of human skin. J Biomed Opt 2010;15:1–15. [DOI] [PubMed] [Google Scholar]
- 15.Lurie KL, Smith GT, Khan SA, et al. Three-dimensional, distendable bladder phantom for optical coherence tomography and white light cystoscopy. J Biomed Opt 2014;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang PC, Chaney EJ, Shelton RL, et al. Magnetomotive displacement of the tympanic membrane using magnetic nanoparticles: toward enhancement of sound perception. IEEE Trans Biomed Eng 2018;65:2837–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinez-Molinos S, Martínez-Molina A, Gomar-Sancho C, et al. A collaborative clinical simulation model for the development of competencies by medical students. Med Teach 2017;39:195–202. [DOI] [PubMed] [Google Scholar]
- 18.Agha RA, Fowler AJ. The role and validity of surgical simulation. Int Surg 2015;100:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bando Y, Kuratate T, Nishita T. A simple method for modeling wrinkles on human skin. In: 10th Pacific Conference on Computer Graphics and Applications 2002:166–175. [Google Scholar]
- 20.Sifakis E, Hellrung J, Teran J, et al. Local flaps: a real-time finite element based solution to the plastic surgery defect puzzle. Stud Health Technol Inform 2009;142:313–318. [PubMed] [Google Scholar]
- 21.Lapeer RJ, Gasson PD, Karri V. A hyperelastic finite-element model of human skin for interactive real-time surgical simulation. IEEE Trans Biomed Eng 2011;58:1013–1022. [DOI] [PubMed] [Google Scholar]
- 22.Bimber O, Raskar R. Spatial Augmented Reality: Merging Real and Virtual Worlds. Boca Raton, FL: Taylor & Francis; 2005. [Google Scholar]
- 23.Hochreiter J, Daher S, Nagendran A, et al. Touch sensing on non-parametric rear-projection surfaces: a physical-virtual head for hands-on healthcare training. IEEE Virtual Reality (VR) 2015;69–74. [Google Scholar]
- 24.Daher S, Hochreiter J, Schubert R, et al. The physical-virtual patient simulator: a physical human form with virtual appearance and behavior. Simul Healthc 2020;15:115–121. [DOI] [PubMed] [Google Scholar]
- 25.Punpongsanon P, Iwai D, Sato K. SoftAR: visually manipulating haptic softness perception in spatial augmented reality. IEEE Trans Vis Comput Graph 2015;21:1279–1288. [DOI] [PubMed] [Google Scholar]
- 26.Cortes G, Marchand E, Brincin G, et al. MoSART: Mobile spatial augmented reality for 3D interaction with tangible objects. Front Robot AI 2018;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonar HA, Paik J. Soft pneumatic actuator skin with piezoelectric sensors for vibrotactile feedback. Front Robot AI 2016;2:1–11. [Google Scholar]
- 28.Suh C, Margarit JC, Song Y, et al. Soft pneumatic actuator skin with embedded sensors. In: 2014. IEEE/RSJ International Conference on Intelligent Robots and Systems 2014:2783–2788. [Google Scholar]
- 29.Martinez RV, Fish CR, Chen X, et al. Elastomeric origami: programmable paper-elastomer composites as pneumatic actuators. Adv Funct Mater 2012;22:1376–1384. [Google Scholar]
- 30.Ilievski F, Mazzeo AD, Shepherd RF, et al. Soft robotics for chemists. Angew Chem Int Ed 2011;50:1890–1895. [DOI] [PubMed] [Google Scholar]
- 31.Tao MW, Malik J, Ramamoorthi R. Sharpening out of focus images using high-frequency transfer. Comput Graph Forum 2013;32:489–498. [Google Scholar]


