Abstract
Critical metals, identified from supply, demand, imports, and market factors, include rare earth elements (REE), platinum group metals, precious metals, and other valuable metals such as lithium, cobalt, nickel, and uranium. Extraction of metals from U.S. saline aqueous, emphasizing saline, sources is explored as an alternative to hardrock ore mining. Potential aqueous sources include seawater, desalination brines, oil-and-gas produced waters, geothermal aquifers, and acid mine drainage, among others. A feasibility assessment reveals opportunities for recovery of lithium, strontium, magnesium, and several REE from select sources, in quantities significant for U.S. manufacturing and for reduction of U.S. reliance on international supply chains. This is a conservative assessment given that water quality data are lacking for a significant number of critical metals in certain sources. The technology landscape for extraction and recovery of critical metals from aqueous sources is explored, identifying relevant processes along with knowledge gaps. Our analysis indicates that aqueous mining would result in much lower environmental impacts on water, air, and land than ore mining. Preliminary assessments of the economics and energy consumption of recovery show potential for recovery of critical metals.
Keywords: Critical Metals, Saline Water Sources, Mining Impacts, Extraction Technologies
INTRODUCTION AND BACKGROUND
This paper proposes a sustainable approach for aqueous-phase recovery of metals from saline water sources and brines. Many metals are used as components in manufacturing of a range of products and devices. Some have been designated as critical to the U.S. economy and national security based on domestic consumption, domestic supply, import reliance, and market dynamics; these include many rare earth elements (REE), platinum group metals (PGM), precious metals (including some PGM), and other metals such as lithium, cobalt, nickel, and uranium. The conventional approach to obtaining these metals is by hardrock mining of ores to separate and later refine metal-containing minerals. However, traditional surface and sub-surface mining of minerals is associated with significant environmental impacts and reliance on international supply chains (importation). A secondary approach is recycling of metal components from discarded products and devices but there are economic and logistical (e.g., collection) constraints. This paper explores the feasibility of extracting critical metals from domestic (U.S.) aqueous resources as an alternative to traditional hardrock mining, with an emphasis is on saline sources, both naturally-occurring (e.g., seawater) and anthropogenic (e.g., oil-and-gas produced waters), because of their high dissolved mineral (and potentially metal) content. Our focus is on metals with most elements classified as metals.
The importance of metals criticality today is evidenced by recent federal government efforts, including the founding of the U.S Department of Energy Critical Minerals Institute (CMI) in 2013 to focus on eliminating supply chain disruptions created due to increased reliance on REE and PGM. In 2017, 2020, and 2021, three U.S. Presidential Executive Orders were issued. The first one in 2017 was to develop a Federal Strategy to Ensure Secure and Reliable Supplies of Critical Minerals (No.13817 to “reduce the Nation’s vulnerability to disruptions in the supply of critical minerals” and “identify new sources of critical minerals.”1 Pursuant to this order, the United States Geological Survey (USGS) identified 35 minerals that (1) are “essential to the economic and national security of the United States,” (2) have supply chains that are “vulnerable to disruption,” and (3) serve “an essential function in the manufacturing of a product, the absence of which would have significant consequences for our economy or our national security.”2 In 2020, an “Executive Order on Addressing the Threat to the Domestic Supply Chain from Reliance on Critical Minerals from Foreign Adversaries” (No. 13953) declared a national emergency to deal with that threat and expressed the emergence of secure critical minerals supply chains that do not depend on resources or processing from foreign adversaries.3 In 2021, a “Executive Order on America’s Supply Chains” (No. 14017) requested the Secretary of Defense (as the National Defense Stockpile Manager), in consultation with the heads of appropriate agencies, submit a report identifying risks in the supply chain for critical minerals, including rare earth elements, and policy recommendations to address these risks.4 To date, criticality has largely been conceptualized and defined from the perspective of traditional mining of minerals and metals from ores, an activity associated with high environmental consequences and significant concerns about supply chain sovereignty.
Recovery of dissolved critical metals from saline water sources represents a new paradigm as an alternative to and/or augmentation of traditional hardrock mining of ores. This new approach of aqueous mining would significantly reduce environmental and human health impacts associated with land degradation, water pollution, and air emissions as well as lower water consumption of traditional ore mining. Moreover, the mining of proximate saline water sources would reduce reliance on international supply chains and the need for importation of some critical metals. Also, there is no direct equivalent outcome to the conventional mining industry’s problem of legacy mine remediation, although residual brines may present a significant disposal problem.
ASSESSMENT OF METAL CRITICALITY
Economic importance and supply risk are the two most important factors that have been used to assess the criticality of minerals, elements, and metals in the literature.5 A raw material is critical if both of these dimensions reach a given threshold.6 The primary focus of economic importance is market volatility (i.e., variation of the trade price of metal over time). Also, added value and job creation are considered in the formulation of economic importance.5 The formulation of supply risk includes both the dependency of economic systems on specific metals and the likelihood of supply disruptions (vulnerability of supply chains).5,7 For instance, excessive reliance on imports, with a high risk of not being adequate to meet industry demand, will risk the continuity of production – thus, of supply – if substitutes cannot be found.5 Disruptions of supply can have severe outcomes for essential sectors such as defense, information, and communication infrastructure.8 The environmental dimension (including human health and ecosystems implications) has been involved in several criticality assessments in addition to vulnerability to supply restriction (including importance, substitutability, and susceptibility) and the supply risk (including geologic, technologic, economic risks; social and regulatory risks; and geopolitical risks).9–12 Globally, two terms are being used synonymously, critical and strategic, based on our definition of criticality above; in our discussion we uniformly use the former term.”
The geographical scope of criticality assessments differs in the literature: from a single corporation13 to industrial categories14–17 as well as from national16,18–21 to multinational19,22 to global assessments.11,23 Among the national criticality assessment methodologies, the most recognized ones are those of the U.S. National Research Council, the U.S. Department of Energy, the European Commission, and the British Geological Survey.11
Metals and minerals included in these studies also range in scope from a single commodity18,24 to a set of commodities to differentiate the level of criticality.6,25 In addition to metals and minerals, non-food and non-energy bio-based raw materials6,25 as well as the water have been included26 in some assessments. Metals examined within the criticality literature have included manganese (Mn), gallium (Ga), niobium (Nb), indium (In), rhodium (Rh), palladium (Pd), and platinum (Pt).8 Metals that have been denoted as critical through analysis in past studies include platinum group metals (PGMs – Pt, Pd, Rh, ruthenium (Ru), iridium (Ir), osmium (Os)), precious metals (PMs – gold (Au) and silver (Ag)), and REEs (cerium (Ce), dysprosium (Dy), erbium (Er), europium (Eu), gadolinium (Gd), holmium (Ho), lanthanum (La), lutetium (Lu), neodymium (Nd), praseodymium (Pr), promethium (Pm), samarium (Sm), scandium (Sc), terbium (Tb), thulium (Tm), ytterbium (Yb), and yttrium (Y); given that Pm isotopes are radioactive, it does not occur naturally but from decay of uranium-238)).
The recent list of “35 Minerals Deemed Critical to U.S. National Security and the Economy”, published in 2018 by the United Stated Geological Survey (USGS)2, includes key elements and metals; with criticality based on the criteria of supply, demand, and concentration of production; for different products or uses such as PGMs (used for catalytic agents), lithium (Li; used for batteries), uranium (U; used for nuclear fuels), REEs (primarily used in batteries and electronics), and potassium (K; potash used as a fertilizer). The 35 minerals included a few non-metals and lumped together both REEs and PGMs as individual entities. The main focus of this study was on key elements and metals for which the U.S. relies on imports from foreign adversaries to maintain country’s economic and military strength, while its main objective was “to reduce the Nation’s vulnerability to disruptions in the supply of critical minerals.”
In contrast to the U.S.-centric USGS study above27, a study by Graedel et al.23 assessed the level of criticality of 62 metals and metalloids at the global level, based on supply risk, environmental implications, and vulnerability to supply restriction. According to the study: (i) the risk of supply was highest for elements that are crucial for emerging electronics (i.e., Ga and selenium (Se)), (ii) the most vulnerable elements for supply restriction were steel alloying elements (i.e., chromium (Cr) and Nb) and elements used in high-temperature alloys (i.e., tungsten (W) and molybdenum (Mo)), and (iii) the elements with the most elevated environmental implications are PGMs, Au, and mercury (Hg). The most vulnerable products and applications for countries’ security interests and for the smooth functioning of global supply chains are permanent magnets, advanced ceramics, superalloys, catalysts, refractories, chemicals, passenger cars and light trucks, batteries, capacitors, cemented carbides, catalytic converters, metal alloys, integrated circuits, electronics, LED lights, lasers, aerospace alloys, fiberoptics, and tin alloys.27,28
Our study aims to estimate a multi-dimensional criticality index for metals that are critical to the U.S. national security and economy. Our analysis starts with an assessment of previously defined critical metals for the country. The 58 metals presented in Table 1 are extracted from two mono-dimensional prominent studies focusing on US mineral and metal criticality: (i) “35 Minerals Deemed Critical to U.S. National Security and the Economy”, by USGS2 and (ii) an import reliance assessment by the CMI.29 Similar to the USGS, the focus of the CMI is to eliminate supply chain disruptions created as a result of increased reliance on REEs and precious metals. While the USGS mainly focuses on sectoral vulnerability, the CMI focuses on import reliance for the materials. These 58 metals include 50 metals (17 REEs, six PGMs, and 27 other metals) which were found to be critical by the USGS in 2018,2 with all REEs and PGMs assumed to be critical, as well as eight additional metals that had greater than 50% or 80% import reliance, thus were deemed to be critical by the CMI in 2020.29 In Table 1, an element or mineral shows a ✓, when a criterion is validated.
Table 1.
| USGS Critical Mineral | Import Reliance >50% | Import Reliance>80% | USGS Critical Mineral | Import Reliance >50% | Import Reliance>80% | USGS Critical Mineral | Import Reliance >50% | Import Reliance>80% | USGS Critical Mineral | Import Reliance >50% | Import Reliance>80% | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | · | ✓ | · | Ge | ✓ | ✓ | · | Pb | · | ✓ | · | Tb1 | ✓ | · | ✓ |
| Al | ✓ | ✓ | · | Hf | ✓ | · | · | Pd2 | ✓ | · | ✓ | Te | ✓ | ✓ | · |
| As | ✓ | · | ✓ | Hg | · | ✓ | · | Pm1 | ✓ | · | · | Th | · | · | ✓ |
| Be | ✓ | · | · | Ho1 | ✓ | · | ✓ | Pr1 | ✓ | · | ✓ | Ti | ✓ | ✓ | · |
| Bi | ✓ | · | ✓ | In | ✓ | · | ✓ | Pt2 | ✓ | ✓ | · | Tl | · | ✓ | · |
| Cd | · | ✓ | · | Ir2 | ✓ | · | ✓ | Rb | ✓ | · | ✓ | Tm1 | ✓ | · | ✓ |
| Ce1 | ✓ | · | ✓ | La1 | ✓ | · | ✓ | Re | ✓ | · | ✓ | U | ✓ | ✓ | · |
| Co | ✓ | ✓ | · | Li | ✓ | ✓ | · | Rh2 | ✓ | · | ✓ | V | ✓ | · | ✓ |
| Cr | ✓ | ✓ | · | Lu1 | ✓ | · | ✓ | Ru2 | ✓ | · | ✓ | W | ✓ | ✓ | · |
| Cs | ✓ | · | ✓ | Mg | ✓ | · | · | Sb | ✓ | · | ✓ | Y1 | ✓ | · | ✓ |
| Dy1 | ✓ | · | ✓ | Mn | ✓ | · | ✓ | Sc1 | ✓ | · | ✓ | Yb1 | ✓ | · | ✓ |
| Er1 | ✓ | · | ✓ | Nb | ✓ | · | ✓ | Sm1 | ✓ | · | ✓ | Zn | · | · | ✓ |
| Eu1 | ✓ | · | ✓ | Nd1 | ✓ | · | ✓ | Sn | ✓ | ✓ | · | Zr | ✓ | · | · |
| Ga | ✓ | · | ✓ | Ni | · | ✓ | · | Sr | ✓ | · | ✓ | ||||
| Gd1 | ✓ | · | ✓ | Os2 | ✓ | · | ✓ | Ta | ✓ | ✓ | · |
REEs,
PGMs
We next further evaluated the criticality for the 58 metals in Table 1, using the U.S. National Science and Technology Council’s three-dimensional index,30 which has been used to calculate global criticality values for metals and minerals;31 we now extend this approach by focusing on a USA-centric analysis. The U.S. National Science and Technology Council estimates the metal criticality index (C, ranging from 0 to 1.0) based upon the geometric average of three dimensions: (i) supply risk, (ii) consumption growth, and (iii) market dynamics:30
| (1) |
where R= Supply risk indicator (Net import reliance)
G=Consumption growth indicator (Average growth rate of consumption)
M=Market dynamics indicator (Price volatility)
Figure 1 presents a USA-centric index for critical metals and their level of criticality, based on the above equation and the most recent data provided by the USGS32 in a 2020 Mineral Commodity Summary reporting 2018 data. Based on an early-warning threshold value of C=0.3, as defined by McCullough and Nassar,33 criticality is observed for REEs, some PGMs (Pt, Ag, Pd), Li, U, and Co among others. REEs are shown lumped together in Figure 1 because USGS did not tabulate necessary 2018 data for individual REEs, although individual PGMs were tabulated. It is important to note that all of the metals shown in Figure 1 are, by definition critical. Their respective C values indicate their level of criticality; however, not all can be feasibly supplied by aqueous sources, an issue explored in a later section. Criticality indexes were not calculated for nine metals (Cd, Cs, Hf, Ir, Os, Rb, Rh, Te, and W) for which necessary data were lacking as well as for two metals (Th and Zr) for which the U.S. was a net exporter - therefore net import reliance was equal to zero in 2018.
Figure 1.
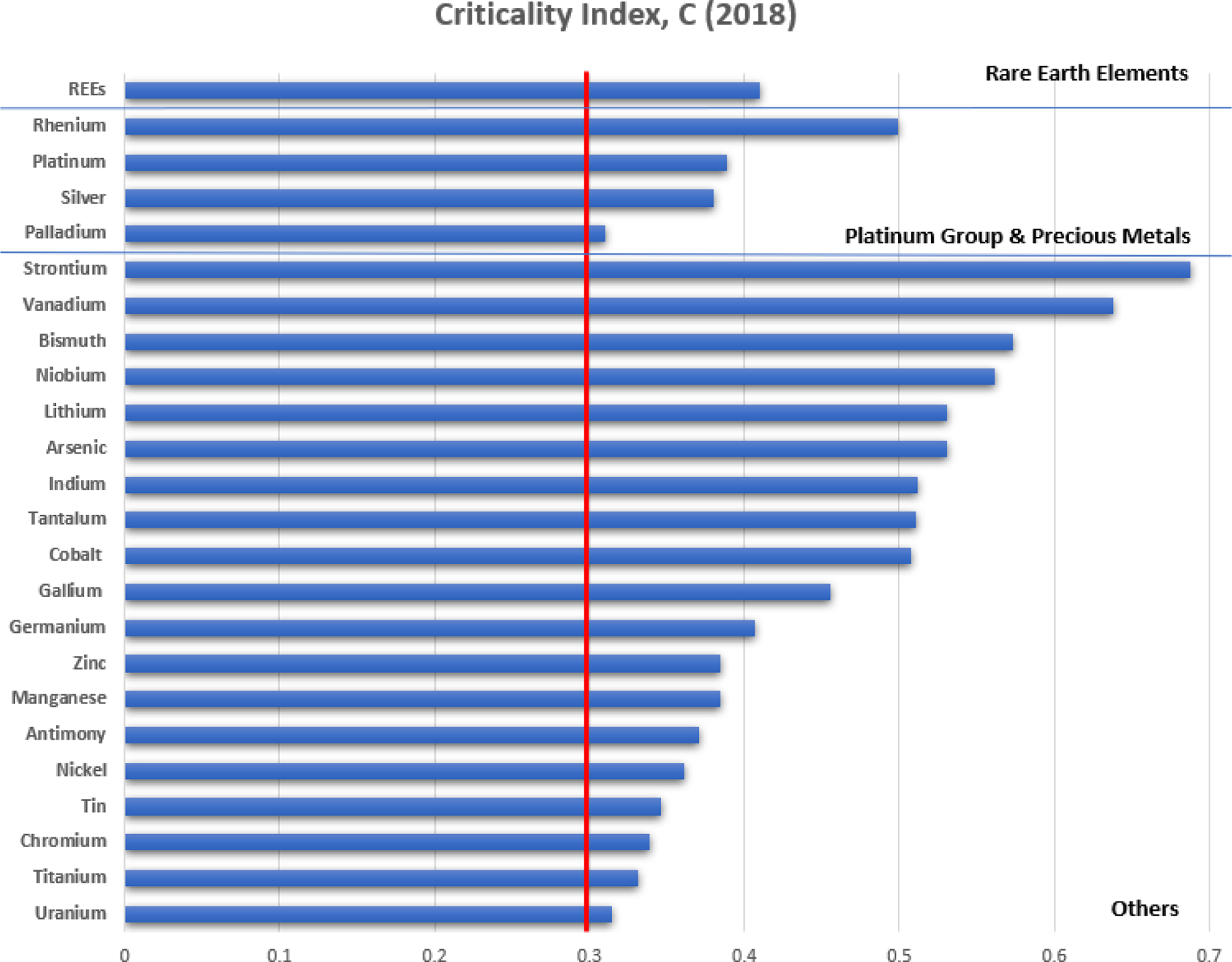
USA-Centric Criticality Index, C (2018 Data).
OCCURRENCE OF CRITICAL METALS IN SALINE AQUEOUS SOURCES
Saline water sources represent a potential alternative to the hardrock ore sources used in traditional mining, for the extraction of critical metals; our focus is on saline (high salinity) sources because they are rich in dissolved metals. While there is no consensus on a definition of saline sources, Mayer et al. identified aqueous sources that contain 2,000 ppm or more of total dissolved salts (TDS) per liter as saline sources.34 As illustrated in Figure 2, there are abundant natural and anthropogenic saline water sources, occurring ubiquitously and covering a range of salinities and constituent compositions, to mine as aqueous feedstocks. These include: open ocean, brines from seawater desalination, brackish groundwater (inland & coastal aquifers), brines from brackish groundwater desalination, produced waters from conventional and non-conventional oil-and-gas recovery, geothermal aquifers, traditional mining and ore-processing wastewaters, acid mine drainage (from mineral/metal mines and coal mines), coal flue-gas scrubber waters, coal ash ponds, agricultural return flows, cooling tower blowdown (including seawater), inorganic industrial waste streams, and salt lakes. While almost all of these sources are clearly saline, arguably, a few, e.g., agricultural return flows, may only be marginally classified as saline. Given their higher water temperatures, geothermal aquifers and produced waters also contain heat as energy, a potential energy source to drive extraction technologies.
Figure 2.
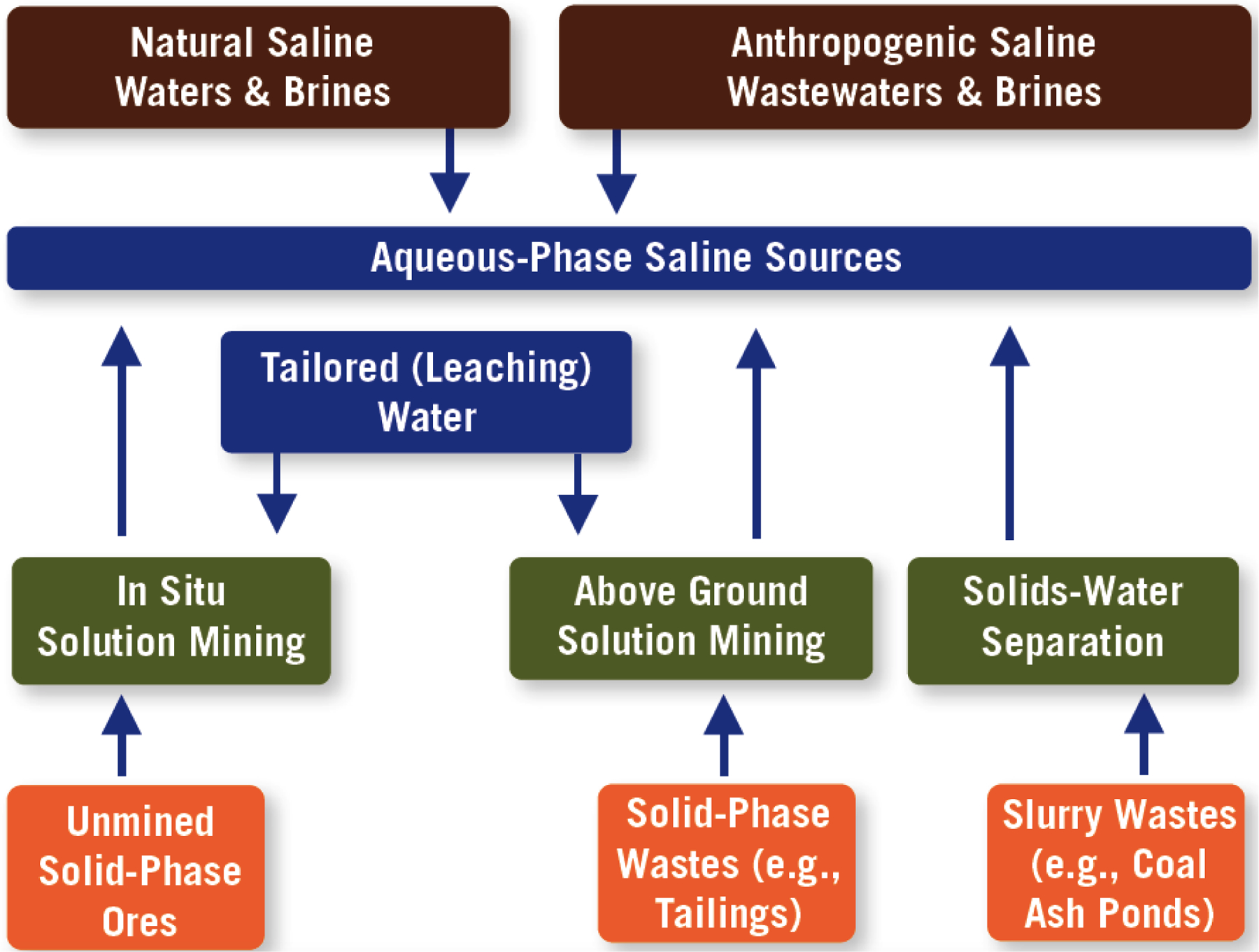
Aqueous-Phase Saline Sources for Mining of Critical Elements.
While most of these sources are truly aqueous, several (e.g., mine tailings ponds) represent slurry (semi-aqueous) sources containing solid-phase particulates whereby process separation of water from solids would provide an aqueous-phase feedstock. In addition, solution mining of solids with tailored-quality leaching water would represent another approach to create aqueous-phase feedstocks, e.g., leachate from in situ solution mining of subsurface ores or above-ground solution mining of solid-phase wastes (e.g., mine tailings). While considerable solution mining is already practiced, there is an opportunity to develop additional selective leaching agents to target specific critical constituents. Another opportunity is recycling of components from discarded products including aqueous (e.g.., electrolytes from batteries) as well hydro-metallurgically-extracted (e.g., Pt and Pd from catalytic converters) components, diminishing the need for primary production and tying into the circular economy.
Valuable metals potentially recovered from saline water sources may include traditional-mining constituents (e.g., Li, U, and Co), PGMs (e.g., Pt, Pd, and Rh), and REEs (e.g., Dy, Nd, and Y). In addition to mining of naturally-occurring aqueous sources, the emerging narrative of the circular economy (product manufacturing with waste valorization and minimization, and reduction of new material feedstocks by recycling) can be advanced through recovery of metal constituents from waste brines and recycling of aqueous components from electronic devices and wastes. Table 2 summarizes the magnitude (volume) available for several select aqueous sources, emphasizing U.S.-relevant sources.
Table 2.
Volumes of Saline Aqueous Sources Available Within or Proximate to the U.S.
| Saline Water Source | Availability (millions of cubic meters, Mm3) | References/Assumptions |
|---|---|---|
| Seawater - All Oceans | 1.39 × 1012 | 35 |
| Seawater - U.S. Continental Shelf | 8 × 107 | 36 |
| Seawater Brine from Ten Hypothetical U.S. 100 MGD (0.379 Mm3/day) Desalination Facilities | 1.39 × 103/year | *Assuming 1000 MGD (10 × 100 MGD) of desalinated water will produce 1000 MGD (3.79 Mm3/day) of brine (with extraction of 2000 MGD of seawater by intake). |
| Produced Water - Conventional - U.S. Total | 3.34 × 103/year | 37 |
| Produced Water – Unconventional - U.S. Total | 79.5/year | 38 |
| Geothermal Water Associated with Geothermal Energy Facilities - U.S. Total | 381/year | 39,40 |
| Acid Mine Drainage - Coal* - U.S. Total | 70/year | 41 |
| Acid Mine Drainage - Hard Rock** - U.S. Total | 7.5/year | 42 |
| Mine Process Water – Copper Mine Raffinate - U.S. Total | 70/year | 43 |
| Coal Power - Flue Gas Desulfurization Wastewater - Avg. of 100 U.S. Plants*** | 62/year | 44 |
| Coal Power - Combustion Residuals Leachate - Avg. of 110 U.S. Plants**** | 14/year | 44 |
Volume estimated from a single, maximum-flow measured in an inventory of 140 abandoned coal mines in Pennsylvania. Additional volume available from dispersed sources.
Volume estimate is for acid mine drainage from 48 features in the Bonita Peak Mining District Superfund Site producing 20,000 m3 per day. Additional volume available from dispersed sources.
Average discharge of steam electric power facilities is 1,710 m3 per day per plant (n=100).
Average discharge of steam electric power facilities is 300–340 m3 per day per plant(n=100–110); assumed 340 m3 per day per plant from 110 plants.
Table 3 summarizes reported concentrations of most of the critical metals that appear in Table 1 and/or Figure 1 within select USA-centric saline water-source categories (blank cells indicate no data available). Also reported are overall salinity (TDS) levels. Metal concentrations vary significantly between sources as well as within the same source, depending on location (e.g., produced waters). Blank cells indicate no data for a metal/source combination. It is important to note that a number of previously-defined critical metals; Be, Bi, Co, Ga, Ge, In, Nb, Pd, Pt, Sn, Ta, Ti, and V; do not appear in Table 3 due to a lack of water quality data, with further source characterization being an important research need.
Table 3. Metal Concentrations in Select Aqueous Sources Within or Proximate to the USA (ppm).
(Values reported represent either a range or single value for individual study cited, or a collective range for multiple studies cited).
| Metal | Produced Water (Conventional)45,46 | Produced Water (Unconventional)47–49* | Seawater50–52 | Geothermal Sources39,53 | Acid Mine Drainage54 |
|---|---|---|---|---|---|
| TDS | 70,000 | 120,000 – 300,000 | 35,000 | 46,000 – 50,000 | 13,000 |
| Ag | 1.40 | ||||
| Al | 0.50 | 4.20 | 300.00 | ||
| As | 0.09 | 1.10 | 0.01 – 0.02 | 12.00 | |
| Ba | 8.00 – 342 | 1.00 – 3,390 | 0.02 – 0.05 | 235 | 0.01 |
| Cd | 0.00 – 0.01 | ||||
| Ce | 1.07 | ||||
| Cr | 0.02 – 0.03 | ||||
| Cs | 14.0 | ||||
| Dy | 0.17 | ||||
| Er | 0.11 | ||||
| Eu | 0.04 | 0.03 | |||
| Gd | 0.16 | ||||
| Hg | 0.01 | ||||
| Ho | 0.04 | ||||
| K | 200 – 11,600 | 516 – 1,040 | 329 – 392 | 17,500 | 9.40 |
| La | 0.27 | ||||
| Li | 7.00 – 400 | 19.1 | 0.10 – 0.17 | 215 −440 | |
| Lu | 0.02 | ||||
| Mg | 100 – 26,000 | 781 – 1,190 | 1,290 – 1,320 | 54.0 | 504 |
| Mn | 0.50 – 7.00 | 0.68 | 0.00 – 0.01 | 1,400 – 1,560 | 231 |
| Nd | 0.49 | ||||
| Ni | 0.03 – 0.42 | 0.02 | 0.00 – 0.01 | ||
| Pb | 0.00–0.05 | 102 | |||
| Pr | 0.11 | ||||
| Rb | 0.12 – 0.20 | 135 | |||
| Sb | 0.01 | 0.40 | |||
| Sc | 0.04 | 0.03 | |||
| Sm | 0.12 | ||||
| Sr | 7.00 – 4,500 | 237 – 277 | 8.10 – 13.0 | 400 | |
| Tb | 0.03 | ||||
| Tm | 0.02 | ||||
| U | 0.00 – 0.02 | 0.85 | |||
| Y | 0.89 | ||||
| Yb | 0.10 | ||||
| Zn | 0.10 – 26.0 | 0.00 | 540 | 645 |
This reference is the only non-U.S. centric source but is included because of its wealth of information on REEs.
FEASIBILITY ASSESSMENT FOR COMBINATIONS OF METALS AND SOURCES
A fundamental question is whether there is adequate availability of critical metals in nationally proximate aqueous sources to satisfy demand. This section highlights the following saline water sources: produced waters (PW), geothermal aquifers (GA), seawater desalination brines (SB) (based on the scenario of 10 large desalination plants described in Table 2), and acid mine drainage (AMD). The availability of critical metals in these sources is compared with current U.S. and global consumption levels. Table 4 provides a preliminary evaluation of combinations of critical metals and select saline source waters that have the potential for yielding globally or domestically (USA) significant quantities. The feasibility score is the percentage of global or domestic consumption that is available in the scenarios described in this analysis for U.S. produced waters, geothermal aquifers, coastal seawater from desalination plants, and acid mine drainage. This preliminary analysis suggests several feasible scenarios for recovery of a number of critical metals from some U.S. sources, at levels that can provide a meaningful resource for the United States. Among source waters, Table 4 suggests significant opportunities for AMD and PW while opportune minerals and metals include Li, Sr, Mg, and some REEs. It is important to note that this feasibility assessment does not include critical metals which did not appear in Table 3 because of a lack of water quality data (see previous list) as well as only considering select aqueous sources among those identified; thus, this is a conservative feasibility assessment with other future opportunities to emerge as more water quality data are created and/or more sources assessed.
Table 4.
Feasibility Analysis of Critical Metals and Saline Water Sources (Produced Water (PW); Geothermal Aquifer (GA); Seawater Brine (SB); and Acid Mine Drainage (AMD).
| Metal | Criticality Index (C) | Feasibility Score | Source(s) | |
|---|---|---|---|---|
| Global Demand % | USA Demand % | |||
| REEs | ||||
| Dysprosium | 0.42 | 15 | NA | AMD |
| Gadolinium | 0.42 | 4 | NA | AMD |
| Holmium | 0.42 | 41 | NA | AMD |
| Lutetium | 0.42 | 17 | NA | AMD |
| Neodymium | 0.42 | 5 | NA | AMD |
| Samarium | 0.42 | 1 | NA | AMD |
| Scandium | 0.42 | 18 | NA | AMD |
| Terbium | 0.42 | 30 | NA | AMD |
| Thulium | 0.42 | 3 | NA | AMD |
| Ytterbium | 0.42 | 22 | NA | AMD |
| Yttrium | 0.42 | 0.7 | 19.7 | AMD |
| Other Critical Metals | ||||
| Arsenic | 0.53 | 5 | 9 | PW, GA |
| Lithium | 0.53 | 59 | 1880 | PW, GA, SB |
| Magnesium | 0.16 | 721 | 15600 | PW, SB |
| Strontium | 0.68 | 186 | 1760 | PW, SB, GA |
Figure 3 portrays an overall periodic table representation of the feasibility to meet global demand of critical metals from U.S. aqueous sources. Identified in the periodic table are non-critical metals, metals whose criticality could not be determined based on a criticality index, critical metals whose feasibility cannot be determined, and a range of feasibility for critical metals. The following discussion further illustrates several higher opportunities among select constituents and/or sources.
Figure 3.
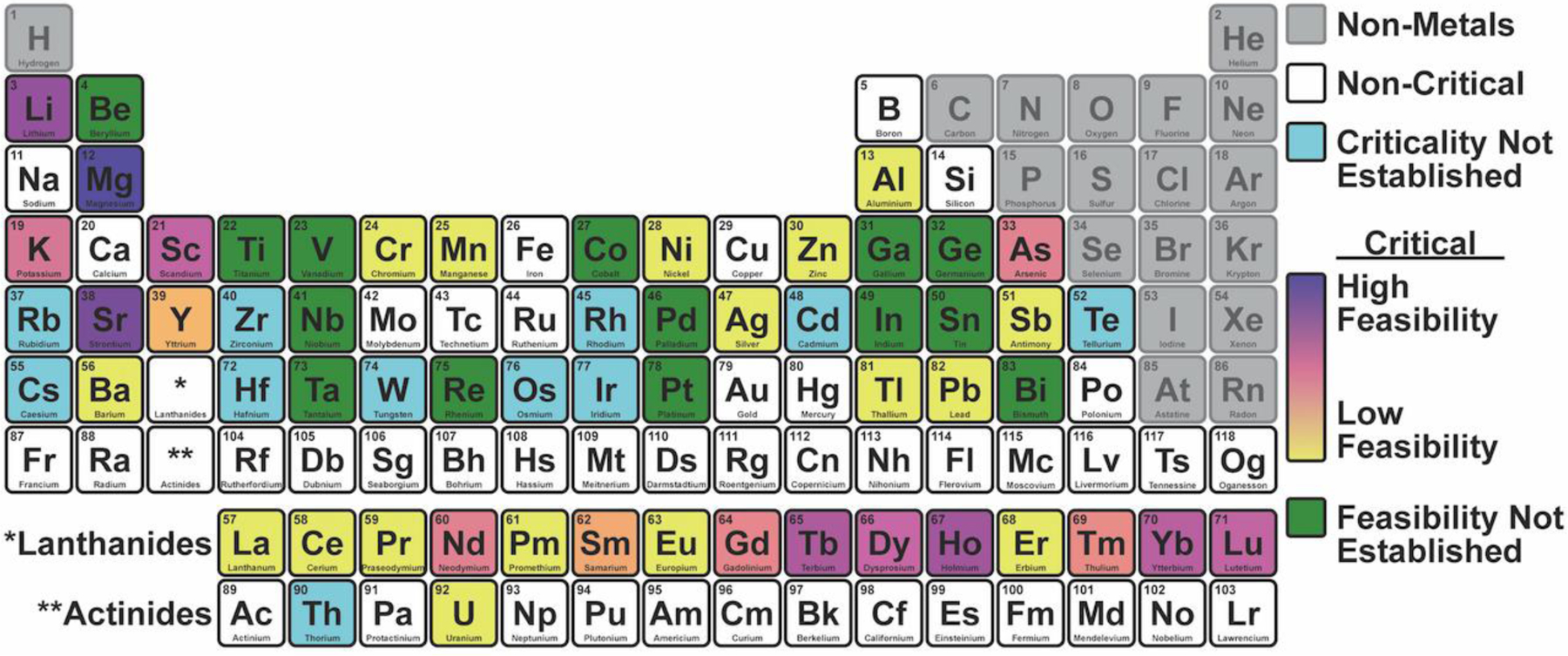
Feasibility to Meet Global Demand of Critical Metals from Aqueous Sources
Produced waters provide opportunities for recovery of some critical metals. Of particular interest are two important oil-and-gas regions: the Permian Basin, which is the largest petroleum producing basin in the U.S., and the Marcellus Basin, which is the largest hydraulically fractured shale gas field of the country. Among five critical metals (Ba, K, Li, Mg, Sr) reported to have significant concentrations (>300ppm) in these two regions, Li is attractive for extraction based on its level and value. The estimated annual Li capacity in the U.S. inland conventional produced waters is 4.7 × 104 tons, based on 35 mg/L in the Permian Basin45 and 14 mg/L in California oil fields,46 exceeding the U.S. annual Li demand, although more data is needed on the ranges of Li in other produced waters. Likewise, Sr is found to be significantly available in both Permian Basin (180 mg/L45) and Marcellus shale region (32 mg/L47), and the available inland produced water capacity exceeds the annual demand both nationally and globally. U.S. produced water concentrations for Mg, a marginally critical element in Figure 1, is found to be sufficient to exceed both the global and national demand (based on levels between 100 mg/L46 and 26,000 mg/L45).
Geothermal aquifers also provide some opportunities for critical metal recovery. The literature for critical metals in geothermal aquifers was first studied by Blake in 1974.53 The focus of the study was the extractable levels of critical minerals including metals from geothermal brines in Niland, Imperial Valley (CA). Considering the current levels of geothermal water use of country (varying between 103 and 243 billion liters per year55), his findings suggest that aqueous mining of critical metals in geothermal aquifers would be able to provide the entire Li and Sr demand globally (based on concentrations of 215 mg/L and 400 mg/L, respectively). Focusing only on the Salton Sea, we found that the current concentrations of geothermal brines will be enough to satisfy the demand for Sr, Li, and Mg domestically and for Sr globally (Table 4). In addition, the Salton Sea geothermal brines will be able to provide 60% of the global Li demand annually.
Considering seawater, the estimated mass of Li in seawater along the U.S. continental shelf (volume: 8 × 1013 m3) is 1.4 × 1010 kg, based on a concentration of 0.17 mg/L in the open ocean. While practically difficult to recover, the associated mass would exceed annual demand of the U.S. for Li of 3×106 kg/year;32 moreover, proximate coastal seawater may be considered a “renewable” source for metals like Li, given its dynamic replacement by the open ocean. Considering a more practical recovery scenario, for a seawater desalination facility producing 100 MGD (379,000 m3/day) of desalted water, the resultant brine of 100 MGD (379,000 m3/day) based on 50% recovery would contain 0.34 mg/L of Li,50 corresponding to 47,000 kg/year of potentially-recoverable Li; hypothetically, brine from ten such plants constructed in water-scarce regions of the U.S. (e.g., CA, TX, and FL) would satisfy more than 15% of annual Li demand. Similarly, brines from ten such seawater desalination plants would satisfy more than 96% of the annual demand for Sr with a seawater concentration ranging between 8.1 mg/L and 13 mg/L.
The U.S. currently imports almost all of its REEs, an important issue considering recent consumer product tariffs that affect REE availability and affordability. Aqueous REEs have been shown to occur at trace levels in produced waters and geothermal brines, sometimes exceeding seawater REE concentrations by a factor of 1,000.49 A recent study reported that Eu, an REE used in LED lights, was present at 27 ug/L in fracking-produced waters in China.49 For the US, data on concentrations of REE are not available for most aqueous sources. Based on available U.S. data, AMD is the most promising anthropogenic and natural water source. Even though the national consumption data is not currently accessible for all REEs, the current REE concentrations in AMD emerge as promising sources which have capacity to provide 41% of the world’s Ho (AMD concentration: 0.04 mg/L) demand, as well as 30% and 22% of Tb (AMD concentration:0.03 mg/L) and Yb (AMD concentration: 0.10 mg/L) demands, respectively. The current AMD concentration levels of Sc (0.03 mg/L), Lu (0.02 mg/L) and Dy (0.17 mg/L) also provides capacity to satisfy more than 15% of global annual demand.
TECHNOLOGIES FOR EXTRACTION OF METALS FROM AQUEOUS SOURCES
Landscape of Existing and Emerging Separation Technologies
There are existing and emerging separation technologies that can extract targeted inorganic constituents (e.g., metals), valuable or problematical, from complex aqueous mixtures as practiced within several application domains: water and wastewater treatment, aqueous-stream processing by the mining industry, and legacy mining site remediation including acid mine drainage as well as more recent direct investigations on critical metals extraction from a range of aqueous saline sources. The relevance and adaptation of each of these domains is discussed below.
Drinking Water and Wastewater Treatment Processes.
Removal of problematical inorganic contaminants from water sources can be achieved during water and wastewater treatment through processes such as sorption (including electro-sorption and ion exchange), membrane separation, and chemical precipitation, with targeted-contaminant selectivity being a desired attribute of all. These water/wastewater treatment technologies can potentially be adapted to extraction of valuable constituents from aqueous sources of dissolved minerals and metals. However, a major distinction for this domain is that problematical contaminants that are removed must be safely disposed of, considering their form (e.g., a solid-phase precipitate, an aqueous membrane concentrate, or adsorbed molecules on the surface of a solid-phase sorbent) whereas for valuable constituents, there must be a means of recovery. For example, adsorption of a valuable constituent must be followed by an effective desorption step to obtain a concentrated aqueous solution. Moreover, in a complex mixture, there may be competing constituents which can reduce the efficacy of targeted-contaminant removal, a phenomenon also affecting extraction of valuable constituents.
Hydrometallurgy in Mining Industry.
Another approach would be technology adaptation from the traditional mining industry practice of hydrometallurgy, an approach in extractive metallurgy in which aqueous solutions are used for the recovery of metals from ores (e.g., Cu) or naturally occurring brines (e.g., Li extraction from brines in Chile). Given the three steps involved in hydrometallurgy; leaching, solution concentration and purification, and metal or metal compound recovery; there are intermediate aqueous streams after extraction for further processing by steps potentially adaptable to our paradigm. Another potentially relevant mining practice involving hydrometallurgy is solution mining whereby a selective leaching solution is introduced into an ore deposit to create a targeted constituent-rich aqueous leachate. In some cases, a ligand may be introduced for enhanced metal dissolution by complex formation (e.g., cyanide (CN−) complexation of gold (Au+)). Solution mining of U and Cu has long been practiced whereby acids may first be introduced to dissolve U and Cu from simple compounds, followed later by ion exchange for U recovery and solvent extraction or chemical precipitation for Cu recovery. Some of these aqueous-phase processing steps may be of potential relevance to our concept. Moreover, an opportunity area is the development of selective ligands for dissolution and recovery of metals.
Legacy Mining Site Remediation Including Acid Mine Drainage.
There has been much work done on remediation of improperly-closed legacy mining sites, focusing on clean-up of surface water pollution from acid mine drainage and subsurface contamination of groundwater. Exposure of acid mine drainage to the atmosphere leads to oxidation of metal sulfides with the acidic character of acid mine drainage enhancing the solubility of toxic heavy metals like Cu and nickel (Ni) as well as trace metals like arsenic (As). Treatment techniques include neutralization with lime, chemical precipitation with sulfide, and ion exchange. It is noteworthy that acid mine drainage also represents a saline source of valuable constituents. Groundwater proximate to mining operations may occur in hard-rock aquifers with treatment/remediation often involving a pump-and-treat approach for surface treatment and reinjection, although some work has been done on in situ permeable reactive barriers.
Direct Studies on Extraction of Valuable Metals from Saline Waters.
While a wealth of information exists on potentially adaptable water-treatment and mining-practice processes, there has also been some recent direct investigations of processes for recovery of valuable metals from aqueous sources, largely focused on opportune sources (e.g., seawater, desalination brines, and produced waters) and targeted critical metals (e.g., Li). In some cases, there may be opportunities to recover a group of chemically-similar valuable metals (e.g., REEs). A tabular summary of selected direct studies appears in Table 5 showing potential technologies, targeted metals, and present limiting factors/barriers/gaps.
Table 5.
Technologies Directly Considered for Extraction of Valuable Metals from Saline Aqueous Sources.
| Technology | Target Metals | Limiting Factor/Barrier/Gap | References |
|---|---|---|---|
| Selective Capacitive Deionization (CDI) | Lithium, Uranium, Cesium | CDI Electrode Selectivity and Capacity | 58 |
| Selective Solvent Extraction | Lithium | Low Recovery, Alterative Solvents | 59 |
| Biosurfactant-Ligand Complexation | REEs, Uranium | Low Selectivity Among REEs | 60,61 |
| Ionic liquids (ILs) Extraction | REEs | IL Extraction Mechanism, Functionality of ILs | 62 |
| Specific Ion Exchange (IX) Resins | Lithium | Presence of Interfering Cations; Incomplete IX Regeneration | 63 |
| Fractionation Crystallization Process | Magnesium | Mg Salt Product Purity | 64 |
| Polymeric Adsorbents | Uranium | Decreased Capacity with Increasing Desorption Cycles | 65 |
| Chelating Resins and Membrane Adsorbers | Copper, Cobalt, Platinum, Palladium, Silver, Gold, Uranium | Expense of Dendrimers as Chelating Agents | 36 |
| Polymeric Adsorbent | Uranium | Significant Impact of Capacity Degradation on Cost | 66 |
| Carbon Nanotube-Iron Oxide Adsorbent | Cobalt | Reduced Capacity by Background Organic Matter | 67 |
| Functionalized Adsorbents | Europium | Competition by Divalent Cations | 49 |
| Magnetic and Functionalized Nanoparticle Sorbents | Uranium | Limited Work Done on Desorption | 68 |
| Selective Sorbents and Exchangers | Cesium, Rubidium, Lithium, Uranium | Constituent- and Sorbent-Specific Capacity | 69 |
| Bio-Electrochemical Systems (BES) | Chromium, Selenium | Application of BES to metalloids (chromate, selenate) | 70 |
| Membrane Crystallization (MCr) | Barium, Strontium, Magnesium, Lithium, Manganese, Copper | Requires NF Reject Stream from SWRO Brine | 71 |
| Nanofiltration (NF) Fractionation of Multi- and Poly-Valent Cations | Lithium versus Magnesium | Fate of Non-Targeted Mono- and Poly-Valent Ions | 72 |
| Bipolar Membranes | Lithium | Improved Membrane Performance | 73 |
Scrutiny of the tabular summary reveals that there is a significant body of work with outcomes ranging from proof-of-concept to translational research to overcome technology limitations as well as identified barriers and/or gaps along the extraction and recovery pathway. The technology readiness levels (TRLs) range from TRL 3 (proof-of-concept) to TRL 5 (validation under laboratory and environmental conditions), to the need for translation research and piloting (TRL ≥ 6). Key goals are improved technology performance as well as demonstration of economic viability and environmental sustainability (discussed in subsequent sections). Given the conventional and emerging technology landscape, we believe that the most attractive extraction technology approach is sorption, either in the form of more conventional fixed-bed adsorbers with granular media, more innovative porous membrane adsorbers, or possibly electro-sorption. Among the most notable limiting factors/barriers/gaps are low selectivity, low capacity, and low recovery/regeneration/desorption, all of which are constrained by a lack of knowledge on sorption/desorption reaction mechanisms. A major challenge of selective sorption is extraction of a targeted dilute solute in a complex water quality matrix containing competing solutes. Luo et al. have shown that it it possible to recover Li from a complex water matrix but much more work is needed to scale up the process.56,57
Technology Enabling Considerations
Important technology enabling considerations affecting process and system efficacy and performance include: concentration(s) of targeted metal(s) in feed waters; influential properties of targeted metals and separation media; and post-extraction processing and purification.
Concentration of Feed Streams.
For valuable metals extraction from an aqueous feed stream, depending on constituent level, a first prior step may be necessary to achieve a higher constituent concentration through volume reduction, using existing brine concentration technologies (e.g., membrane distillation, forward osmosis, evaporation). While higher constituent levels will provide a higher extraction-reaction driving force, the impacts of higher salinity (ionic strength) on activity coefficients will increase ionic constituent solubility, however, the former will dominate. There are emerging membrane-based brine concentration technologies (e.g., forward osmosis) that are energetically more favorable than thermal evaporation. Another approach to constituent concentration would be fractionation of a feed stream by nanofiltration (NF) using a softening NF membrane to create a dilute monovalent-rich steam (permeate) and a concentrated polyvalent-rich stream (reject). Safe environmental disposal of any final residual concentrated brine after extraction would be an unintended consequence to be addressed; one option could be incorporation into building/construction materials (e.g., bricks).
Metal Constituent and Separation Media Properties.
Constituent properties such as hydrated radius, charge, and solubility will influence extraction process and efficacy. Constituent interactions with separation media will be influenced by their properties including surface area and charge for sorbents and pore size and surface charge for membranes as well as water quality conditions (e.g., pH effects on solubility of precipitates).
Downstream Processing and Purification.
Extraction by sorption/ion exchange (IX) (aqueous phase to solid phase sorbent) must be followed by an effective desorption step with a concentrated regenerant stream (e.g., a salt, acid, or base) to obtain a concentrated constituent-rich solution. Extraction by membrane separation will create a constituent-rich reject stream or, depending on constituent-membrane interactions, permeate stream. After a concentrated solution of the targeted constituent is obtained, if a solid-phase material is required as a marketable product, precipitation may follow, e.g., precipitation of Li ion (Li+) as a carbonate salt (Li2CO3(s)). Figure 4 is a schematic illustrating extraction and concentration technologies for constituent recovery by ion-selective sorption, electro-sorption, and membrane processes. It is important to recognize that metal salt solubility, e.g., Li2CO3, is affected by ionic strength (salinity) and, in the case of elevated temperature sources (e.g., geothermal), by temperature. Determination of solubilities from solubility products in brines requires adjustment using activity coefficients. For Li2CO3, Cheng et al.74 observed an initial increase to a maximum value and then a gradual decrease with increasing NaCl concentration as well as a decrease in solubility with temperature. Finally, a key issue is product purity, affected by the degree of constituent selectivity.
Figure 4.
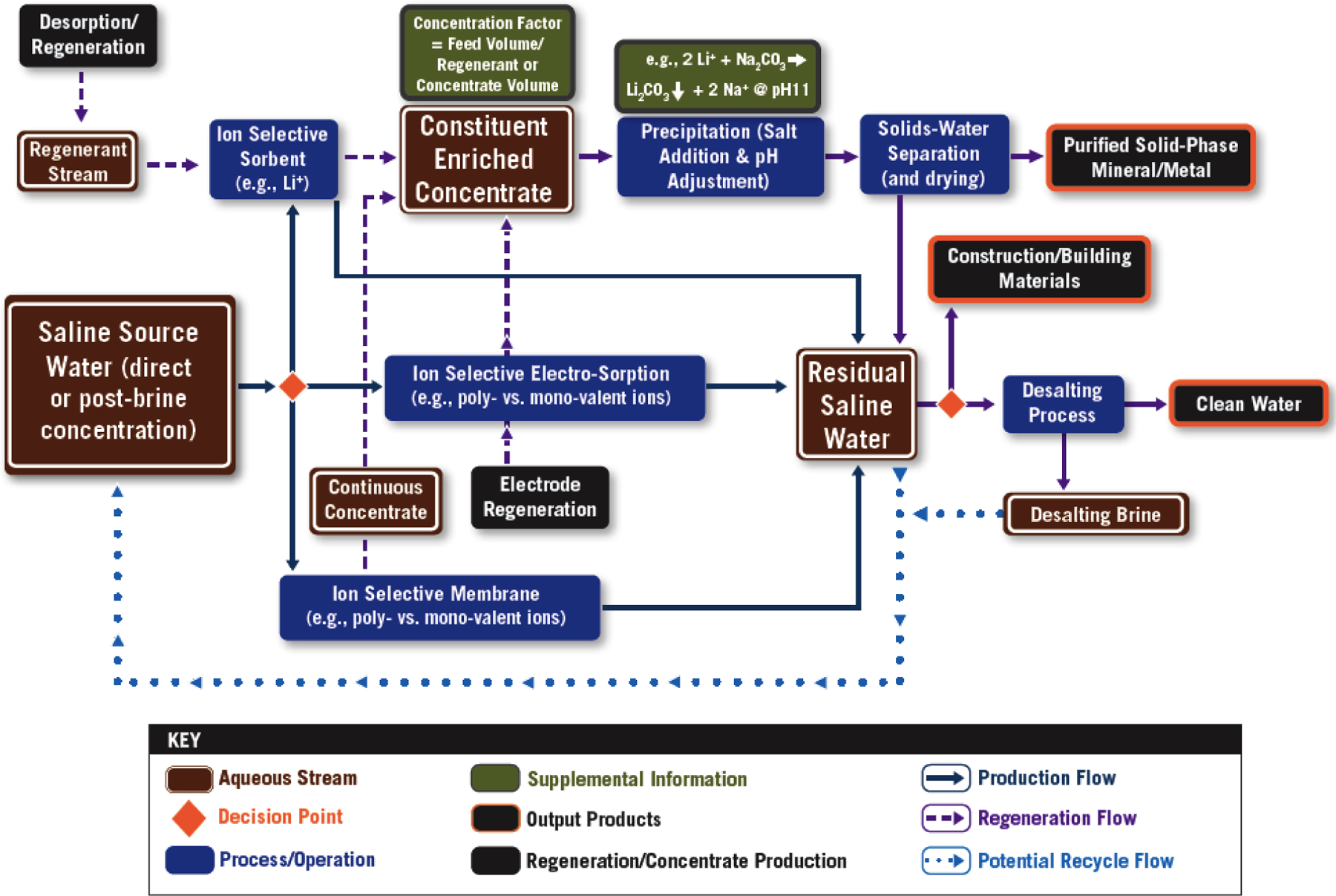
Extraction and Concentration of Targeted Constituents by Ion-Selective Sorption, Electro-Sorption, and Membrane Processes.
ENVIRONMENTAL IMPACT AND SUSTAINABILITY
This section describes the environmental impact of both legacy and modern mineral and metal mining with a focus on water issues to highlight the potential environmental benefits of “mining” non-ore alternative resources, i.e., saline water sources, to meet critical metal needs. Environmental impacts are considered for both legacy mines within the context of possible recovery of metals associated with acid mine drainage (AMD) releases and for modern mines in the context of the major resources, including water and energy, needed for continued operation as well as associated land, air, and water pollution.
Legacy Mines and the Potential for Metal Recovery
The environmental impacts of a single legacy mining operation can be minor or severe, local or far reaching; the determinants of the impacts are primarily factors which are unique to each site: the target metal, the size of the site (e.g., small scale artisanal versus industrial scale), the type of disturbance (e.g., pit mining versus underground versus in situ), environmental setting (climate, mineralogy, etc.), and the era in which active mining occurred. Considering only abandoned mining lands; 22,625 mine features across federal lands;75 one associated hazard at many of these sites is the metal-rich sulfuric acid (H2SO4) AMD solution that is released from mine tunnels, shafts, open pits, and waste rock piles due to the rapid oxidation of iron-bearing sulfide minerals.76 The actual amount of AMD released in the western U.S. is not known,42 however, it is estimated that more than 40 percent of watersheds have been impacted by AMD streams.77
To conceptualize the scale of this issue, consider the Bonita Peak Mining District which was added to the Superfund National Priorities list following the prominent 2015 Gold King Mine Spill—a rapid single release of three million gallons of AMD into Colorado’s Animas River. Although this District encompasses only 48 mining sites, including the Gold King Mine, it collectively produces 5.4 MGD (20,400 m3/day) of AMD per day.42 This problem is not unique to the American West. A national stream survey of the eastern U.S. identified >10,000 km of streams impacted by AMD from primarily coal-related abandoned mining lands.78 In addition to AMD, legacy sites can be a source of environmentally detrimental metal contamination due to wind and water dispersal of metal-laden tailing materials and waste rock from earlier, inefficient metal extraction technologies.75 As highlighted further below, the presence of metals already in solution offers an opportunity to recover these metals for beneficial re-use and prevent them from entering environmental compartments.
Environmental and Resource Costs of Modern Mining
In stark contrast to legacy mining, modern mining occurs at relatively few sites (126 domestic metal mines79) under strict environmental regulation. Even so, the environmental impact of these operations is immense due to their large size and extended lifespan. To begin enumerating the environmental costs that are incurred by the development of new traditional resources, it is useful to consider a case example to highlight to impacts of a single mine. The proposed Rosemont Copper mine in southern Arizona will extract an estimated 550 million tons of ore during its lifetime. The associated environmental impact statement (EIS) required for the project has identified 20 categories of impacts for the proposed project, many related to surface water and groundwater quality and ecosystem health.80 Estimated impacts include removal of 1.66 billion tons of rock and disturbance of 22.7 km2 of soil. Air emissions in Pima County, AZ would increase for CO2 by 165,464 tons per year, volatile organic carbon by 78 tons per year, and NOx by 1,088 tons per year. Groundwater recharge would be impaired by 43,000 m3 per year (in perpetuity), and the mine would extract 123,000,000 m3 of groundwater to supplement the 2,280,000 m3 captured from de-watering activities. Stormwater flows to watersheds would be reduced by 45.8% (post closure). The loss of 7 springs with another 69 impacted would be paired with the destruction or disturbance of 711 hectare of riparian areas; 39 km of streams could transition from intermittent/perennial flow to ephemeral flow from this disturbance. A pit lake with acidic, metal rich water would develop upon closure which would lead to a further evaporative water loss of up to 460,000 m3 per year (in perpetuity), or the equivalent of 3% of the current basin recharge. These impacts are neither all-inclusive of the impacts listed in the EIS nor an exhaustive assessment of potential impacts, rather they demonstrate the potential impacts from a single mine on a local scale.
Globally, metal mining has increased 3.5 times from 1970 to 2017, reaching 9.1 billion tons in 2017. Extracting and processing these metals represent (in shares of total global impact) 10% of climate change impacts, 12% of particulate matter health impacts, 3% of water stress, and 1% of land-use related biodiversity loss.81 These impacts occur in the process of liberating minute amounts of metal from a stable, solid matrix into some labile, manageable form. The primary advantage of saline aqueous source mining would be that target metal(s) are already present in a labile form ripe for collection. To better understand how saline mining can be advantageous compared to conventional mining approaches, it is important to enumerate some of the current impacts of mining, as discussed below for water, land, and energy/greenhouse gas emissions.
Water.
Mining requires water to suppress dust, concentrate ores, extract metals, transport materials, and for domestic purposes.82,83 In an analysis of 8314 data points from 359 mining company reports, Northey et al.84 found that water withdrawal, use, and discharge varied greatly between mining operations, but found 90% of operations withdrew between 0.13 and 17.29 m3, used 0.34 and 6.27 m3 (for mining and mineral processing), and discharged 0.03 to 9.94 m3 of water per tonne of ore. Water consumption by metal shows requirements of 172 m3/t for Cu, 116 m3/t for Cu-Au, 716 m3/kg for Au, 107 m3/t for Ni (sulfide), 260 m3/kg for PGMs, 505 m3/t for uranium oxide, and 29.2 m3/t for Zn.85 The average water use for REEs was 11.2 m3/t with a range of 3.8 m3/t for Sm and Gd to 29.9 m3/t for Y.86 Mudd also found wide variations among and across metal commodities but found little evidence of water-use benefits due to ‘economies-of-scale’ for base metals and bulk minerals;85 however, efficiencies tended to be greater for the precious metals operations with greater throughput. They also found little evidence that water-use efficiency was increasing over time, but there is evidence otherwise.87 Water usage on this scale is concerning because mining frequently occurs in regions (the Southwestern U.S., Chile, Australia, China, India, and South Africa) facing growing water risks according to measures such as the water stress index, water depletion index, and water deprivation potential.88
Given that solution mining starts with a saline source, the process results in a large reduction in water consumption and practically eliminates potential water reuse due to contamination or pollution. In the case of remediation applications necessitated by existing or future contamination, saline-water mining offers an opportunity to create added value products (i.e., economic benefits) to remediation applications as part of the clean-up measures. Saline-water mining removes contaminants from (or prevents them from entering) environmental compartments and introduces them into “industrial metabolism” for productive use. Such inputs will be an essential component within the circular economy for critical materials. Added value products could also be mined from desalination brines in areas where desalination plants are operated to supply water needs of conventional mining operations, e.g., Chile.89 A further extension of the added value concept is the future commoditization of water resources. Water scarcity is a global and growing problem that can be addressed by clean-up of marginal resources61. Thus, the niche for innovative remediation technology for metal-contaminated water resources (i.e., saline-water mining technology) is expected to grow with increasing stress on the available potable water resources and increasing need for domestic use, agriculture, and industry due to climate warming and population growth in urban regions. Further, such technologies are needed to mitigate the toxicity of metal contaminants now ubiquitous in many water sources and supply clean water.
Land Use.
Conventional mining disrupts large land areas for operational and processing facilities (e.g., roads, conveyors, crushers, processing plants, water treatment plants), material extraction (e.g., ore pits), waste (overburden) dumps, leaching pads, and tailings ponds.90 The global land area utilized for mining is an estimated 57,300 km2 (0.04% global land area) and 6,400 km2 (0.07% of land area) in the U.S..91 Land area requirements for Cu (surface mining) are reported to be 4.3 m2/t Cu.90 Murguía and Bringezu92 report land disturbances (per million metric ton of ore extracted) of 7.98 hectare (ha) for bauxite (Al), 6.70 ha for Au, 5.53 ha for Ag, 4.5 ha for Cu, and 4.25 ha for Fe. The average disruption for surface mining was less than underground mining with 5.05 ha and 11.85 ha land disturbed per million metric tons, respectively.
Disturbing large land areas is costly due to the capital investments that must be made to permit, open, and operate a traditional mine. Mining of saline waters requires significantly less investment in capital because the land disruption can be limited to an extraction facility. Thus, one advantage to saline-water mining operations is that they would be less risk-averse to market fluctuations that may make large mining operations uneconomical to operate—after a significant portion of their environmental impact has already occurred accessing orebodies. A saline-water mining approach is particularly relevant for critical materials with smaller markets such as REE. The relatively small REE market makes mining operations sensitive to price fluctuations (see the history of the Mountain Pass, CA REE project93). Currently, there are 10 new rare earth mining and exploration projects in the U.S. being considered to meet demand for REE domestically, and any of these operations risks collapsing REE prices by oversupplying the market.93 In lieu of opening new mines with significant environmental impacts, mining of saline waters for REE would both secure a domestic supply while supplementing current domestic REE resources (i.e., the existing Mountain Pass Mine). Additionally, if market forces make processing activities uneconomical, saline-water mining can simply cease, whereas traditional mining operations would suspend operations but continue to monitor/manage their large-scale environmental disturbances.
Energy Use and Greenhouse Emissions.
Disturbing large land areas and extracting their metal constituents is energy intensive process with accompanying greenhouse gas emissions. In 2008, mining was responsible for 9.5% of global energy use (49 EJ/yr) and emitted 3.4 Gt CO2-eq.94 In a comprehensive life cycle analysis of 63 metals, Nuss and Eckelman found a strong correlation between cumulative energy use and global warming potential (measured in CO2-eq).94 The precious metals (including Au and the PGMs Ru, Rh, Pd, Os, Ir, and Pt) account for the highest environmental impacts per kilogram of metal while the major industrial metals (e.g., Fe, Mn, Ti) account for the lowest. When reviewed on an annual production basis, however, Fe and Al account for the largest impacts (see also van der Voet et al.95). The total energy demand and global warming potential of the critical materials identified in Figure 1 and select other metals are provided in Table 6. Nuss and Eckelman also describe the relative environmental implications of each metal by life cycle stage from mining to refined metal; the environmental impacts are primarily derived from the purification (smelting) and refining stages.
Table 6.
Cradle-to-gate cumulative energy demand and global warming potentials per kilogram of select metals.94
| Category | Metal | Cumulative Energy Demand (MJ-eq/kg) | Global Warming Potential (kg CO2-eq/kg) |
|---|---|---|---|
| REE | |||
| Cerium | 252 | 12.9 | |
| Dysprosium | 1,170 | 59.6 | |
| Erbium | 954 | 48.7 | |
| Europium | 7,750 | 395 | |
| Gadolinium | 914 | 46.6 | |
| Holmium | 4,400 | 226 | |
| Lanthanum | 215 | 11.0 | |
| Lutetium | 17,600 | 896 | |
| Neodymium | 344 | 17.6 | |
| Praseodymium | 376 | 19.2 | |
| Samarium | 1,160 | 59.1 | |
| Scandium | 97,200 | 5,710 | |
| Terbium | 5,820 | 297 | |
| Thulium | 12,700 | 649 | |
| Ytterbium | 2,450 | 125 | |
| Yttrium | 295 | 15.1 | |
| PGM | |||
| Palladium | 72,700 | 3,880 | |
| Platinum | 243,000 | 12,500 | |
| Other | |||
| Aluminum | 131 | 8.2 | |
| Antimony | 141 | 12.9 | |
| Arsenic | 5.0 | 0.3 | |
| Beryllium | 1,720 | 122 | |
| Indium | 1,720 | 102 | |
| Bismuth | 697 | 58.9 | |
| Cobalt | 128 | 8.3 | |
| Chromium | 40.2 | 2.4 | |
| Gallium | 3,030 | 205 | |
| Germanium | 2,890 | 170 | |
| Lead | 18.9 | 1.3 | |
| Lithium | 125 | 7.1 | |
| Magnesium | 18.8 | 5.4 | |
| Manganese | 23.7 | 1.0 | |
| Nickel | 111 | 6.5 | |
| Niobium | 172 | 12.5 | |
| Rhenium | 9,040 | 450 | |
| Rhodium | 683,000 | 35,100 | |
| Silver | 3,280 | 196 | |
| Strontium | 48.8 | 3.2 | |
| Tantalum | 4,360 | 260 | |
| Thallium | 5,160 | 376 | |
| Tin | 321 | 17.1 | |
| Titanium | 115 | 8.1 | |
| Uranium | 1,270 | 90.7 | |
| Vanadium | 516 | 33.1 | |
| Zinc | 52.9 | 3.1 |
Substantial reductions in energy expenditures and greenhouse gas emissions shown in Table 6 could be achieved by engaging in saline-water mining because ore mining and processing steps can be eliminated and the complexity of metal-extraction processes reduced since metals are already present in the saline water as a mobile and labile, capturable species. Energy use by source and additional energy benefits of saline-water mining are further explored in the next section.
In summary, increasing global resource demands,96 decreasing ore grades,97 and increasing orebody complexity (e.g., depth, finer-grained mineralogy)98,99 are likely to exacerbate many of the environmental impacts discussed above, in addition to those not discussed (e.g., ecotoxicity, human toxicity, resource depletion, acidification/eutrophication issues, ozone depletion, etc.). Water use in Western Australia is projected to increase from a relatively steady 508 GL in 2008 to 810–940 GL.100 Modeling indicates energy requirements for the comminution of main metal ores will quadruple from current rates by 2030 (from 1970 to 8705 PJ/y).98 Increased production of lower grade ores will also require moving larger volumes of material which directly translates into increases in water use, energy demand, greenhouse gas emissions, and land disruption (accessing ore and storing waste).87,101 Advances in mining technology and process efficiency along with the increased use of renewable energy resources will be needed to offset some the impacts of conventional mining,98,101 but these offsets may not be sufficient to prevent increased impacts.99 Furthermore, mining is a major source of trace metal emissions to the environment with impacts to human and environmental health,102–104 so increases in production will also lead to increased trace metal emissions. Owing to the absence of mining, grinding, floating, leaching and/or smelting processes that are foundational to conventional mining, saline-water solution mining has the potential to be a major alternative source to meet metal demands while reducing land degradation, air emissions, and water demand and pollution. We acknowledge two caveats with respect to aqueous mining. The first is that while aqueous mining may have lower environmental impact, it is recognized that this technology may not be able to fully replace the need for conventional mining. The second is that we do not consider here new approaches to ore mining (e.g., bioleaching) that may reduce environmental (water) impacts as they are outside the scope of this review.
ECONOMICS OF EXTRACTION
The economics of production of materials are governed by a number of factors: quality of the resource (e.g., ore grade); capital and infrastructure costs; operating costs (e.g., labor); and environmental costs (e.g., treatment and remediation, as discussed in the previous section.105 Data on production costs can be found from supply curves from companies which rank cost of individual mines, resources, or whole countries.
Recovery costs of different materials range over five orders of magnitude. Of interest for aqueous mining, costs for Li recovery from brine are around double that of hard rock sources. However, these production cost estimates do not include environmental costs, such as site remediation or treatment/disposal of waste streams, which for aqueous sources can constitute a major barrier to recover. The U.S. Government Accountability Office (GAO) recent report on abandoned hardrock mines states that there are no comprehensive estimates for environmental cleanup of hardrock mines on federal lands,75,106 but between 2008–2017 federal agencies spent a total of $2.9B addressing physical and environmental hazards at abandoned mines with the majority being spent by the U.S. EPA (80%) for environmental hazards only (88%). Over the same period, 13 mining states spent a total of $117M to address abandoned mines, with 78% on environmental hazards. Federal agencies estimate that future costs could be around $11.6B to address hazards associated with abandoned hardrock mines, with 58% for environmental hazards.
Plotted in Figure 5 is the range of market values as well as production costs for the period 1991–2018.33 In some cases, the costs of certain metals (e.g., tin) may be low due to the value of hitchhiker (i.e., co-product) metals. The high market value of some of these metals could improve the economic feasibility of other related processes such as geothermal electricity generation with geothermal aquifers and desalination with seawater. Li extraction from geothermal brines has been studied extensively (e.g., Paranthaman et al.107) and many projects are currently in operation worldwide.108 Ortiz-Albo et al. undertook an analysis of resource recovery from desalination brines, highlighting technology readiness level (TRL), recovery rate, cost, and market price and size.109 Li recovery is hampered by lower recovery yields and lower market price, although this may change as demand increases.
Figure 5.
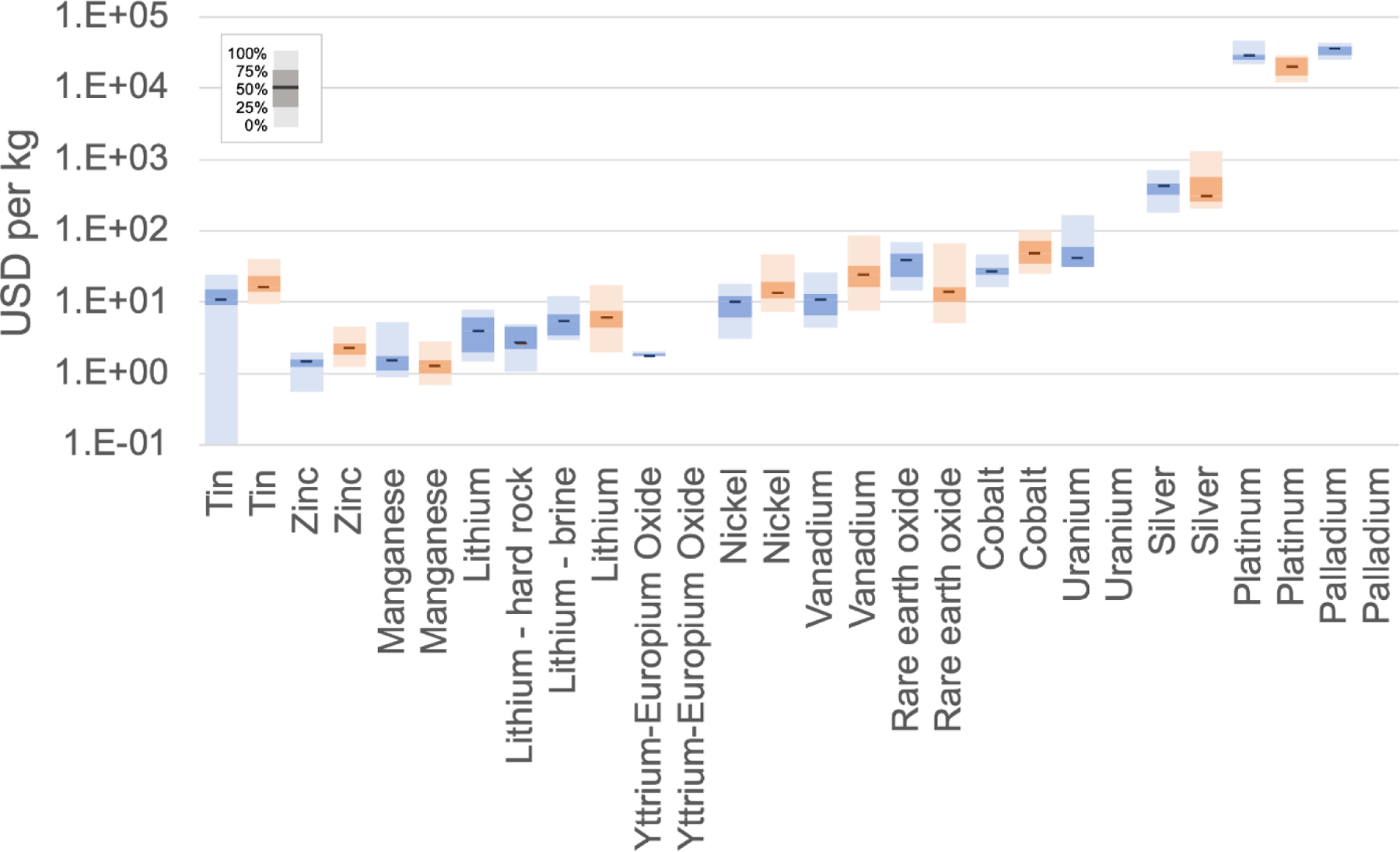
Production cost (blue) and market price (red) of critical metals (within 0 – 100 % scale are median values (solid line) and 25, 50, 75, and 100 percentile values).
ENERGY OF EXTRACTION AND INTEGRATION OF AQUEOUS SOURCE HEAT
Traditional mining and mineral production can be very energy intensive, which comprises a large environmental impact for many of these materials, both in terms of embodied energy but also associated impacts such as global warming. Based on data from the life cycle inventory database ecoinvent,110 in Figure 6 we present energy inputs per kg of produced material to mining and mineral production processes, globally, broken out by electricity, heat, gas, liquid (e.g., diesel), and solid fuels (e.g., coke). Note that where we show different stages in production (e.g., Li brine and Li2CO3) we do not account for primary material needed to produce the secondary material (i.e., cannot sum the values to calculate embodied energy). For example, over 10 tons of Pt metal concentrate are required to produce each kg of Pt. As with production cost, we can see that energy inputs span many orders of magnitude. Energy inputs for treatment of traditional mining wastes, e.g., sulfidic tailing from gold mining, are also large, around 0.8 MJ per kg of tailing110 (or 286 GJ per kg of Au recovered), of which the majority (96%) is in the form of electricity.
Figure 6.
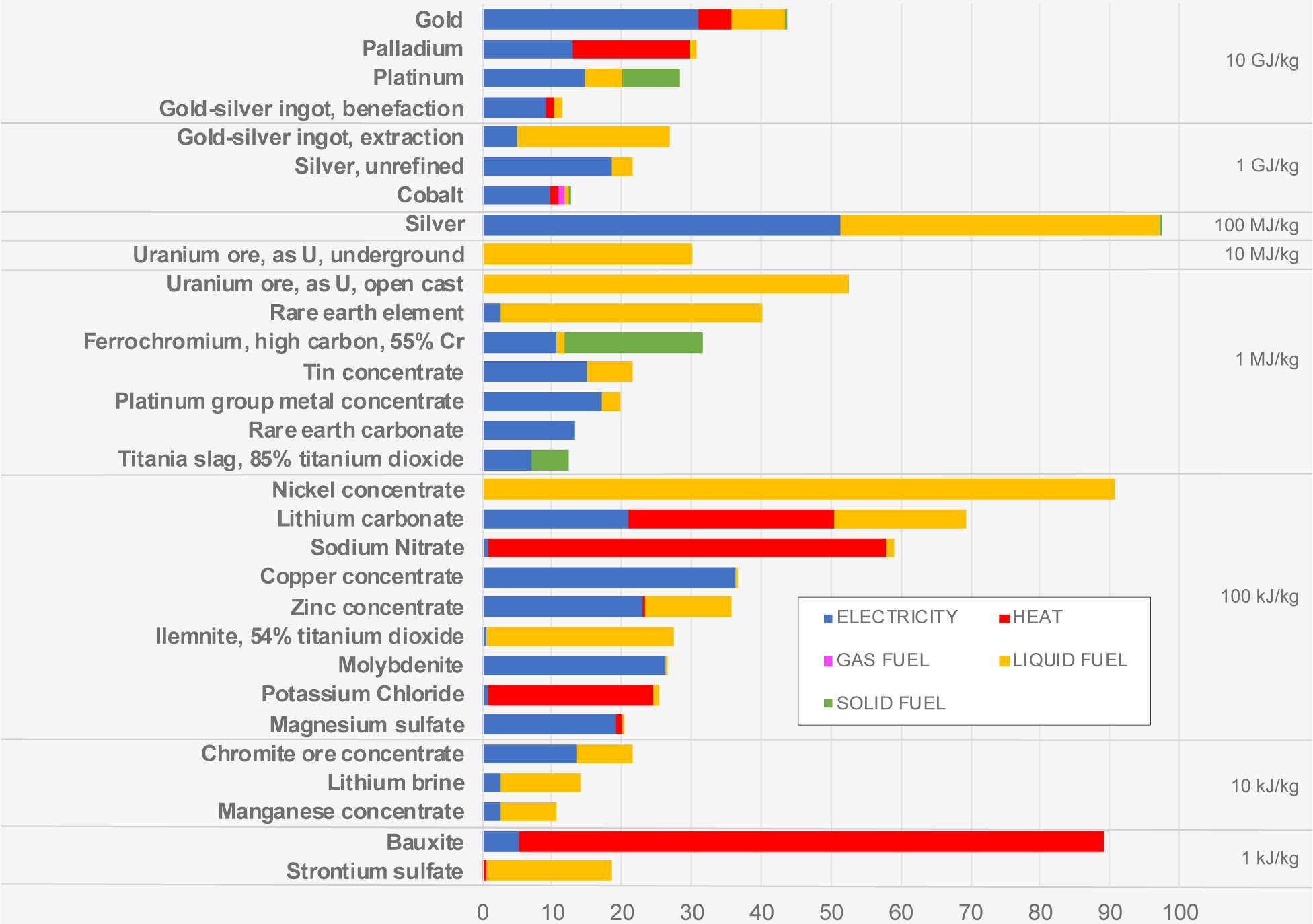
Energy inputs to mining and processing of minerals and metals (data from110).
In mining of saline aqueous sources, an ancillary goal may be to harvest embedded energy from aqueous sources and use it to drive minerals/metals recovery. Theoretical salinity gradient energy potentials for seawater and seawater desalination brine are 0.5 and 1.0 kWh/m3, respectively.111 Given that many geothermal aquifers are brackish or saline,112 high-temperature geothermal sources already being utilized by existing geothermal facilities for heat recovery can be repurposed for recovery of both energy and minerals. For low-temperature geothermal sources, low-grade heat can be recovered to partially offset energy requirements of minerals/metals recovery. High (> 150 °C) and low (90–150°C) temperature geothermal sources in the U.S. have energy potentials of 120 and 30 GW, respectively.113 Produced water from conventional and unconventional oil and gas recovery is elevated in temperature, depending on oil reservoir/shale formation depth, with typical values ranging from about 60 – 110°C.114,115 Potential Integration of geothermal energy (heat) is discussed below.
Yari analyzed exergy destruction within different types of geothermal plants,116 finding that between 51–66% of the exergy in the incoming geothermal fluid is destroyed, including exergy lost in the reinjected fluid. Since we would not want to cannibalize the geothermal reservoir by reinjecting lower temperature fluid, ignoring this exergy loss leaves between 30–45% exergy destruction within the plant itself. Some of this exergy destruction includes such losses as working fluids lost in the condenser, which would be nearly impossible to capture, but other losses, such as turbine and pre-heater-vaporizer losses, have the potential for recapture as low-grade heat for driving metal recovery processes.
It is important to recognize that some critical metals have relevance to energy systems, e.g., U as a fuel source and Li as an energy storage component.
CONCLUSIONS AND RECOMMENDATIONS
There are a number of metals, essential for manufacturing of products in the U.S. economy, that have been deemed as critical, based on their inadequate and/or low-grade occurrence in national mining ores, and associated reliance on international supply chains. Many of these, including REEs and PGMs, occur in an aqueous phase in various saline water sources within or proximate to the U.S., suggesting an alternative to, or augmentation of, traditional hardrock mining for their recovery. Specifically, this review reveals several high-criticality metals that could be recovered from saline water sources including: traditional-mining constituents (e.g., Li, U, and Co), PGMs (e.g., Pt, Pd, and Rh), and REEs (e.g., Dy, Nd, and Y). Following identification of these metals, a feasibility assessment revealed that there are several opportune combinations of metal and source (e.g., Li in produced water, geothermal aquifers, and seawater desalination brines; REEs in AMD). Moreover, processing of such aqueous feedstocks provides not only the opportunity for mitigating significant environmental and public health impacts associated, but also reduces reliance on international supply chains.
Another finding is that while critical metals in seawater (and associated desalination brines) have been well characterized, there is a significant need for a comprehensive analytical characterization of other potential sources. Such a survey would serve to reveal occurrence and quantities of critical metals in a wide variety of sources including produced waters, acid mine drainage, and geothermal aquifers.
Finally, this review reveals a number of existing potential technologies for recovery of critical metals. One limitation to many of these technologies is that if pre-concentration using brine concentration technologies is needed, there will a significant increase in energy consumption and cost. Further, many of these technologies are based on selective extraction (e.g., sorption). However, in order to address the technology challenge of developing processes for selective extraction of targeted dissolved constituents from dilute mixed-matrix sources, additional research is needed to further elucidate sorption/desorption reaction mechanisms to increase process selectivity, capacity, and metal recovery by regeneration/desorption.
SYNOPSIS:
Extraction and recovery of critical metals from aqueous saline sources to reduce environmental impacts and increase supply-chain sovereignty compared to hardrock ore mining.
ACKNOWLEDGEMENT
This work was supported in part by the National Institute of Environmental and Health Sciences (NIEHS) Superfund Program (SRP) grant P42 ES004940.
Biographies
Serife E. Can Sener

Serife Elif Can Sener received her B.A. in economics from Galatasaray University (Turkey), her M.A. in development, environment, and societies at Université Catholique de Louvain (Belgium), and her Ph.D. in policy studies (specialized in environmental and natural resource policy) from Clemson University (USA). Currently, Elif is an Assistant Professor in her home country (Turkey) and a Visiting Scholar at the Department of Environmental Engineering at Clemson University. With her multidisciplinary background in socioeconomics of the environment, Elif’s research mainly focuses on environment–economics nexus, emphasizing the interpretation of the empirical relationship between energy and societal benefits.
Valerie M. Thomas

Valerie M. Thomas is the Anderson Interface Chair of Natural Systems in the H. Milton Stewart School of Industrial and Systems Engineering at the Georgia Institute of Technology, College of Engineering. She holds a secondary appointment as Professor of Public Policy. She received a B.A. in physics from Swarthmore College, a Ph.D. in high-energy physics from Cornell University, and postdoctoral training at Carnegie Mellon University in the Department of Engineering and Public Policy. Her research is in environmental lifecycle analysis, energy systems analysis, industrial ecology, and sustainability.
David E. Hogan

David E. Hogan received a B.S. in microbiology from New Mexico State University. He received his Ph.D. in environmental science from the University of Arizona. He is now an Assistant Research Professor in the Department of Environmental Science and a member of UArizona’s Center for Environmentally Sustainable Mining. He is an interdisciplinary Environmental Scientist whose core focus is developing remediation technologies utilizing biosurfactants and bioinspired synthetic surfactants. Dr. Hogan is particularly interested in remediating mining-impacted aqueous solutions for the protection of environmental and human health. Technologies derived from his research aim to clean metalliferous waters, enable beneficial water reuse, and recover metal constituents for either responsible disposal or the generation of saleable products.
Raina M. Maier

Raina M. Maier is a Professor of environmental microbiology in the Department of Environmental Science at the University of Arizona. She has a B.A. in biology/chemistry from the University of Minnesota, and her Ph.D. from Rutgers University is in microbiology. Her research program focuses on understanding how we can exploit microbes and their activities and products to benefit human health and the environment. She has been studying microbially produced surfactants (biosurfactants) since she joined the University of Arizona. These amazing molecules have been the basis for several research discoveries and patents in environmental cleanup and metal recovery.
Michael Carbajales-Dale

Michael Carbajales-Dale joined Clemson University in August 2014 as an Assistant Professor in the environmental engineering & earth sciences department. Before joining Clemson, Mik was an Energy Systems Analyst with Stanford’s Environmental Assessment & Optimization Lab and the Global Climate & Energy Project (GCEP). Before this, Mik undertook his Ph.D. in mechanical engineering with the Advanced Energy and Material Systems (AEMS) Laboratory at the University of Canterbury, New Zealand. His research focuses on the long-term, large-scale evolution and dynamics of the energy–economy system, primarily how the development of energy resources affects social development and the effects of a future transition from fossil fuels to renewable energy technologies.
Mark D. Barton

Mark D. Barton is Director of the Lowell Institute for Mineral Resources and Professor of geosciences at the University of Arizona. Mark earned B.S. and M.S. degrees from Virginia Tech (1977, 1978) and a Ph.D. from the University of Chicago (1981). Following a postdoctoral fellowship at the Geophysical Laboratory (Carnegie Institution of Washington), he taught at the University of California, Los Angeles (UCLA), before joining the faculty at the University of Arizona. He cofounded the Lowell Institute for Mineral Resources (soon to become a new multicollege school), a state-funded, industry-funded, and privately funded interdisciplinary organization. The Institute catalyzes and facilitates research and education related to mineral resources spanning science and engineering to public health and policy. His research interests span many aspects of energy and mass transfer in the Earth’s lithosphere and their applications to mineral deposits.
Tanju Karanfil

Tanju Karanfil is a Professor of environmental engineering and earth sciences at Clemson University and a Registered Professional Engineer in South Carolina, a Board-Certified Environmental Engineer by the American Academy of Environmental Engineers, and a Fellow of the International Water Association. His research interests are in the fundamentals and applications of physicochemical processes in natural and engineered environmental systems to improve water quality and treatment technologies and public health. Karanfil received his B. Sc. in environmental engineering from Istanbul Technical University and his M.Sc. and Ph.D. in environmental engineering from the University of Michigan.
John C. Crittenden

John C. Crittenden is the director of the Brook Byers Institute for Sustainable Systems and a Professor in the School of Civil and Environmental Engineering at the Georgia Institute of Technology. John Crittenden holds the Hightower Chair and is a Georgia Research Alliance Eminent Scholar in Environmental Technologies. John Crittenden received his bachelor’s degree in chemical engineering and a master’s degree and Ph.D. degree in civil and environmental engineering from the University of Michigan. He was elected to the U.S. National Academy of Engineering in 2002, the Chinese Academy of Engineering in 2013, and the European Academy of Sciences in 2019. He is the 2015 Clarke Prize laureate and received the 2020 Simon W. Freese Environmental Engineering Award and Lecture for using fundamental scientific principles and current research findings to solve the most challenging water quality problems (American Society of Civil Engineers). His research focuses on sustainable urban infrastructure, membrane technologies, adsorption, and advanced oxidation, including electrochemical advanced oxidation.
Gary L. Amy

Gary L. Amy is a Dean’s Distinguished Professor in the College of Engineering and Science at Clemson University and Visiting Professor at the National University of Singapore. He is the Former Director of the Water Desalination and Reuse Center (WDRC) at the King Abdullah University of Science and Technology (KAUST) in Saudi Arabia. Before KAUST, he was Professor of Water Supply Engineering at the UNESCO-IHE Institute for Water Education in The Netherlands, Professor of Environmental Engineering at the University of Colorado at Boulder, and earlier at the University of Arizona.
REFERENCES
- (1).The White House. Federal Strategy to Ensure Secure and Reliable Supplies of Critical Minerals https://www.whitehouse.gov/presidential-actions/presidential-executive-order-federal-strategy-ensure-secure-reliable-supplies-critical-minerals/ (accessed Dec 22, 2020).
- (2).USGS. 35 Minerals Deemed Critical to U.S. National Security and the Economy; 2018.
- (3).The White House. Executive Order on Addressing the Threat to the Domestic Supply Chain from Reliance on Critical Minerals from Foreign Adversaries https://www.govinfo.gov/content/pkg/FR-2020-10-05/pdf/2020-22064.pdf (accessed Jun 30, 2021).
- (4).The White House. Executive Order on America’s Supply Chains https://www.whitehouse.gov/briefing-room/presidential-actions/2021/02/24/executive-order-on-americas-supply-chains/ (accessed Jun 30, 2021).
- (5).Blengini GA; Nuss P; Dewulf J; Nita V; Talens Peiró L; Vidal-Legaz B; Latunussa C; Mancini L; Blagoeva D; Pennington D; Pellegrini M; Van Maercke A; Solar S; Grohol M; Ciupagea C EU Methodology for Critical Raw Materials Assessment: Policy Needs and Proposed Solutions for Incremental Improvements. Resour. Policy 2017, 53, 12–19. 10.1016/j.resourpol.2017.05.008. [DOI] [Google Scholar]
- (6).European Commission. Report on Critical Raw Materials 2014; 2014.
- (7).Buijs B; Sievers H; Espinoza LAT Limits to the Critical Raw Materials Approach. Proc. Inst. Civ. Eng. Waste Resour. Manag 2012, 165 (4), 201–208. 10.1680/warm.12.00010. [DOI] [Google Scholar]
- (8).Erdmann L; Graedel TE Criticality of Non-Fuel Minerals: A Review of Major Approaches and Analyses. Environmental Science and Technology. Environ Sci Technol; September 15, 2011, pp 7620–7630. 10.1021/es200563g. [DOI] [PubMed] [Google Scholar]
- (9).Nassar NT; Barr R; Browning M; Diao Z; Friedlander E; Harper EM; Henly C; Kavlak G; Kwatra S; Jun C; Warren S; Yang M-Y; Graedel TE Criticality of the Geological Copper Family. Environ. Sci. Technol 2012, 46 (2), 1071–1078. 10.1021/es203535w. [DOI] [PubMed] [Google Scholar]
- (10).Harper EM; Kavlak G; Burmeister L; Eckelman MJ; Erbis S; Sebastian Espinoza V; Nuss P; Graedel TE Criticality of the Geological Zinc, Tin, and Lead Family. J. Ind. Ecol 2015, 19 (4), 628–644. 10.1111/jiec.12213. [DOI] [Google Scholar]
- (11).Ciacci L; Nuss P; Reck BK; Werner TT; Graedel TE Metal Criticality Determination for Australia, the US, and the Planet-Comparing 2008 and 2012 Results. Resources 2016, 5 (4). 10.3390/resources5040029. [DOI] [Google Scholar]
- (12).Kosmol J; Müller F; Keßler H The Critical Raw Materials Concept: Subjective, Multifactorial and Ever-Developing; 2018; pp 71–92. 10.1007/978-3-319-50079-9_5. [DOI] [Google Scholar]
- (13).Duclos SJ; Otto JP; Konitzer DG Design in an Era of Constrained Resources. Mech. Eng 2010, 132 (9), 36–40. 10.1115/1.2010-sep-3. [DOI] [Google Scholar]
- (14).Buchert M; Schuler D; Bleher D Critical Metals for Future Sustainable Technologies and Their Recycling Potential Sustainable Innovation and Technology Transfer Industrial Sector Studies http://www.unep.fr/shared/publications/pdf/DTIx1202xPA-Critical Metals%0A and their Recycling Potential.pdf (accessed Oct 9, 2020).
- (15).Eggert R Materials, Critical Materials and Clean-Energy Technologies. In EPJ Web of Conferences; EDP Sciences, 2017; Vol. 148. 10.1051/epjconf/201714800003. [DOI] [Google Scholar]
- (16).Goe M; Gaustad G Identifying Critical Materials for Photovoltaics in the US: A Multi-Metric Approach. Appl. Energy 2014, 123, 387–396. 10.1016/j.apenergy.2014.01.025. [DOI] [Google Scholar]
- (17).Hache E; Seck GS; Simoen M; Bonnet C; Carcanague S Critical Raw Materials and Transportation Sector Electrification: A Detailed Bottom-up Analysis in World Transport. Appl. Energy 2019, 240, 6–25. 10.1016/j.apenergy.2019.02.057. [DOI] [Google Scholar]
- (18).Beylot A; Villeneuve J Assessing the National Economic Importance of Metals: An Input-Output Approach to the Case of Copper in France. Resour. Policy 2015, 44, 161–165. 10.1016/j.resourpol.2015.02.007. [DOI] [Google Scholar]
- (19).Glöser-Chahoud S; Espinoza LT; Walz R; Faulstich M Taking the Step towards a More Dynamic View on Raw Material Criticality: An Indicator Based Analysis for Germany and Japan. Resources 2016, 5 (4). 10.3390/resources5040045. [DOI] [Google Scholar]
- (20).Kosai S; Hashimoto S; Matsubae K; McLellan B; Yamasue E Comprehensive Analysis of External Dependency in Terms of Material Criticality by Employing Total Material Requirement: Sulfuric Acid Production in Japan as a Case Study. Minerals 2018, 8 (3). 10.3390/min8030114. [DOI] [Google Scholar]
- (21).Moss RL; Tzimas E; Willis P; Arendorf J; Tercero Espinoza L Critical Metals in the Path towards the Decarbonisation of the EU Energy Sector; 2011. 10.2790/46338. [DOI] [Google Scholar]
- (22).Eheliyagoda D; Zeng X; Li J A Method to Assess National Metal Criticality: The Environment as a Foremost Measurement. Humanit. Soc. Sci. Commun 2020, 7 (1). 10.1057/s41599-020-00537-4. [DOI] [Google Scholar]
- (23).Graedel TE; Harper EM; Nassar NT; Nuss P; Reck BK; Turner BL Criticality of Metals and Metalloids. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (14), 4257–4262. 10.1073/pnas.1500415112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rosenau-Tornow D; Buchholz P; Riemann A; Wagner M Assessing the Long-Term Supply Risks for Mineral Raw Materials--a Combined Evaluation of Past and Future Trends. Resour. Policy 2009, 34 (4), 161–175. [Google Scholar]
- (25).European Commission. Report on Critical Raw Materials 2010; 2010.
- (26).Sonderegger T; Pfister S; Hellweg S Criticality of Water: Aligning Water and Mineral Resources Assessment. Environ. Sci. Technol 2015, 49 (20), 12315–12323. 10.1021/acs.est.5b02982. [DOI] [PubMed] [Google Scholar]
- (27).Fortier SM; Nassar NT; Mauk JL; Hammarstrom JM; Day WC; Seal RR Mınıng Engıneerıng USGS Critical Minerals Review A Report Titled “A Federal Strategy to Ensure; 2019.
- (28).Nassar NT; Brainard J; Gulley A; Manley R; Matos G; Lederer G; Bird LR; Pineault D; Alonso E; Gambogi J; Fortier SM Evaluating the Mineral Commodity Supply Risk of the U.S. Manufacturing Sector. Sci. Adv 2020, 6 (8), eaay8647. 10.1126/sciadv.aay8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Taufour V Periodic Table of Elements Their Abundances, Prices, Global Sources https://www.ameslab.gov/cmi/research-highlights/critical-materials-periodic-table (accessed Nov 11, 2020).
- (30).U.S. National Science and Technology Council. Assessment of Critical Minerals: Screening Methodology and Initial Application; 2016.
- (31).Hayes SM; McCullough EA Critical Minerals: A Review of Elemental Trends in Comprehensive Criticality Studies. Resour. Policy 2018, 59, 192–199. 10.1016/j.resourpol.2018.06.015. [DOI] [Google Scholar]
- (32).USGS. Mineral Commodity Summaries 2020; 2020.
- (33).McCullough E; Nassar NT Assessment of Critical Minerals: Updated Application of an Early-Warning Screening Methodology. Miner. Econ 2017, 30 (3), 257–272. 10.1007/s13563-017-0119-6. [DOI] [Google Scholar]
- (34).Mayer X; Ruprecht J; Bari M Stream Salinity Status and Trends in South-West Western Australia; 2005.
- (35).National Oceanic and Atmospheric Administration. How much water is in the ocean? https://oceanservice.noaa.gov/facts/oceanwater.html (accessed Dec 20, 2020).
- (36).Diallo MS; Kotte MR; Cho M Mining Critical Metals and Elements from Seawater: Opportunities and Challenges. Environ. Sci. Technol 2015, 49 (16), 9390–9399. 10.1021/acs.est.5b00463. [DOI] [PubMed] [Google Scholar]
- (37).Clark CE; Veil JA Produced Water Volumes and Management Practices in the United States; 2009.
- (38).Lucas T HOW MUCH WATER DOES U.S. FRACKING REALLY USE? DUKE TODAY. 2015. [Google Scholar]
- (39).Neupane G; Wendt DS Assessment of Mineral Resources in Geothermal Brines in the US. In 42nd Workshop on Geothermal Reservoir Engineering; 2017. [Google Scholar]
- (40).Neupane G; Wendt DS Potential Economic Values of Minerals in Brines of Identified Hydrothermal Systems in the US; 2017.
- (41).Cravotta CA Dissolved Metals and Associated Constituents in Abandoned Coal-Mine Discharges, Pennsylvania, USA. Part 1: Constituent Quantities and Correlations. Appl. Geochemistry 2008, 23 (2), 166–202. 10.1016/j.apgeochem.2007.10.011. [DOI] [Google Scholar]
- (42).US Environmental Protection Agency. Frequent Questions Related to Gold King Mine Response.
- (43).Office of Solid Waste. Identification and Description of Mineral Processing Sectors and Waste Streams; 1998.
- (44).Office of Water. Technical Development Document for the Effluent Limitations Guidelines and Standards for the Steam Electric Power Generating Point Source Category; Washington, DC, 2015. https://doi.org/EPA-821-R-15-007. [Google Scholar]
- (45).Neff J; Lee K; DeBlois EM Produced Water: Overview of Composition, Fates, and Effects. In Produced Water; Springer; New York, 2011; pp 3–54. 10.1007/978-1-4614-0046-2_1. [DOI] [Google Scholar]
- (46).Stringfellow WT; Camarillo MK Flowback Verses First-Flush: New Information on the Geochemistry of Produced Water from Mandatory Reporting. Environ. Sci. Process. Impacts 2019, 21 (2), 370–383. 10.1039/c8em00351c. [DOI] [PubMed] [Google Scholar]
- (47).Cluff MA; Hartsock A; Macrae JD; Carter K; Mouser PJ Temporal Changes in Microbial Ecology and Geochemistry in Produced Water from Hydraulically Fractured Marcellus Shale Gas Wells. Environ. Sci. Technol 2014, 48 (11), 6508–6517. 10.1021/es501173p. [DOI] [PubMed] [Google Scholar]
- (48).Rodriguez AZ; Wang H; Hu L; Zhang Y; Xu P Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12 (3), 770. 10.3390/w12030770. [DOI] [Google Scholar]
- (49).Tian L; Chang H; Tang P; Li T; Zhang X; Liu S; He Q; Wang T; Yang J; Bai Y; Vidic RD; Crittenden JC; Liu B Rare Earth Elements Occurrence and Economical Recovery Strategy from Shale Gas Wastewater in the Sichuan Basin, China. ACS Sustain. Chem. Eng 2020, 8 (32), 11914–11920. 10.1021/acssuschemeng.0c04971. [DOI] [Google Scholar]
- (50).Turekian KK Oceans; Prentice-Hall, 1968. [Google Scholar]
- (51).Weiss HV; Fresco J Platinum Group Elements in Seawater. I. Palladium. Can.J.Chem 1982, 61, 734–736. [Google Scholar]
- (52).Piper DZ; Isaacs CM Geochemistfy of Minor Elements in the Monterey Formation, California; U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER; 1566; 1995. [Google Scholar]
- (53).Blake RL Extracting Minerals from Geothermal Brines. Literature Survey; U.S. Department of Energy, 1974. 10.2172/5208644. [DOI] [Google Scholar]
- (54).Gammons CH; Wood SA; Jonas JP; Madison JP Geochemistry of the Rare-Earth Elements and Uranium in the Acidic Berkeley Pit Lake, Butte, Montana. Chem. Geol 2003, 198 (3–4), 269–288. 10.1016/S0009-2541(03)00034-2. [DOI] [Google Scholar]
- (55).Union of Concerned Scientists. Environmental Impacts of Geothermal Energy https://www.ucsusa.org/resources/environmental-impacts-geothermal-energy (accessed Feb 10, 2021).
- (56).Luo X; Zhang K; Luo J; Luo S; Crittenden J Capturing Lithium from Wastewater Using a Fixed Bed Packed with 3-D MnO2 Ion Cages. Environ. Sci. Technol 2016, 50 (23), 13002–13012. 10.1021/acs.est.6b02247. [DOI] [PubMed] [Google Scholar]
- (57).Luo X; Guo B; Luo J; Deng F; Zhang S; Luo S; Crittenden J Recovery of Lithium from Wastewater Using Development of Li Ion-Imprinted Polymers. ACS Sustain. Chem. Eng 2015, 3 (3), 460–467. 10.1021/sc500659h. [DOI] [Google Scholar]
- (58).Zhang X; Zuo K; Zhang X; Zhang C; Liang P Selective Ion Separation by Capacitive Deionization (CDI) Based Technologies: A State-of-the-Art Review. Environmental Science: Water Research and Technology. Royal Society of Chemistry; February 1, 2020, pp 243–257. 10.1039/c9ew00835g. [DOI] [Google Scholar]
- (59).Jang E; Jang Y; Chung E Lithium Recovery from Shale Gas Produced Water Using Solvent Extraction. Appl. Geochemistry 2017, 78, 343–350. 10.1016/j.apgeochem.2017.01.016. [DOI] [Google Scholar]
- (60).Hogan DE; Curry JE; Pemberton JE; Maier RM Rhamnolipid Biosurfactant Complexation of Rare Earth Elements. J. Hazard. Mater 2017, 340, 171–178. 10.1016/j.jhazmat.2017.06.056. [DOI] [PubMed] [Google Scholar]
- (61).Hogan DE; Curry JE; Maier RM Ion Flotation of La3+, Cd2+, and Cs+ Using Monorhamnolipid Collector. Colloids and Interfaces 2018, 2 (4), 43. 10.3390/colloids2040043. [DOI] [Google Scholar]
- (62).Wang K; Adidharma H; Radosz M; Wan P; Xu X; Russell CK; Tian H; Fan M; Yu J Recovery of Rare Earth Elements with Ionic Liquids. Green Chemistry. Royal Society of Chemistry; October 2, 2017, pp 4469–4493. 10.1039/c7gc02141k. [DOI] [Google Scholar]
- (63).Arroyo F; Morillo J; Usero J; Rosado D; El Bakouri H Lithium Recovery from Desalination Brines Using Specific Ion-Exchange Resins. Desalination 2019, 468. 10.1016/j.desal.2019.114073. [DOI] [Google Scholar]
- (64).Cipollina A; Misseri A; Staiti GDA; Galia A; Micale G; Scialdone O Integrated Production of Fresh Water, Sea Salt and Magnesium from Sea Water. Desalin. Water Treat 2012, 49 (1–3), 390–403. 10.1080/19443994.2012.699340. [DOI] [Google Scholar]
- (65).Das S; Oyola Y; Mayes RT; Janke CJ; Kuo LJ; Gill G; Wood JR; Dai S Extracting Uranium from Seawater: Promising AI Series Adsorbents. Ind. Eng. Chem. Res 2016, 55 (15), 4103–4109. 10.1021/acs.iecr.5b03135. [DOI] [Google Scholar]
- (66).Dungan K; Butler G; Livens FR; Warren LM Uranium from Seawater – Infinite Resource or Improbable Aspiration? Progress in Nuclear Energy. Elsevier Ltd August 1, 2017, pp 81–85. 10.1016/j.pnucene.2017.04.016. [DOI] [Google Scholar]
- (67).Wang Q; Li J; Chen C; Ren X; Hu J; Wang X Removal of Cobalt from Aqueous Solution by Magnetic Multiwalled Carbon Nanotube/Iron Oxide Composites. Chem. Eng. J 2011, 174 (1), 126–133. 10.1016/j.cej.2011.08.059. [DOI] [Google Scholar]
- (68).Singhal P; Jha SK; Pandey SP; Neogy S Rapid Extraction of Uranium from Sea Water Using Fe 3 O 4 and Humic Acid Coated Fe 3 O 4 Nanoparticles. J. Hazard. Mater 2017, 335, 152–161. 10.1016/j.jhazmat.2017.04.043. [DOI] [PubMed] [Google Scholar]
- (69).Petersková M; Valderrama C; Gibert O; Cortina JL Extraction of Valuable Metal Ions (Cs, Rb, Li, U) from Reverse Osmosis Concentrate Using Selective Sorbents. Desalination 2012, 286, 316–323. 10.1016/j.desal.2011.11.042. [DOI] [Google Scholar]
- (70).Nancharaiah YV; Mohan SV; Lens PNL Removal and Recovery of Metals and Nutrients from Wastewater Using Bioelectrochemical Systems. In Biomass, Biofuels, Biochemicals: Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier, 2018; pp 693–720. 10.1016/B978-0-444-64052-9.00028-5. [DOI] [Google Scholar]
- (71).Quist-Jensen CA; Macedonio F; Drioli E Integrated Membrane Desalination Systems with Membrane Crystallization Units for Resource Recovery: A New Approach for Mining from the Sea. Crystals 2016, 6 (4). 10.3390/cryst6040036. [DOI] [Google Scholar]
- (72).Sun SY; Cai LJ; Nie XY; Song X; Yu JG Separation of Magnesium and Lithium from Brine Using a Desal Nanofiltration Membrane. J. Water Process Eng 2015, 7, 210–217. 10.1016/j.jwpe.2015.06.012. [DOI] [Google Scholar]
- (73).Jiang C; Wang Y; Wang Q; Feng H; Xu T Production of Lithium Hydroxide from Lake Brines through Electro-Electrodialysis with Bipolar Membranes (EEDBM). Ind. Eng. Chem. Res 2014, 53 (14), 6103–6112. 10.1021/ie404334s. [DOI] [Google Scholar]
- (74).Cheng W; Li Z; Cheng F Solubility of Li2CO3 in Na-K-Li-Cl Brines from 20 to 90 °c. J. Chem. Thermodyn 2013, 67, 74–82. 10.1016/j.jct.2013.07.024. [DOI] [Google Scholar]
- (75).U.S. Government Accountability Office. Abandoned Hardrock Mines: Information on Number of Mines, Expenditures, and Factors That Limit Efforts to Address Hazards; Washington, DC, 2020. https://doi.org/GAO-20-238. [Google Scholar]
- (76).Bigham JM; Cravotta CA Acid Mine Drainage. In Encyclopedia of Soil Science; Lal R, Ed.; CRC Press: Boca Raton, FL, 2017; pp 6–10. 10.1081/E-ESS3-120053867. [DOI] [Google Scholar]
- (77).Office of Water. Liquid Assets 2000: America’s Water Resources at a Turning Point; Washington, DC, 2000. https://doi.org/EPA-840-B-00-001. [Google Scholar]
- (78).Herlihy AT; Kaufmann PR; Mitch ME; Brown DD Regional Estimates of Acid Mine Drainage Impact on Streams in the Mid-Atlantic and Southeastern United States. Water. Air. Soil Pollut 1990, 50 (1–2), 91–107. 10.1007/BF00284786. [DOI] [Google Scholar]
- (79).Osborne SE 2015 Minerals Yearbook: Mining and Quarrying Trends [Advance Release]; 2020.
- (80).U.S. Forest Service. Final Environmental Impact Statement for the Rosemont Copper Project: A Proposed Mining Operation Coronado National Forest Pima County, Arizona Volume 1. U.S. Department of Agriculture; 2013. https://doi.org/MB-R2-05-6. [Google Scholar]
- (81).International Resource Panel. Global Resources Outlook 2019 Fact Sheet: Natural Resources for the Future We Want; Nairobi, Kenya, 2019. [Google Scholar]
- (82).Bleiwas DI Estimated Water Requirements for the Conventional Flotation of Copper Ores: U.S. Geological Survey Open-File Report 2012–1089; Reston, VA, 2012. [Google Scholar]
- (83).Singh MM Water Consumption at Copper Mines in Arizona, Special Report 29; Phoenix, AZ, 2010. [Google Scholar]
- (84).Northey SA; Mudd GM; Werner TT; Haque N; Yellishetty M Sustainable Water Management and Improved Corporate Reporting in Mining. Water Resour. Ind 2019, 21, 100104. 10.1016/j.wri.2018.100104. [DOI] [Google Scholar]
- (85).Mudd GM Sustainability Reporting and Water Resources: A Preliminary Assessment of Embodied Water and Sustainable Mining. Mine Water Environ. 2008, 27 (3), 136–144. 10.1007/s10230-008-0037-5. [DOI] [Google Scholar]
- (86).Browning C; Northey S; Haque N; Bruckard W; Cooksey M Life Cycle Assessment of Rare Earth Production from Monazite BT. In REWAS 2016: Towards Materials Resource Sustainability; Kirchain RE, Blanpain B, Meskers C, Olivetti E, Apelian D, Howarter J, Kvithyld A, Mishra B, Neelameggham NR, Spangenberger J, Eds.; Springer International Publishing: Cham, 2016; pp 83–88. 10.1007/978-3-319-48768-7_12. [DOI] [Google Scholar]
- (87).Lagos G; Peters D; Videla A; Jara JJ The Effect of Mine Aging on the Evolution of Environmental Footprint Indicators in the Chilean Copper Mining Industry 2001–2015. J. Clean. Prod 2018, 174, 389–400. 10.1016/j.jclepro.2017.10.290. [DOI] [Google Scholar]
- (88).Northey SA; Mudd GM; Saarivuori E; Wessman-Jääskeläinen H; Haque N Water Footprinting and Mining: Where Are the Limitations and Opportunities? Journal of Cleaner Production. Elsevier Ltd November 2016, pp 1098–1116. 10.1016/j.jclepro.2016.07.024. [DOI] [Google Scholar]
- (89).Montes C; Cantallopts J Forecast for Water Consumption in the Copper Mining Industry, 2018–2029; 2018.
- (90).Martens PN; Ruhrberg M; Mistry M Assessing Global Land Requirement for Surface Copper Mining. Miner. Resour. Eng 2002, 11 (4), 337–348. [Google Scholar]
- (91).Maus V; Giljum S; Gutschlhofer J; da Silva DM; Probst M; Gass SLB; Luckeneder S; Lieber M; McCallum I A Global-Scale Data Set of Mining Areas. Sci. Data 2020, 7 (1), 289. 10.1038/s41597-020-00624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Murguía DI; Bringezu S Measuring the Specific Land Requirements of Large-Scale Metal Mines for Iron, Bauxite, Copper, Gold and Silver. Prog. Ind. Ecol. An Int. J 2016, 10 (2/3), 264. 10.1504/PIE.2016.082142. [DOI] [Google Scholar]
- (93).Barakos G; Mischo H; Gutzmer J A Forward Look Into the US Rare-Earth Industry; How Potential Mines Can Connect to the Global REE Market. Min. Eng 2018, 70 (8), 30–37. [Google Scholar]
- (94).Nuss P; Eckelman MJ Life Cycle Assessment of Metals: A Scientific Synthesis. PLoS One 2014, 9 (7), e101298. 10.1371/journal.pone.0101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).van der Voet E; Salminen R; Eckelman M; Mudd G; Norgate T; Hischier R Environmental Risks and Challenges of Anthropogenic Metals Flows and Cycles, A Report of the Working Group on the Global Metal Flows to the International Resource Panel; 2013.
- (96).Watari T; Nansai K; Nakajima K Major Metals Demand, Supply, and Environmental Impacts to 2100: A Critical Review. Resources, Conservation and Recycling. Elsevier B.V. January 2021, p 105107. 10.1016/j.resconrec.2020.105107. [DOI] [Google Scholar]
- (97).Calvo G; Mudd G; Valero A; Valero A Decreasing Ore Grades in Global Metallic Mining: A Theoretical Issue or a Global Reality? Resources 2016, 5 (4), 36. 10.3390/resources5040036. [DOI] [Google Scholar]
- (98).Norgate T; Jahanshahi S Reducing the Greenhouse Gas Footprint of Primary Metal Production: Where Should the Focus Be? Miner. Eng 2011, 24 (14), 1563–1570. 10.1016/j.mineng.2011.08.007. [DOI] [Google Scholar]
- (99).Memary R; Giurco D; Mudd G; Mason L Life Cycle Assessment: A Time-Series Analysis of Copper. J. Clean. Prod 2012, 33, 97–108. 10.1016/j.jclepro.2012.04.025. [DOI] [Google Scholar]
- (100).Prosser I; Wolf L; Littleboy A Water in Mining and Industry. In Water: Science and Solutions for Australia Series; Prosser I, Ed.; CSIRO Publishing: Collingwood, VIC, AU, 2011; pp 135–146. [Google Scholar]
- (101).Rötzer N; Schmidt M Historical, Current, and Future Energy Demand from Global Copper Production and Its Impact on Climate Change. Resources 2020, 9 (4), 44. 10.3390/resources9040044. [DOI] [Google Scholar]
- (102).Althaus HJ; Classen M Life Cycle Inventories of Metals and Methodological Aspects of Inventorying Material Resources in Ecoinvent. Int. J. Life Cycle Assess 2005, 10 (1), 43–49. 10.1065/lca2004.11.181.5. [DOI] [Google Scholar]
- (103).Hong J; Chen Y; Liu J; Ma X; Qi C; Ye L Life Cycle Assessment of Copper Production: A Case Study in China. Int. J. Life Cycle Assess. 2017 239 2017, 23 (9), 1814–1824. 10.1007/S11367-017-1405-9. [DOI] [Google Scholar]
- (104).Streicher-Porte M; Althaus HJ China and Global Markets: Copper Supply Chain Sustainable Development: A Life Cycle Assessment Study; 2011.
- (105).Camm TW IC 9298: ANNEX Simplified Cost Models For Prefeasibility Mineral Evaluations; 1991.
- (106).GAO. ABANDONED HARDROCK MINES Information on Number of Mines, Expenditures, and Factors That Limit Efforts to Address Hazards United States Government Accountability Office; 2020.
- (107).Paranthaman MP; Li L; Luo J; Hoke T; Ucar H; Moyer BA; Harrison S Recovery of Lithium from Geothermal Brine with Lithium-Aluminum Layered Double Hydroxide Chloride Sorbents. Environ. Sci. Technol 2017, 51 (22), 13481–13486. 10.1021/acs.est.7b03464. [DOI] [PubMed] [Google Scholar]
- (108).EGEC. Geothermal Lithium in Europe; 2020.
- (109).Ortiz-Albo P; Torres-Ortega S; González Prieto M; Urtiaga A; Ibañez R Techno-Economic Feasibility Analysis for Minor Elements Valorization from Desalination Concentrates. Separation and Purification Reviews. Taylor and Francis Inc. July 3, 2019, pp 220–241. 10.1080/15422119.2018.1470537. [DOI] [Google Scholar]
- (110).Wernet G; Bauer C; Steubing B; Reinhard J; Moreno-Ruiz E; Weidema B The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess 2016, 21 (9), 1218–1230. 10.1007/s11367-016-1087-8. [DOI] [Google Scholar]
- (111).Yip NY; Brogioli D; Hamelers HVM; Nijmeijer K Salinity Gradients for Sustainable Energy: Primer, Progress, and Prospects. Environ. Sci. Technol 2016, 50 (22), 12072–12094. 10.1021/acs.est.6b03448. [DOI] [PubMed] [Google Scholar]
- (112).Kristmannsdóttir H Types of Scaling Occurring by Geothermal Utilization in Iceland. Geothermics 1989, 18 (1–2), 183–190. 10.1016/0375-6505(89)90026-6. [DOI] [Google Scholar]
- (113).Green BD; Nix RG Geothermal-The Energy Under Our Feet Geothermal Resource Estimates for the United States Innovation for Our Energy Future; 2006.
- (114).Bader MSH Seawater versus Produced Water in Oil-Fields Water Injection Operations. Desalination 2007, 208 (1–3), 159–168. 10.1016/j.desal.2006.05.024. [DOI] [Google Scholar]
- (115).Mastouri R; Borghei SM; Nadim F; Roayaei E The Investigation of Induced Gas Flotation at Elevated Temperatures and High Total Dissolved Solids (TDS) Produced Water. In 16th Annual Petroleum and Biofuels Environmental Conference [IPEC]; Houston, 2009. [Google Scholar]
- (116).Yari M Exergetic Analysis of Various Types of Geothermal Power Plants. Renew. Energy 2010, 35 (1), 112–121. 10.1016/j.renene.2009.07.023. [DOI] [Google Scholar]


