Abstract
Interleukin-2 (IL-2) activates several different families of tyrosine kinases, but precisely how these kinases interact is not completely understood. We therefore investigated the functional relationships among Jak3, Lck, and Syk in IL-2 signaling. We first observed that in the absence of Jak3, both Lck and Syk had the capacity to phosphorylate Stat3 and Stat5a. However, neither supported IL-2-induced STAT activation, nor did dominant negative alleles of these kinases inhibit. Moreover, pharmacological abrogation of Lck activity did not inhibit IL-2-mediated phosphorylation of Jak3 and Stat5a. Importantly, ligand-dependent Syk activation was dependent on the presence of catalytically active Jak3, whereas Lck activation was not. Interestingly, Syk functioned as a direct substrate of Jak1 but not Jak3. Additionally, Jak3 phosphorylated Jak1, whereas the reverse was not the case. Taken together, our data support a model in which Lck functions in parallel with Jak3, while Syk functions as a downstream element of Jaks in IL-2 signaling. Jak3 may regulate Syk catalytic activity indirectly via Jak1. However, IL-2-mediated Jak3/Stat activation is not dependent on Lck or Syk. While the essential roles of Jak1 and Jak3 in signaling by γc-utilizing cytokines are clear, it will be important to dissect the exact contributions of Lck and Syk in mediating the effects of IL-2 and related cytokines.
Interleukin-2 (IL-2) is a growth factor that is important for proliferation and homeostasis of lymphocytes (22). The IL-2 receptor (IL-2R) contains the following three subunits: the α chain (IL-2Rα), which is expressed only on activated lymphocytes; the β chain (IL-2Rβ); and the γ common chain (IL-2Rγc), a subunit shared by several cytokines (14, 23, 38). The cytoplasmic domains of IL-2R subunits do not possess intrinsic enzymatic activity; rather, IL-2 binding induces oligomerization of IL-2R, which initiates activation of several protein tyrosine kinases (PTKs) and subsequent phosphorylation of the IL-2R complex (14, 46).
Among these kinases, Lck was the first reported to be associated with the cytoplasmic domain of IL-2Rβ (11, 13, 28, 46). It constitutively associates with the acidic region (A region; amino acids [aa] 313 to 382) of IL-2Rβ via the amino-terminal region of its kinase domain (12, 28). Mutation of the A region abrogated Lck binding, and the mutant receptor failed to mediate activation of Ras and the induction of c-Fos and c-Jun (6, 12, 28, 44, 45). However, IL-2-induced mitogenesis was not impaired (6, 19), and lack of the A region resulted in the enhanced proliferation of primary T cells (8). The interpretation of these studies is complicated, however, by the fact that the Shc binding site (Y338) also resides within the A region (6, 25, 40). Thus, the distinct contributions of Lck and Shc are not clear with these mutants. Later, studies on Lck−/− mice demonstrated that Lck deficiency was associated with defects in T-cell development, but that T cells from Lck−/− mice exhibited a normal proliferative response to IL-2 (33). These data suggested that although Lck is essential for T-cell receptor (TCR) signaling, it is dispensable for some IL-2-induced events.
Shortly thereafter, Syk kinase was shown to constitutively associate with IL-2Rβ and to be activated upon IL-2 stimulation (30, 39, 46). Syk binds the serine-rich region (S region; aa 267 to 322) of IL-2Rβ; this region was reported to be required for IL-2-mediated DNA synthesis and induction of c-Myc, Bcl-2, Bcl-xL, and Bax (30, 32). Therefore, IL-2-mediated activation of Syk has been implicated in two important functions: IL-2-induced proliferation and antiapoptosis. Further analysis, however, demonstrated that the S region alone was insufficient for IL-2-induced proliferation, and both A and C-terminal (aa 383 to 525) regions of IL-2Rβ were required for optimal IL-2-induced proliferation (6, 7, 8, 44). Additionally, although Syk−/− mice had severe defects in the development and function of B cells, T cells from Syk−/− mice could support a relatively normal IL-2 response (48). IL-2 activation of Lck and Syk was unable to fully account for IL-2 signals, which suggested that other PTKs were primarily responsible for IL-2 signaling.
The Janus kinases (Jaks) are the most recently identified family of PTKs involved in IL-2 signaling (16, 38, 51). Jak3 constitutively binds the cytoplasmic domain of IL-2Rγc and is activated by γc-utilizing cytokines, IL-2, IL-4, IL-7, IL-9, and IL-15 (16, 17, 34, 42). Jak1, by contrast, binds the ligand-specific subunit of these receptors (31, 42). For instance, it constitutively interacts with IL-2Rβ. But in addition, Jak3 also binds and phosphorylates IL-2Rβ upon IL-2 stimulation (16, 31, 42). Recent studies have shown that the membrane-proximal region of IL-2Rβ, which contains box 1 (aa 251 to 259) and box 2 (aa 296 to 306), is vital for binding both Jaks (55). Moreover, both S and A regions of IL-2Rβ are also differentially required for binding to Jak1 (aa 300 to 350) and Jak3 (aa 330 to 362). Thus, some of the abnormalities seen with mutations of IL-2Rβ that were initially attributed to interference with Lck and Syk might also interfere with the function of Jak1 or Jak3. In contrast to Lck and Syk, the absence of Jak1 or Jak3 has very clear and dramatic effects on signaling via γc-utilizing cytokines. Deficiency of either IL-2Rγc or Jak3 completely abrogates IL-2 signaling and results in severe combined immunodeficiency (SCID) in humans and mice (27, 35, 36, 43, 47). Moreover, deficiency of Jak1 also produces SCID in mice (41), demonstrating the importance of the Jaks in signaling by IL-2 and other γc-utilizing cytokines, especially IL-7.
The ligand-induced phosphorylation of receptor subunits by Jaks is thought to create a docking site for a family of transcription factors, termed signal transducers and transcriptional activators (STATs), by virtue of their SH2 domains (4, 15). Subsequently, STATs are phosphorylated by Jaks on conserved tyrosine residues, which are required for dimer formation, nuclear translocation, DNA binding, and transactivation of target genes (5, 23). However, whereas several studies suggested that Jaks are responsible for the phosphorylation and activation of STATs in IL-2 signaling (1, 18, 37), Src family kinases and not Jaks were reported to be required for Stat3 activation in IL-3 signaling (2). In light of the conflicting reports in different systems, it was important to understand which PTK is central for IL-2-induced STAT activation and if Jak3, Lck, and Syk functionally interact each other.
In this study, we first determined the contributions of Jak3, Lck, and Syk to IL-2-induced STAT activation. We also investigated whether a hierarchy exists in PTK activation during IL-2 signaling; that is, whether Lck or Syk activation was Jak3 dependent or Jak3 activation was Lck/Syk dependent. Our findings indicate that whereas Lck activation was independent of Jak3 in IL-2 signaling, Syk activation required Jak3, probably indirectly via activation of Jak1. IL-2-mediated STAT activation, however, was not dependent on Lck or Syk but was entirely dependent on Jak3.
MATERIALS AND METHODS
Cells and antibodies.
COS-7 cells, 3T3-αβγ cells, U4A cells, NK3.3 cells, YT cells, and primary human T lymphocytes were maintained as previously described (3, 17, 21, 24, 29). Patients with suspected Jak3 deficiency were admitted to the NIH Clinical Center and apheresed under an Institutional Review Board-approved protocol. Epstein-Barr virus (EBV)-transformed human B cells from a healthy donor and a Jak3-SCID patient have been previously described (37). The antiphosphotyrosine monoclonal antibody (MAb) 4G10 and rabbit antiserum against human Lck were purchased from Upstate Biotechnology (Lake Placid, N.Y.). The antiphosphotyrosine MAb PY20 was purchased from ICN (Costa Mesa, Calif.), and anti-Lck MAb was purchased from Transduction Laboratories (Lexington, Ky.). Both rabbit antiserum and MAb against human Syk were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.), and the rabbit antiserum against human Stat3 was kindly provided by Andrew Larner (Cleveland Clinic Foundation, Cleveland, Ohio). Rabbit antisera against human Jak3 and Stat5a were generated by our laboratory as described elsewhere (3, 16, 21).
Plasmid, mutagenesis, and transfection.
The expression cDNA constructs pME18SJak3 and pME18SK855A were constructed as described previously (54) and are referred to as wild-type Jak3 (Jak3-wt) and kinase-inactive Jak3 (K855A), respectively. The cDNA constructs for human Lck-505 (kinase-active Lck), Lck-wt, and Lck-kd (kinase-inactive Lck) were kindly provided by Lawrence Samelson (National Cancer Institute, Bethesda, Md.). The cDNA constructs for murine Stat3 and Stat5a were provided by Andrew Larner and Lothar Hennighausen (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md.), respectively. The cDNA constructs of murine Syk-wt and Syk-kd were subcloned into pRc/CMV vector (Invitrogen, Carlsbad, Calif.). For transient transfection, U4A cells were transfected with 5 μg of each cDNA by a LipofectAMINE method (GIBCO BRL, Gaithersburg, Md.), while COS-7 cells were transfected with 5 μg of each cDNA by a DEAE-dextran method (Promega, Madison, Wis.), according to the manufacturer's instructions.
To measure STAT transcriptional activity, 3 × 105 3T3-αβγ cells were transfected with 0.4 μg of a STAT reporter gene (p3xIRF-luc, a luciferase reporter gene driven by three copies of the STAT binding site of the IRF-1 gene, kindly provided by Richard Pine, Public Health Research Institute, New York, N.Y.) alone or cotransfected with the STAT reporter gene plus other indicated cDNAs (0.5 μg) by a LipofectAMINE method. One day later, cells were starved overnight and incubated with or without IL-2 (1,000 U/ml; kindly provided by C. Reynolds, National Cancer Institute, Frederick, Md.) for 5 h at 37°C. Luciferase activity was measured with a luciferase assay system (Promega). In each experiment, DNA amounts were normalized by addition of plasmid DNA and samples were analyzed in triplicate.
Kinase inhibition assay.
Log-phase YT cells (107 cells/ml in RPMI with 1% fetal calf serum) were added to 24-well plates (0.5 ml each) and incubated with or without kinase inhibitor (CP-118556 or staurosporine) in 0.6% dimethyl sulfoxide for 30 min at 37°C. After IL-2 stimulation (1,000 U/ml) for 15 min at 37°C, the cells were washed with ice-cold phosphate-buffered saline containing 10 mM sodium orthovanadate and 10 mM EDTA and then lysed for immunoprecipitation and immunoblotting.
Immunoprecipitation, immunoblotting, and immune complex kinase assay.
Cells were lysed on ice as previously described (54). Cell debris was removed by centrifugation for 15 min at 14,000 rpm, and the supernatants were immunoprecipitated with anti-Jak3, anti-Lck, anti-Syk, anti-Stat3, and anti-Stat5a antisera as indicated. The immunoprecipitates were washed three times with lysis buffer and then eluted from the beads by boiling the beads in the sample buffer. For in vitro kinase assays, the immune complexes of Jak3, Lck, or Syk were washed one additional time with 100 mM NaCl and 10 mM HEPES (pH 7.5) and resuspended in 50 μl of kinase reaction buffer (20 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 5 mM MnCl2, 1 μM ATP) containing 1 μCi of [γ-32P]ATP (Amersham, Arlington Heights, Ill.). The in vitro kinase reactions of Jak3 were performed on ice, and others were performed at room temperature for the times indicated. The reactions were terminated by addition of 50 μl of ice-cold lysis buffer containing 50 mM EDTA. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and subjected to autoradiography or immunoblotted with indicated antibodies. The radioactivity incorporated by Lck, Syk, and Jak3 was quantitated using a STORM PhosphorImager (Molecular Dynamics), and protein levels were quantitated by densitometric scanning of the film (54).
Tyrosine kinase ELISA.
Kinases used for in vitro analysis of inhibitors were purified proteins, containing the catalytic domain of Jak3 or Lck fused to glutathione S-transferase (K. S. Magnuson et al., unpublished data). Nunc Maxi-Sorp 96-well flat-bottom plates were coated with 100 μg of the random copolymer l-glutamic acid and tyrosine (4:1; Sigma) per ml dissolved in Dulbecco's PBS (D-PBS) and incubated overnight at 37°C. Prior to a kinase enzyme-linked immunosorbent assay (ELISA), coated plates were washed three times in washing buffer (D-PBS plus 0.5% Tween 20). In addition to the assay buffer (50 mM HEPES [pH 7.3], 125 mM NaCl, 24 mM MgCl2, 1 mM sodium orthovanadate), an appropriate concentration of ATP (0.2 μM for Jak3 and 0.3 μM for Lck) was added to each well, and dilutions of the kinase inhibitors and tyrosine kinases (approximately 100 ng enzyme/well) were added as described in the relevant figure legend. The assay plates were shaken at room temperature for 30 min and washed three times in wash buffer. Antiphosphotyrosine antibody PY20 (50 μl, diluted 1:1,700 in D-PBS plus 3% bovine serum albumin) was added to each well. Plates were again shaken at room temperature for 25 min, followed by three washes with washing buffer. The horseradish peroxidase-conjugated PY20 antibody was detected by adding 50 μl of tetramethylbenzidine (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), and color development was stopped by adding 0.09 M H2SO4 to each well. Absorbance was read as optical density at 450 nm on a SpectroMax 340 96-well plate reader (Molecular Devices, Sunnyvale, Calif.).
RESULTS
Lck can phosphorylate Stat3 and Stat5a when coexpressed.
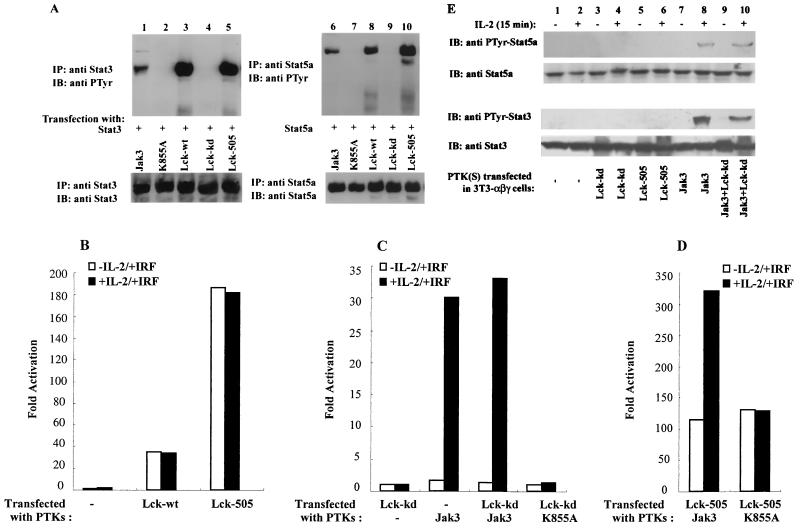
For IL-3 signaling, it has been argued that c-Src and not Jaks are critical for ligand-dependent STAT activation (2). To determine if Lck plays a role in IL-2-induced STAT activation, we first examined its capacity to phosphorylate Stat3 and Stat5a in an overexpression system. To this end, COS cells were transfected with cDNAs encoding Stat3 (Fig. 1A, lanes 1 to 5) or Stat5a (Fig. 1A, lanes 6 to 10) with catalytically active or inactive forms of Jak3 or Lck and immunoblotted with antiphosphotyrosine MAb (Fig. 1A, upper panels). Consistent with previous observations (54), phosphorylation of Stat3 and Stat5a was observed upon coexpression with Jak3-wt (upper panels, lanes 1 and 6) but not with the catalytically inactive mutant K855A (upper panels, lanes 2 and 7). Additionally, both Lck-wt (upper panels, lanes 3 and 8) and the constitutively active form Lck-505 (upper panels, lanes 5 and 10) phosphorylated Stat3 and Stat5a when coexpressed. As a negative control, catalytically inactive Lck (Lck-kd) was also analyzed and, as expected, did not phosphorylate either Stat3 or Stat5a (upper panels, lanes 4 and 9), confirming that the catalytic activity of Lck was required to phosphorylate the STATs. To ensure that equivalent levels of Stat3 or Stat5a were analyzed, the membranes were stripped and reblotted with anti-Stat3 (lower panel, lanes 1 to 5) or anti-Stat5a (lower panel, lanes 6 to 10) antiserum, and similar amounts of STAT proteins were detected in each sample. These data demonstrated that Lck could phosphorylate Stat3 and Stat5a in the absence of Jak3, suggesting that Lck might play a role in IL-2-induced STAT activation.
FIG. 1.
Lck can phosphorylate and activate STATs but does not mediate IL-2-dependent phosphorylation and activation. (A) COS-7 cells were transfected with either Stat3 (lanes 1 to 5) or Stat5a (lanes 6 to 10), together with cDNAs encoding Jak3, K855A, Lck-wt, Lck-kd, or Lck-505 as indicated. Two days later, cell lysates were immunoprecipitated with anti-Stat3 (lanes 1 to 5) or anti-Stat5a (lanes 6 to 10) antiserum. The immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antiphosphotyrosine (anti PTyr) MAb 4G10 (upper panels) or anti-Stat3 and anti-Stat5a antisera (lower panels). (B to D) 3T3-αβγ cells were transfected with the STAT reporter construct p3xIRF-luc alone or with one or more cDNAs encoding catalytically active or inactive versions of Lck and Jak3 as indicated. Two days later, the cells were incubated without or with IL-2 for 5 h, and luciferase activity was determined. (E) 3T3-αβγ cells were transfected with one or more cDNAs encoding catalytically active or inactive versions of Lck and Jak3 as indicated. Two days later, the cells were incubated without or with IL-2 for 15 min, and phosphorylation of Stat5a and Stat3 was visualized.
Catalytically active Jak3 is required for IL-2-induced STAT activation, and catalytically inactive Lck does not block Jak3-dependent STAT activation.
Since Lck could phosphorylate Stat3 and Stat5a when overexpressed in COS cells, we next investigated whether Lck played a functional role in IL-2-induced STAT activation. We used 3T3-αβγ cells, fibroblasts which express all three IL-2R subunits and Jak1 but which lack Jak3 and Lck (3, 29). This system allows for evaluation of each of these kinases separately or in combination in mediating IL-2-induced STAT activation using a luciferase reporter construct (p3xIRF-luc). As shown in Fig. 1B, without the transfected kinases, there was no transactivation of the reporter gene in 3T3-αβγ cells. These results are consistent with previous studies of cells from Jak3-SCID patients and further confirmed that Jak1 cannot support IL-2-induced transactivation of STAT in the absence of Jak3 (3, 37). Consistent with the STAT phosphorylation seen in COS cells (Fig. 1A), expression of Lck-wt or Lck-505 in 3T3-αβγ cells resulted in constitutive transactivation. However, although Lck had the capacity to phosphorylate and activate STATs, IL-2-induced activation did not occur in the absence of Jak3.
To further ascertain whether IL-2-induced STAT activation was dependent on Lck, we next expressed Lck-kd alone or with Jak3 in 3T3-αβγ cells to determine if the former could block IL-2 signaling. As is evident in Fig. 1C, Lck-kd did not support STAT-mediated transactivation, demonstrating that catalytic activity of Lck is essential for the constitutive STAT activation. More important, expression of Lck-kd had no effect on the IL-2-mediated STAT activation that occurs in the presence of Jak3; Lck-kd and Jak3 resulted in STAT activation equivalent to that seen when Jak3 was expressed alone upon IL-2 stimulation of cells. Thus, the catalytically inactive Lck did not block Jak3-dependent IL-2-induced STAT activation, indicating that Lck is likely not required for this aspect of IL-2 signaling.
We were next interested in determining whether Lck could functionally cooperate with Jak3. Figure 1D shows that the combination of Lck-505 and Jak3 increased basal STAT transactivation and the maximal level of IL-2-inducible transactivation. However, the magnitude of ligand-dependent induction was actually less when Lck-505 was present (3-fold increase when Jak3 and Lck-505 were coexpressed [Fig. 1D] but 15-fold increase when Jak3 was expressed alone [Fig. 1C]). This experiment also illustrates that absence of IL-2 responsiveness upon Lck-505 expression was not simply because the system was saturated (Fig. 1B); in the presence of Jak3, further IL-2-dependent enhancement was observed (Fig. 1D). Catalytic activity of Jak3 was required because Lck-505 did not increase the IL-2-induced STAT activation when cotransfected with the catalytically inactive mutant K855A (Fig. 1D). Finally, we examined the effects of Lck on IL-2-induced STAT phosphorylation (Fig. 1E). Our results show that Jak3 (lanes 7 and 8), but not Lck (lanes 5 and 6), permitted IL-2-dependent STAT phosphorylation and catalytically inactive Lck did not block Jak3-mediated STAT phosphorylation (lanes 9 and 10). At this level of expression, little constitutive STAT phosphorylation was apparent (lanes 5 and 6). The discrepancy between these results (Fig. 1E) and Lck-mediated constitutive activation of STAT reporter gene is presumably indicative of the sensitivity of the luciferase assay (Fig. 1B), because at higher levels of expression of Lck in COS cells, constitutive STAT phosphorylation was readily detected by immunoblotting (Fig. 1A, lanes 3, 5, 8, and 10). Taken together, our data support the idea that the catalytic activity of Jak3 is essential for IL-2-induced STAT phosphorylation and activation. The data also suggest that Lck might affect the basal level of STAT activation but not IL-2-dependent STAT activation.
The Lck-specific inhibitor does not block IL-2-induced phosphorylation of Jak3 and Stat5a.
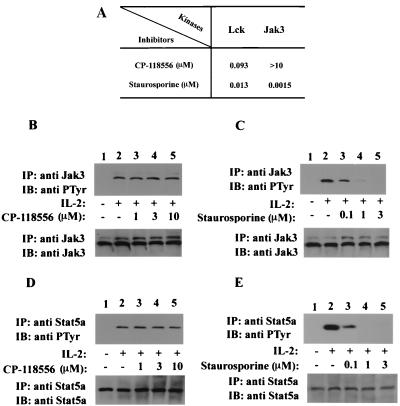
The preceding experiments showed that catalytically inactive Lck did not block Jak3-dependent IL-2-induced STAT activation, suggesting that IL-2-induced Jak3/Stat activation is not dependent on Lck. To confirm this idea, we turned to a second line of inquiry, namely, the effect of a well-characterized Src family kinase inhibitor, CP-118556 (10), on IL-2-induced Jak3 and Stat5a activation. We first ascertained that this inhibitor did not directly affect the catalytic activity of Jak3. The activity of purified fusion proteins of Lck and Jak3 was measured in the presence of CP-118556 or staurosporine using a kinase ELISA (Fig. 2A). The results show that CP-118556 potently inhibited the catalytic activity of Lck at nanomolar concentrations but had no effect on Jak3 catalytic activity up to a concentration of 10 μM. In contrast, the nonspecific kinase inhibitor staurosporine blocked the catalytic activity of both Jak3 and Lck at nanomolar concentrations.
FIG. 2.
Inhibition of Lck does not block IL-2-induced phosphorylation of Jak3 and Stat5a. (A) In vitro catalytic activity of Lck and Jak3 was detected by ELISA using random copolymers of l-glutamic acid and tyrosine as the substrate. Prior to the kinase assay, each well was incubated with an appropriate concentration of ATP and dilutions of the indicated kinase inhibitors, after which recombinant kinase domains of Lck and Jak3 were added. Values represent 50% inhibitory concentrations of CP-118556 and staurosporine for Lck and Jak3. (B to E) YT cells were incubated without (lanes 1 and 2) or with (lanes 3 to 5) inhibitors at 37°C (30 min), followed by IL-2 stimulation (15 min, lanes 2 to 5). The cells were lysed and immunoprecipitated with anti-Jak3 (B and C) or anti-Stat5a (D and E) antiserum. The immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antiphosphotyrosine (anti PTyr) MAb 4G10 (B to E, upper panels), anti-Jak3 antiserum (B and C, lower panels), and anti-Stat5a antiserum (D and E, lower panels).
Given that CP-118556 inhibited Lck but not Jak3, we next determined the effect of this compound on IL-2-induced phosphorylation of Jak3 and Stat5a. To this end, YT cells were preincubated with CP-118556 (Fig. 2B and D) or staurosporine (Fig. 2C and E) and then treated with IL-2. As shown in Fig. 2B, phosphorylation of Jak3 was evident upon IL-2 stimulation (upper panel, lane 2), and the IL-2-induced phosphorylation of Jak3 was not affected by the Lck inhibitor up to a concentration of 10 μM (upper panel, lanes 3 to 5). Phosphorylation of Stat5a was also detected upon IL-2 stimulation (Fig. 2D, upper panel, lane 2), and IL-2-induced phosphorylation of Stat5a was not altered in the presence of CP-118556 (upper panel, lanes 3 to 5). These results indicated that inhibition of Lck kinase had no effect on IL-2-induced Jak3 catalytic activity and subsequent STAT activation. In contrast, we have shown that CP-118556 can inhibit anti-CD3-induced proliferation of primary human peripheral blood lymphocytes with a potency of 500 nM (10). As a further control, we used the nonspecific kinase inhibitor staurosporine and found that it inhibited phosphorylation of both Jak3 and Stat5a (Fig. 2C and E, upper panel, lanes 3 to 5). In each case, the membranes were stripped of detecting antibody and reprobed for Jak3 and Stat5a to confirm equal loading. We concluded from these experiments that activation of Jak3 and subsequent STAT activation are not dependent on Lck kinase or other Src family kinases.
IL-2-mediated Lck activation is not dependent on Jak3 catalytic activity.
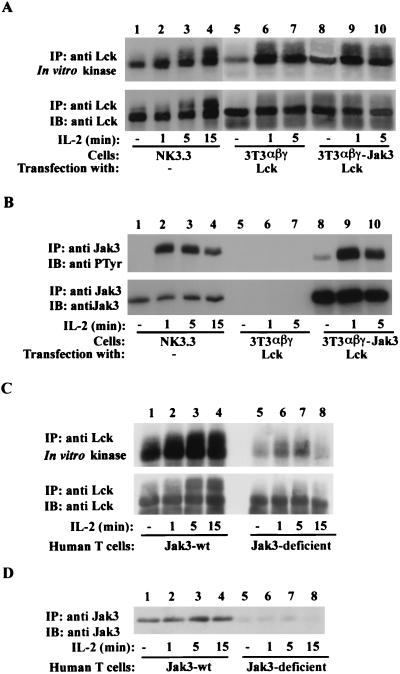
Our data showed that Lck has no effect on the IL-2-induced phosphorylation and activation of Jak3 and Stat5a, but these studies did not address whether IL-2-induced activation of Lck requires Jak3. To investigate this issue, we next analyzed the ligand-dependent activation of Lck in the presence or absence of Jak3. Cells were incubated with IL-2, and Lck catalytic activity and Jak3 phosphorylation were determined (Fig. 3A and B). As shown in Fig. 3A, IL-2 augmented Lck catalytic activity in the positive control, NK cells, with its kinase activity increasing after 1 min of IL-2 stimulation (upper panel, lanes 1 to 4). Interestingly, IL-2-mediated Lck activation was observed in both Jak3-expressing (upper panel, lanes 9 and 10) and Jak3-deficient (upper panel, lanes 6 and 7) fibroblasts. Consistent with observations in NK cells, Lck catalytic activity was enhanced about twofold upon 1 min of IL-2 stimulation, and the level of Lck activation was comparable in the absence or presence of Jak3, suggesting that Jak3 plays little role in Lck activation. IL-2-induced Jak3 phosphorylation was readily observed in both NK cells and 3T3-αβγJak3Lck cells upon IL-2 stimulation (Fig. 3B, upper panel, lanes 2, 3, 4, 9, and 10). As expected, Jak3 phosphorylation was not seen in 3T3-αβγLck cells due to their lack of this protein (Fig. 3B, upper panel, lanes 5 and 7). Additionally, consistent with the experiments using the Lck inhibitor (Fig. 2B), Jak3 phosphorylation was unaffected by the presence or absence of Lck (data not shown). Again, the membranes were blotted for Lck (Fig. 3A, lower panel) or Jak3 (Fig. 3B, lower panel) to confirm equivalent amounts of protein. These results demonstrate that IL-2-mediated activation of Lck is not dependent on Jak3 and suggest that Lck does not function downstream of Jak3 in IL-2 signaling.
FIG. 3.
IL-2-dependent activation of Lck does not require Jak3. (A and B) 3T3-αβγ (lanes 5 to 7) and 3T3-αβγJak3 (lanes 8 to 10) cells were transfected with cDNA encoding Lck-505 and then incubated with or without IL-2. NK3.3 cells were used as a positive control for IL-2-mediated activation of Jak3 and Lck (lanes 1 to 4). (C and D) Primary human T lymphocytes (107 cells per point) from a healthy individual (lanes 1 to 4) or a patient with Jak3 mutation (lanes 5 to 8) were activated with phytohemagglutinin for 3 days, starved overnight, and then incubated without (lanes 1 and 5) or with IL-2 for 1 (lanes 2 and 6), 5 (lanes 3 and 7), and 15 (lanes 4 and 8) min. Cells were lysed and immunoprecipitated with anti-Lck (A and C) or anti-Jak3 (B and D) antiserum. Immune complex kinase assays of Lck were performed in the presence of [γ-32P]ATP for 30 min and then stopped by the addition of 1× lysis buffer. Immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, subjected to autoradiography (A and C, upper panels), and subsequently immunoblotted with anti-Lck MAb (A and C, lower panels). Anti-Jak3 immune complexes were immunoblotted with antiphosphotyrosine (anti PTyr) MAb 4G10 (B, upper panel) and then probed with anti-Jak3 antiserum (B, lower panel, and D).
Since the above results were obtained using nonlymphoid cells, we next further investigated whether IL-2-induced activation of Lck requires Jak3 in human T lymphocytes. We analyzed primary human T lymphocytes from a healthy individual (lanes 1 to 4) and a Jak3-deficient individual who produced some T cells (lanes 5 to 8 and unpublished data) and analyzed the ligand-dependent activation of Lck in both circumstances (Fig. 3C). Cells were incubated with or without IL-2, and Lck catalytic activity was then determined. IL-2-mediated Lck activation was observed in both normal (Fig. 3C, upper panel, lanes 2 to 4) and Jak3-deficient (upper panel, lanes 6 to 8) T lymphocytes. In contrast, IL-2-dependent STAT phosphorylation was dramatically reduced in Jak3-deficient T lymphocytes (data not shown). As expected, Jak3 was readily detected in normal T lymphocytes (Fig. 3D, lanes 1 to 4), but little was seen in Jak3-deficient T lymphocytes (lanes 6 to 8). Higher levels of Lck expression as well as constitutive kinase activity were also detected in normal T lymphocytes (lower panel, lanes 1 to 4) compared to the Jak3-deficient T lymphocytes (lower panel, lanes 5 to 8). We do not know why the basal activity of Lck from the Jak3-deficient patient is reduced. However, Jak3-deficient patients typically do not produce T lymphocytes (23, 27, 43), so we do not know for certain whether the low level of Lck is a consistent finding. Nonetheless, the activation of Lck in Jak3-deficient cells is of about 1.5- to 2.5-fold in response to IL-2 (13, 19). Taken together, these results argue that IL-2-mediated Lck activation is independent of Jak3. These findings are consistent with a previous report in which IL-2-induced Lck activation in resting T cells was interpreted to be independent of Jak3 activation as well (9).
Based on preceding experiments, we conclude the following regarding the function of Lck in IL-2 signal transduction: (i) Lck activation is not Jak3 dependent, (ii) Jak3 activation is not Lck dependent, and (iii) IL-2-induced STAT activation is Jak3 dependent but not dependent on Lck or other Src PTKs. Thus, Jak3 and Lck function in parallel in IL-2 signaling.
Syk phosphorylates Stat3 and Stat5a when coexpressed but is not required for IL-2-induced STAT activation.
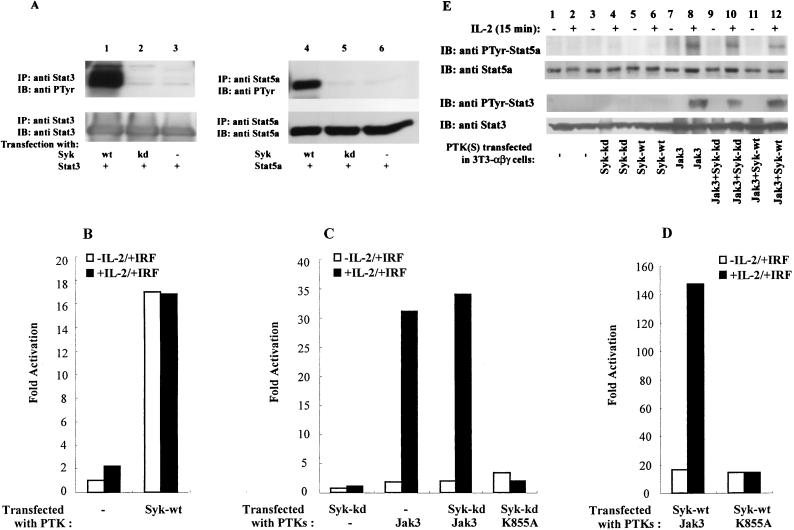
Syk has been shown to interact with the S region of IL-2Rβ, an interaction required for IL-2-mediated activation of the kinase and subsequent IL-2-mediated proliferation (30, 32, 46). However, it has been previously unclear whether Syk contributes to STAT activation. To address this issue, as in our studies of Lck, we first investigated the capacity of Syk to phosphorylate Stat3 and Stat5a in an overexpression system (Fig. 4A). COS cells were transfected with Stat3 or Stat5a in the absence (lanes 3 and 6) or presence (lanes 1 and 4) of Syk. As shown in Fig. 4A, upon overexpression, Syk phosphorylated both Stat3 and Stat5a (upper panels, lanes 1 and 4), whereas no phosphorylation was observed in its absence or in the presence of catalytically inactive Syk (upper panels, lanes 2, 3, 5, and 6). Immunoblotting revealed that STAT proteins were equally loaded in each sample (lower panels). These data indicate that Syk has the capacity to phosphorylate STAT proteins in the absence of Jak3 and thus may play a role in IL-2-induced STAT activation.
FIG. 4.
Syk can phosphorylate and activate STATs but does not mediate IL-2-dependent phosphorylation and activation. (A) COS-7 cells were transfected with either Stat3 (lanes 1 to 3) or Stat5a (lanes 4 to 6), together with cDNAs encoding Syk-wt. Syk-kd as indicated. Two days later, cells were lysed and immunoprecipitated with anti-Stat3 (lanes 1 to 3) or anti-Stat5a (lanes 4 to 6) antiserum. The immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antiphosphotyrosine (anti PTyr) MAb 4G10 (upper panels) or anti-Stat3 and anti-Stat5a antisera (lower panels). (B to D) As indicated, 3T3-αβγ cells were transfected with the STAT reporter construct p3xIRF-luc and cDNAs encoding Syk-wt, Syk-kd, Jak3, and K855A separately or in combination. Two days later, the cells were incubated without or with IL-2 (5 h), and luciferase activity was measured. (E) 3T3-αβγ cells were transfected with one or more cDNAs encoding catalytically active or inactive versions of Syk and Jak3 as indicated. Two days later, the cells were incubated without or with IL-2 for 15 min, and phosphorylation of Stat5a and Stat3 was visualized.
To test this hypothesis, we again used 3T3-αβγ cells, which lack Syk kinase (3, 29). 3T3-αβγ cells were transfected with the STAT reporter construct along with cDNAs encoding catalytically active Syk. Our results showed that expression of Syk did not allow for an IL-2-induced STAT activation in the absence of Jak3 but caused an increased basal level of STAT activation (Fig. 4B). This increased basal level of STAT activation was not observed when Syk was absent in 3T3-αβγ cells (Fig. 4B) and was consistent with the observations in COS cells (Fig. 4A).
To further define the function of Syk in IL-2-induced STAT activation, we next assessed whether catalytically inactive Syk (Syk-kd) could block IL-2-induced STAT activation. As shown in Fig. 4C, coexpression of Syk-kd with Jak3 gave a similar level of IL-2-induced STAT transactivation compared to cells in which Jak3 was expressed alone, and thus the catalytically inactive Syk did not block Jak3-dependent IL-2-induced STAT activation. These data suggest that Syk, like Lck, is not required for IL-2-induced STAT activation.
Having shown that Syk cannot facilitate IL-2-induced STAT activation in the absence of Jak3, we next investigated whether Syk could influence IL-2-induced STAT activation in the presence of Jak3. As observed in Fig. 4D, expression of Syk enhanced IL-2-induced STAT activation in the presence of Jak3, but the magnitude of ligand-dependent induction was not greater when Syk was present (7-fold increase when Jak3 and Syk were coexpressed [Fig. 4D] but 15-fold increase when Jak3 was expressed alone [Fig. 4C]). Furthermore, the augmentation of IL-2-induced STAT activation by Syk required the catalytic activity of Jak3, as Syk did not increase the IL-2-induced STAT activation in the presence of catalytically inactive Jak3 (Fig. 4D). Examination of the effects of Syk on IL-2-induced STAT phosphorylation (Fig. 4E) revealed that IL-2-induced STAT phosphorylation was also dependent on Jak3 (lanes 7 and 8) but not Syk (lanes 5 and 6); moreover, catalytically inactive Syk did not block IL-2-induced STAT phosphorylation (lanes 9 and 10). Like Lck (Fig. 1E, lanes 5 and 6), Syk expression also did not constitutively phosphorylate STAT at this level of expression (Fig. 4E, lanes 5 and 6). Therefore, the discrepancy between these results (Fig. 4E) and Syk-mediated constitutive activation of STAT reporter gene (Fig. 4B) might be also a reflection of the sensitivity of the luciferase assay, given that constitutive STAT phosphorylation was readily detected at higher levels of expression of Syk in COS cells (Fig. 4A). Taken together, these data further support the contention that the catalytic activity of Jak3 is essential for IL-2-induced STAT activation.
IL-2-mediated activation of Syk is dependent on Jak3.
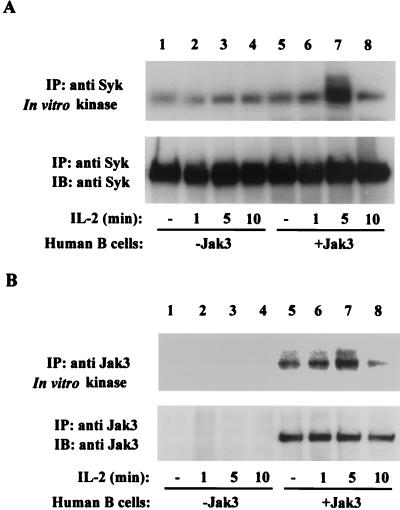
In previous studies, the S region of IL-2Rβ has been shown to be critical for IL-2-mediated activation of Syk (30, 32, 46). These studies, however, did not investigate the requirement of Jak3 in this event. To define the role of Jak3 in IL-2-mediated activation of Syk, we used EBV-transformed healthy human B cells and B cells obtained from a Jak3-deficient patient, as B cells normally express IL-2R constitutively and respond to this cytokine (37). As seen in Fig. 5A, normal human B cells have low levels of catalytically active Syk prior to IL-2 stimulation (upper panel, lane 5); however, IL-2 markedly but transiently augmented its kinase activity in Jak3-containing cells, reaching a maximal level upon 5 min of IL-2 stimulation (about 3.2-fold increase; upper panel, lane 7). In contrast, when IL-2-induced kinase activity of Syk was evaluated in Jak3-deficient B cells, augmentation of Syk kinase activity was abrogated (upper panel, lanes 1 to 4). Figure 5B shows the IL-2-mediated regulation of Jak3 catalytic activity. Jak3 kinase activity was present prior to IL-2 stimulation (Fig. 5B, upper panel, lane 5), but its kinase activity was enhanced upon IL-2 stimulation, reaching a maximal level upon 5 min of IL-2 stimulation (about twofold increase; upper panel, lane 7). For comparison, Jak3-deficient cells are shown in lanes 1 to 4. As expected, no Jak3 was detected in these cells (Fig. 5B, lower panel). Thus, the catalytic activities of both Jak3 and Syk were activated upon IL-2 stimulation, and activation of Syk requires Jak3.
FIG. 5.
Activation of Syk by IL-2 is dependent on Jak3. EBV-transformed human B cell lines from a healthy individual (lanes 5 to 8) or a patient with Jak3 deficiency (lanes 1 to 4) were incubated without (lanes 1 and 5) or with IL-2 for 1 (lanes 2 and 6), 5 (lanes 3 and 7) and 10 (lanes 4 and 8) min. Cell lysates were immunoprecipitated with anti-Syk (A) or anti-Jak3 (B) antiserum. In vitro kinase assays of Syk and Jak3 were performed on the immune complexes at room temperature for 5 min. Samples were then separated by SDS-PAGE, transferred to nitrocellulose, and subjected to autoradiography (A and B, upper panels) or immunoblotted with anti-Syk MAb (A, lower panel) or anti-Jak3 antiserum (B, lower panel).
Jak1, but not Jak3, phosphorylates Syk.
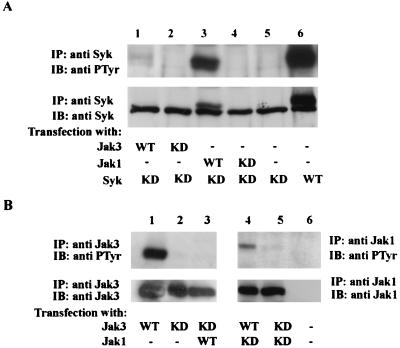
Since our data suggested that IL-2-mediated Syk activation requires Jak3, we next investigated possible mechanisms by which this might occur. We first tested if Syk is the direct substrate of either Jak by overexpressing catalytically active or inactive versions of the various kinases (Fig. 6A). As expected, phosphorylation of Syk-wt was readily observed (upper panel, lane 6), whereas no phosphorylation of catalytically inactive Syk was seen (upper panel, lane 5). Interestingly, coexpression of catalytic active Jak3 did not result in Syk phosphorylation (upper panel, lane 1), whereas coexpression of catalytic active Jak1 clearly did (upper panel, lane 3). When we reprobed the membrane with anti-Syk antibody, the phosphorylated Syk appeared as a slower-migrating band (lower panel, lanes 3 and 6). Taken together, these results demonstrate that although Jak3 is required for Syk activation, Syk may be a direct substrate of Jak1.
FIG. 6.
Phosphorylation of Syk by Jak1 but not by Jak3. (A) COS-7 cells were transfected with one or more cDNAs encoding catalytically active or inactive versions of Syk, Jak1, or Jak3 as indicated. Two days later, cells were lysed and immunoprecipitated with an anti-Syk antibody. The immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antiphosphotyrosine (anti PTyr) MAb 4G10 (upper panel) or anti-Syk antibody (lower panel). (B) U4A cells were transfected with one or more cDNAs encoding catalytically active or inactive versions of Jak1 and Jak3 as indicated. The immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antiphosphotyrosine MAb 4G10 (upper panels), followed by immunoblotting with anti-Jak3 (lanes 1 to 3, lower panel) and anti-Jak1 antibody (lanes 4 to 6, lower panel).
If Syk is not a direct substrate of Jak3, we next reasoned that perhaps Jak1 is activated by Jak3 and activated Jak1 in turn phosphorylates Syk. To determine whether Jak3 and Jak1 could phosphorylate each other, we expressed the catalytically active or inactive versions of Jak1 or Jak3 in U4A cells, a somatic mutant human fibrosarcoma cell line lacking both Jak1 and Jak3 (24). Our results showed that Jak1 is a good substrate for Jak3 (Fig. 6B, upper panel, lane 4), but the reverse is not the case (upper panel, lane 3). These results are in agreement with a previous report that IL-2 activation of Jak1 functions downstream of Jak3 (52). In related experiments, we also examined whether Syk could phosphorylate Jak1 or Jak3, but did not find this to be the case (data not shown). Thus, our findings support the notion that Jak3 regulates Syk kinase indirectly via activation of Jak1 upon IL-2 stimulation.
In summary, these experiments indicated the following: (i) Syk has the capacity to phosphorylate STAT transcription factors, (ii) in the absence of Jak3, Syk does not support IL-2-induced STAT phosphorylation and activation, (iii) Syk is not required for IL-2-induced STAT phosphorylation and activation, (iv) Syk activation is Jak3 dependent, and (v) Jak3 is necessary, but not sufficient, to mediate Syk activation.
DISCUSSION
In this study, we have investigated the functional interactions among Jak3, Lck, and Syk in IL-2 signaling with an emphasis on the activation of STAT transcription factors. Our data indicate that Lck or Syk alone has the capacity to phosphorylate Stat3 and Stat5a in COS cells, but neither supports IL-2-induced STAT activation in the absence of Jak3. Furthermore, the absence of either Lck or Syk, or the overexpression of their catalytically inactive versions, does not block IL-2-induced STAT phosphorylation or activation as long as Jak3 is present. More importantly, we find that IL-2-mediated activation of Syk is dependent on Jak3, whereas the activation of Lck is Jak3 independent.
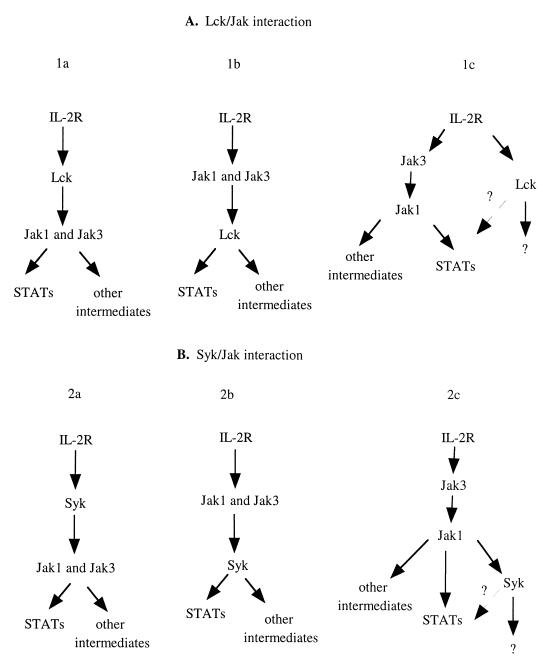
At the outset of our studies, we considered several possibilities regarding the interactions of Lck, Syk, and Jaks in IL-2 signaling. These simple models are diagrammed in Fig. 7. The data generated in our studies argue that with respect to Lck and Jak3 interactions, models 1a and 1b are not likely to be correct (Fig. 7A). That is, in the absence of Lck, expression of a catalytically inactive Lck or addition of a pharmacological inhibitor of Lck failed to block Jak3 activation and IL-2-induced STAT activation. This supports the contention that Jak3 is not dependent on Lck either for its activation or for its ability to activate STAT transcription factors. Interestingly, the data provided by this and previous studies clearly indicate that Lck has the capacity to activate STAT proteins (26, 50, 53). So, does Lck play any role in IL-2 induced STAT activation? Our data suggest that Lck is clearly not required, in sharp contrast with studies on IL-3 signaling in which Src kinases and not Jaks were found to be required for ligand-induced STAT activation (2). Though our results suggest that Lck has little effect on IL-2-induced STAT activation, they do indicate that Lck might influence the set point of STAT activation, an action possibly mediated by a receptor other than the IL-2R. Since a recent study indicated that TCR-dependent signaling can indeed lead to Stat 5 activation (50), it is quite possible that Lck could contribute to STAT activation in this manner.
FIG. 7.
Proposed models for interactions between Lck, Syk, and Jaks in IL-2 signaling.
Our data also indicate that IL-2 mediated activation of Lck is Jak3 independent (Fig. 7A, model 1c). In a previous study, which examined IL-2 signaling in unactivated lymphocytes, the authors concluded that IL-2 could activate Lck without activation of Jaks (9). These cells expressed low levels of Jak3, but it is clearly not absent in unactivated T cells. Our results in 3T3-αβγ cells that completely lack Jak3 and Jak3-deficient T lymphocytes support this conclusion.
A number of functions have been attributed to Lck in IL-2 signaling, but some of these conclusions pertaining to Lck were derived from the studies of IL-2Rβ mutations (8, 12, 28, 45, 46). We now know that the A region of the IL-2Rβ is important for binding not only Lck but also Jak1, Jak3, and Shc (6, 40, 55). Thus, it is more difficult now to relate the consequences of deleting this domain with actions of any specific intermediate that binds this region.
Nonetheless, several studies have argued for the importance of Lck in suppressing apoptosis. One study concluded that activated Lck (Lck-505) could cooperate with either c-Myc or Bcl-2 in suppression of apoptosis, suggesting a role of Lck in transmitting antiapoptosis signals (32). Another study analyzed IL-2 signaling in resting T cells and showed IL-2-dependent Lck activation in the absence of detectable Jak phosphorylation. This correlated with phosphatidylinositol-3 kinase (PI-3K) activation and PI-3K-dependent induction of Bcl-xL (9). Taken together, one function of Lck, independent of Jak3 in IL-2 signaling, might be suppression of apoptosis. This signal might be especially important early during lymphocyte activation when Jak3 expression is very low, since Lck can evidently be activated irrespective of the presence or absence of Jak3.
Although IL-2 has been shown to activate Syk (30, 39, 46), the data provided by our study argue against models in which Syk contributes to Jak3 activation in IL-2 signaling (Fig. 7B, models 2a). The converse appears to be true: Jak3 is required for Syk activation (model 2c). Syk, however, does not appear to be a direct Jak3 substrate, but it does have the capacity to be a Jak1 substrate. In addition, our data and data from a previous study demonstrate that Jak1 functions as a direct substrate of Jak3, but the reverse is not the case (24, 37, 52); furthermore, we found that Syk did not phosphorylate either Jak1 or Jak3 (data not shown). Taken together, our observations support the idea that IL-2-mediated Jak activation functions upstream of Syk. Ligand-induced aggregation of IL-2R results in juxtapositioning Jak1 and Jak3, which facilitates Jak3 transphosphorylation of Jak1. Our data suggest that Jak1 then phosphorylates Syk. Therefore, Jak3 is necessary, but not sufficient, to effect Syk activation. While Syk appears to be downstream of Jaks, it is clearly not required for IL-2-induced STAT phosphorylation and transactivation (model 2b). That is, it does not appear to be an intermediate between the Jaks and STATs, since the dominant negative mutant Syk did not block Jak3 dependent IL-2-induced STAT activation.
As in studies of Lck, several functions have been attributed to Syk, as various mutations of IL-2Rβ disrupt the ability to associate and activate Syk, resulting in various functional defects in IL-2 signaling (30, 32, 46). The inference has been made that Syk is responsible for various functions including c-Myc induction and cellular proliferation. However, it is clear now that the S region of the IL-2Rβ is important not only for binding Syk but also for binding Jak1 and Jak3 (55). Thus, it is also difficult to correlate the consequences of deleting this S region with actions of a single PTK. Moreover, since T cells from Syk−/− mice exhibit a relatively normal IL-2 response (48), the role of Syk in IL-2 signaling remains an open question. Our results indicated that Syk clearly could influence STAT activation via an action possibly mediated by a receptor other than the IL-2R. Interestingly, like Lck, Syk is activated by TCR and BCR occupancy (49), and so it too may influence STAT activation by a non-IL-2-mediated mechanism.
In conclusion, we have provided functional and biochemical evidence pertaining to the interactions of Jak1, Jak3, Lck, and Syk in IL-2 signaling with a focus on STAT activation. Our data favor a model for PTK activation in IL-2 signaling in which Jak3 is dependent on neither Syk nor Lck for its activation of STATs (Fig. 7). Lck activation is not dependent on Jak3; it may be viewed as functioning in parallel with Jak3. Lck clearly can contribute to the absolute level of STAT activation but does not mediate IL-2-induced phosphorylation and activation. Indeed, it is also possible that the contribution is via a receptor other than the IL-2R. Syk activation appears to be dependent on Jak3, and Jak3 probably regulates Syk kinase indirectly via activation of Jak1. However, Syk is not required for IL-2-induced STAT activation. The target genes dependent on Lck and Syk have been hitherto inferred from studies of receptor mutants. These mutants however, disrupt the ability to activate not only these specific kinases but other kinases and pathways as well. Therefore, it will be essential to define the key downstream substrates of each kinase in order to understand the distinct contributions of these molecules in mediating the effects of IL-2.
ACKNOWLEDGMENTS
We thank L. E. Samelson, R. Pine, and A. C. Larner for providing useful reagents. We thank Y. Minami, T. Taniguchi, and R. Visconti for critically reading the manuscript. We are grateful to B. J. Fowlkes, J. Rivera, M. Aringer, and C. Sudarshan for technical assistance.
REFERENCES
- 1.Beadling C, Guschin D, Witthuhn B A, Ziemiecki A, Ihle J N, Kerr I M, Cantrell D A. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994;13:5605–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi P, Reddy M V, Reddy E P. Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene. 1998;16:1749–1758. doi: 10.1038/sj.onc.1201972. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Cheng A, Chen Y Q, Hymel A, Hanson E P, Kimmel L, Minami Y, Taniguchi T, Changelian P S, O'Shea J J. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc Natl Acad Sci USA. 1997;94:6910–6915. doi: 10.1073/pnas.94.13.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 6.Evans G A, Goldsmith M A, Johnston J A, Xu W, Weiler S R, Erwin R, Howard O M, Abraham R T, O'Shea J J, Greene W C, et al. Analysis of interleukin-2-dependent signal transduction through the Shc/Grb2 adapter pathway. Interleukin-2-dependent mitogenesis does not require Shc phosphorylation or receptor association. J Biol Chem. 1995;270:28858–28863. doi: 10.1074/jbc.270.48.28858. [DOI] [PubMed] [Google Scholar]
- 7.Friedmann M C, Migone T S, Russell S M, Leonard W J. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc Natl Acad Sci USA. 1996;93:2077–2082. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii H, Ogasawara K, Otsuka H, Suzuki M, Yamamura K, Yokochi T, Miyazaki T, Suzuki H, Mak T W, Taki S, Taniguchi T. Functional dissection of the cytoplasmic subregions of the IL-2 receptor beta chain in primary lymphocyte populations. EMBO J. 1998;17:6551–6557. doi: 10.1093/emboj/17.22.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Garcia A, Merida I, Martinez-A C, Carrera A C. Intermediate affinity interleukin-2 receptor mediates survival via a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 1997;272:10220–10226. doi: 10.1074/jbc.272.15.10220. [DOI] [PubMed] [Google Scholar]
- 10.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 11.Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin S D, Perlmutter R M, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama M, Kawahara A, Mori H, Shibuya H, Taniguchi T. C-fos gene induction by interleukin 2: identification of the critical cytoplasmic regions within the interleukin 2 receptor beta chain. Proc Natl Acad Sci USA. 1992;89:2022–2026. doi: 10.1073/pnas.89.6.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horak I D, Gress R E, Lucas P J, Horak E M, Waldmann T A, Bolen J B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci USA. 1991;88:1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihle J N. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 15.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 16.Johnston J A, Kawamura M, Kirken R A, Chen Y Q, Blake T B, Shibuya K, Ortaldo J R, McVicar D W, O'Shea J J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 17.Johnston J A, Wang L M, Hanson E P, Sun X J, White M F, Oakes S A, Pierce J H, O'Shea J J. Interleukins 2, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells. Potential role of JAK kinases. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 18.Johnston J A, Bacon C M, Finbloom D S, Rees R C, Kaplan D, Shibuya K, Ortaldo J R, Gupta S, Chen Y Q, Giri J D, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci USA. 1995;92:8705–8709. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karnitz L, Sutor S L, Torigoe T, Reed J C, Bell M P, McKean D J, Leibson P J, Abraham R T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara A, Minami Y, Miyazaki T, Ihle J N, Taniguchi T. Critical role of the interleukin 2 (IL-2) receptor gamma-chain-associated Jak3 in the IL-2-induced c-fos and c-myc, but not bcl-2, gene induction. Proc Natl Acad Sci USA. 1995;92:8724–8728. doi: 10.1073/pnas.92.19.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura M, McVicar D W, Johnston J A, Blake T B, Chen Y Q, Lal B K, Lloyd A R, Kelvin D J, Staples J E, Ortaldo J R, et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci USA. 1994;91:6374–6378. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard W J. Type I cytokines, interferons and their receptors. In: Paul W E, editor. Fundamental immunology. 4th ed. Philadelphia, Pa: Lippincott-Raven Publisher; 1999. pp. 741–774. [Google Scholar]
- 23.Leonard W J, O'Shea J J. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 24.Liu K D, Gaffen S L, Goldsmith M A, Greene W C. Janus kinases in interleukin-2-mediated signaling: JAK1 and JAK3 are differentially regulated by tyrosine phosphorylation. Curr Biol. 1997;7:817–826. doi: 10.1016/s0960-9822(06)00369-1. [DOI] [PubMed] [Google Scholar]
- 25.Lord J D, McIntosh B C, Greenberg P D, Nelson B H. The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J Immunol. 1998;161:4627–4633. [PubMed] [Google Scholar]
- 26.Lund T C, Garcia R, Medveczky M M, Jove R, Medveczky P G. Activation of STAT transcription factors by herpesvirus saimiri Tip-484 requires p56lck. J Virol. 1997;71:6677–6682. doi: 10.1128/jvi.71.9.6677-6682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macchi P, Villa A, Gillani S, Sacco M G, Frattini A, Porta F, Ugazio A G, Johnston J A, Candotti F, O'Shea J J, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 28.Minami Y, Kono T, Yamada K, Kobayashi N, Kawahara A, Perlmutter R M, Taniguchi T. Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck. EMBO J. 1993;12:759–768. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minami Y, Oishi I, Liu Z J, Nakagawa S, Miyazaki T, Taniguchi T. Signal transduction mediated by the reconstituted IL-2 receptor. Evidence for a cell type-specific function of IL-2 receptor beta-chain. J Immunol. 1994;152:5680–5690. [PubMed] [Google Scholar]
- 30.Minami Y, Nakagawa Y, Kawahara A, Miyazaki T, Sada K, Yamamura H, Taniguchi T. Protein tyrosine kinase Syk is associated with and activated by the IL-2 receptor: possible link with the c-myc induction pathway. Immunity. 1995;2:89–100. doi: 10.1016/1074-7613(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z J, Oishi I, Silvennoinen O, Witthuhn B A, Ihle J N, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, Liu Z J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Permutter R M, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 33.Molina T J, Kishihara K, Siderovski D P, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige C J, Hartmann K U, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 34.Nelson B H, McIntosh B C, Rosencrans L L, Greenberg P D. Requirement for an initial signal from the membrane-proximal region of the interleukin 2 receptor gamma(c) chain for Janus kinase activation leading to T cell proliferation. Proc Natl Acad Sci USA. 1997;94:1878–1883. doi: 10.1073/pnas.94.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noguchi M, Yi H, Rosenblatt H M, Filipovich A H, Adelstein S, Modi W S, McBride O W, Leonard W J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 36.Nosaka T, Van Deursen J M, Tripp R A, Thierfelder W E, Witthuhn B A, McMickle A P, Doherty P C, Grosveld G C, Ihle J N. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 37.Oakes S A, Candotti F, Johnston J A, Chen Y Q, Ryan J J, Taylor N, Liu X, Hennighausen L, Notarangelo L D, Paul W E, Blaese R M, O'Shea J J. Signaling via IL-2 and IL-4 in JAK3-deficient severe combined immunodeficiency lymphocytes: JAK3-dependent and independent pathways. Immunity. 1996;5:605–615. doi: 10.1016/s1074-7613(00)80274-5. [DOI] [PubMed] [Google Scholar]
- 38.O'Shea J J. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 39.Qin S, Inazu T, Yang C, Sada K, Taniguchi T, Yamamura H. Interleukin 2 mediates p72syk activation in peripheral blood lymphocytes. FEBS Lett. 1994;345:233–236. doi: 10.1016/0014-5793(94)00450-1. [DOI] [PubMed] [Google Scholar]
- 40.Ravichandran K S, Igras V, Shoelson S E, Fesik S W, Burakoff S J. Evidence for a role for the phosphotyrosine-binding domain of Shc in interleukin 2 signaling. Proc Natl Acad Sci USA. 1996;93:5275–5280. doi: 10.1073/pnas.93.11.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodig S J, Meraz M A, White J M, Lampe P A, Riley J K, Arthur C D, King K L, Sheehan K C, Yin L, Pennica D, Johnson E M J, Schreiber R D. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 42.Russell S M, Johnston J A, Noguchi M, Kawamura M, Bacon C M, Friedmann M, Berg M, McVicar D W, Witthuhn B A, Silvennoinen O, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 43.Russell S M, Tayebi N, Nakajima H, Riedy M C, Roberts J L, Aman M J, Migone T S, Noguchi M, Markert M L, Buckley R H, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 44.Satoh T, Minami Y, Kono T, Yamada K, Kawahara A, Taniguchi T, Kaziro Y. Interleukin 2-induced activation of Ras requires two domains of interleukin 2 receptor beta subunit, the essential region for growth stimulation and Lck-binding domain. J Biol Chem. 1992;267:25423–25427. [PubMed] [Google Scholar]
- 45.Shibuya H, Yoneyama M, Ninomiya-Tsuji J, Matsumoto K, Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992;70:57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 47.Thomis D C, Gurniak C B, Tivol E, Sharpe A H, Berg L J. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 48.Turner M, Mee P J, Costello P S, Williams O, Price A A, Duddy L P, Furlong M T, Geahlen R L, Tybulewicz V L. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 49.Van Oers N S, Weiss A. The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes. Semin Immunol. 1995;7:227–236. doi: 10.1006/smim.1995.0027. [DOI] [PubMed] [Google Scholar]
- 50.Welte T, Leitenberg D, Dittel B N, al-Ramadi B K, Xie B, Chin Y E, Janeway C A J, Bothwell A L M, Bottomly K, Fu X Y. STAT5 interaction with the T cell receptor complex and stimulation of T cell proliferation. Science. 1999;283:222–225. doi: 10.1126/science.283.5399.222. [DOI] [PubMed] [Google Scholar]
- 51.Witthuhn B A, Silvennoinen O, Miura O, Lai K S, Cwik C, Liu E T, Ihle J N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 52.Witthuhn B A, Williams M D, Kerawalla H, Uckun F M. Differential substrate recognition capabilities of Janus family protein tyrosine kinases within the interleukin 2 receptor (IL2R) system: Jak3 as a potential molecular target for treatment of leukemias with a hyperactive Jak-Stat signaling machinery. Leuk Lymphoma. 1999;32:289–297. doi: 10.3109/10428199909167389. [DOI] [PubMed] [Google Scholar]
- 53.Yu C L, Jove R, Burakoff S J. Constitutive activation of the Janus kinase-STAT pathway in T lymphoma overexpressing the Lck protein tyrosine kinase. J Immunol. 1997;159:5206–5210. [PubMed] [Google Scholar]
- 54.Zhou Y J, Hanson E P, Chen Y Q, Magnuson K, Chen M, Swann P G, Wange R L, Changelian P S, O'Shea J J. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc Natl Acad Sci USA. 1997;94:13850–13855. doi: 10.1073/pnas.94.25.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu M H, Berry J A, Russell S M, Leonard W J. Delineation of the regions of interleukin-2 (IL-2) receptor beta chain important for association of Jak1 and Jak3. Jak1-independent functional recruitment of Jak3 to Il-2Rbeta. J Biol Chem. 1998;273:10719–10725. doi: 10.1074/jbc.273.17.10719. [DOI] [PubMed] [Google Scholar]