Abstract
Two green-sensitive spectrofluorometric methods were investigated for assay of rupatadine (RUP) [method I] and its binary mixture with montelukast (MKT) [method II]. Method I depends on measuring native fluorescence of RUP in the presence of 0.10 M H2SO4 and 0.10%w/v sodium dodecyl sulfate at 455 nm after excitation at 277 nm. The range of the first method was 0.20–2.00 µg ml−1 with detection and quantitation limits of 59.00 and 179.00 ng ml−1, respectively. Method II depends on the first derivative synchronous spectrofluorometry. The derivative intensities were measured for the two drugs in an aqueous solution containing Mcllvaine's buffer pH 2.60 at fixed Δλ of 140 nm. Each drug was estimated at zero-contribution of the other. The intensity was measured at 261 and 371 nm for RUP and MKT, respectively. The method was linear over 0.10–4.00 and 0.20–1.60 µg ml−1 with limits of detection 31.00 and 66.00 ng ml−1 and limits of quantitation 94.00 and 200.00 ng ml−1 for RUP and MKT, respectively. The method was extended to determine this mixture in laboratory-prepared mixtures and combined tablets. Method validation was performed according to ICH guidelines. Statistical interpretation of data revealed good agreement with the comparison method. Method greenness was confirmed by applying three different assessment tools.
Keywords: rupatadine, montelukast, native fluorescence, derivative synchronous fluorescence spectroscopy, pharmaceutical dosage forms
1. Introduction
Rupatadine (RUP) fumarate (figure 1a) is 8-Chloro-11-[1-[(5-methylpyridin-3-yl)methyl]piperidin-4-ylidine]-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine(2E)-but-2-enedioate. Montelukast (MKT) sodium (figure 1b) is sodium[1-[[[1R)-1-[3-(E)-2-(7-choroquinolin-2-yl)ethenyl]phenyl]-3-[2-(1-hydroxy)methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl] acetate [1]. RUP and MKT are official drugs in British Pharmacopeia (B.P) [1] and United States Pharmacopeia [2]. RUP is a second-generation non-sedating antihistamine with platelet-activating factor antagonist activity that is prescribed for the treatment of allergic rhinitis, conjunctivitis and chronic idiopathic urticaria. It is given as the fumarate although doses are expressed in terms of the base; RUP fumarate 12.80 mg is equivalent to about 10.00 mg of RUP. The usual oral dose is the equivalent of 10.00 mg once daily of RUP. MKT is a selective leukotriene receptor antagonist that is approved in cases of allergic rhinitis and chronic asthma. It is also used as a prophylactic agent for exercise-induced asthma [3]. It is always co-administered with corticosteroids. RUP and MKT are co-formulated in tablet dosage forms like (Rupanex M®, Montyrup®) by pharmaceutical ratio 1 : 1. It has also been found that this combination is more effective than a single one in control of allergic rhinitis symptoms.
Figure 1.
Structural formula for (a) RUP fumarate and (b) MKT sodium.
It is commonly known that spectrofluorometric methods are sensitive, selective, economic, accurate, rapid and usually green. However, some selectivity problems appeared, especially in multi-drug analysis due to the overlapping of their excitation and emission spectra as RUP and MKT. Synchronous fluorescence spectroscopy (SFS) solved this problem by providing simple, sharp spectra with high selectivity and low interference. Our method uses a constant difference between wavelengths, and it is a type of SFS known as constant wavelength SFS. So, SFS has an important feature over the conventional fluorescence which in turn improves the spectral resolving and diverging of light. Coupling the SFS technique with derivative amplitude leads to perfect resolution for both drugs [4].
The literature pointed to some reports for RUP estimation like densitometric [5], derivative UV spectrophotometry [6,7], RP-HPLC [8–11], GC-MS [12,13] voltametric techniques [14], RP-UPLC [15], LC-MS/MC [16], capillary zone electrophoresis [17], HPTLC [18], non-aqueous potentiometric titration method [19] and spectrofluorometric derivatization technique [20]. Different analytical methods have been used in determination of MKT such as RP-HPLC [21,22], UV spectrophotometry [23], spectrofluorometric [24], derivative spectrofluorometry [25] and voltametric technique [26]. MKT and RUP were estimated together by RP-UPLC and RP-HPLC methods with UV spectrophotometric detection [10,15]. It is worth mentioning that there is no spectrofluorometric method for determination of RUP alone (conventional) without reactions or with MKT (derivative synchronous fluorometric technique) in combination pharmaceutical dosage forms.
In the current study, we aim to determine RUP alone and in the presence of MKT as their co-formulation in a tablet dosage form. By scanning their native fluorescence spectra, great overlapping between them is so challenging. So, first derivative SFS (FDSFS) is a magnificent way to separate such mixtures qualitatively and quantitatively (method II) while the conventional native fluorescence technique is used for the determination of RUP (method I). These two sensitive spectrofluorometric methods are simple and highly green for the quantification of RUP and MKT in the commercial dosage forms.
2. Experimental
2.1. Apparatus
Cary Eclipse Fluorescence Spectrophotometer equipped with Xenon flash lamp. High-sensitivity mode (800 V), smoothing factor 20.00, slit width 5.00 nm with 1.00 cm quartz cell was manipulated for the conventional spectrofluorometric measuring method.
Synchronous spectrofluorometric measurements were performed at Δλ = 140 nm with scanning in the range of 200–600 nm. Gathering the stored data was achieved by the Cary Eclipse software. The first derivative spectra were manipulated at a filter size of 19.00 and an interval of 1.00 nm. A scan rate of 600 nm min−1 was adopted using 10 nm excitation and emission windows. A pH-meter (Consort, NV P-901, Belgium) was used for adjusting the pH of buffer solutions. A Sonix IV model-SS101H 230 (USA) sonicator was used.
2.2. Material and solvents
Chemicals were of analytical grade and HPLC grade solvents were used. MKT sodium was provided by Hikma Pharma, Giza district, Egypt (batch number:MT17020021), stored in opaque glass vials. RUP fumarate was provided by Mash premiere, Fifth settlement, New Cairo, Egypt. Singulair® tablets contain 10.00 mg MKT (batch no. G23009), produced by Global Nabi Pharmaceuticals, 6th of October, Giza, Egypt, bought from a local pharmacy in Egypt. Hisatrup® tablets each contain 10.00 mg RUP (batch no. M 1044018) produced by Mash premiere, fifth settlement, New Cairo, Egypt, bought from a local pharmacy in Egypt. Laboratory made tablets of RUP and MKT in their commercial ratio 1 : 1 w/w were prepared by mixing these components per one tablet: 10.00 mg of RUP, 10.00 mg of MKT with 15.00 mg lactose, 20.00 mg talc powder, 15.00 mg of maize starch and 10.00 mg magnesium stearate. Methanol, acetonitrile, ethanol and n-propanol were of Sigma-Aldrich products (Germany) HPLC grade, while acetone was from EL-Nasr pharmaceutical chemical co. (ADWIC, Cairo, Egypt) produced at analytical grade. Surfactants like 94% sodium dodecyl sulfate (SDS), carboxy methyl cellulose (CMC), tween 80, cetrimide and β-cyclodextrin (β-CD) were purchased from EL-Nasr pharmaceutical chemical co. (ADWIC, Cairo, Egypt). They were prepared as 0.1% aqueous solutions. Other reagents were also used, such as anhydrous citric acid, disodium hydrogen phosphate, boric acid, sodium hydroxide, sulfuric acid, acetic acid and sodium acetate, which were also purchased from EL-Nasr pharmaceutical chemical co. (ADWIC, Cairo, Egypt). Double-distilled water was also used throughout the whole procedure for both methods. Mcllvaine's buffer covered pH ranging from 2.2 to 6.5 was used by mixing suitable volumes from 0.1 M anhydrous citric acid and 0.20 M disodium hydrogen phosphate. Borate buffer covered pH ranging from 8.5 to 10 was prepared by adding suitable volumes of 0.2 M boric acid and 0.2 M sodium hydroxide; 0.2 M acetate buffer covered pH ranging from 3.50 to 5.50. The buffer was made by mixing appropriate volumes of acetic acid (96%) and sodium acetate trihydrate.
2.3. Preparation of standard solution
Stock standard solutions of RUP and MKT were prepared by dissolving 10.0 mg in a 100 ml volumetric flask using methanol. Appropriate dilutions were then carried out with methanol to obtain solutions containing 20.0 µg ml−1. RUP was stable for 3 days without alteration, while MKT must be freshly prepared as it was stable for 2 h. Both drugs were protected from light by covering them with aluminium foil, particularly MKT as it is photosensitive.
2.4. Procedures
2.4.1. Methods for calibration graphs
2.4.1.1. Method I
Aliquots of RUP standard solutions were transferred into a series of 10 ml volumetric flasks, 1 ml of 0.1 M sulfuric acid and 0.80 ml of 0.10% w/v SDS were added and completed to volume with double-distilled water so that the final concentration of RUP is in the linear range (0.20–2.00 µg ml−1). The fluorescence intensity was recorded at 277/455 nm. A blank experiment was performed, and the relative fluorescence intensity (RFI) was graphed against the corresponding drug concentrations in µg ml−1. The regression equation was then derived.
2.4.1.2. Method II
In a set of 10 ml volumetric flasks, aliquots of RUP or MKT standard solutions covering the studied linear range for each drug (0.10–4.00 µg ml−1 for RUP and 0.20–1.60 µg ml−1 for MKT) were transferred. One millilitre of Mcllvaine's buffer pH 2.60 was added and diluted to the mark with double-distilled water. Synchronous spectra of the solutions were recorded at Δλ = 140 nm and then the first derivative synchronous fluorescence spectra of RUP and MKT were derived using Cary Eclipse software. The peak amplitudes of the first derivative spectra (1D) were recorded at 261 nm for RUP and at 371 nm for MKT. The peak amplitude of the first derivative spectra (1D) was then plotted against the drug concentration in µg ml−1 to construct the calibration graph and the corresponding regression equations.
2.4.2. Analysis of rupatadine/montelukast synthetic mixtures
Synthetic mixtures of RUP and MKT in the concentration range mentioned in table 1 were prepared from their standard stock solutions in the pharmaceutical ratio 1 : 1. The mixtures were treated as under §2.4.1. The peak amplitudes of the first derivative synchronous spectra (Δ1D) and the relative accuracy was calculated concurrently for each drug in the same ratios.
Table 1.
Analytical performance data for the determination of the studied drugs by the proposed methods.
| parameter | method (I) | method (II) |
|
|---|---|---|---|
| drug | RUP | RUP | MKT |
| wavelength (nm) | 277 nm/455 nm | 261 nm | 371 nm |
| linearity range (µg ml−1) | 0.20–2.00 | 0.10–4.00 | 0.20–1.60 |
| intercept (a) | 29.14 | −0.18 | −0.78 |
| slope (b) | 219.54 | 7.44 | 19.38 |
| correlation coefficient (r) | 0.9999 | 0.9999 | 0.9999 |
| S.D. of residuals (Sy/x) | 1.38 | 0.036 | 0.05 |
| S.D. of intercept (Sa) | 0.79 | 0.01 | 0.03 |
| S.D. of slope (Sb) | 0.88 | 0.01 | 0.04 |
| percentage relative standard deviation, % RSD | 0.574 | 0.99 | 0.82 |
| percentage relative error, % Error | 0.235 | 0.40 | 0.31 |
| limits of detection, LOD (ng ml−1) | 59.00 | 31.00 | 66.00 |
| limits of quantitation, LOQ (ng ml−1) | 179.00 | 94.00 | 200.00 |
2.4.3. Analysis of pharmaceutical preparations
2.4.3.1. Single tablets
The contents of either ten Hisatrup® tablets or Singulair® tablets were triturated well individually. A 10.0 mg equivalent amount of powder was weighed then added to 100 ml volumetric flasks completed with methanol. Sonication was applied for 30 min and then samples were filtered. Tablet extracts were diluted as appropriate to reach the working range. Then, the general procedure, designated for calibration graphs, was followed. The contents of tablets were computed from the regression equations.
2.4.3.2. Co-formulated tablets
Ten tablets containing RUP and MKT in pharmaceutical ratio 1 : 1 was mixed and a quantity of the powdered tablets equivalent to 10 mg of each drug was moved to a 100 ml volumetric flask. About 80 ml methanol was added and sonicated for 30 min, completed to the mark with the same solvent and filtered. The procedure explained previously was followed. The regression equations corresponding to each of the two drugs were used to determine the content of tablets.
2.4.4. Comparison method
The comparison method used to evaluate the obtained results is based on RP-HPLC. The chromatographic separation was achieved on HibarR 250–4, C-18 columns (250 × 4.6 mm, 5 um) using a mobile phase consisting of methanol : water (90 : 10 v/v) with 0.1% triethylamine (pH 3.41 adjusted with ortho phosphoric acid) at a flow rate of 1 ml min−1 and a detection wavelength of 260 nm [27].
3. Results and discussion
RUP and MKT exhibit native fluorescence at 277/455 nm and 350/450 nm respectively, as presented in figure 2. The emission spectra of different concentrations of RUP in an aqueous acidic solution containing 0.1% w/v SDS at 455.00 nm are illustrated in figure 3.
Figure 2.
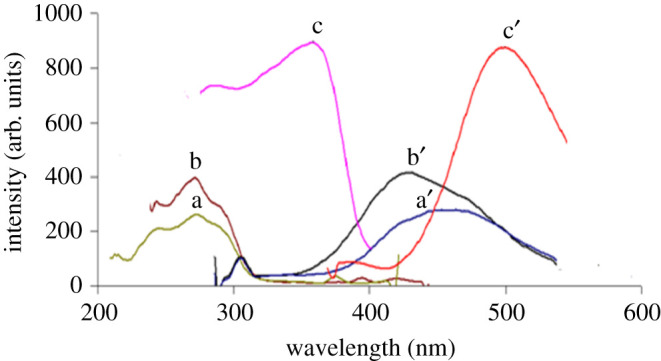
Excitation and the emission spectra of a,a′ 1.6 µg ml−1 RUP in aqueous solution containing Mcllvaine's buffer pH 2.6. b,b′ 1.6 µg ml−1 RUP in acidic aqueous solution containing 0.1% w/v SDS. c,c′ 1.6 µg ml−1 MKT in aqueous solution containing Mcllvaine's buffer pH 2.6.
Figure 3.
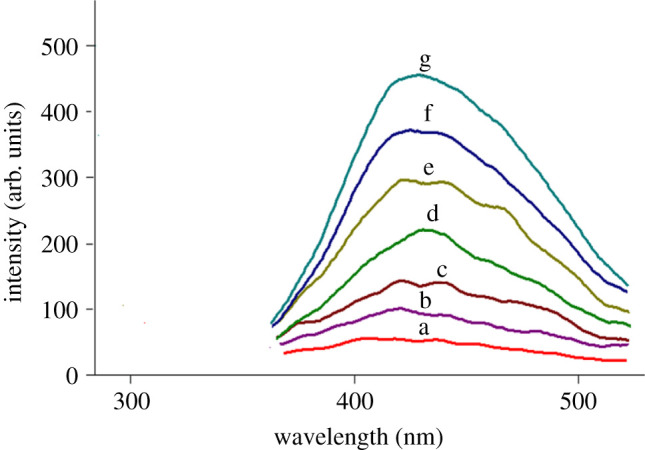
Emission spectra of different concentrations of RUP (a–g) (blank, 0.2, 0.4, 0.8, 1.2, 1.6, 2 µg ml−1) in acidic aqueous solution containing 0.1% w/v SDS at 455 nm after excitation at 277 nm.
A great overlap between the emission spectra of RUP and MKT is impossible to be separated by conventional spectrofluorometry (figure 2). So, the SFS technique is a good alternative for improving the selectivity. Different values of Δλ were examined to improve the resolution of this mixture. electronic supplementary material, figure S1 shows that RUP and MKT synchronous fluorescence spectra were overlapped. Hence, their simultaneous quantification and separation is challenging. So, the FDSFS was adopted to estimate the two drugs simultaneously. The fluorescence spectra of RUP and MKT were well resolved with sharp zero-crossing point for each drug (figure 4). RUP could be well calibrated using FDSFS at 261 nm in the presence of MKT (electronic supplementary material, figure S2-A). As shown in the figure, RUP could be quantitated at 261 nm, 266 nm and 275 nm, and the selected wavelength was 261 nm as the readings obtained at zero-crossing points at 266 nm and 275 nm are not reproducible. MKT could be well quantitated at 371 nm in the presence of RUP as shown in electronic supplementary material, figure S2-B.
Figure 4.
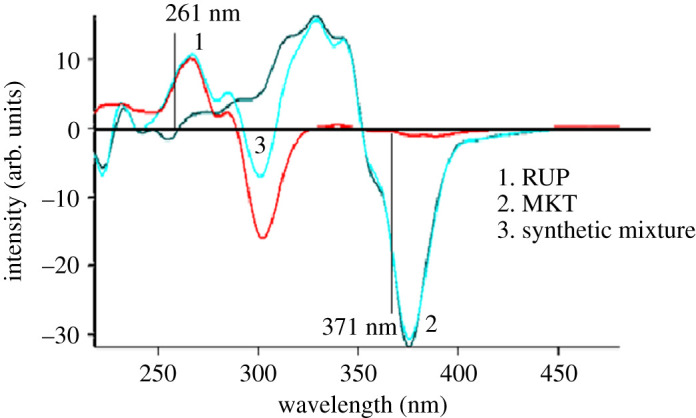
First derivative spectroscopy at Δλ = 140 nm for (1) 1.00 µg ml−1 RUP. (2) 1.00 µg ml−1 MKT. (3) Synthetic mixture of 1 µg ml−1 RUP and 1 µg ml−1 MKT.
3.1. Optimizing the experimental conditions
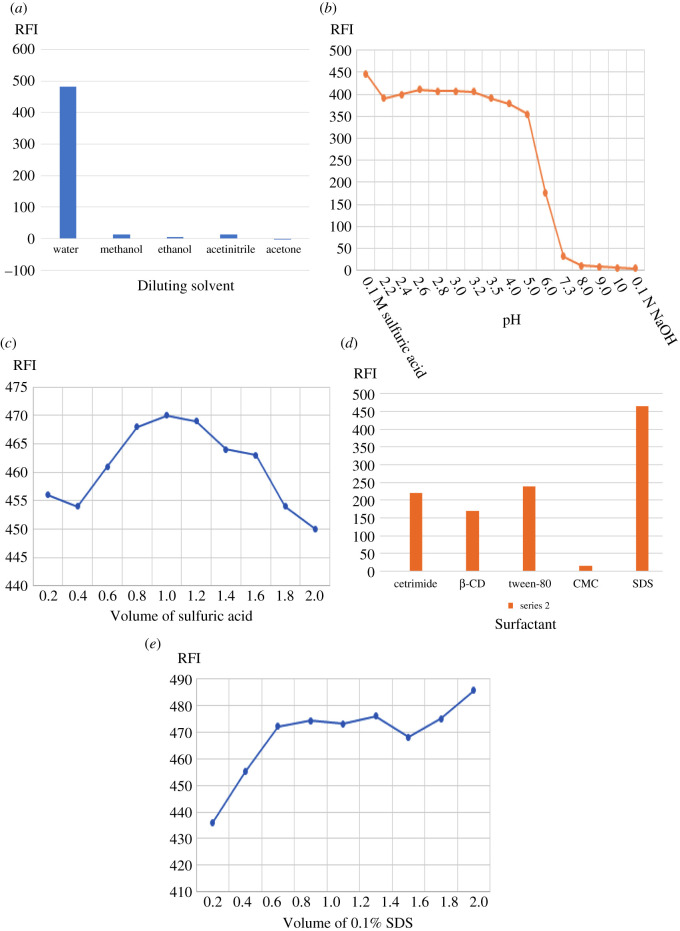
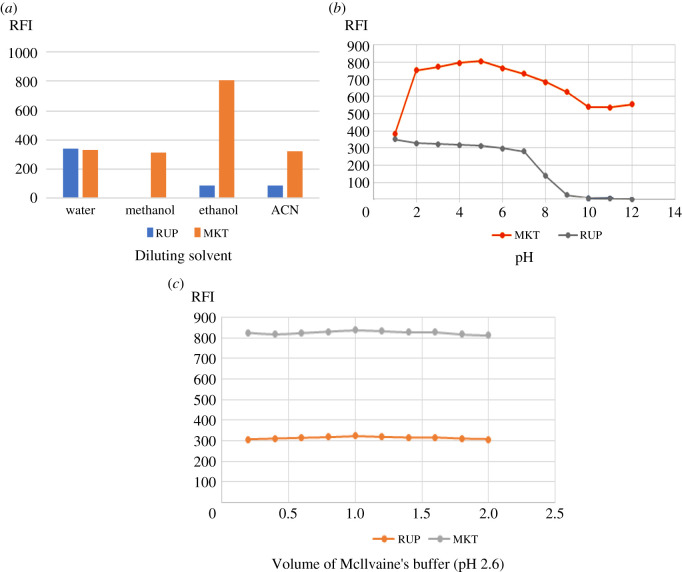
Factors that may affect the fluorescence intensities for both drugs were studied, by changing one parameter while fixing the others. The results of this study are illustrated in figures 5 and 6
Figure 5.
(a) The effect of diluting solvents on the native fluorescence of RUP (2 µg ml−1). (b) The effect of pH on the native fluorescence of RUP (2 µg ml−1). (c) The effect of different volumes of sulfuric acid on the native fluorescence of RUP (2 µg ml−1). (d) The effect of different surfactants on the native fluorescence of RUP (2 µg ml−1). (e) The effect of different volumes of 0.1% SDS on the native fluorescence of RUP (2 µg ml−1).
Figure 6.
(a) The effect of diluting solvents on the native fluorescence of both RUP (2 µg ml−1) and MKT (1.6 µg ml−1). (b) The effect of pH showing that pH 2.6 on the native fluorescence of both RUP (2 µg ml−1) and MKT (1.6 µg ml−1). (c) The effect of different volumes of Mcllvaine's buffer pH 2.6 on the native fluorescence of both RUP (2 µg ml−1) and MKT (1.6 µg ml−1).
Diluting solvents were examined for better sensitivity including double-distilled water, acetonitrile, ethanol, methanol, n-propanol and acetone but double-distilled water was the best diluting solvent in both methods (figures 5a and 6a). It was observed that RUP suffers from low sensitivity compared to MKT which in turn is characterized by high sensitivity. So, water was selected as the optimum diluting solvent to enhance the fluorescence of RUP. Different buffer systems were studied for both methods including 0.2 M acetate buffer covering a range from 3.5 to 5.5, borate buffer covering a range from 8.5 to 10.5, Mcllvaine's buffer covering a range from 2.2 to 6.5, 0.1 N sodium hydroxide for higher pH 12.0 and 0.1 M sulfuric acid pH 1.50 and their volumes were also studied.
For method I, it was clear that the native fluorescence of RUP was enhanced by decreasing pH, so 0.1 M sulfuric acid was chosen as it gave the highest intensity figure 5b. A volume of 1.00 ml of 0.10 M sulfuric acid was selected for this method as it greatly enhanced the intensity of RUP, figure 5c.
For method II, pH was found to have a great effect on the SFS intensity. The FI for MKT is constant from 2.20 up to 5.00 while for RUP, the intensity increases by decreasing pH. Unfortunately, 0.1 M sulfuric acid caused a marked decrease in the fluorescence intensity of MKT. Mcllvaine's buffer pH 2.6 was selected as it gave adequate sensitivity for both drugs, figure 6b. Borate buffer solutions have decreased the sensitivity for both drugs. The highest sensitivity for both drugs was achieved using 1 ml of Mcllvaine's buffer pH 2.6, figure 6c.
Different surfactants were investigated, including cetrimide, SDS, tween 80, CMC and β cyclodextrin.
For method I, SDS was selected as it greatly enhanced the fluorescence intensity of RUP, figure 5d. SDS concentrations such as 0.1–0.5–1.0% w/v were studied and 0.1% w/v SDS was selected for the reproducibility of the results. The volume of SDS was also studied from 0.2–2 ml and 0.80 ml was found to be the best as it gave the highest FI, figure 6e.
For method II, no surfactant was chosen for this study as SDS, β-CD and cetrimide significantly decreased the SFI of MKT. Although high FI was obtained for MKT with tween-80, it's not selected as it slightly improved the FI of RUP but markedly enhanced the SFI of MKT and was always over the range (greater than 1000), so it failed in analysing the studied mixture in the pharmaceutical ratio (1 : 1).
Varying the value of Δλ was performed. A direct relationship was found between choosing an optimum value of Δλ and the sensitivity and resolution in the synchronous fluorescence. To reach the optimal spectra shape, a wide scale of Δλ (20–200 nm) was examined. The best Δλ for RUP and MKT was 140 nm, giving well-defined spectra with minimal spectral interference. Smaller or larger values of Δ λ than the ideal one exhibited low SFI and poor separation.
MKT is photosensitive so its stock solution and calibration standards were protected from light by packaging in aluminium foiled flasks and must be freshly prepared as it is stable for 2 h in the refrigerator. RUP is found to be stable for 3 days in the refrigerator.
3.2. Validation of the developed methods
Both methods were tested to ensure the validation parameters such as linearity, range, selectivity, specificity, accuracy, precision, LOD and LOQ in accordance with ICH Q2(R1) recommendations [28].
Linear ranges were calculated from the calibration graphs depending on the RFI or 1D values with the drug concentrations. The ranges were 0.2–2 µg ml−1 for RUP in conventional fluorometric technique (method I), 0.1–4 µg ml−1 for RUP at 261 nm and 0.1–1.6 µg ml−1 for MKT at 377 nm in the FDSFS technique (method II). Table 1 indicates the results of regression analysis.
The analysis of the data resulted in the regression equations:
and
where RFI = relative fluorescence intensity, 1D = peak amplitude of first derivative spectra, X = drug concentrations in µg ml−1 for RUP and MKT, r = correlation coefficient.
As per ICH Q2(R1) [27], LOQ could be determined as the lowest concentration that would be measured within the accuracy and precision, while the LOD is the lowest concentration that could be detected. Their values were calculated mathematically as per ICH equations [27] and abridged in table 1.
Accuracy was checked by calculating the per cent recoveries as shown in table 2, by determining the two drugs in the pure and pharmaceutical dosage forms through the referred concentrations. By comparing the results of the studied methods with the comparison methods [27], accuracy was successfully guaranteed.
Table 2.
Application of the proposed methods for the determination of the studied drugs in raw materials. The tabulated t- and F-values at 2.77 and 19 at p = 0.05, respectively [29].
| method I |
method II |
comparison method [27] |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RUP at 455 nm |
RUP at 261 nm |
MKT at 371 nm |
RUP |
MKT |
|||||||||
| amount taken µg ml−1 | amount found µg ml−1 | %founda | amount taken µg ml−1 | amount found µg ml−1 | %founda | amount taken µg ml−1 | amount found µg ml−1 | %founda | amount taken µg ml−1 | % founda | amount taken µg ml−1 | % founda | |
| parameters | 0.20 | 0.200 | 100.00 | 0.10 | 0.099 | 99.00 | 0.20 | 0.197 | 98.50 | 20.00 | 100.68 | 20.00 | 101.11 |
| 0.40 | 0.404 | 100.75 | 0.20 | 0.196 | 98.00 | 0.40 | 0.402 | 100.50 | 30.00 | 99.18 | 30.00 | 98.51 | |
| 0.80 | 0.797 | 99.50 | 0.40 | 0.398 | 99.50 | 0.80 | 0.804 | 100.50 | 40.00 | 100.29 | 40.00 | 100.57 | |
| 1.20 | 1.202 | 100.00 | 1.00 | 1.009 | 100.90 | 1.20 | 1.203 | 100.25 | |||||
| 1.60 | 1.590 | 99.75 | 2.00 | 3.998 | 99.90 | 1.30 | 1.299 | 99.92 | |||||
| 2.00 | 2.008 | 100.20 | 4.00 | 3.999 | 99.98 | 1.40 | 1.386 | 99.00 | |||||
| 1.60 | 1.609 | 99.92 | |||||||||||
| mean | 100.09 | 99.55 | 99.89 | 100.05 | 100.06 | ||||||||
| ±S.D. | 0.58 | 0.98 | 0.82 | 0.78 | 1.32 | ||||||||
| % RSD | 0.57 | 0.99 | 0.82 | 0.78 | 1.32 | ||||||||
| % error | 0.24 | 0.40 | 0.31 | 0.45 | 0.79 | ||||||||
| N | 6.00 | 6.00 | 7.00 | ||||||||||
| N | 3.00 | ||||||||||||
| t-test | 0.05 | 0.08 | 0.19 | ||||||||||
| F-value | 1.53 | 1.16 | 1.41 | ||||||||||
amean of three determinations.
Intraday and intermediate precision and interday precision were calculated to achieve the repeatability of the proposed methods through calculating their standard deviation (s.d.), mean, relative standard deviation (RSD) and the percentage relative error (% Error) which is calculated by dividing the RSD over the square root of number of samples and then multiplying by 100. The intraday precision was done by getting three different concentrations and measuring them three successive times in the same day, while the interday precision was assessed by measuring these three concentrations in 3 subsequent days. Table 3 shows the values of assessing the precision.
Table 3.
Precision data for the estimation of studied drugs by the proposed methods. N.B. Each result is the average of three separate determinations.
| parameters drugs (µg ml−1) | method (I) |
method (II) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RUP |
RUP |
MKT |
||||||||
| 0.20 | 0.80 | 2.00 | 0.40 | 1.00 | 4.00 | 0.20 | 1.00 | 1.60 | ||
| intraday | mean | 100.10 | 100.00 | 100.00 | 99.8 | 99.99 | 100.00 | 99.60 | 100.00 | 100.00 |
| ± S.D. | 1.57 | 0.87 | 0.61 | 1.49 | 1.84 | 1.90 | 1.65 | 0.77 | 0.80 | |
| % RSD | 1.57 | 0.866 | 0.61 | 1.50 | 1.84 | 1.90 | 1.66 | 0.78 | 0.80 | |
| % error | 0.91 | 0.50 | 0.35 | 0.86 | 1.06 | 1.01 | 0.96 | 0.45 | 0.46 | |
| interday | mean | 99.33 | 100.00 | 99.99 | 99.63 | 100.00 | 100.00 | 100.20 | 99.56 | 101.03 |
| ± S.D. | 1.03 | 0.53 | 0.80 | 0.81 | 0.90 | 0.40 | 0.87 | 1.37 | 0.61 | |
| % RSD | 1.04 | 0.53 | 0.80 | 0.811 | 0.90 | 0.40 | 0.87 | 1.38 | 0.61 | |
| % error | 0.60 | 0.31 | 0.46 | 0.47 | 0.52 | 0.23 | 0.50 | 0.80 | 0.35 | |
Changing some experimental conditions in both methods was carried out to test robustness. Variations in pH, volume of buffer and surfactant were performed.
For method I, changing the volume of 0.1 M of sulfuric acid and SDS by ±0.20 ml did not affect the FI of RUP. For method II, changing of the volume of Mcllvaine's buffer by ±0.20 ml or the pH 2.6 by ±0.20 did not affect the measured 1D of both drugs, illustrated in table 4.
Table 4.
Robustness testing of the developed methods.
| parameters | mean ± s.d. | % RSD |
|---|---|---|
| method I | ||
| 1 - volume of 0.1 M sulfuric acid 1 ml ± 0.2 | 100.003 ± 0.69 | 0.69 |
| 2 - volume of 0.1% SDS 0.8 ml ± 0.2 | 100.00 ± 0.17 | 0.17 |
| method II | ||
| 1 - changing in Mcllvaine's buffer pH 2.6 ± 0.2 | 100.003 ± 0.66 | 0.66 |
| 2 - volume of Mcllvaine's buffer pH 2.6 (1 ± 0.2 ml) | 99.99 ± 0.35 | 0.35 |
The selectivity was examined by testing the presence of interference from the excipients in the tablets in both methods. No interference was found from lactose, talc or magnesium stearate. In addition, the two drugs could be quantified at the zero crossing of each other without interference.
3.3. Applications
3.3.1. Analysis of rupatadine/montelukast synthetic mixtures
The proposed synchronous method was used to analyse the two drugs in their 1 : 1 synthetic mixture. Electronic supplementary material, table S1 showed acceptable percentage recoveries for both drugs.
3.3.2. Analysis of rupatadine/montelukast combined tablets
The studied drugs in single form or in combined dosage forms were analysed by the two methods. The results were compared with other comparison methods [27] and no significant difference was observed as revealed from student's t-test and variance ratio F-test [29]. Tables 5 and 6 indicated the data of analysis of different formulations.
Table 5.
Application of the proposed methods to determine RUP and MKT in prepared combined tablets. The tabulated t- and F- values at 2.77 and 19 at p = 0.05, respectively [29].
| parameter | proposed method |
comparison method [27] |
||||
|---|---|---|---|---|---|---|
| amount taken (µg ml−1) | amount found (µg ml−1) | percentage founda | amount taken (µg ml−1) | amount found (µg ml−1) | percentage founda | |
| RUP | 0.80 | 0.812 | 101.50 | 20.00 | 20.135 | 100.68 |
| 1.00 | 0.984 | 98.40 | 30.00 | 29.754 | 99.18 | |
| 1.40 | 1.405 | 100.36 | 40.00 | 40.117 | 100.29 | |
| mean | 100.09 | 100.05 | ||||
| ± S.D. | 1.57 | 0.78 | ||||
| % RSD | 1.57 | 0.78 | ||||
| % error | 0.91 | 0.45 | ||||
| t-test | 0.03 | |||||
| F-value | 4.05 | |||||
| MKT | 0.80 | 0.802 | 101.00 | 20.00 | 20.221 | 101.11 |
| 1.00 | 0.997 | 99.30 | 30.00 | 29.552 | 98.51 | |
| 1.40 | 1.401 | 100.14 | 40.00 | 40.226 | 100.57 | |
| mean | 100.14 | 100.06 | ||||
| ± S.D. | 0.85 | 1.37 | ||||
| % RSD | 0.85 | 1.37 | ||||
| % error | 0.49 | 0.79 | ||||
| t-test | 0.08 | |||||
| F-value | 2.60 | |||||
aN.B. mean of three determinations.
Table 6.
Determination of RUP and MKT in pharmaceutical preparations using the proposed methods. The tabulated t and F-values at 2.77 and 19 at p = 0.05, respectively [29].
| parameter | proposed method |
comparison method [27] |
||||
|---|---|---|---|---|---|---|
| amount taken (µg ml−1) | amount found (µg ml−1) | percentage founda | amount taken (µg ml−1) | amount found (µg ml−1) | percentage founda | |
| method (I) | ||||||
| Hisatrup® tablets RUP (10.00 mg) | 0.80 | 0.804 | 100.50 | 20.00 | 20.135 | 100.68 |
| 1.00 | 0.996 | 99.60 | 30.00 | 29.754 | 99.18 | |
| 1.40 | 1.405 | 100.14 | 40.00 | 40.117 | 100.29 | |
| mean | 100.01 | 100.05 | ||||
| ± S.D. | 0.56 | 0.78 | ||||
| % RSD | 0.56 | 0.78 | ||||
| % error | 0.32 | 0.45 | ||||
| t- test | 0.05 | |||||
| F-value | 2.95 | |||||
| method (II) | ||||||
| Hisatrup® tablets RUP (10.00 mg) | 0.80 | 0.804 | 100.5 | 20.00 | 20.135 | 100.68 |
| 1.00 | 0.994 | 99.40 | 30.00 | 29.754 | 99.18 | |
| 1.40 | 1.402 | 100.14 | 40.00 | 40.117 | 100.29 | |
| mean | 100 | 100.05 | ||||
| ± S.D. | 0.56 | 0.78 | ||||
| % RSD | 0.56 | 0.78 | ||||
| % error | 0.32 | 0.45 | ||||
| t-test | 0.06 | |||||
| F-value | 1.92 | |||||
| method (II) | ||||||
| Singulair® tablets MKT (10.00 mg) | 0.80 | 0.804 | 100.50 | 20.00 | 20.221 | 101.11 |
| 1.00 | 0.994 | 99.40 | 30.00 | 29.552 | 98.51 | |
| 1.40 | 1.402 | 100.14 | 40.00 | 40.226 | 100.57 | |
| mean | 100.01 | 100.06 | ||||
| ± S.D. | 0.56 | 1.37 | ||||
| % RSD | 0.56 | 1.37 | ||||
| % error | 0.32 | 0.79 | ||||
| t-test | 0.05 | |||||
| F-value | 5.98 | |||||
aN.B. mean of three determinations.
3.3.3. Assessment of the Green property
Greening an analytical procedure is very challenging in analysis due to the large use of organic solvents. Decreasing the use of these solvents or replacing them with green ones is a method for greenness for any analytical method. Three ways were used to evaluate the greenness of these methods. The green analytical procedure index (GAPI) is a recent method for measuring the greenness, first introduced by Plotka–Wasylka [30]. It follows all the stages of the method starting from the sample collection to the waste treatment. It offers a thorough assessment of each step in the analytical method by including 15 items to be examined using three levels of colour: green, yellow or red. The green profiles for the proposed spectrofluorometric methods using the GAPI tool are presented in table 7 and electronic supplementary material, table S2. MKT must be kept in aluminium foil and in the refrigerator under normal conditions, so the fourth parameter was yellow shaded in method II. The 5th parameter is shaded yellow as there was a bit sample preparation as filtration in both methods. The two pictograms (10,11) related to the reagents and solvents were yellow shaded; due to the use of some hazardous chemicals, such as methanol, sulfuric acid, SDS and Mcllvaine's buffer pH 2.6 even their usage by small volume therefore GAPI evaluation may oppress some methods. The amount of waste was between 1 to 10 ml shaded yellow, with no treatment of the waste indicated by red shading, covering field no. 15 in both methods.
Table 7.
Results for the evaluation of the developed conventional method by the three green chemistry tools (method I &II).
| 1 - green analytical procedure index (GAPI) | |||
| method I | method II | ||
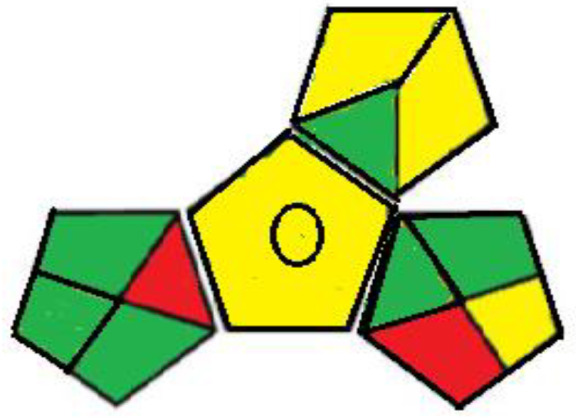 |
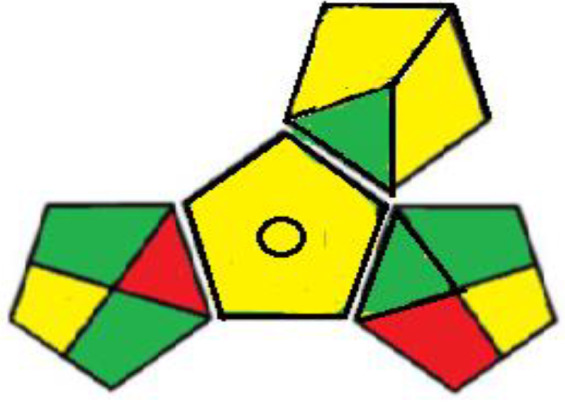 |
||
| 2 - analytical Eco scale score | |||
| A - method I | |||
| reagents/instruments | |||
| reagent, volume (ml) | no. pictograms | word sign [31] | penalty points |
| methanol, 1 ml | 3 | danger | 6 |
| 0.1% SDS, 0.8 ml | 1 | warning | 1 |
| 0.1 M sulfuric acid, 1 ml | 1 | danger | 2 |
| water | 0 | ||
| item | penalty points | ||
| spectrofluorometer | <0.1 k w h per sample | 0 | |
| waste | no treatment | 3 | |
| occupational hazards | analytical process hermitization | 0 | |
| total penalty points | ⅀ 12 | ||
| analytical eco scale score | 100 – 12 = 88 | ||
| B - method II | |||
| reagents/instruments | |||
| reagent, volume (ml) | no. Pictograms | word Sign [31] | penalty points |
| methanol, 1 ml | 3 | danger | 6 |
| Mcllvaine's buffer pH 2.6, 1 ml | 2 | warning | 2 |
| water | 0 | ||
| item | penalty points | ||
| spectrofluorometer | <0.1 k w h per sample | 0 | |
| waste | no treatment | 3 | |
| occupational hazards | analytical process hermitization | 0 | |
| total penalty points | ⅀ 11 | ||
| analytical eco scale score | 100 – 11 = 89 | ||
| 3 - NEMI pictogram | |||
 |
 |
||
Analytical eco scale is another quantitative tool for assessment that is published by Van-Aken et al. [31]. Ranking the greenness of the method depends on the penalty point score. The score of penalty points is recorded according to the signal word and no. of pictograms presented in ‘The Globally Harmonized System of Classification and Labelling of Chemicals’ (GHS) and the safety label data sheet for each chemical or solvent and then subtracted from 100. Excellent green methods scored 75 or more but acceptable green methods scored 50 or more as shown in table 7. The conventional method scored 88 while the synchronous method scored 89. Both methods are excellent regarding the analytical eco scale criteria. The penalty points were calculated from the national fire protection association [32].
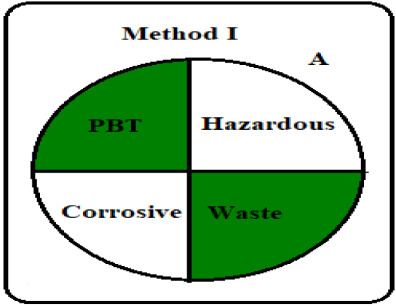
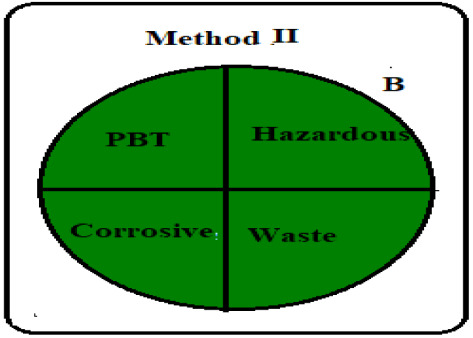
The last qualitative tool is the old one called the national environmental method index (NEMI) [33]. It describes the greenness through a pictogram divided into four quadrants as in table 7 in which the first quadrant shows reagents that are not persistent, bio-accumulative or toxic. The second one (Hazardous) includes reagents that are not hazard; the third one, called corrosive, includes pH less than 2 and more than 12 while the last quadrant, called waste, includes overall waste less than 50 gm or 50 ml. The conventional developed method I fulfills NEMI criteria as the first and fourth quadrants were green while in the second and third quadrants the used pH is 1.5 which deviated from the selected range due to the usage of the sulfuric acid which was considered corrosive and hazardardous, while the synchronous method successfully fulfills NEMI criteria as all quadrants are green. One quick look will indicate whether the method is green or not.
It is obvious that the developed methods are well matched with the three green analytical chemistry tools which shows that these methods are eco-friendly; moreover, they are simple, sensitive and rapid.
4. Conclusion
A green, simple and highly sensitive conventional fluorometric method is established to quantify RUP in pharmaceutical dosage forms. Moreover, a FDSFS is used as a simple, selective and green technique to determine RUP and MKT in pure form and in their pharmaceuticals. The two methods are validated according to pharmacopeial guidelines. Owing to the simplicity and sensitivity of the proposed methods, they can be an excellent alternative to other sophisticated techniques in quality control units.
Supplementary Material
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.kwh70rz43 [34].
Authors' contributions
R.G. carried out the laboratory work, participated in data analysis and participated in the design of the study; M.I.E. and M.M.T. drafted the manuscript, carried out the statistical analyses, conceived of the study and followed up the experimental work; F.I. coordinated the study participated in data analysis and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.BP. 2013. British pharmacopoeia, electronic version. London, UK: The stationary office. [Google Scholar]
- 2.USP. 2009. The United States Pharmacopeia 32 Electronic Version, US Pharmacopeial Convention, Rockville,The National Formulary 27, 2009.
- 3.Sweetman SC. 2011. Martindale: the complete drug reference, 37th edn. London, UK: Pharmaceutical Press, 576,7,11126. [Google Scholar]
- 4.Ibrahim FA, Elmansi H, El-Awady MI, El Abass SA. 2020. Investigation of micellar enhancement in simultaneous assay of rosuvastatin and amlodipine in their fixed-dose combined tablets. Microchem. J. 158, 105207. ( 10.1016/j.microc.2020.105207) [DOI] [Google Scholar]
- 5.Czerwinska K, Wyszomirska EL, Kublin EL, Malanowic E, Mazurek AP. 2016. Identification and determination of rupatadine and fexofenadine by densitometric method. Acta Pol. Pharm. 73, 1467-1474. [PubMed] [Google Scholar]
- 6.Rele RV, Tiwatane PP. 2014. UV spectrophotometric estimation of rupatadine fumarate by second order derivative method in bulk and pharmaceutical dosage form. Asian J. Res. Chem. 7, 859-862. [Google Scholar]
- 7.Patel PG, Vaghela VM, Rathi SG, Rajgor NB, Bhaskar VH. 2009. Derivative spectrophotometry method for simultaneous estimation of rupatadine and montelukast in their combined dosage form. J. Young Pharmacists 1, 354. ( 10.4103/0975-1483.59327) [DOI] [Google Scholar]
- 8.Wang HH, Ge P, Zhao W, Hang TJ, Li TA. 2009. RP-HPLC determination of rupatadine fumarate and its related substances. Chinese J. Pharmaceut. Analysis 29, 791-794. [Google Scholar]
- 9.Vekaria H, Limbasiya V, Patel P. 2013. Development and validation of RP-HPLC method for simultaneous estimation of montelukast sodium and fexofenadine hydrochloride in combined dosage form. J. Pharmacy Res. 6, 134-139. ( 10.1016/j.jopr.2012.11.028) [DOI] [Google Scholar]
- 10.Redasani VK, Kothawade AR, Surana SJ. 2014. Stability indicating RP-HPLC method for simultaneous estimation of rupatadine fumarate and montelukast sodium in bulk and tablet dosage form. J. Anal. Chem. 69, 384-389. ( 10.1134/S1061934814040121) [DOI] [Google Scholar]
- 11.Kanthiaha S, Kannappana V. 2016. Development, optimization and validation of new liquid chromatographic method for the simultaneous determination H1 receptor blockers in bulk and their pharmaceutical formulations by applying D-optimal mixture design methodology. Der Pharmacia Lettre 8, 94-105. [Google Scholar]
- 12.Katselou M, Athanaselis S, Nikolaou P, Qammaz S, Dona A, Spiliopoulou C, Papoutsis I. 2018. A fully validated method for the simultaneous determination of 11 antihistamines in breast milk by gas chromatography–mass spectrometry. Biomed. Chromatogr. 32, e4260. ( 10.1002/bmc.4260) [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Liu H, Jing WU, Fang X, Song R. 2016. Determination of residual solvents in rupatadine fumarate by headspace gas chromatography. J. China Pharmacist 19, 1024-1026. [Google Scholar]
- 14.Devnani H, Singh P, Saxena S, Satsangee SP. 2014. Voltammetric determination of rupatadine at graphene modified glassy carbon electrode. J. Int. J. Pharmaceut. Sci. Res. 5, 4792. [Google Scholar]
- 15.Kumar N, Sangeetha D, Sunil Reddy P. 2013. Development and validation of a stability indicating RP-UPLC method for simultaneous determination of rupatadine and montelukast in pharmaceutical formulation. Curr. Pharmaceut. Analysis 9, 61-68. ( 10.2174/157341213804806133) [DOI] [Google Scholar]
- 16.Sun C, Li Q, Pan L, Liu B, Gu P, Zhang J, Ding L, Wu C. 2015. Development of a highly sensitive LC–MS/MS method for simultaneous determination of rupatadine and its two active metabolites in human plasma: application to a clinical pharmacokinetic study. J. Pharmaceut. Biomed. Analysis 111, 163-168. ( 10.1016/j.jpba.2015.03.025) [DOI] [PubMed] [Google Scholar]
- 17.Dalmora SL, dos Santos Butzge C, Machado FT, Walter ME, de Ávila Dalmora ME, Souto RB. 2012. Stability-indicating capillary zone electrophoresis method for the assessment of recombinant human granulocyte-macrophage colony-stimulating factor and its correlation with reversed-phase liquid chromatography method and bioassay. J. Talanta 94, 1-7. ( 10.1016/j.talanta.2012.03.015) [DOI] [PubMed] [Google Scholar]
- 18.Patil MT, Ankalgi AD. 2013. Deep simultaneous estimation of montelukast sodium and rupatadine fumarate in tablet formulation by HPTLC method. J. Curr. Pharma Res. 3, 791. ( 10.33786/JCPR.2013.v03i02.003) [DOI] [Google Scholar]
- 19.Rele RV, Mahimkar SA, Sawant SA. 2009. A validated simple titrimetric method for the quantitative determination of rupatadine as rupatadine fumarate from pharmaceutical dosages. Anal. Chem. Indian J. 8, 561-564. [Google Scholar]
- 20.Almahri A, Abdel-Lateef MA, Samir E, Derayea SM, El Hamd MA. 2021. Resonance Rayleigh scattering and spectrofluorometric approaches for the selective determination of rupatadine using erythrosin B as a probe: application to content uniformity test. J. Luminescence 36, 651-677. ( 10.1002/bio.3983) [DOI] [PubMed] [Google Scholar]
- 21.Rana NS, Rajesh K, Patel Nikita N, Patel PR, Limbachiya U, Pasha TY. 2013. Development and validation of rp-hplc method for the simultaneous estimation of montelukast sodium and ebastine in tablet dosage form. Indian J. Pharmaceut. Sci. 75, 599. [PMC free article] [PubMed] [Google Scholar]
- 22.Rashmitha N, Raj T, Srinivas CH, Srinivas N, Ray UK, Sharma HK, Mukkanti K. 2010. A Validated RP-HPLC method for the determination of impurities in montelukast sodium. J. Chem. 7, 555-563. ( 10.1155/2010/156593) [DOI] [Google Scholar]
- 23.Arayne MS, Sultana N, Hussain F. 2009. Spectrophotometric method for quantitative determination of montelukast in bulk, pharmaceutical formulations and human serum. J. Anal. Chem. 64, 690-695. ( 10.1134/S1061934809070065) [DOI] [Google Scholar]
- 24.Alsarra I, Khalil N, Sultan M, Al-Ashban R, Belal F. 2005. Spectrofluorometric determination of montelukast in dosage forms and spiked human plasma. Die Pharmazie-An Int. J. Pharmaceut. Sci. 60, 823-826. ( 10.1016/j.farmac.2005.04.004) [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim FA, El-Enany N, El-Shaheny RN, Mikhail IE. 2015. Simultaneous determination of desloratadine and montelukast sodium using second-derivative synchronous fluorescence spectrometry enhanced by an organized medium with applications to tablets and human plasma. Luminescence 30, 485-494. ( 10.1002/bio.2764) [DOI] [PubMed] [Google Scholar]
- 26.Alghamdi AF. 2014. Voltammetric analysis of montelukast sodium in commercial tablet and biological samples using the hanging mercury drop electrode. Portugaliae Electrochim. Acta 32, 51-64. ( 10.4152/pea.201401051) [DOI] [Google Scholar]
- 27.Jani A, Jasoliya J, Vansjalia D. 2014. Method development and validation of stability indicating RP-HPLC for simultaneous estimation of rupatadine fumarate and montelukast sodium in combined tablet dosage form. J. Int. Pharm. Pharm. Sci. 6, 229-233. [Google Scholar]
- 28.ICH Harmonized Tripartite Guideline. 2005. Validation of Analytical Procedures: Text and Methodology, Q2(R1), Current Step 4 Version,Parent Guidelines on Methodology. www.ich.org/page/quality-guidelines. Acessed 01-4-2021 Dated November 6 1996, Incorporated in November 2005. [PubMed]
- 29.Miller JC, Miller JN. 2005. Pearson education limited, harlow, england, statistics and chemometrics for analytical chemistry, 5th edn. Harlow, UK: Pearson Education Limited. [Google Scholar]
- 30.Płotka-Wasylka J. 2018. A new tool for the evaluation of the analytical procedure: green analytical procedure index. J. Talanta 181, 204-209. ( 10.1016/j.talanta.2018.01.013) [DOI] [PubMed] [Google Scholar]
- 31.Van Aken K, Strekowski L, Patiny L. 2006. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. J. Beilstein J. Org. Chem. 2, 3. ( 10.1186/1860-5397-2-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NFPA1852. 2019. standard on selection, Care, and Maintanence of open-circuit self contained Breathing Apparatus (SCBA).
- 33.Marek T. 2016. Metrics for green analytical chemistry. J. Analytical Methods 8, 2993-2999. ( 10.1039/C6AY00478D) [DOI] [Google Scholar]
- 34.El-Awady M, Ghonim R, Tolba M, Ibrahim F.. 2021. Green quantitative spectrofluorometric analysis of rupatadine and montelukast at nanogram scale using direct and synchronous techniques. Dryad, Dataset. ( 10.5061/dryad.kwh70rz43) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- El-Awady M, Ghonim R, Tolba M, Ibrahim F.. 2021. Green quantitative spectrofluorometric analysis of rupatadine and montelukast at nanogram scale using direct and synchronous techniques. Dryad, Dataset. ( 10.5061/dryad.kwh70rz43) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.kwh70rz43 [34].